Abstract
Objective
Curcumin (capable of inhibiting angiogenic growth of human umbilical vein endothelial cells [HUVECs]), can be employed in vitro as a model of pathogenesis of corneal neovascularization (CRNV). The aim of this study was to explore regulatory mechanisms of microRNA (miR) levels after curcumin treatment.
Materials and methods
Expression profiles of miRs in curcumin‐treated HUVECs were investigated by miR microassay. Specific mimics and inhibitors of miR‐1275 or miR‐1246 were transfected into HUVECs. Then, their target genes, vascular endothelial growth factor B (VEGFB) and nuclear transcription factor kappa B acting protein (NKAP) were detected by quantitative real‐time PCR, Western blotting assay or immunofluorescence assay. Cell proliferation and cell cycle parameters were measured with the help of CCK‐8 assay and flow cytometry.
Results
MiR‐1275 and miR‐1246 expression levels were up‐regulated by curcumin. Administration of the specific mimics and inhibitors of the two miRs led to significant changes in expression of VEGFB and NKAP as well as the indicators related to angiogenesis. Anti‐angiogenic effect of curcumin depended on expression patterns of the two miRs in that inhibition of either miR interfered with the effect of curcumin. Furthermore, overexpression of NKAP interrupted effects of curcumin on the cells.
Conclusion
Collectively, our findings demonstrate that curcumin inhibited HUVEC proliferation by up‐regulation of miR‐1275 and miR‐1246.
1. Introduction
Many corneal neovascularization (CRNV)‐related eye diseases, such as infectious keratitis caused by fungi, bacteria, acanthamoeba, etc., pose a diagnostic dilemma to public health, especially in developing countries. If adequate control measures are not instituted in time, the disorder causes severe ocular morbidity and blindness.1 In healthy eyes, cornea is an avascular organ.2 However, once infected or stimulated by angiogenic factors, new blood vessels start to develop from the vessel of limbal area, reach on the top of the cornea and support the recruitment of inflammatory cells.3 These inflammatory cells, especially neutrophils, produce many angiogenic factors such as NF‐кB and VEGF and finally cause blindness. These steps are common in the pathogenesis of most ocular diseases, including infectious keratitis.4, 5, 6 Therefore, antagonizing new blood vessel formation is a promising therapy for the treatment of CRNV‐related eye diseases.
Three major pathways through which new vessels form in both health and disease include vasculogenesis, angiogenesis and intussusception.7 Among the three modes, angiogenesis—the growth of new blood vessels from an existing vasculature—is the major driving force in many severe human disorders.8 This process is also associated with the differential recruitment of supporting cells to different segments of the vasculature.9 In adults, angiogenesis occurs by the mobilization of endothelial cells from preexisting of capillaries. Moreover, the onset of angiogenesis requires degradation of extracellular matrix, cell migration, cell proliferation and cell organization in new capillary networks.10 Persistent and up‐regulated angiogenesis is pathological and is often found to be associated with conditions such as arthritis, cancer, diabetic retinopathy or atherosclerosis.11, 12
The functionality of endothelial cells is fundamental for the homeostasis of the vascular system. Endothelial cells also serve as the primary sensor of blood flow‐mediated mechanotransduction.13 Based on these studies, a classical type of endothelia cells, human umbilical vein endothelial cells (HUVECs), has been employed as an in vitro model to study angiogenesis. The cells are capable of forming capillary like structures called tubes in response to appropriate stimuli.14 Application of HUVECs in angiogenesis‐related studies has contributed to the discovery of numerous compounds with therapeutic potency against different types of devastating diseases.15, 16, 17 Curcumin, also known as “diferuloylmethane,” is a principal active component of turmeric (Curcuma Longa Linn). The compound has anti‐inflammatory and antioxidant activities and has been widely used for centuries in indigenous medicine for the treatment of a variety of diseases.18, 19, 20, 21 Regarding the effect of curcumin on angiogenesis, several studies have verified the powerful function of the agent to inhibit the angiogenic differentiation of HUVECs.15, 22, 23, 24 Moreover, it has been demonstrated that curcumin suppresses the angiogenesis through NF‐κB pathways.23 Thus, it is reasonable to take curcumin as a promising treatment modality for angiogenesis‐related disorders, i.e., corneal neovascularization.
In our previous studies on the anti‐angiogenic effect of curcumin, we found that the administration of curcumin on HUVECs not only influenced pathways but also significantly changed the expression profiles of multiple micro‐RNAs (miRs) (unpublished data). MiRs have recently come into focus as a powerful mechanism to control gene expression after transcription. They are short, single‐stranded RNA molecules transcribed from non‐coding genes. Members of miRs enter into the RNA interference pathway by imperfectly binding to the 3′UTR of mRNAs and inhibit translation processes.25, 26, 27 In recent years, emerging evidence confirms the key role of miRs in vascular and endothelial cell biology.13 The highly expressed let‐7f and miR‐27b exert a pro‐angiogenic effect as evidenced by the blockade of in vitro angiogenesis with 2′‐O‐methyl oligonucleotide inhibitors.28 Moreover, among the types of miRs in HUVECs, miR‐221 and miR‐222 were proved to have anti‐angiogenic effect.29 However, even with the evident roles of curcumin and miRs in the angiogenesis process of multiple diseases, the interaction between the two factors is scarcely reported. Therefore, an investigate targeting the association between curcumin and miRs in the anti‐angiogenic process is reasonable for development of novel therapeutic strategies for angiogenesis‐related disorders, i.e., corneal neovascularization.
In our study, it was hypothesized that miRs were involved in the posttranscriptional modulation of angiogenesis by curcumin in corneal neovascularization. To validate the hypothesis, HUVECs were employed as an in vitro model to mimic the vessel formation process in the pathogenesis of keratitis. The miR expression profile in HUVECs was extensively investigated and most significantly dysexpressed miRs were selected to study their putative role in the anti‐angiogenic effect of curcumin on HUVECs. In particular, this is the first study to demonstrate that the miR‐1275 and miR‐1246 can modulate the activities of several angiogenesis‐related factors during the effect of curcumin on HUVECs by suppressing their target genes, vascular endothelial growth factor B (VEGFB) and nuclear factor kappa B acting protein (NKAP), which provides a new insight into the protective effect of curcumin against CRNV.
2. Materials and methods
2.1. Chemicals and cell culture
Curcumin (Sigma‐Aldrich, St. Louis, MO, USA) was dissolved in DMSO. Specific mimics and inhibitors for miR‐1275 and miR‐1246 and non‐targeting mimics and inhibitors were obtained from GenePharma, China. HUVECs were routinely collected from umbilical cords of newborns30 and cultured in DEMD medium supplemented with 20% foetal bovine serum (FBS) and 1% (v/v) antibiotics mixture in an atmosphere of 95% air and 5% CO2 at 37°C.
2.2. Determination of curcumin employed in assays
To determine the concentration of curcumin used for all the subsequent experiments, HUVECs were incubated with curcumin at concentration of 20, 40, 80 and 160 μm for 96 hours. The inhibiting effect of curcumin on proliferation of HUVECs was determined using CCK‐8 assay, which showed a concentration over 40 μm can significantly inhibit the proliferation of HUVECs (Fig. 1a) and thus employed as the concentration for subsequent detections.
Figure 1.
