Abstract.
We examine the cell proliferation activity and expression of cyclin‐dependent kinase inhibitors of the Cip/Kip family, p21Cip1, p27Kip1 and p57Kip2, in foetal hamster lungs to determine the expression patterns of the cyclin‐dependent kinase inhibitors and to clarify the relationship between expression of the cyclin‐dependent kinase inhibitors and lung development. Foetal hamster lungs on gestational days 12.5–16 (the day of birth) and adult lungs were fixed in 4% paraformaldehyde. Frozen sections were immunostained for the cyclin‐dependent kinase inhibitors, and examined by immunostaining for Ki‐67 and bromodeoxyuridine to determine the proliferation activity of the foetal lungs. During the foetal period, cell proliferation activity, as analysed by Ki‐67 or bromodeoxyuridine labelling, decreased with development of the lung. In contrast to the gradual decrease of cell proliferation activity, cells with p27Kip1 immunoreactivity increased with development. On the other hand, p21Cip1‐positive cells were most prominent around gestational day 14.5, while after birth positive cells decreased markedly. A few p57Kip2‐positive cells were detected in the bronchiolar epithelium on gestational day 14.5. Western blotting analyses confirmed these immunostaining patterns. Thus, the levels of the cyclin‐dependent kinase inhibitors of the Cip/Kip family are modulated in the lungs during the foetal period, and each shows a unique expression pattern. The cyclin‐dependent kinase inhibitors may play roles not only in regulating cell proliferation activity but also in regulating other functions such as differentiation in the lung during the foetal period.
Introduction
The mammalian lung epithelium is derived from the foregut endoderm. Development of the respiratory system starts in the trachea and progresses as a wave distally to include the developing bronchi, bronchioles and alveoli (Sorokin 1965; Cutz 1987; Ito et al. 1990). Development of the foetal lungs involves intricate processes regulated by various factors, which play roles in morphogenesis, cell proliferation, cell differentiation and cell motility. The ontogenic sequence of these events in lung organogenesis must be well coordinated.
Cell proliferation is controlled by complex signal transduction pathways along with cell‐cycle exit and cell differentiation during the foetal developmental period. Lung epithelial cells undergo repeated rounds of proliferation, and proceed through characteristic cell differentiation and maturation pathways towards terminal differentiation to form various cell types in the different anatomical locations of the lungs. The cell cycle is positively regulated by the binding of cyclin‐dependent kinases (CDKs) to cyclins and resultant phosphorylation, whereas progression of the cell cycle is negatively controlled by the binding of cyclin‐dependent kinase inhibitors (CDKIs) to the cyclin/CDK complex (Sherr 1994). CDKIs are categorized into two families based on their functions and structures: the Cip/Kip family, consisting of p21Cip1, p27Kip1 and p57Kip2, and the Ink4 family, consisting of p16Ink4a, p15Ink4b, p18Ink4c and p19Ink4d (Vogt & Reed 1998). The Cip/Kip family inhibits all G1 cyclin/CDK complexes, and thereby prevents phosphorylation of Rb protein and brings about cell‐cycle arrest (Harper & Elledge 1996). These CDKIs show tissue‐specific expression during foetal development of mammals (Nakayama & Nakayama 1998), but morphological localization of the CDKIs has not been intensively studied in foetal lungs. Furthermore, the functional roles of these CDKIs are largely unknown in developing foetal lungs, although a recent study of mice with double gene deficiencies for p21Cip1 and p57Kip2 showed that these CDKIs have a potential cooperative role in foetal lung development, as shown by the abnormal developmental phenotype of these mice (Zhang et al. 1999). In the present study, we have immunohistochemically characterized expression of the CDKIs of the Cip/Kip family in the foetal developing lungs, and compared their immunostaining patterns with the cell proliferation activity as evaluated by Ki‐67 and bromodeoxyuridine (BrdUrd) immunostainings. We studied Syrian golden hamsters (Mesocricetus auratus) because this species has a very short gestational period (16 days) and marked morphological changes can be observed in the lungs on a daily basis before birth (Ito et al. 1990).
