Abstract
Objectives
Textiles used to make clothing can represent a source, often ignored, of chemicals potentially noxious to both skin and the whole organism. Among the most frequently produced potentially noxious chemical manufacturing by‐products are formaldehyde (FA), nickel (Ni) and hexavalent chromium (Cr); they are of potential clinical interest as all are known to be carcinogenic to humans and to be potent skin sensitizers. The aim of this study was to investigate, in vitro, effects of these potentially dangerous compounds on two different melanoma cell lines. In particular, attention was focused on A375P, a poorly metastatic and low invasive cell line and SK‐MEL‐28, a highly metastatic cell line.
Materials and methods
Effects of these compounds was evaluated on A375P and SK‐MEL‐28 cells. FA (1–5 × 10−5 m), NiSO 4 (10−6–10−3 m), K2Cr2O7 (10−7–10−6 m) effects on cell proliferation were evaluated by cell counting, while ERK pathway involvement was evaluated by Western blot analysis.
Results
Low concentrations of the chemicals, covering a range that corresponds to commonly accepted limits in textile production, induced a significant increase in cell proliferation concomitant with transient activation of phosphorylated ERK expression.
Conclusions
Data obtained suggest that increasing attention must be focused on these by‐products' potentially harmful effects in chemical manufacturing of clothes and accessories, that remain for long periods of time, in contact with human skin.
Introduction
The skin is the largest organ of the body in vertebrates and represents a barrier against exogenous substances, pathogens and mechanical stress. It also plays a pivotal role in permitting organism/external environment interactions, thanks to receptors and nerve endings. As any other organ in human body, skin can develop benign as well as malignant tumours. Among malignant skin cancers, great attention is focused on malignant melanoma. This tumour is one of the most aggressive skin cancers, and according to World Health Organization (WHO) estimates, numbers of melanoma cases worldwide are increasing faster than of any other cancer type. It has an estimated doubling of incidence every 10–20 years, becoming a major public health concern in many countries 1, 2. Recent epidemiological data highlight that in the United States, melanoma incidence is of at least 50 000 new cases/year and represents about 3% of all newly diagnosed cancers 1. Malignant melanoma represents a great medical challenge as tumour lesions can remain undetectable or asymptomatic for long periods of time 3. It is well known that this malignancy displays high metastatic potential and strong resistance to currently available clinical treatment, finally resulting in poor prognosis 1, 4, 5.
Acting as a physical barrier between the internal body and external environment, skin is constantly in contact with clothing. Textiles used to make clothes can therefore represent a source, often ignored, of potentially noxious chemicals for both skin and the whole organism. Prolonged contact with potentially harmful compounds derived from clothes and accessories (where they are present as manufacturing by‐products), could conceivably damage skin integrity. In particular, in the short term, they can induce allergic or sensitization reactions, while over long periods, there is fear that they might induce overproliferation of neoplastic cells. Current literature does not report comprehensive studies on these aspects, thus a preliminary investigation is desirable.
Among the most frequently used potentially noxious chemical manufacturing by‐products, are formaldehyde (FA), nickel (Ni) and hexavalent chromium (Cr VI). They are of potential clinical interest, as all these are known to be carcinogenic to humans and to be potent skin sensitizers. Recently, a number of in vitro studies has highlighted that these compounds could induce different cell effects, in both normal and tumourigenic cell lines, ranging from apoptosis to enhanced cell proliferation, depending on their concentrations 6, 7, 8, 9, 10, 11. Moreover, FA, Ni and Cr have been described to be able to activate the ERK signalling pathway in both normal and tumour cell models 6, 12, 13, 14.
Due to their recognized potentially harmful effects on human health (even if there are still no mandatory regulations), many standards or voluntary labels have introduced limits to regulate FA, Ni and Cr presence in textiles: European Ecolabel, National Technical Report UNI/TR 11359 and many further private trademarks (Oeko‐Tex, Bluesigned, Aafa RLS, and more).
The aim of this study was to evaluate, in an in vitro model, effects of the three potentially noxious compounds that are known to represent common manufacturing by‐products of clothing and accessory industry (formaldehyde, nickel and hexavalent chromium) on two different melanoma cell lines. In particular, our attention was focused on A375P, a poorly metastatic and low invasive cell line 15, 16 and SK‐MEL‐28, a highly metastatic cell line 1.
