Abstract
Neuroendocrine/Aggressive Variant Prostate Cancers are lethal variants of the disease, with an aggressive clinical course and very short responses to conventional therapy. The age-adjusted incidence rate for this tumor sub-type has steadily increased over the past 20 years in the United States, with no reduction in the associated mortality rate. The molecular networks fueling its emergence and sustenance are still obscure; however, many factors have been associated with the onset and progression of neuroendocrine differentiation in clinically typical adenocarcinomas including loss of androgen-receptor expression and/or signaling, conventional therapy, and dysregulated cytokine function. “Tumor-plasticity” and the ability to dedifferentiate into alternate cell lineages are central to this process. Epithelial-to-mesenchymal (EMT) signaling pathways are major promoters of stem-cell properties in prostate tumor cells. In this review, we examine the contributions of EMT-induced cellular-plasticity and stem-cell signaling pathways to the progression of Neuroendocrine/Aggressive Variant Prostate Cancers in the light of potential therapeutic opportunities.
Keywords: variant prostate cancers, plasticity, cancer stem cells, neuroendocrine trans-differentiation
1. Introduction:
Neuroendocrine prostate cancer (NEPC) is a histological variant of prostate cancer that is associated with an aggressive and lethal clinical course [1, 2]. While primary/de novo NEPC is rare with an incidence of <2%, it has been found upon autopsy in up to 20% of men who have died of castration-resistant prostate cancer (CRPC) [2–7]. In addition, a subset of men with advanced prostate cancers present with atypical clinical features frequently associated with NEPC such as lytic bone disease, exclusive visceral disease, and early castration resistance [2] but without the histological features of NEPC. These have been termed aggressive variant prostate carcinomas (AVPC) [8] or castration-resistant prostate cancer-neuroendocrine type (CRPC-NE) [9]. Both AVPC and CRPC-NE have poor prognosis, respond to platinum-based chemotherapies, and have molecular profiles of histologically defined NEPC [5]. NEPCs and AVPC/CRPC-NE (henceforth jointly referred to in this review as “NEPC”) are thought to account for upto a third of all prostate cancer-related deaths [2–5, 10] (FIG. 1).
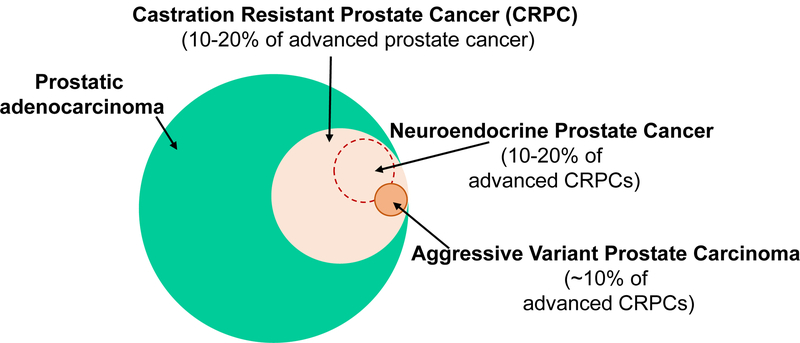
Figure 1. Incidence of AVPCs (Aggressive Variant Prostate Cancers) and NEPCs (Neuroendocrine Prostate Cancers).
Around 10–20% of advanced prostate cancer patients develop CRPCs (castration resistant prostate cancers). Among these, AVPCs are observed in about 10% of CRPC patients, and NEPCs are observed in 10–20%. AVPCs and NEPCs are highly aggressive and may express neuroendocrine markers. These tumors are characterized by clinical androgen indifference, and simultaneous loss of AR (androgen receptor) and PSA (prostate-specific antigen) expression.
