Abstract
Abstract. Resveratrol, a phytochemical present in grapes, has been demonstrated to inhibit tumourigenesis in animal models. However, the specific mechanism by which resveratrol exerts its anticarcinogenic effect has yet to be elucidated. In the present study, the inhibitory effects of resveratrol on cell proliferation and apoptosis were evaluated in the human leukaemia cell line HL‐60 and the human hepatoma derived cell line HepG2. We found that after a 2 h incubation period, resveratrol inhibited DNA synthesis in a concentration‐dependent manner. The IC50 value was 15 µm in both HL‐60 and HepG2 cells. When the time of treatment was extended, an increase in IC50 value was observed; for example, at 24 h the IC50 value was 30 µm for HL‐60 cells and 60 µm for HepG2 cells. Flow cytometry revealed that cells accumulated in different phases of the cell cycle depending on the resveratrol concentration. Furthermore, an increase in nuclear size and granularity was observed in the G1 and S phases of HL‐60 treated and HepG2‐treated cells. Apoptosis was also stimulated by resveratrol in a concentration‐dependent manner in HL‐60 and HepG2 cells. In conclusion, resveratrol inhibits cell proliferation in a concentration‐ and time‐dependent manner by interfering with different stages of the cell cycle. Furthermore, resveratrol treatment causes stimulation of apoptosis as well as an increase in nuclear size and granularity.
INTRODUCTION
Resveratrol (3,5,4′‐trihydroxystilbene, the trans‐isoform) is a phytoalexin naturally occurring in many plants, some of which are used for human consumption (Aggarwal et al. 2004). In 1976, the compound was identified in grapes (Langcake & Pryce 1976), where it is produced primarily in the skin in response to external stress factors such as injury, ultraviolet radiation and fungus infection (Soleas et al. 1997; Pervaiz 2003). Resveratrol is also found in high concentrations in one of the end products of grapes, red wine (Siemann & Creasy 1992; Stervbo et al. 2006).
The first report on cancer preventive effects of resveratrol was published by Jang et al. (1997). The authors evaluated the effect of resveratrol on tumourigenesis by a two‐stage mouse skin cancer model and found a 98% reduction of skin tumours. Since this first study concerning the inhibitory effect of resveratrol on tumourigenesis, several reports have indicated that it may reduce tumour number (Carbo et al. 1999; Schneider et al. 2001; Kapadia et al. 2002) and tumour size (Caltagirone et al. 2000; Kimura & Okuda 2001; Liu et al. 2003; Chen et al. 2004; Wyke et al. 2004; Zhou et al. 2005) in vivo.
The mechanisms by which resveratrol exerts its anticarcinogenic effects are not well understood. In vitro studies have shown that it reduces cell proliferation and modulates the cell cycle in different cell lines, among which are HL‐60 and HepG2 cells (Latruffe et al. 2002; Liang et al. 2003; Roberti et al. 2003; Ahmad et al. 2004; Fulda & Debatin 2004; Opipari et al. 2004; Castello & Tessitore 2005). Modulation of the cell cycle may be mediated by an increase in levels of the cyclin‐dependent kinase inhibitor, cdkn1a and the stabilization of p53 (Hsieh et al. 1999; Kuo et al. 2002; Kim et al. 2003; Fulda & Debatin 2004). Resveratrol appears to stimulate caspase‐dependent and caspase‐independent apoptosis, as inhibition of caspase‐3 results in only a reduction in resveratrol‐stimulated cell death (Clement et al. 1998; Roman et al. 2002; Estrov et al. 2003; Roberti et al. 2003; Opipari et al. 2004). Thus, several cellular mechanisms can contribute to the chemopreventive effects of resveratrol.
The purpose of the present work was to study resveratrol's time‐ and concentration‐dependent effects with respect to modulation of the cell cycle and stimulation of apoptosis in the two human cell lines, HL‐60 and HepG2. Effects on the cell cycle were studied using [3H]dT and flow cytometry on propidium iodine (PI)‐stained nuclei. Apoptosis was detected by alterations in nuclear morphology, as assessed by fluorescence microscopy, and binding of Annexin V. as well as DNA fragmentation, evaluated by flow cytometry.
MATERIALS AND METHODS
Chemicals and reagents
Resveratrol, camptothecin, 4′,6‐diamidino‐2‐phenylindole (DAPI), PI, Triton X‐100, and dimethylsulfoxide (DMSO) were all obtained from Sigma (Copenhagen, Denmark). Roswell Park Memorial Institute medium (RPMI)‐1640, L‐glutamine, foetal calf serum (FCS) and Glutamax were from Invitrogen (Taastrup, Denmark). Phosphate buffer saline (PBS), penicillin‐streptomycin‐glutamine mix (10 000 units/ml penicillin, 10 000 µg/ml streptomycin and 29.2 mg/ml L‐glutamine) and trypsin‐ethylenediaminetetraacetic acid (EDTA) were purchased from Gibco (Taastrup, Denmark). Dulbecco's modified eagle's medium (DMEM) was obtained from Cambrex (Vallensbaek Strand, Denmark) and 4% paraformaldehyde in phosphate buffer, pH 7.4, was from Bie & Berntsen (Rodovre, Denmark).
Resveratrol was diluted in DMSO at millimolar concentrations, kept at −20 °C until use and stored for no more than 6 months. All cell culture flasks as well as 96‐well microtitre plates and six‐well multiwell dishes were from Nunc (Roskilde, Denmark), unless otherwise specified.
Cell lines
HL‐60 cells, a human promyelocytic leukaemia cell line, were grown in suspension, and HepG2 cells, an epithelial human hepatoma‐derived cell line, were obtained from the American Tissue Culture Collection. The HL‐60 cells were cultured in RPMI‐1640 supplemented with 200 mm L‐glutamine and 10% heat‐inactivated FCS. The HepG2 cells were cultured in DMEM supplemented with 1% penicillin‐streptomycin‐glutamine mix and 10% heat‐inactivated FCS. All cells were maintained in a humidified 37 °C, 5% CO2 incubator. When cell density reached approximately 80% confluence, the cells were subcultured. HL‐60 cells were subcultured by diluting cells in fresh culture medium to approximately 2 × 105 cells/ml. HepG2 cells were detached by trypsin‐EDTA and were seeded at 4 × 104 cells/cm2. Cell lines were subcultured no more than 30 times.