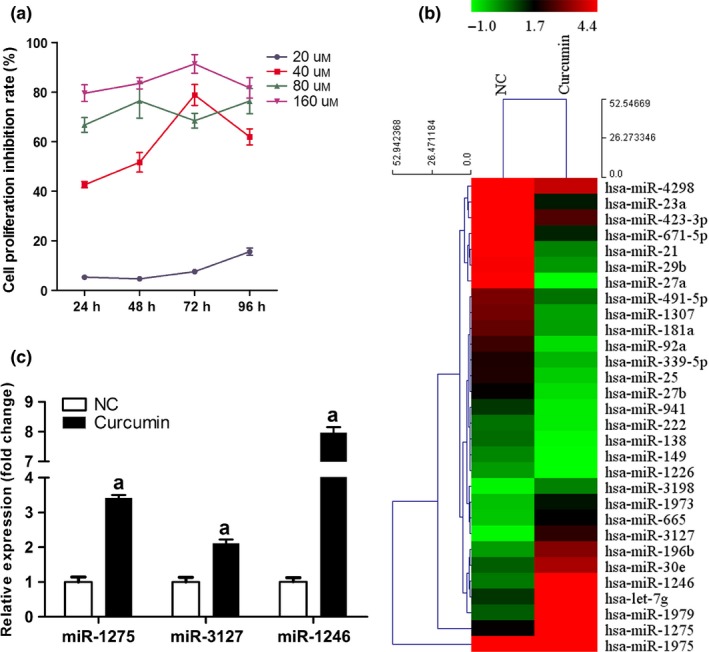
Administration of curcumin influenced the expression profiles of miRs in HUVECs. (a) Determination of curcumin concentration employed in this study with CCK‐8 methods. (b) Heat map of miR expression profiles of miRs in HUVECs. (c) Quantitative analysis results of the expression levels of miR‐1275, miR‐3127 and miR‐1246 in curcumin‐treated HUVECs with qRT‐PCR (U6 was employed as an internal reference gene, “a,” Significantly different from NC group, P<.05)
2.3. MiR microassay
Healthy HUVCEs were seeded into six‐well plates and adjusted to a concentration of 1 × 105 cell/mL. HUVCEs were randomly divided into two groups: (A) NC group, healthy HUVCEs; (B) Curcumin group, HUVCEs administrated with 40 μm curcumin for 24 hours. Thereafter, whole RNA in HUVCEs was collected and used for microassay analysis by GeneChip miRNA Arrays 2.0 (Affymetrix, Sacramento, CA, USA). The target genes of differentially expressed miRNAs were analysed using three miRNA target databases: TargetScan, TarBase and Miranda.
2.4. Transfection of specific mimics into HUVECs
To analyse the effect of miR‐1275 and miR‐1246 on the angiogenic proliferation of HUVCEs, HUVCE cells were randomly divided into four groups and transfection of different mimics were conducted using Lipofectamine‐2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol: (A) NC group, HUVCEs transfected with NC mimics; (B) miR‐1275 mimics group, HUVCEs transfected with specific miR‐1275 mimics; (C) miR‐1246 mimics group, HUVCEs transfected with specific miR‐1246 mimics; (D) miR‐1275 mimics+ miR‐1246 mimics, HUVCEs co‐transfected with miR‐1275 and miR‐1246 mimics. The effect of miR overexpression on the cell viability, cell cycle distribution and expression of specific indicators related to angiogenesis were assessed.
2.5. Dual luciferase assay
Fragments of the 3′UTR of VEGFB and NKAP and mutant 3′UTR of VEGFB and NKAP cDNAs were inserted into psiCHECK‐2 vector (vector containing firefly luciferase under the control of SV40 promoter; Promega, Madison, WI, USA) to form different WT and MUT versions of plasmids. Concentration of HUVCEs was adjusted to 1 × 104/mL and incubated for 24 hours before co‐transfected with different combinations of vectors using Lipofectamine 2000 reagent (Invitrogen), and subsequent selection was conducted using 400 μg/mL ampicillin. For assessment of action of miR‐1275 on VEGFB, cells were grouped as following: (A) WT+NC group, cells co‐transfected with WT VEGFB plasmid and NC mimics; (B) WT+miR‐1275 mimics, cells co‐transfected with WT VEGFB plasmid and miR‐1275 mimics group; (C) MUT+NC group, cells co‐transfected with MUT VEGFB plasmid and NC mimics; (D) MUT+miR‐1275 mimics group, cells co‐transfected with MUT VEGFB plasmid and miR‐1275 mimics. For assessment of action of miR‐1246 on NKAP, HUVCEs were grouped as follows: (A) WT+NC group, cells co‐transfected with WT NKAP plasmid and NC mimics; (B) WT+miR‐1246 mimics, cells co‐transfected with WT NKAP plasmid and miR‐1246 mimics group; (C) MUT+NC group, cells co‐transfected with MUT NKAP plasmid and NC mimics; (D) MUT+miR‐1246 mimics group, cells co‐transfected with MUT VEGFB plasmid and miR‐1246 mimics. Dual luciferase assay was conducted to measure the firefly luciferase activity using Luciferase Report Gene Assay Kit (Promega).
2.6. Transfection of specific inhibitors into HUVECs
To evaluate the function of miR‐1275 and miR‐1246 in the anti‐angiogenic effect of curcumin, HUVECs pre‐treated with 40 μm curcumin for 24 hours were administered with different miR inhibitors using Lipofectamine 2000 (Invitrogen) as described in the following: (A) curcumin+NC group, curcumin‐treated HUVCEs transfected with NC inhibitor; (B) curcumin+miR‐1275 inhibitor group, curcumin‐treated HUVCEs transfected with specific miR‐1275 inhibitor; (C) curcumin+miR‐1246 inhibitor group, curcumin‐treated HUVCEs transfected with specific miR‐1246 inhibitors; (D) curcumin+miR‐1275 inhibitor+miR‐1246 inhibitors, curcumin‐treated HUVCEs co‐transfected with miR‐1275 and miR‐1246 inhibitors. The effect of miR inhibition on the cell viability, cell cycle distribution and expression of specific indicators related to angiogenesis were assessed.
2.7. Exploration of the interaction between miR‐1275 and miR‐1246
To investigate the possible interaction between miR‐1275 and miR‐1246 in the proliferation of HUVECs, HUVECs were divided into three groups: (A) NC group, HUVCEs co‐transfected NC mimics and inhibitors; (B) miR‐1246 mimics+miR‐1275 inhibitor group, HUVCEs co‐transfected with specific miR‐1246 mimics and miR‐1275 inhibitors; (C) miR‐1275 mimics miR‐1246 inhibitor group, HUVCEs transfected with 1275 mimics and miR‐1246 inhibitors. The effect of different treatments on the cell viability, cell cycle distribution and expression of specific indicators related to angiogenesis were assessed.
2.8. Overexpression of NKAP in HUVECs
To further elucidate the mechanism through which curcumin inhibited the growth of HUVECs, an expression vector encoding NKAp was constructed. Two groups were set up for subsequent analyses: (A) curcumin+NC group, curcumin‐treated HUVCEs transfected with blank vector; (B) curcumin+NKAP group, curcumin‐treated HUVCEs transfected with NKAP expression vector. The effect of overexpression of NKAP on the cell viability, cell cycle distribution and expression of specific indicators related to angiogenesis were assessed.
