Abstract
Abstract. Objectives: The use of platelets and platelet products has become increasingly popular clinically as a means of accelerating endosseous wound healing. It is likely that growth factors released by activated platelets at the site of injury play a role in periodontal regeneration by regulating cellular activity. The purpose of this study was to evaluate the biological effects of platelet‐rich plasma (PRP) on human periodontal ligament cells (hPDLCs) in vitro. Materials and methods: Primary cultures of hPDLCs were obtained from healthy premolars. PRP was isolated by two‐step centrifugation. Two main growth factors present in the thrombin‐activated PRP (platelet‐derived growth factor [PDGF‐AB] and transforming growth factor‐β1 [TGF‐β1]) were evaluated using ELISA assay. Activated PRP or the combination of recombined human TGF‐β1 (rhTGF‐β1) and PDGF‐AB (rhPDGF‐AB) were added to hPDLCs in different concentrations to assess cell proliferation and osteogenic differentiation. Results: PRP contained high levels of TGF‐β1 and PDGF‐AB. Cell attachment, proliferation and ALP activity were enhanced by addition of PRP or rhTGF‐β1 and rhPDGF‐AB combination to the cell cultures, while the stimulatory potency of PRP was much greater than the latter. These stimulatory effects presented in a dose‐dependant manner, it seemed that PRP with 50~100ng/ml TGF‐β1 was an ideal concentration. Conclusions: PRP can enhance hPDLC adhesion, proliferation and induce the differentiation of hPDLC into mineralized tissue formation cell; thereby contribute to the main processes of periodontal tissue regeneration. For economical and biological reasons, PRP has more clinical beneficial than analogous growth factors.
INTRODUCTION
Periodontitis is a worldwide disease, often resulting in severe bone loss around teeth. The most desirable goal of therapy is to achieve the regeneration of tissues destroyed by periodontitis, including alveolar bone, cementum and periodontal ligament. Periodontal ligament cells (PDLC) play the most important role in the regeneration of periodontal attachment (Lekic et al. 2001). According the principles of tissue engineering, periodontal tissue regeneration might be accessed by using human periodontal ligament cells (hPDLC) as cell seed plus assisting growth factors alone or in combination with suitable allograft/alloplast as scaffold.
Growth factors are a class of natural biologic mediators that regulate key cellular events in tissue repair, including cell proliferation, differentiation and extracellular matrix synthesis. Previous studies have demonstrated that local application of growth factors alone or mixed with bone allograft is capable of increasing bone growth, accelerating healing of soft tissue and facilitating periodontal repair in animal and human studies (reviewed by Cochran & Wozney 1999). One way of delivering concentrated amounts of growth factor to the wound site is via the use of platelet‐rich plasma (PRP). In humans, PRP is a concentration of platelets in a small volume of plasma. In addition to haemostasis and inflammation, platelets are involved in wound healing and repair of mineralized tissue (Gentry 1992). Platelet α‐granules contain growth factors such as platelet‐derived growth factor (PDGF), transforming growth factor‐β (TGF‐β), insulin‐like growth factor (IGF), epidermal growth factor (EGF) and more. For treatment, they can provide an autologous source of growth factors. By concentrating platelets and delivering them locally, a higher level of growth factors might be reached which could stimulate the healing process in bone in a more physiological way rather than delivery of a single growth factor (Marx et al. 1998; Kim et al. 2001). Recent clinical and histological findings suggest that the use of platelet concentrates has technical benefits and may enhance bone regeneration when used in conjunction with autologous bone grafts, freeze‐dried bone allografts and alloplastic bone substitution substance. PRP has been applied and well studied in the field of orthopaedics, plastic surgery, maxillofacial surgery and oral implant surgery. Recently, it has been introduced to the field of periodontal surgery for the purpose of periodontal tissue regeneration.
Although individual, purified growth factors have been extensively studied in vitro prior to use in vivo, PRP has only recently started to be assessed in a similar manner. However, studies investigating the effect of PRP on cell function in vitro have utilized a wide variety of cell types and have obtained conflicting results (Liu et al. 2002; Lucarelli et al. 2003; Gruber et al. 2004; Kilian et al. 2004; Soffer et al. 2004; Kanno et al. 2005). Limited information is currently available concerning the interaction of platelet concentrates with PDLCs, and more basic research is required to understand the mechanisms in detail. In particular, it is still uncertain which concentrations of PRP are optimal in promoting wound healing and regeneration. It is assumed that PRP would act in a similar manner to individual growth factors and that preparations containing maximal concentration of growth factors are ideal. However, there is little evidence to support this assumption, which may be flawed on the basis that PRP is a combination of different growth factors, each of which exerts a unique influence on the complex cascade of events that occurs during wound healing and tissue regeneration. Thus, in order to provide clear evidence for the clinical use of PRP, a controlled in vitro study has been designed to assess the effect of different concentrations of supernatant obtained after PRP activation and centrifugation, on the proliferation and differentiation of hPDLCs.
