Abstract
Objectives
We were interested in determining whether epidermal growth factor gene‐transfected mesenchymal stem cells (EGF‐MSC) would accelerate fibroblast migration and proliferation.
Materials and methods
Fibroblasts were cultured in serum‐free conditioned media from EGF‐MSC; RT‐PCR was performed to detect expression of EGF gene in EGF‐MSCs. EGF protein levels in cell culture supernatants from EGF‐MSC were assayed by ELISA and proliferation of EGF‐MSC‐treated fibroblasts was performed using MTT assay. Effects of EGF‐MSC on fibroblast migration were evaluated using scratch wound and transmigration assays. Cell adhesion molecules, cell dynamics molecules and phospho‐(Ser) kinase substrate expressions of EGF‐MSC‐treated fibroblasts were evaluated by western blotting.
Results
EGF gene expression increased in EGF‐MSCs and viability of EGF‐MSC‐treated fibroblasts was elevated. EGF‐MSC‐treated fibroblasts showed increased migration compared to controls. Expressions of cell adhesion molecules (β‐catenin, N‐cadherin), cell dynamics molecules (cofilin, ezrin) and phospho‐(Ser) kinase substrates (phospho‐MAPK/CDK substrate, phospho‐Arg‐(Ser)‐X‐Tyr/Phe‐X‐pSer motif) increased in EGF‐MSC‐treated fibroblasts. These results imply that EGF‐MSCs contributed to enhancing the wound healing process by increased cell adhesion, dynamic effects, fibroblast migration, and proliferation.
Conclusions
This study indicates that EGF‐MSCs had a positive influence on fibroblast migration and proliferation and EGF‐MSC may provide a useful strategy for wound healing.
Introduction
Skin is an essential organ maintaining homeostasis, as it protects the body against toxins, microorganisms and further external potential insult. Healing of cutaneous wounds requires complex interactions of dermal cells, epidermal cells and the extracellular matrix (ECM) 1, 2. During the course of wound healing, fibroblasts differentiate into myofibroblasts 3, and myofibroblasts synthesize the ECM. As wound healing progresses, myofibroblasts begin to die by apoptosis and are replaced by a second wave of fibroblasts from surrounding tissue 4. Successful wound healing involves a number of processes including cell migration, cell proliferation and matrix deposition 5. In particular, migration and proliferation are critical and are driven by growth factors released at the injury site. Epidermal growth factor (EGF) facilitates epidermal cell regeneration and plays an essential role in the process of dermal wound healing by stimulating proliferation and migration of keratinocytes. EGF promotes formation of granulation tissue, stimulates fibroblast motility and has been widely employed in studies to promote epidermal wound healing.
Mesenchymal stem cells (MSC) are plastic‐adherent cells with fibroblast‐like morphology and have the capacity for multipotent differentiation in vitro 6. Umbilical cord blood (UCB) has been identified as a source of MSCs, and human UCB‐derived MSCs can serve as an alternative source of bone marrow‐derived MSCs. Wound healing mechanisms include differentiation of MSCs and paracrine effects of MSCs on epidermal and dermal cells 7, 8. Beneficial effects of MSCs are more likely to be mediated by their secretion of soluble factors than by their long‐term presence in repaired tissue 9. For example, Chen et al. demonstrated that growth medium conditioned with murine bone marrow‐derived MSCs contains high levels of secreted cytokines and is sufficient to enhance cutaneous wound healing in Balb/C mice 10. In other studies, Kim et al. have shown that adipose‐derived MSCs stimulate human fibroblasts via paracrine effects, significantly reducing wound size, and promoting re‐epithelialization, in an in vivo model 11. Conditioned media (CM) are media in which cells have been cultured for some time; they contain many mediator substances such as growth factors and cytokines.
The focus of this study was to clarify effects of EGF gene‐transfected MSCs (EGF‐MSC) on wound healing. EGF‐MSC CM was used to verify interactions between EGF‐MSCs and fibroblasts. A scratch wound assay was performed to determine whether EGF‐MSCs would influence fibroblast mobility and western blotting was performed to detect expression of cell adhesion molecules, cell dynamics molecules and phospho‐(Ser) kinase substrates as increased expression of these substrates may be involved in wound healing by EGF‐MSCs. These proteins play an important role in EGF‐MSC‐induced wound healing by fibroblasts.