Thymidine incorporation assay
Exponentially growing HL‐60 cells were diluted in fresh culture medium to 6 × 104 cells/ml. The cell suspension was seeded in a 96‐well microtitre plates, 100 µl/well. Resveratrol solution or vehicle solution (0.1%) in culture medium were made in centrifuge tubes (Nalgene Labware, Albertslund, Denmark) in concentrations double that required for final concentration and were kept in the dark in a standard incubator. At different time points prior to cell harvest, 100 µl resveratrol stock solution was passed to each well of the 96‐well microtitre plate to a final volume of 200 µl/well leading to the required final concentration.
Exponentially growing HepG2 cells were diluted in fresh culture medium to 3 × 104 cells/ml and were seeded in a 96‐well microtitre plate, 200 µl/well. All outer wells received 200 µl PBS. The cells were allowed to adhere overnight. On the day of the experiment, resveratrol solution or vehicle solution (0.1%) in culture medium were made in centrifuge tubes (Nalgene Labware) at the required final concentrations and were kept in the dark in the incubator. At different time points prior to cell harvest, the medium was removed by gently inverting the plates and 200 µl resveratrol solutions were added to each well. In all experiments, outer wells received 200 µl PBS and the plates were left in the incubator for the desired incubation time.
Two hours before harvest, 20 µl 6.25 µCi/ml [3H]dT (Amersham Biosciences, Hillerod, Denmark) was added to each well, and the plates were left to incubate. Subsequently, the cells were transferred to filter plates (PerkinElmer, Allerod, Denmark) using a cell harvester (Packard, Greve, Denmark) and were left to dry overnight at room temperature. To each well on the dried filter plates, 30 µl MicroScint O scintillation liquid (PerkinElmer) was added. The intensity of the radioactivity was measured in a TopCount NXT scintillation counter (Packard). The TopCount NXT was controlled by the topcount nxt version 1.06 (Packard) external software on Windows 95.
Analysis by flow cytometry
The distribution of cells in the cell cycle as well as apoptosis was analysed by the CycleTEST Plus DNA Reagent Kit (BD Biosciences, Brondby, Denmark). The assay is based on the method by Vindelov et al. (1983) where nuclei are isolated using a mixture of a non‐ionic detergent and trypsin. The DNA is subsequently stained with DNA‐binding dye, PI.
Exponentially growing HepG2 cells were seeded (1 × 106 cells in a T25 flask) one day prior to resveratrol treatment. On the day of treatment, the culture medium was replaced with culture medium containing resveratrol or vehicle solution (0.1%). HL‐60 cells were diluted in fresh culture medium. Resveratrol or vehicle solution (0.1%) was added, and 1 × 106 cells were added to each well of a six‐well multiwell dish. After a treatment period of 24 h, the cells were harvested and the assays were performed according to the manufacturer's protocol. Stained cells were analysed as described in succeeding discussions using the FL2‐H parameter. The size and granularity of nuclei were investigated using the FSC‐H (particle size) and SSC‐H (particle complexity) parameters of the flow cytometer.
Evaluation of resveratrol‐induced apoptosis was performed on HL‐60 cells using the Annexin V‐enhanced green fluorescent protein (V‐EGFP) Apoptosis Detection Kit (Stratech Scientific, Suffolk, UK). Using DNA‐binding dye PI, the cells with compromised membranes could be excluded and those treated with 10 µm camptothecin for 4 h were used as positive controls.
Exponentially growing HL‐60 cells were diluted in fresh culture medium and 1 × 106 cells were transferred to each well of a six‐well multiwell dish. Resveratrol or vehicle solution was added at the appropriate concentration (0.1% vehicle). The cells were incubated for 18 h and were treated according to the manufacturer's protocol. Immediately prior to analysis, 5 µl PI was added to each sample. Stained cells were analysed measuring Annexin‐V and PI using FL1‐H and FL3‐H, respectively.
All the cells were analysed by flow cytometry using a FACSCalibur (Becton Dickinson, Brondby, Denmark) equipped with a standard 488‐nm laser, and at least 20 000 events were counted. The flow cytometer was controlled by CellQuestPro version 4.0.2 (BD Biosciences) external software on Mac OS 9.
DAPI staining
To visualize changes in nuclear morphology, the cells were stained with DNA‐binding dye DAPI and were observed using fluorescence microscopy. HL‐60 cells were treated with 10‐µm camptothecin for 4 h and were used as positive controls. Exponentially growing HL‐60 cells were diluted in fresh culture medium and 1 × 106 cells were transferred to each well of a six‐well multiwell dish. Resveratrol or vehicle solution was added to obtain the appropriate concentration (0.1% vehicle). The cells were incubated for 18 h.
A stock solution of 1 mg/ml DAPI was made from DAPI dissolved in dH2O. This solution was further diluted to 1 µg/ml in PBS. Aliquots of 500 µl cell suspensions (1 × 105 cells) were centrifuged (400 g at room temperature for 5 min) onto coated glass slides (Thermo Electron, Copenhagen, Denmark) using a Cytospin 4 Cytocentrifuge (Thermo Electron). The slides were left to dry completely for at least 30 min to allow full adhesion. A hydrophobic barrier was drawn around the dried cells using a DakoCytomation Pen (DakoCytomation, Glostrup, Denmark). The cells were fixed with 4% paraformaldehyde in phosphate buffer (pH 7.4) at room temperature for 15 min and were washed with cold PBS in a Hellendahl‐type staining jar (Bie & Berntsen) for approximately 3 min. The fixed cells were permeabilized with ice‐cold 0.1% Triton X‐100 at room temperature for 4 min and then washed twice with ice‐cold PBS. Permeabilized cells were stained with a volume of 1 µg/ml DAPI appropriate to fully cover the cells. The slides were left in the dark at room temperature for 5 min and were washed once with cold PBS. Coverslips were mounted using Vectashield mounting solution (Vector Laboratories, Rodovre, Denmark). Stained cells were visualized on a Leica DMR light microscope (Leica, Herlev, Denmark). Images of at least three random positions on the slide were captured with a Leica DC200 camera (Leica) using the Leica im50 version 1.20 release 19 (Leica) external software on Windows 2000.