MATERIALS AND METHODS
Cell isolation and culture
Human periodontal ligament cells (hPDLC) were obtained according to previously published methods. Briefly, hPDLCs were prepared from extracted teeth that were removed for orthodontic reasons in young healthy volunteers. The periodontal ligament tissues attached to the middle one‐third of the roots were removed by a surgical scalpel, were minced, placed in 35 mm culture dishes in Dulbecco's modified minimum essential medium (DMEM; Gibco BRL, Grant Island, NY, USA) supplemented with 10% (v/v) foetal bovine serum (FBS; Hyclone, Logan, UT, USA), 100 U/ml penicillin G, 100 µg/ml streptomycin and were overlaid with sterile cover slips. Cultures were maintained at 37 °C in an incubator with an atmosphere consisting of 95% air, 5% CO2 and 100% relative humidity. Cells outgrowing from the tissue pieces were harvested with a standard trypsin (Sigma, St. Louis, MO, USA) procedure when the culture had reached 80% confluence. Then cells were sub‐cultured in fresh DMEM containing 10% FBS in the standard incubation condition. Cells of the second and the third passage were used for the experiments described below. The study was conducted under informed consent of volunteer subjects and approved by the Ethics Committee of the Peking University Health Science Center, Beijing, China.
Preparation of activated platelet‐rich plasma
Two whole blood samples of healthy adults were obtained commercially from a blood bank, in samples of 200 ml. The anticoagulant used was citrate‐phosphate‐dextrose (CPD). PRP was prepared by a two‐step centrifugation procedure as soon as possible after the blood being drawn. In brief, whole blood was initially centrifuged at 220 g for 15 min. The plasma was decanted up to the red blood cell sediment. After discarding the red blood cell fraction, the plasma was again centrifuged at 980 g for 10 min. The platelet pellet accumulates at the bottom, the platelet‐poor plasma (PPP) on top. The PPP was drawn off to separate PRP from PPP. Before and after the preparation of PRP, an aliquot was removed and the platelets in whole blood and PRP were counted. Bovine thrombin (Sigma) was combined with 10% calcium chloride in a proportion of 1000 units thrombin/1 ml CaCl2. Release of platelet products into the supernatant was induced by adding 300 IU‐activated thrombin into each millilitre of PRP sample. The mixture was allowed to undergo maximal clot retraction at 4 °C overnight and then was centrifuged at 3000 g for 10 min. The supernatant was stored at −20 °C until used.
Determination of TGF‐β and PDGF levels in PRP
The TGF‐β1 and PDGF‐AB levels in supernatant of activated PRP were determined by a commercially available sandwich enzyme‐linked immunosorbent assay technique kits from R&D System (Quantikine, R&D System, Inc., Minneapolis, MN, USA). Growth factor concentrations were measured according to the manufacturer's instructions. Triplicates were performed for all assays.
Effects of different PRP concentrations on hPDLC proliferation and alkaline phosphatase activity
The hPDLCs were seeded in 96‐well plates (Corning Life Sciences, Acton, MA, USA) at a density of 1 × 104 cells/well and were incubated for 24 h in 100 µl DMEM containing 10% FBS. The medium was then replaced with 100 µl DMEM containing 5% FBS. After 24 h, at the baseline of the experiment, the cells were divided into three different groups: (i) PRP group, in which the cells were cultured in serum‐free DMEM medium treated with the supernatant of activated PRP; (ii) growth factors group, in which the cells were cultured in serum‐free DMEM medium containing the combination of rhTGF‐β1 and rhPDGF‐AB (CytoLab/PeproTech Asia, CytoLab Ltd., Rehovot, Israel) and (iii) negative control group, in which the cells were cultured in serum‐free DMEM medium. Seven subgroups were set in the PRP group and in the growth factor group. Concentrations of growth factors in each subgroup are shown in Table 1.
Table 1.
Growth factors’ concentration in seven subgroups of PRP and growth factor group
| Growth factor (ng/ml) | SG1 | SG2 | SG3 | SG4 | SG5 | SG6 | SG7 |
|---|---|---|---|---|---|---|---|
| TGF‐β1/rhTGF‐β1 | 0.5 | 1.0 | 5.0 | 10.0 | 50.0 | 100.0 | 200.0 |
| PDGF‐AB/rhPDGF‐AB | 0.17 | 0.34 | 1.70 | 3.40 | 17.0 | 34.0 | 72.0 |
PDGF, platelet derived growth factor; PRP, platelet‐rich plasma; SG, subgroups; TGF, transforming growth factor.
Study 1: 3‐(4,5‐dimethylthiazole‐2‐yl)‐2,5‐diphenylterazolium bromide (MTT) assay
Cell proliferation analysis was performed using the MTT assay. After culturing for 3 days in 100 µl culture medium in the absence, and in the presence of varied concentrations of test substances, the MTT assay was carried out according to the cell proliferation kit protocol (Sigma). In the MTT test, tetrazolium salts were transformed by active enzymes of the cells into intracellular formazan deposits; amount of colour produced was directly proportional to the number of viable cells. Absorbance was determined at 490 nm with a microplate reader (Bio‐Rad Model 550, Hercules, CA, USA). All reported values are the means of triplicate samples. This test was repeated twice.
Study 2: Detection of alkaline phosphatase activity
Alkaline phosphatase (ALP) activity in the cell layer was measured by p‐nitrophenyl phosphate (p‐NPP) substrate reactions using Sigma ALP assay reagents. In brief, after culturing for the indicated periods, cells were washed twice with PBS, shaking in 50 µl of 0.2% Triton X‐100 with a plate‐shaker for 20 min. Then, the cell layers were incubated with 100 µl substrate (10 mmol/l p‐NPP, 1 mmol/l MgCl2) for 30 min at 37 °C. The reaction was stopped by adding 100 µl of 1 mol/l NaOH. p‐Nitrophenol formed was spectrophotometrically measured at 405 nm using a microplate reader (Bio‐Rad Model 550).