There is significant controversy regarding the definition of these variant tumors and their classification. An ideal approach to identifying/studying these tumors should involve an integrated consideration of clinical-, morphological- as well as molecular parameters. We therefore provide here a compilation of widely accepted definition criteria based on all these parameters. The most commonly used pathological (morphological) classification for these tumors stems from the efforts of the Prostate Cancer Foundation working committee of 2013 [10]. This classification scheme includes 6 groups and consists of (1) usual prostate adenocarcinoma, (2) adenocarcinoma with Paneth cell NE differentiation, (3) carcinoid tumor, (4) small cell carcinoma, (5) large cell NE carcinoma and (6) mixed (small or large cell) NE carcinoma-acinar adenocarcinoma. The 2016 World Health Organization classification [11] includes the following categories for neuroendocrine tumors – (1) adenocarcinoma with neuroendocrine differentiation, (2) well-differentiated neuroendocrine tumor, (3) small cell neuroendocrine carcinoma and (4) large cell neuroendocrine carcinoma. Histologically, NEPCs often lack expression of prostate luminal epithelial genes such as those encoding androgen receptor (AR) and its target gene prostate-specific-antigen (PSA), which are expressed in typical AR+ adenocarcinomas. NEPCs are instead characterized by immunoreactivity to clinical neuroendocrine (NE) markers such as chromogranin A, synaptophysin, CD56, and neuron-specific enolase (NSE), although these are not specific. At the molecular level, these tumors are often characterized by RB1 and PTEN losses and TP53 mutations or deficiencies, which have been associated in preclinical studies with androgen independence [8, 12, 13], as well as increased expression/activity of aurora kinase A and N-Myc [6, 14, 15]. Therefore, while most castration-resistant adenocarcinomas (adeno-CRPCs) remain dependent on AR signaling [16], clinical and preclinical data support the androgen-independence of NEPCs [4, 5]. This presents a major therapeutic challenge since androgen signaling inhibition remains the mainstay of prostate cancer treatment. Clinical features of these “anaplastic” prostate carcinomas are also distinct (outlined in [5]), and include CRPC with at least one of the following 7 criteria: (1) histological evidence of small-cell prostate carcinoma (pure or mixed), or (2) exclusively visceral metastasis, or (3) radiographically predominant lytic bone metastasis by plain x-ray or CT scan, or (4) bulky (≥5 cm) lymphadenopathy or bulky (≥5 cm) high-grade (Gleason≥8) tumor mass in prostate/pelvis, or (5) low PSA (≤10 ng/mL) at initial presentation (before ADT or at symptomatic progression in the castrate setting) plus high volume (≥20) bone metastases, or (6) presence of neuroendocrine markers on histology (positive staining of chromogranin A or synaptophysin) or in serum (abnormal high serum levels for chromogranin A or GRP) at initial diagnosis or at progression. Plus any of the following in the absence of other causes: (6A) elevated serum LDH (≥2 X IULN); (6B) malignant hypercalcemia; (6C) elevated serum CEA (≥2 X IULN), or (7) short interval (≤6 months) to androgen-independent progression following the initiation of hormonal therapy. Clinically, although most NEPC and AVPC/CRPC-NE tumors respond to platinum-based chemotherapies, these responses are short-lived and most patients die within 12 to 24 months of diagnosis [2, 5, 17]. There are no targeted treatments available. Much of the “NEPCs” referred to in recent publications is in the context of CRPC, and includes “anaplastic” tumors in patients that present with a wide range of clinical features and variable pathological features. The inherent heterogeneity in these tumors, the lack of understanding of the cellular signaling networks that drive the NEPC and the AVPC/CRPC-NE, and the inconsistency in biomarker expression in these tumors, underscore the urgent need for their further study.
2. Neuroendocrine trans-differentiation in prostate cancer evolution and the origin of NEPC:
As the name suggests, NE cells have functions of both neurons and endocrine cells. In the normal physiological context, NE cells of the prostate are post-mitotic, terminally differentiated AR− cells, and their main role is to maintain tissue homeostasis by providing paracrine signals to neighboring epithelial cells through neuropeptide secretions [18, 19]. In prostate cancer, NE cells can either continue to prevail and influence the proliferation rates of surrounding AR+ adenocarcinoma cells or, in theory, can acquire stem cell properties and potentiate metastatic competence. However, there is no substantial evidence in literature suggesting that NEPC could evolve from preexisting NE cells in the prostate. Clinically, NEPCs are only rarely seen in the absence of prior therapy (de novo), and are most frequently detected during the emergence of treatment resistance (and are hence termed treatment-induced NEPCs or tNEPCs) [2, 20, 21]. This has led to the proposal that the process of dedifferentiation to a neuroendocrine state in prostate cancer cells is an adaptive mechanism for hormonal escape or resistance to androgen-targeting agents [9, 22, 23] (FIG. 2).
