Abstract
Objectives
Previous studies have shown alterations in bone marrow cell proliferation in malnourished rats, during lactation. The objective of this study was to determine in vivo effects of moderate and severe malnutrition on spleen cell proliferation in 21‐day‐old rat pups.
Materials and methods
Spleen cell proliferation was determined following administration of bromodeoxyuridine (BrdUrd) over a time course of 2, 4, 6 and 8 h. Incorporation of BrdUrd was detected using FITC‐conjugated anti‐BrdUrd monoclonal antibodies and total DNA content was detected and evaluated using propidium iodide using flow cytometry.
Results
Proportions of cells in S and G2/M were reduced in the rats with moderate (MN2nd) and severe (MN3rd) malnutrition. BrdUrd incorporation was lower in both groups of malnourished rat. In cells of MN2nd individuals, length of G1 became shorter, while length of S‐phase increased. In contrast, fraction of cells in proliferation was significantly lower in both groups of malnourished rat, with MN3rd group having lowest percentage of cell population growth. In this study, severe malnutrition did not significantly affect duration of phases of the cell cycle, although fractions of proliferating cells were dramatically reduced.
Conclusion
Moderate malnutrition increased time of cells in DNA synthesis and time of total cell cycle and severe malnutrition reduced growth fraction of spleen cells in malnourished rats during lactation.
Introduction
Malnutrition has a major negative impact on immune function of children, rendering them more vulnerable to diseases that increase risk of death 1, 2, 3. Numbers of authors have suggested that malnutrition is the primary cause of immunodeficiency worldwide 4, 5, 6.
In mammals, the spleen is the largest secondary lymphoid organ. Antigens enter the body and circulate to the spleen where lymphocytes are organized into structures that improve cell interactions and promote pathogen removal 7, 8. The key function of lymphoid organs is to provide a suitable microenvironment for sequential interaction of different sub‐populations of lymphocytes and non‐lymphoid cells, to achieve an adaptive immunological response 9, 10.
The immune system is compromised in malnourished children and animal models of experimental malnutrition. Atrophy of lymphoid organs has been described in animal models, and the thymus in particular has been termed to be the barometer for malnutrition, by Prentice 11. This phenomenon is due primarily to reduction in lymphoid compartments 12, 13. Alterations in other lymphoid tissues, such as the spleen and lymphatic ganglia, have also been observed 14.
In rats, reduction in absolute and relative weight of the spleen and thymus after malnutrition has been induced in the prenatal phase and during lactation 15. In addition to alterations in various cell subpopulations, thymic atrophy has been observed in adults 16. Ortiz et al. 17 demonstrated that spontaneous apoptosis is increased in the thymus of malnourished rats during lactation and the same group demonstrated that double‐negative thymocytes (CD4−, CD8−) are the most susceptible populations, although increased apoptosis was also observed in CD4+ and CD8+ subpopulations 18.
In the spleen, CD3+ and CD4+ lymphocyte subpopulations were shown to be reduced in malnourished rats 19 and in the same study, moderate and severe malnutrition were shown to reduce proportions of CD71+ and CD25+ cells, in cultures stimulated with phytohemagglutinin (PHA). Prolongation of the cell cycle was observed in bone marrow cells of malnourished rats during lactation 20, and reduction in proportions of proliferative cells in vivo and in vitro have also been shown 21, 22. DNA damage has been widely studied in humans and in animal models of malnutrition, and many studies have shown relationships between malnutrition and genetic damage 23, 24.
The objective of this study was to determine in vivo effects of moderate and severe malnutrition on proliferation of spleen cells in 21‐day‐old rat pups.
Materials and methods
Experimental malnutrition during lactation
Wistar rats were obtained from a closed breeding colony from the División de Ciencias Biológicas y de la Salud at the Universidad Autónoma Metropolitana, Iztapalapa (UAM‐I) in Mexico City, Mexico. They were maintained under 12‐h controlled light–dark cycles at temperatures of 22–25 °C with 45% relative humidity. Nursing mothers used in the study had given birth once prior to the litter under study, and here were fed ad libitum with a balanced rodent diet (Teklad Global 2018S; Harlan, Madison, WI, USA). Experiments were performed according to guidelines of the Universidad Autónoma Metropolitana (UAM), the Official Mexican Guidelines (Norma Oficial Mexicana NOM‐062‐ZOO‐1999) and the National Institutes of Health (NIH).
