Abstract
Objectives
To evaluate anti‐proliferative as well as apoptotic activities of compounds identified in chloroform extract of Juglans regia leaves, on human breast and oral cancer cell lines (MCF‐7 and BHY).
Materials and methods
Column chromatography, MTT assay, flowcytometry and western blotting have all been used in the study.
Results
Bioassay‐guided fractionation of chloroform extract of J. regia afforded isolation of 5‐hydroxy‐3,7,4′‐trimethoxyflavone [1], lupeol [2], daucosterol [3], 4‐hydroxy‐α‐tetralone [4], β‐sitosterol [5], 5,7‐ dihydroxy‐3,4′‐dimethoxyflavone [6] and regiolone [7]. Structures of the compounds were established on the basis of spectroscopic analyses [Nuclear magnetic resonance (NMR) and mass]. All compounds inhibited proliferation of MCF‐7 (human breast adenocarcinoma) and BHY (human oral squamous carcinoma) cells in a concentration‐dependent manner. Compounds 6 and 7 had potent cytotoxic effects on both MCF‐7 and BHY cells (IC 50 21–51 μm), yet were not toxic to normal cells. MCF‐7 growth inhibition was attributed to apoptosis; population of apoptotic cells increased from 1.12% in controls to 5.64 and 8.1% after 48‐h treatment with compounds 6 and 7, indicating their potential at inducing early and late apoptosis. The caspase cascade was not activated, as indicated by only insignificant cleavage of caspase‐3.
Conclusions
Our results suggest that compounds 6 and 7 can induce apoptosis in MCF‐7 cells through the caspase‐3 independent pathway.
Introduction
Cancer, with more than 11 million deaths every day, is the principal cause of mortality in economically developed countries, and the second leading cause of death in developing countries 1. It is estimated that there will be 16 million new cases of cancer‐related demise each year, by 2020 2. Thus, discovering successful treatment for cancer is a vital objective. Major issues relating to conventional anti‐cancer chemotherapy are, occurrence of side effects induced by non‐specific targeting of both normal and cancer cells 3, 4, and emergence of drug‐resistant cancer cells 5. Hence, there is an urgent need for novel treatment options with improved characteristics. In this regard, plants have played a decisive role as a source of effective anti‐cancer agents 6, 7. Many plant‐derived compounds (such as paclitaxel, vinblastine, vincristine), and semi‐synthetic derivatives of natural products (such as etoposide and teniposide) are used as anti‐cancer drugs 8, 9. Anti‐cancer effects of natural agents and herbal products may affect cells by many different mechanisms, for example by preventing initiation and promotion of carcinogenesis or by inducing apoptosis 10. Thus, it is very important to isolate and screen apoptotic inducers from natural sources, especially plants, which synthesize a vast diversity of compounds 11.
Juglans regia L. (Juglandaceae) commonly known as the Persian or common walnut, is an important deciduous tree found primarily in temperate areas and commercially cultivated in the United States, western South America, Asia, Central and Southern Europe 12. Walnut leaves are considered to be a source of therapeutic compounds and have been used in traditional medicine for treatment of various maladies, and for anti‐diarrhetic, anti‐helminthic, depurative and astringent properties 13, 14, 15. Previous investigations have reported walnut leaves as a rich source of flavonoids and steroids, as well as of terpenoids, and have demonstrated considerable anti‐proliferative activities against various cancer cell lines 12, 16, 17. In our soluble partitioning experiment for anti‐proliferative activity of J. regia leaves, highest activity was recorded using the chloroform extract, which inhibited population growth of BHY (human oral squamous carcinoma) and MCF‐7 (human breast adenocarcinoma) cells with IC50 values ranging between 0.36 and 0.81 mg/ml 18.
Considering potential cytotoxic activity of chloroform fraction of J. regia leaves, chloroform extract was selected for detailed phytochemical–biological analysis to identify its anti‐proliferative constituents.
Materials and methods
Plant material and preparation of extracts
Leaves of J. regia were collected from suburbs of Tehran, Iran, in June 2009. A voucher specimen (no. 6727 THE) was deposited at the Herbarium of the Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. Air‐dried leaves (900 g) were pulverized and mixed with n‐hexane using the percolation method, for 24 h. Extraction continued for three consecutive days and all extracts were then filtered and concentrated to dryness using a rotary evaporator. After removal of hexane under reduced pressure, residual powder was suspended then extracted with CHCl3 to provide the chloroform fraction. This was subjected to chromatography for further purification.
Chromatography
Column chromatography is one of the most practical methods for separation and purification of both solids and liquids, when carrying out small‐scale experiments; here it was performed on the chloroform fraction using silica gel and Sephadex LH‐20 as the stationary phases. Isolated compounds were purified by re‐column chromatography and re‐crystallization and were characterized by spectral techniques 1H‐NMR, 13C‐NMR and LC MS/MS.