Cell dynamics and cell adhesion are essential for cell migration. Additionally, phospho‐(Ser) kinase substrates are highly conserved proteins involved in regulation of cell survival, apoptosis and proliferation.
Materials and methods
MSCs
Cryopreserved MSCs isolated from UCB were purchased at passage 3 from Medipost Co. (Seoul, Korea); they were grown in MEM‐α supplemented with 2 mm l‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% foetal bovine serum (FBS), and seeded at 2 × 105 cells in 100‐mm dishes. After 7 days population expansion, the cells were trypsinized and cryopreserved at passage 6. They were then maintained at low density at 37 °C in 5% CO2, and passages 9–12 were used for subsequent experiments.
Human skin fibroblasts
Human skin fibroblasts were obtained at passage 3 from KCLB (Seoul, Korea) and were grown in MEM‐α supplemented with 2 mm l‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS. They were seeded at 2 × 105 cells in 100‐mm dishes and maintained at 37 °C in 5% CO2. Passages 8–12 were used for all subsequent experiments. Fibroblasts were cultured in MEM‐α without FBS, and medium was exchanged for MSC CM, vector‐MSC CM or EGF‐MSC CM, 1 day later.
Plasmids
Total RNA was isolated from HT29 human normal colorectal cells. Total RNA was used to synthesize EGF‐cDNA using Maxime RT Premix Kit (Intron, Sungnam, Korea). Polymerase chain reaction (PCR) analyses were carried out using specific primers for human EGF. PCR primers for EGF‐cDNA were as follows:
Forward primer: 5′‐GCTAGCTAGCGATGCTGCTCACTCTTATC‐3′;
Reverse primer: 5′‐CGCCTCGAGCTGAGTCAGCTCCATTTG‐3′.
Genomic fragments of EGF were cloned into the pEGFP‐N1 plasmid using the following steps: (i) for cloning EGF, amplified product was ligated into NheI‐ and XhoI‐cut pEGFP‐N1; (ii) Ligation mixtures were transformed into chemically competent Escherichia coli strains DH5α (Gibco, Sparks, MD, USA) with SOC medium supplementation (2% tryptone, 0.5% yeast extract, 10 mm NaCl, 2.5 mm KCl, 10 mm MgCl2, 10 mm MgSO4, 20 mm glucose) for 90 min, with shaking at room temperature. Bacterial transformants were then spread on to an LB medium agar plate (Sigma, St Louis, MO, USA) with 25 mg/ml ampicillin, and incubated at 37 °C for 1–2 days. EGF‐transformed pEGFP‐N1 (pEGFP‐N1‐EGF) was precipitated using the Qiagen Plasmid Kit (Qiagen, Venlo, the Netherlands). pEGFP‐N1‐EGF was sequenced to confirm its identity using dideoxynucleotide sequencing (data not shown). EGF in pEGFP‐N1‐EGF was identified by cutting with the EcoRI restriction enzyme.
Transfection
Mesenchymal stem cells were seeded in 100‐mm dishes with MEM‐α supplemented with 2 mm l‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin and 10% FBS. In total, 20 μg of plasmid pEGFP‐N1 and pEGFP‐N1‐EGF was transfected into MSCs using Lipofectamine‐2000 (Invitrogen, Paisley, UK). Medium was changed 5 h after transfection.
Total RNA isolation
Total RNA was extracted from EGF‐CM‐treated cells using RNAiso (Takara, Otsu, Japan). Fibroblasts were washed once in PBS to remove cell debris, and 2 ml RNAiso and DNase I were added to them, cultured in EGF‐MSC CM. Cells were gathered using a cell scraper and transferred to 15 ml conical tubes to incubate for 5 min; after 4 ml of chloroform was added to the tubes, they were centrifuged. Supernatant was removed and placed into new 1.7 ml centrifuge tubes. Supernatant was washed with isopropanol, and total RNA was precipitated at 12 000 g for 10 min at 4 °C.