Experimental malnutrition was induced by food competition 25. One‐day‐old rats from different litters were randomly assigned to either the experimental malnourished group or the control group. In the control group, pups were fed by nursing dams that suckled six to eight pups. In the malnourished group, nursing dams fed 16 pups. Pups were weighed from the first day until weaning (from 1 to 21 days after birth).
Well‐nourished rats were selected from different control litters, and malnourished rats were selected from different experimental litters. In the experimental group, degree of malnutrition was established according to the classification used for children described by Gómez et al. 22. Malnutrition was categorized by weight deficit compared to weight of age‐matched control rats (WN): moderate or second degree (MN2nd) when weight deficit was 25–40%; and severe or third degree (MN3rd) when weight deficit was greater than 40%. Rat pups had other physical signs of malnutrition, such as sparse hair, bone fragility and low activity levels.
Biochemical measurements of malnutrition
Total serum proteins were quantified in 16 MN3rd rats, in 11 MN2nd rats and in 16 WN control rats, using the method previously described by Lowry et al. 26. Serum cholesterol and triglyceride levels were measured using Reflotron Plus System (Roche Diagnostics, Basel, Switzerland).
BrdUrd labelling
Rats were injected 1 mg/g body weight, i.p., BrdUrd (5‐bromo‐2′‐deoxyuridine; Sigma, St Louis, MO, USA). At 2, 4, 6 and 8 h after injection, animals were sacrificed by cervical dislocation and spleens were dissected; cells were obtained by sieving tissues through a nylon screen. Cells were resuspended in phosphate buffered saline solution (PBS, pH = 7.4; Microlab, Mexico City, Mexico.) and were fixed in cold 70% ethanol (Gly 5 mm, pH = 2; Sigma) and stored at −20 °C for 24 h; they were then washed in PBS. BrdUrd labelling was detected using 5‐bromo‐2′‐deoxyuridine Labelling and Detection Kit I (Roche, Mannheim, Germany). Cells were incubated in an anti‐BrdUrd monoclonal antibody and nuclease, for 60 min at 37 °C and 30 min with secondary FITC‐labelled goat anti‐mouse monoclonal antibody. Propidium iodide (Sigma, 5 μg/ml, final concentration) was added followed by incubation for 15 min. Cells were fixed in paraformaldehyde (1% in PBS). A total of 20 000 cells were analysed by flow cytometry (FACSscan model; Becton Dickinson, San Jose, CA, USA). Contour graphs were utilized to establish percentages of BrdUrd‐positive cells (Fig. 1). CellQuest Software (V 3.0.1; Becton Dickinson) was used for data acquisition and analysis.
Figure 1.
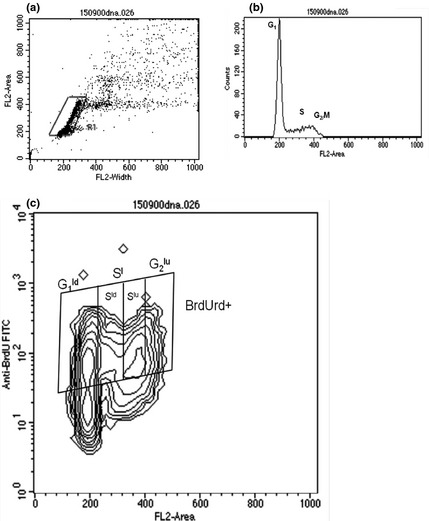
(a) Dot plot of IP‐W versus IP‐A parameters (DNA content) to select population of spleen cells, discriminating doublets. (b) Histogram of DNA content versus cell frequency where peaks correspond to cells in G1, S and G2/M phases. (c) Contour plot indicating cells in different phases of the cell cycle and cells that incorporated BrdUrd during the process of DNA synthesis. Figure is of cells from WN rat pups.