Cell culture
MCF‐7, BHY and NIH‐3T3 (mouse embryonic fibroblasts) were obtained from the Cell Bank of the Pasteur Institute of Iran. They were preserved in DMEM high‐glucose medium containing l‐glutamine with 10% foetal bovine serum, 1% penicillin–streptomycin and sodium bicarbonate in 5% CO2 at 37 °C.
Anti‐proliferation assay
Cytotoxicity was determined to MCF‐7 and BHY cells using MTT assay [3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5 diphenyltetrazolium bromide] then IC50 values were calculated 19. Briefly, cells were plated in 96‐well plates and treated with a range of concentrations (10–80 μm) of compounds [1–7] by dissolving them in DMSO. After incubation for 24, 48 and 72 h at 37 °C in a humidified incubator, MTT (5 mg/ml) was added to each well and incubated for another 4 h. After careful removal of medium, 0.2 ml DMSO was added to each well and plates were shaken. Absorbance was recorded at 545 nm. Effects of compounds on population growth inhibition were assessed as percentage of cell cytotoxicity, where vehicle‐treated cells were taken as 100% viable. Final concentration of DMSO was 0.5% in all treatment protocols.
Identification of apoptosis by annexin‐V/PI staining
This assay was performed to detect apoptosis using an annexin‐V‐FLUOS apoptosis detection kit (Roche Applied Science, Indianapolis, IN, USA), following the manufacturer's instructions. In brief, harvested MCF‐7 cells were resuspended in 100 μl annexin‐V‐FLUOS labelling solution containing 2 μl annexin‐V‐FLUOS labelling agent, 2 μl propidium iodide solution and 1 ml incubation buffer, to achieve a concentration of 106 cells/ml, and incubated at 37 °C. Finally, each tube was diluted with buffer before the cells were analysed by flow cytometry.
Western blot analysis
MCF‐7 cells were treated with compounds 6 and 7 at their IC50 concentrations for 48 h. Proteins were collected and lysed in lysis buffer [Tris 62.5 mm, pH 6.8; dithiothreitol, 50 mm; sodium dodecyl sulphate, 10%; glycerol, 10%; bromophenol blue, 0.25% (w/v)] in the presence of protease inhibitor. Eighty micrograms of protein was separated by 12% SDS‐PAGE and electroblotted to polyvinylidene fluoride membrane (GE Health Care Life Sciences, Buckinghamshire, UK) using semi‐dry blotting apparatus (Bio‐Rad, Hercules, CA, USA). After blocking with 1% casein, membranes were incubated overnight at 4 °C with primary antibody (anti‐caspase‐3, 1:500), followed by labelling with secondary antibody (1:5000). Protein bands were visualized using ECL advanced western blotting detection kit. Glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) was used as endogenous control and control cells were cultured in complete medium without experimental compounds (control cells were treated with DMSO only, at highest level used in the experiment).
Statistical analysis
IC50 values were calculated by non‐linear regression analysis with the GraphPad Prism®5 (Version 5.01, GraphPad Software, Inc., San Diego, CA, USA). Results were expressed as mean ± SE in at least triplicate, and statistical comparisons were based on ANOVA followed by Tukey's post‐test. P < 0.05 was considered significant.
Results
Extraction, isolation and purification of compounds
Bioassay‐guided fractionation of chloroform extract of J. regia leaves afforded isolation of seven known compounds [1–7] (Fig. 1), whose structures are presented in Fig. 2. Structures of these compounds were identified by comparing spectroscopic data with those reported as 5‐hydroxy‐3,7,4′‐trimethoxyflavone [1] 20, 21; lupeol [2] 22; daucosterol [3] 23; 4‐hydroxy‐α –tetralone [4] 24, 25; β‐sitosterol [5] 25, 26; 5,7‐ dihydroxy‐3,4′‐dimethoxyflavone [6] 27; and regiolone [7] 28. Among the isolated compounds, 5,7‐ dihydroxy‐3,4′‐dimethoxyflavone [6] was isolated from this genus for the first time, whereas 4‐hydroxy‐α –tetralone [4] and regiolone [7] have been reported as constituents of J. mandshurica, J. nigra 24, 25 and green husks of J. regia 29. In addition, compounds 3 and 5 have previously been isolated from J. cathayensis, J. mandshurica and leaves of J. regia 30, 31, 32. Lupeol [2] and 5‐hydroxy‐3,7,4′‐trimethoxyflavone [1] have also been identified in the Juglandacea genus in previous studies 22, 29, 33.
Figure 1.