2.9. Immunofluorescence assay
The expressions of NAKP in different groups were further detected using immunofluorescence microscopy. To examine the action of curcumin on NKAP, HUVECs were classified into two groups: (A) NC group, HUVECs; (B) curcumin group, HUVECs incubated with 40 μm curcumin for 24 hours. To illustrate the regulation of NKAP by miR‐1246, HUVCEs were divided into two groups: (A) NC group, HUVECs transfected with NC mimics; (B) 1246 mimic group, HUVECs transfected with miR‐1246 mimics. Cells were seeded into 14‐well chambers, washed with PBS, fixed with 4% paraformaldehyde for 15 minutes. Then, the cells were permeabilized with 0.5% Triton X‐100 for 30 minutes. After being washed with PBS for three cycles, 5 minutes for each cycle, the cells were blocked in 10% goat serum for 15 minutes. Primary rabbit polyclonal antibodies (1:200) to NKAP (Catl. No. ab121121; Abcam, Cambridge, MA, USA) was then added, and the cells were incubated overnight at 4°C in 1% goat serum. Staining was performed by incubating the cells with fluorescein isothiocyanate secondary antibody (1:200) (Catl. No. A0516; Beyotime Biotechnology, Shanghai, China) for 1 hours. After incubation with the secondary antibody, cells were washed and then stained with DAPI for 5 minutes at room temperature. After three cycles of 5 minutes washes with PBS buffer, the slides were fixed and imaged on a Carl Zeiss Apotome Axiovert 200 M Fluorescence Microscope (Carl Zeiss, Jena, Germany) with an Axiocam HRm camera at 400× magnification.
2.10. Quantitative real‐time PCR (qRT‐PCR)
Following RNA simple Total RNA Kit (TIANGEN, Peking, China), total RNA extracted from different samples was reverse transcribed and subjected to PCR. The final qRT‐PCR reaction mixture of volume 20 μL consisted of 10 μL SYBR GREEN mastermix, 0.5 μL each primers (Table 1), 1 μL cDNA template and 8 μL Rnase free H2O. Thermal cycling parameters were set up as a denaturation at 94°C for 2 minutes, followed by 40 cycles at 95°C for 20 seconds, 58°C for 20 seconds and 72°C for 20 seconds. Relative expression level of target gene was calculated with Mx3000P (Agilent Technologies, Santa Clara, CA, USA) according to the expression of 2−△△ct.
Table 1.
Primers for quantitative real‐time PCR
| Name | Sequence(5′‐3′) |
|---|---|
| U6 F | CTCGCTTCGGCAGCACA |
| U6 R | AACGCTTCACGAATTTGCGT |
| β‐actin F | ATCGTGCGTGACATTAAGGAGAAG |
| β‐actin R | AGGAAGGAAGGCTGGAAGAGTG |
| TNF‐a F | CCTCTCTCTAATCAGCCCTCTG |
| TNF‐a R | GAGGACCTGGGAGTAGATGAG |
| IL‐1 F | AGCTACGAATCTCCGACCAC |
| IL‐1 R | CGTTATCCCATGTGTCGAAGAA |
| NF‐kB p65 F | TGCACCCTGATATGTATGAGAGA |
| NF‐kB p65 R | AAATGGCAGTGATCCAGTAGC |
| CyclinD1 F | GCTGCGAAGTGGAAACCATC |
| CyclinD1 R | CCTCCTTCTGCACACATTTGAA |
| VEGFB F | GAGATGTCCCTGGAAGAACACA |
| VEGFB R | GAGTGGGATGGGTGATGTCAG |
| MMP‐9 F | GGGACGCAGACATCGTCATC |
| MMP‐9 R | TCGTCATCGTCGAAATGGGC |
| has‐miR‐3127 | ATCAGGGCTTGTGGAATGGGAAG |
| has‐miR‐3127 RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCTTCCCA |
| has‐miR‐3127F | ACACTCCAGCTGGGATCAGGGCTTGTGGA |
| hsa‐miR‐1246 | AATGGATTTTTGGAGCAGG |
| hsa‐miR‐1246 RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCTGCTC |
| hsa‐miR‐1246 F | ACACTCCAGCTGGGGAATGGATTTTTGGAG |
| hsa‐miR‐1275 | GTGGGGGAGAGGCTGTC |
| hsa‐miR‐1275 RT | CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGACAGCC |
| hsa‐miR‐1275 F | ACACTCCAGCTGGGGTGGGGGAGAGGC |
| ALL R | CTCAACTGGTGTCGTGGA |
2.11. CCK‐8 assay
For proliferation measurement, HUVECs in different treatments were incubated routinely. Cell sampling was conducted every 24 hours and each time point was represented by at least three replicates. The cell viability was measured using a CCK‐8 method; cell cultures were incubated with 100 μL CCK‐8 solution at 37°C for 1 hour and OD at 450 nm was recorded using a Mircoplate Reader.
2.12. Flow cytometry
For determination of cell cycle distribution in different groups, cells were collected by centrifugation (581.36g, 5 minutes) and stained with propidium iodide (PI)‐FITC after fixation with 70% alcohol (4°C, 2 hours) and analysed on Accuri™ C6 flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA).
2.13. Western blotting assay
Protein of different samples was extracted using Whole Protein Extraction Kit (Wanleibio, Shenyang, China), and nuclear NF‐кB subunit p65 was extracted using Nuclear and Cytoplasm Protein Extraction kit (Wanleibio). Total protein concentration was estimated by BCA method. Equal amounts of protein were subjected to 10% SDS‐PAGE and electrically transferred onto PVDF membranes. Subsequently, the membranes were washed with TTBS for 5 minutes, blocked for 1 hour with 5% skim milk and probed with specific primary antibodies like anti‐NF‐кB p65 (1:5000), anti‐Cyclin D1 (1:2000), anti‐MMP‐9 (1:2000), anti‐VEGFB (1:5000), anti‐NA PK (1:1000) and anti‐GAPDH (1:5000) (Abcam, Cambridge, UK) followed by incubation with IgG tagged with HRP (Santa Cruz Biotechnology, Santa Cruz, CA, USA; 1:2000) for 45 minutes at 37°C. The blots were developed using Beyo ECL Plus reagent and observed in the Gel Imaging System. The relative expression levels of protein in different groups were calculated with Gel‐Pro‐Analyzer (Media Cybernetics, Maryland, MD, USA).
2.14. Statistical analysis
All the data were expressed in the form of mean ± SD. Student t‐test and multiple comparisons using LSD method were performed with a significant level of 0.05 with GraphPad Prism 6 (GraphPad Software, La Jolla, CA, USA).
3. Results
3.1. MiR expression profiling in HUVECs during curcumin administration
HUCECs were incubated with curcumin for 24 hours before subjecting to miRNA expression microarrays. Among 576 types of human miRs that could be detected by the probes, the expression levels of 30 miRs were significantly changed in curcumin group compared with those in the NC group (Fig. 1b), 11 miRs were up‐regulated and 19 miRs were down‐regulated. Based on the data of fold changing (unpublished data), three members, including miR‐1275, miR‐3127 and miR‐1246, were selected for qRT‐PCR validation. We observed that the relative expression levels of miR‐1275 and miR‐1246 changed more than that of miR‐3127 (Fig. 1c).
