Abstract
Autophagy is an evolutionarily conserved lysosomal mechanism implicated in a wide variety of pathological processes, such as cancer. Autophagy can be regulated by a limited number of autophagy‐related genes (Atgs) such as oncogenic Bcl‐2/Bcl‐X L, mTORC1, Akt and PI3KCI, and tumour suppressive proteins PI3KCIII, Beclin‐1, Bif‐1, p53, DAPKs, PTEN and UVRAG, which play their crucial roles in regulating autophagy‐related cancer. As autophagy has a dual role in cancer cells, with tumour‐promoting and tumour‐suppressing properties, it has become an attractive target for a series of emerging small molecule drugs. In this review, we reveal new discoveries of related small molecules or chemical compounds that can regulate autophagic pathways and lead to pro‐death or pro‐survival autophagy, in different types of cancer. We discuss the knots between autophagic targets and candidate drugs, in the hope of shedding new light on exploiting new anti‐tumour small molecule drugs for future cancer therapy.
Introduction
Autophagy is a genetically programmed, multi‐step lysosomal process that enables degradation and recycling of long‐lived proteins or damaged organelles. It is activated during stress conditions such as amino acid starvation, unfolded protein response or viral infection 1, 2. There are three primary forms of autophagy: chaperone‐mediated autophagy, microautophagy and macroautophagy, which are different in physiological function and modes of cargo delivery to the lysosome 3. Macroautophagy (hereafter referred to as autophagy) is the most prevalent form, by which a cytosolic double‐membrane vesicle termed the autophagosome sequesters portions of cytoplasm and then fuses with a lysosome or vacuole for degradation and recycling of the contents 4.
Autophagy can be dissected into five different steps, induction, vesicle nucleation, vesicle elongation and completion, docking and fusion, and degradation & recycling 5. A limited number of autophagy‐related genes (Atg) have been reported to be crucial in autophagosome formation and regulation, and have been known to be associated with cancer initiation and progression 6, 7. A variety of oncogenic pathways involved in Bcl‐2/Bcl‐XL, mammalian target of rapamycin (mTOR), class I phosphatidylinositol 3‐kinase (PI3KCI), Akt and mitogen‐activated protein kinases (MAPKs), as well as tumour suppressive pathways of Beclin‐1, class III phosphatidylinositol 3‐kinase (PI3KCIII), Bif‐1, ultraviolet irradiation resistance‐associated gene (UVRAG), death‐associated protein kinases (DAPKs), PTEN and p53, all have distinct influences on different types of cancer 8, 9. Functional autophagy prevents necrosis and inflammation, leading to genetic instability, whereas it might also promote tumour progression by providing energy during unfavourable metabolic circumstances 10. Thus, autophagy may act as tumour promoter or tumour suppressor, under different circumstances.
The janus‐faced role of autophagy in determining destiny of cancer cells makes it an attractive target for a number of small molecular compounds. Some anti‐cancer drugs induce autophagy which acts as a survival mechanism and leads to drug resistance. In this case, application of combined treatment with inhibitors of autophagy may be a useful therapeutic strategy. However, there are other drugs that lead to autophagic cell death in cancer cells. Thus, induction of pro‐death autophagy has become a new strategy for cancer treatment. Both approaches have significant potential to be translated into ongoing clinical trials that could shed light on exploiting new anti‐tumour small molecule drugs for future cancer therapy. In this review, we provide an account of some related small molecule drugs or chemical compounds, which can regulate oncogenic/tumour suppressive autophagic pathways in different types of cancer. Also, we discuss the knot between autophagic targets and these candidate drugs, in the hope of shedding new light on exploiting new anti‐tumour small molecule drugs for future cancer therapy.
Stages of autophagy and the involved molecular regulators
Autophagy has previously been dissected into five different steps, induction, vesicle nucleation, vesicle elongation and completion, docking and fusion, and degradation and recycling 5 (Fig. 1).
Figure 1.
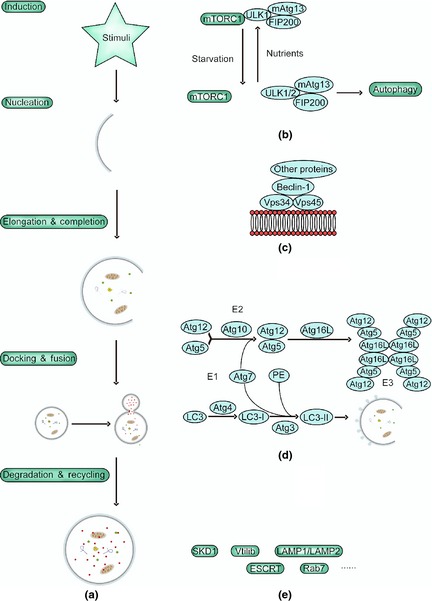
Stages of autophagy and the involved molecular regulators. The complete autophagic flow is a highly regulated process that can generally be divided into the following five stages: (a) induction, (b) vesicle nucleation, (c) vesicle elongation (d) completion, docking and fusion, and (e) degradation and recycling.
Induction is modulated by ULK‐Atg13‐FIP200 complexes comprised of Unc‐51‐like kinase 1/2(ULK1/2), Atg13 and focal adhesion kinase family interacting protein of 200 kDa (FIP200) 11. Under nutrient‐rich conditions, mTORC1 phosphorylates and subsequently inactivates ULK1/2 and Atg13, whereas under nutrient starvation, mTORC1 phosphorylation of ULK is suppressed, which enhances kinase activity of ULK1/2 and triggers phosphorylation of Atg13 and FIP200, and autophosphorylation of ULK. This results in induction of autophagy 12.
Vesicle nucleation is dependent on Beclin‐1‐Vps34/PI3KIII‐Vps15 core complexes and other Beclin‐1 binding proteins such as Atg14L, ultraviolet irradiation resistance‐associated gene (UVRAG), Bif‐1, Ambra1, high‐mobility group box 1 (HMGB1), Survivin, PTEN‐induced putative kinase 1 (PINK1), Bcl‐2, Bcl‐XL and Rubicon, which can mediate PI3KIII activity by interacting with Beclin‐1, thus regulating autophagy 13, 14.
Vesicle elongation and completion is mediated by two ubiquitin‐like systems that together promote assembly of the Atg12–Atg5–Atg16L complex and processing of LC3. The first is an Atg12–Atg5 covalently conjugating system, in which Atg12 is catalysed by E1 protein Atg7 and E2 protein Atg10 fused with Atg5; then the Atg12–Atg5 conjugate interacts with Atg16L to form an Atg12–Atg5–Atg16L complex. In conjunction with Atg12–Atg5, Atg16L directs the complex to elongating autophagic isolation membranes 15, 16. The second is an Atg8/LC3 system, in which Atg8(LC3) is converted to LC3‐I when carboxy‐terminal Arg residue of Atg8 is cleaved away by Atg4. LC3‐I is then covalently conjugated to phosphatidylethanolamine (PE). The PE‐conjugated form is called LC3‐II, an important marker of autophagy 16. In this way, Atg8 proteins recruit lipid molecules to expand autophagosome membranes 17. In addition, Atg12–Atg5 conjugate has E3‐like activity for Atg8 lipidation 18. Once autophagosome expansion is complete, Atg8 detaches from PE by Atg4 and then is released to the cytosol 19.
Docking and fusion refer to maturation of autolysosomes and are mediated by LC3, Belclin‐1, GTP‐binding protein Rab7, lysosomal‐associated proteins 1/2(LAMP1/LAMP2), ATPase SKD1, Vtil 1b and the ESCRT complex 20, 21. At the final stage, autophagosomal cargoes are digested, and nutrients and energy are recycled.
Autophagic signalling pathways in cancer treatment
mTORC1 serves as the main negative regulator of autophagy in cancer cells. Three major mTORC1‐inducing pathways have been identified (Fig. 2). The PI3K–Akt pathway and the extracellular signal‐regulated kinase (ERK)–90 kDa ribosomal S6 kinase (RSK)–death‐associated protein kinase (DAPK) pathway are the first two and activate mTORC1 and suppress autophagy. The third is the AMP‐activated protein kinase (AMPK) pathway, which inhibits mTORC1 and positively regulates autophagy 22. Most signalling pathways regulate TSC2/TSC1 complex, the key point upstream of mTORC1, and suppress mTORC1 by inactivating mTORC1‐interacting protein, Ras homologue enriched in brain (Rheb) 22, 23, 24. Under conditions of nutrient starvation, mTORC1 phosphorylation of ULK is suppressed, which enhances kinase activity of ULK1/2, triggers phosphorylations of Atg13 and FIP200, and autophosphorylation of ULK, and results in autophagic induction 12. Moreover, mTORC1 regulates autophagy by mediating protein translation by phosphorylating 4E‐binding protein 1 (4E‐BP1) and p70S6K. Phosphorylation of 4E‐BP1 leads to its detachment from eukaryotic translation initiation factor 4E (eIF4E), and up‐regulates cap‐dependent translation. Phosphorylation of p70S6K by mTORC1 allows it to phosphorylate downstream targets, including 40S ribosomal protein S6, a pro‐apoptotic BH3‐only protein Bad and eukaryotic elongation factor 2 kinase (eEF2K). Phosphorylation of eEF2K inhibits its activity, making it relieve eEF2 from inhibition by eEF2K and promoting autophagy 22, 25.
Figure 2.
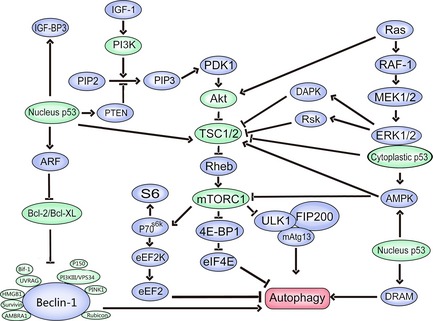
Autophagic signalling pathways in cancer treatment.
Beclin‐1, the mammalian homolog of Atg6, a Bcl‐2 interacting coiled‐coil protein, is mediated by positive regulators Atg14L, UVRAG, Bif‐1, Ambra1, HMGB1, Survivin, and PINK1, as well as negative regulators Bcl‐2, Bcl‐XL and Rubicon 13. Atg14L enhances Vps34 lipid kinase activity and up‐regulates autophagy 26. Furthermore, Atg14L co‐localizes with Atg5 and Atg16L1 on isolation membranes during autophagy 27. UVRAG promotes autophagosome formation by activation of the Beclin‐1 complex 28 and Bif‐1 interacts with Beclin‐1 through UVRAG, and functions as a positive mediator of autophagy 29. AMBRA1 promotes Beclin‐1 interaction with its target Vps34/PI3KIII, thus mediating autophagosome nucleation 30 and HMGB1 directly interacts with autophagy protein Beclin‐1 displacing Bcl‐2 31. A further positive regulator is Survivin, a member of the inhibitor of apoptosis protein family, which presents a possible mechanism underlying cross‐talk between autophagy and apoptosis, by Beclin‐1‐mediated degradation 32. PINK1, a serine/threonine protein kinase that localizes to mitochondria, interacts with Beclin‐1 and enhances autophagy 33. Bcl‐2 and Bcl‐XL, the well‐characterized guards of apoptosis, appear to be important factors in autophagy, binding to Beclin‐1 to inhibit it 34. Rubicon negatively regulates autophagy, possibly by inhibition of autophagosome maturation 26.
p53, the well‐known tumour suppressor protein, becomes activated in response to stresses such as DNA damage and oxidative stress 35. p53 plays a dual regulatory role in autophagy, depending on its subcellular localization, which dictates whether p53 willcontributs to cancer cell survival or to cell death 36. Nuclear p53 activates AMPK to inhibit mTOR and promote autophagy. Also, nuclear p53 promotes autophagy by transactivating autophagy‐promoting factors, including AMPK β1/β2 subunits damage‐regulated autophagy modulator, pro‐apoptotic Bcl‐2 proteins (Bad, Bax, BNIP3, PUMA and more), PTEN, Sestrin1/2 and TSC2 37. In contrast to nuclear p53, cytoplasmic p53 inhibits autophagy by reducing phosphorylation of AMPKα, ACCα and TSC2, as well as by increasing phosphorylation of p70S6K 38.