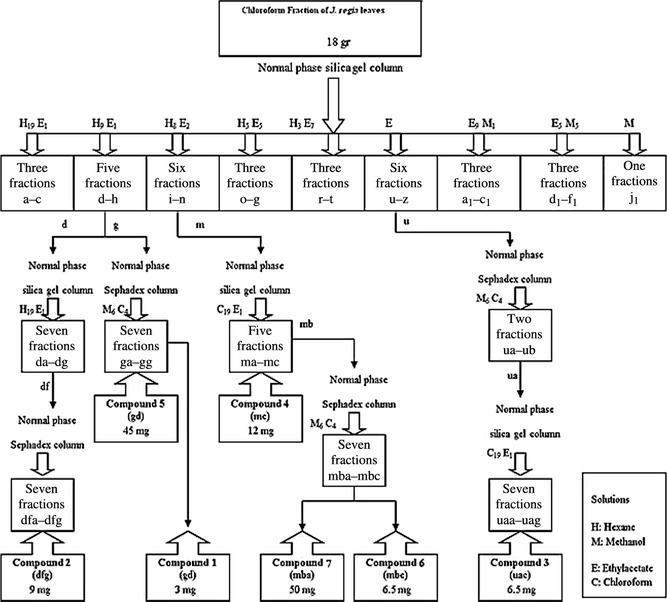
Isolation and purification of compounds [1–7] from the chloroform fraction of Juglans regia leaves.
Figure 2.
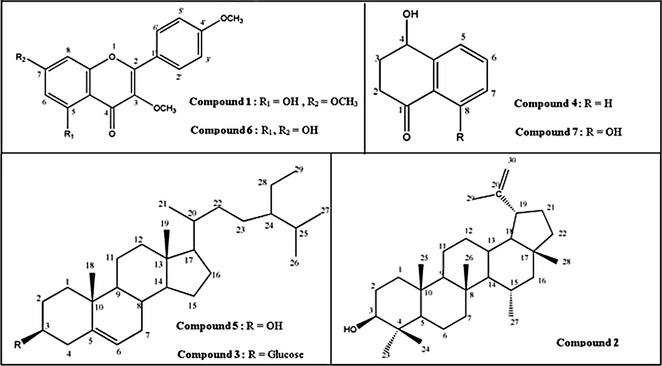
Structures of compounds isolated from chloroform fraction of Juglans regia leaves.
Anti‐cancer activity
Compounds [1–7] were evaluated for cell population growth inhibitory activity using MTT assay with human cancer cell lines, MCF‐7 and BHY, as well as normal mouse fibroblast cell line (NIH‐3T3), at 24, 48 and 72 h. All compounds exhibited cytotoxic activities in a concentration‐dependent manner at different times (data not shown). Best anti‐proliferative effect was obtained after 72‐h treatment; IC50 results are summarized in Table 1.
Table 1.
Antiproliferative effects (IC50, μm) of the isolated compounds [1–7] against MCF‐7 and BHY at 72 h.a
| Cell line/Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| MCF‐7 | 46.59 ± 1.5 | 38.93 ± 3.2 | 36.45 ± 3.61 | 57.14 ± 1.3 | 9.05 ± 0.4 | 50.98 ± 1.8 | 21.30 ± 3.5 |
| BHY | 46.04 ± 2.6 | 57.13 ± 2.5 | 74.14 ± 5.62 | 56.02 ± 1.5 | 56.20 ± 2.2 | 40.55 ± 2.00 | 48.58 ± 2.3 |
Values were determined from at least three independent experiments each performed in triplicate and expressed as mean ± SE.
Although compounds [1–5] have previously been evaluated for cytotoxic activity to several cancer cell lines 21, 34, 35, 36, 37, we still tested them along with the other previously non‐evaluated isolated compounds [6,7], on both MCF‐7 and BHY cells. Additionally, there has been no report to indicate cytotoxic effects of the isolated compounds [1–7] on oral squamous carcinoma cell line, BHY. Our results demonstrated that compounds 5 and 7 were significantly cytotoxic to MCF‐7 cells, whereas compounds 1, 6 and 7 revealed considerable inhibition to BHY cell growth. Considering IC50 values, the most sensitive cell line to almost all compounds was MCF‐7 (Table 1). Interestingly, compounds 6 and 7 were found for the first time to be active against MCF‐7 and BHY cells. Together, compounds 6 and 7 were selectively active against both cancer cell types but were much less effective against normal cells compared to the other isolated compounds (Table 2). Based on our observations of activities of compounds 6 and 7 on malignant and normal cells, as well as novelty of the cytotoxicity report of these two compounds, we selected them for further studies.
Table 2.