Materials and methods
Cell culture
Human melanoma cell lines A375P and SK‐MEL‐28 were a kind gift of Prof. Daniela Taverna from Molecular Biotechnology Center, Department of Molecular Biotechnology and Health Sciences, University of Turin. A375P is a poorly metastatic and lowly invasive cell line 15, 16, while SK‐MEL‐28 is highly metastatic 1. A375P cells were grown in culture flasks (75 cm2) in DMEM (Euroclone, Milan, Italy) supplemented with 10% heat inactivated foetal bovine serum (FBS) (Euroclone), penicillin (100 U/ml), streptomycin (100 mg/ml), l‐glutamine (2 mm), non‐essential aminoacids, vitamins, Hepes (25 mm) and sodium pyruvate (1 mm) (Euroclone) in a humidified atmosphere containing 5% CO2 at 37 °C. SK‐MEL‐28 cells were grown in culture flasks (75 cm2) in DMEM (Euroclone) supplemented with 10% heat inactivated foetal bovine serum (FBS) (Euroclone), penicillin (100 U/ml), streptomycin (100 mg/ml), l‐glutamine (2 mm), Hepes (25 mm) and sodium pyruvate (1 mm) (Euroclone) in a humidified atmosphere containing 5% CO2 at 37 °C.
Cell treatments
Cells were treated with growing concentrations of formaldehyde (FA) (Sigma Aldrich, St. Louis, MO, USA), nickel (NiSO4) (Sigma Aldrich) and hexavalent chromium (K2Cr2O7) (Carlo Erba, Milan, Italy) aqueous solutions. Starting aqueous solutions were diluted in DMEM without FBS just before cell treatment, to obtain intermediate concentrations, and cell treatment was performed in complete medium. Tested concentrations were decided considering both compounds IC50 and limits described in private label Oeko‐Tex and in volunteer European label (Ecolabel) rules for these compounds in textiles. Formaldehyde was used at concentrations of 10, 25, 50 μm, with a limit of 20 ppm generally accepted in textiles (corresponding to 1 mg/l or 3.33 × 10−5 m in the elution solution) and IC50 of 3.03 × 10−4 m 17. NiSO4 was used at 10−6–10−3 m concentration range; NiSO4 IC50 was at 6.22 × 10−4 m 18, while the nickel limit generally accepted for textiles is 1 mg/kg (corresponding to 0.05 mg/l or 8.52 × 10−7 m in the elution solution). K2Cr2O7 was used at 10−7 and 10−6 m; K2Cr2O7 IC50 is 3.23 × 10−5 m 18, while hexavalent chromium limit generally accepted for textiles is 0.5 mg/kg (corresponding to 0.025 mg/l or 4.81 × 10−7 m in the elution solution). Correlations between concentrations of the investigated chemicals on textiles and concentrations of elution solutions were referred to values obtained by acid sweat extraction method performed on fabrics, with liquor ratio 1:20. To evaluate formaldehyde, nickel and chromium effects on cell proliferation, depending on observed cell duplication time (around 24 h for A375P and 72 h for SK‐MEL‐28), A375P cells were exposed overnight to test chemicals, while SK‐MEL‐28 cells were treated for 3 days.
Cell proliferation
To evaluate formaldehyde, nickel and chromium effects on cell proliferation, 1 × 103 cells were seeded in multiwell plates (35 cells/mm2) and allowed to adhere overnight. Non‐adherent cells were then removed by gentle washing in phosphate buffer (PBS, pH = 7.4) and test chemicals were added in complete cell culture medium. At the end of the incubation time, cells were fixed in cold methanol, and stained with crystal violet; tested samples were photographed at ×4 magnification using an optical microscope (Leica ICC50HD). Each experiment was performed three times in triplicate. Counting was performed by two researchers, blinded to experimental groups, to assess reproducibility of the analysis. Interindividual variation was less than or equal to 20%, thus counting data from both researchers were analysed. Cell proliferation was evaluated by counting cells in each microscopic field in at least three fields for each experimental condition. Cell density was expressed as percentage of control values ± standard deviation (SD).
Western blot analysis
For time course experiments, 2 × 105 cells were treated for different times (0, 5, 15, 30, 60, 120 min) with formaldehyde, nickel or chromium compound, at concentrations providing the maximum proliferation result. Cells were lysed in PBS containing 0.5% Triton X‐100 and protease inhibitor cocktail (Roche Diagnostics GmbH, Mannheim, Germany). Total protein content of each sample was determined by means of BCA assay (Pierce, Rockford, IL, USA). Of proteins, 15 μg were separated onto a 12% SDS‐PAGE gel under reducing conditions and blotted onto nitrocellulose membrane (Amersham Biosciences, Little Chalfont, UK) using standard methods. Membranes were blocked in 7% milk in PBS with 0.1% Tween‐20 at room temperature for 2 h. Membranes were then incubated with primary specific anti‐ERK1/2 antibody (1:1000; Cell Signaling Technology cat #9102, Cell Signalling Inc, Beverly, MA, USA) or anti‐phospho‐ERK1/2 antibody (1:5000; Cell Signaling Technology cat #9106) overnight at 4 °C. As control for protein loading, actin was detected on membranes using anti‐actin antibody purchased from Sigma Aldrich.