Reverse transcriptase‐PCR (RT‐PCR)
Total RNA was used to synthesize cDNA using Maxime RT Premix kit (Intron). PCR reactions were carried out using specific primers for human EGF. PCR primers for EGF cDNA were as follows:
Forward primer: 5′‐CGATATGGACGTACACACGA‐3′;
Reverse primer: 5′‐TGGGTTAGCTGATAGGAGAG‐3′.
PCR products were obtained after 35 cycles amplification at denaturation temperature 92 °C, annealing temperature 57 °C and extension temperature 68 °C.
Elisa
Epidermal growth factor protein level in cell culture supernatants from EGF‐MSCs was assayed using human EGF immunoassay kit (R&D, Minneapolis, MN, USA). After transfection, conditioned medium was collected from EGF‐MSCs. CM and EGF‐MSC CM was added to each well and incubated for 2 h at room temperature. EGF was determined by addition of 200 μl peroxidase‐conjugated anti‐human antibody for 2 h. Plates were washed four times in TBST; 100 μl substrate was added to each well and incubated for 15 min at room temperature. The reaction was stopped using 100 μl of 2 n sulphuric acid, and absorbance was measured at 450 nm using the Dynex opsys MR ELISA reader (Chantilly, VA, USA).
Cell viability assay
Human skin fibroblasts were seeded at 5 × 104 cells/well in MEM‐α and grown for 2 days. Cell viability assay was initiated by adding EGF‐MSC CM, vector‐MSC CM, or MSC CM. After 24‐h incubation, 20 μl 3‐(4,5‐dimethylthiazol‐2y)‐2,5‐diphenyl‐2H‐tetrazolium bromide (MTT) was added to each well, and cells were incubated for 4 h. Supernatant was removed and dimethyl sulphoxide was added to each well. Samples were shaken to dissolve the formazan and absorbance was measured at 570 nm with a uQuant microplate reader.
Cell migration assay
We analysed effects of MSCs on fibroblast migration using a scratch wound assay. The underside of each well was scribed vertically with an edge blade to generate two guidelines in a row through the middle of the well, to define reference points for image capture. Fibroblasts were then seeded in the scribed plate at 2 × 104 cells/well in MEM‐α, and maintained to 80–90% confluence. The fibroblast monolayer was scratched using a p200 pipette tip running the full length of the well between the guidelines. Each well was washed once in growth medium to remove cell debris, then MSC CM, vector‐MSC CM or EGF‐MSC CM was added to the scratched fibroblast cultures. Images were captured along etched scratch guidelines to record scratch wound closure, this being monitored by collecting digitized images at selected time intervals. Digitized images were captured on a Nikon Eclipse TE300 inverted microscope then acquired using T‐view software, and saved as TIFF files. Cell‐transmigration assay was performed in a co‐culture system using an upper cell culture insert (SPL, Gyeonggi‐do, Korea) with polycarbonate membrane (8 μm pore size, 1 × 105 pores/cm2) and a companion 24‐well plate beneath. Briefly, fibroblasts were centrifuged at a low speed (250 × g for 5 min at 24 °C), resuspended in fresh medium (1 × 105 cells/ml), placed in upper inserts and incubated for 2 h at 37 °C; lower plates were filled with MSC CM, vector‐MSC CM or EGF‐MSC CM. The upper inserts and lower plates were assembled and incubated in 70% humidity and 5% CO2 at 37 °C for 12, 24 and 48 h. After incubation, fibroblasts in upper inserts were removed by scraping. Membranes were then removed from upper inserts, placed on slides and stained with trypan blue (Sigma). Numbers of transmigrated cells in nine fixed fields were determined using light microscopy at 200× magnification.
Western blotting
Protein in cell lysates was separated by 12% sodium dodecyl sulphate polyacrylamide gel electrophoresis, and protein was transferred to nitrocellulose membranes (Bio‐Rad, Hercules, CA, USA), probed with antibodies specific to β‐catenin, N‐cadherin, pan‐cadherin phospho‐(Ser) CDK substrate, phospho‐MAPK/CDK substrate, phospho‐(Ser)‐Arg‐X‐Tyr/Phe‐X‐pSer motif, phospho‐(Ser) 14‐3‐3 binding motif, cofilin, and p‐ezrin (Cell Signaling Technology, Danvers, MA, USA) diluted in TBST buffer (10 mm, pH 7.5 Tris‐HCl, 100 mm NaCl and 0.1% Tween‐20), followed by goat antirabbit IgG‐horseradish peroxidase (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Protein labelled with antibody was detected using enhanced chemiluminescence kits (Cell Signaling Technology).