MATERIALS AND METHODS
Animals
Eight‐week‐old Syrian golden hamsters were purchased from Japan SLC (Shizuoka, Japan), housed three per cage, given food and water ad libitum, and maintained under standard conditions in a room with a 12‐h light/dark cycle. The time of conception was established as the date when sperm was recognized in the vagina, and designated as gestational day 1. On each of gestational days 12.5, 13.5, 14.5, 15.5 and 16 (the day of birth), five pregnant hamsters were deeply anaesthetized with an overdose of intraperitoneal pentobarbital and the foetuses were removed from the uterus. The lungs of the foetuses were removed and fixed with phosphate‐buffered 4% paraformaldehyde for 1 day. For the study of adult lungs, five 8‐week‐old male hamsters were deeply anaesthetized with pentobarbital. After the adult lungs were instilled with the fixative through the trachea and removed, the lung tissues were fixed with the fixative for 1 day. One hour before sacrifice, the pregnant female and adult male animals received an intraperitoneal injection of BrdUrd (Sigma Chemical, St. Louis, MO, USA) at a dose of 2 mg/kg body weight. Additionally, for Western blotting analysis, 15 pairs of foetal lungs from each gestational day and three pairs of adult lungs were frozen with liquid nitrogen.
This animal study project was approved by the Animal Care Committee of Yokohama City University School of Medicine (#99–20).
Immunostaining
For immunohistochemistry, 5‐µm‐thick frozen sections were made with a Leitz 1720 Digital Cryostat (Leica, Wetzlar, Germany). The sections, except for those used for BrdUrd immunostaining, were heated at 97 °C in a citrate buffer (pH 6) in a microwave oven (model MFW‐2, Nissin‐EM, Tokyo, Japan) for 15 min. After cooling at room temperature and blockage of endogenous peroxidase with 0.3% H2O2, sections were treated overnight at 4 °C with each of the primary antibodies, which included mouse monoclonal antibodies against Ki‐67 antigen (MIB5, Immunotech, Marseille, France), p21Cip1 (F‐5, Santa Cruz Biotechnology, Santa Cruz, CA, USA), p27Kip1 (Transduction Laboratories, Lexington, Kentucky, USA), and a rabbit polyclonal antibody against p57Kip2 (H‐91, Santa Cruz). The sections were processed using the streptavidin–biotin peroxidase complex method using a kit (Dako, Carpinteria, CA, USA), developed with a diaminobenzidine–H2O2 substrate complex, and counterstained with haematoxylin. For immunostaining for BrdUrd, the sections were treated with 0.5% pepsin in 4 N HCl for 10 min, and incubated overnight at 4 °C with a monoclonal antibody against BrdUrd (Br‐3, Caltag, San Francisco, CA, USA). Thereafter, the sections were processed in a manner similar to that used for the sections immunostained with the other antibodies. Negative controls were incubated with mouse or rabbit non‐specific immunoglobulin for the primary antibodies, followed by an immunostaining procedure similar to that described above. Adult hamster intestines were used for positive controls for immunostaining for Ki‐67 and BrdUrd.
The labelling indices of the sections for each immunostaining were calculated as the percentage of positive nuclei in 300 cells counted in different lung areas, including the lobar bronchus (LB), distributing bronchiole (DB) and alveolus (AL) in the left lungs.
Spearman’s rank correlation coefficient was used to determine the correlations between labelling index values for Ki‐67, BrdUrd, p21Cip1, p27Kip1 and p57Kip2.