Autophagy‐related drugs/chemical compounds, in different types of cancer
The majority of anti‐cancer drugs, as well as ionizing radiation (IR), affect autophagy, mainly by increasing it, in tumour cells. Moreover, autophagy causes cell death after drug treatment, although it may promote or inhibit cell death under different circumstances. Here, we focus on new discoveries in drugs and chemical compounds related to autophagy in some representative types of cancer, in the hope of illuminating exploitation of new anti‐tumour small molecule drugs to improve cancer therapy in the future.
Leukaemia
The lymphomas comprise a group of blood cell tumours that develop from lymphocytes; they are the most common form of haematological malignancy. Given the potential function of autophagy in cell survival and death, its manipulation alone may provide a critical means to treat leukaemia or be used together with chemotherapeutic agents to tackle resistance to chemotherapy (Table 1).
Table 1.
Autophagy‐related drugs or chemical compounds in leukaemia
| Type of cancer | Drugs or chemical compounds | Autophagic pathways | Cell lines and primary cells | Autophagy role pro‐death | References |
|---|---|---|---|---|---|
| ALL CLL AML | APO866 | – | ALL, CLL, AML cell lines and primary cells | Pro‐death | 38 |
| ALL | Resveratrol | Inhibition of Akt/mTOR/p70S6K/4E‐BP1 and activation of p38‐MAPK signalling pathways | Glucocorticoid (GC)‐resistant Molt‐4/Jurkat/CEM‐C1‐15/CEM‐C7‐14 cells | Pro‐survival | 39 |
| CML | AMPK activation and JNK‐mediated p62/SQSTM1 expression | IM‐S/IM‐R K562 cell line, Ba/f3p210BCR‐ABL, p210BCR‐ABL T315I | Pro‐death | 40 | |
| ALL | I3M | – | JM1 cell line | – | 43 |
| CML | K562 cell line | ||||
| AML | As2O3 | Activation of the MEK/ERK pathway and down‐regulation of Bcl‐2 | U937 cell line | Pro‐death | 41, 42 |
| CML |
KT1 cell line K562 cell line |
||||
|
AML CML |
Platonin | BNIP3 overexpression and mTOR/p70S6K inhibition | HL‐60/U937 cell line | Pro‐death | 44 |
| K562 cell line | |||||
| ALL | RAD001 | mTOR inhibition and Beclin‐1 overexpression | Primary cells | Pro‐death | 45, 46 |
| ALL | MK‐2206 | Akt inhibition | Primary cells | – | 48 |
| ALL | NVP‐BEZ235 | PI3K/mTOR inhibition | Jurkat/MOLT‐4/CEM‐S/CEM‐R/RPMI‐8402/BE‐13 cell line | – | 47 |
| ALL | Obatoclax (GX15‐070) | Dissociation of beclin‐1 from Bcl‐2 and inhibition of mTOR | GC‐resistant CCM‐C7‐14/C1‐C15 cells | Pro‐death | 51 |
| ALL | Dexamethasone | Bcl‐2 down‐regulation and PML/AKT inhibition | RS4; 697, Reh and SUP‐B15 cell line | Pro‐death | 50 |
| ALL | Triciribine | Akt1/Akt2/mTORC1 inhibition | T‐ALL human cell lines Jurkat, MOLT‐4, CEM‐S and CEM‐R, drug‐resistant cells overexpressing P‐gp | Pro‐survival | 49 |
| CML | Rapamycin | mTOR/p70S6K/4E‐BP1 inhibition | K562 cell line | Pro‐death | 53 |
| CML | IM | ER stress and Ca2+ release | CML blast crisis cell lines, CML primary cells, p210BCR/ABL‐expressing myeloid precursor cells | Pro‐survival | 52 |
| AML | Vitamin D3 | Beclin‐1 up‐regulation | HL‐60 cell line | Pro‐death | 54 |
| AML | Eupalinin A | Inhibition of GSH synthesis | HL‐60 cell line | Pro‐death | 55 |
| AML | Vitamin K2 | – | HL‐60 cell line | Pro‐death | 56 |
A number of drugs has been reported to trigger autophagy in various types of human leukaemia. APO866, a nicotinamide adenine dinucleotide biosynthesis inhibitor, is reported to lead to autophagic cell death in acute myeloid leukaemia (AML), acute lymphoblastic leukaemia (ALL) and chronic lymphocytic leukaemia 39. Resveratrol inhibits proliferation, and induces apoptosis and protective autophagy in T‐cell ALL (T‐ALL) cells in a dose and time‐dependent manner by inhibiting Akt/mTOR/p70S6K/4E‐BP1 and activating p38‐MAPK signalling pathways 40. Resveratrol also triggers autophagic cell death in CML cells, via both AMPK activation and JNK‐mediated p62/SQSTM1 expression 41. Arsnic trioxide (As2O3) is a potent inducer of autophagy in AML and CML cells by activation of the MEK/ERK pathway and down‐regulation of Bcl‐2, but is unrelated to AKT/mTOR or JNK pathways 42, 43. Indirubin‐3′‐monoxime (I3M) is a derivative of indirubin, an active component of a Chinese traditional medicine with known anti‐cancer function. I3M can reduce cell viability significantly and dose‐dependently, and induce autophagy in ALL and also CML, but the role of autophagy has not been confirmed 44. Platonin is capable of inducing caspase‐independent autophagy‐associated cell death in AML and CML by BNIP3 over‐expression and mTOR/p70S6K inhibition 45.
Autophagy plays a critical role in ALL and Everolimus (RAD001), an mTORC1 inhibitor, reduces cell viability and induces cytotoxic autophagy in ALL and B‐precursor ALL (B‐pre ALL) 46, 47. The novel dual PI3K/mTOR inhibitor NVP‐BEZ235, an orally bioavailable imidazoquinoline derivative, induces autophagy and displays strong cytotoxic activity against T‐ALL cells. Autophagy plays an unknown role in this case as inhibition of autophagy does not significantly affect cell proliferation. Moreover, combinations of NVPBEZ235 with conventional anti‐T‐ALL chemotherapeutic agents (cyclophosphamide, cytarabine and dexamethasone) has shown strong synergistic activity, implying that NVP‐BEZ235‐based combinations could be feasible in the clinic 48. An autophagy and apoptosis inducer, MK‐2206, reduces T‐ALL cell line viability by Akt inhibition, but the role of autophagy has not been further investigated 49. Akt inhibitor, triciribine, induces autophagy in T‐ALL cell lines by Akt1/Akt2/mTORC1 inhibition, which could be a defensive mechanism as the autophagy inhibitor chloroquine (CQ) increases triciribine‐induced apoptosis 50. Dexamethasone, a glucocorticoid fundamental drug used in treatment of leukaemia, has fatal consequences for ALL cells by autophagy prior to apoptosis by Bcl‐2 down‐regulation and PML/AKT inhibition 51. Combination of obatoclax (GX15‐070) and dexamethasone triggers cell death by autophagy but not apoptosis in GC‐resistant ALL cells. This effect is associated with dissociation of Beclin‐1 from Bcl‐2 and inhibition of mTOR 52.
Autophagy‐related drugs in CML, for example Imatinib mesylate (IM), a potent inhibitor of BCR/ABL tyrosine kinase, has become standard first‐line therapy for CML patients and can induce apoptosis and autophagy in CML cells. IM‐induced autophagy is associated with endoplasmic reticulum (ER) stress and Ca2+ release. Based on suppression of autophagy enhanced cell death induced by IM, autophagy may play a pro‐survival role in this case 53. Treatment of CML cell line K56 with rapamycin caused significant reduction in cell viability in a dose‐dependent manner by mTOR/4E‐BP1/p70S6K inhibition 54.
Autophagic drugs related to AML, have also been demonstrated. In addition to triggering differentiation and inhibiting apoptosis, vitamin D3 up‐regulates Beclin‐1 to trigger autophagic cell death in HL‐60 AML cells 55. Eupalinin A (a sesquiterpene lactone found in Eupatorium chinense L), induces autophagy‐mediated cell death in AML cells by inhibition of GSH synthesis 56. Vitamin K2 has also been reported to trigger autophagic cell death in AML but its mechanism of action needs further investigation 57.
Glioblastoma
Glioblastoma (GBM), the most common primary malignant brain tumour in adults, is typically associated with dismal prognosis and poor quality of life. Apoptosis (type I cell death) is reported to be the main mechanism by which most chemotherapeutic drugs and radiation kill GBM cells. Poor prognosis for patients suffering from GBMs is strongly related to apoptosis resistance of GBM cells. Inhibition of protective autophagy in chemotherapy and induction of autophagic cell death (type II cell death) become new concepts to trigger glioma cell death and overcome resistance to apoptosis for development of novel glioma therapies (Table 2).
Table 2.
Autophagy‐related drugs or chemical compounds in CBM
| Drugs or chemical compounds | Autophagic pathways | Cell lines and primary cells | Autophagy role | References |
|---|---|---|---|---|
| Autophagy inducers | ||||
| Quercetin | – | U373MG cell line | Pro‐survival | 67 |
| Bortezomib | – | U251 and U87 cell lines | Pro‐survival | 68 |
| Perifosine | – | U87MG and U251MG cell lines | Pro‐survival | 65 |
| Vodiamine | Calcium‐mediated JNK activation | U87MG cell line | Pro‐survival | 62 |
| NVP‐BEZ235 | PI3K/mTOR inhibition | LN229, SF763, U373 and U87 cell lines and human primary glioma GS2 cells | Pro‐survival | 63 |
| Erlotinib | – | U87MG cell line | Pro‐survival | 69 |
| EMAP‐II | PI3K/Akt/FoxO1 axis Inhibition | U87‐MG cell line | Pro‐death | 70 |
| TMZ | – | U‐87 cell line | Pro‐death | 71 |
| Imipramine | PI3K/Akt/mTOR inhibition | U87‐MG cell line | Pro‐death | 73 |
| CM | – | U‐87MG and GBM8401 cell lines | – | 74 |
| Sulfinlsine | – | NCI‐H460 and U87 cell lines | – | 75 |
| Concanavalin‐A | Up‐regulation of BNIP3 and Atg3/Atg12/Atg16L | U‐87 cell line | – | 76 |
| EDL‐291 | – | C6, U87 and U251 cell lines | – | 77 |
| Bromopyruvate | – | GL‐15 cell line | – | 78 |
| Curcumin | – | Glioma‐initiating cells from human GBM samples | – | 79 |
| Autophagy inhibitors | ||||
| Ceramide (C6) | – | U87MG and U251MG cell lines | Pro‐survival | 65 |
| ZD6474 | PI3K/Akt/mTOR inhibition | U251 and U87MG cell lines | Pro‐survival | 66 |
| TQ | – | T98G and U87MG cell lines | Pro‐survival | 58 |
In some cases, autophagy promotes tumour cell survival and adaptation to anti‐cancer treatment in GBM. One randomized, double‐blind, placebo‐controlled trial has revealed that CQ may improve mid‐term survival when given in addition to conventional therapy for GBM 58; a further study has shown that GBM cells are sensitive to growth inhibition by CQ 59. These findings suggest that GBM cells may be dependent on the autophagic pathway for survival. Cucurbitacin I, a natural selective inhibitor of JAK2/STAT3, triggers protective autophagy through the AMPK/mTOR/p70S6K pathway and down‐regulates HIF‐1α 60. However, a further study has shown that quercetin has no effect on autophagic induction in human GBM multiforme T98G cells 61. Piperlongumine (PL), a natural alkaloid present in the fruit of the long pepper, stimulates autophagy by inhibition of Akt/mTORC1. Using CQ, level of PL‐induced cell death was significantly increased, indicating a pro‐survival role for autophagy 62 and vodiamine, a plant alkaloid, induces calcium/mitochondria‐mediated apoptosis and calcium/JNK‐mediated autophagy, which is pro‐survival, in human GBM cells 63. NVP‐BEZ235 promotes cell death and induces autophagy by inhibiting the PI3K/mTOR pathway and autophagy seems to be cytoprotective in this situation 64. Alkylphospholipids, including perifosine, edelfosine, erucylphosphocholine and hexadecylphosphocholine, have been found to induce autophagy in GBM cells 65. A further study has shown that perifosine induces significant protective autophagy, which inhibited cell apoptosis induction. It also showed that inhibition of autophagy by short‐chain ceramide (C6) restored perifosine‐induced apoptosis and cytotoxicity in GBM cells, without affecting Akt/mTOR signalling 66. In addition to traditional autophagy inhibitors including 3‐methyladenine, wortmanin and CQ, other drugs or chemical compounds have recently been reported to be able to inhibit autophagy in GBM. ZD6474 inhibits autophagy by PI3K/Akt/mTOR inhibition and enhances pro‐apoptotic effects of glioblastoma 67 and thymoquinone, the most abundant bioactive component of the Nigella sativa (black cumin) seed oil, inhibits autophagy and selectively induces cathepsin‐mediated, caspase‐independent cell death 59; also, quercetin, bortezomib and erlotinib are capable of inducing autophagy and cell death. Autophagy seems to be pro‐survival as inhibition of autophagy induces significant increase in erlotinib‐induced cell death 68, 69, 70.