Software used
Cycle distribution was analysed by modfitlt version 2.0 (Verity Software House) for Mac OS 9. Graphs were prepared by grace 5.1.x series and images were manipulated by Gimp version 2.2.x and were prepared for presentation with Xfig version 3.2.5 Alpha on an appropriate Linux system. Weasel version 2.2.3, unlicensed (Walter and Eliza Hall Institute), was utilized for analysis of flow cytometric data. Image analysis was performed with Imagej version 1.35d (Abràmoff et al. 2004). Both applications were run using the Java 2 Runtime Environment 5.0, build 5 for Linux.
Statistical analysis
Statistical analysis was performed using Student's two‐tailed paired t‐test.
RESULTS
Resveratrol induces a concentration‐dependent inhibition of thymidine incorporation in HL‐60 and HepG2 cells
Treatment of HL‐60 cells with resveratrol increased the IC50 value for incorporation of [3H]dT in a concentration‐ and time‐dependent manner (Fig. 1a). IC50 increased from 15 µm resveratrol at 12 h to 30 µm resveratrol at 24 h. Between 2, 4, 8 and 12 h, no significant differences in IC50 values were observed. At 48 h, the IC50 value remained identical to value at 24 h. This result shows that resveratrol inhibits [3H]dT incorporation in a concentration‐dependent manner, and that the IC50 increases as a function of time up to 24 h.
Figure 1.

Resveratrol induces a time‐ and concentration‐dependent reduction in thymidine incorporation in HL‐60 and HepG2 cells. Resveratrol was added to HL‐60 (a) and HepG2 (b) cells at different time points, and all cells were harvested at the same time after the specified treatment times. Values are presented relative to untreated cells. The experiment was performed on three occasions and the results of a typical experiment are presented as mean ± SD, n = 6 (a) and n = 6 (b). Overlapping data points are annotated with the lowest common P value (**P 0.01, ***P 0.001 compared to vehicle‐treated cells).
A similar pattern was observed for the HepG2 cells. When treated with resveratrol for 2–48 h, an increase in IC50 values of [3H]dT incorporation in HepG2 cells was observed (Fig. 1b). The IC50 values ranged from 15 µm at 2 h to 50 µm at 12–24 h. However, at 48 h, the IC50 value decreased to 35 µm. This indicated that the modification of the IC50 value is a function of resveratrol concentration and incubation time when incubation time is less than 24 h. For incubation times greater than 24 h, IC50 is modified as a function of concentration and an inverse function of time in HepG2 cells.
The lowest concentration necessary for significant inhibition of incorporation of [3H]dT in HL‐60 cells was 5 µm for up to 12 h of treatment, and 20 µm for 24 and 48 h of treatment (Fig. 1a). For HepG2 cells, the pattern was more complex (Fig. 1b). The lowest inhibitory concentration is 20 µm for 2 and 4 h of treatment, 40 µm for 8, 12 and 48 h of treatment and 80 µm for 24 h of treatment.
Cell cycle progression is altered by resveratrol in a concentration‐dependent manner
To further explore the effects of resveratrol‐induced inhibition of cell growth measured by [3H]dT incorporation, the cell cycle modulating properties of resveratrol were examined.
After treatment with resveratrol for 24 h, a clear concentration‐dependent cell cycle accumulation was observed in HL‐60 cells (Fig. 2a). At low concentrations (20 µm), the number of HL‐60 cells increased in the S phase. At higher resveratrol concentrations (40 and 80 µm), there was a considerable accumulation of cells in the G1 phase.
Figure 2.
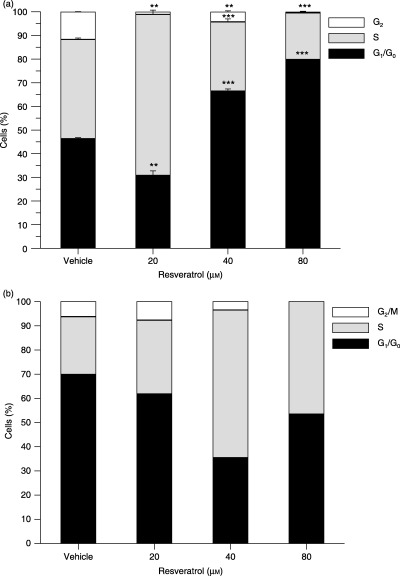
Resveratrol induces cell cycle arrest in HL‐60 and HepG2 cells. HL‐60 (a) and HepG2 (b) cells were treated with resveratrol for 24 h. The nuclei were stained with PI and DNA content was examined by flow cytometry. All data are presented relative to untreated cells. The experiments were performed on three occasions and the results of a typical experiment are shown. Data are presented as mean ± SD, n = 3 (a) and n = 1 (b). Overlapping data points are annotated with the lowest common P value (**P 0.01, ***P 0.001 compared to vehicle treated cells).
The effect on cell cycle progression in HepG2 cells (Fig. 2b) appeared to follow the same pattern as was observed with HL‐60 cells, although at slightly higher concentrations. Incubation with 20 µm resveratrol for 24 h did not affect the cell cycle distribution whereas 40 µm increased the number of HepG2 cells in the S phase. At increased concentrations (80 µm), resveratrol also caused accumulation of cells in the S phase, but to a lesser extent than was observed for 40 µm.
These results demonstrated that resveratrol modulated the cell cycle in vitro and the effect on the cell cycle was concentration dependent.
Modulation of the size and granularity of cell nuclei
Based on the observed cell cycle modulating effects of resveratrol, we expected a slight increase in the size of the nucleus in cells treated with resveratrol at concentrations causing accumulation in the S phase. Indeed, modulation of cell cycle progression of HL‐60 cells caused by resveratrol was demonstrated to correlate with a relative increase in the size of the nucleus of 20% at 20 µm resveratrol (Fig. 3a). Enlarged nuclei were observed in resveratrol‐treated cells mainly in the G1 and S phases, but cells in the G2+ M phase were also affected (data not shown). Increased resveratrol concentration reduced the size of the nucleus compared to control cells (8% decrease at 80 µm resveratrol for 24 h).
Figure 3.