Western blotting
The lung tissues homogenized on ice with ice‐cold homogenizing buffer [5 mm Tris, 0.25 m sucrose, 2 mm EDTA, 2 mm ethylene glycol bis (2‐aminoethyl ether)‐tetraacetic acid (EGTA)] containing protease inhibitors phenylmethane sulphonyl fluoride (PMSF, 0.5 mm), dithiothreitol (DTT, 0.5 mm) and leupeptin (5 µg/ml) were clarified by centrifugation and resuspended in the lysate buffer (50 mm Tris, 150 mm NaCl, 25 mm NaF, 25 mmβ‐glycerophosphate, 2 mm EDTA, 2 mm EGTA, 0.3% NP‐40, 0.5 mm PMSF, 0.5 mm DTT, 5 µg/ml leupeptin). The lysate was centrifuged and the concentration of the supernatant protein extract was determined using the Bio‐Rad protein assay kit (Bio‐Rad Laboratories, CA, USA). Aliquots of approximately 40 µg of protein were subjected to SDS‐PAGE (in 8–12% gels), and the proteins were then transferred to nitrocellulose membranes. The blots were blocked with 5% non‐fat milk in phosphate‐buffered saline solution. Antibodies against p21Cip1, p27Kip1 and p57Kip2, which were the same as the antibodies used for immunohistochemistry, were used for Western blotting. The filters were incubated with primary antibody, and then with horseradish peroxidase‐conjugated secondary antibody, and the stained proteins were visualized with an enhanced chemiluminescence detection kit (Amersham Pharmacia Biotech, Uppsala, Sweden).
RESULTS
A summary of the nuclear labelling indices of cell proliferation markers (Ki‐67 and BrdUrd) and the CDKIs of the Cip/Kip family (p21Cip1, p27Kip1 and p57Kip2) using immunohistochemistry is shown in Fig. 1. Cell proliferation activity as revealed by Ki‐67 or BrdUrd labelling index decreased with age (1, 2, 3). Many Ki‐67‐positive cells were seen in the airway and alveolar epithelia and in the mesenchyme in the early developing lungs (Fig. 3a). Later during the gestational period, positive cells decreased (Fig. 3b), and in adults, only a few positive cells were seen in all anatomical locations of the lung (Fig. 3c). The incidence of BrdUrd‐incorporating cells also tended to change with a pattern similar to that of Ki‐67‐positive cells (Fig. 2), although the number of BrdUrd‐positive cells was approximately a half to a third of that of Ki‐67‐positive cells (1, 2, 3).
Figure 1.
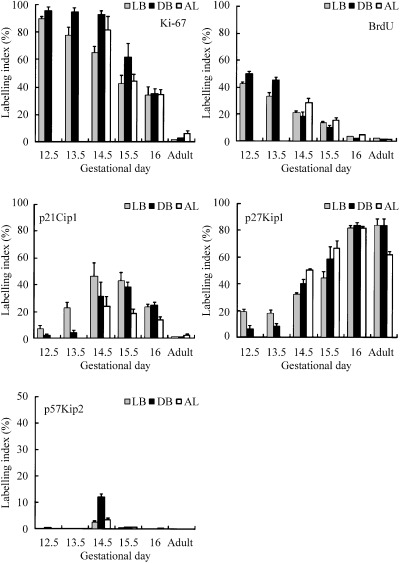
Profile of nuclear labelling index of cell proliferation markers (Ki‐67 and BrdUrd) and cyclin‐dependent kinase inhibitors of the Cip/Kip family (p21Cip1, p27Kip1 and p57Kip2) in the lobar bronchus (LB), distributing bronchiole (DB) and alveolus (AL) in foetal Syrian golden hamsters on gestational days 12.5–16 (the day of birth) and in adult hamsters.
Figure 2.
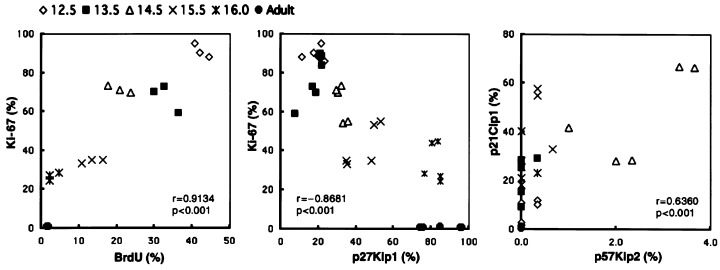
Correlations between labelling index values of Ki‐67 and BrdUrd (left), Ki‐67 and p27Kip1 (centre), and p21Cip1 and p57Kip2 (right) in the lobar bronchi of hamsters.