The cytotoxicity effects of compounds [1–7] on normal cell line (NIH‐3T3).
| Compounds | Concentration (μm)a | Cytotoxicity (%) |
|---|---|---|
| 1 | 50 | 77.27 ± 2.7 |
| 2 | 40 | 93.60 ± 1.4 |
| 60 | 88.26 ± 1.3 | |
| 3 | 40 | 87.56 ± 1.0 |
| 80 | 83.15 ± 2.0 | |
| 4 | 60 | 81.73 ± 2.0 |
| 5 | 10 | 31.11 ± 1.7 |
| 60 | 84.35 ± 0.6 | |
| 6 | 40 | 12.56 ± 1.0 |
| 50 | 18.51 ± 1.7 | |
| 7 | 25 | 5.43 ± 2.1 |
| 50 | 9.22 ± 1.3 |
Data are presented as (%) of three independent experiments.
Cells were treated with the IC50 equivalent concentrations of compounds.
Apoptosis determination with annexin‐V/propidium iodide staining
To explore whether compounds 6 and 7 were cytotoxic to MCF‐7 cells by inducing apoptosis, cells were stained with annexin‐V/PI and analysed by flow cytometry. In dual parameter fluorescent dot plots, cells in early apoptosis (annexin V+/PI−, lower right quadrant), in late apoptosis (annexin V+/PI+, upper right quadrant) and necrotic (annexin V−/PI+, upper left quadrant) were counted (Fig. 3). As shown in Table 3, 0.43% cells were annexin V+/PI− and 0.69% were annexin V+/PI+ in untreated cells. After treatment with compound 6 at 50.98 μm for 48 h, number annexin V+/PI−, annexin V+/PI+ and annexin V−/PI+ cells increased remarkably, while late‐stage apoptotic MCF‐7 cells augmented by 5% after treatment with compound 7 at 48 h. Meanwhile, the necrotic population (annexinV−/PI+) increased significantly – by 20.8% in compound 7‐treated cells. Our results collectively demonstrated that compounds 6 and 7 had anti‐cancer activities, mainly by inducing late apoptosis.
Figure 3.
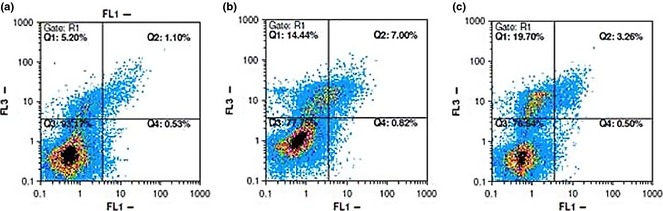
Flow cytometric analysis of annexin‐V/PI to quantify compound‐induced apoptosis in MCF ‐7 cells. (a) Dot plot of MCF‐7 cells with DMSO treatment for 48 h. (b) Dot plot of MCF‐7 cells with compound 6 treatment at 50.98 μm for 48 h. (c) Dot plot of MCF‐7 cells with compound 7 treatment at 21.30 μm for 48 h. Results shown are representative of three independent experiments. Quadrant 3, living cells An−/PI−; quadrant 4, early apoptotic cells An+/PI−; quadrant 2, late apoptotic cells An+/PI+; quadrant 1, necrotic cells An−/PI+.
Table 3.
Percentage of breast adenocarcinoma cells in each state.a
| Com‐pound | Vital cells (%) An−/PI− | Early apoptosis (%) An+/PI− | Late apoptosis (%) An+/PI+ | Necrosis (%) An−/PI+ |
|---|---|---|---|---|
| 6 | 78.6 ± 3.4* | 1.6 ± 0.41* | 6.5 ± 1.1* | 13.3 ± 2.2 |
| 7 | 73.5 ± 4.2* | 0.64 ± 0.19 | 5.00 ± 1.8 | 20.8 ± 2.3** |
| DMSO | 94.2 ± 2.4 | 0.43 ± 0.17 | 0.69 ± 0.25 | 4.63 ± 2.05 |
Cells treated with compounds 6 and 7 at 50.98 and 21.30 μm, respectively for 48 h of incubation. Data represent means of triplicate experiment.
*P < 0.05, **P < 0.01, ***P < 0.001 relative to solvent.
Cell death via the caspase‐3 independent pathway
Activation of an apoptosis pathway is a key mechanism by which cytotoxic drugs kill cancer cells 38; activation of caspases is the major event in apoptotic cell death 39. Apoptosis can be triggered by two types of apoptotic caspase, namely initiator caspases (caspase‐2, ‐8, ‐9 and ‐10) and effector caspases (caspase‐3, ‐6, and ‐7) 40. Thus, we next determined whether apoptosis induced by compounds 6 and 7 would be related to increased activation of the caspase pathway, in MCF‐7 cells. We analysed caspase‐3 activity in response to compounds 6 and 7 at their IC50 concentrations and found no increase in activity in comparison to controls at 48 h (Fig. 4).
Figure 4.