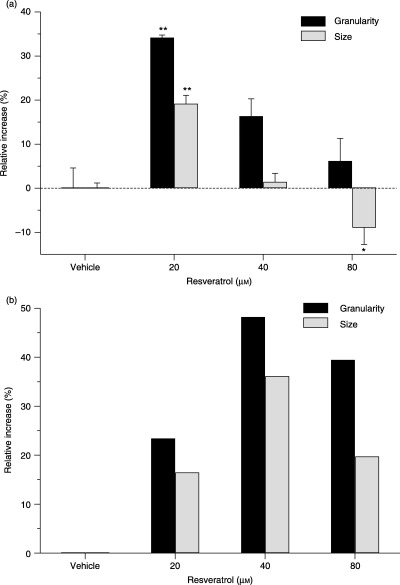
Resveratrol stimulates an increase in the nuclear size and granularity in HL‐60 and HepG2 cells. HL‐60 (a) and HepG2 (b) cells were treated with resveratrol for 24 h, and the size and granularity of the nuclei was examined by flow cytometry. All data are presented relative to untreated cells. The experiments were performed on three occasions and the results of a typical experiment are shown. Data are presented as mean ± SD, n = 3 (a) and n = 1 (b) (*P ≤ 0.05, **P ≤ 0.01 compared to vehicle‐treated cells).
HL‐60 cells treated with resveratrol also displayed an increased granularity of the cell nucleus (Fig. 3a). Cells in all phases of the cell cycle were affected by the treatment. The effect of resveratrol on the granularity of the nucleus appeared to be highest at 20 µm (Fig. 3a).
The size and granularity of HepG2 nuclei also increased when the cells were treated with resveratrol (Fig. 3b). Cells in all phases of the cell cycle appeared affected (data not shown). The peak effect appeared to be at 40 µm resveratrol (Fig. 3b).
It was not possible to observe the resveratrol‐induced alterations of nuclear size in HL‐60 and HepG2 cells measuring the area of DAPI‐stained nuclei visualized with fluorescent microscopy (data not shown).
Resveratrol stimulates apoptosis in HL‐60 cells
The ability of resveratrol to modulate apoptosis was initially studied by examination of nuclear morphological changes associated with apoptosis. In a preliminary study, it was found that resveratrol treatment for 12–18 h in HL‐60 cells resulted in optimal stimulation of apoptosis (data not shown). When treated with resveratrol for 18 h, there was an obvious concentration‐dependent increase in the number of HL‐60 nuclei with a condensed and fragmented morphology (Fig. 4). This demonstrated a concentration‐dependent stimulation of apoptosis.
Figure 4.
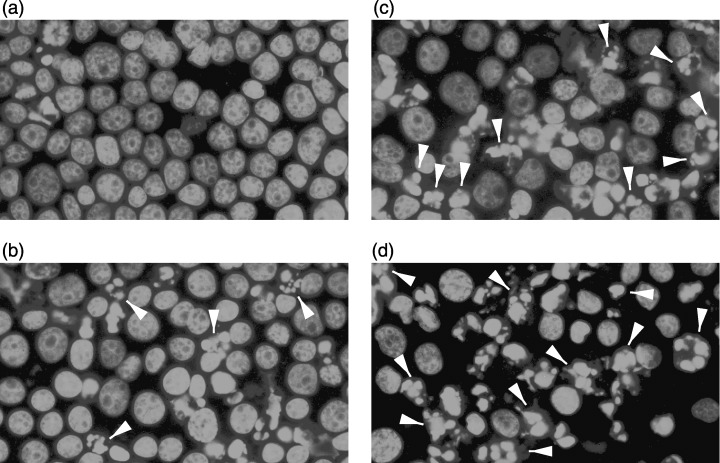
Resveratrol stimulates morphological changes of the cell nucleus of HL‐60 in a concentration‐dependent manner. Cells were treated with vehicle (a), 20 µm (b) or 40 µm (c) resveratrol for 18 h. After treatment, cells were stained with DAPI and the nuclei were visualized under a fluorescence microscope. Camptothecin 10 µm, 4 h incubation (d) was used as positive control. White arrowheads indicate morphological changes. The experiments were performed on four occasions and the results of a typical experiment are shown.
To further study the apoptosis‐modulating effect in HL‐60 cells, the binding of EGFP conjugated Annexin V was evaluated (Fig. 5a). Early apoptotic cells in this assay bind Annexin V but exclude the nuclear binding dye PI, that is, cells with lost membrane asymmetry but intact membranes.
Figure 5.
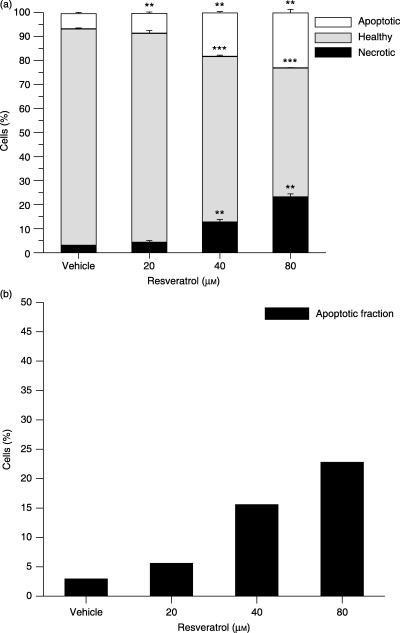
Resveratrol stimulates apoptosis in a concentration‐dependent manner in HL‐60 and HepG2 cells. Cells were treated with resveratrol for 18 h (a) or 24 h (b). In (a), HL‐60 cells were double stained with Annexin V and PI, and were examined by flow cytometry after resveratrol treatment. In (b), HepG2 cells were stained with PI and the sub‐G1 population was examined by flow cytometry. All data are presented relative to untreated cells. The experiments were performed on three occasions and results of a typical experiment are shown. They are presented as mean ± SD, n = 3 (a) and n = 1 (b). Overlapping data points are annotated with the lowest common P value (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001 compared to vehicle‐treated cells).
HL‐60 cells treated with resveratrol for 18 h showed a concentration‐dependent increase in Annexin V‐positive/PI‐negative cells (Fig. 5a). Only a minor effect of 20 µm resveratrol on HL‐60 cells was found, whereas higher concentrations greatly stimulated Annexin V binding. Treatment with 80 µm resveratrol resulted in 23% of the HL‐60 cells being early apoptotic and 23% being late apoptotic or necrotic. The same degree of concentration‐dependent apoptosis was observed in HepG2 cells (Fig. 5b).