Although autophagy mainly plays a pro‐survival role in GBM cells, there are still some drugs associated with autophagic cell death. Endothelial‐monocyte activating polypeptide‐II has been shown to be an effective anti‐cancer agent for GBM therapy; it can reduce cell viability in GBM stem cells (GSCs) through impaired autophagy mediated by the PI3K/Akt/FoxO1 axis 71. Temozolomide (TMZ) causes autophagy‐mediated cell death, markedly reinforced and prolonged by addition of RAD001 72; however, one further study has revealed that resveratrol potentiates the cytotoxic effect of TMZ on GBM cells, but while resveratrol increases autophagy induced by TMZ, autophagy does not affect acute cell death 73. Imipramine exerts anti‐tumour effects on glioma cells by inducing autophagic cell death through inhibiting PI3K/Akt/mTOR signalling 74. Other drugs and chemical compounds, including sulphinosine, Cordyceps militaris (CM), concanavalin‐A, EDL‐291, curcumin and bromopyruvate, have also been reported to be capable of inducing autophagy in GBM 75, 76, 77, 78, 79, 80. However, the role of autophagy in these cases requires further study.
Non‐small cell lung cancer
Non‐small cell lung cancer (NSCLC) represents in the order of 80–90% of lung cancers and is among the leading causes of cancer death. Here, we discuss pro‐survival or pro‐death role of autophagy of various drugs and chemical compounds in NSCLC, in the hope of illuminating exploration of effective therapeutic strategies for NSCLC treatment (Table 3).
Table 3.
Autophagy‐related drugs or chemical compounds in NSCLC
| Drugs or chemical compounds | Autophagic pathways | Cell lines and primary cells | Autophagy role | References |
|---|---|---|---|---|
| Ouabain | JNK‐dependent decrease of Bcl‐2 | A549 and H1975 cell lines | Pro‐death | 81 |
| Digoxin | Activation of AMPK and ERK1/2 | A549 and H460 cell lines | Pro‐death | 80 |
| Harmol | Activation of ERK1/2 | A549 cell line | Pro‐death | 82 |
| PM02734 | Inhibition of the Akt/mTOR pathway, and activation of DAPK | H322 and A549 cell lines | Pro‐death | 83 |
| OB | Down‐regulation of Nrf2 | A549 cell line | Pro‐death | 84 |
| SU11274 | p53 activation | A549 cell line | Pro‐death | 85 |
| TW01001 | – | A549 cell line | Pro‐death | 86 |
| Erlotinib | p53 nuclear translocation, AMPK activation and mTOR suppression | HCC827 and HCC4006 cell lines | Pro‐survival | 87 |
| β‐Elemene | PI3K/Akt/mTOR/p70S6K1 inhibition | A549 cell line | Pro‐survival | 90 |
| Salinomycin | Akt1‐mTOR inhibition | A549 cell line | Pro‐survival | 91 |
| Sodium selenite | ROS | A549 cell line | Pro‐survival | 92 |
| NVP‐BEZ235 | PI3K/mTOR inhibition | NSCLC cell lines A549, H522, H460 and H1792, etc. | Pro‐survival | 93 |
| AT101 | – | A549 cell line | Pro‐survival | 94 |
| 5‐FU | – | A549 cell line | Pro‐survival | 95 |
| MEMC | – | NCI‐H460 cell line | Pro‐survival | 96 |
| Plumbagin | Inhibition of PI3K/Akt/mTOR | A549 and H23 cells | – | 97 |
| Calyxin Y | JNK activation and overexpression of Atg proteins | NCI‐H460 cell line | – | 98 |
| OP‐B | Inhibition of PI3K/Akt | NSCLC cell lines H460, NCI‐H460 and NCI‐H157 etc. | – | 99 |
First, drugs which represent a pro‐death role of autophagy. Two representative cardiac glycosides, digoxin and ouabain, induce autophagy and autophagy‐mediated cell death in A549 and H460 cell lines by activation of AMPK and ERK1/2 81. Further research has also indicated a mechanism involving JNK‐dependent reduction of Bcl‐2 in ouabain 82. β‐carboline alkaloid harmol, induces cell death via autophagy without involving apoptosis in human non‐small cell lung cancer A549 cells, the mechanism possibly relating to activation of the ERK1/2 pathway 83. PM02734 (elisidepsin) has been reported to be capable of causing autophagic cell death by a complex mechanism involving inhibition of the Akt/mTOR pathway and activation of DAPK 84. Oleifolioside B (OB) induces apoptosis and triggers autophagy by down‐regulation of NF‐E2‐related factor 2 (Nrf2); OB‐induced autophagy functions as a death mechanism in this case 85; SU11274 leads to autophagic cell death in NSCLC A549 cells. It has been demonstrated that p53 can be activated after SU11274 treatment, while interruption of p53 activity reduces SU11274‐induced autophagy 86. Moreover, TW01001 has the ability to induce pro‐death autophagy, although at the moment its mechanism is uncertain 87.
Concerning cytoprotective mechanisms of autophagy of further drugs, erlotinib induces both apoptosis and autophagy in NSCLC cells via p53 nuclear translocation, AMPK activation and mTOR suppression. Autophagy seems to be pro‐survival in this case as CQ increases cytotoxicity of erlotinib 88, 89. In addition, further research has shown that combination of erlotinib–cisplatin induces synergistic cell death, and a significant reduction in autophagy in erlotinib‐resistant lung cancer. However, the pro‐survival role of autophagy was not confirmed as being by inhibition of autophagy 90. β‐elemene inhibits activity of the PI3K/Akt/mTOR/p70S6K1 signalling pathway in human NSCLC A549 cells, resulting in apoptosis as well as protective autophagy 91 while salinomycin induces pro‐survival autophagy in NSCLC cells via Akt1‐mTOR inhibition 92. Sodium selenite treatment of A549 cells appears to trigger both apoptosis and cytoprotective autophagy, both mediated by reactive oxygen species (ROS) 93 and the novel dual PI3K/mTOR inhibitor NVP‐BEZ235 also induces apoptosis and protective autophagy 94. Also, methylene chloride extracts of the Mori cortex root, AT‐101 and 5‐fluorouracil (5‐FU), are reported to induce protective autophagy in NSCLC cells, but with unknown mechanisms 95, 96, 97.
A variety of drugs and chemical compounds have recently been shown to induce autophagy, but where its role remains unknown. Plumbagin is cytotoxic to NSCLC cells and able to induce apoptosis and autophagy by inhibition of the PI3K/Akt/mTOR pathway, but the role of autophagy in this case is unclear 98. Calyxin Y (isolated from a folk anti‐tumour medicine Alpinia katsumadai), induces autophagy and apoptosis via JNK activation and over‐expression of Atg proteins, but whether calyxin Y‐induced autophagy is protective or pro‐death remains controversial 99. Ophiopogonin B (OP‐B), a bioactive component of Chinese traditional medicine Radix Ophiopogon japonicus, also has the ability to induce autophagy by inhibition of PI3K/Akt 100.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC), the most common liver cancer and major cause of cancer death worldwide, is significantly relevant to autophagy. Autophagy‐related markers such as Belclin‐1 and LC3 have been reported to have prognostic significance in HCC. Regulation of autophagy may be a useful therapeutic strategy for HCC (Table 4).
Table 4.
Autophagy‐related drugs or chemical compounds in HCC
| Drugs or chemical compounds | Autophagic pathways | Cell lines and primary cells | Autophagy role | References |
|---|---|---|---|---|
| Autophagy inducers | ||||
| Nilotinib | AMPK activation | PLC5, Huh‐7 and Hep3B cell lines | Pro‐death | 100 |
| RAD001 | mTORC1 inhibition | SK‐Hep1 cells | Pro‐death | 101 |
| Andrographolide | Disruption of MTP and elevation of ROS | Huh‐7, QGY‐7703 and Bel‐7402 cell lines | Pro‐death | 102 |
| Bufalin | Inhibition of Akt/mTOR | SK‐HEP‐1 cell line | Pro‐death | 103 |
| Oroxylin A | Inhibition of PI3K‐PTEN‐Akt‐mTOR | HepG2 and SMMC‐7721 cell lines | Pro‐death | 104 |
| SB203580 | Activation of AMPK and DAPK | HepG2, Hep3B, Huh‐7, PLC/PRF/5 and SK‐Hep‐1 cell lines | Pro‐death | 105 |
| Galangin | Increase of p53 expression | HepG2 cell line | Pro‐death | 106 |
| Fangchinoline | Activation of p53/Sestrin2/AMPK signalling | HepG2 and PLC/PRF/5 cell lines | Pro‐death | 107 |
| Baicalin | – | SMMC‐7721 cell line | Pro‐death | 108 |
| Cisplatin | Down‐regulation of miR‐199a‐5p | Huh‐7 cells, HepG2 cell lines | Pro‐survival | 109 |
| NVP‐BEZ235 | – | Hep3B and PLC/PRF/5 cell lines | Pro‐survival | 110 |
| C6‐ceramide | – | HepG2 cell line | Pro‐survival | 111 |
| Bevacizumab | – | SMMC‐7721 and Hep3B cell lines | Pro‐survival | 112 |
| Bortezomib | – | PLC/PRF/5, Huh‐7, MHCC‐97H cell lines | Pro‐survival | 113 |
| MG‐132 | ||||
| AS‐6 | – | HepG2 cell line | – | 117 |
| Panobinostat | – | Huh7, Hep3B and HepG2 cell lines | – | 118 |
| Neferin | – | Hep3B cell line | – | 114 |
| Sorafenib | – | HepG2 cell line | – | 115 |
| Regorafenib | – | Hep3B, HepG2 and PLC/PRF/5 cell line | – | 116 |
| Autophagy inhibitors | ||||
| 5‐FU | ER stress | Sk‐Hep1 cell line | Pro‐survival | 119 |
Considering drugs which have autophagy‐mediated anti‐tumour activity, Nilotinib has been reported to induce pro‐death autophagy, but not apoptosis in HCC by AMPK activation 101. mTOR inhibitor RAD001 augments radiation‐induced growth inhibition of HCC cells, and induction of autophagy may account for this effect 102. Andrographolide (a diterpenoid lactone isolated from Andrographis paniculata), induces autophagic cell death distinct from apoptosis, by disruption of mitochondrial transmembrane potential (MTP) and elevation of ROS in HCC cells 103. Bufalin, a major component of Chan‐Su (a traditional Chinese medicine), is able to trigger autophagic cell death by the AKT/mTOR signalling pathway in SK‐HEP‐1 HCC cells 104 and oroxylin A exhibits autophagy‐mediated anti‐tumour activity in a dose‐ and time‐dependent manner in vivo and in vitro, induction of autophagy being associated with suppression of the PI3K‐PTEN‐Akt‐mTOR signalling pathway 105. SB203580 induces autophagy via activating AMPK and DAPK, but is independent of p38‐MAPK; induction of autophagy can thus account for the anti‐proliferative effect of SB203580 in HCC cells 106. Treatment with polyphenolic compound, galangin, may inhibit cell proliferation and induce autophagy by increasing p53 expression in HepG2 cells 107 and fangchinoline (a highly specific anti‐tumour agent) induces autophagic cell death via p53/Sestrin2/AMPK signalling in HepG2 and PLC/PRF/5 cells 108. Baicalin, which can significantly inhibit viability of SMMC‐7721 cells in a dose‐ and time‐dependent manner, induces apoptosis and pro‐death autophagy, however its mechanism of autophagy remains to be revealed 109.