Statistical analysis
Data were obtained from at least three independent experiments and values are expressed as mean ± standard deviation. Student's t‐test was used for statistical analyses, and P < 0.05 was considered significant.
Results
EGF expression in EGF gene‐transfected MSCs
Restriction enzyme cutting was performed using EcoRI to investigate whether the EGF cDNA was cloned into pEGFP‐N1 and the restriction enzyme cut product was confirmed by 1.5% agarose gel electrophoresis (Fig. 1); 3.7 kbp EGF cDNA band was confirmed. pEGFP‐N1‐EGF was transfected into MSCs. EGF mRNA was clearly expressed in pEGFP‐N1‐EGF‐transfected MSCs (EGF‐MSC) using RT‐PCR (Fig. 2a). To measure EGF expression in EGF‐MSCs, ELISA was performed. Expression of EGF protein was found to be higher in EGF‐MSCs (Fig. 2b).
Figure 1.
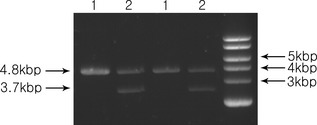
Determination of epidermal growth factor gene in EGF gene‐transfected pEGFP‐N1 plasmid. pEGFP‐N1 and pEGFP‐N1‐EGF were cut with EcoRI; digested products were loaded on a 1.5% agarose gel. 1, digested pEGFP‐N1 was loaded in the first and third lanes. 2, digested pEGFP‐N1‐EGF was loaded in the second and fourth lanes. 3.7 kbp EGF cDNA band and 4.8 kbp pEGFP‐N1 band were detected.
Figure 2.
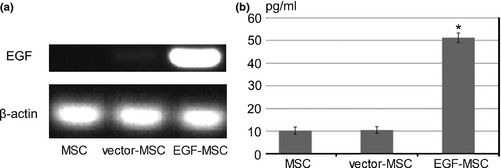
Expression of EGF in EGF‐MSC. (a) RT‐PCR was performed using total RNA isolated from MSC, vector‐MSC, and EGF‐MSC. RT‐PCR products were loaded on a 1.5% agarose gel for electrophoresis. No EGF mRNA was detected in MSC, low expression in the vector‐MSCs, and high expression in EGF‐MSC. (b) EGF levels from MSC, vector‐MSC, EGF‐MSC were measured in CM by ELISA. Concentration of EGF in EGF‐MSC was upregulated compared to that of MSC and vector‐MSC. Data represent means ± SEM, n = 3. *P < 0.05 versus MSC.
Effects of EGF‐MSC on fibroblast viability
We cultured human skin fibroblasts with EGF‐MSC CM for 24 h, and MTT assay was performed to investigate effects of EGF‐MSCs on fibroblast viability. Fibroblasts were cultured with MSC CM, pEGFP‐N1‐transfected‐MSC (vector‐MSC) CM or EGF‐MSC CM. EGF‐MSC CM significantly increased fibroblast viability compared to MSC CM or vector‐MSC CM (Fig. 3).
Figure 3.
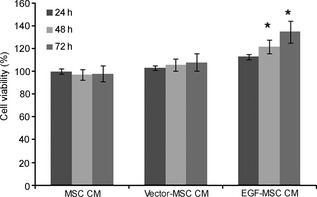
Effects of EGF ‐ MSC s on fibroblast viability. Human skin fibroblasts were seeded at 5 × 104 cell/well in MEM‐α and grown for 2 days. Cell viability assay was conducted by adding MSC CM, vector‐MSC CM or EGF‐MSC CM; MTT assay was performed after 24, 48 and 72 h incubation. Cell viability of EGF‐MSC CM‐treated fibroblasts increased significantly. Data represent means ± SEM, n = 3. *P < 0.05 versus MSC CM‐treated fibroblasts.