Signals were revealed using appropriate secondary peroxidase‐conjugated antibodies, and bands were visualized by chemoluminescence (Amersham Biosciences). Western blotting experiments were quantified by densitometric analysis using ImageJ software (U. S. National Institutes of Health, Bethesda, Maryland, USA).
Statistical analysis
Unpaired Student's t‐tests were performed for statistical analysis. Statistical procedures were performed with the Prism 4.0 statistical software (GraphPad Software Inc., La Jolla, CA, USA). Probability values of P < 0.05 were considered statistically significant. Statistical analysis was performed comparing cell density observed at each concentration tested, to control.
Results
Formaldehyde‐mediated effects
Formaldehyde, at 10−5 and 2.5 × 10−5 m (two concentrations that virtually correspond to fabric content lower than limits normally accepted by standards and labels (20 ppm, 1 mg/l, 3.33 × 10−5 m) and compound IC50 (3.03 × 10−4 m) 17), increased both A375P and SK‐MEL‐28 cell proliferation (Fig. 1), in a statistically significant manner. In particular, maximum cell proliferation increase was observed at lowest concentrations tested (10−5 m) in the poorly metastatic and low invasive A375P cell line (Fig. 1, white bars, P < 0.001), while in the highly metastatic SK‐MEL‐28 cell line, maximum proliferation peak was obtained with 2.5 × 10−5 m stimulation (Fig. 1, grey bars, P < 0.05). Highest concentration tested (5 × 10−5 m) induced an initial toxic effect on A375P cells (Fig. 1, white bars, P < 0.001), while this had no statistically significant effect on SK‐MEL‐28 cell proliferation (Fig. 1, grey bars). As shown in Fig. 2, formaldehyde‐induced increase in A375P and SK‐MEL‐28 cell proliferation was mediated by a transient increase in ERK (extra‐cellular signalling‐regulated kinase) activation. Considering the less aggressive A375P cell line, ERK activation occurred between 30 and 60 min stimulation, as highlighted by phospho‐ERK/ERK ratio (Fig. 2, white bars, P < 0.001). On the other hand, in the more aggressive SK‐MEL‐28 cell line, statistically significant ERK activation started after 5 min stimulation and reached maximum after 60 min, as highlighted by phospho‐ERK/ERK ratio (Fig. 2, grey bars, P < 0.001). In both cell lines, after 120 min stimulation, ERK activation returned to control levels.
Figure 1.
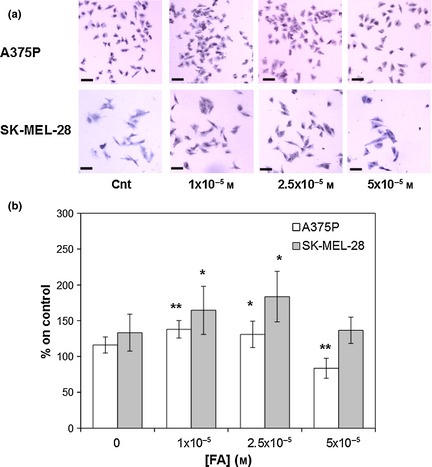
Formaldehyde ( FA ) effects on A375P and SK ‐ MEL ‐28 cell lines after 24 and 72 h incubation respectively. (a) Representative images (magnification 4×). (b) Quantification of FA effects on A375P (white bars) and SK‐MEL‐28 (grey bars) cell proliferation. Results represent the mean values obtained from three independent experiments and are expressed as mean values ± standard deviation (SD). *P < 0.05, **P < 0.001 compared to control samples.
Figure 2.
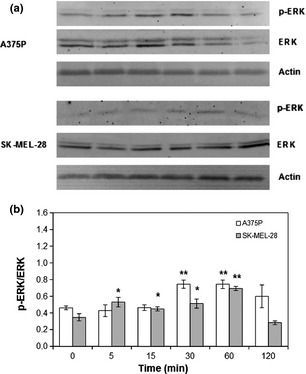
FA ‐induced ERK phosphorylation. A375P cells were treated with 10−5 m FA, while SK‐MEL‐28 cells were treated with 2.5 × 10−5 m FA. (a) Representative western blot images. (b) Densitometric quantification of ERK phosphorylation. Results represent mean values obtained from three independent experiments and are expressed as mean values ± standard deviation (SD). White bars represent A375P cells, while grey bars are referred to SK‐MEL‐28 cells.