Assessment of the effect of PRP on cell attachment
Two groups were set. In the test group, 12.5 µl PRP was added to each well of 96‐well plates and in the control group, 12.5 µl serum‐free DMEM medium was added to the cells. hPDLC density was adjusted to 2 × 104/ml and 87.5 µl was added to each well of both groups. After incubating 4 h in standard incubation conditions, cell viability was detected by MTT assay according to the cell proliferation kit protocol as described above. Amount of colour produced was directly proportional to the number of attached viable cells; non‐attached cells had been washed off. All reported values are the means of triplicate samples. This test was repeated twice.
Assessment of the time‐dependent effects of PRP on hPDLC proliferation and ALP activity
hPDLCs were seeded in 96‐well plates at a density of 2 × 103 cells/well and were incubated for 24 h in DMEM containing 10% FBS. The medium was then replaced with DMEM containing 5% FBS. After 24 h, at the baseline of the experiment, the cells were treated with 12.5% PRP (which contained 50 ng/ml TGF‐β1 and 17 ng/ml PDGF‐AB), rhTGF‐β1 and rhPDGF‐AB combination at the same concentration or serum‐free DMEM medium. hPDLCs were further incubated in the standard culture condition. After 0, 1, 2, 3, 4, 5 and 6 days of culture, cell viability was evaluated by MTT assay and ALP activity was measured by p‐NPP substrate reactions. The reported values are the means of triplicate samples. At the same time, cells in two wells of each group cells were digested with 0.25% trypsin solution, and then cell numbers were evaluated using a haemacytometer. ALP activity data were converted to units of ALP per 104 cells.
Statistical analysis
Data were analysed using SPSS version 10.0 (Chicago, IL, USA). Statistical analysis of the data was performed by one‐way analysis of variance (anova) and post‐hoc test for multiple comparisons was carried out using the Fisher LSD test. The effect of PRP on hPDLC attachment was analysed by Student's t‐test. For all tests, statistical significances were accepted for P values lower than 0.05.
RESULTS
Platelet counts and levels of growth factors
Platelet counts and growth factor levels in the two PRP preparations are shown in Table 2. Counts in the PRP preparations were 15.11 × 105 and 18.97 × 105, respectively. In contrast, platelet counts in non‐concentrated plasma were 1.13 × 105 and 1.79 × 105, respectively. Thus, the concentration of the platelet in PRP was increased by 10.6‐ and 13.4‐fold, respectively. Supernatant of activated PRP contains high levels of TGF‐β1 and PDGF‐AB (Table 2). The TGF‐β1 and PDGF‐AB level in sample A were 403.95 ng/ml and 136.84 ng/ml, respectively. The TGF‐β1 and PDGF‐AB level in sample B were 407.50 ng/ml and 151.92 ng/ml, respectively.
Table 2.
Platelet counts and growth factor levels in PRP
| Samples | Whole blood (108/ml) | PRP (108/ml) | PRP/whole blood ration | TGF‐β1 (ng/ml) | PDGF‐AB (ng/ml) |
|---|---|---|---|---|---|
| A | 1.13 | 15.11 | 13.4 | 403.95 | 136.84 |
| B | 1.79 | 18.97 | 10.6 | 407.50 | 151.92 |
PDGF, platelet derived growth factor; PRP, platelet‐rich plasma; TGF, transforming growth factor.
Dose‐dependent effect of PRP on hPDLC proliferation
The effects of different PRP concentrations on hPDLC proliferation at 72 h are shown in Fig. 1. There was a statistically significant increase in hPDLC proliferation when the sample contained PRP 1–200 ng/ml TGF‐β1 compared to the negative control. The addition of rhTGF‐β1 and rhPDGF‐AB combination stimulated hPDLC proliferation only when the rhTGF‐β1 level was within the range of 50–200 ng/ml. rhTGF‐β1 and rhPDGF‐AB combination was less stimulatory than PRP. When TGF‐β1 in PRP was between 10 and 200 ng/ml, PRP induced a statistically significant increase in proliferation compared to rhTGF‐β1 and rhPDGF‐AB combinations. Furthermore, when TGF‐β1 level in PRP was between 50 and 200 ng/ml, the increase in cell proliferation induced by PRP was statistically higher than with other PRP concentrations. Close to a 1.5–2‐fold increase in the number of hPDLCs was observed in PRP treated groups at these concentrations compared to negative control groups after 3 days in culture.
Figure 1.