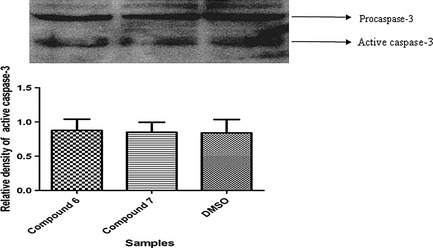
Cleaved caspase‐3 level: – MCF ‐7 cells were treated with compounds 6 and 7 for 48 h. Proteins, transferred to polyvinylidene fluoride (PVDF) membrane probed with rabbit anti‐caspase 3; then filtres were stripped and re‐probed with anti‐rabbit‐ GAPDH antibody, to show equal amounts of loading. Data are the mean ± SE of three separate experiments.
Discussion
Cancer is a multi‐mechanistic disease that has many features and requires a wide‐range of approaches for its treatment, control and prevention 41, 42. Given that cancer is a multi‐factorial disorder resulting in unlimited division of cells, remedial strategies that target tumour cells while causing fewer side effects on normal cells are desired 2. In such cases, plants have been historically used for prevention and treatment of human diseases such as cancer without serious side effects 43. Juglans species as therapeutic plants used in folk medicine have been asserted to possess anti‐cancer activities. Yang et al. 44 demonstrated strong anti‐proliferative activity of walnut seed extract against human HepG2 liver and Caco‐2 colon cancer cells and its anti‐cancer property was attributed to its chemical composition 45. Extract of root, bark, fruit and leaves of Juglans species also demonstrated anti‐proliferative effects against different cancer cell lines such as MDA‐MB‐231 human breast cancer cells 46.
Results of our previously performed cytotoxic screening of different fractions obtained from J. regia leaves indicated that the chloroform fraction had the most potent activity on breast cancer cells. Results obtained encouraged us to investigate chemical compositions of this fraction, which led to the isolation of 5‐hydroxy‐3,7,4′‐trimethoxyflavone [1], lupeol [2], daucosterol [3], 4‐hydroxy‐α‐tetralone [4], β‐sitosterol [5], 5,7‐ dihydroxy‐3,4′‐dimethoxyflavone [6] and regiolone [7] by the use of column chromatography and preparative TLC. Compounds were identified by means of LC/MS/MS and NMR spectroscopy (1H‐NMR, 13C‐NMR), and comparison of spectral data with data from the literature (available on request). To our knowledge, among the seven isolated compounds, compound 6 was identified for the first time in Juglans species.
Effects of isolated compounds [1–7] on cell population growth of breast adenocarcinoma, MCF‐7 and oral squamous cancer BHY were investigated by the MTT method. As indicated in Table 1, among the seven tested compounds, compounds 5, 6 and 7 were found to have the strongest anti‐proliferative activity against both of the cell lines with IC50 values ranging between 9.05 and 50.98 μm for MCF‐7 cells and 40.55 and 56.20 μm for BHY cells. In addition, compound 5 had cytotoxic activity on NIH‐3T3 mouse embryonic fibroblasts, with 31% and 84% cytotoxicity at IC50 equivalent concentration, on MCF‐7 and BHY cells, whereas compounds 6 and 7 exhibited weak cytotoxic activities over periods of 72 h at their IC50 equivalent concentrations on both cell lines (Table 2). On the basis of IC50 values and cytotoxicity data (Tables 1, 2), compounds 6 and 7 appeared to be worthy of further study as potential anti‐cancer agents. Moreover, there was no prior information concerning these two chemicals having anti‐cancer properties. One of the compounds [6] is a flavonoid (secondary metabolites of large numbers of plants known to be chemopreventive agents) 47, 48, 49. Compound 7 is a plant naphthoquinone (reported in various plants), and has shown remarkable anti‐cancer activities 50. Thus, the present study focused on a flavonoid and a naphtoquinone, providing valuable insight into their anti‐cancer potentials, and abilities to kill cancer cells. To examine whether anti‐proliferative activities by compounds 6 and 7, against MCF‐7 breast cancer cells, are related to apoptosis induction – the cells were stained with annexin‐V/PI and analysed by flow cytometry.
Cell death can be classified into at least two types, necrosis ‘accidental cell death’ and apoptosis ‘programmed cell death’. Induction of tumour cell apoptosis has long been used as an important indicator to detect the ability of chemotherapeutic drugs to inhibit tumour growth 51. In this study, to examine whether apoptosis or necrosis was the dominant cause of cell death induced by compounds 6 and 7 at 48 h, we performed more detailed experiments using annexin‐V and PI. As can be seen from Fig. 3 and Table 3, both compounds induced cell death through apoptosis and necrosis. Compared to solvent‐treated cells, which had a total of 5.8% background cell death (1.1% apoptosis and 4.6% necrosis), MCF‐7 cells treated with compounds 6 and 7 at 50.98 and 21.3 μm indicated 8.1 and 5.6% total apoptosis as well as 13.3 and 20.8% necrosis after 48‐h treatment. In other words, compound 6 significantly increased numbers of apoptotic cells at early and late stages after 48‐h incubation; however, compound 7 was able to induce late‐apoptosis by this time point. In addition, the ability of compound 7 to induce MCF‐7 necrosis was significantly higher than that of compound 6. These levels are in agreement with the results of the MTT experiment and suggest that compounds 6 and 7 may be potential anti‐cancer agents for human breast cancer investigation.