Nickel‐mediated effects
Nickel effects on the two melanoma cell lines under investigation were assessed using increasing concentrations of NiSO4 in aqueous solution. As shown in Fig. 3, lowest NiSO4 concentration tested (10−6 m, corresponding to presence of nickel similar to limits normally accepted for this metal on textiles) induced a statistically significant increase in A375P cell proliferation (Fig. 3, white bars, P < 0.05), while increasing concentrations (10−5, 10−4 m) had no effect on proliferation of this poorly metastatic melanoma cell line. On the other hand, when the tested concentration was higher than compound IC50 (6.22 × 10−4 m) 18, toxic effects were observed (Fig. 5, white bars, P < 0.001). As shown in the same figure, lowest concentrations tested (10−6, 10−5 m) enhanced highly metastatic SK‐MEL‐28 melanoma cell proliferation, with proliferation peak at 10−5 m (Fig. 3, grey bars, P < 0.001). While 10−4 m concentration had no statistically significant effect on SK‐MEL‐28 cell proliferation, the highest concentration tested (10−3 m) induced toxic effects (Fig. 3, grey bars, P < 0.001). As shown in Fig. 4, NiSO4‐induced increase in A375P and SK‐MEL‐28 cell proliferation was associated with a transient increase in ERK activation. In particular, in the poorly metastatic A375P cell line, ERK activation occurred between 5 and 60 min of stimulation, as demonstrated by increase in phospho‐ERK/ERK ratio (Fig. 4, white bars, P < 0.05). On the other hand, in the highly metastatic SK‐MEL‐28 cell line, a statistically significant increase in ERK activation was observed between 5 and 30 min, with maximum activation peak at 15 min, as demonstrated by increase in phospho‐ERK/ERK ratio (Fig. 4, grey bars, P < 0.001).
Figure 3.
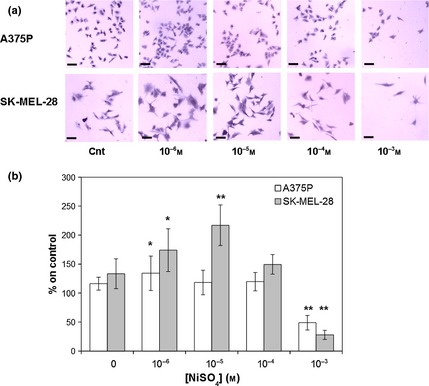
Nickel (Ni SO 4 ) effects on A375P and SK ‐ MEL ‐28 cell lines after 24 and 72 h incubation respectively. (a) Representative images (magnification 4×). (b) Quantification of NiSO 4 effects on A375P (white bars) and SK‐MEL‐28 (grey bars) cell proliferation. Results represent mean values obtained from three independent experiments and are expressed as mean values ± standard deviation (SD). *P < 0.05, **P < 0.001 compared to control samples.
Figure 5.
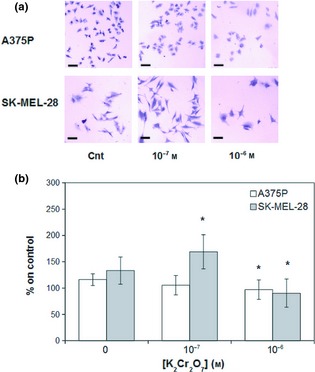
Hexavalent chromium (K 2 Cr 2 O 7 ) effects on A375P and SK ‐ MEL ‐28 cell lines after 24 and 72 h incubation respectively. (a) Representative images (magnification 4×). (b) Quantification of K2Cr2O7 effects on A375P (white bars) and SK‐MEL‐28 (grey bars) cell proliferation. Results represent mean values obtained from three independent experiments and are expressed as mean values ± standard deviation (SD). *P < 0.05, **P < 0.001 compared to control samples.
Figure 4.
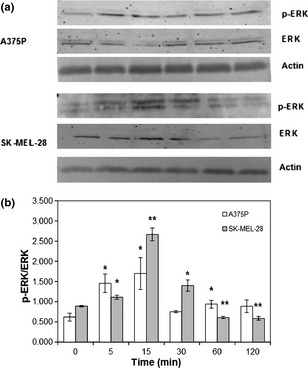
Ni SO 4 ‐induced ERK phosphorylation. A375P cells were treated with 10−6 m NiSO 4, while SK‐MEL‐28 cells were treated with 10−5 m NiSO 4. (a) Representative western blot images. (b) Densitometric quantification of ERK phosphorylation. Results represent mean values obtained from three independent experiments and are expressed as mean values ± standard deviation (SD). White bars represent A375P cells, while grey bars are referred to SK‐MEL‐28 cells.