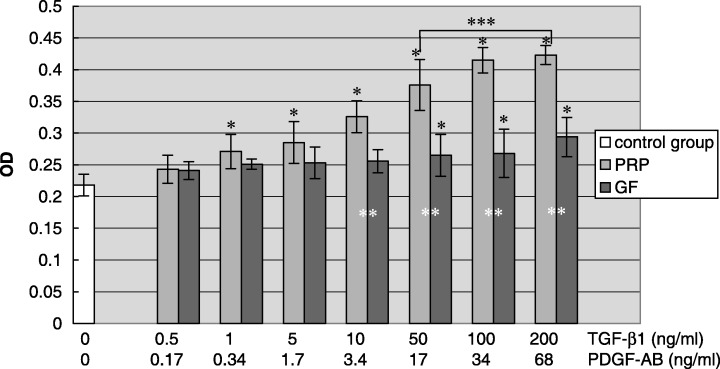
Effect of different PRP concentrations on hPDLC proliferation. The result represents the means ± SD of three replicates. Control group, serum‐free DMEM medium; PRP, serum‐free DMEM medium with the supernatant of activated platelet‐rich plasma; growth factor, serum‐free DMEM medium containing the combination of rhTGF‐β1 and rhPDGF‐AB. *P < 0.05 vs. control group; **P < 0.05 PRP group vs. growth factor group; ***P < 0.05 vs. other concentration subgroups among PRP group.
Dose‐dependent effect of PRP on ALP activity in hPDLCs
The ALP activity of hPDLCs in response to different PRP concentrations was increased in a dose‐dependent mode of action, as indicated in Fig. 2. Following 72 h of exposure to the various concentrations of PRP, there was a statistically significant increase in ALP activity of hPDLC treated with PRP containing 5–200 ng/ml TGF‐β1. The highest cell ALP activity was obtained with PRP containing 50 ng/ml TGF‐β1, ALP activity of hPDLCs was increased up to 2‐fold compared to serum free medium control and was significantly higher than other PRP concentrations. rhTGF‐β1 and rhPDGF‐AB concentration containing 50–200 ng/ml TGF‐β1 also increased ALP activity in hPDLCs, although to a much lesser extent than PRP.
Figure 2.
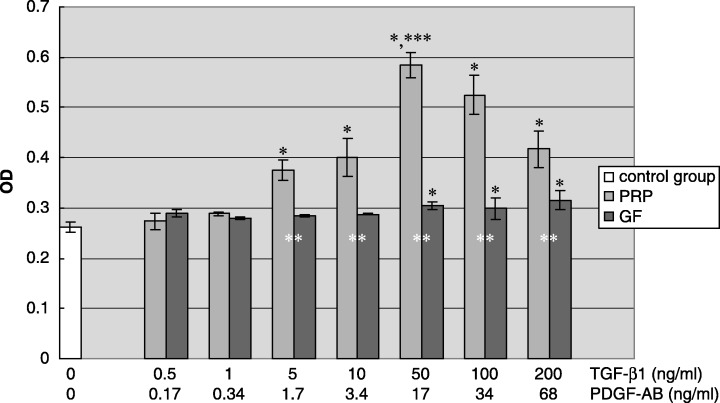
Effect of different PRP concentrations on ALP activity of hPDLC. The result represents the means ± SD of three replicates. Control group, serum‐free DMEM medium; PRP, serum‐free DMEM medium with the supernatant of activated platelet‐rich plasma; growth factor, serum‐free DMEM medium containing the combination of rhTGF‐β1 and rhPDGF‐AB. *P < 0.05 vs. control group; **P < 0.05 PRP group vs. growth factor group; ***P < 0.05 vs. other concentration subgroups among PRP group.
Effect of PRP on hPDLC attachment
Platelet‐rich plasma (PRP) containing 50 ng/ml TGF‐β1 promoted hPDLC attachment by around 1.4‐fold compared to serum free medium (Fig. 3).
Figure 3.
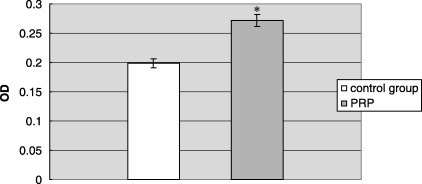
Effect of PRP on hPDLC attachment. The result represents the means ± SD of three replicates. Control group, serum‐free DMEM medium; PRP, 12.5% (v/v) platelet‐rich plasma in serum‐free DMEM medium. The end concentration of TGF‐β1 and PDGF‐AB was 50 ng/ml and 17 ng/ml, respectively. *P < 0.05 PRP group vs. control group.
Time‐dependent effects of PRP on hPDLC proliferation
Human periodontal ligament cell cultured with serum‐free medium alone did not show any obvious signs of cell proliferation, whereas hPDLC incubated with PRP or rhTGF‐β1 and rhPDGF‐AB combination resulted in an increase in proliferation (Fig. 4). Close to a 2.5‐fold and 1.5‐fold increase in the number of proliferating cells was observed in the PRP group when compared to control and growth factor groups, respectively. In groups treated with PRP instead of rhTGF‐β1 and rhPDGF‐AB, at parallel time points, around a 1.5‐fold increase in the number of cells was measured in PRP groups compared to growth factor groups.
Figure 4.