In the case of pro‐survival autophagic drugs, cisplatin increases drug resistance by activating autophagy due to down‐regulation of miR‐199a‐5p in HCC cells 110. NVP‐BEZ235, nanoliposomal C6‐ceramide, bevacizumab, bortezomib and MG‐132 act as autophagy inducers and reduce cell viability of this cell type. This autophagy may be a survival mechanism that counteracts their anti‐cancer effects as combination with autophagy inhibitors synergistically reduces cell viability 111, 112, 113, 114.
4‐O‐carboxymethyl ascochlorin (AS‐6), panobinostat, sorafenib, fluoro‐sorafenib (Regorafenib) and neferine (a major bisbenzylisoquinoline alkaloid derived from embryos of Nelumbo nucifera), exhibit cytotoxicity against HCC cells and are able to induce autophagy. However, neither the role nor mechanisms of autophagy in these examples has been described 115, 116, 117, 118, 119.
Except for traditional autophagy inhibitors, 5‐FU has been reported to activate apoptotic cell death and induce ER stress to suppress protective autophagy 120.
Carcinoma of the pancreas
Pancreatic cancer is one of the most aggressive human malignancies with very low 5‐year survival level. Experimental evidence has pointed out that autophagy acts as a mechanism that pancreatic cancer cells survive under adverse conditions, or as a cytoprotective mechanism, that favours pancreatic cancer cell resistance to treatment. It has been revealed that Beclin‐1 over‐expression and increase in alteration of autophagy‐related proteins, are independently associated with poor prognosis 121. Thus, detection and inhibition of protective autophagy becomes a crucial strategy to fight pancreatic cancer (Table 5).
Table 5.
Autophagy‐related drugs or chemical compounds in pancreatic cancer
| Drugs or chemical compounds | Autophagic pathways | Cell lines and primary cells | Autophagy role | References |
|---|---|---|---|---|
| Autophagy inducers | ||||
| CP‐31398 | p53 activation | PaCa3 and PANC‐1 cell lines | Pro‐survival | 121 |
| RITA | ||||
| ROT | Inhibition of PI3K/Akt/mTOR | Human pancreatic cancer stem cells | Pro‐survival | 122 |
| 5‐FU | – | PANC‐1 and BxPC‐3 cell lines | Pro‐survival | 123 |
| Gemcitabine | ||||
| EPA | – | MIA‐PaCa‐2 and Capan‐2 cell lines | Pro‐survival | 124 |
| Calix[6]arene | PI3K/mTOR inhibition | PANC‐1 cells | Pro‐death | 128 |
| DRE | – | BxPC‐3 and PANC‐1 cell lines | Pro‐death | 127 |
| Cannabinoids | AMPK activation | PANC‐1 cell line | – | 129 |
| Autophagy inhibitors | ||||
| Manzamine A | The accumulation of p62 | PANC‐1, AsPC‐1, MIA PaCa‐2 and BxPC3 cell lines | Pro‐survival | 125 |
| TP421 | The accumulation of p62 | MIA PaCa‐2, PANC‐1, BxPC‐3 and HPAC pancreatic cancer cell lines | – | 126 |
In most cases, autophagy acts as a survival mechanism. Two p53‐reactivating molecules, CP‐31398 and RITA, have been reported to reduce cell viability and induce pro‐survival autophagy in human pancreatic cancer stem cells 122. Rottlerin (ROT) induces protective autophagy followed by induction of apoptosis via inhibition of the PI3K/Akt/mTOR pathway 123. 5‐fluorouracil, gemcitabine and eicosapentaenoic acid induce autophagy in pancreatic cancer cells, and induction of autophagy diminishes its ability to induce cell death, but its mechanism here remains unclear 124, 125.
Autophagy inhibitors have been discovered to inhibit protective autophagy in pancreatic cells. Treatment with manzamine A (a member of the manzamine alkaloids), results in inhibition of autophagy and cell viability increase 126. TP421 has been shown to have the ability to inhibit autophagy and inhibit cell proliferation in a panel of pancreatic cancer cell lines where its mechanisms seem to depend on accumulation of p62 127. Dandelion root extract has also been reported to trigger pro‐death autophagy in pancreatic cancer cells with the mechanism requiring further investigation 128.
Although autophagy mainly plays a pro‐survival role, some drugs have been reported to possibly induce autophagic cell death in pancreatic cancer. Calix[6]arene has been shown to be more potent in reducing Panc‐1 cell viability than gemcitabine and 5‐fluorouracil, with autophagy being the mode of cell death not apoptosis, by inhibiting the PI3K/mTOR pathway 129. Treated by cannabinoids, pancreatic cancer cells have exhibited strong induction of autophagy and reduction in viability. The mechanism of autophagy lies in AMPK activation, but clear relevance between autophagy and other mechanisms of cell death has not been clarified 130. Moreover, antroquinonol has been reported to be capable of inducing autophagy in pancreatic cancer cells, but with the role of autophagy being unclear 131.
Carcinoma of the breast
Breast cancer is an ancient disease and a leading malignancy for women. Its incidence is high in developed countries, while being low but increasing in developing countries. Research has demonstrated the close relationship between autophagy and breast cancer. Inhibition of protective autophagy and induction of pro‐death autophagy has become a new strategy for breast cancer treatment (Table 6).
Table 6.
Autophagy‐related drugs or chemical compounds in breast cancer
| Drugs or chemical compounds | Autophagic pathways | Cell lines and primary cells | Autophagy role | References |
|---|---|---|---|---|
| Rottlerrin | Activation of AMPK | Primary cells | Pro‐death | 131 |
| IR/SAHA combination | – | 4T1, MCF‐7 and MDA‐MB‐231 cell lines | Pro‐death | 132 |
| RY10‐4 | Inhibition of Akt/mTOR/p70S6K | MCF‐7 cell line | Pro‐death | 133 |
| Lapatinib | Akt/mTOR/p70S6K inhibition and AMPK activation | BT474 and AU565 cell lines | Pro‐death | 134 |
| SD | Endoplasmic reticulum stress | MCF‐7 and MDA‐MB‐231 cell lines | Pro‐death | 135 |
| DHEA/EPEA | PPARg activation | MCF‐7 cell line | Pro‐death | 136 |
| Gefitinib | Down‐regulation of AKT and ERK1/2 | Cell lines SKBR3, BT474 and JIMT‐1 etc. | Pro‐survival | 137 |
| CuO NPs | – | MCF‐7 cell line | Pro‐survival | 138 |
| Salinomycin | – | MDA‐MB‐468 cell lines | Pro‐survival | 139 |
| Rapamycin | PI3K/mTOR/Akt inhibition | T‐47D cell line | Pro‐survival | 141 |
| HIMOXOL | – | MDA‐MB‐231 (ER‐) cell lines | – | 142 |
| γ‐Tocotrienol | – | MCF‐7 and MDA‐MD‐231 cell lines | – | 143 |
| Parthenolide | Activation of JNK and PI3K inhibition | MDA‐MB231 cell line | – | 144 |
| Papuamine | Mitochondria damage and JNK activation | MCF‐7 cell line | – | 145 |
| Sulforaphane | Inhibition of Akt and S6K1 kinases | MDA MB 231, MCF‐7, SKBR‐3 and MDA MB 468 cell lines | – | 146 |
| Obatoclax | – | MCF‐7 cell line | – | 147 |
| Celastrus paniculatus | – | MCF‐7 cell line | – | 148 |
| Clionamine B | – | MCF‐7 cell line | – | 149 |
| C19 | – | MCF‐7 cell line | – | 150 |
Many drugs and chemical compounds have recently revealed the ability of inducing autophagic cell death. Treatment with ROT induces dose‐ and time‐dependent cell population growth inhibition, and triggers cell death by apoptosis and pro‐death autophagy by activation of the AMPK pathway 132. Breast cancer cells treated with IR and suberoylanilide hydroxamic acid (SAHA, an inhibitor of histone deacetylase) combination result in significantly enhanced toxicity compared to SAHA or IR treatment; autophagy plays a pro‐death role in this case 133. A novel protoapigenone analogue RY10‐4, is able to induce breast cancer MCF‐7 cell death by autophagy via inhibition of the Akt/mTOR/p70S6K pathway 134. Lapatinib, an oral dual tyrosine kinase inhibitor, is a potent inducer of autophagy, thus may be pro‐death mechanism, and stimulates apoptosis, with the mechanism of autophagy being Akt/mTOR/p70S6K inhibition and AMPK activation 135. Saxifragifolin D inhibits proliferation of breast cancer cells significantly by interplay between apoptosis and pro‐death autophagy, through ROS‐mediated ER stress 136. The two ethanolamide derivatives of DHA and eicosapentaenoic acid, docosahexaenoylethanolamine and eicosapentaenoylethanolamine, induce inhibition of cell proliferation triggering autophagy by PPARg activation 137.
Some research has put an emphasis on pro‐survival autophagy in breast cancer. Gefitinibautophagy, a small molecule inhibitor of the epidermal growth factor receptor EGFR tyrosine kinase, has been reported to induce autophagy in breast cancer cells by down‐regulation of AKT and ERK1/2 signalling, the role of autophagy being pro‐survival 138. Copper oxide nanoparticles (CuO NPs) and salinomycin have been reported to trigger autophagy counteracting cell death, however with unclear mechanisms 139, 140. However, a further study indicates that salinomycin may act as an autophagy inhibitor in breast cancer cells. Interplay between apoptosis and autophagy seems to be based on ROS‐mediated ER stress 141; traditional autophagy inducer rapamycin is capable of inducing autophagy in T‐47D breast carcinoma cells, and its effect seems to be pro‐survival 142.
In addition, other drugs and chemical compounds including HIMOXOL (methyl 3‐hydroxyimino‐11‐oxoolean‐12‐en‐28‐oate), γ‐tocotrienol, parthenolide, papuamine, sulforaphane, obatoclax,celastrus paniculatus, clionamine B and 2‐ethyl‐3‐O‐sulphamoyl‐estra‐1,3,5(10)16‐tetraene(C19), have been reported to be associated with induction of autophagy, but the relationship between autophagy and other types of cell death in breast cancer cells has not been revealed 143, 144, 145, 146, 147, 148, 149, 150, 151.
The knot between autophagic targets and candidate drugs in cancer therapy
Autophagy, as we know, is closely associated with both specific autophagic pathways and non‐specific ones. Small molecule drugs and chemical compounds can affect autophagy, and induce autophagic cell death or cell survival, through both specific autophagic pathways and non‐specific autophagic pathways (Table 7).
Table 7.