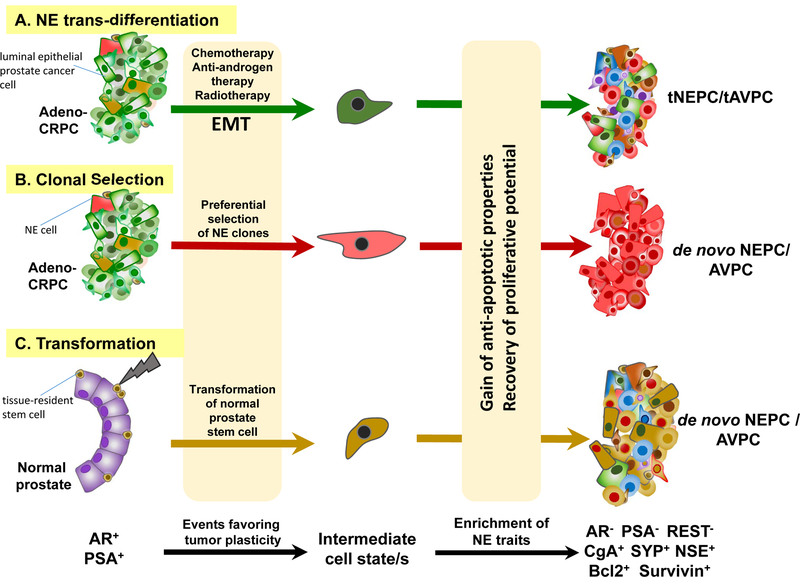
Figure 2. Models explaining potential evolution of AVPCs/NEPCs.
This schematic depicts potential origin of cells that possibly populate various NEPC tumors. The cellular origin of de novo AVPCs/NEPCs is still debated. There is considerable consensus that widespread use of chemotherapy as well as secondary anti-androgen (abiraterone or enzalutamide) therapy of CRPCs results in trans-differentiation of epithelial adenocarcinoma cells into treatment-induced aggressive cancers (tNEPCs/tAVPCs) that lack AR-, PSA- or REST expression, but instead express various neuroendocrine markers (CgA, SYP, or NSE) as well as anti-apoptotic gene products such as Bcl2 and Survivin. Several recent mechanistic studies have shown that the EMT program plays a critical role in bestowing tumor cells with “plasticity” required to successfully adopt alternate states such as the neuroendocrine phenotype. Prostate cancer cells may transition through multiple intermediary cell states (of varying degrees of stemness/differentiation) during neuroendocrine tumor evolution. The luminal epithelial AR+ adenocarcinoma (AR+/PSA+/REST+) and the neuroendocrine AR− variants (AR−/PSA−/REST−/CgA+/SYP+/NSE+/Bcl2+/Survivin+) represent the two ends of this continuum. While distinct molecular markers are often associated with the two extreme cell states, it is important to note that not all of these markers are simultaneously expressed/absent in every case. Clonal selection within adenocarcinomas may also preferentially enrich for neuroendocrine stem-cell traits. There is lesser evidence that these aggressive variants might potentially arise via transformation of normal adult prostate tissue stem cells. The role of radiotherapy in this context is also not substantiated in the clinic. The colors depicted in the NEPC tumors in this schematic (on the right) are meant to reflect on potential origin of cells responsible for populating the ultimate NEPC tumor bulk. Green represents adeno-CRPC cells; Red represents NE cells; Brown represents tissue-resident stem-cells. AR, androgen receptor; PSA, prostate-specific antigen; REST, Repressor Element-1 Silencing Transcription factor; adeno-CRPC, castration-resistant adenocarcinomas; AVPC, aggressive variant prostate carcinoma; NE, neuroendocrine; NEPC, neuroendocrine prostate cancer; CRPC, castration-resistant prostate cancer; EMT, epithelial-mesenchymal transition; NSE, neuron-specific enolase; CgA, chromogranin A; SYP, synaptophysin.