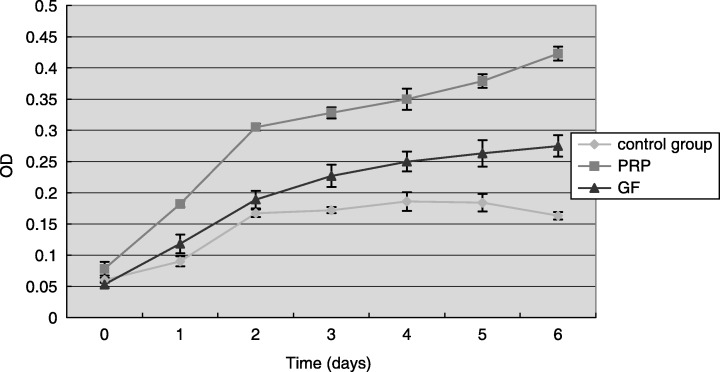
Time‐dependent effect of PRP on hPDLC proliferation. The result represents the means ± SD of three replicates. Control group, serum‐free DMEM medium; PRP, 12.5% (v/v) platelet‐rich plasma in serum‐free DMEM medium, the end concentration of TGF‐β1 and PDGF‐AB was 50 ng/ml and 17 ng/ml, respectively; growth factor, serum‐free DMEM medium containing the combination of 50 ng/ml rhTGF‐β1 and 17 ng/ml rhPDGF‐AB.
Time‐dependent effects of PRP on hPDL cells ALP activity
Human periodontal ligament cell cultured with PRP showed a significantly increase of ALP activity compared to control and growth factor groups (Fig. 5). ALP activity of hPDLCs reached their maximum after 3 days of culture with PRP, which was 2.5‐fold higher compared to control groups and 2‐fold compared to growth factor groups. ALP activity of hPDLCs cultured with PRP maintained significantly higher than control levels through the 6‐day culture time, while the ALP activity of hPDLC cultured with the rhTGF‐β1 and rhPDGF‐AB combination was only significantly higher than the control group in the second and third days.
Figure 5.
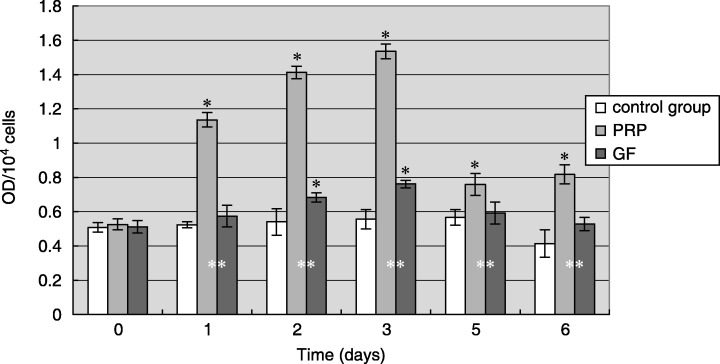
Time‐dependent effect of PRP on ALP activity of hPDLC. The result represents the means ± SD of three replicates. Control group, serum‐free DMEM medium; PRP, 12.5% (v/v) platelet‐rich plasma in serum‐free DMEM medium, the end concentration of TGF‐β1 and PDGF‐AB was 50 ng/ml and 17 ng/ml, respectively; growth factor, serum‐free DMEM medium containing the combination of 50 ng/ml rhTGF‐β1 and 17 ng/ml rhPDGF‐AB. *P < 0.05 vs. control group; **P < 0.05 PRP group vs. GF group.
DISCUSSION
In recent years in periodontics, the use of PRP has gained considerable popularity for the purpose of delivering growth factors to a wound‐healing site, in order to promote tissue regeneration. Because PRP is prepared in the dental surgery from autogenous blood, it confers several advantages over other products and techniques, most notably in terms of simplicity, safety and cost‐effectiveness. Although its therapeutic effectiveness is still under investigation by many research groups, several teams have recently reported significant increase in bone formation and maturation rates (Marx et al. 1998; Kassolis et al. 2000; Kim et al. 2002; Mazor et al. 2004), while others found no additional benefit (Aghaloo et al. 2002; Froum et al. 2002; Jensen et al. 2004; Roldán et al. 2004). By now, the clinical usefulness of PRP still remains controversial (Sanchez et al. 2003; Tozum & Demiralp 2003; Marx 2004).
Due to variation in procedures for centrifugation of the original blood samples (e.g. force, time), platelet density in PRP preparations was different. In different studies, percentage increase in platelet density was from 174% to 1000%, and there were significant variations in growth factor concentrations between individuals, platelet collection efficiency, platelet and white blood cell content and degree of platelet activation by the production process. For example, Weibrich et al. (2003) recently evaluated concentrated levels of TGF‐β1 and PDGF‐AB in PRP obtained from the same whole blood sample by two different methods. TGF‐β1 levels were determined as 467 ng/ml and 80 ng/ml, respectively, and PDGF‐AB levels were 252 ng/ml and 314 ng/ml, respectively. In further work (Okuda et al. 2003), the TGF‐β1 and PDGF‐AB levels in PRP were 140.9 ng/ml and182.0 ng/ml, respectively. Platelet concentration and growth factor levels in PRP preparation are important aspects to be considered in evaluation of the validity of a method and are crucial to being able to compare studies of PRP. We believed that varying methods of PRP preparation and consequently, concentrations of growth factor levels may explain the inconsistent clinical results.
Platelet‐rich plasma are mitogenic for a variety of cell types, including bone cells released from embryonic tissue (Slater et al. 1995), bone cells from adults (Gruber et al. 2002), AG1518 FBs (Liu et al. 2002), rat bone marrow‐derived cells (Oprea et al. 2003), rat calvarial bone cells (Soffer et al. 2004), human mesenchymal progenitor cells (Lucarelli et al. 2003; Gruber et al. 2004), calf periosteum‐derived cells (Gruber et al. 2003), endothelial cells (Kilian et al. 2004; Fréchette et al. 2005), commercially available human gingival fibroblast (Gin‐1), osteoblast MG63 and rat osteoblast UMR106 cell lines (Okuda et al. 2003), and human osteosarcoma cell lines HOS and SaOS‐2 (Kanno et al. 2005). However, little data exist with regard to cells of human periodontal ligament (Okuda et al. 2003). Our studies have indicated that PRP also stimulated attachment and proliferation of hPDLCs, which is consistent with findings reported above.