The knot between autophagic targets and candidate drugs in cancer therapy
| Autophagic pathways | Autophagy role | Drugs or chemical compounds | Chemical structure |
|---|---|---|---|
|
PI3KCI/Akt/mTORC1 Inhibition |
Dual | Rapamycin |
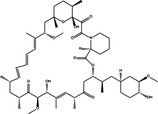
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Dual | Resveratrol |
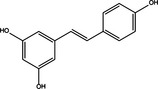
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐survival | NVP‐BEZ235 |
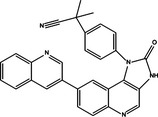
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐survival | Triciribine |
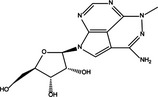
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐survival | ZD6474 |
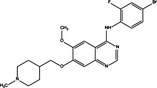
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐survival | β‐Elemene |
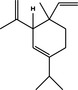
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐survival | Salinomycin |
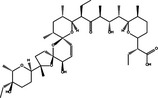
|
|
PI3KCI/Akt/mTORC1 Inhibition |
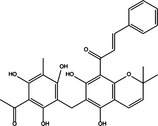
Pro‐survival | ROT |
|
|
PI3KCI/Akt/mTORC1 Inhibition |
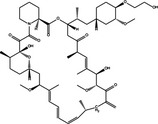
Pro‐death | RAD001 |
|
|
PI3KCI/Akt/mTORC1 Inhibition |
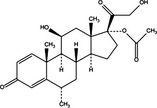
Pro‐death | Dexamethasone |
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐death | Imipramine |
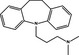
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐death | PM02734 |
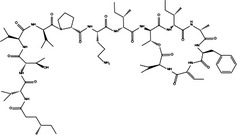
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐death | Bufalin |
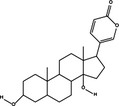
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐death | Oroxylin A |
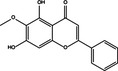
|
|
PI3KCI/Akt/mTORC1 Inhibition |
Pro‐death | Calix[6]arene |
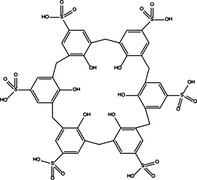
|
| Beclin‐1 up‐regulation | Pro‐death | Vitamin D3 |
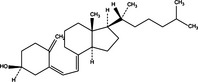
|
| Beclin‐1 up‐regulation | Pro‐death | RAD001 |
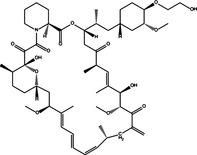
|
| Bcl‐2/Bcl‐XL down‐regulation | Pro‐death | Dexamethasone |
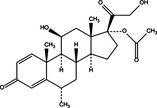
|
| Bcl‐2/Bcl‐XL down‐regulation | Pro‐death | Ouabain |
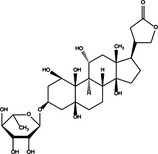
|
| Beclin‐1/Bcl‐2 detachment | Pro‐death | Obatoclax |
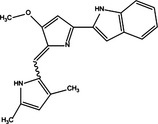
|
| p53 up‐regulation | Pro‐survival | Erlotinib |
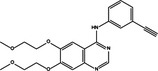
|
| p53 up‐regulation | Pro‐survival | CP‐31398 |
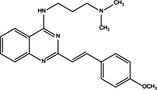
|
| p53 up‐regulation | Pro‐death | SU11274 |
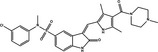
|
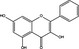
| p53 up‐regulation | Pro‐death | Galangin |
|
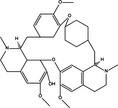
| p53 up‐regulation | Pro‐death | Fangchinoline |
|
The PI3KCI/Akt/mTORC1 pathway, main negative regulator of autophagy in cancer cells, is the major target for candidate drugs and plays a dual role in cell proliferation, by inducing cell survival or cell death, under different circumstances. Small molecule drugs and chemical compounds such as NVP‐BEZ235, triciribine, ZD6474, β‐elemene, salinomycin and ROT have been reported to induce autophagic cell survival by using the PI3KCI/Akt/mTORC1 pathway 50, 64, 67, 91, 92, 94, 123, 140. Other candidates such as RAD001, dexamethasone, imipramine, PM02734, bufalin, oroxylin A and calix[6]arene have been shown to have the ability to induce pro‐death autophagy through the PI3KCI/Akt/mTORC1 pathway 46, 47, 51, 74, 84, 102, 104, 105, 129. In addition, some candidate drugs such as rapamycin and resveratrol also have a double‐faced role, by inducing pro‐death or pro‐survival autophagy in different cancer cells 40, 41, 54, 142. Exact mechanisms of the different effects that different candidate drugs cause by regulating the PI3KCI/Akt/mTORC1 pathway remains unclear and calls for considerable further investigation.
Beclin‐1 can positively regulate pro‐death autophagy and is mediated by positive regulators such as Atg14L, UVRAG, Bif‐1, Ambra1, HMGB1, Survivin, and PINK1, as well as negative regulators Bcl‐2, Bcl‐XL and Rubicon. Up‐regulating Beclin‐1 becomes an attractive target to induce pro‐death autophagy. Candidate drugs obatoclax, RAD001 and vitamin D3 have been reported to induce autophagic cell death by up‐regulating Beclin‐1 in leukaemia cells 46, 47, 52, 55. Bcl‐2 and Bcl‐XL binds to Beclin‐1 to inhibit autophagy and they have their key roles in cancer cell survival. Down‐regulating Bcl‐2/Bcl‐XL is also an attractive target for candidate drugs to induce autophagic cell death. Dexamethasone and quabain have been reported to induce autophagic cell death by Bcl‐2/Bcl‐XL down‐regulation 51, 82. Also, some candidate drugs such as obatoclax may also induce pro‐death autophagy by inducing dissociation of Beclin‐1 from Bcl‐2 52. Although other Beclin‐1 cofactors such as Atg14L, UVRAG, Bif‐1, Ambra1, HMGB1, Survivin, PINK1 and Rubicon are capable of mediating Beclin‐1 activity and have the potential to regulate autophagy, candidate drugs targeting other Beclin‐1 cofactors have drawn less attention in recent areas of research.
p53 plays a dual regulatory role in autophagy depending on its subcellular localization, as nuclear p53 boosts autophagy and cytoplasmic p53 reduces it, independent of its transcriptional activity. This may dictate whether p53 contributes to cancer cell survival or death. Recent research has shown that candidate drugs such as erlotinib, CP‐31398, SU11274, galangin and fangchinoline induce pro‐death or pro‐survival autophagy by regulating p53, but the key point to determine cell fate remains unclear 86, 107, 108, 122.
Moreover, autophagy is closely associated with other pathological processes. Some candidate drugs such as IM, eupalinin A, sodium selenite, andrographolide and 5‐FU are reported to affect autophagy by regulating pathological processes such as apoptosis, ER stress, oxidative stress and inflammatory responses 53, 56, 92, 103, 120. Non‐specific autophagic pathways may provide potential targets to regulate autophagy in cancer cells.
Conclusions
Autophagy, an evolutionarily conserved lysosomal degradation process, has a Janus face in cancer: it acts as a survival mechanism or leads to autophagic cell death under different conditions. Autophagy can be modulated by some key oncogenes (Bcl‐2, PI3KCI, Akt and mTORC1) and tumour suppressors (Beclin‐1,PI3KCIII, Bif‐1, UVRAG, DAPKs, PTEN and p53), which determine the fate of the cancer cells.
Many anti‐cancer drugs affect autophagy. In recent years, many new discoveries of drugs and chemical compounds related to autophagy in different types of cancer have been made. However, for some newly discovered autophagy inducers, neither the role nor the mechanism of autophagy is clear, thus calling for further studies in the future. Moreover, due to the complicated Janus nature of autophagy, establishing the dual role of autophagy in tumour survival versus death may help to improve cancer therapeutic potential. Some anti‐cancer drugs have the ability to induce pro‐survival autophagy, which diminishes its ability to induce death of tumour cells. Thus, application of combined treatment with autophagy inhibitors may overcome drug resistance and become a new strategy to increase therapeutic effect. However, some drugs are reported to be associated to autophagic cell death, and its induction has become a new concept to trigger tumour cell death. Both strategies have significant potential to be translated into ongoing clinical trials that may shed light on exploiting new anti‐tumour small molecule drugs for future cancer therapy.
Small molecule drugs and chemical compounds can affect autophagy, and induce autophagic cell death or cell survival through both specific autophagic pathways and non‐specific ones. The PI3KCI/Akt/mTORC1 pathway is a major target for candidate drugs and plays a dual role in cell proliferation, by inducing cell survival or cell death under different circumstances. Exact mechanisms to determine cell fate remain unclear and require further investigation. Up‐regulating Beclin‐1, down‐regulating Bcl‐2/Bcl‐XL and dissociating Beclin‐1 from Bcl‐2 are three major mechanisms for candidate drugs which target the Beclin‐1 pathway to induce autophagic cell death in recent research, although, candidate drugs regulating other Beclin‐1 cofactors may be of potential research value. Candidate drugs targeting p53 have been reported to cause autophagic cell death and survival under different circumstances, but mechanisms of the different effects remain unclear. In addition, non‐specific autophagic pathways may provide potential targets to regulate autophagy in cancer cells.
Conflict of interest
We declare that none of the authors have a financial interest related to this work.
Acknowledgements
This work was supported by grants from the Key Projects of the National Science and Technology Pillar Program (no. 2012BAI30B02), National Natural Science Foundation of China (nos U1170302, 81202403, 81160543, 81260628, 81303270, 81172374 and 81473091).
References
- 1. Chen Y, Fu LL, Wen X, Liu B, Huang J, Wang JH et al (2014) Oncogenic and tumor suppressive roles of microRNAs in apoptosis and autophagy. Apoptosis 19, 1177–1189. [DOI] [PubMed] [Google Scholar]
- 2. Fu LL, Xie T, Zhang SY, Liu B (2014) Eukaryotic elongation factor‐2 kinase (eEF2K): a potential therapeutic target in cancer. Apoptosis 19, 1527–1531. [DOI] [PubMed] [Google Scholar]
- 3. Liu B, Wen X, Cheng Y (2013) Survival or death: disequilibrating the oncogenic and tumor suppressive autophagy in cancer. Cell Death Dis. 4, e892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wen X, Wu J, Wang F, Liu B, Huang C, Wei Y (2013) Deconvoluting the role of reactive oxygen species and autophagy in human diseases. Free Radic. Biol. Med. 65, 402–410. [DOI] [PubMed] [Google Scholar]
- 5. Feng Y, He D, Yao Z, Klionsky DJ (2014) The machinery of macroautophagy. Cell Res. 24, 24–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yang Z, Klionsky DJ (2010) Eaten alive: a history of macroautophagy. Nat. Cell Biol. 12, 814–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132, 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu JJ, Lin M, Yu JY, Liu B, Bao JK (2011) Targeting apoptotic and autophagic pathways for cancer therapeutics. Cancer Lett. 300, 105–114. [DOI] [PubMed] [Google Scholar]
- 9. Fu LL, Cheng Y, Liu B (2013) Beclin‐1: autophagic regulator and therapeutic target in cancer. Int. J. Biochem. Cell Biol. 45, 921–924. [DOI] [PubMed] [Google Scholar]
- 10. Wang SY, Yu QJ, Zhang RD, Liu B (2011) Core signaling pathways of survival/death in autophagy‐related cancer networks. Int. J. Biochem. Cell Biol. 43, 1263–1266. [DOI] [PubMed] [Google Scholar]