The cellular precursor of de novo NEPC is yet uncertain. However, autopsies investigating classic adeno-CRPC tumors from patients treated with androgen-deprivation therapy (ADT) revealed the co-existence of NEPC in up to 20% of patients, suggesting a common clonal origin for NEPC from adeno-CRPC that is induced in response to first-line treatment [5, 24]. This hypothesis is supported by genetic analyses of human prostate tumors that exhibit concurrent adeno-CRPC and NEPC demonstrating that TP53 mutations and the classic prostate-specific TMPRSS2-ERG genetic rearrangements are equally shared by both subtypes of tumors [25, 26] and by more recent evidence demonstrating that adeno-CRPC and NEPC can indeed be functionally derived from a common epithelial precursor [14]. A comprehensive study evaluating whole exome sequencing data of 114 metastatic and normal biopsy pairs from patients revealed significant genetic overlap between these tumor subtypes and provided evidence for divergent clonal evolution of the NEPC phenotype that can be traced back to an adeno-CRPC precursor [9].
Thus, currently available data indicate that NEPC may indeed involve true trans-differentiation of adeno-prostate cancer cells into the NEPC phenotype, rather than selection and preferential expansion of a few rare NE cells already existing in the prostate. The former may involve intermediate step/s prior to attaining the NE phenotype. The prevalence of an NE state (even among advanced adeno-CRPC) has been proposed to reflect an altered biochemistry adopted by AR+ luminal prostate cancer cells in order to fuel growth and survival due to the following evidence: (i) NE cells express increased levels of anti-apoptotic gene products such as Bcl-2 [27] and survivin [28] and (ii) NE secretory products stimulate growth of neighboring non-NE cells [19, 22, 29], thereby contributing to both tumor progression as well as therapy resistance. A recent study quantifying NE marker expression in circulating tumor cells isolated from serial samples of patients treated with abiraterone acetate or enzalutamide demonstrated a direct correlation between percent synaptophysin expression on these cells and emergence of drug resistance [30].
3. Role of tumor plasticity in neuroendocrine trans-differentiation and NEPC:
Evidence indicates that there may be a spectrum of cellular differentiation states as prostate tumors progress from AR+ adenocarcinomas to AR− therapy-resistant NEPCs. The epithelial luminal AR+ state and the AR− state with expression of neural progenitor markers represent the two ends of this cellular differentiation continuum. The existence of “transit amplifying cell populations” with intermediate phenotypes co-expressing markers of both AR+ and AR− cells, confirms the possibility of inter-conversion of one cell type to the other, in response to specific signals [31]. This capacity to alternate between distinct cell states is referred to as ‘cellular plasticity’.
Recent studies by Aggarwal et al. further attest to the possible existence of intermediate states as prostate tumors transition from AR+ adenocarcinomas to AR− NEPCs [32]. The newly proposed “Intermediate Atypical Adenocarcinomas” are a morphologically distinct set of small-cell NEPCs that retain AR expression and activity similar to adeno-CRPCs but are associated with shortened survival. Though at this point in time, there aren’t enough studies yet to corroborate this distinct intermediate state, if substantiated, these NEPC cells may indeed represent the pathological counterpart of at least a subset of the clinically defined AVPCs that retain AR expression and yet exhibit an aggressive clinical course. Attainment of the NE phenotype is presumably dependent on an extensive network of transcriptional reprogramming events that provide for the expression and maintenance of neuronal features.
3A. Down-regulation of AR and/or AR-mediated signaling can induce NE trans-differentiation:
In LNCaP cells, a widely used androgen-responsive cell line model of human prostate cancer, silencing of AR using small interfering RNA is sufficient to induce an NE phenotype with the outgrowth of neuron-like processes [33]. This suggests that AR actively represses an intrinsic NE trans-differentiation program in androgen-responsive prostate cancer cells. In vivo studies also support a protective role for AR in NE plasticity. Castration of mice bearing AR-dependent CWR22 or LNCaP xenografts significantly increased the quantity of cells positive for NE markers within the tumor [34]. Further, adenocarcinoma cells derived from primary patient tumor tissue implanted under the renal capsule of castrated mice exhibited profound NE differentiation, suggesting that NE plasticity is favored by an adaptive response to the loss of androgens [23]. These findings suggest that the widely used androgen ablation therapy may promote NEPCs by inducing plasticity in tumor cells.