Although the biological rationale for the use of PRP is theoretically sound, precise methodology that will result in the optimal benefit from this therapy is yet to be elucidated. In particular, the precise platelets and growth factor concentrations that would be ideal are yet to be determined. Some recent studies have shown a PRP dose‐dependent proliferation increase in the cells under investigation (Lucarelli et al. 2003; Gruber et al. 2004; Kilian et al. 2004; Soffer et al. 2004), with increasing PRP concentrations resulting in enhanced cell proliferation. However, these studies varied greatly in the methodology that was utilized, including length of observation time, method of PRP preparation, composition of the PRP preparations (co‐incubated with 1–2% foetal calf serum (FCS)) and the type of cells used. Our study has suggested that the increase in cell proliferation induced by PRP containing 50–200 ng/ml TGF‐β1 (12.5%∼50% in v/v) was statistically significantly higher than other PRP concentration, but there was no statistical difference within the range of these concentrations. Graziani's study (2006) found that although the effect of PRP on oral gingival fibroblasts and osteoblast proliferation was dose dependent, increasing PRP concentrations did not result in increasing cell proliferation. Optimal results were obtained by final concentration of 16.5% (at a platelet concentration of 2.5‐fold). Liu et al. (2002) investigated the effect of different platelet concentrations on fibroblasts. They prepared maximally concentrated platelet preparations and diluted them in media to final concentrations of 8.8%, 17.5% and 35%. They found that superior proliferation was obtained with the 8.8% and 17.5% preparations compared to the 35% concentration, because high PRP concentrations resulted in pH changes that negatively affected cell proliferation. Similarly, our study has found the high level of cell proliferation was obtained by diluting about 12.5–50 µl of the maximally concentrated PRP to 100 µl with serum free DMEM, giving a final concentration of 12.5%∼50% (v/v).
Aside from proliferation, this study also examined osteogenic differentiation of hPDLCs following exposure to different PRP concentrations, by assessing ALP activity, a representative marker of bone‐forming. When TGF‐β1 concentration in PRP was between 5 and 200 ng/ml, PRP promoted ALP activity significantly in the absence of dexamethasone, and the highest ALP activity was obtained when the TGF‐β1 level was 50 ng/ml (12.5% in v/v and platelet concentration approximately 1.9∼2.4 × 105/µl). Interestingly, there have been different reports regarding the osteogenic differentiation potential of PRP, with some studies indicating stimulation (Kilian et al. 2004; Kawase et al. 2005a; Graziani et al. 2006) and others showing inhibition (Arpornmaeklong et al. 2004; 2006, 2004). Our findings are similar to those of Weibrich et al. (2004) and Graziani et al. (2006). Weibrich et al. (2004) evaluated the in vivo effect of different concentrations of platelets on the bone regeneration histologically, around implants placed in femurs of rabbits. They found that the use of platelet concentrate had a positive effect on bone regeneration only within an ‘intermediate’ concentration range, which is approximately 503 000–1 729 000/µl. In fact, the use of highly concentrated platelet preparations (6–11 times the basal platelet count) appeared to have an inhibitory effect on healing. In Graziani's study (2006), PRP‐max (33%) stimulated osteoclastogenesis and osteoblast differentiation. This indicates that high concentrations of platelets may lead to an increase in osteoblast differentiation at the cost of cell proliferation. 2005a, 2005b) also found that PRP time dependently increases ALP activity and transmission electron microscopy revealed that mineralized spicules were initially deposited onto PRP‐derived platelet aggregates. They suggested that PRP provided platelet aggregates with nuclei on which to initiate mineralization.
From the above results of our study, it seems that the ideal PRP concentration is when the TGF‐β1 is between 50 and 100 ng/ml (12.5%∼25% in v/v). If more were known about the mechanisms and ideal concentration of platelets and growth factors in the platelet concentrate fraction, it would easily be possible to vary the concentration by increasing or reducing the amount of plasma left in the platelet concentrate fraction.
It was considered that PRP actions were essentially identical to those of TGF‐β and PDGF. However, our study has shown that compared to rhTGF‐β1 and rhPDGF‐AB concentration at the same concentration alone, PRP more potently promoted hPDLC proliferation and ALP activity. Also, TGF‐β1 and PDGF‐AB concentrations for this stimulation effect were much lower in PRP than in the growth factor group and the stimulatory effect appear earlier in PRP group. Therefore, we speculate that other identified and unidentified components in PRP may be directly or indirectly involved in modulation of PDLC proliferation and differentiation. According to already published data, it is likely that some other growth factors (such as IGF and EGF) contained in PRP, could also promote hPDLC proliferation and ALP activity. On the other hand, it seems likely that other particular components can effectively facilitate hPDLC proliferation and ALP activity in the presence of lower levels of TGF‐β1 and PDGF‐AB. Recently, Kawase et al. (2005a) suggested that beside concentrated TGF‐β1 in PRP, fibrinogen‐mediated actions also played an important role in PDLC proliferation and the promotion of ALP activity. PRP produced a number of potent effects on hPDLCs that do not solely reflect simple combination of its major known growth factors.