Figure 3.
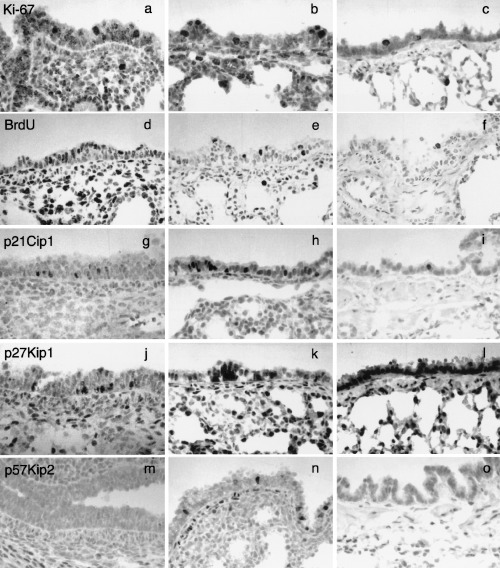
Immunostainings for Ki‐67 (a–c), BrdUrd (d–f), p21Cip1 (g–i), p27Kip1 (j–l) and p57Kip2 (m–o) in lobar bronchus (a–o) and alveolus (b, c, e, f, h, i, k, l, n, o) of foetal (a, b, d, e, g, h, j, k, m, n) and adult (c, f, i, l, o) hamster lungs. Counterstained with haematoxylin. × 400. (a–c) Ki‐67 immunostaining of foetal (a, gestational day 12.5; b, day 16) and adult (c) hamster lungs. (d–f) BrdUrd immunostaining of foetal (d, gestational day 13.5; e, day 15.5) and adult (f) hamster lungs. (g–i) p21Cip1 immunostaining of foetal (g, gestational day 13.5; h, day 15.5) and adult (i) hamster lungs. (j–l) p27Kip1 immunostaining of foetal (j, gestational day 12.5; k, day 15.5) and adult (l) hamster lungs. Note strong staining of a cell cluster, presumably corresponding to neuroendocrine cells (arrow). (m–o) p57Kip2 immunostaining of foetal (m, gestational day 12.5; n, day 14.5) and adult (o) hamster lungs.
p21Cip1, p27Kip1 and p57Kip2 showed various patterns of expression in the developing lungs (1, 2, 3). The number of p21Cip1‐positive cells was low in the early gestational period, but increased and became maximal on gestational days 14.5 and 15.5 in all epithelial levels (1, 3). Thereafter, p21Cip1‐positive cells decreased, and were scarcely detected in the adult lungs (1, 3).
p27Kip1‐positive cells increased with age in all areas of the epithelium in the developing foetal hamster lungs (Fig. 1), and there was a significant negative correlation between labelling index values of p27Kip1 and Ki‐67 or BrdUrd for all airway levels (Fig. 2). In the early gestational period, some p27Kip1‐positive cells appeared in the airway epithelium (1, 3), and the number of such cells increased in the developing epithelium as gestation proceeded (1, 3). Furthermore, in the adult lungs, expression of p27Kip1 was seen in almost all airway and alveolar epithelial cells (Fig. 3l). Besides diffuse p27Kip1 staining in lung epithelial cells, a strong expression of p27Kip1 was observed in pulmonary neuroendocrine cells (Fig. 3k; Ito 1999). On the other hand, p57Kip2‐positive cells were seen in foetal lungs only in the middle of the gestational period, and on day 14.5, some positive cells were seen in the bronchioles (1, 3), while they were rarely seen in other lung epithelial tissues and in other gestational periods (1, 3). The maximum labelling of p57Kip2 on day 14.5 coincided with that of p21Cip1 (1, 2).