Chromium‐induced effects
Chromium effects on the two melanoma cell lines under investigation were assessed using increasing concentrations of K2Cr2O7 aqueous solution. As shown in Fig. 5, the lowest K2Cr2O7 concentration tested (10−7 m, a concentration corresponding to a presence of hexavalent chromium lower than the limit normally accepted on textiles) had no statistically significant effect on A375P cell proliferation, while increasing concentrations (10−6 m), even if below the compound IC50 (3.23 × 10−5 m) 18, but higher than Oeko‐Tex limit (4.81 × 10−7 m) started to display a toxic effect (Fig. 5, white bars, P < 0.05). Cell treatment with K2Cr2O7 concentrations similar to compound IC50 caused an increasingly toxic effect (Fig. 5, white bars, P < 0.001). As shown in the same figure, lowest K2Cr2O7 concentration tested (10−7 m) was accompanied by a statistically significant increase in SK‐MEL‐28 cell proliferation (Fig. 5, grey bars, P < 0.05), while increasing concentrations (10−6 m) had a statistically significant toxic effect (Fig. 5, grey bars, P < 0.05). Even if K2Cr2O7 did not induce a statistically significant effect on cell proliferation (Fig. 5, white bars), it was sufficient to induce a transient increase in ERK activation. On the other hand, K2Cr2O7 induced, in SK‐MEL‐28, a transient increase in ERK activation associated with the observed increase in cell proliferation. In particular, in A375P cell line, increase in phospho‐ERK/ERK ratio was maximum after 5 min stimulation (Fig. 6, white bars, P < 0.05), while in SK‐MEL‐28 cell line, maximum increase in phospho‐ERK/ERK ratio was observed after 15 min stimulation (Fig. 6, grey bars, P < 0.001).
Figure 6.
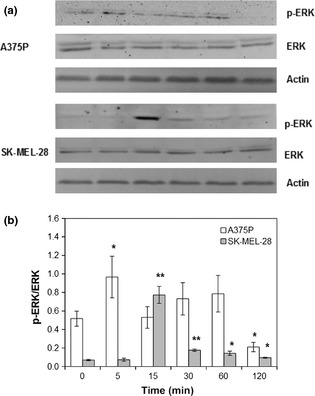
K 2 Cr 2 O 7 ‐induced ERK phosphorylation. A375P cells were treated with 10−7 m K2Cr2O7, while SK‐MEL‐28 cells were treated with 10−7 m K2Cr2O7. (a) Representative western blot images. (b) Densitometric quantification of ERK phosphorylation. Results represent mean values obtained from three independent experiments and are expressed as mean values ± standard deviation (SD). White bars represent A375P cells, while grey bars are referred to SK‐MEL‐28 cells.
Discussion
Clothes and accessories, due to their prolonged contact with skin might represent a source, often ignored, of potentially noxious substances. Some chemicals that can be potentially harmful to human health, such as formaldehyde, nickel and chromium, can be found in textiles and accessories as manufacturing by‐products, and can be released on sweating. Sweat, thanks to its aqueous nature, can induce chemical by‐product solubilization from clothes and accessories, making them more easily accessible to cells.
Despite these chemicals being recognized as potentially carcinogenic to humans, there are no mandatory legal norms regulating chemical manufacturing by‐product levels in either clothing or accessories. To overcome this lack in international legislation, many standards or voluntary labels have introduced limits to regulate FA, Ni and Cr presence in textiles, such as the European Ecolabel, the National Technical Report UNI/TR 11359 and other private trademarks (Oeko‐Tex, Bluesigned, Aafa RLS, and others).
The aim of this study was to evaluate effects of FA, Ni and Cr, three potentially noxious compounds known to be manufacturing by‐products of the clothing and accessories industries, on cell proliferation and phospho‐ERK expression in two melanoma cell lines.
To compare concentrations of solutions used in this study with limits normally accepted in the textile market, it has been speculated an extraction of these chemicals by an acid sweat extraction method, with a liquor ratio 1:20. However, it must be added that the above method is normally adopted for heavy metals (such as Ni and Cr VI), while, in the case of FA, the limit of 20 ppm refers to a water extraction of hydrolysed FA.
Formaldehyde is a volatile, colourless, pungent‐smelling, highly water soluble chemical that can reach living organisms as an exogenous agent or an endogenous metabolite 8, 19. It is widely used as preservative and disinfectant, as well as in consumer products such as textiles, where it is mainly used in anti‐crease treatments 8, 13.
It is also known that direct contact with diluted formaldehyde solutions may cause inflammation and allergic reactions, such as contact dermatitis 8. Recently, formaldehyde has been classified by IARC as being “carcinogenic to humans” (group 1) 11, 13, 20. Many experimental in vitro studies have highlighted that formaldehyde biological reactions are largely dose‐dependent; when administered in very high doses, it induces necrotic cell death, while in low doses, it is able to enhance cell proliferation in cultured cells 8. Cell stimulation with low doses of formaldehyde, by enhancing cell proliferation and inhibiting apoptosis, can sustain neoplastic processes, as suggested by Tyihák and coworkers, that tumour cells are more responsive than normal ones to this kind of stimulation 6, 11.
Nickel and chromium are metals ubiquitously present in the environment and, in the last century, industrialization and modern living have resulted in increased cutaneous exposure to them and hence in increase in metal allergies, such as incidence of systemic contact dermatitis (SCD) and allergic contact dermatitis (ACD) 21.