In summary, our study has demonstrated that PRP can serve as a source for representative growth factors such as TGF‐β and PDGF. PRP is an effective stimulator of hPDLC attachment and cell proliferation and PRP can increase ALP activity as well as stimulate osteoblastic differentiation of hPDLC to a certain extent. These two actions in combination would effectively promote bone repair and periodontal regeneration at the site of periodontal tissue injury more potently than PDGF or TGF each alone or both together. Furthermore, these data indicate that these effects follow a dose‐dependent mode. The ideal concentration of PRP is that which contains 50–100 ng/ml TGF‐β1, which provided the most optimal environment for periodontal regeneration through a closer to ideal balance between proliferation and differentiation. These findings therefore provide convincing evidence and useful data for the clinical application of PRP as a potent tool to facilitate periodontal regeneration, and suggest that future clinical studies should be designed with the understanding that PRP concentrations may affect clinical outcome.
ACKNOWLEDGEMENTS
This research was supported by the Special Fund for Promotion of Education, Ministry of Education P.R.C.; Clinical Research Fund, Ministry of Health P.R.C.
REFERENCES
- Aghaloo TL, Moy PK, Freymiller EG (2002) Investigation of platelet‐rich plasma in rabbit cranial defects: a pilot study. Int. J. Oral Maxillofac. Surg. 60, 1176–1181. [DOI] [PubMed] [Google Scholar]
- Arpornmaeklong P, Kochel M, Depprich R, Kubler NR, Wurzler KK (2004) Influence of platelet‐rich plasma (PRP) on osteogenic differentiation of rat bone marrow stromal cells. An in vitro study. Int. J. Oral Maxillofac. Surg. 33, 60–70. [DOI] [PubMed] [Google Scholar]
- Cochran DL, Wozney JM (1999) Biological mediators for periodontal regeneration. Periodontol. 2000 19, 40–58. [DOI] [PubMed] [Google Scholar]
- Fréchette JP, Martineau I, Gagnon G (2005) Platelet‐rich plasmas: growth factor content and roles in wound healing. J. Dent. Res. 84, 434–439. [DOI] [PubMed] [Google Scholar]
- Froum SJ, Wallace SS, Tarnow DP, Cho SC (2002) Effect of platelet‐rich plasma on bone growth and osseointegration in human maxillary sinus grafts: three bilateral case reports. Int. J. Periodontics Restorative Dent. 22, 45–53. [PubMed] [Google Scholar]
- Gentry PA (1992) The mammalian blood platelet: its role in haemostasis: inflammation and tissue repair. J. Comp. Pathol. 107, 243–270. [DOI] [PubMed] [Google Scholar]
- Graziani F, Ivanovski S, Cei S, Ducci F, Tonetti M, Gabriele M (2006) The in vitro effect of different PRP concentrations on osteoblasts and fibroblasts. Clin. Oral Implants Res. 17, 212–219. [DOI] [PubMed] [Google Scholar]
- Gruber R, Kandler B, Fischer MB, Watzek G (2006) Osteogenic differentiation induced by bone morphogenetic proteins can be suppressed by platelet‐released supernatant in vitro . Clin. Oral Implants Res. 17, 188–193. [DOI] [PubMed] [Google Scholar]
- Gruber R, Karreth F, Frommlet F, Fischer MB, Watzek G (2003) Platelets are mitogenic for periosteum‐derived cells. J. Orthop. Res. 21, 941–948. [DOI] [PubMed] [Google Scholar]
- Gruber R, Karreth F, Kandler B, Fuerst G, Rot A, Fischer MB, Watzek G (2004) Platelet‐released supernatants increase migration and proliferation, and decrease osteogenic differentiation of bone marrow‐derived mesenchymal progenitor cells under in vitro conditions. Platelets 15, 29–35. [DOI] [PubMed] [Google Scholar]
- Gruber R, Varga F, Fischer MB, Watzek G (2002) Platelets stimulate proliferation of bone cells: involvement of platelet‐derived growth factor, microparticles and membranes. Clin. Oral Implants Res. 13, 529–535. [DOI] [PubMed] [Google Scholar]
- Jensen TB, Rahbek O, Overgaard S, Søballe K (2004) Platelet rich plasma and fresh frozen bone allograft as enhancement of implant fixation An experimental study in dogs. J. Orthop. Res. 22, 653–658. [DOI] [PubMed] [Google Scholar]
- Kanno T, Takahashi T, Tsujisawa T, Ariyoshi W, Nishihara T (2005) Platelet‐rich plasma enhances human osteoblast‐like cell proliferation and differentiation. Int. J. Oral Maxillofac. Surg. 63, 362–369. [DOI] [PubMed] [Google Scholar]
- Kassolis JD, Rosen PS, Reynolds MA (2000) Alveolar ridge and sinus augmentation utilizing platelet‐rich plasma in combination with freezedried bone allograft: case series. J. Periodontol. 71, 1654–1661. [DOI] [PubMed] [Google Scholar]