DISCUSSION
The purpose of this study was to characterize the effect of resveratrol on DNA synthesis and cell cycle progression. In addition, the apoptosis‐stimulating properties of resveratrol were also studied. Inhibition of cell proliferation and stimulation of apoptosis by resveratrol may be key responses in relation to the putative cancer chemopreventive effect of this product.
The concentration‐dependent effect on cell growth measured as [3H]dT incorporation after 2 h was unexpected, and this very early effect indicates resveratrol's interference with cell replication machinery in vitro. To the best of our knowledge, this is the first report showing that such short‐term treatment with resveratrol affects DNA‐synthesis in HL‐60 and HepG2 cells in culture. The observation has only been paralleled by a single observation using the human prostate cancer cell line LNCap (Kuwajerwala et al. 2002).
In HL‐60 cells, the IC50 value at 8, 24 and 48 h for inhibition of incorporation of thymidine in the DNA is in concordance with earlier reports (Surh et al. 1999; Roy et al. 2002; Horvath et al. 2005). The experiments presented here show that resveratrol inhibits thymidine incoporation in HepG2 cells. This finding is in contrast to studies by Goldberg et al. (1995) and Delmas et al. (2000), who did not observe an inhibitory effect of resveratrol. This difference may be caused by variations between experimental conditions.
The putative interference with cell replication machinery in HL‐60 and HepG2 cells after such a short treatment time could be either a direct or an indirect inhibition of formation or movement of DNA polymerase. Resveratrol has been shown to interfere with DNA polymerase expression in vitro and ex vivo (Sun et al. 1998; Stivala et al. 2001) and inhibits the ribonucleotide reductase in vitro and ex vivo (Fontecave et al. 1998; Pozo‐Guisado et al. 2002). Also, resveratrol has been shown to reduce intracellular levels of dNTPs in HL‐60 cells (Horvath et al. 2005). The mechanism by which resveratrol inhibits DNA synthesis is not yet fully understood, but resveratrol is likely to interact with DNA polymerase (Stivala et al. 2001), whereas the assembly of replication complexes is not affected (Stivala et al. 2001).
Increase in IC50 values of incorporated [3H]dT in both cell lines strongly suggests a role of cellular activity, such as metabolism or removal of resveratrol. Metabolism of resveratrol in cells has not been studied in detail and results diverge (Allen et al. 2001; Raucy 2003). Preliminary studies in our laboratory indicate that removal of resveratrol may indeed be the cause of increased IC50 values, and further studies are required to fully investigate this phenomenon.
Increase in the size and granularity of the cell nuclei was observed in both cell lines. DAPI staining of nuclei indicates that this effect is not correlated to changes in the spatial arrangement of the DNA. An increase in nuclear size has previously been reported with two HIV protease inhibitors in adipose cells and with palmitate in cardiomyocytes (Kong & Rabkin 1999; Caron et al. 2003). To the best of our knowledge, no compound has been reported to stimulate nuclear enlargement in leukaemia and hepatoma cells, and further studies are needed to elucidate this novel property of resveratrol.
Nuclear granularity has only been investigated in a few cases, whereas increased cell granularity is a well known link to apoptosis. The peak of the effect of resveratrol appeared to be 20 and 40 µm for HL‐60 and HepG2 cells, respectively. These concentrations correlate to resveratrol concentrations needed to stimulate accumulation in the S phase of the cell cycle of the respective cell lines. We therefore propose that S phase arrest stimulated by resveratrol correlates with morphological changes in the surface structure of the nuclei.
Resveratrol has previously been reported to stimulate accumulation of HepG2 cells in the S phase of the cell cycle (Delmas et al. 2000) and HL‐60 cells in the G1 or S phase (Ragione et al. 1998; Joe et al. 2002; Kang et al. 2003; Ahmad et al. 2004). A striking result of treating HL‐60 and HepG2 cells with different concentrations of resveratrol is the concentration‐dependent phase specific accumulation of cells (Fig. 3e,f). Treatment with 20 µm resveratrol for 24 h resulted in nearly a doubling of the number of cells in the S phase compared to untreated HL‐60 cells. The same was observed for 40 µm in HepG2 cells. The apparent accumulation of cells in the S phase of the cell cycle may be attributed to apoptotic G2+ M phase cells. However, our preliminary studies show no indication of phase specific stimulation of apoptosis by resveratrol. Results presented here thus demonstrate that low concentrations of resveratrol prolong the S phase.
Treatment with 80 µm doubles the number of cells in the G1 phase compared to HL‐60 and HepG2 cells treated with 20 and 40 µm, respectively. This result clearly suggests that high concentrations of resveratrol stimulate accumulation of HL‐60 cells in the G1 phase and thus prevent cells from entering the S phase. This finding is in concordance with observations where resveratrol inhibited the production of polyamines by interfering with ornithine decarboxylase (Schneider et al. 2000), which catalyses the rate‐limiting step in polyamine production.
The observed concentration‐dependent cell cycle arrest in HL‐60 and HepG2 cells is in agreement with results found in a range of other cell lines (Table 1). In general, Table 1 and our observations indicate that in experiments where a concentration‐dependent effect on cell‐cycle distribution by resveratrol are observed, lower resveratrol concentrations cause accumulation of cells in the S phase, whereas higher concentrations cause accumulation of the cells in the G0/G1 phase or G2+ M phases. This may indicate that resveratrol has more than one target for the inhibition of cell division modulated at different concentrations, as proposed in prostate cells by Kuwajerwala et al. (2002).
Table 1.