- 11. Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J et al (2009) ULK‐Atg13‐FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol. Biol. Cell 20, 1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jung CH, Ro SH, Cao J, Otto NM, Kim DH (2010) mTOR regulation of autophagy. FEBS Lett. 584, 1287–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kang R, Zeh HJ, Lotze MT, Tang D (2011) The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 18, 571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Funderburk SF, Wang QJ, Yue Z (2010) The Beclin 1‐VPS34 complex–at the crossroads of autophagy and beyond. Trends Cell Biol. 20, 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mizushima N, Kuma A, Kobayashi Y, Yamamoto A, Matsubae M, Takao T et al (2003) Mouse Apg16L, a novel WD‐repeat protein, targets to the autophagic isolation membrane with the Apg12‐Apg5 conjugate. J. Cell Sci. 116, 1679–1688. [DOI] [PubMed] [Google Scholar]
- 16. Mizushima N, Yoshimori T, Ohsumi Y (2003) Role of the Apg12 conjugation system in mammalian autophagy. Int. J. Biochem. Cell Biol. 35, 553–561. [DOI] [PubMed] [Google Scholar]
- 17. Ichimura Y, Kirisako T, Takao T, Satomi Y, Shimonishi Y, Ishihara N et al (2000) A ubiquitin‐like system mediates protein lipidation. Nature 408, 488–492. [DOI] [PubMed] [Google Scholar]
- 18. Geng J, Klionsky DJ (2008) The Atg8 and Atg12 ubiquitin‐like conjugation systems in macroautophagy. ‘Protein modifications: beyond the usual suspects’ review series. EMBO Rep. 9, 859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kirisako T, Ichimura Y, Okada H, Kabeya Y, Mizushima N, Yoshimori T et al (2000) The reversible modification regulates the membrane‐binding state of Apg8/Aut7 essential for autophagy and the cytoplasm to vacuole targeting pathway. J. Cell Biol. 151, 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Metcalf D, Isaacs AM (2010) The role of ESCRT proteins in fusion events involving lysosomes, endosomes and autophagosomes. Biochem. Soc. Trans. 38, 1469–1473. [DOI] [PubMed] [Google Scholar]
- 21. Eskelinen EL (2005) Maturation of autophagic vacuoles in Mammalian cells. Autophagy 1, 1–10. [DOI] [PubMed] [Google Scholar]
- 22. Liu B, Bao JK, Yang JM, Cheng Y (2013) Targeting autophagic pathways for cancer drug discovery. Chin. J. Cancer. 32, 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Inoki K, Li Y, Zhu T, Wu J, Guan KL (2002) TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat. Cell Biol. 4, 648–657. [DOI] [PubMed] [Google Scholar]
- 24. Avruch J, Long X, Ortiz‐Vega S, Rapley J, Papageorgiou A, Dai N (2009) Amino acid regulation of TOR complex 1. Am. J. Physiol. Endocrinol. Metab. 296, E592–E602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu B, Cheng Y, Liu Q, Bao JK, Yang JM (2010) Autophagic pathways as new targets for cancer drug development. Acta Pharmacol. Sin. 31, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong Y, Wang QJ, Li X, Yan Y, Backer JM, Chait BT et al (2009) Distinct regulation of autophagic activity by Atg14L and Rubicon associated with Beclin 1‐phosphatidylinositol‐3‐kinase complex. Nat. Cell Biol. 11, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Itakura E, Kishi C, Inoue K, Mizushima N (2008) Beclin 1 forms two distinct phosphatidylinositol 3‐kinase complexes with mammalian Atg14 and UVRAG. Mol. Biol. Cell 19, 5360–5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH et al (2006) Autophagic and tumour suppressor activity of a novel Beclin1‐binding protein UVRAG. Nat. Cell Biol. 8, 688–699. [DOI] [PubMed] [Google Scholar]
- 29. Takahashi Y, Coppola D, Matsushita N, Cualing HD, Sun M, Sato Y et al (2007) Bif‐1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 9, 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fimia GM, Stoykova A, Romagnoli A, Giunta L, Di Bartolomeo S, Nardacci R et al (2007) Ambra1 regulates autophagy and development of the nervous system. Nature 447, 1121–1125. [DOI] [PubMed] [Google Scholar]
- 31. Tang D, Kang R, Livesey KM, Cheh CW, Farkas A, Loughran P et al (2010) Endogenous HMGB1 regulates autophagy. J. Cell Biol. 190, 881–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Niu TK, Cheng Y, Ren X, Yang JM (2010) Interaction of Beclin 1 with survivin regulates sensitivity of human glioma cells to TRAIL‐induced apoptosis. FEBS Lett. 584, 3519–3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lenzi P, Marongiu R, Falleni A, Gelmetti V, Busceti CL, Michiorri S et al (2012) A subcellular analysis of genetic modulation of PINK1 on mitochondrial alterations, autophagy and cell death. Arch. Ital. Biol. 150, 194–217. [DOI] [PubMed] [Google Scholar]
- 34. Zhou F, Yang Y, Xing D (2011) Bcl‐2 and Bcl‐xL play important roles in the crosstalk between autophagy and apoptosis. FEBS J. 278, 403–413. [DOI] [PubMed] [Google Scholar]
- 35. Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, Kroemer G (2010) Autophagy regulation by p53. Curr. Opin. Cell Biol. 22, 181–185. [DOI] [PubMed] [Google Scholar]
- 36. Levine B, Abrams J (2008) p53: the Janus of autophagy? Nat. Cell Biol. 10, 637–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sui X, Jin L, Huang X, Geng S, He C, Hu X (2011) p53 signaling and autophagy in cancer: a revolutionary strategy could be developed for cancer treatment. Autophagy 7, 565–571. [DOI] [PubMed] [Google Scholar]
- 38. Tasdemir E, Maiuri MC, Galluzzi L, Vitale I, Djavaheri‐Mergny M, D'Amelio M et al (2008) Regulation of autophagy by cytoplasmic p53. Nat. Cell Biol. 10, 676–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nahimana A, Attinger A, Aubry D, Greaney P, Ireson C, Thougaard AV et al (2009) The NAD biosynthesis inhibitor APO866 has potent antitumor activity against hematologic malignancies. Blood 113, 3276–3286. [DOI] [PubMed] [Google Scholar]
- 40. Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG et al (2013) Resveratrol induces apoptosis and autophagy in T‐cell acute lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating p38‐MAPK. Biomed. Environ. Sci. 26, 902–911. [DOI] [PubMed] [Google Scholar]
- 41. Puissant A, Robert G, Fenouille N, Luciano F, Cassuto JP, Raynaud S et al (2010) Resveratrol promotes autophagic cell death in chronic myelogenous leukemia cells via JNK‐mediated p62/SQSTM1 expression and AMPK activation. Cancer Res. 70, 1042–1052. [DOI] [PubMed] [Google Scholar]
- 42. Goussetis DJ, Altman JK, Glaser H, McNeer JL, Tallman MS, Platanias LC (2010) Autophagy is a critical mechanism for the induction of the antileukemic effects of arsenic trioxide. J. Biol. Chem. 285, 29989–29997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cheng J, Wei HL, Chen J, Xie B (2012) Antitumor effect of arsenic trioxide in human K562 and K562/ADM cells by autophagy. Toxicol. Mech. Methods 22, 512–519. [DOI] [PubMed] [Google Scholar]
- 44. Lee MY, Liu YW, Chen MH, Wu JY, Ho HY, Wang QF et al (2013) Indirubin‐3’‐monoxime promotes autophagic and apoptotic death in JM1 human acute lymphoblastic leukemia cells and K562 human chronic myelogenous leukemia cells. Oncol. Rep. 29, 2072–2078. [DOI] [PubMed] [Google Scholar]
- 45. Chen YJ, Huang WP, Yang YC, Lin CP, Chen SH, Hsu ML et al (2009) Platonin induces autophagy‐associated cell death in human leukemia cells. Autophagy 5, 173–183. [DOI] [PubMed] [Google Scholar]
- 46. Neri LM, Cani A, Martelli AM, Simioni C, Junghanss C, Tabellini G et al (2014) Targeting the PI3K/Akt/mTOR signaling pathway in B‐precursor acute lymphoblastic leukemia and its therapeutic potential. Leukemia 28, 739–748. [DOI] [PubMed] [Google Scholar]
- 47. Crazzolara R, Bradstock KF, Bendall LJ (2009) RAD001 (Everolimus) induces autophagy in acute lymphoblastic leukemia. Autophagy 5, 727–728. [DOI] [PubMed] [Google Scholar]
- 48. Chiarini F, Grimaldi C, Ricci F, Tazzari PL, Evangelisti C, Ognibene A et al (2010) Activity of the novel dual phosphatidylinositol 3‐kinase/mammalian target of rapamycin inhibitor NVP‐BEZ235 against T‐cell acute lymphoblastic leukemia. Cancer Res. 70, 8097–8107. [DOI] [PubMed] [Google Scholar]
- 49. Simioni C, Neri LM, Tabellini G, Ricci F, Bressanin D, Chiarini F et al (2012) Cytotoxic activity of the novel Akt inhibitor, MK‐2206, in T‐cell acute lymphoblastic leukemia. Leukemia 26, 2336–2342. [DOI] [PubMed] [Google Scholar]
- 50. Evangelisti C, Ricci F, Tazzari P, Chiarini F, Battistelli M, Falcieri E et al (2011) Preclinical testing of the Akt inhibitor triciribine in T‐cell acute lymphoblastic leukemia. J. Cell. Physiol. 226, 822–831. [DOI] [PubMed] [Google Scholar]
- 51. Laane E, Tamm KP, Buentke E, Ito K, Kharaziha P, Oscarsson J et al (2009) Cell death induced by dexamethasone in lymphoid leukemia is mediated through initiation of autophagy. Cell Death Differ. 16, 1018–1029. [DOI] [PubMed] [Google Scholar]
- 52. Bonapace L, Bornhauser BC, Schmitz M, Cario G, Ziegler U, Niggli FK et al (2010) Induction of autophagy‐dependent necroptosis is required for childhood acute lymphoblastic leukemia cells to overcome glucocorticoid resistance. J. Clin. Invest. 120, 1310–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bellodi C, Lidonnici MR, Hamilton A, Helgason GV, Soliera AR, Ronchetti M et al (2009) Targeting autophagy potentiates tyrosine kinase inhibitor‐induced cell death in Philadelphia chromosome‐positive cells, including primary CML stem cells. J. Clin. Invest. 119, 1109–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Li J, Xue L, Hao H, Han Y, Yang J, Luo J (2012) Rapamycin provides a therapeutic option through inhibition of mTOR signaling in chronic myelogenous leukemia. Oncol. Rep. 27, 461–466. [DOI] [PubMed] [Google Scholar]
- 55. Wang J, Lian H, Zhao Y, Kauss MA, Spindel S (2008) Vitamin D3 induces autophagy of human myeloid leukemia cells. J. Biol. Chem. 283, 25596–25605. [DOI] [PubMed] [Google Scholar]
- 56. Itoh T, Ohguchi K, Nozawa Y, Akao Y (2009) Intracellular glutathione regulates sesquiterpene lactone‐induced conversion of autophagy to apoptosis in human leukemia HL60 cells. Anticancer Res. 29, 1449–1457. [PubMed] [Google Scholar]
- 57. Yokoyama T, Miyazawa K, Naito M, Toyotake J, Tauchi T, Itoh M et al (2008) Vitamin K2 induces autophagy and apoptosis simultaneously in leukemia cells. Autophagy 4, 629–640. [DOI] [PubMed] [Google Scholar]
- 58. Sotelo J, Briceno E, Lopez‐Gonzalez MA (2006) Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double‐blind, placebo‐controlled trial. Ann. Intern. Med. 144, 337–343. [DOI] [PubMed] [Google Scholar]
- 59. Racoma IO, Meisen WH, Wang QE, Kaur B, Wani AA (2013) Thymoquinone inhibits autophagy and induces cathepsin‐mediated, caspase‐independent cell death in glioblastoma cells. PLoS One 8, e72882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yuan G, Yan SF, Xue H, Zhang P, Sun JT, Li G (2014) Cucurbitacin I induces protective autophagy in glioblastoma in vitro and in vivo. J. Biol. Chem. 289, 10607–10619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jakubowicz‐Gil J, Langner E, Badziul D, Wertel I, Rzeski W (2013) Apoptosis induction in human glioblastoma multiforme T98G cells upon temozolomide and quercetin treatment. Tumour Biol. 34, 2367–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Makhov P, Golovine K, Teper E, Kutikov A, Mehrazin R, Corcoran A et al (2014) Piperlongumine promotes autophagy via inhibition of Akt/mTOR signalling and mediates cancer cell death. Br. J. Cancer 110, 899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Liu AJ, Wang SH, Chen KC, Kuei HP, Shih YL, Hou SY et al (2013) Evodiamine, a plant alkaloid, induces calcium/JNK‐mediated autophagy and calcium/mitochondria‐mediated apoptosis in human glioblastoma cells. Chem. Biol. Interact. 205, 20–28. [DOI] [PubMed] [Google Scholar]