Effects of EGF‐MSC on fibroblast migration
Effects of EGF‐MSCs on human skin fibroblast migration were determined using scratch wound assay. Images of scratch wounds at 24, 48 and 72 h after scratching showed that fibroblast migration into the wound area was accelerated in the presence of EGF‐MSC CM (Fig. 4a) and scratch wound closure was complete by 72 h with this. Increased cell migration contributed to accelerated wound closure in EGF‐MSC CM‐treated fibroblasts. After they had been cultured for 3 days, fibroblasts were cultured with EGF‐MSC CM, vector‐MSC CM or MSC CM to determine their transmigration activity. Their incubation in EGF‐MSC CM produced progressive increase in cell transmigration in a time‐dependent manner (Fig. 4b). Results of transmigration assay show that number of transmigrated EGF‐MSC CM‐treated fibroblasts was 3‐fold (P < 0.05) greater than MSC CM‐treated or vector‐MSC CM‐treated fibroblasts.
Figure 4.
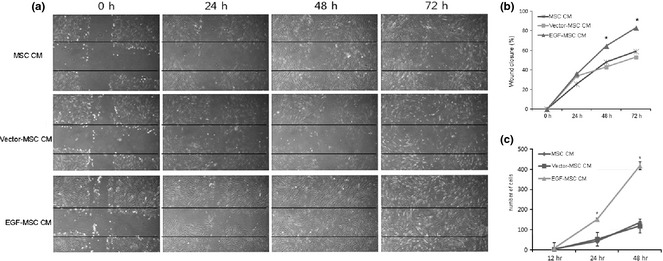
Effects of EGF ‐ MSC s on fibroblast migration. (a) Scratch wound assay was conducted in the presence of MSC CM, vector‐MSC CM and EGF‐MSC CM for 24, 48 and 72 h. Images were captured along scratch guidelines to record scratch wound closure which was monitored by collecting digitized images. Results showed that number of cells migrating increased amongst EGF‐MSC CM‐treated fibroblast. Images were taken at 100× magnification. (b) Enhanced wound closure was detected in EGF‐MSC CM‐exposed fibroblasts and percentage of wound closure in scratch wound assays was determined. Data represent means ± SEM, n = 3. *P < 0.05 versus the MSC CM fibroblasts. (c) Fibroblast transmigration was performed in a modified Boyden chamber using MSC CM, vector‐MSC CM and EGF‐MSC CM. Fibroblasts were seeded on 8 μm pore size filters. Trypan blue staining was used to assess numbers of migrating cells. Numbers of transmigrating fibroblasts was higher amongst EGF‐MSC CM‐exposed fibroblasts.
Effects of EGF‐MSC on expression of cell adhesion molecules, cell dynamics molecules and phospho‐(Ser) kinase substrates of fibroblasts
We investigated whether EGF‐MSC‐induced fibroblast migration was dependent on cell adhesion and cell dynamics molecules. As shown in Fig. 5, expressions of cell adhesion molecules (β‐catenin, N‐cadherin, and pan‐cadherin) as well as cell dynamics molecules (cofilin and p‐Ezrin) were increased in EGF‐MSC‐treated fibroblasts. These results suggest that EGF‐MSCs may enhance migration by increasing expression of adhesion molecules and cell dynamics molecules on fibroblasts. Phospho‐(Ser) kinase substrates are highly conserved proteins involved in regulation of cell survival, apoptosis, and proliferation. We investigated whether EGF‐MSC‐induced fibroblast proliferation was dependent on phospho‐(Ser) kinase substrates. As shown in Fig. 6, expressions of phospho‐(Ser) CDK substrate, phospho‐MAPK/CDK substrate, phospho‐(Ser)‐Arg‐X‐Tyr/Phe‐X‐pSer motif, and phospho‐(Ser) 14‐3‐3 binding motif were increased in EGF‐MSC‐treated fibroblasts. These results suggest that EGF‐MSCs enhanced fibroblast proliferation by increasing expression of phospho‐(Ser) kinase substrates.
Figure 5.