Several different AR+/PSA+ prostate cancer cell lines can be reprogrammed to intermediary “metastable” states with cancer stem cell (CSC) properties (exemplified by NE differentiation markers, an invasive phenotype, and robust tumor-initiating potential) upon anti-androgen treatment [35, 36]. These states, however, are transient and can be reversed to AR+ states by stopping the treatment. A gene signature derived from such reprogrammed prostate cancer cells was able to distinguish tumors from patients with lethal prostate cancer from less aggressive tumors [35], further arguing that tumor cell plasticity acquired during NE trans-differentiation is a major blockade for the clinical management of this disease. Interestingly, in some cases reprogramming of cells back to an AR+ state by the withdrawal of treatment did not restore sensitivity to ADT [35]. Many independent studies (below) have confirmed this concept of “acquired resistance”.
One key distinguishing factor between the two extreme states in the differentiation spectrum of prostate cancer cells (i.e., AR+ adeno-CRPC vs. AR− NEPC) is their epigenetic profile. The histone deacetylase EZH2 is highly expressed in NEPC, and its increased expression in clinically localized prostate tumors is associated with poorer prognosis [6, 37, 38]. In PC3, prostate cancer cells with high metastatic potential and neuroendocrine properties, as well as in LNCaP cells, which have lower metastatic potential, EZH2 was found to be upregulated selectively within the CD44+/CD133+ population [39], and inducing its degradation using DZNep (both a S-adenosyl-homocysteine synthesis inhibitor, and a histone methyl-transferase EZH2 inhibitor), significantly diminished the number of tumor-initiating cells, as well as attenuated the growth of AR-/low DU145 cells [40]. Studies using patient-derived xenografts revealed that in NEPC, the AR promoter is enriched in silencing histone modifications such as H3K27me3 [41]. Again in this context, EZH2 inhibition resulted in the restoration of AR expression and growth inhibition in the AR− NEPC cells. Accumulation of these epigenetic alterations by AR+ adenocarcinoma cells over time may, therefore, facilitate cellular plasticity seen during the trans-differentiation process.
As discussed above, NEPC predominantly occurs and is clinically recognized in the context of CRPC. While approaches exist (albeit with short-term efficacy) to antagonize androgen biosynthesis or even nuclear translocation of full length AR in prostate cancer cells, the contribution of AR-V7 (the truncated splice variant of AR that remains unaffected by enzalutamide/abiraterone) to therapy-resistant disease progression cannot be minimized [42]. Targeted inhibition of the AR-V7 has remained a challenge. However, since resistance to ADT has been integrally associated with epigenetic modifications and aberrant tyrosine kinase signaling, development of suitable epigenetic inhibitors likely holds promise for the future in this context. Recent reports have demonstrated the contribution of the non-receptor tyrosine kinase ACK1 in the epigenetic activation of the AR gene locus [43–45]. Targeting ACK1 using an epigenetic inhibitor not only reduced full-length AR levels, but also reduced AR-V7 levels to overcome stemness-associated enzalutamide-resistance, and curb castration-resistant prostate tumor growth [43, 44].
AR-dependent control of NE plasticity is further witnessed in its ability to directly influence the neural differentiation program. The neural transcription factor Brn2 was identified as an AR-suppressed driver of NEPC proliferation both in vitro and in vivo [46]. In the context of enzalutamide treatment, Brn2 suppression by AR is lost, and this drives NE differentiation. In patients, high Brn2 expression is a characteristic of NEPC and is correlated with low serum PSA levels [46]. Interestingly, AR has also been shown to interact directly with a related neuronal transcription factor Brn3a (a player known for its anti-apoptotic functions) to promote the expression of the Nav1.7 voltage-gated sodium channel SCN9A, which is correlated with tumor progression [47–49]. However, AR directly repressed the expression of the NEPC self-renewal marker Sox2, and inhibiting AR activity via enzalutamide treatment robustly upregulated Sox2 expression in multiple prostate cancer cell lines [50]. The absence of circulating gonadal androgens is also known to induce the expression of adrenomedullin in epithelial LNCaP cells, which, in turn, potentiates neuroendocrine differentiation and associated neuritic outgrowths with increased serum NSE levels [51].
Gradual acquisition of a neuroendocrine phenotype has been proposed as a counter-response of prostate cancer cells to radiotherapy. LNCaP cells undergo massive NE differentiation following fractionated ionizing radiation treatment, with significant increases in NSE and chromogranin A levels, as well as resistance to androgen depletion-induced growth inhibition [52]. Although early clinical investigations indicated that chromogranin A levels are increased in the serum or tumors of patients following radiotherapy [53, 54], the role of radiotherapy in NEPC remains to be substantiated in the clinic.