- 64. Fan QW, Cheng C, Hackett C, Feldman M, Houseman BT, Nicolaides T et al (2010) Akt and autophagy cooperate to promote survival of drug‐resistant glioma. Sci. Signal. 3, ra81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rios‐Marco P, Martin‐Fernandez M, Soria‐Bretones I, Rios A, Carrasco MP, Marco C (2013) Alkylphospholipids deregulate cholesterol metabolism and induce cell‐cycle arrest and autophagy in U‐87 MG glioblastoma cells. Biochim. Biophys. Acta 1831, 1322–1334. [DOI] [PubMed] [Google Scholar]
- 66. Qin LS, Yu ZQ, Zhang SM, Sun G, Zhu J, Xu J et al (2013) The short chain cell‐permeable ceramide (C6) restores cell apoptosis and perifosine sensitivity in cultured glioblastoma cells. Mol. Biol. Rep. 40, 5645–5655. [DOI] [PubMed] [Google Scholar]
- 67. Shen J, Zheng H, Ruan J, Fang W, Li A, Tian G et al (2013) Autophagy inhibition induces enhanced proapoptotic effects of ZD6474 in glioblastoma. Br. J. Cancer 109, 164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kim H, Moon JY, Ahn KS, Cho SK (2013) Quercetin induces mitochondrial mediated apoptosis and protective autophagy in human glioblastoma U373MG cells. Oxid. Med. Cell. Longev. 2013, 596496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zhang X, Li W, Wang C, Leng X, Lian S, Feng J et al (2014) Inhibition of autophagy enhances apoptosis induced by proteasome inhibitor bortezomib in human glioblastoma U87 and U251 cells. Mol. Cell. Biochem. 385, 265–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Eimer S, Belaud‐Rotureau MA, Airiau K, Jeanneteau M, Laharanne E, Veron N et al (2011) Autophagy inhibition cooperates with erlotinib to induce glioblastoma cell death. Cancer Biol. Ther. 11, 1017–1027. [DOI] [PubMed] [Google Scholar]
- 71. Liu J, Liu L, Xue Y, Meng F, Li S, Wang P et al (2014) Anti‐neoplastic activity of low‐dose endothelial‐monocyte activating polypeptide‐II results from defective autophagy and G2/M arrest mediated by PI3K/Akt/FoxO1 axis in human glioblastoma stem cells. Biochem. Pharmacol. 89, 477–489. [DOI] [PubMed] [Google Scholar]
- 72. Josset E, Burckel H, Noel G, Bischoff P (2013) The mTOR inhibitor RAD001 potentiates autophagic cell death induced by temozolomide in a glioblastoma cell line. Anticancer Res. 33, 1845–1851. [PubMed] [Google Scholar]
- 73. Filippi‐Chiela EC, Thome MP, Bueno e Silva MM, Pelegrini AL, Ledur PF, Garicochea B et al (2013) Resveratrol abrogates the temozolomide‐induced G2 arrest leading to mitotic catastrophe and reinforces the temozolomide‐induced senescence in glioma cells. BMC Cancer 13, 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Jeon SH, Kim SH, Kim Y, Kim YS, Lim Y, Lee YH et al (2011) The tricyclic antidepressant imipramine induces autophagic cell death in U‐87MG glioma cells. Biochem. Biophys. Res. Commun. 413, 311–317. [DOI] [PubMed] [Google Scholar]
- 75. Yang CH, Kao YH, Huang KS, Wang CY, Lin LW (2012) Cordyceps militaris and mycelial fermentation induced apoptosis and autophagy of human glioblastoma cells. Cell Death Dis. 3, e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Dacevic M, Isakovic A, Podolski‐Renic A, Isakovic AM, Stankovic T, Milosevic Z et al (2013) Purine nucleoside analog–sulfinosine modulates diverse mechanisms of cancer progression in multi‐drug resistant cancer cell lines. PLoS One 8, e54044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Pratt J, Roy R, Annabi B (2012) Concanavalin‐A‐induced autophagy biomarkers requires membrane type‐1 matrix metalloproteinase intracellular signaling in glioblastoma cells. Glycobiology 22, 1245–1255. [DOI] [PubMed] [Google Scholar]
- 78. Wang XD, Freeman NE, Patil R, Patil SA, Mitra S, Orr WE et al (2012) EDL‐291, a novel isoquinoline, presents antiglioblastoma effects in vitro and in vivo. Anticancer Drugs 23, 494–504. [DOI] [PubMed] [Google Scholar]
- 79. Davidescu M, Sciaccaluga M, Macchioni L, Angelini R, Lopalco P, Rambotti MG et al (2012) Bromopyruvate mediates autophagy and cardiolipin degradation to monolyso‐cardiolipin in GL15 glioblastoma cells. J. Bioenerg. Biomembr. 44, 51–60. [DOI] [PubMed] [Google Scholar]
- 80. Zhuang W, Long L, Zheng B, Ji W, Yang N, Zhang Q et al (2012) Curcumin promotes differentiation of glioma‐initiating cells by inducing autophagy. Cancer Sci. 103, 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Wang Y, Qiu Q, Shen JJ, Li DD, Jiang XJ, Si SY et al (2012) Cardiac glycosides induce autophagy in human non‐small cell lung cancer cells through regulation of dual signaling pathways. Int. J. Biochem. Cell Biol. 44, 1813–1824. [DOI] [PubMed] [Google Scholar]
- 82. Trenti A, Grumati P, Cusinato F, Orso G, Bonaldo P, Trevisi L (2014) Cardiac glycoside ouabain induces autophagic cell death in non‐small cell lung cancer cells via a JNK‐dependent decrease of Bcl‐2. Biochem. Pharmacol. 89, 197–209. [DOI] [PubMed] [Google Scholar]
- 83. Abe A, Yamada H, Moriya S, Miyazawa K (2011) The beta‐carboline alkaloid harmol induces cell death via autophagy but not apoptosis in human non‐small cell lung cancer A549 cells. Biol. Pharm. Bull. 34, 1264–1272. [DOI] [PubMed] [Google Scholar]
- 84. Ling YH, Aracil M, Zou Y, Yuan Z, Lu B, Jimeno J et al (2011) PM02734 (elisidepsin) induces caspase‐independent cell death associated with features of autophagy, inhibition of the Akt/mTOR signaling pathway, and activation of death‐associated protein kinase. Clin. Cancer Res. 17, 5353–5366. [DOI] [PubMed] [Google Scholar]
- 85. Jin CY, Yu HY, Park C, Han MH, Hong SH, Kim KS et al (2013) Oleifolioside B‐mediated autophagy promotes apoptosis in A549 human non‐small cell lung cancer cells. Int. J. Oncol. 43, 1943–1950. [DOI] [PubMed] [Google Scholar]
- 86. Liu Y, Yang Y, Ye YC, Shi QF, Chai K, Tashiro S et al (2012) Activation of ERK‐p53 and ERK‐mediated phosphorylation of Bcl‐2 are involved in autophagic cell death induced by the c‐Met inhibitor SU11274 in human lung cancer A549 cells. J. Pharmacol. Sci. 118, 423–432. [DOI] [PubMed] [Google Scholar]
- 87. Chen MC, Chen CH, Liu YN, Wang HP, Pan SL, Teng CM (2013) TW01001, a novel piperazinedione compound, induces mitotic arrest and autophagy in non‐small cell lung cancer A549 cells. Cancer Lett. 336, 370–378. [DOI] [PubMed] [Google Scholar]
- 88. Li YY, Lam SK, Mak JC, Zheng CY, Ho JC (2013) Erlotinib‐induced autophagy in epidermal growth factor receptor mutated non‐small cell lung cancer. Lung Cancer 81, 354–361. [DOI] [PubMed] [Google Scholar]
- 89. Zou Y, Ling YH, Sironi J, Schwartz EL, Perez‐Soler R, Piperdi B (2013) The autophagy inhibitor chloroquine overcomes the innate resistance of wild‐type EGFR non‐small‐cell lung cancer cells to erlotinib. J. Thorac. Oncol. 8, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lee JG, Wu R (2012) Combination erlotinib‐cisplatin and Atg3‐mediated autophagy in erlotinib resistant lung cancer. PLoS One 7, e48532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Liu J, Hu XJ, Jin B, Qu XJ, Hou KZ, Liu YP (2012) beta‐Elemene induces apoptosis as well as protective autophagy in human non‐small‐cell lung cancer A549 cells. J. Pharm. Pharmacol. 64, 146–153. [DOI] [PubMed] [Google Scholar]
- 92. Li T, Su L, Zhong N, Hao X, Zhong D, Singhal S et al (2013) Salinomycin induces cell death with autophagy through activation of endoplasmic reticulum stress in human cancer cells. Autophagy 9, 1057–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park SH, Kim JH, Chi GY, Kim GY, Chang YC, Moon SK et al (2012) Induction of apoptosis and autophagy by sodium selenite in A549 human lung carcinoma cells through generation of reactive oxygen species. Toxicol. Lett. 212, 252–261. [DOI] [PubMed] [Google Scholar]
- 94. Xu CX, Zhao L, Yue P, Fang G, Tao H, Owonikoko TK et al (2011) Augmentation of NVP‐BEZ235's anticancer activity against human lung cancer cells by blockage of autophagy. Cancer Biol. Ther. 12, 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mei H, Lin Z, Wang Y, Wu G, Song Y (2014) Autophagy inhibition enhances pan‐Bcl‐2 inhibitor AT‐101‐induced apoptosis in non‐small cell lung cancer. Neoplasma 61, 186–192. [DOI] [PubMed] [Google Scholar]
- 96. Pan X, Zhang X, Sun H, Zhang J, Yan M, Zhang H (2013) Autophagy inhibition promotes 5‐fluorouraci‐induced apoptosis by stimulating ROS formation in human non‐small cell lung cancer A549 cells. PLoS One 8, e56679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Park SH, Chi GY, Eom HS, Kim GY, Hyun JW, Kim WJ et al (2012) Role of autophagy in apoptosis induction by methylene chloride extracts of Mori cortex in NCI‐H460 human lung carcinoma cells. Int. J. Oncol. 40, 1929–1940. [DOI] [PubMed] [Google Scholar]
- 98. Li YC, He SM, He ZX, Li M, Yang Y, Pang JX et al (2014) Plumbagin induces apoptotic and autophagic cell death through inhibition of the PI3K/Akt/mTOR pathway in human non‐small cell lung cancer cells. Cancer Lett. 344, 239–259. [DOI] [PubMed] [Google Scholar]
- 99. Zhang C, Yang L, Wang XB, Wang JS, Geng YD, Yang CS et al (2013) Calyxin Y induces hydrogen peroxide‐dependent autophagy and apoptosis via JNK activation in human non‐small cell lung cancer NCI‐H460 cells. Cancer Lett. 340, 51–62. [DOI] [PubMed] [Google Scholar]
- 100. Chen M, Du Y, Qui M, Wang M, Chen K, Huang Z et al (2013) Ophiopogonin B‐induced autophagy in non‐small cell lung cancer cells via inhibition of the PI3K/Akt signaling pathway. Oncol. Rep. 29, 430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Yu HC, Lin CS, Tai WT, Liu CY, Shiau CW, Chen KF (2013) Nilotinib induces autophagy in hepatocellular carcinoma through AMPK activation. J. Biol. Chem. 288, 18249–18259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Altmeyer A, Josset E, Denis JM, Gueulette J, Slabbert J, Mutter D et al (2012) The mTOR inhibitor RAD001 augments radiation‐induced growth inhibition in a hepatocellular carcinoma cell line by increasing autophagy. Int. J. Oncol. 41, 1381–1386. [DOI] [PubMed] [Google Scholar]
- 103. Chen W, Feng L, Nie H, Zheng X (2012) Andrographolide induces autophagic cell death in human liver cancer cells through cyclophilin D‐mediated mitochondrial permeability transition pore. Carcinogenesis 33, 2190–2198. [DOI] [PubMed] [Google Scholar]
- 104. Tsai SC, Yang JS, Peng SF, Lu CC, Chiang JH, Chung JG et al (2012) Bufalin increases sensitivity to AKT/mTOR‐induced autophagic cell death in SK‐HEP‐1 human hepatocellular carcinoma cells. Int. J. Oncol. 41, 1431–1442. [DOI] [PubMed] [Google Scholar]
- 105. Zou M, Lu N, Hu C, Liu W, Sun Y, Wang X et al (2012) Beclin 1‐mediated autophagy in hepatocellular carcinoma cells: implication in anticancer efficiency of oroxylin A via inhibition of mTOR signaling. Cell. Signal. 24, 1722–1732. [DOI] [PubMed] [Google Scholar]
- 106. Zhang H, Chen GG, Zhang Z, Chun S, Leung BC, Lai PB (2012) Induction of autophagy in hepatocellular carcinoma cells by SB203580 requires activation of AMPK and DAPK but not p38 MAPK. Apoptosis 17, 325–334. [DOI] [PubMed] [Google Scholar]
- 107. Wen M, Wu J, Luo H, Zhang H (2012) Galangin induces autophagy through upregulation of p53 in HepG2 cells. Pharmacology 89, 247–255. [DOI] [PubMed] [Google Scholar]