3.2. Activation of miR‐1275 and miR‐1246 inhibited the angiogenic proliferation of HUVECs
To explore the roles of miR‐1275 and miR‐1246 in the proliferation of HUVECs, cells were administrated with different combinations of specific mimics of the two miRs. As illustrated in Fig. 2a, respective administration of two mimics both inhibited the proliferation of HUVECs. Moreover, concatenated administration of the two mimics exhibited a synergistic effect to reduce the viability of HUVECs (Fig. 2a). The qRT‐PCR and Western blotting results showed that NF‐кB p65, Cyclin D1, VEGFB, MMP‐9 responded to miR‐1275 and miR‐1246 administration, while it had no impact on the production of angiogenesis inducing indicators, TNF‐α and IL‐1 (Fig. 2b–d). Based on these results, the target genes of the two miRs were predicted with bioinformatics method, and it was found that the potential targets of miR‐1275 and miR‐1246 were VEGFB and NAKP, respectively (unpublished data). The flow cytometry detection result showed reduction in the proportion of cells distributed in S phase following the treatment with miR mimics which further confirms the role of miR‐1257 and miR‐1246 in proliferation of HUVECs (Fig. 2e).
Figure 2.
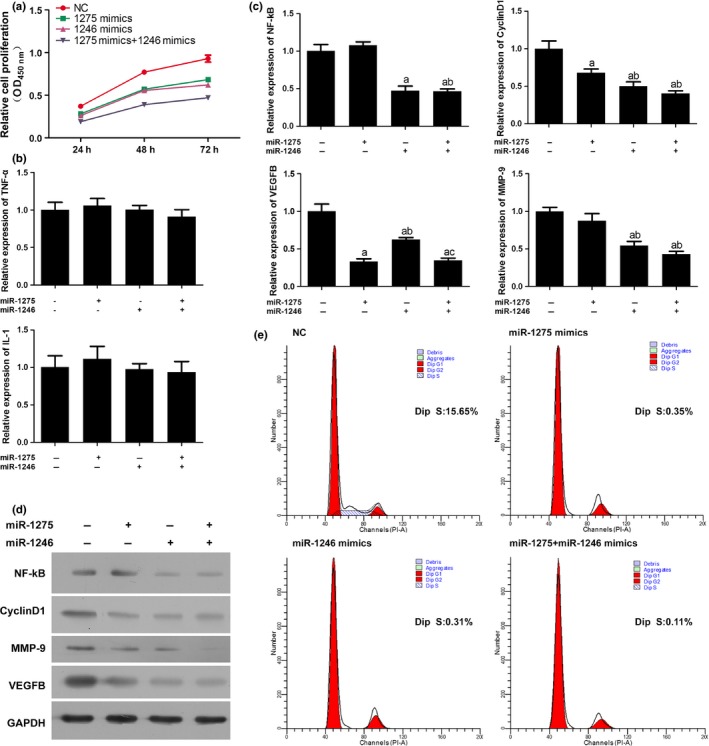
Administration of miR‐1275 and miR‐1246 mimics inhibited the proliferation of HUVECs. (a) Cell viability of HUVECs was inhibited by miR‐1275 and miR‐1246 mimics. (b) Quantitative analysis results of the expression levels of TNF‐α and IL‐1 in HUVECs as detected by qRT‐PCR. Administration of miR‐1275 and miR‐1246 mimics had no effect on levels of both molecules. (b, c) Quantitative analysis results of the expression levels of NF‐κB, Cyclin D1, VEGFB and MMP‐9 in HUVECs as detected by qRT‐PCR (β‐actin was employed as an internal reference gene). Administration of miR‐1275 and miR‐1246 mimics inhibited the expression of all the molecules. (d) Representative images of the expression levels of NF‐κB, Cyclin D1, VEGFB and MMP‐9 in HUVECs as detected by Western blotting. Administration of miR‐1275 and miR‐1246 mimics inhibited the expression of all the molecules. (e) Representative images of cell cycle distribution as detected by flow cytometry. Administration of miR‐1275 and miR‐1246 mimics decreased the number of cell distributed in S phase. “a,” Significantly different from NC group, P<.05. “b,” Significantly different from 1275 mimics group, P<.05. “c,” Significantly different from 1246 mimics group, P<.05
3.3. VEGFB and NAKP are the target genes of miR‐1275 and miR‐1246
The direct regulating effect of the two miRs on VEGFB and NKAP was verified by a dual luciferase assay (Fig. 3). Reporter assay revealed that overexpression of miR‐1275 significantly suppressed the activity of WT VEGFB plasmid in HUVECs (P<.05) without change in luciferase activity of MUT VEGF plasmid (Fig. 3c), which indicated that miR‐1275 directly modulate VEGFB expression by binding to 3′UTR of VEGFB in keloid cells. Similar conclusion was drawn for detection with miR‐1246 and NKAP (Fig. 3d). The results of dual luciferase assay uncovered the regulating targets of the two miRs in angiogenesis and further supported the possible involvement of the two miRs in the anti‐angiogenic effect of curcumin.
Figure 3.
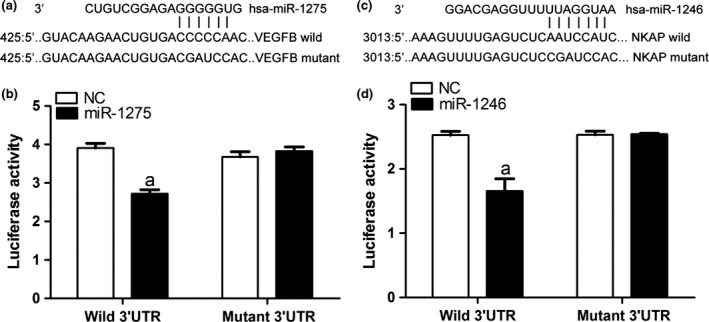
VEGFB and NKAP are the potential target genes of miR‐1275 and miR‐1245. (a) Quantitative analysis results of dual luciferase assay of miR‐1275 and VEGFB. (b) Quantitative analysis results of dual luciferase assay of miR‐1246 and NKAP. “a,” Significantly different from NC group, P<.05
3.4. The anti‐angiogenic effect of curcumin depended on the actions of miR‐1275 and miR‐1246
To uncover the involvement of miR‐1275 and miR‐1246 in the anti‐angiogenic effect of curcumin, the curcumin‐treated HUVCEs were administrated with specific inhibitors against these miRs, which significantly inhibited the proliferation of HUVECs (Fig. 4a), but the effect was interrupted by both miR inhibitors. Furthermore, it seemed that the function of the two miRs in curcumin's anti‐angiogenic effect was synergistic; the concatenated administration of two inhibitors further interrupted the effect of curcumin and induced the proliferation of HUVECs (Fig. 4a). The results of qRT‐PCR and Western blotting assay demonstrated the changing pattern of molecules participating in the angiogenic proliferation of HUVECs. Administration of specific inhibitors induced the activation of the target genes of the two miRs; the expression levels of VEGFB and NKAP were both up‐regulated at mRNA and protein levels (Fig. 4b,c). Correspondingly, activation of NKAP activated NF‐кB p65 as well as its downstream effectors, Cyclin D1 and MMP‐9 (Fig. 4c). Moreover, VEGFB was also closely associated with the activity of NF‐кB p65, which might provide a possible explanation for the synergistic effect of the two miRs. Similar results were also detected by the flow cytometry assay. The proportion of cells distributed in S phase was increased in inhibitor‐treated groups (Fig. 4d), which indicates that the anti‐angiogenic effect of curcumin is miR‐1275 and miR‐1246 dependent.
Figure 4.