Percentages of cells that incorporated BrdUrd were calculated from the total number of analysed cells. Time in phases G1 (T G1), S (T S), G2 (T G2) and total cycle time (T C) were obtained from incorporation data after 8 h based on the method proposed by Eidukevicius et al. 27. Total number of cells labelled in S‐phase (S l) during a pulse can be estimated at any time interval shorter than duration of the cell cycle as follows:
Total number of dividing cells labelled in G1 (G 1 ld), in S‐phase (S ld); non‐divided cells in S‐phase (S lu) and non‐divided cells in G2 were analysed using contour graphs and histograms of positive cells. Equations used to establish timing of each phase of the cycle, T C , growth fraction (GF), labelling index of proliferating cells (LIP) and labelling index (LI) are shown in Table 1.
Table 1.
Equations to establish the timing of each phase of the cycle
| Cell cycle parameter | Equations |
|---|---|
| Sl | S lu + G 2 lu + ½G 1 ld + ½S ld |
| TS | (S l/(G 2 lu + ½(G 1 ld + S ld)) × t |
| TG2 | (½(G 1 ld + S ld)/S l) × t |
| TG1 | t × T G2 − (½S ld/S l) × T S |
| TC | T G1 + T S + T G2 |
| GF (%) | (LI/LIP) × 100 |
| LIP | (T S/T C) × T S |
| LI (%) | (S l/n t − ½(G 1 ld + S ld) − (G 2 − G 2 lu)) × 100 |
G 1 ld, labelled divided G1 cells; G 2 lu, labelled undivided G2 cells; GF, growth fraction; LI, labelling index of the whole population; LIP, labelling index of proliferating cells; n t, total number of BrdUrd positive and negative cells per sample; S l, labelled S cells; S lu, labelled undivided S cells; t, time of BrdUrd incorporation; T, time of phase cycle.
Statistical analysis
Body weight and weight of spleens, numbers of cells, weight deficits and biochemical measurements are expressed as mean ± standard deviation. BrdUrd incorporation results are expressed as mean ± standard error of four rats by incorporation time. Statistics were calculated using BioEstat 5 software (Manuel Ayres, Universidade Federal do Pará, Pará, Brasil). All groups were compared using the non‐parametric Kruskal–Wallis and Dunnett's tests. Level of significance was set at P < 0.05; at least 15 000 cells/sample were analysed.
Results
Table 2 shows average weight of body and spleen and standard deviation of control rat pups and malnourished animals (MN) with second and third degree severities. 21 days after birth, observed weight deficits of MN2nd and MN3rd rats compared to WN group were 34.5% and 51.9%, respectively. Significantly lower cholesterol and triglyceride levels were observed in serum of both groups of malnourished rats (P < 0.05).
Table 2.
Body and spleen weights of well‐nourished and malnourished rat pups
| Variable | WN | MN2nd | MN3rd |
|---|---|---|---|
| Body weight (g) | 54.4 ± 2.3 | 36.2 ± 2.0 | 26.2 ± 3.3* |
| Body weight deficit | — | 34.5 ± 3.7 | 51.9 ± 6.0 |
| Spleen weight (mg) | 308.7 ± 71.9 | 154.7 ± 40.0 | 117.1 ± 43.4 |
| Spleen weight deficit (%) | — | 49.9 ± 13.0 | 61.7 ± 14.2 |
| Spleen weight (mg)/body weight (g) | 5.7 ± 1.4 | 4.3 ± 1.0* | 4.4 ± 1.4* |
| Cell number (×106)/spleen | 196.4 ± 49.4 | 113.7 ± 34.8* | 79.7 ± 39.3* , ** |
| Cell number/spleen deficit | — | 45.7 ± 22.4 | 62.9 ± 13.8 |
| Cell number (×106)/100 mg spleen | 73.5 ± 17.1 | 78.2 ± 28.8 | 68.1 ± 22.4 |
| Total serum proteins (mg/ml) | 57.1 ± 15.5 | 40.1 ± 2.2 | 27.9 ± 10.4* |
| Serum triglycerides (mg/dl) | 272.6 ± 22.5 | 165.4 ± 22.6 | 175.3 ± 20.0* |
| Serum cholesterol (mg/dl) | 129.0 ± 5.9 | 108.1 ± 3.0 | 115.0 ± 5.9* |
Values are the mean ± standard deviation. *Significant differences in the WN group, P < 0.05; **Significant differences MN2nd versus MN3rd, P < 0.05. WN, n = 16; MN2nd, n = 11; MN3rd, n = 16. The cholesterol serum concentration was <100 mg/dl in ten of the MN3rd rats.