The important role of mitochondria in promoting caspase activation has been a major focus of apoptosis research; however, it is also clear that mitochondria can contribute to cell death by caspase‐independent mechanisms 52. For evaluation of the type of apoptosis induced by compounds 6 and 7, cleaved caspase‐3 was determined by western blot analysis. As shown in Fig. 4, no activation of caspase‐3 was observed in MCF‐7‐treated cells with eithe compound 6 or 7 for 48 h; that favoured apoptosis to be the result of a caspase‐3 independent pathway.
In conclusion, our bioassay‐guided study indicated that the strong inhibitory effect of CHCl3 extract of J. regia on population growth of cultured human tumour cell lines MCF7 and BHY may be attributed mainly to a flavonoid [6] and naphtoquinone [7]. However, favourable interaction between chemicals could be suggested, responsible for overall anti‐proliferative action of the extract. Our study also demonstrated that anti‐proliferative effects of compounds 6 and 7 as anti‐cancer agents could be based not only on their cytotoxicity, but also on their ability to induce apoptosis via a caspase‐3‐independent pathway. Additional studies will be necessary to obtain clear understanding of the molecular mechanisms involved.
Acknowledgements
This study was financially supported by grants from the Pasteur Institute of Iran (597).
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Rather MA, Bhat BA, Qurishi MA (2013) Multicomponent phytotherapeutic approach gaining momentum: is the “one drug to fit all” model breaking down? Phytomedicine 21, 1–14. [DOI] [PubMed] [Google Scholar]
- 3. Gurung RL, Lim SN, Khaw AK, Soon JFF, Shenoy K, Mohamed Ali SM et al (2010) Thymoquinone induces telomere shortening, DNA damage and apoptosis in human glioblastoma cells. PLoS One 5, e12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Head J, Johnston SRD (2000) New targets for therapy in breast cancer – farnesyltransferase inhibitors. Breast Cancer Res. 6, 262–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gibbs JB (2000) Mechanism‐based target identification and drug discovery in cancer research. Science 287, 1969–19735. [DOI] [PubMed] [Google Scholar]
- 6. Butler MS (2004) The role of natural product chemistry in drug discovery. J. Nat. Prod. 67, 2141–2153. [DOI] [PubMed] [Google Scholar]
- 7. Paterson I, Anderson EA (2005) The renaissance of natural products as drug candidates. Science 310, 451–453. [DOI] [PubMed] [Google Scholar]
- 8. Koehn FE, Carter GT (2005) The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4, 206–220. [DOI] [PubMed] [Google Scholar]
- 9. Dar MY, Shah WA, Mubashir S, Rather MA (2012) Chromatographic analysis, antiproliferative and radical scavenging activity of Pinus wallichina essential oil growing in high altitude areas of Kashmir, India. Phytomedicine 19, 1228–1233. [DOI] [PubMed] [Google Scholar]
- 10. Alshatwi AA, Hasan TN, Shafi G, Ahmed Syed N, Al‐Assaf AH, Alamri MS et al (2012) Validation of the antiproliferative effects of organic extracts from the green husk of Juglans regia L. on PC‐3 human prostate cancer cells by assessment of apoptosis related genes. Evid. Based Complement. Alternat. Med. 2012, 103026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wani BA, Ramamoorthy D, Rather MA, Arumugam N, Hamid A, Ganie AS et al (2013) Induction of apoptosis in human pancreatic MiaPaCa‐2 cells through the loss of mitochondrial membrane potential (ΔΨm) by Gentiana kurroo root extract and LC‐ESI‐MS analysis of its principal constituents. Phytomedicine 20, 723–733. [DOI] [PubMed] [Google Scholar]
- 12. Carvalho M, Ferreira PJ, Mendes VS, Silva R, Pereira JA, Jeronimo C et al (2010) Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 48, 441–447. [DOI] [PubMed] [Google Scholar]
- 13. Rather MA, Dar BA, Dar MY, Wani BA, Shah WA, Bhat BA et al (2012) Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phyomedicine 19, 1185–1190. [DOI] [PubMed] [Google Scholar]