Collectively, all these studies point to a reciprocal relationship between the normal AR signaling axis and the acquisition of NE plasticity that facilitates chemotherapy or radiotherapy resistance. Importantly, many of these reports also suggest that NEPC pathophysiology is reversible and highlight the need to identify agents that can undo AR silencing while simultaneously countering therapy resistance. It should be stressed that NE markers themselves are unlikely to be significant drivers of disease progression. Rather, they appear to reflect underlying cellular processes like the epithelial-mesenchymal-transition (EMT) and are likely downstream of neural and stem cell markers that drive disease progression.
3B. EMT-induced plasticity promotes neuroendocrine trans-differentiation and NEPC:
The term EMT is commonly used to refer to a broad set of cell biological alterations that dictate the conversion of cells from an epithelial state to a mesenchymal state. In the epithelial state, cells are immobile and non-invasive, performing specialized tissue functions, and can be biochemically defined by the expression of markers such as E-cadherin or microRNA miR200. When epithelial cells convert to the mesenchymal state, they gain migratory and invasive properties. Mesenchymal cells are characterized by the expression of markers such as N-cadherin, vimentin, or fibronectin. EMT plays a fundamental role during normal mammalian development as primitive embryonic cells undergo dramatic changes over time to define the three germ layers. Not surprisingly, EMT also plays a decisive role in promoting migration of cancer cells to distant sites within the body [55]. The EMT program equips cancer cells with stem cell properties [56] necessary to survive the harsh environments at primary and secondary tumor sites as well as in circulation. Following EMT, epithelial cells also attain the capability of trans-differentiating into other lineages such as adipocytes, astrocytes, and chondrocytes [57].
Recently, a critical “master-switch” capable of mediating the core EMT program [58] was identified to induce NE differentiation in epithelial prostate cancer cells [59]. A growing mass of evidence suggests that untoward activation of EMT pathways may facilitate the development of prostate cancer and hasten progression to the advanced therapy-resistant state [59–64]. Many studies also support the view that ADT or inhibition of AR signaling can actually promote the onset of EMT [65, 66]. Arguably, the ability of tumor cells to transition from the epithelial to the mesenchymal state equips them with the ability to mount an adaptive response to a variety of signals – one manifestation of this phenomenon is drug resistance.
At the cellular level, EMT in prostate cancer cells is accomplished by a myriad of transcription factors that each contribute to an interrelated signaling network that ultimately charges the cell with plasticity. Well-recognized EMT inducers in prostate tumorigenesis include Twist, Snail, Slug, and E-cadherin repressors (such as Zeb1/2), which are also known to play roles in regulating NE differentiation. Overexpression of Snail in epithelial LNCaP cells results in robust induction of EMT with simultaneous expression of markers of terminal NE differentiation [59, 67]. LNCaP C-33 cells, which bear a neuroendocrine phenotype, endogenously express high levels of Snail. Further, inhibition of Snail expression in neuroendocrine-like PC3 cells abolishes NE differentiation attributes and also significantly down-regulates the pluripotency factor Sox2 [68]. Similarly, loss of Slug (a related zinc finger transcription factor) is sufficient to block EMT in PC3 cells [69].
The T-box EMT factor Brachyury (an independent biomarker of poor prognosis in prostate cancer patients) has also been shown to promote neuroendocrine differentiation in conjunction with resistance to cytotoxic drugs [70]. Interestingly, EMT has also been implicated downstream of the TMPRSS2-ERG gene fusion event, which is by far the most commonly observed chromosomal rearrangement in prostate tumors [71, 72]. In this event, the androgen-responsive TMPRSS2 promoter is juxtaposed with the erythroblast transformation-specific transcription factor ERG. Although data from genomic studies suggest that ERG-positive cancers are luminal, this molecular rearrangement is observed in approximately 50% of NEPCs [73]. TMPRSS2-ERG-overexpressing prostate cells undergo robust EMT, with upregulation of transcription factors Zeb 1 and Zeb2. Leshem et al. demonstrated that TMPRSS2-ERG directly binds to the promoter of Zeb1, as well as promoters of the Zeb2 modulators IL1R2 and SPINT1 [71].