To confirm the results of this immunohistochemical study, we performed a Western blotting study of p21Cip1, p27Kip1 and p57Kip2 (Fig. 4). A strong expression of p21Cip1 was detected on days 14.5, 15.5 and 16, a weak expression on days 12.5 and 13.5, and only a trace expression in the adult lungs. The expression of p27Kip1 gradually became stronger with age. p57Kip2 could only be weakly detected in the lung on day 14.5.
Figure 4.
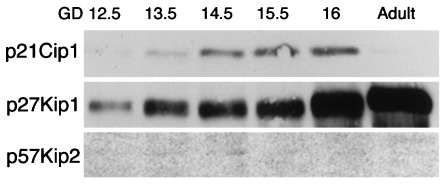
Western blotting for p21Cip1, p27Kip1 and p57Kip2 in foetal developing lung of hamsters.
DISCUSSION
In this study, we showed that the Cip/Kip family of CDKIs displayed different expression patterns, although the labelling indices of proliferative markers such as Ki‐67 and BrdUrd showed gradual decreases with development. It has been reported that Ki‐67 antigen is expressed in G1, S, G2 and M, but not in G0 (Gerdes et al. 1984), and that monoclonal antibody against Ki‐67 antigen (clone MIB5) was useful for staining of cells during the relevant phases of the cell cycle in rodent tissues (Gerlach et al. 1997; Ito et al. 1998). The expression patterns of these proliferative markers are similar to each other, and the number of BrdUrd‐positive cells is approximately a half to a third of that of Ki‐67‐positive cells, as reported in a study of chemically induced murine lung adenocarcinomas (Ito et al. 1998). Ki‐67‐ and BrdUrd‐positive cells decreased with age and were rarely seen in the lungs at birth and in the adult lungs, suggesting that many of the epithelial cells of the developing foetal lungs exited from the cell cycle before birth and underwent differentiation.
p21Cip1 was discovered as a protein that binds to Cdk2 (Hunter 1993), and has a binding domain that interacts with proliferating cell nuclear antigen, resulting in the prevention of DNA synthesis (Flores‐Rozas et al. 1994; Waga et al. 1994). Moreover, it has been reported that p21Cip1 is involved in terminal differentiation in various tissues such as intestinal epithelial cells and muscle cells (Halevy et al. 1995; Tian & Quaroni 1999). In the present study, the number of p21Cip1‐positive cells was low during the early development of the foetal lungs, increased in the middle of the developing period, decreased before birth and was extremely low in adult lungs. As p21Cip1 expression decreased along with reduced cell proliferation activity in the lungs during the late foetal period, p21Cip1 may have some roles other than cell‐cycle control in organogenesis of the lung. Two regulatory pathways of expression of p21Cip1 have been reported: one is dependent on p53 tumour‐suppressor protein, and the other is p53‐independent (Hengst & Reed 1998). In our previous study of lung cell injury in adult mice, bleomycin treatment induced elevated expression of p21Cip1 with enhanced expression of p53 but without apoptosis in the mouse lungs. However, bleomycin did not induce p21Cip1 expression in the lungs from p53‐gene‐deficient mice, and we assume that p21Cip1 could work during cell repair processes after injury in adult lungs (Okudela et al. 1999). We did not study whether p21Cip1 expression in the foetal lung is p53‐dependent or independent.
p27Kip1 is thought to have various functions, including regulation of the cell cycle, suppression of tumour cell proliferation, promotion of apoptosis and regulation of differentiation of various tissues and cells (Lloyd et al. 1999). In the present study, p27Kip1 expression increased in the lungs during foetal development, and the change was inversely related to cell proliferation activity. In a human airway epithelial cell line, contact inhibition induced high levels of p27Kip1 expression and cell‐cycle arrest (Yatabe et al. 1998). These observations suggest that p27Kip1 may be one of the main suppressors for lung cell proliferation during the foetal period and also in adulthood.