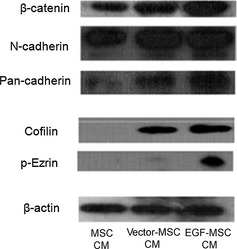
Effects of EGF ‐ MSC s on cell adhesion molecules and cell dynamics molecules in fibroblasts. Fibroblasts were treated with MSC CM, vector‐MSC CM or EGF‐MSC CM for 24 h. Expression of cell adhesion molecules (β‐catenin, N‐cadherin and pan‐cadherin) increased in EGF‐MSC CM‐treated fibroblasts. Expression of cell dynamics molecules (cofilin and p‐ezrin) increased in EGF‐MSC CM‐treated fibroblasts.
Figure 6.
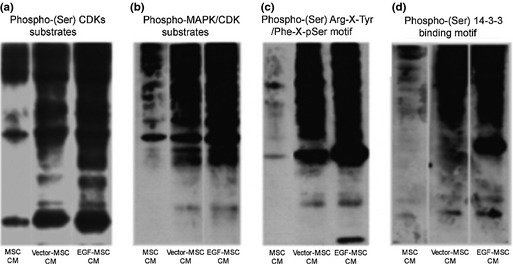
Effects of EGF ‐ MSC s on phospho‐(Ser) kinase substrates of fibroblasts. Fibroblasts were treated with MSC CM, vector‐MSC CM or EGF‐MSC CM for 24 h. Exposure to EGF‐MSC CM‐treated fibroblasts resulted in increased expression of phospho‐(Ser) kinase substrates. (a) Expression of phospho‐(Ser) CDKs substrates, cell cycle‐related proteins, increased in EGF‐MSC CM‐treated fibroblasts. (b) Expression of phospho‐MAPK/CDK substrates, cell cycle and cell signalling‐related proteins increased in EGF‐MSC CM‐treated fibroblasts. (c) Expression of phospho‐(Ser) Arg‐X‐Tyr/Phe‐X‐pSer motif, cell survival and proliferation regulation‐related proteins increased in EGF‐MSC CM‐treated fibroblasts. (d) Expression of phospho‐(Ser) 14‐3‐3 binding motif, cell survival and proliferation regulation‐related proteins increased in EGF‐MSC CM‐treated fibroblasts.
Discussion
Skin is as an essential barrier to protect organisms from their environment. It is composed of epidermis (forming the barrier) and dermis – which provides support and nutrition for the epidermis. During wound healing, fibroblast migration and proliferation help form a provisional matrix as the basis for granulation tissues 8.
Fibroblasts are essential for cutaneous wound repair 10, 11 and are responsible for scar formation of a wound. Further essential roles of fibroblasts during wound healing are extracellular matrix deposition and tissue remodelling. Fibroblast responses to injury are dysregulated during abnormal wound repair; for example, hypertrophic scars are characterized by excessive extracellular matrix deposition and wound contraction due to increased number of myofibroblasts. Because wound healing is associated with fibroblasts and the extracellular matrix in the dermis and epidermis, fibroblasts were used in this study.
Mesenchymal stem cells are often used in wound‐healing investigations. Recent studies have demonstrated that treating cutaneous wounds with MSCs accelerates wound healing kinetics, increases epithelialization and increases angiogenesis 7, 8, 12, 13, 14, 15, 16, 17. EGF is an essential growth factor needed for epithelial cell proliferation and fibroblast wound healing and the role of EGF has been extensively investigated during normal and pathological wound healing. EGF is implicated in keratinocyte migration, fibroblast function and formation of granulation tissue. More studies have focused on promoting wound healing by application of cytokines and some studies have found that external EGF accelerates wound healing 18, 19. Moreover, recent studies have demonstrated that soluble epidermal growth factor could be used to increase MSC numbers while not affecting differentiation of these cells in the absence or presence of external differentiation cues 20. Similarly, externally applied EGF promotes healing of skin incisions and prevents excessive scars. However, after huge losses of skin, such as by burns or ulcers, few living cells remain in local tissues. Considering this disadvantage, pEGFP‐N1‐EGF transfected MSCs were used in this study.
RT‐PCR showed that EGF mRNA was clearly expressed in EGF‐MSCs; however, we found low expression of EGF mRNA in controls and pEGFP‐N1 transfected MSCs, suggesting that higher expression contributed to extraneous EGF expression.