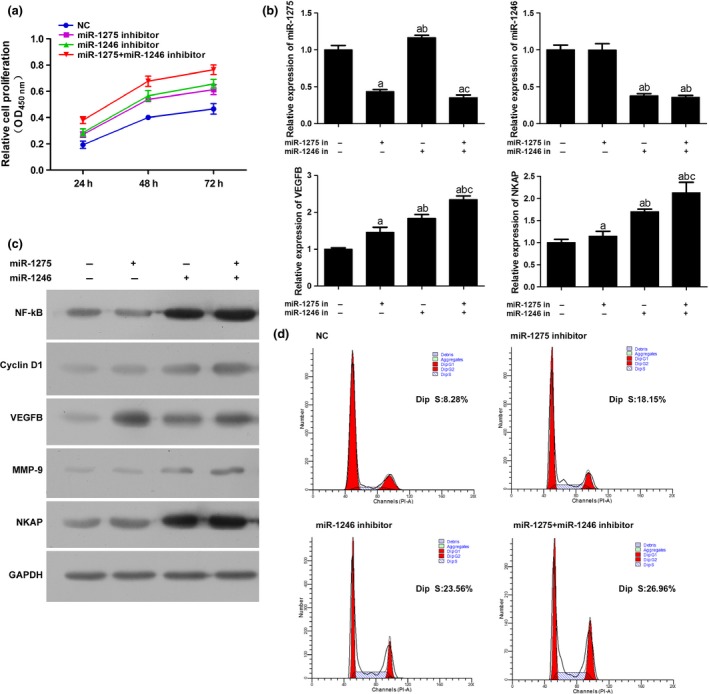
Administration of miR‐1275 and miR‐1246 inhibitors induced the proliferation of curcumin‐treated HUVECs via NF‐κB signalling. (a) Cell viability of HUVECs was increased by miR‐1275 and miR‐1246 inhibitors. (b) Quantitative analysis results of the expression levels of miR‐1275, miR‐1246, VEGFB and NKAP in HUVECs as detected by qRT‐PCR (U6 was employed as an internal reference gene for miR‐1275 and miR‐1246, and β‐actin was employed as an internal reference gene for VEGFB and NKAP). Administration of miR‐1275 and miR‐1246 inhibitors induced the expression of all the molecules. (c) Representative images of the expression levels of NF‐κB, Cyclin D1, VEGFB, MMP‐9, NKAP in HUVECs as detected by Western blotting. Administration of miR‐1275 and miR‐1246 inhibitors induced the expression of all the molecules. (d) Representative images of cell cycle distribution as detected by flow cytometry. Administration of miR‐1275 and miR‐1246 inhibitors increased the number of cell distributed in S phase. “a,” Significantly different from NC group, P<.05. “b,” Significantly different from 1275 mimics group, P<.05. “c,” Significantly different from 1246 mimics group, P<.05
3.5. MiR‐1275 and miR‐1246 affect synergistically on anti‐angiogenic effect of curcumin
The above assays also infer a synergism between the two miRs. To investigate the influence of the two miRs on the effect of curcumin, HUVECs were incubated with different combinations of mimics and inhibitors. The CCK‐8 assay showed that the miR‐1246 inhibition and miR‐1275 activation induced the proliferation of HUVECs more strongly than that of miR‐1275 inhibition and miR‐1246 activation (Fig. 5a). Activation of miR‐1246 not only influenced the expression of its target gene NKAP more strongly than miR‐1275 but also powerfully suppressed the expression of VEGFB even with the inhibition of miR‐1275 (Fig. 5b,c). Similar conclusion can be drawn from the detection of flow cytometry, in which number of cells distributed in S phase in miR‐1246 inhibited group was higher than that of miR‐1275 inhibited group (Fig. 5b).
Figure 5.
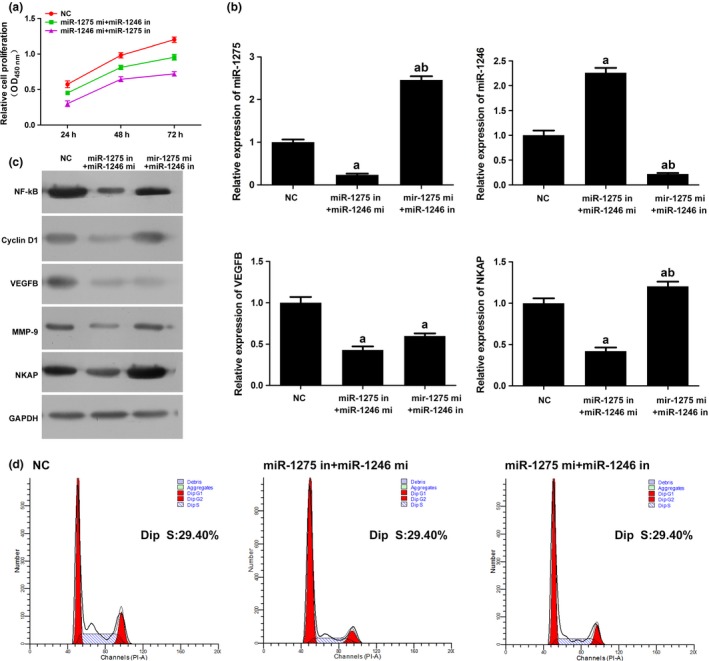
MiR‐1275 and miR‐1246 affect the proliferation of HUVECs synergistically via NF‐κB signalling. (a) Cell viability of HUVECs was inhibited by concatenated administration of mimics and inhibitors of the two miRs. Moreover, miR‐1245 mimics could confound the effect of miR‐1275 inhibitor to some extent. (b) Quantitative analysis results of the expression levels of miR‐1275, miR‐1246, VEGFB and NKAP in HUVECs as detected by qRT‐PCR (U6 was employed as an internal reference gene for miR‐1275 and miR‐1246, and β‐actin was employed as an internal reference gene for VEGFB and NKAP). Administration of concatenated administration of mimics and inhibitors of the two miRs inhibited the expression of all the molecules. MiR‐1245 mimics could confound the effect of miR‐1275 inhibitor on the expression of VEGFB to some extent. (c) Representative images of the expression levels of NF‐κB, Cyclin D1, VEGFB, MMP‐9, NKAP in HUVECs as detected by Western blotting. Administration of concatenated administration of mimics and inhibitors of the two miRs inhibited the expression of all the molecules. MiR‐1245 mimics could confound the effect of miR‐1275 inhibitor to some extent. (d) Representative images of cell cycle distribution as detected by flow cytometry. Administration of concatenated administration of mimics and inhibitors of the two miRs decreased the number of cell distributed in S phase. MiR‐1245 mimics could confound the effect of miR‐1275 inhibitor to some extent. “a,” Significantly different from NC group, P<.05. “b,” Significantly different from 1275 mimics group, P<.05. “c,” Significantly different from 1246 mimics group, P<.05
3.6. NKAP played a key role in the anti‐angiogenic function of curcumin
From the above findings, it is evident that miR‐1275 and miR‐1246 modulates HUVECs through VEGFB and NKAP; NKAP induces the activation of VEGFB through NF‐кB signalling. From the results of CCK‐8 assay an increased cell viability was observed, suggesting that the overexpression of NKAP blocked the anti‐angiogeneis effect of curcumin (Fig. 6a). Also, overexpression of NKAP induced the activation of NF‐кB p65 and a series of effectors of NF‐кB p65, including Cyclin D1, MMP‐9 and VEGFB, which all contributed to the angiogenesis process (Fig. 6b). The flow cytometry assays showed that the proportion of cell distributed in S phase were decreased by the overexpression of NKAP (Fig. 6c). Moreover, the results of immunofluorescence assay further confirmed the restricted production and distribution of NKAP in curcumin or miR‐1246 mimics treated HUVECs (Fig. 7), supporting our conclusion that curcumin exerted its function on HUVECs through a miR‐1246‐mediated NKAP inhibition manner.