Weights of spleens were lower by approximately 44% in MN2nd group and by 63% in MN3rd group. This observation was corroborated by the cell number analysis as cellularity in malnourished animals was 43% and 52% lower compared to the WN group. Ratios between spleen and body weights were clearly lower in both malnourished groups compared to the control group (P < 0.05 for both). However, statistically significant differences were not observed between numbers of cells and spleen‐to‐weight ratios. This result indicates that cell density was maintained in spleens of malnourished and well‐nourished rats, although sizes of spleens was less.
Percentages of cells in different phases of the cell cycle were analysed using DNA content as marker, based on fluorescence of propidium iodide (Fig. 1a and 1b). Figure 2 shows cell distribution in each phase of the cycle. In all phases, significant differences in numbers of cells were observed, between malnourished rats compared to WN pups (Fig. 2) (P < 0.05). BrdUrd incorporation increased over time in well‐nourished rats, indicating active cell proliferation. However, cells of malnourished animals had lower rates of BrdUrd incorporation after 2 h compared to WN cells, reflecting reduced proliferation (Fig. 3).
Figure 2.
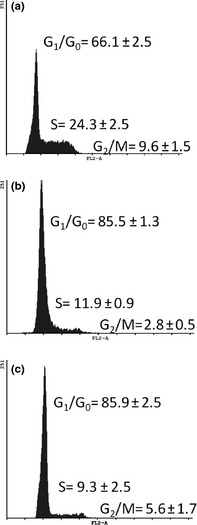
Distribution of cells in each phase of the cell cycle. Data for each group are shown as average ± standard error (SE). (a) WN (n = 9) rat spleen cells. (b) MN2nd (n = 8) rat spleen cells. (c) MN3rd (n = 10) rat spleen cells. Both malnourished groups had significantly lower percentage of cells in S‐phase and G2/M compared to well‐nourished rats (P < 0.05).
Figure 3.
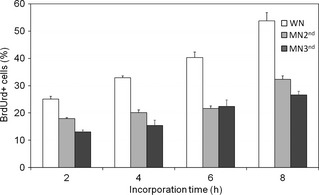
Percentage of BrdUrd+ cells in vivo in spleen of well‐nourished (WN) rats and moderately (MN2nd) and severely (MN3rd) malnourished pups after 2, 4, 6 and 8 h. Results indicate minor incorporation of BrdUrd in both groups of malnourished rats. (P < 0.05).
Figure 4 shows distribution of BrdUrd‐positive cells in each of the cell cycle phases. In both malnourished groups, percentages of BrdUrd‐positive cells was lower in each phase of the cycle. In MN2nd animals, change in distribution of cells was observed after 8 h, with increase in percentages of positive cells in S‐phase and reduction in G1. Table 3 shows labelling index and duration of each of the phases of the cell cycle. T G1 was lower in the MN2nd group (3.4 h ± 0.3) compared to WN group (5.4 h ± 0.5), whereas T G1 in MN3rd group (5.5 h ± 0.4) was similar to the WN group. T S was significantly higher in MN2nd group (9.6 h ± 1.1) compared to WN group (3.6 h ± 1.1), and non‐significant increase was observed in the MN3rd group (4.3 h ± 1.1). The T G2 was similar for all the groups (WN 1.4 h ± 0.3, MN2nd 1.4 h ± 0.1 and MN3rd 1.2 h ± 1.1). T C was significantly higher in MN2nd group, and increase was also observed in MN3rd group, although it did not reach significance. GF was significantly lower in the MN3rd group (19.9 ± 1.3%) compared to MN2nd and WN groups (30.9 ± 1.8% and 47.0 ± 4.4%, respectively).
Figure 4.