To determine fibroblast cell viability, colorimetric MTT metabolic activity assay was used; results indicate that viability of EGF‐MSC CM‐treated fibroblasts was increased. Results from scratch assays revealed that migrating cells increased in number in EGF‐MSC CM‐treated groups (Fig. 4a).
These experiments have demonstrated that EGF‐MSCs enhanced fibroblast proliferation and migration. EGF‐MSC‐induced fibroblast proliferation and migration are consistent with previously published reports demonstrating mitogenic and chemoattractive effects of CM from bone marrow‐derived MSCs on keratinocytes and endothelial cells 22. EGF‐MSCs affect cell adhesion molecules such as β‐catenin, N‐cadherin and pan‐cadherin as well as actin reorganization molecules such as cofilin and p‐ezrin. Recent studies have revealed that β‐catenin, N‐cadherin, pan‐cadherin, cofilin and p‐ezrin play essential roles in fibroblast migration. Poon et al. have shown that β‐catenin signalling is activated in fibroblasts during wound healing, and that it positively regulates fibroblast proliferation 21. Akitaya et al. have shown regulation of localization and function of N‐cadherin and pan‐cadherin during migration of neural crest cells 22 and Dawe et al. showed that actin filament disassembly is necessary for protrusion of lamellipodia during fibroblast migration, and that cofilin is essential for catalysis of filament disassembly in fibroblasts 23. Lamb et al. revealed that p‐ezrin plays an important part in pseudopodial extension in fibroblasts and maintenance of cell shape 24. In this study, increased expression of β‐catenin, N‐cadherin, pan‐cadherin, cofilin and p‐ezrin was detected in EGF‐MSC CM‐treated fibroblasts (Fig. 5), suggesting that increased expression of cell adhesion molecules and actin reorganization molecules may contribute to fibroblast migration.
Phospho‐(Ser) kinase substrates are highly conserved proteins involved in regulation of cell survival, apoptosis and proliferation. As shown in Fig. 6, elevated expression of phospho‐(Ser) CDK substrates and phospho‐MAPK/CDK substrates, cell cycle and cell signalling‐related substrates was observed in EGF‐MSC CM‐treated fibroblasts. Expression of phospho‐(Ser) Arg‐X‐Tyr/Phe‐X‐pSer motif and phospho‐(Ser) 14‐3‐3 binding motif, and cell survival and proliferation regulation‐related protein increased in EGF‐MSC CM‐treated fibroblasts. These results suggest that EGF‐MSC CM affected the cell cycle, cell signalling, cell survival and proliferation in fibroblasts by enhanced expression of phospho‐(Ser) kinase substrates.
In summary, we investigated interactions between EGF‐MSCs and fibroblasts as an application of EGF‐MSCs for wound healing. Increased expression of cell adhesion molecules, actin reorganization‐related proteins and phospho‐(Ser) kinase substrates suggested that EGF‐MSCs had positive influence on fibroblast migration and proliferation. Considering these effects, EGF‐MSCs can be considered to be effective for promoting wound healing. Further mechanistic studies may clarify the roles of more EGF‐MSCs factors.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010‐0007191) and partial funding from Han Cell Inc (2008‐1‐1). This work was also supported by the Gachon University research fund of 2012 (GCU‐2012‐M037).