Nickel is a heavy metal belonging to the transition metals group that is abundant in the natural environment. Once within a cell, it can bind several biological components and alter cellular functions, morphology and ultrastructure 10, 22. In the clothing industry, nickel is contained in pigments and in metal complex dyes and is also contained in metal components such as zip fasteners and buckles.
It is a well‐known toxic element, described as being carcinogen to humans, but is also a common allergen and potent sensitizer, recognized as one of the main causes of irritant skin reactions and ACD, whose incidence within the general population is 20–30% 10, 22, 23, 24. Nickel‐mediated biological effects mostly depend on its concentration; in vitro studies have highlighted that, depending on the experimental model, it can induce totally different cellular effects, ranging from apoptosis in oral endothelial cells to enhanced cell proliferation in human primary cultured keratinocytes and HaCaT cells 23.
In the environment, chromium exists in two stable oxidation states: +3 (trivalent chromium, Cr(III)) and +6 (hexavalent chromium, Cr(VI)) 25. In the clothing industry, hexavalent chromium is contained in chrome dyes and in metal complex dyes and it is known to be genotoxic and carcinogenic in a number of experimental systems 14; it is also recognized as being one of the main causes of ACD, SCD and skin irritant reactions 10, 21, 26. Once entered into the mammalian cell environment, Cr(VI) is reduced via the redox system to form intermediate, unstable forms (Cr(V) and Cr(IV)) that finally yield Cr(III) that, being kinetically inert, is considered less toxic than Cr(VI) 27, 28, 29. Hexavalent chromium reduction to trivalent chromium generates (mainly via Fenton and Haber–Weiss type reactions 27) a wide spectrum of reactive oxygen species (ROS), such as superoxide anion, hydrogen peroxide and hydroxyl radicals 7, 30. These reactive intermediates interfere with intracellular signalling pathways, cell proliferation, cytokine and transcription factor activation, and apoptosis 29, 30. An in vitro study using a HLF (foetal human lung fibroblasts) cellular model, has highlighted that a narrow range of chromium concentrations (5‐30 × 10−6 m) can induce various cellular effects, ranging from cell cycle re‐entry (non‐toxic concentration, 5 × 10−6 m) to growth arrest (sub toxic concentration, 15 × 10−6 m) and apoptosis (toxic concentration, 30 × 10−6 m) 7.
It is acknowledged that cell proliferation is a critical factor in carcinogenesis induced by chemicals 19. In this study, attention has been focused on the ability of formaldehyde, nickel and chromium to increase cell proliferation in two different melanoma cell lines. As shown in the results section, all tested chemicals were able to increase cell proliferation in the chosen melanoma cell lines. In particular, low invasive and poorly metastatic A375P cell line 15, 16 responded with increased cell proliferation, to lower formaldehyde and nickel concentrations (10−5 m and 10−6 m, respectively) compared to the highly metastatic SK‐MEL‐28 melanoma cell line (2.5 × 10−5 m and 10−5 m, respectively) 1. In the light of these results, further studies have been performed to identify molecular mechanisms involved in the observed phenomena. Attention was focused on MAPKs (mitogen activated protein kinases), as it is known that this intracellular signalling pathway controls many aspects of mammalian cell physiology, including cell growth, differentiation and death, acting by phosphorylating downstream transcription factors 14. Among MAP kinases, the ERK signalling pathway is critical in regulating cell mitogenesis and differentiation; in particular, transient ERK activation leads to cell proliferation, while persistent activation mediates growth arrest and differentiation 14.
Data presented in the results section highlight ERK activation involvement in the observed formaldehyde‐, nickel‐ and hexavalent chromium‐induced increases in cell proliferation. These results confirm previously described formaldehyde, nickel and chromium involvement in ERK activation in various normal and tumour cell models 6, 12, 13, 14. The observed results are even more interesting considering that both A375P and SK‐MEL‐28 cells harbour BRAF(V600E) mutation, which is associated with enhanced sensitivity to MEK inhibition, finally resulting in both cyclin D1 protein expression down‐regulation and G1 cell cycle arrest 31, 32.
Formaldehyde, nickel and chromium metabolism have been described as generating ROS 22, 30, 33. It is widely accepted that redox balance is essential to maintain cell and tissue homoeostasis and that impaired oxidation can affect cell growth and differentiation, along with cell signalling and apoptosis 30. Many of these physiological events are mediated by MAPKs and, as increased ROS production has been described to induce MAPKs activation 34, oxidative alterations of this signalling pathway may provide a plausible explanation for the observed melanoma cell proliferation increase.
In conclusion, data presented herein show that melanoma cell exposure to formaldehyde, nickel and chromium, three chemical compounds present in clothing and accessories industry manufacturing by‐products, can induce, even at low concentrations, an increase in tumour cell proliferation ability.