Resveratrol stimulates cell cycle arrest in several cell lines in a concentration‐dependent manner
| Model | Phase accumulation | Concentration (µm) | Treatment time (h) | Reference |
|---|---|---|---|---|
| Human embryonic fibroblasts | ||||
| S | 30 | 24 | (Stivala et al. 2001) | |
| G1/S | 90 | 24 | ||
| MCF7* (mammary cells) | ||||
| S | 50 | 36 | (Pozo‐Guisado et al. 2002) | |
| G0/G1 | 100, 150 | 36 | ||
| CEM‐C7H2 (leukaemia cells) | ||||
| S | 10, 20 | 24 | (Bernhard et al. 2000) | |
| G1/S | 40, 100 | 24 | ||
| LNCap (prostate cells) | ||||
| S | 10 | 24 | (Kuwajerwala et al. 2002) | |
| G1 | 20, 25 | 24 | ||
| A2780 (ovarian cells) | ||||
| S | 50 | 24 | (Opipari et al. 2004) | |
| G1 | 100 | 24 | ||
| HCT‐116 (colonic cells) | ||||
| S | 50 | 24 | (Wolter et al. 2001) | |
| G2 + M | 100 | 24 | ||
| almost none | 200 | 24 | ||
| SW480 (colonic cells) | ||||
| S | 30, 50, 100 | 24/48 | (Delmas et al. 2002) | |
| S | 30 | 72 | ||
| G2 + M | 50, 100 | 72 | ||
| Caco2 (colonic cells) | ||||
| S | 25 | 16/24 | (Schneider et al. 2000) | |
| G2 + M | 25 | 40 | ||
| none | 25 | 48 | ||
| S | 25 | 64 | ||
| BPAE (endothelial cells) | ||||
| S/G2 + M | 50 | 72 | (Hsieh et al. 1999) | |
| S | 100 | 72 | ||
| CHL (Chinese hamster lung) | ||||
| S | 40 | 24 | (Matsuoka et al. 2001) | |
| S | 80 | 24 | ||
| G1/S | 40 | 48 | ||
| G1 | 80 | 48 | ||
Synchronized cells.
In HL‐60 cells where 20 µm resveratrol‐stimulated S‐phase arrest, incorporation of [3H]dT was reduced only by 30%. In HepG2 cells, where 40 µm resveratrol showed maximal S‐phase arrest, no significant decrease in incorporation of [3H]dT was observed. This suggests that accumulation in the S phase was caused by a slow down of progression through the cell cycle or an inhibition of S to G2 phase transition as suggested by Latruffe et al. (2002). The effect of resveratrol on the dynamics of this prolongation of the cell cycle is, to the best of our knowledge, not well studied and deserves closer examination.
Change in nuclear morphology and loss of membrane asymmetry are two hallmarks of apoptosis, and these were used to study apoptosis‐stimulating effects of resveratrol. The study of nuclear condensation and fragmentation, number of cells in the sub‐G1 fraction and exposure of phosphatidylserine identified a concentration‐dependent effect of resveratrol. DAPI staining identified a possible pro‐apoptotic effect of 20 µm resveratrol as seen by nuclear condensation. However, the same observation could not be made with the Annexin V binding assay, although higher concentrations resulted in a correlation between the results of DAPI staining and Annexin V binding. Nuclear condensation and exposure of phosphatidylserine are both controlled by caspase‐3 activation (Hirata et al. 1998). Thus, the difference may caused by a sequential process with loss of membrane asymmetry following nuclear condensation and fragmentation. In both cell lines, concentration‐dependent stimulation of apoptosis is in concordance with previous reports (Kuo et al. 2002; Roberti et al. 2003).
In conclusion, short‐term treatment with resveratrol results in concentration‐dependent interference with DNA‐synthesis and later cell proliferation. This early effect of resveratrol is previously unreported. Treatment of HL‐60 and HepG2 cells with resveratrol for 24 h causes a complex pattern of cell phase accumulation. Low concentrations cause a slowdown in progression through the cell cycle, whereas higher concentrations induce a phase‐specific cell cycle arrest. For the first time, resveratrol was shown to stimulate an increase in nuclear size of HL‐60 cells. An increase in granularity HL‐60 and HepG2 nuclei was also observed. These increases in nuclear size and granularity are correlated to the phases of the cell cycle. Furthermore, resveratrol stimulates apoptosis in a concentration‐dependent manner.
REFERENCES
- Abràmoff MD, Magalhães PJ, Ram SJ (2004) Image processing with Image. Biophotonics Int. 11, 36–42. [Google Scholar]
- Aggarwal BB, Bhardwaj A, Aggarwal RS, Seeram NP, Shishodia S, Takada Y (2004) Role of resveratrol in prevention and therapy of cancer: preclinical and clinical studies. Anticancer Res. 24, 2783–2840. [PubMed] [Google Scholar]
- Ahmad KA, Clement MV, Hanif IM, Pervaiz S (2004) Resveratrol inhibits drug‐induced apoptosis in human leukemia cells by creating an intracellular milieu nonpermissive for death execution. Cancer Res. 64, 1452–1459. [DOI] [PubMed] [Google Scholar]
- Allen SW, Mueller L, Williams SN, Quattrochi LC, Raucy J (2001) The use of a high‐volume screening procedure to assess the effects of dietary flavonoids on human CYP1A1 expression. Drug Metab. Dispos. 29, 1074–1079. [PubMed] [Google Scholar]
- Bernhard D, Tinhofer I, Tonko M, Hubl H, Ausserlechner MJ, Greil R, Kofler R, Csordas A (2000) Resveratrol causes arrest in the S‐phase prior to fas‐independent apoptosis in CEM‐C7H2 acute leukemia cells. Cell Death Differ. 7, 834–842. [DOI] [PubMed] [Google Scholar]
- Caltagirone S, Rossi C, Poggi A, Ranelletti FO, Natali PG, Brunetti M, Aiello FB, Piantelli M (2000) Flavonoids apigenin and quercetin inhibit melanoma growth and metastatic potential. Int. J. Cancer 87, 595–600. [DOI] [PubMed] [Google Scholar]
- Carbo N, Costelli P, Baccino FM, Lopez‐Soriano FJ, Argilos JM (1999) Resveratrol, a natural product present in wine, decreases tumour growth in a rat tumour model. Biochem. Biophys. Res. Commun. 254, 739–743. [DOI] [PubMed] [Google Scholar]