Figure 6.
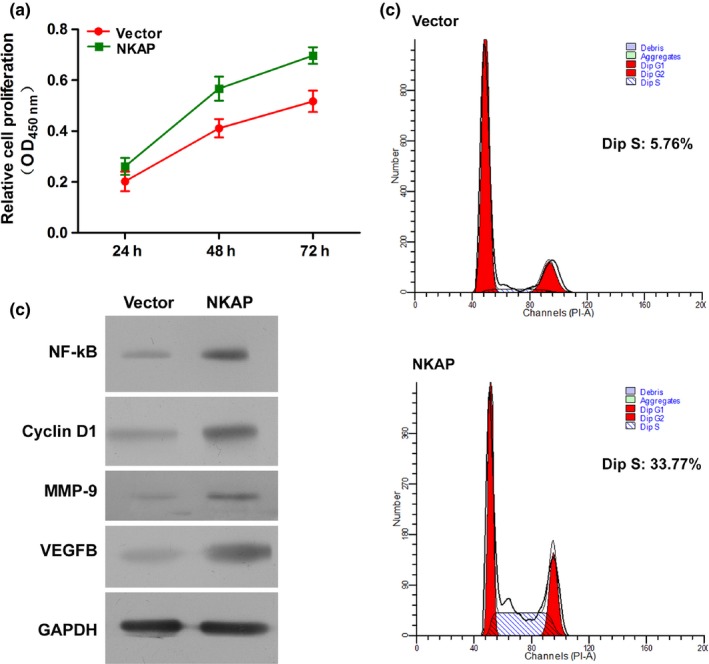
Overexpression of NKAP restored the proliferation ability of curcumin‐treated HUVECs. (a) Cell viability of HUVECs was increased by overexpression of NKAP. (b) Representative images of the expression levels of NF‐κB, Cyclin D1, VEGFB, MMP‐9 and NKAP in HUVECs as detected by Western blotting. Overexpression of induced the expression of all the molecules. (c) Representative images of cell cycle distribution as detected by flow cytometry. Overexpression of NKAP increased the number of cell distributed in S phase. “a,” Significantly different from NC group, P<.05. “b,” Significantly different from 1275 mimics group, P<.05. “c,” Significantly different from 1246 mimics group, P<.05
Figure 7.
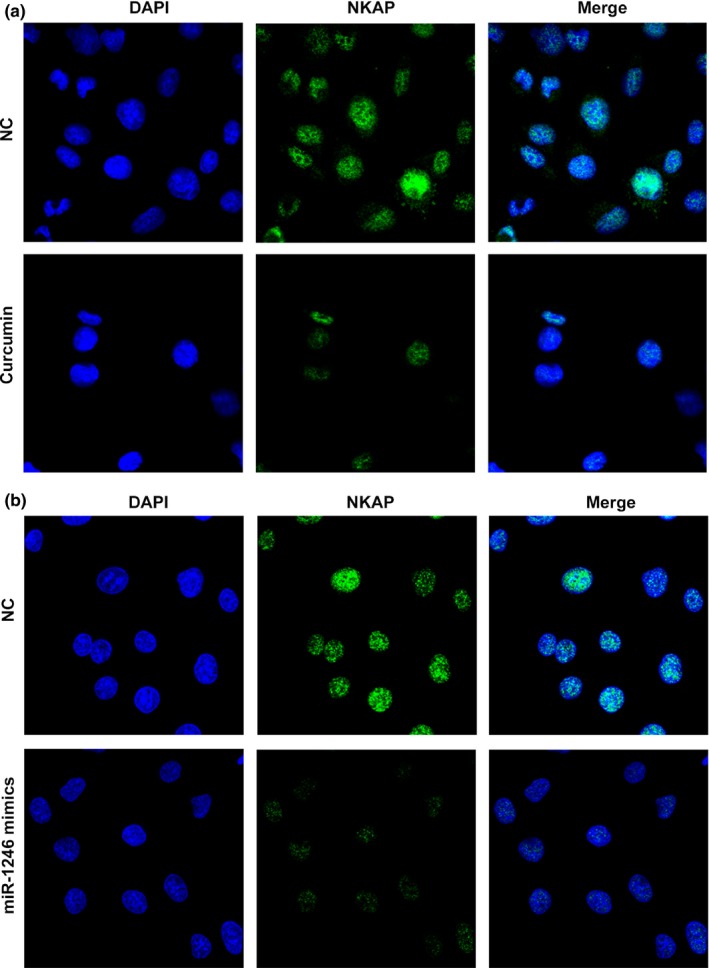
Activity of NKAP was inhibited in HUVECs by curcumin and miR‐1246 as illustrated by immunofluorescence assay at 400× magnification. (a) Expression and distribution of NKAP was inhibited by curcumin in HUVECs. (b) Expression and distribution of NKAP was inhibited by miR‐1246 in HUVECs
4. Discussion
Infectious keratitis has been recognized as an emergencies incidence disease in recent decades. According to an investigation in 2000, fungal keratitis accounts for the first in the incidence of infectious corneal disease.31 Thus, the disease has got lots of attention for its potential to lead to blindness. In most cases, infectious keratitis is treated by corneal transplantation; however, it being expensive and due to few corneal donations, many people with infectious keratitis are forced to be eyeball enucleated. A more economical and effective approach to treatment of the disease is imperative.
The onset of angiogenesis involves a complicated cascade of events, and multiple factors that stimulate this process, i.e., VEGF32 and TNF‐α,33 have been identified. The anti‐angiogenic potential of some compounds targeting these indicators has been investigated extensively.34, 35, 36 Of types of anti‐angiogenic compounds, curcumin, which is previously employed as a tumour inhibitor,37, 38 is proved to possess great potency to antagonize angiogenesis‐related disorders.15, 39, 40 Given the clinical value of curcumin, comprehensive research aiming to uncover the mechanism, through which curcumin antagonizing angiogenesis, is being conducted in our group. Based on our data, curcumin administration evidently influences the miR expression profiles in HUVECs, which revealed a mechanism driving the anti‐angiogenic effect of curcumin.
The data of miR microassay showed that administration of curcumin changed the expression pattern of 30 members of human miRs, and changes in miR‐1275 and miR‐1246 expression were most significant. MiR‐1275 was reported to contribute to the establishment of tissue heterogeneity via epigenetic mechanisms.41 MiR‐1246 is multi‐functional, it is capable of enhancing the migration of hepatocellular carcinoma42 and have inhibitory role in the cell growth of hepatocellular carcinoma.43 It has been reported that the miR‐1246 promotes angiogenesis by activating Smad signalling in endothelial cells.44 In our study, levels of both miRs in HUVECs were up‐regulated by curcumin administration. Subsequent treatment of HUVECs with specific mimics showed that augmented expression of the two miRs not only inhibited the cell proliferation of HUVECs but also suppressed the expression of NF‐кB p65 and its downstream effectors. However, overexpression of miR‐1275 and miR‐1246 had no effect on the expression of TNF‐α and IL‐1. According to the study by Gururaj et al., it is inferred that curcumin suppresses the angiogenesis by regulating the activity of NF‐кB signalling transduction.39 Therefore, it was hypothesized in this study that the effect of curcumin on NF‐кB signalling was mediated through a miR‐dependent manner.