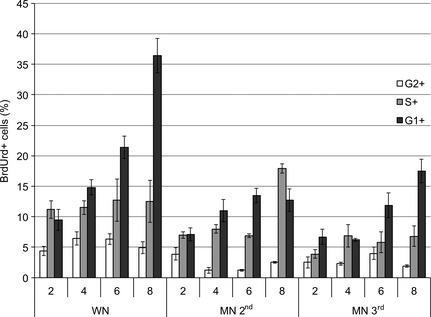
Distribution of cells in each phase of the cell cycle that incorporated BrdUrd in vivo in well‐nourished rats (WN) and in moderately (MN2nd) and severely (MN3rd) malnourished pups at 2, 4, 6 and 8 h. Results indicate minor incorporation of BrdUrd in both groups of malnourished rats, P < 0.05.
Table 3.
Estimated duration of the cell cycle phases in the spleen cells of well‐nourished and malnourished rats using the Eidukevicius method after 8 h of a BrdUrd pulse
| T G1 | T S | T G2 | T C | GF (%) | |
|---|---|---|---|---|---|
| WN | 5.4 ± 0.5 | 3.6 ± 1.1 | 1.4 ± 0.3 | 10.5 ± 0.7 | 47.0 ± 4.4 |
| MN2nd | 3.4 ± 0.3* , ** | 9.6 ± 1.1* , ** | 1.4 ± 0.1 | 14.4 ± 0.9* , ** | 30.9 ± 1.8* |
| MN3rd | 5.5 ± 0.4 | 4.3 ± 1.1 | 1.2 ± 1.1 | 11.0 ± 0.7 | 19.9 ± 1.3* |
Values are the mean ± standard error. Times are given in hours. Different superscript letters are statistically significant; *significant differences in the well‐nourished (WN) group, P < 0.05; **significant differences in the moderately (MN2nd) and severely (MN3rd) malnourished rats, P < 0.05.
Discussion
This study has evaluated effects of malnutrition on in vivo proliferation of rat pup spleen cells during lactation, representing time of accelerated growth when effects of malnutrition are more significant than in later stages. Malnutrition at lower age has been shown to create permanent effects 28.
Malnutrition reduced spleen‐to‐body weight ratio in both of malnourished rat pup groups compared to healthy control animals. Numbers of cells in spleens were lower in malnourished animals, but values of cell density remained very similar among the three groups. This result indicates that cell numbers determines weight of the spleen. However, other studies have reported that proportions of cell subpopulations in the spleen are altered as a result of malnutrition, with reduced number of T lymphocytes and higher numbers of non‐lymphoid cells 19. Results of this study show changes in cell proliferation with different effects depending on degree of malnutrition. Proportions of cells in the various phases of the cell cycle were reduced in both groups of malnourished rats. These data provide evidence that the cell cycle was altered as a result of malnutrition. In vivo BrdUrd incorporation enables evaluation of proliferation kinetics and effects induced by malnutrition 21.
The method of Eidukevicius et al. 27 was selected to establish cell kinetics as it allows calculation of periods of time cells are in G1, S and G2 phases. The assumption is that the method used can estimate total number of cells labelled in S phase during BrdUrd after any time interval shorter than duration of the cell cycle. The method described by Begg et al. 29 and cubic method 30 were also tested to calculate T G2+M, T s and T pot. However, the main disadvantage of these two methods is that it is impossible to calculate lengths of time of all phases using the same method. In addition, it was not possible to obtain reliable results using data from all of samples (results not shown). An advantage of the selected method is the option to calculate growth fraction (GF%), valuable in this study. Time spent in M‐phase is absent in the equation proposed by Eidukevicius et al. 27 as the technique used to isolate nuclei does not allow cells to be captured in mitosis. The impact of this omission on results of the study should be negligible, regarding estimation of total number of labelled cells.
BrdUrd incorporation results demonstrate that there were fewer proliferating cells in both malnourished groups of pups and that cells from those with severe malnutrition had lowest BrdUrd incorporation out of all three of groups. Alterations in duration of phases were observed in the moderately malnourished group; T G1 was diminished and T S and T C were increased compared to WN rats. Results from rats in the severe malnutrition group indicated that the effect was produced primarily by an important reduction in fraction of growing cells (GF%), with non‐significant increase in T S and T C. Winick and Noble 28 reported that malnutrition during lactation affects spleen growth due to reduced cell division, and that this decrease was observed even after nutritional recovery up until 133 days of age. Our results confirm that severe malnutrition diminished spleen cell proliferation in rats, due to differences in competence during lactation (from birth to 21 days).