- Caron M, Auclair M, Sterlingot H, Kornprobst M, Capeau J (2003) Some HIV protease inhibitors alter lamin A/C maturation and stability, SREBP‐1 nuclear localization and adipocyte differentiation. AIDS 17, 2437–2444. [DOI] [PubMed] [Google Scholar]
- Castello L, Tessitore L (2005) Resveratrol inhibits cell cycle progression in U937 cells. Oncol. Rep. 13, 133–137. [PubMed] [Google Scholar]
- Chen Y, Tseng SH, Lai HS, Chen WJ (2004) Resveratrol‐induced cellular apoptosis and cell cycle arrest in neuroblastoma cells and antitumor effects on neuroblastoma in mice. Surgery 136, 57–66. [DOI] [PubMed] [Google Scholar]
- Clement MV, Hirpara JL, Chawdhury SH, Pervaiz S (1998) Chemopreventive agent resveratrol, a natural product derived from grapes, triggers CD95 signaling‐dependent apoptosis in human tumor cells. Blood 92, 996–1002. [PubMed] [Google Scholar]
- Delmas D, Jannin B, Malki MC, Latruffe N (2000) Inhibitory effect of resveratrol on the proliferation of human and rat hepatic derived cell lines. Oncol. Rep. 7, 847–852. [DOI] [PubMed] [Google Scholar]
- Delmas D, Passilly‐Degrace P, Jannin B, Malki MC, Latruffe N (2002) Resveratrol, a chemopreventive agent, disrupts the cell cycle control of human SW480 colorectal tumor cells. Int. J. Mol. Med. 10, 193–199. [PubMed] [Google Scholar]
- Estrov Z, Shishodia S, Faderl S, Van Harris DQ, Kantarjian HM, Talpaz M, Aggarwal BB (2003) Resveratrol blocks interleukin‐1beta‐induced activation of the nuclear transcription factor NF‐kappaB, inhibits proliferation, causes S‐phase arrest, and induces apoptosis of acute myeloid leukemia cells. Blood 102, 987–995. [DOI] [PubMed] [Google Scholar]
- Fontecave M, Lepoivre M, Elleingand E, Gerez C, Guittet O (1998) Resveratrol, a remarkable inhibitor of ribonucleotide reductase. FEBS Lett. 421, 277–279. [DOI] [PubMed] [Google Scholar]
- Fulda S, Debatin KM (2004) Sensitization for tumor necrosis factor‐related apoptosis‐inducing ligand‐induced apoptosis by the chemopreventive agent resveratrol. Cancer Res. 64, 337–346. [DOI] [PubMed] [Google Scholar]
- Goldberg DM, Hahn SE, Parkes JG (1995) Beyond alcohol: beverage consumption and cardiovascular mortality. Clin. Chim. Acta 237, 155–187. [DOI] [PubMed] [Google Scholar]
- Hirata H, Takahashi A, Kobayashi S, Yonehara S, Sawai H, Okazaki T, Yamamoto K, Sasada M (1998) Caspases are activated in a branched protease cascade and control distinct downstream processes in Fas‐induced apoptosis. J. Exp. Med. 187, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath Z, Saiko P, Illmer C, Madlener S, Hoechtl T, Bauer W, Erker T, Jaeger W, Fritzer‐Szekeres M, Szekeres T (2005) Synergistic action of resveratrol, an ingredient of wine, with Ara‐C and tiazofurin in HL‐60 human promyelocytic leukemia cells. Exp. Hematol. 33, 329–335. [DOI] [PubMed] [Google Scholar]
- Hsieh TC, Juan G, Darzynkiewicz Z, Wu JM (1999) Resveratrol increases nitric oxide synthase, induces accumulation of p53 and p21 (WAF1/CIP1), and suppresses cultured bovine pulmonary artery endothelial cell proliferation by perturbing progression through S and G2 . Cancer Res. 59, 2596–2601. [PubMed] [Google Scholar]
- Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, Pezzuto JM (1997) Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science 275, 218–220. [DOI] [PubMed] [Google Scholar]
- Joe AK, Liu H, Suzui M, Vural ME, Xiao DH, Weinstein IB (2002) Resveratrol induces growth inhibition, S‐phase arrest, apoptosis, and changes in biomarker expression in several human cancer cell lines. Clin. Cancer Res. 8, 893–903. [PubMed] [Google Scholar]
- Kang JH, Park YH, Choi SW, Yang EK, Lee WJ (2003) Resveratrol derivatives potently induce apoptosis in human promyelocytic leukemia cells. Exp. Mol. Med. 35, 467–474. [DOI] [PubMed] [Google Scholar]
- Kapadia GJ, Azuine MA, Tokuda H, Takasaki M, Mukainaka T, Konoshima T, Nishino H (2002) Chemopreventive effect of resveratrol, sesamol, sesame oil and sunflower oil in the Epstein–Barr virus early antigen activation assay and the mouse skin two‐stage carcinogenesis. Pharmacol. Res. 45, 499–505. [DOI] [PubMed] [Google Scholar]
- Kim YA, Lee WH, Choi TH, Rhee SH, Park KY, Choi YH (2003) Involvement of p21WAF1/CIP1, pRB, Bax and NF‐kappa B in induction of growth arrest and apoptosis by resveratrol in human lung carcinoma A549 cells. Int. J. Oncol. 23, 1143–1149. [PubMed] [Google Scholar]
- Kimura Y, Okuda H (2001) Resveratrol isolated from Polygonum cuspidatum root prevents tumor growth and metastasis to lung and tumor‐induced neovascularization in Lewis lung carcinoma‐bearing mice. J. Nutr. 131, 1844–1849. [DOI] [PubMed] [Google Scholar]
- Kong JY, Rabkin SW (1999) Palmitate induces structural alterations in nuclei of cardiomyocytes. Tissue Cell 31, 473–479. [DOI] [PubMed] [Google Scholar]
- Kuo PL, Chiang LC, Lin CC (2002) Resveratrol‐induced apoptosis is mediated by p53‐dependent pathway in Hep G2 cells. Life Sci. 72, 23–34. [DOI] [PubMed] [Google Scholar]
- Kuwajerwala N, Cifuentes E, Gautam S, Menon M, Barrack ER, Reddy GP (2002) Resveratrol induces prostate cancer cell entry into S phase and inhibits DNA synthesis. Cancer Res. 62, 2488–2492. [PubMed] [Google Scholar]