References
- 1. Harding KG, Morris HL, Patel GK (2002) Science, medicine and the future: healing chronic wounds. BMJ 324, 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Metcalfe AD, Ferguson MW (2007) Tissue engineering of replacement skin: the crossroads of biomaterials, wound healing, embryonic development, stem cells and regeneration. J. R. Soc. Interface 4, 413–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Serini G, Bochaton‐Piallat ML, Ropraz P, Geinoz A, Borsi L, Zardi L et al (1998) The fibronectin domain ED‐A is crucial for myofibroblastic phenotype induction by transforming growth factor‐beta1. J. Cell Biol. 142, 873–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sorrell JM, Caplan AI (2004) Fibroblast heterogeneity: more than skin deep. J. Cell Sci. 117, 667–675. [DOI] [PubMed] [Google Scholar]
- 5. Martin P (1997) Wound healing–aiming for perfect skin regeneration. Science 276, 75–81. [DOI] [PubMed] [Google Scholar]
- 6. Friedenstein AJ, Gorskaja JF, Kulagina NN (1976) Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 4, 267–274. [PubMed] [Google Scholar]
- 7. Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H (2008) Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J. Immunol. 180, 2581–2587. [DOI] [PubMed] [Google Scholar]
- 8. Wu Y, Chen L, Scott PG, Tredget EE (2007) Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 25, 2648–2659. [DOI] [PubMed] [Google Scholar]
- 9. Liu TM, Martina M, Hutmacher DW, Hui JH, Lee EH, Lim B (2007) Identification of common pathways mediating differentiation of bone marrow‐ and adipose tissue‐derived human mesenchymal stem cells into three mesenchymal lineages. Stem Cells 25, 750–760. [DOI] [PubMed] [Google Scholar]
- 10. Chen L, Tredget EE, Wu PY, Wu Y (2008) Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 3, e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim WS, Park BS, Sung JH, Yang JM, Park SB, Kwak SJ et al (2007) Wound healing effect of adipose‐derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J. Dermatol. Sci. 48, 15–24. [DOI] [PubMed] [Google Scholar]
- 12. Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N et al (2007) Autologous bone marrow‐derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 13, 1299–1312. [DOI] [PubMed] [Google Scholar]
- 13. Javazon EH, Keswani SG, Badillo AT, Crombleholme TM, Zoltick PW, Radu AP et al (2007) Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen. 15, 350–359. [DOI] [PubMed] [Google Scholar]
- 14. Jin H, Xia B, Yu N, He B, Shen Y, Xiao L et al (2012) The effects of autologous bone marrow mesenchymal stem cell arterial perfusion on vascular repair and angiogenesis in osteonecrosis of the femoral head in dogs. Int. Orthop. 36, 2589–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin CD, Allori AC, Macklin JE, Sailon AM, Tanaka R, Levine JP et al (2008) Topical lineage‐negative progenitor‐cell therapy for diabetic wounds. Plast. Reconstr. Surg. 122, 1341–1351. [DOI] [PubMed] [Google Scholar]
- 16. McFarlin K, Gao X, Liu YB, Dulchavsky DS, Kwon D, Arbab AS et al (2006) Bone marrow‐derived mesenchymal stromal cells accelerate wound healing in the rat. Wound Repair Regen. 14, 471–478. [DOI] [PubMed] [Google Scholar]
- 17. Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS et al (2010) Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp. Cell Res. 316, 48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alemdaroglu C, Degim Z, Celebi N, Zor F, Ozturk S, Erdogan D (2006) An investigation on burn wound healing in rats with chitosan gel formulation containing epidermal growth factor. Burns 32, 319–327. [DOI] [PubMed] [Google Scholar]
- 19. Jahovic N, Guzel E, Arbak S, Yegen BC (2004) The healing‐promoting effect of saliva on skin burn is mediated by epidermal growth factor (EGF): role of the neutrophils. Burns 30, 531–538. [DOI] [PubMed] [Google Scholar]
- 20. Tamama K, Fan VH, Griffith LG, Blair HC, Wells A (2006) Epidermal growth factor as a candidate for ex vivo expansion of bone marrow‐derived mesenchymal stem cells. Stem Cells 24, 686–695. [DOI] [PubMed] [Google Scholar]
- 21. Poon R, Nik SA, Ahn J, Slade L, Alman BA (2009) Beta‐catenin and transforming growth factor beta have distinct roles regulating fibroblast cell motility and the induction of collagen lattice contraction. BMC Cell Biol. 10, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Akitaya T, Bronner‐Fraser M (1992) Expression of cell adhesion molecules during initiation and cessation of neural crest cell migration. Dev. Dyn. 194, 12–20. [DOI] [PubMed] [Google Scholar]
- 23. Dawe HR, Minamide LS, Bamburg JR, Cramer LP (2003) ADF/cofilin controls cell polarity during fibroblast migration. Curr. Biol. 13, 252–257. [DOI] [PubMed] [Google Scholar]
- 24. Lamb RF, Ozanne BW, Roy C, McGarry L, Stipp C, Mangeat P et al (1997) Essential functions of ezrin in maintenance of cell shape and lamellipodial extension in normal and transformed fibroblasts. Curr. Biol. 7, 682–688. [DOI] [PubMed] [Google Scholar]