p57Kip2 expression was detected on day 14.5, which coincided with the period showing the maximum expression of p21Cip1. p57Kip2‐positive cells were low in number but predominantly occurred in the bronchiolar epithelium. In the reports on gene‐targeted mice for p21Cip1, p27Kip1 or p57Kip2, there were no obvious abnormalities in their lungs (Deng et al. 1995; Fero et al. 1996; Zhang et al. 1997), but malformation of the lung was seen in the mice doubly deficient for p21Cip1 and p57Kip2 (Zhang et al. 1999). Based on the studies using the mice deficient for CDKI genes and our histochemical study, p21Cip1 and p57Kip2 may work cooperatively to regulate normal lung development.
The present study revealed that each of the CDKIs of the Cip/Kip family showed a specific spatio‐temporal expression pattern in the lungs during the foetal gestational period, suggesting that these CDKIs could work to ensure proper sequential organogenesis of the lung. The functions of p21Cip1, p27Kip1 and p57Kip2 in lung development in vivo may differ because the relationship between the modulation of cell proliferation activity and the incidence and localization of each of them varied. Among the Cip/Kip family, one of the CDKIs that must be involved mostly in cell‐cycle regulation for lung development is p27Kip1, although we did not study the expression of CDKIs belonging to the Ink4 family and thus we cannot speculate about whether the Cip/Kip and INK4 families cooperate to induce cell‐cycle arrest of lung epithelial cells (Reynisdottir & Massague 1997). Furthermore, the different expression patterns of the member of the Cip/Kip family of CDKIs suggest the existence of different regulatory pathways inducing expression of the CDKIs, although, for example, p21Cip1 and p27Kip1 are both induced by transforming growth factor beta for induction (Polyak et al. 1994; Datto et al. 1995).
Acknowledgements
This study was supported in part by grants from the Smoking Research Foundation of Japan and by a Grant‐in‐Aid for Scientific Research (No. 11 670 222) from the Japan Society for the Promotion of Science.
References
- Cutz E (1987) Cytomorphology and differentiation of airway epithelium in developing human lung In: McDowell EM, ed. Lung Carcinomas, p. 1 Edinburgh: Churchill‐Livingstone. [Google Scholar]
- Datto MB, Panus JF, Howe DJ, Xiong Y, Wang XF (1995) Transforming growth factor beta induces the cyclin‐dependent kinase inhibitor p21 through a p53‐independent mechanism. Proc. Natl. Acad. Sci. USA 92, 5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Zhang P, Harper JW, Elledge SJ, Leder P (1995) Mice lacking p21Cip1/Waf1 undergo normal development, but are defective in G1 checkpoint control. Cell 82, 675. [DOI] [PubMed] [Google Scholar]
- Fero ML, Rivkin M, Tasch M et al. (1996) A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27 (Kip1)‐deficient mice. Cell 85, 733. [DOI] [PubMed] [Google Scholar]
- Flores‐Rozas H, Kelman Z, Dean FB et al. (1994) Cdk‐interacting protein 1 directly binds with proliferating cell nuclear antigen and inhibits DNA replication catalyzed by the DNA polymerase delta holoenzyme. Proc. Natl. Acad. Sci. USA 91, 8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J, Lemke H, Baish H, Wacher H‐H, Schwab U, Stein H (1984) Cell cycle analysis of a cell proliferation‐associated human nuclear antigen defined by the monoclonal antibody Ki‐67. J. Immunol. 133, 1710. [PubMed] [Google Scholar]