Further studies have shown that malnutrition affects different phases of the cell cycle in different types of tissues. Rose et al. 31 showed that cells of intestinal mucosa have prolonged S‐phase in rats on a restricted protein diet, whereas delay in the G2 and S‐phases were observed in hair follicles and epidermis of rats malnourished from birth 32. Spermatogonia and Sertoli cells of rats with caloric deficiencies during lactation have been observed to have delayed G1 and S‐phases 33. Proliferation of cells in the bone marrow of malnourished rats is slower than in cells of well‐nourished rats and reduced percentage of proliferating cells has also been observed 20, 21, 22. Gomez et al. 22 showed that the cell cycle is prolonged in malnourished rats. G1 +1/2M phases are more sensitive to malnutrition, whereas no differences have been found in S and G2 +1/2M phases. It has been proposed that malnutrition makes it difficult for cells to complete G1, which could be related to deficiency of essential nutrients required for synthesis of proteins necessary for activities specific to G1, and to subsequent progression to S‐phase 34. In malnourished mice, numbers of progenitor and CD5+ cells from bone marrow, in G0 increased, with reduction in number of cycling cells 35, 36.
Studies evaluating DNA damage in lymphoid tissue cells in malnourished rats have reported that malnutrition is associated with increased DNA damage in the spleen, bone marrow and blood lymphocytes, which could be related to lower capacity to repair damage 37. Evaluation of micronuclei in cells of malnourished children has shown that DNA damage in children with moderate malnutrition is lower than DNA damage in children with severe malnutrition, and may be related to availability of micronutrients and levels of oxidative stress 24. The cell cycle has checkpoints that evaluate DNA integrity. If the cell is damaged, cycle cannot progress 38, 39, 40, 41. It is possible that levels of DNA damage would have different effects on the cell cycle. Thus, additional studies are required to evaluate relation of levels of micronutrients and oxidative stress with levels of DNA damage and the cell cycle. Length of the cell cycle was higher in cells of rat pups with moderate malnutrition, as more time was needed to complete S‐phase, probably related to the increased DNA damage and induction of the repair mechanisms. Severe malnutrition reduces numbers of proliferating cells and restricts initiation of the cell cycle to a small fraction of the cell population, probably related to deficiency of nutrients and increased levels of DNA damage.
In conclusion, moderate malnutrition increased time required for DNA synthesis and time of the total cycle, and severe malnutrition reduced growth fraction of spleen cells in malnourished rats during lactation.
Acknowledgements
This work was supported in part by CONACyT (México): grants 50804 and 118848. We thank to Pedro Rojas, Nancy Palacios, Biól. Alma Arellano and MISP Gloria Ruiz for their technical assistance.