- 14. Bruneton J (2009) Pharmacognosie, Phytochimie, Plantes Médicinales, pp. 841–842. Paris: Tec & Doc/Lavoisier. [Google Scholar]
- 15. Wichtl M, Anton R, Bernard M (1999) Plantes thérapeutiques: tradition, pratique officinale, science et thérapeutique, pp. 291–293. Paris: Tec & Doc/Lavoisier. [Google Scholar]
- 16. Martinez ML, Labuckas DO, Lamarque AL, Maestri DM (2010) Walnut (Juglans regia L.):genetic resources, chemistry, by‐products. J. Sci. Food Agric. 90, 1959–1967. [DOI] [PubMed] [Google Scholar]
- 17. Pauli GF, Friesen JB, Godecke T, Farnsworth NR, Glodny B (2010) Occurrence of progesterone and related animal steroids in two higher plants. J. Nat. Prod. 73, 338–345. [DOI] [PubMed] [Google Scholar]
- 18. Salimi M, Majd A, Sepahdar Z, Azadmanesh K, Irian S, Ardestaniyan MH et al (2012) Cytotoxicity effects of various Juglans regia (walnut) leaf extracts in human cancer cell lines. Pharm. Biol. 50, 1416–1422. [DOI] [PubMed] [Google Scholar]
- 19. Rashid A, Rather MA, Shah WA, Bhat BA (2013) Chemical composition, antimicrobial, cytotoxic and antioxidant activities of the essential oil of Artemisia indica Wild. Food Chem. 138, 693–700. [DOI] [PubMed] [Google Scholar]
- 20. Murillo JI, Encarnacion‐Dimayuga R, Malmstrom J, Christophersen C, Franzblau SG (2003) Antimycobacterial flavones from Haplopappus sonorensis . Fitoterapia 74, 226–230. [DOI] [PubMed] [Google Scholar]
- 21. Rivero‐Cruz JF, Chai HB, Kardono LB, Setyowati FM, Afriatini JJ, Riswan S et al (2004) Cytotoxic constituents of the twigs and leaves of Aglaia rubiginosa . J. Nat. Prod. 67, 343–347. [DOI] [PubMed] [Google Scholar]
- 22. Yang H, Jeong EJ, Kim J, Sung SH, Kim YC (2011) Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC‐T6 cells. J. Nat. Prod. 74, 751–756. [DOI] [PubMed] [Google Scholar]
- 23. Li J, Xu KP, Zou H, Long HP, Zou ZX, Kuang JW et al (2013) Chemical constituents in green peel of Juglans mandshurica Maxim. Zhongna Yaoxue 11, 1–3. [Google Scholar]
- 24. Machida K, Matsuoka E, Kasahara T, Kikuchi M (2005) Studies on the constituents of Juglans species. I. Structural determination of (4S)‐ and (4R)‐4‐hydroxy‐alpha‐tetralone derivatives from the fruit of Juglans mandshurica MAXIM. var. sieboldiana MAKINO. Chem. Pharm. Bull. 53, 934–937. [DOI] [PubMed] [Google Scholar]
- 25. Lal C, Raja ASM, Pareek PK, Shakyawar DB, Sharma KK, Sharma MC (2011) Juglans nigra: chemical constitution and its application on Pashmina (cashmere) fabric as a dye. J. Nat. Prod. Plant Resour. 1, 13–19. [Google Scholar]
- 26. Bada J, León‐Camacho M, Prieto M, Copovi P, Alonso L (2010) Characterization of walnut oils (Juglans regia L.) from Asturias, Spain. J. Am. Oil Chem. Soc. 87, 1469–1474. [Google Scholar]
- 27. Kim BG, Kim H, Kim JH, Lim Y, Ahn J (2006) Synthesis of ermanin, 5, 7‐dihydroxy‐3, 4′‐dimethoxyflavone from kaempferol, 3, 5, 7, 4′‐tetrahydroxyflavone with two Omethyltransferases expressed in E. coli . Bull. Korean Chem. Soc. 27, 357–358. [Google Scholar]
- 28. Talapatra SKB, Karmacharya SCD, Talapatra B (1998) (‐)‐Regiolone, an [alpha]‐tetralone from Juglans regia: structure, stereochemistry and conformation. Phytochemistry 27, 3929–3932. [Google Scholar]
- 29. Liu J, Meng M, Li C, Huang X, Di D (2008) Simultaneous determination of three diarylheptanoids and an alpha‐tetralone derivative in the green walnut husks (Juglans regia L.) by high‐performance liquid chromatography with photodiode array detector. J. Chromatogr. A 1190, 80–85. [DOI] [PubMed] [Google Scholar]
- 30. Chen C, Hu Y, Sun JX, Pi HF, Zhang P, Ruan HL (2011) Chemical constituents from leaves of Juglans cathayensis . Chin. Trad. Herb. Drugs 42, 2177–2180. [Google Scholar]
- 31. Zhang JB, Liu J, Zha F, Di D (2009) Chemical constituents in green walnut husks of Juglans regia . Zhongcaoyao 40, 847–849. [Google Scholar]
- 32. Wang JL, Zhang SX, Li TJ, Zhang WQ, Wang JJ, Zhang SJ (2008) Chemical constituents from bark of Juglans mandshurica . Zhongcaoyao 39, 490–493. [Google Scholar]