- Langcake P, Pryce RJ (1976) Production of resveratrol by Vitis vinifera and other members of Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 9, 77–86. [Google Scholar]
- Latruffe N, Delmas D, Jannin B, Malki MC, Passilly‐Degrace P, Berlot JP (2002) Molecular analysis on the chemopreventive properties of resveratrol, a plant polyphenol microcomponent. Int. J. Mol. Med. 10, 755–760. [PubMed] [Google Scholar]
- Liang YC, Tsai SH, Chen L, Lin‐Shiau SY, Lin JK (2003) Resveratrol‐induced G2 arrest through the inhibition of CDK7 and p34CDC2 kinases in colon carcinoma HT29 cells. Biochem. Pharmacol. 65, 1053–1060. [DOI] [PubMed] [Google Scholar]
- Liu HS, Pan CE, Yang W, Liu XM (2003) Antitumor and immunomodulatory activity of resveratrol on experimentally implanted tumor of H22 in Balb/c mice. World. J. Gastroenterol. 9, 1474–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka A, Furuta A, Ozaki M, Fukuhara K, Miyata N (2001) Resveratrol, a naturally occurring polyphenol, induces sister chromatid exchanges in a Chinese hamster lung (CHL) cell line. Mutat. Res. 494, 107–113. [DOI] [PubMed] [Google Scholar]
- Opipari AW, Tan LJ, Boitano AE, Sorenson DR, Aurora A, Liu JR (2004) Resveratrol‐induced autophagocytosis in ovarian cancer cells. Cancer Res. 64, 696–703. [DOI] [PubMed] [Google Scholar]
- Pervaiz S (2003) Resveratrol: from grapevines to mammalian biology. FASEB J. 17, 1975–1985. [DOI] [PubMed] [Google Scholar]
- Pozo‐Guisado E, Alvarez‐Barrientos A, Mulero‐Navarro S, Santiago‐Josefat B, Fernandez‐Salguero PM (2002) The antiproliferative activity of resveratrol results in apoptosis in MCF‐7 but not in MDA‐MB‐231 human breast cancer cells: cell‐specific alteration of the cell cycle. Biochem. Pharmacol. 64, 1375–1386. [DOI] [PubMed] [Google Scholar]
- Ragione FD, Cucciolla V, Borriello A, Pietra VD, Racioppi L, Soldati G, Manna C, Galletti P, Zappia V (1998) Resveratrol arrests the cell division cycle at S/G2 phase transition. Biochem. Biophys. Res. Commun. 250, 53–58. [DOI] [PubMed] [Google Scholar]
- Raucy JL (2003) Regulation of CYP3A4 expression in human hepatocytes by pharmaceuticals and natural products. Drug Metab. Dispos. 31, 533–539. [DOI] [PubMed] [Google Scholar]
- Roberti M, Pizzirani D, Simoni D, Rondanin R, Baruchello R, Bonora C, Buscemi F, Grimaudo S, Tolomeo M (2003) Synthesis and biological evaluation of resveratrol and analogues as apoptosis‐inducing agents. J. Med. Chem. 46, 3546–3554. [DOI] [PubMed] [Google Scholar]
- Roman V, Billard C, Kern C, Ferry‐Dumazet H, Izard JC, Mohammad R, Mossalayi DM, Kolb JP (2002) Analysis of resveratrol‐induced apoptosis in human B‐cell chronic leukaemia. Br. J. Haematol. 117, 842–851. [DOI] [PubMed] [Google Scholar]
- Roy M, Chakraborty S, Siddiqi M, Bhattacharya RK (2002) Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac. J. Cancer Prev. 3, 61–67. [PubMed] [Google Scholar]
- Schneider Y, Vincent F, Duranton B, Badolo L, Gosse F, Bergmann C, Seiler N, Raul F (2000) Anti‐proliferative effect of resveratrol, a natural component of grapes and wine, on human colonic cancer cells. Cancer Lett. 158, 85–91. [DOI] [PubMed] [Google Scholar]
- Schneider Y, Duranton B, Gosse F, Schleiffer R, Seiler N, Raul F (2001) Resveratrol inhibits intestinal tumorigenesis and modulates host‐defense‐related gene expression in an animal model of human familial adenomatous polyposis. Nutr. Cancer 39, 102–107. [DOI] [PubMed] [Google Scholar]
- Siemann EH, Creasy LL (1992) Concentration of the phytoalexin resveratrol in wine. Am. J. Enol. Viticult. 43, 49–52. [Google Scholar]
- Soleas GJ, Diamandis EP, Goldberg DM (1997) Resveratrol: a molecule whose time has come? and gone? Clin. Biochem. 30, 91–113. [DOI] [PubMed] [Google Scholar]
- Stervbo U, Vang O, Bonnesen C (2006) A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem. [Epub ahead of print].
- Stivala LA, Savio M, Carafoli F, Perucca P, Bianchi L, Maga G, Forti L, Pagnoni UM, Albini A, Prosperi E, Vannini V (2001) Specific structural determinants are responsible for the antioxidant activity and the cell cycle effects of resveratrol. J. Biol. Chem. 276, 22586–22594. [DOI] [PubMed] [Google Scholar]
- Sun NJ, Woo SH, Cassady JM, Snapka RM (1998) DNA polymerase and topoisomerase II inhibitors from Psoralea corylifolia. J. Nat. Prod. 61, 362–366. [DOI] [PubMed] [Google Scholar]
- Surh YJ, Hurh YJ, Kang JY, Lee E, Kong G, Lee SJ (1999) Resveratrol, an antioxidant present in red wine, induces apoptosis in human promyelocytic leukemia (HL‐60) cells. Cancer Lett. 140, 1–10. [DOI] [PubMed] [Google Scholar]
- Vindelov LL, Christensen IJ, Nissen NI (1983) A detergent‐trypsin method for the preparation of nuclei for flow cytometric DNA analysis. Cytometry 3, 323–327. [DOI] [PubMed] [Google Scholar]
- Wolter F, Akoglu B, Clausnitzer A, Stein J (2001) Downregulation of the cyclin D1/Cdk4 complex occurs during resveratrol‐induced cell cycle arrest in colon cancer cell lines. J. Nutr. 131, 2197–2203. [DOI] [PubMed] [Google Scholar]
- Wyke SM, Russell ST, Tisdale MJ (2004) Induction of proteasome expression in skeletal muscle is attenuated by inhibitors of NF‐kappaB activation. Br. J. Cancer 91, 1742–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou HB, Chen JJ, Wang WX, Du Cai JT, Q (2005) Anticancer activity of resveratrol on implanted human primary gastric carcinoma cells in nude mice. World J. Gastroenterol. 11, 280–284. [DOI] [PMC free article] [PubMed] [Google Scholar]