References
- 1. Rodriguez L, Cervantes E, Ortiz R (2012) Malnutrition and gastrointestinal and respiratory infections in children: a public health problem. Int. J. Environ. Res. Public Health 8, 1174–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woodward B (1998) Protein, calories, and immune defenses. Nutr. Rev. 56, S84–S92. [DOI] [PubMed] [Google Scholar]
- 3. Rice AL, Sacco L, Hyder A, Black RE (2000) Malnutrition as an underlying cause of childhood deaths associated with infectious diseases in developing countries. Bull. World Health Organ. 78, 1207–1221. [PMC free article] [PubMed] [Google Scholar]
- 4. Chandra RK (1999) Nutrition and immunology: from the clinic to cellular biology and back again. Proc. Nutr. Soc. 58, 681–683. [DOI] [PubMed] [Google Scholar]
- 5. Dai G, Phalen S, McMurray DN (1998) Nutritional modulation of host responses to mycobacteria. Front Biosci. 3, e110–e122. [DOI] [PubMed] [Google Scholar]
- 6. Ortiz R, Rodríguez L (2008) Effects of malnutrition on immunologic function. Curr. Res. Immunology. 2, 1–25. [Google Scholar]
- 7. Cyster JG (1999) Chemokines and cell migration in secondary lymphoid organs. Science 286, 2098–2102. [DOI] [PubMed] [Google Scholar]
- 8. Fu YX, Chaplin DD (1999) Development and maturation of secondary lymphoid tissues. Annu. Rev. Immunol. 17, 399–433. [DOI] [PubMed] [Google Scholar]
- 9. Janeway CA, Travers P, Walport M, Shlomchik M (2001) Immunobiology, 5th edn New York and London: Garland Science. [Google Scholar]
- 10. Pabst O, Herbrand H, Bernhardt G, Forster R (2004) Elucidating the functional anatomy of secondary lymphoid organs. Curr. Opin. Immunol. 16, 394–399. [DOI] [PubMed] [Google Scholar]
- 11. Prentice AM (1999) The thymus: a barometer of malnutrition. Br. J. Nutr. 81, 345–347. [PubMed] [Google Scholar]
- 12. Savino W (2002) The thymus gland is a target in malnutrition. Eur. J. Clin. Nutr. 56(Suppl. 3), S46–S49. [DOI] [PubMed] [Google Scholar]
- 13. Desai M, Crowther NJ, Lucas A, Hales CN (1996) Organ‐selective growth in the offspring of protein‐restricted mothers. Br. J. Nutr. 76, 591–603. [DOI] [PubMed] [Google Scholar]
- 14. Chandra RK (1991) Interactions between early nutrition and the immune system. Ciba Found. Symp. 156, 77–89; discussion‐92 [PubMed] [Google Scholar]
- 15. Menendez‐Patterson A, Fernandez S, Diaz F, Marin B (1987) Malnutrition in rats during pregnancy and lactation period: a study on body, spleen and thymus weights and hematologic parameters in dams and their offspring. Rev. Esp. Fisiol. 43, 287–296. [PubMed] [Google Scholar]
- 16. Barone KS, O'Brien PC, Stevenson JR (1993) Characterization and mechanisms of thymic atrophy in protein‐malnourished mice: role of corticosterone. Cell. Immunol. 148, 226–233. [DOI] [PubMed] [Google Scholar]
- 17. Ortiz R, Cortés L, González‐Marquez H, Gómez JL, González C, Cortés E (2001) Flow cytometric analysis of spontaneous and dexamethasone‐induced apoptosis in thymocytes from severely malnourished rats. Br. J. Nutr. 86, 545–548. [DOI] [PubMed] [Google Scholar]
- 18. Ortiz R, Cortés L, Cortés E, Medina H (2009) Malnutrition alters the rates of apoptosis in splenocytes and thymocyte subpopulations of rats. Clin. Exp. Immunol. 155, 96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cortés‐Barberena E, González‐Márquez H, Gómez‐Olivares JL, Ortiz‐Muniz R (2008) Effects of moderate and severe malnutrition in rats on splenic t lymphocyte subsets and activation assessed by flow cytometry. Clin. Exp. Immunol. 152, 585–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ortiz R, Betancourt M (1984) Cell proliferation in bone marrow cells of severely malnourished animals. J. Nutr. 114, 472–476. [DOI] [PubMed] [Google Scholar]
- 21. Betancourt M, Ortiz R, González C (1992) Proliferation index in bone marrow cells from severely malnourished rats during lactation. Mutat. Res. 283, 173–177. [DOI] [PubMed] [Google Scholar]
- 22. Gómez JL, Campos C, Rangel P, Ortiz R (1996) Cell cycle phase duration in bone marrow cells from malnourished rats during suckling. Mutat. Res. 352, 57–60. [DOI] [PubMed] [Google Scholar]