- Gerlach C, Golding M, Larue L, Alison MR, Gerdes J (1997) Ki‐67 immunoexpression is a robust marker of proliferative cells in the rat. Lab. Invest 77, 697. [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB et al. (1995) Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science 267, 1018. [DOI] [PubMed] [Google Scholar]
- Harper JW & Elledge SJ (1996) Cdk inhibitors in development and cancer. Curr. Opin. Genet. Dev. 6, 56. [DOI] [PubMed] [Google Scholar]
- Hengst L & Reed SI (1998) Inhibitors of the Cip/Kip family In: Vogt PK, Reed SI, eds. Cyclin Dependent Kinase Inhibitors, p. 25 Berlin: Springer. [Google Scholar]
- Hunter T (1993) Braking the cycle. Cell 75, 839. [DOI] [PubMed] [Google Scholar]
- Ito T (1999) Differentiation and proliferation of pulmonary neuroendocrine cells. Prog. Histochem. Cytochem. 110, 245. [DOI] [PubMed] [Google Scholar]
- Ito T, Newkirk C, Strum JM, McDowell EM (1990) Changes in glycoconjugates revealed by lectin staining in the developing airways of Syrian golden hamsters. Anat. Rec. 228, 151. [DOI] [PubMed] [Google Scholar]
- Ito T, Mitsui H, Udaka N et al. (1998) Ki‐67 (MIB 5) immunostaining of mouse lung tumors induced by 4‐nitroquinoline 1‐oxide. Histochem. Cell. Biol. 110, 589. [DOI] [PubMed] [Google Scholar]
- Lloyd RV, Erickson LA, Jin L et al. (1999) p27Kip1: a multifunctional cyclin‐dependent kinase inhibitor with prognostic significance in human cancers. Am. J. Pathol. 154, 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K‐I & Nakayama K (1998) Cip/Kip cyclin‐dependent kinase inhibitors: brakes of the cell cycle engine during development. Bioessays 20, 1020DOI: [DOI] [PubMed] [Google Scholar]
- Okudela K, Ito T, Mitsui H et al. (1999) The role of the p53 in bleomycin‐induced DNA damage in the lung: a comparative study with the small intestine. Am. J. Pathol. 155, 1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyak K, Lee MH, Erdjument‐Bromage H, et al. (1994) Cloning of p27Kip1, a cyclin dependent kinase inhibitor and a potential mediator of extracellular antimitogenic signals. Cell 78, 59. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I & Massague J (1997) The subcellular localization of p15Ink4b and p27Kip1 coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 11, 492. [DOI] [PubMed] [Google Scholar]
- Sherr CJ (1994) G1 phase progression: cycling on cue. Cell 79, 551. [DOI] [PubMed] [Google Scholar]
- Sorokin S (1965) Recent work on developing lungs In: DeHaan RL, Upsprung H, eds. Organogenesis, p. 467 New York: Holt, Reinhart & Winston. [Google Scholar]
- Tian JQ & Quaroni A (1999) Involvement of p21 (WAF1/Cip1) and p27 (Kip1) in intestinal epithelial cell differentiation. Am. J. Physiol. 276, C1245. [DOI] [PubMed] [Google Scholar]
- Vogt PK & Reed SI (1998) Cyclin Dependent Kinase (CDK) Inhibitors, Berlin: Springer. [Google Scholar]
- Waga S, Hannon GJ, Beach D, Stillman B (1994) The p21 inhibitor of cyclin‐dependent kinases controls DNA replication by interaction with PCNA. Nature 369, 574. [DOI] [PubMed] [Google Scholar]
- Yatabe Y, Masuda A, Koshikawa T et al. (1998) p27KIP1 in human lung cancers: differential changes in small cell and non‐small cell carcinomas. Cancer Res. 58, 1042. [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C et al. (1997) Altered cell differentiation and proliferation in mice lacking p57kip2 indicates a role in Beckwith–Wiedemann syndrome. Nature 387, 151. [DOI] [PubMed] [Google Scholar]
- Zhang P, Wong C, Liu D, Finegold M, Harper JW, Elledge SJ (1999) 4 p21cip1 and p57kip2 control muscle differentiation at the myogenin step. Genes Dev. 13, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]