- 108. Wang N, Pan W, Zhu M, Zhang M, Hao X, Liang G et al (2011) Fangchinoline induces autophagic cell death via p53/sestrin2/AMPK signalling in human hepatocellular carcinoma cells. Br. J. Pharmacol. 164, 731–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J (2012) Autophagy induced by baicalin involves downregulation of CD147 in SMMC‐7721 cells in vitro. Oncol. Rep. 27, 1128–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xu N, Zhang J, Shen C, Luo Y, Xia L, Xue F et al (2012) Cisplatin‐induced downregulation of miR‐199a‐5p increases drug resistance by activating autophagy in HCC cell. Biochem. Biophys. Res. Commun. 423, 826–831. [DOI] [PubMed] [Google Scholar]
- 111. Chang Z, Shi G, Jin J, Guo H, Guo X, Luo F et al (2013) Dual PI3K/mTOR inhibitor NVP‐BEZ235‐induced apoptosis of hepatocellular carcinoma cell lines is enhanced by inhibitors of autophagy. Int. J. Mol. Med. 31, 1449–1456. [DOI] [PubMed] [Google Scholar]
- 112. Adiseshaiah PP, Clogston JD, McLeland CB, Rodriguez J, Potter TM, Neun BW et al (2013) Synergistic combination therapy with nanoliposomal C6‐ceramide and vinblastine is associated with autophagy dysfunction in hepatocarcinoma and colorectal cancer models. Cancer Lett. 337, 254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Guo XL, Li D, Sun K, Wang J, Liu Y, Song JR et al (2013) Inhibition of autophagy enhances anticancer effects of bevacizumab in hepatocarcinoma. J. Mol. Med. 91, 473–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hui B, Shi YH, Ding ZB, Zhou J, Gu CY, Peng YF et al (2012) Proteasome inhibitor interacts synergistically with autophagy inhibitor to suppress proliferation and induce apoptosis in hepatocellular carcinoma. Cancer 118, 5560–5571. [DOI] [PubMed] [Google Scholar]
- 115. Yoon JS, Kim HM, Yadunandam AK, Kim NH, Jung HA, Choi JS et al (2013) Neferine isolated from Nelumbo nucifera enhances anti‐cancer activities in Hep3B cells: molecular mechanisms of cell cycle arrest, ER stress induced apoptosis and anti‐angiogenic response. Phytomedicine 20, 1013–1022. [DOI] [PubMed] [Google Scholar]
- 116. Yi P, Higa A, Taouji S, Bexiga MG, Marza E, Arma D et al (2012) Sorafenib‐mediated targeting of the AAA(+) ATPase p97/VCP leads to disruption of the secretory pathway, endoplasmic reticulum stress, and hepatocellular cancer cell death. Mol. Cancer Ther. 11, 2610–2620. [DOI] [PubMed] [Google Scholar]
- 117. Carr BI, Cavallini A, Lippolis C, D'Alessandro R, Messa C, Refolo MG et al (2013) Fluoro‐Sorafenib (Regorafenib) effects on hepatoma cells: growth inhibition, quiescence, and recovery. J. Cell. Physiol. 228, 292–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kang JH, Chang YC, Maurizi MR (2012) 4‐O‐carboxymethyl ascochlorin causes ER stress and induced autophagy in human hepatocellular carcinoma cells. J. Biol. Chem. 287, 15661–15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lachenmayer A, Toffanin S, Cabellos L, Alsinet C, Hoshida Y, Villanueva A et al (2012) Combination therapy for hepatocellular carcinoma: additive preclinical efficacy of the HDAC inhibitor panobinostat with sorafenib. J. Hepatol. 56, 1343–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Yadunandam AK, Yoon JS, Seong YA, Oh CW, Kim GD (2012) Prospective impact of 5‐FU in the induction of endoplasmic reticulum stress, modulation of GRP78 expression and autophagy in Sk‐Hep1 cells. Int. J. Oncol. 41, 1036–1042. [DOI] [PubMed] [Google Scholar]
- 121. Ko YH, Cho YS, Won HS, Jeon EK, An HJ, Hong SU et al (2013) Prognostic significance of autophagy‐related protein expression in resected pancreatic ductal adenocarcinoma. Pancreas 42, 829–835. [DOI] [PubMed] [Google Scholar]
- 122. Fiorini C, Menegazzi M, Padroni C, Dando I, Dalla Pozza E, Gregorelli A et al (2013) Autophagy induced by p53‐reactivating molecules protects pancreatic cancer cells from apoptosis. Apoptosis 18, 337–346. [DOI] [PubMed] [Google Scholar]
- 123. Singh BN, Kumar D, Shankar S, Srivastava RK (2012) Rottlerin induces autophagy which leads to apoptotic cell death through inhibition of PI3K/Akt/mTOR pathway in human pancreatic cancer stem cells. Biochem. Pharmacol. 84, 1154–1163. [DOI] [PubMed] [Google Scholar]
- 124. Muilenburg D, Parsons C, Coates J, Virudachalam S, Bold RJ (2014) Role of autophagy in apoptotic regulation by Akt in pancreatic cancer. Anticancer Res. 34, 631–637. [PMC free article] [PubMed] [Google Scholar]
- 125. Fukui M, Kang KS, Okada K, Zhu BT (2013) EPA, an omega‐3 fatty acid, induces apoptosis in human pancreatic cancer cells: role of ROS accumulation, caspase‐8 activation, and autophagy induction. J. Cell. Biochem. 114, 192–203. [DOI] [PubMed] [Google Scholar]
- 126. Kallifatidis G, Hoepfner D, Jaeg T, Guzman EA, Wright AE (2013) The marine natural product manzamine A targets vacuolar ATPases and inhibits autophagy in pancreatic cancer cells. Mar. Drugs 11, 3500–3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Shabaik YH, Millard M, Neamati N (2013) Mechanistic evaluation of a novel small molecule targeting mitochondria in pancreatic cancer cells. PLoS One 8, e54346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ovadje P, Chochkeh M, Akbari‐Asl P, Hamm C, Pandey S (2012) Selective induction of apoptosis and autophagy through treatment with dandelion root extract in human pancreatic cancer cells. Pancreas 41, 1039–1047. [DOI] [PubMed] [Google Scholar]
- 129. Pelizzaro‐Rocha KJ, de Jesus MB, Ruela‐de‐Sousa RR, Nakamura CV, Reis FS, de Fatima A et al (2013) Calix[6]arene bypasses human pancreatic cancer aggressiveness: downregulation of receptor tyrosine kinases and induction of cell death by reticulum stress and autophagy. Biochim. Biophys. Acta 1833, 2856–2865. [DOI] [PubMed] [Google Scholar]
- 130. Dando I, Donadelli M, Costanzo C, Dalla Pozza E, D'Alessandro A, Zolla L et al (2013) Cannabinoids inhibit energetic metabolism and induce AMPK‐dependent autophagy in pancreatic cancer cells. Cell Death Dis. 4, e664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Yu CC, Chiang PC, Lu PH, Kuo MT, Wen WC, Chen P et al (2012) Antroquinonol, a natural ubiquinone derivative, induces a cross talk between apoptosis, autophagy and senescence in human pancreatic carcinoma cells. J. Nutr. Biochem. 23, 900–907. [DOI] [PubMed] [Google Scholar]
- 132. Kumar D, Shankar S, Srivastava RK (2013) Rottlerin‐induced autophagy leads to the apoptosis in breast cancer stem cells: molecular mechanisms. Mol. Cancer. 12, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Chiu HW, Yeh YL, Wang YC, Huang WJ, Chen YA, Chiou YS et al (2013) Suberoylanilide hydroxamic acid, an inhibitor of histone deacetylase, enhances radiosensitivity and suppresses lung metastasis in breast cancer in vitro and in vivo. PLoS One 8, e76340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Zhang X, Wei H, Liu Z, Yuan Q, Wei A, Shi D et al (2013) A novel protoapigenone analog RY10‐4 induces breast cancer MCF‐7 cell death through autophagy via the Akt/mTOR pathway. Toxicol. Appl. Pharmacol. 270, 122–128. [DOI] [PubMed] [Google Scholar]
- 135. Zhu X, Wu L, Qiao H, Han T, Chen S, Liu X et al (2013) Autophagy stimulates apoptosis in HER2‐overexpressing breast cancers treated by lapatinib. J. Cell. Biochem. 114, 2643–2653. [DOI] [PubMed] [Google Scholar]
- 136. Shi JM, Bai LL, Zhang DM, Yiu A, Yin ZQ, Han WL et al (2013) Saxifragifolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS‐dependent endoplasmic reticulum stress. Biochem. Pharmacol. 85, 913–926. [DOI] [PubMed] [Google Scholar]
- 137. Rovito D, Giordano C, Vizza D, Plastina P, Barone I, Casaburi I et al (2013) Omega‐3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARgamma activation in MCF‐7 breast cancer cells. J. Cell. Physiol. 228, 1314–1322. [DOI] [PubMed] [Google Scholar]
- 138. Dragowska WH, Weppler SA, Wang JC, Wong LY, Kapanen AI, Rawji JS et al (2013) Induction of autophagy is an early response to gefitinib and a potential therapeutic target in breast cancer. PLoS One 8, e76503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Laha D, Pramanik A, Maity J, Mukherjee A, Pramanik P, Laskar A et al (2014) Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Biochim. Biophys. Acta 1840, 1–9. [DOI] [PubMed] [Google Scholar]
- 140. Jangamreddy JR, Ghavami S, Grabarek J, Kratz G, Wiechec E, Fredriksson BA et al (2013) Salinomycin induces activation of autophagy, mitophagy and affects mitochondrial polarity: differences between primary and cancer cells. Biochim. Biophys. Acta 1833, 2057–2069. [DOI] [PubMed] [Google Scholar]
- 141. Yue W, Hamai A, Tonelli G, Bauvy C, Nicolas V, Tharinger H et al (2013) Inhibition of the autophagic flux by salinomycin in breast cancer stem‐like/progenitor cells interferes with their maintenance. Autophagy 9, 714–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Moad AI, Muhammad TS, Oon CE, Tan ML (2013) Rapamycin induces apoptosis when autophagy is inhibited in T‐47D mammary cells and both processes are regulated by Phlda1. Cell Biochem. Biophys. 66, 567–587. [DOI] [PubMed] [Google Scholar]
- 143. Lisiak N, Paszel‐Jaworska A, Bednarczyk‐Cwynar B, Zaprutko L, Kaczmarek M, Rybczynska M (2014) Methyl 3‐hydroxyimino‐11‐oxoolean‐12‐en‐28‐oate (HIMOXOL), a synthetic oleanolic acid derivative, induces both apoptosis and autophagy in MDA‐MB‐231 breast cancer cells. Chem. Biol. Interact. 208, 47–57. [DOI] [PubMed] [Google Scholar]
- 144. Tiwari RV, Parajuli P, Sylvester PW (2014) gamma‐Tocotrienol‐induced autophagy in malignant mammary cancer cells. Exp. Biol. Med. 239, 33–44. [DOI] [PubMed] [Google Scholar]
- 145. D'Anneo A, Carlisi D, Lauricella M, Puleio R, Martinez R, Di Bella S et al (2013) Parthenolide generates reactive oxygen species and autophagy in MDA‐MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 4, e891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Kanno S, Yomogida S, Tomizawa A, Yamazaki H, Ukai K, Mangindaan RE et al (2013) Papuamine causes autophagy following the reduction of cell survival through mitochondrial damage and JNK activation in MCF‐7 human breast cancer cells. Int. J. Oncol. 43, 1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pawlik A, Wiczk A, Kaczynska A, Antosiewicz J, Herman‐Antosiewicz A (2013) Sulforaphane inhibits growth of phenotypically different breast cancer cells. Eur. J. Nutr. 52, 1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Schwartz‐Roberts JL, Shajahan AN, Cook KL, Warri A, Abu‐Asab M, Clarke R (2013) GX15‐070 (obatoclax) induces apoptosis and inhibits cathepsin D‐ and L‐mediated autophagosomal lysis in antiestrogen‐resistant breast cancer cells. Mol. Cancer Ther. 12, 448–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Weng JR, Yen MH, Lin WY (2013) Cytotoxic constituents from Celastrus paniculatus induce apoptosis and autophagy in breast cancer cells. Phytochemistry 94, 211–219. [DOI] [PubMed] [Google Scholar]
- 150. Forestieri R, Donohue E, Balgi A, Roberge M, Andersen RJ (2013) Synthesis of clionamine B, an autophagy stimulating aminosteroid isolated from the sponge Cliona celata. Org. Lett. 15, 3918–3921. [DOI] [PubMed] [Google Scholar]
- 151. Nkandeu DS, Mqoco TV, Visagie MH, Stander BA, Wolmarans E, Cronje MJ et al (2013) In vitro changes in mitochondrial potential, aggresome formation and caspase activity by a novel 17‐beta‐estradiol analogue in breast adenocarcinoma cells. Cell Biochem. Funct. 31, 566–574. [DOI] [PubMed] [Google Scholar]