- 23. Ortiz R, Medina H, Cortes E, Cervantes E, Rodriguez L (2011) Trimethoprim‐sulfamethoxazole increase micronuclei formation in peripheral blood from weanling well‐nourished and malnourished rats. Environ. Mol. Mutagen. 52, 673–680. [DOI] [PubMed] [Google Scholar]
- 24. Cervantes‐Rios E, Ortiz‐Muniz R, Martinez‐Hernandez AL, Cabrera‐Rojo L, Graniel‐Guerrero J, Rodriguez‐Cruz L (2012) Malnutrition and infection influence the peripheral blood reticulocyte micronuclei frequency in children. Mutat. Res. 731, 68–74. [DOI] [PubMed] [Google Scholar]
- 25. Ortiz R, Cortés E, Pérez L, González C, Betancourt M (1996) Assessment of an experimental method to induce malnutrition by food competition during lactation. Med. Sci. Res. 24, 843–846. [Google Scholar]
- 26. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- 27. Eidukevicius R, Characiejus D, Janavicius R, Kazlauskaite N, Pasukoniene V, Mauricas M et al (2005) A method to estimate cell cycle time and growth fraction using bromodeoxyuridine‐flow cytometry data from a single sample. BMC Cancer 5, 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Winick M, Noble A (1966) Cellular response in rats during malnutrition at various ages. J. Nutr. 89, 300–306. [DOI] [PubMed] [Google Scholar]
- 29. Begg AC, McNally NJ, Shrieve DC, Karcher H (1985) A method to measure the duration of DNA synthesis and the potential doubling time from a single sample. Cytometry 6, 620–626. [DOI] [PubMed] [Google Scholar]
- 30. White RA, Meistrich ML, Pollack A, Terry NH (2000) Simultaneous estimation of t(g2 + m), t(s), and t(pot) using single sample dynamic tumor data from bivariate DNA‐thymidine analogue cytometry. Cytometry 41, 1–8. [PubMed] [Google Scholar]
- 31. Rose PM, Hopper AF, Wannemacher RW Jr (1971) Cell population changes in the intestinal mucosa of protein‐depleted or starved rats. I. Changes in mitotic cycle time. J. Cell Biol. 50, 887–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mathur M, Doe MG (1976) Kinetics of proliferation and differentiation in the hair follicle and epidermis in neonatally undernourished rats. Am. J. Pathol. 82, 9–24. [PMC free article] [PubMed] [Google Scholar]
- 33. Bansal‐Rajbanshi M, Mathur M (1985) Testicular morphology and cell proliferation kinetics of immature germ cells and sertoli cells in suckling undernourished rats. Cell Tissue Kinet. 18, 183–191. [DOI] [PubMed] [Google Scholar]
- 34. Murray LE, Singer RA, Fenwick RG Jr, Johnston GC (1991) The g1 interval in the mammalian cell cycle: dual control by mass accumulation and stage‐specific activities. Cell Prolif. 24, 215–228. [DOI] [PubMed] [Google Scholar]
- 35. Borelli P, Blatt S, Pereira J, de Maurino BB, Tsujita M, de Souza AC et al (2007) Reduction of erythroid progenitors in protein‐energy malnutrition. Br. J. Nutr. 97, 307–314. [DOI] [PubMed] [Google Scholar]
- 36. Fock RA, Blatt SL, Beutler B, Pereira J, Tsujita M, de Barros FE et al (2009) Study of lymphocyte subpopulations in bone marrow in a model of protein‐energy malnutrition. Nutrition 26, 1021–1028. [DOI] [PubMed] [Google Scholar]
- 37. Cortés E, González C, Betancourt M, Ortiz R (2001) Assessment of DNA damage in spleen, bone marrow, and peripheral blood from malnourished rats by single cell gel electrophoresis assay. Teratog. Carcinog. Mutagen. 21, 231–247. [DOI] [PubMed] [Google Scholar]
- 38. Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB (1992) Wild‐type p53 is a cell cycle checkpoint determinant following irradiation. Proc. Natl Acad. Sci. USA 89, 7491–7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elledge SJ (1996) Cell cycle checkpoints: preventing an identity crisis. Science 274, 1664–1672. [DOI] [PubMed] [Google Scholar]
- 40. Latif C, Harvey SH, O'Connell MJ (2001) Ensuring the stability of the genome: DNA damage checkpoints. Scientific World Journal 1, 684–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Langerak P, Russell P (2011) Regulatory networks integrating cell cycle control with DNA damage checkpoints and double‐strand break repair. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 366, 3562–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]