Another recognized feature in the process of NE differentiation of prostate tumor cells is the down-regulation of REST (Repressor Element-1 Silencing Transcription factor) [74], an event that also promotes EMT. REST is a transcriptional repressor for neuron-specific genes and is often referred to as the master negative regulator of neurogenesis. Reduction in REST expression has been causally associated with NE differentiation of prostate tumor cells [75, 76], is observed in tissue from relapsed prostate cancer patients, and has been shown to predict early recurrence of the disease [74]. Also notably, long-term loss in REST expression has been shown to facilitate EMT and stemness properties when prostate cancer cells gain NE plasticity (with enhanced expression of Twist and CD44) [77]. As a corollary, enforced expression of REST in hormone-refractory CWR22Rv1 prostate cancer cells transcriptionally repressed Twist1 and CD44 expression with consequent reduction in cell migration and sphere formation potential.
4. The relevance of “stemness” in NEPC initiation and progression:
Normal tissue stem cells have long been viewed as “targets for transformation” in the origin/expansion of many solid tumors including the prostate [78, 79]. The prostate epithelium is composed of at least three different types of cell populations that could potentially foster normal tissue stem cells – luminal, basal, and neuroendocrine. Luminal (secretory) cells typically express high AR and PSA and low molecular weight keratins (K8 and K18), whereas basal cells have undetectable AR and PSA and are rich in high molecular weight keratins (K5 and K14) as well as in p63. NE cells are a rare subpopulation named for their morphology (neural-like outgrowths) and their secretions (neuropeptides). They are defined immunohistochemically by the presence of cytoplasmic markers (chromogranin A, synaptophysin, or NSE), and show dense granules at the ultrastructural level. The luminal and basal cells of the prostate derive from the urogenital sinus (UGS), whereas NE cells are believed to have a dual origin including the UGS and the neural crest [80].
Although early experiments alluded to the existence of a “stem cell-like” population within the normal prostate with marked capacity for both self-renewal and differentiation [81, 82], their origin and tissue-residence is a highly debated topic. It is unclear which cell type supports the continued existence and function of normal tissue-resident stem cells, and it is also unclear which cell type spurs tumorigenesis in the prostate. This is obscured by the lack of clear-cut markers, and also because of the existence of intermediate states as cells transition over time to a more pathological phenotype. The selective endurance of basal cells to androgen-ablation has favored the view that prostate stem cells subsist in the basal layer of the prostate [81, 83]. In further support of this, K5/K14/p63-expressing multipotent basal progenitors were found to give rise to basal, luminal, and NE lineages of the prostate [84, 85]. Additionally, Sca-1-expressing basal cells have robust self-renewal capacity as well as prostate-regeneration potential [86, 87]. Markers such as Trop2, CD166, and CD49f further enrich for the stem cell activity of Sca-1+ cells [86, 88]. Slow-cycling basal cells displaying the antigenic profile of Lin−Sca-1+CD44+CD133+CD117+ successfully regenerate prostatic tissue, further supporting the hypothesis of the basal origin of the prostate tissue stem cell [89]. However, long-term label-retaining experiments suggest that stem cells may originate from either the basal or luminal layer of the prostate [90]. In a more recent study using a lineage-tracing approach, a rare luminal population of castration-resistant cells expressing NKX3.1 (a member of the NK family of homeobox genes) were shown to contribute to the expansion of multiple lineages of prostate cells [91]. Also, single luminal epithelial cells expressing NKX3.1 robustly generate prostate organoids exhibiting functional AR signaling and normal tissue architecture containing both luminal and basal cells [92]. Luminal cell-derived organoids more closely resemble prostate glands than do organoids derived from basal cells [93].
The role of NE prostate cells as normal tissue-resident stem cells is largely speculative. Some early studies performed in transgenic mouse models, which employed regulatory elements from the cryptdin-2 gene to express SV40 large T antigen in prostatic NE cells, indicate that transformation of normal prostatic NE cells can possibly contribute to NEPC tumorigenesis [94], however, this view is yet to be substantiated in the clinic. Instead, overwhelming evidence supports the alternate model of trans-differentiation of classical luminal adenocarcinomas to the NEPC phenotype.