Data obtained suggest that increasing attention must be focused on potential harmful effects of chemical manufacturing by‐products in clothing and accessories that have to remain for long periods of time in contact with human skin. Future studies of more physiological settings, such as organotypic human skin and skin‐melanoma models, and on actual release of such chemicals by clothes and accessories on human skin, are required to be able to suggest safe cut‐off levels for these chemicals.
Conflict of interest
The authors declare they have no conflict of interest.
Acknowledgements
Dr. Manuela Rizzi is supported by a “Fondazione Edo ed Elvo Tempia” fellowship.
References
- 1. Jeong JB, Hong SC, Koo JS, Jeong HJ (2011) Induction of apoptosis and acetylation of histone H3 and H4 by arctigenin in the human melanoma cell line SK‐MEL‐28. Food Nutr. Sci. 2, 128–132. [Google Scholar]
- 2. Mueller DW, Bosserhoff AK (2009) Role of miRNAs in the progression of malignant melanoma. Br. J. Cancer 101, 551–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Soengas MS, Lowe SW (2003) Apoptosis and melanoma chemoresistance. Oncogene 22, 3138–3151. [DOI] [PubMed] [Google Scholar]
- 4. Ma J, Han H, Liu D, Ii W, Feng X, Xue X et al (2013) HER2 as a promising target for cytotoxicity T cells in human melanoma therapy. PLoS ONE 2013, 8, e73261. doi: 10.1371/journal.pone.0073261. eCollection 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Buommino E, Baroni A, Canozo N, Petrazzullo M, Nicoletti R, Vozza A et al (2009) Artemisin reduces human melanoma cell migration by down‐regulating αVβ3 integrin and reducing metalloproteinase 2 production. Invest. New Drugs 27, 412–418. [DOI] [PubMed] [Google Scholar]
- 6. Aĭzenshtadt AA, Burova EB, Zenin VV, Bubkov DE, Kropacheva IV, Pinaev GP (2012) Effect of formaldehyde at low concentration on proliferation and organization of cytoskeleton of cultured cells. Cell Tissue Biol. 6, 147–153. [PubMed] [Google Scholar]
- 7. Asatiani N, Abuladze M, Kartvelishvili T, Kulikova N, Asanishvili L, Holman H‐Y et al (2010) Response of antioxidant defense system to chromium (VI)‐induced cytotoxicity in human diploid cells. Biometals 23, 161–172. [DOI] [PubMed] [Google Scholar]
- 8. Szende B, Tyihák E (2010) Effect of formaldehyde on cell proliferation and death. Cell Biol. Int. 34, 1273–1282. [DOI] [PubMed] [Google Scholar]
- 9. Marchese C, Visco V, Aimsti L, Cardinali G, Kovacs D, Buttari B et al (2003) Nickel induced keratinocyte proliferation and up‐modulation of the keratinocyte growth factor receptor expression. Exp. Dermatol. 12, 497–505. [DOI] [PubMed] [Google Scholar]
- 10. Ermolli M, Menné C, Pozzi G, Serra MA, Clerici LA (2001) Nickel, cobalt and chromium‐induced cytotoxicity and intracellular accumulation in human hacat keratinocytes. Toxicology 159, 23–31. [DOI] [PubMed] [Google Scholar]
- 11. Tyihák E, Bosci J, Timár F, Rácz G, Szende B (2001) Formaldehyde promotes and inhibits the proliferation of cultured tumor and endothelial cells. Cell Prolif. 34, 135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ding J, He G, Gong W, Wen W, Sun W, Ning B et al (2009) Effects of nickel on cyclin expression, cell cycle progression and cell proliferation in human pulmonary cells. Cancer Epidemiol. Biomarkers Prev. 18, 1720–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freick P, Haas SRL, Singer MV, Böcker U (2006) Low‐dose exposure of intestinal epithelial cells to formaldehyde results in MAP kinase activation and molecular alteration of the focal adhesion protein paxillin. Toxicology 219, 60–72. [DOI] [PubMed] [Google Scholar]
- 14. Chuang SM, Liou GY, Yang JL (2000) Activation of JNK, p38 and ERK mitogen‐activated protein kinases by chromium(VI) is mediated through oxidative stress but does not affect cytotoxicity. Carcinogenesis 21, 1491–1500. [PubMed] [Google Scholar]