- 33. Wollenweber E, Doerr M (2009) Exudate flavonoids of some Juglandaceae. Nat. Prod. Commun. 4, 211–212. [PubMed] [Google Scholar]
- 34. Yenjai C, Prasanphen K, Daodee S, Wongpanich V, Kittakoop P (2004) Bioactive flavonoids from Kaempferia parviflora . Fitoterapia 75, 89–92. [DOI] [PubMed] [Google Scholar]
- 35. Saleem M (2009) Lupeol, a novel anti‐inflammatory and anti‐cancer dietary triterpene. Cancer Lett. 285, 109–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Amico V, Barresi V, Condorelli D, Spatafora C, Tringali C (2006) Antiproliferative terpenoids from almond hulls (Prunus dulcis): identification and structure‐activity relationships. J. Agric. Food Chem. 54, 810–814. [DOI] [PubMed] [Google Scholar]
- 37. Awad AB, Barta SL, Fink CS, Bradford PG (2008) Beta‐Sitosterol enhances tamoxifen effectiveness on breast cancer cells by affecting ceramide metabolism. Mol. Nutr. Food Res. 52, 419–426. [DOI] [PubMed] [Google Scholar]
- 38. Debatin KM (2004) Apoptosis pathways in cancer and cancer therapy. Cancer Immunol. Immunother. 53, 153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Salvesen GS, Riedl SJ (2008) Caspase mechanisms. Adv. Exp. Med. Biol. 615, 13–23. [DOI] [PubMed] [Google Scholar]
- 40. Broker LE, Kruyt FA, Giaccone G (2005) Cell death independent of caspases: a review. Clin. Cancer Res. 11, 3155–3162. [DOI] [PubMed] [Google Scholar]
- 41. Marimuthu P (2008) Projection of cancer incidence in five cities and cancer mortality in India. Indian J. Cancer 45, 4–7. [DOI] [PubMed] [Google Scholar]
- 42. Ludwiczuk A, Saha A, Kuzuhara T, Asakawa Y (2010) Bioactivity guided isolation of anticancer constituents from leaves of Alnussieboldiana (Betulaceae). Phytomedicine 18, 491–498. [DOI] [PubMed] [Google Scholar]
- 43. Dar MY, Shah WA, Rather MA, Qurishi Y, Hamid A (2011) Chemical composition, in vitro cytotoxic and antioxidant activities of the essential oil and major constituents of Cymbopogon jawarancusa (Kashmir). Food Chem. 129, 1606–1611. [Google Scholar]
- 44. Yang J, Liu RH, Halim L (2009) Antioxidant and antiproliferative activities of common edible nut seeds. Food Sci. Technol. 42, 1–8. [Google Scholar]
- 45. Yanez J, Vicente V, Alcaraz M, Castillo J, Benavente‐Garcia O, Canteras M et al (2004) Cytotoxicity and antiproliferative activities of several phenolic compounds against three melanocytes cell lines: relationship between structure and activity. Nutr. Cancer 49, 191–199. [DOI] [PubMed] [Google Scholar]
- 46. Yang H, Cho HJ, Sim SH, Chung YK, Kim DD, Sung SH et al (2012) Cytotoxic terpenoids from Juglans sinensis leaves and twigs. Biorg. Med. Chem. Lett. 22, 2079–2083. [DOI] [PubMed] [Google Scholar]
- 47. Deschner EE, Ruperto J, Wong G, Newmark HL (1991) Quercetin and rutin as inhibitors of azoxymethanol‐induced colonic neoplasia. Carcinogenesis 12, 1193–1196. [DOI] [PubMed] [Google Scholar]
- 48. Heo MY, Sohn SJ, Au WW (2001) Anti‐genotoxicity of galangin as a cancer chemopreventive agent candidate. Mutat. Res. 488, 135–150. [DOI] [PubMed] [Google Scholar]
- 49. Yang K, Lamprecht SA, Liu Y, Shinozaki H, Fan K, Leung D et al (2000) Chemoprevention studies of the flavonoids quercetin and rutin in normal and azoxymethane‐treated mouse colon. Carcinogenesis 21, 1655–1660. [DOI] [PubMed] [Google Scholar]
- 50. Kuete V, Wabo HK, Eyong KO, Feussi MT, Wiench B, Krusche B et al (2011) Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS One 6, e21762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang T, Chen X, Qu L, Wu J, Cui R, Zhao Y (2004) Chrysin and its phosphate ester inhibit cell proliferation and induce apoptosis in Hela cells. Bioorg. Med. Chem. 12, 6097–6105. [DOI] [PubMed] [Google Scholar]
- 52. Pradelli LA, Bénéteau M, Ricci JE (2010) Mitochondrial control of caspase‐dependent and independent cell death. Cell. Mol. Life Sci. 67, 1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]