To validate our hypothesis, the target genes of miR‐1275 and miR‐1246 were predicted with bioinformatics means and it was found that VEGFB and NKAP are the targets of miR‐1275 and miR‐1246, respectively. The direct regulating effect on the two indicators by miR‐1275 and miR‐1246 was verified with a dual luciferase assay. VEGFB is a growth factor for endothelial cells with similar structure to VEGF. When co‐expressed, VEGFB will form cell‐surface‐associated disulphide‐linked homodimers and heterodimerized with VEGF.45 The indicator is a key member in the NF‐кB mediated angiogenesis and its suppression inhibits the angiogenic growth of HUVECs.46 As for target of miR‐1246, NKAP, its role in angiogenesis is seldom reported, but down‐regulation of NKAP by antisense RNA significantly inhibited the TNF‐α and IL‐1 induced NF‐кB activation.47 The conclusion was also proved in this study: increased expression of NKAP by miR‐1246 inhibitor induced the activation of NF‐кB p65. And up‐regulated NF‐кB p65 level enhanced the expression of Cyclin D1, MMP‐9 and VEGFB, which subsequently induced the angiogenesis process in HUVECs.46 Nevertheless, the function of miR‐1246 in the anti‐angiogenesis effect of curcumin was contrary to the previous study by Yamada et al., in which miR‐1246 promotes angiogenesis via activation Smad pathways.44 However, the conclusion was derived based on the transfer of colorectal cancer cell‐derived microvesicles (CRC‐MVs) to HUVECs, which was different from the research system of this study. Given the multiple effect of miR‐1246 in different organs,42, 43, 44 the function of miR‐1246 might be tissue specific and needed to be further validated with more research systems.
Additionally, the role of NKAP in the anti‐angiogenic effect of curcumin was further explored. By transfecting HUVECs with a NKAP expression vector, it was found that HUVECs restored the proliferation ability that was impaired by curcumin treatment. Moreover, the suppression of NF‐кB signalling transduction by curcumin was also alleviated. NF‐кB is a key oxidative stress‐response transcription factor of eukaryotic cells, and the anti‐angiogenesis function of curcumin might be associated with the antioxidant capability of the agent, which needed to be further explored in the future.48
This is the first study that infers the centre role of NKAP in the effect of curcumin on angiogenesis. Combined with direct modulation of miR‐1246, miR‐1275 and miR‐1246 synergistically mediated the anti‐angiogenic effect of curcumin on HUVECs. However, compared with miR‐1246, miR‐1275 exerted its action through the activity of VEGFB that is a down‐stream effector of NF‐кB signalling. Thus, the function of miR‐1275 in angiogenesis might be under the regulation of miR‐1246 as well. Such hypothesis was preliminarily investigated by treating HUVECs with different combination of miR mimics and inhibitors, and the data showed that regulation of miR‐1246 would confound the effect of miR‐1275 regulation to some extent. However, the exact interaction between miR‐1275 and miR‐1246 or the interaction between the two miRs and other factors still remained unrevealed and needed further research.
Multiple attempts to alleviate infectious keratitis through vascularization process were made.6, 49 Compared with these studies, our study is not only a complement to the mechanism driving the anti‐angiogenesis in cornea but also confirms the therapeutic potential of curcumin against infectious keratitis. Findings outlined in our study demonstrated that the curcumin dramatically influenced the miRs expression profiles in HUVECs. Two miR members, miR‐1275 and miR‐1246, played determinant roles in the anti‐angiogenic effect of curcumin by targeting molecules involved in NF‐кB signalling transduction. Moreover, for the first time, our study inferred the involvement of the target gene of miR‐1246, NKAP, in the inhibition of curcumin on angiogenesis, which could be employed as a novel target for development of treatment strategies for CRNV‐related eye diseases.
References
- 1. Solanki S, Rathi M, Khanduja S, Dhull CS, Sachdeva S, Phogat J. Recent trends: medical management of infectious keratitis. Oman J Ophthalmol. 2015;8:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lee S, Zheng M, Kim B, Rouse BT. Role of matrix metalloproteinase‐9 in angiogenesis caused by ocular infection with herpes simplex virus. J Clin Investig. 2002;110:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Banerjee K, Biswas PS, Kim B, Lee S, Rouse BT. CXCR2−/− mice show enhanced susceptibility to herpetic stromal keratitis: a role for IL‐6‐induced neovascularization. J Immunol. 2004;172:1237–1245. [DOI] [PubMed] [Google Scholar]
- 4. Deshpande SP, Zheng M, Lee S, Rouse BT. Mechanisms of pathogenesis in herpetic immunoinflammatory ocular lesions. Vet Microbiol. 2002;86:17–26. [DOI] [PubMed] [Google Scholar]
- 5. Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. [DOI] [PubMed] [Google Scholar]
- 6. Zheng M, Deshpande S, Lee S, Ferrara N, Rouse BT. Contribution of vascular endothelial growth factor in the neovascularization process during the pathogenesis of herpetic stromal keratitis. J Virol. 2001;75:9828–9835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ribatti D, Djonov V. Intussusceptive microvascular growth in tumors. Cancer Lett. 2012;316:126–131. [DOI] [PubMed] [Google Scholar]
- 8. Wang Z, Dabrosin C, Yin X, et al. Broad targeting of angiogenesis for cancer prevention and therapy. Semin Cancer Biol. 2015;35(suppl):S224–S243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pandya NM, Dhalla NS, Santani DD. Angiogenesis—a new target for future therapy. Vascul Pharmacol. 2006;44:265–274. [DOI] [PubMed] [Google Scholar]
- 10. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. [DOI] [PubMed] [Google Scholar]
- 11. Pober JS, Cotran RS. The role of endothelial cells in inflammation. Transplantation. 1990;50:537–544. [DOI] [PubMed] [Google Scholar]
- 12. Ross R, Harker L. Hyperlipidemia and atherosclerosis. Science. 1976;193:1094–1100. [DOI] [PubMed] [Google Scholar]
- 13. Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. [DOI] [PubMed] [Google Scholar]
- 14. Matsui J, Wakabayashi T, Asada M, Yoshimatsu K, Okada M. Stem cell factor/c‐kit signaling promotes the survival, migration, and capillary tube formation of human umbilical vein endothelial cells. J Biol Chem. 2004;279:18600–18607. [DOI] [PubMed] [Google Scholar]
- 15. Singh AK, Sidhu GS, Deepa T, Maheshwari RK. Curcumin inhibits the proliferation and cell cycle progression of human umbilical vein endothelial cell. Cancer Lett. 1996;107:109–115. [DOI] [PubMed] [Google Scholar]