- Kawase T, Okuda K, Saito Y, Yoshie H (2005a) In vitro evidence that the biological effects of platelet‐rich plasma on periodontal ligament cells is not mediated solely by constituent transforming‐growth factor‐beta or platelet‐derived growth factor. J. Periodontol. 76, 760–767. [DOI] [PubMed] [Google Scholar]
- Kawase T, Okuda K, Saito Y, Amizuka N, Suzuki H, Yoshie H (2005b) Platelet‐rich plasma provides nucleus for mineralization in cultures of partially differentiated periodontal ligament cells. In Vitro Cell. Dev. Biol. Anim. 41, 171–176. [DOI] [PubMed] [Google Scholar]
- Kilian O, Flesch I, Wenisch S, Taborski B, Jork A, Schnettler R, Jonuleit T (2004) Effects of platelet growth factors on human mesenchymal stem cells and human endothelial cells in vitro . Eur J. Med. Res. 9, 337–344. [PubMed] [Google Scholar]
- Kim ES, Park EJ, Choung PH (2001) Platelet concentration and its effect on bone formation in calvarial defects: an experimental study in rabbits. J. Prosthet. Dent. 86, 428–433. [DOI] [PubMed] [Google Scholar]
- Kim SG, Chung CH, Kim YK, Park JC, Lim SC (2002) Use of particulate dentin‐plaster of Paris combination with/without platelet‐rich plasma in the treatment of bone defects around implants. Int. J. Oral Maxillofac. Imp. 17, 86–94. [PubMed] [Google Scholar]
- Lekic PC, Rajshankar D, Chen H, Tenenbaum H, McCulloch CA (2001) Transplantation of labelled periodontal ligament cells promotes regeneration of alveolar bone. Anat. Rec. 262, 193–202. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kalen A, Risto O, Wahlstrom O (2002) Fibroblast proliferation due to exposure to a platelet concentrate in vitro is pH dependent. Wound Repair Regen. 10, 336–340. [DOI] [PubMed] [Google Scholar]
- Lucarelli E, Beccheroni A, Donati D, Sangiorgi L, Cenacchi A, Del Vento AM, Meotti C, Bertoja AZ, Giardino R, Fornasari PM, Mercuri M, Picci P (2003) Platelet‐derived growth factors enhance proliferation of human stromal stem cells. Biomaterials 24, 3095–3100. [DOI] [PubMed] [Google Scholar]
- Marx RE (2004) Platelet‐rich plasma: evidence to support its use. Int. J. Oral Maxillofac. Surg. 62, 489–496. [DOI] [PubMed] [Google Scholar]
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR (1998) Platelet rich plasma: growth factor enhancement for bone grafts. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 85, 638–646. [DOI] [PubMed] [Google Scholar]
- Mazor Z, Peleg M, Garg AK, Luboshitz J (2004) Platelet‐rich plasma for bone graft enhancement in sinus floor augmentation with simultaneous implant placement: patient series study. Implant Dent. 13, 65–72. [DOI] [PubMed] [Google Scholar]
- Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, Wolff LF, Yoshie H (2003) Platelet‐rich plasma contains high levels of platelet‐derived growth factor and transforming growth factor‐beta and modulates the proliferation of periodontally related cells in vitro. J. Periodontol. 74, 849–857. [DOI] [PubMed] [Google Scholar]
- Oprea WE, Karp JM, Hosseini MM, Davies JE (2003) Effect of platelet releasate on bone cell migration and recruitment in vitro . J. Craniofac Surg. 14, 292–300. [DOI] [PubMed] [Google Scholar]
- Roldán JC, Jepsen S, Miller J, Freitag S, Rueger DC, Açil Y, Terheyden H (2004) Bone formation in the presence of platelet‐rich plasma vs. bone morphogenetic protein‐7. Bone 34, 80–90. [DOI] [PubMed] [Google Scholar]
- Sanchez AR, Sheridan PJ, Kupp LI (2003) Is platelet‐rich plasma the perfect enhancement factor? A current review. Int. J. Oral Maxillofac. Imp. 18, 93–103. [PubMed] [Google Scholar]
- Slater M, Patava J, Kingham K, Mason RS (1995) Involvement of platelets in stimulating osteogenic activity. J. Orthop. Res. 13, 655–663. [DOI] [PubMed] [Google Scholar]
- Soffer E, Ouhayoun JP, Dosquet C, Meunier A, Anagnostou F (2004) Effects of platelet lysates on select bone cell functions. Clin. Oral Implants Res. 15, 581–588. [DOI] [PubMed] [Google Scholar]
- Tozum TF, Demiralp B (2003) Platelet‐rich plasma: a promising innovation in dentistry. J. Can. Dent. Assoc. 69, 664. [PubMed] [Google Scholar]
- Weibrich G, Hansen T, Kleis W, Buch R, Hitzler WE (2004) Effect of platelet concentration in platelet‐rich plasma on peri‐implant bone regeneration. Bone 34, 665–671. [DOI] [PubMed] [Google Scholar]
- Weibrich G, Kleis Wilfried KG, Buch R, Hitzler WE, Hafner G (2003) The Harvest Smart PRePTM system versus the Friadent‐Schütze platelet‐rich plasma kit: Comparison of a semiautomatic method with a more complex method for the preparation of platelet concentrates. Clin. Oral Implants Res. 14, 233–239. [DOI] [PubMed] [Google Scholar]