- 15. Penna E, Orso F, Cimino D, Tenaglia E, Lembo A, Quaglino E et al (2011) microRNA‐214 contributes to melanoma tumor progression through suppression of TFAP2C. EMBO J. 30, 1990–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sriramarao P, Bourdon MA (1996) Melanoma cell invasive and metastatic potential correlates with endothelial cell reorganization and tenascin expression. Endothelium 4, 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sakaguchi H, Miyazawa M, Yoshida Y, Ito Y, Suzuki H (2007) Prediction of preservative sensitization potential using surface marker CD86 and/or CD54 expression on human cell line THP‐1. Arch. Dermatol. Res. 298, 427–437. [DOI] [PubMed] [Google Scholar]
- 18. An S, Kim S, Huh Y, Lee TR, Kim HK, Park KL et al (2009) Expression of surface markers on the human monocytic leukemia cell line, THP‐1, as indicators for the sensitizing potential of chemicals. Contact Dermatitis 60, 185–192. [DOI] [PubMed] [Google Scholar]
- 19. Starr TB, Gibson JE (1985) The mechanistic toxicology of formaldehyde and its implications for quantitative risk estimation. Annu. Rev. Pharmacol. Toxicol. 25, 745–767. [DOI] [PubMed] [Google Scholar]
- 20. Bosetti C, McLaughlin JK, Tarone RE, Pira E, La Vecchia C (2008) Formaldehyde and cancer risk: a quantitative review of cohort studies through 2006. Ann. Oncol. 19, 29–43. [DOI] [PubMed] [Google Scholar]
- 21. Yoshihisa Y, Shimizu T (2012) Metal allergy and systemic contact dermatitis: an overview. Dermatol. Res. Pract. 2012, doi: 10.1155/2012/749561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Trombetta D, Mondello MR, Cimino F, Cristani M, Pergolizzi S, Saija A (2005) Toxic effect of nickel in an in vitro model of human oral epithelium. Toxicol. Lett. 159, 219–225. [DOI] [PubMed] [Google Scholar]
- 23. Gursoy UK, Sokucu O, Uitto VJ, Aydin A, Demirer S, Toker H et al (2007) The role of nickel accumulation and epithelial cell proliferation in orthodontic treatment‐induced gingival overgrowth. Eur. J. Orthod. 29, 555–558. [DOI] [PubMed] [Google Scholar]
- 24. Jia W, Beatty MW, Reinhardt RA, Petro TM, Cohen DM, Maze CR et al (1999) Nickel release from orthodontic arch wires and cellular immune response to various nickel concentrations. J. Biomed. Mater. Res. (Appl. Biomater) 48, 488–495. [DOI] [PubMed] [Google Scholar]
- 25. Mc Carroll N, Keshava N, Chen J, Akerman G, Kligerman A, Rinde E (2010) An evaluation of the mode of action framework for mutagenic carcinogens case study II: chromium (VI). Environ. Mol. Mutagen. 51, 89–111. [DOI] [PubMed] [Google Scholar]
- 26. Henshaw FN, Morris BW, Mac Neil S (1999) Differentiation of normal human keratinocytes influences hexavalent chromium uptake and distribution and the ability of cells to withstand Cr(VI) cytotoxicity. Br. J. Dermatol. 141, 211–217. [DOI] [PubMed] [Google Scholar]
- 27. Rudolf E, Červinka M (2006) The role of intracellular zinc in chromium(VI)‐induced oxidative stress, DNA damage and apoptosis. Chem. Biol. Interact. 162, 212–227. [DOI] [PubMed] [Google Scholar]
- 28. Carroll S, Wood EJ (2000) Exposure of human keratinocytes and fibroblasts in vitro to nickel sulphate ions induces synthesis of stress proteins Hsp72 and HSp90. Acta Derm. Venereol. 80, 94–97. [PubMed] [Google Scholar]
- 29. Raghnathan VK, Grant MH, Ellis EM (2010) Changes in protein expression associated with chronic in vitro exposure of hexavalent chromium to osteoblasts and monocytes: a proteomic approach. J. Biomed. Mater. Res., Part A 92, 615–625. [DOI] [PubMed] [Google Scholar]
- 30. Asatiani N, Kartvelishvili T, Abuladze M, Asanishvili L, Sapojnikova N (2011) Chromium (VI) can activate and impair antioxidant defense system. Biol. Trace Elem. Res. 142, 388–397. [DOI] [PubMed] [Google Scholar]
- 31. Solit DB, Garraway LA, Pratilas CA, Sawai A, Getz G, Basso A et al (2006) BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim YK, Ahn SK, Lee M (2012) Differential sensitivity of melanoma cell lines with differing B‐Raf mutational status to the new oncogenic B‐Raf kinase inhibitor UI‐152. Cancer Lett. 320, 215–224. [DOI] [PubMed] [Google Scholar]
- 33. Pongsavee M (2013) Changes to oxidative stress levels following exposure to formaldehyde in limphocytes. World Acad. Sci. Eng. Technol. 79, 1538–1540. [Google Scholar]
- 34. Son Y, Cheong YK, Kim NH, Chung HT, Kang DG, Pae HO (2011) Mitogen‐activated protein kinases and reactive oxygen species: how can ROS activate MAPK pathways? J. Signal Transduct. 2011, doi: 10.1155/2011/792639. [DOI] [PMC free article] [PubMed] [Google Scholar]