- 16. Tang JY, Li S, Li ZH, et al. Calycosin promotes angiogenesis involving estrogen receptor and mitogen‐activated protein kinase (MAPK) signaling pathway in zebrafish and HUVEC. PLoS ONE. 2010;5:e11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xiao D, Singh SV. Phenethyl isothiocyanate inhibits angiogenesis in vitro and ex vivo. Cancer Res. 2007;67:2239–2246. [DOI] [PubMed] [Google Scholar]
- 18. Satoskar RR, Shah SJ, Shenoy SG. Evaluation of anti‐inflammatory property of curcumin (diferuloyl methane) in patients with postoperative inflammation. Int J Clin Pharmacol Ther Toxicol. 1986;24:651–654. [PubMed] [Google Scholar]
- 19. Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem Pharmacol. 1976;25:1811–1812. [DOI] [PubMed] [Google Scholar]
- 20. Shizuo T, Toshio M, Hideko A, Hisayuki T, Yoshio T. Natural antioxidants. III. Antioxidative components isolated from rhizome of Curcuma longa L. Chem Pharm Bull. 1985;33:1725–1728. [DOI] [PubMed] [Google Scholar]
- 21. Srimal RC, Dhawan BN. Pharmacology of diferuloyl methane (curcumin), a non‐steroidal anti‐inflammatory agent. J Pharm Pharmacol. 1973;25:447–452. [DOI] [PubMed] [Google Scholar]
- 22. Adams BK, Ferstl EM, Davis MC, et al. Synthesis and biological evaluation of novel curcumin analogs as anti‐cancer and anti‐angiogenesis agents. Bioorg Med Chem. 2004;12:3871–3883. [DOI] [PubMed] [Google Scholar]
- 23. Panahi Y, Darvishi B, Ghanei M, Jowzi N, Beiraghdar F, Varnamkhasti BS. Molecular mechanisms of curcumins suppressing effects on tumorigenesis, angiogenesis and metastasis, focusing on NF‐κB pathway. Cytokine Growth Factor Rev. 2016;28:21–29. [DOI] [PubMed] [Google Scholar]
- 24. Thaloor D, Singh AK, Sidhu GS, Prasad PV, Kleinman HK, Maheshwari RK. Inhibition of angiogenic differentiation of human umbilical vein endothelial cells by curcumin. Cell Growth Differ. 1998;9:305–312. [PubMed] [Google Scholar]
- 25. Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. [DOI] [PubMed] [Google Scholar]
- 26. Kim VN. MicroRNA biogenesis: coordinated cropping and dicing. Nat Rev Mol Cell Biol. 2005;6:376–385. [DOI] [PubMed] [Google Scholar]
- 27. Pillai RS, Bhattacharyya SN, Artus CG, et al. Inhibition of translational initiation by Let‐7 MicroRNA in human cells. Science. 2005;309:1573–1576. [DOI] [PubMed] [Google Scholar]
- 28. Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. [DOI] [PubMed] [Google Scholar]
- 29. Poliseno L, Tuccoli A, Mariani L, et al. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. [DOI] [PubMed] [Google Scholar]
- 30. Oettel A, Lorenz M, Stangl V, Costa S‐D, Zenclussen AC, Schumacher A. Human umbilical vein endothelial cells foster conversion of CD4+ CD25− Foxp3− T cells into CD4+ Foxp3+ regulatory T cells via transforming growth factor‐β. Sci Rep. 2016;6:23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tanure MAG, Cohen EJ, Sudesh S, Rapuano CJ, Laibson PR. Spectrum of fungal keratitis at Wills eye hospital, Philadelphia, Pennsylvania. Cornea. 2000;19:307–312. [DOI] [PubMed] [Google Scholar]
- 32. Pepper MS, Ferrara N, Orci L, Montesano R. Vascular endothelial growth factor (VEGF) induces plasminogen activators and plasminogen activator inhibitor‐1 in microvascular endothelial cells. Biochem Biophys Res Commun. 1991;181:902–906. [DOI] [PubMed] [Google Scholar]
- 33. Montrucchio G, Lupia E, Battaglia E, et al. Tumor necrosis factor alpha‐induced angiogenesis depends on in situ platelet‐activating factor biosynthesis. J Exp Med. 1994;180:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985;230:1375–1378. [DOI] [PubMed] [Google Scholar]
- 35. Li WW, Casey R, Gonzalez EM, Folkman J. Angiostatic steroids potentiated by sulfated cyclodextrins inhibit corneal neovascularization. Invest Ophthalmol Vis Sci. 1991;32:2898–2905. [PubMed] [Google Scholar]
- 36. Pepper MS, Vassalli JD, Orci L, Montesano R, Wilks JW, Schweigerer L. Modulation of bovine microvascular endothelial cell proteolytic properties by inhibitors of angiogenesis. J Cell Biochem. 1994;55:419–434. [DOI] [PubMed] [Google Scholar]
- 37. Azuine MA, Bhide SV. Chemopreventive effect of turmeric against stomach and skin tumors induced by chemical carcinogens in Swiss mice. Nutr Cancer. 1992;17:77–83. [DOI] [PubMed] [Google Scholar]
- 38. Huang MT, Wang ZY, Georgiadis CA, Laskin JD, Conney AH. Inhibitory effects of curcumin on tumor initiation by benzo [a] pyrene and 7, 12‐dimethylbenz [a] anthracene. Carcinogenesis. 1992;13:2183–2186. [DOI] [PubMed] [Google Scholar]
- 39. Gururaj AE, Belakavadi M, Venkatesh DA, Marme D, Salimath BP. Molecular mechanisms of anti‐angiogenic effect of curcumin. Biochem Biophys Res Commun. 2002;297:934–942. [DOI] [PubMed] [Google Scholar]
- 40. Pendurthi UR, Williams JT, Rao LV. Inhibition of tissue factor gene activation in cultured endothelial cells by curcumin. Suppression of activation of transcription factors Egr‐1, AP‐1, and NF‐kappa B. Arterioscler Thromb Vasc Biol. 1997;17:3406–3413. [DOI] [PubMed] [Google Scholar]
- 41. Katsushima K, Shinjo K, Natsume A, et al. Contribution of microRNA‐1275 to Claudin11 protein suppression via a polycomb‐mediated silencing mechanism in human glioma stem‐like cells. J Biol Chem. 2012;287:27396–27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sun Z, Meng C, Wang S, et al. MicroRNA‐1246 enhances migration and invasion through CADM1 in hepatocellular carcinoma. BMC Cancer. 2014;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Q, Cao LY, Cheng SJ, Zhang AM, Jin XS, Li Y. p53‐induced microRNA‐1246 inhibits the cell growth of human hepatocellular carcinoma cells by targeting NFIB. Oncol Rep. 2015;33:1335–1341. [DOI] [PubMed] [Google Scholar]
- 44. Yamada N, Tsujimura N, Kumazaki M, et al. Colorectal cancer cell‐derived microvesicles containing microRNA‐1246 promote angiogenesis by activating Smad 1/5/8 signaling elicited by PML down‐regulation in endothelial cells. Biochim Biophys Acta. 2014;1839:1256–1272. [DOI] [PubMed] [Google Scholar]
- 45. Olofsson B, Pajusola K, Kaipainen A, et al. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc Natl Acad Sci. 1996;93:2576–2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tabruyn SP, Griffioen AW. A new role for NF B in angiogenesis inhibition. Cell Death Differ. 2007;14:1393–1397. [DOI] [PubMed] [Google Scholar]
- 47. Chen D, Li Z, Yang Q, Zhang J, Zhai Z, Shu H‐B. Identification of a nuclear protein that promotes NF‐κB activation. Biochem Biophys Res Commun. 2003;310:720–724. [DOI] [PubMed] [Google Scholar]
- 48. Aggarwal BB, Harikumar KB. Potential therapeutic effects of curcumin, the anti‐inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int J Biochem Cell Biol. 2009;41:40–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kim B, Tang Q, Biswas PS, et al. Inhibition of ocular angiogenesis by siRNA targeting vascular endothelial growth factor pathway genes: therapeutic strategy for herpetic stromal keratitis. Am J Pathol. 2004;165:2177–2185. [DOI] [PMC free article] [PubMed] [Google Scholar]