5. Stem cell signaling pathways fuel tumor plasticity in NEPC:
Conservatively, a prostate tumor cell can be considered a cancer stem cell if it is capable of both initiating a tumor and generating the cellular heterogeneity for long-term sustenance of the tumor. Absolute demonstration of such a population of cells remains a challenge in patients with NEPCs. However, prostate cancer cells with stem-cell properties have long been implicated in tumor initiation and/or therapy resistance. Many of the commonly accepted “stem cell” markers employed in analysis of other cancers have also been deemed to identify CSCs in NEPC. For example, Sox2 has been proposed as a critical prerequisite to sustain the replicative potential of prostate CSCs [95] and to foster metastasis [96]. In 2009, Huang and co-workers reported the selective association of CD44 expression with NE cells of human prostate tumors [97]. In fact, while 100% (n=11) of the prostatic small-cell NE carcinomas examined exhibited CD44 expression, only a minority of NE carcinomas of other organs (from a total n of 47) shared a similar profile [98]. CD44+ PSA-/low cells were also shown to be tumor-initiating and castration-resistant, to be capable of giving rise to CD44− cells, and to express NE markers [59, 99], collectively suggesting that CD44 can uniquely index human NEPCs as a stem cell marker. The pluripotency factor Oct4 has also been demonstrated to mark cells that express chromogranin A in human NEPC [100].
Studies from several labs have shown that epithelial tumor cells that have passaged through EMT gain potent stem cell properties including metastatic competence and drug-resistance [56, 59, 101–104]. The Forkhead transcription factor FOXC2 was recently proposed as a unique identifier of the stem cell phenotype in NEPC, with acquisition of FOXC2 by AR+ prostate cancer cells correlating with NE trans-differentiation as well as with resistance to enzalutamide (ADT) and docetaxel [59]. Loss of FOXC2 function appears to be sufficient to bring about a phenotypic conversion from NE to luminal epithelial with concomitant restoration of AR and PSA expression as well as sensitivity to ADT both in vitro and in vivo [59].
Many stem cell-associated signaling cascades are reported to facilitate acquisition of “NE status” in prostate tumor cells (FIG. 3). Cultured androgen-dependent LNCaP cells, as well as the highly metastatic androgen-independent PC3-M cells gain NE characteristics in vitro in response to treatment with physiological and pharmacological agents that elevate intracellular cAMP (phosphodiesterase inhibitors, epinephrine, isoproterenol, forskolin, and dibutyryl cAMP), cytokines (IL6), or growth factors (EGF, HB-EGF) [29, 105, 106]. LNCaP tumors growing in castrated male nude mice have significantly increased numbers of chromogranin A+ NE cells, supporting the hypothesis that some of these signaling factors may indeed be functional in the tumor microenvironment [107]. Similar results were obtained with C4–2 cells, which are representative of later stages of the disease [29], suggesting that NE differentiation can occur at multiple stages of prostate tumor progression. Interestingly, withdrawal of these inducing agents rapidly resulted in loss of the NE phenotype as well as mitogenic activity, suggesting that NEPC can be arrested or even reversed.
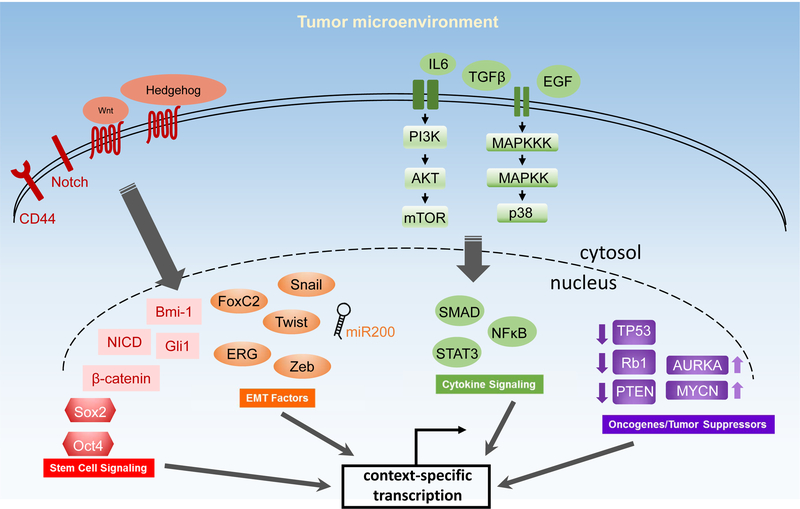
Figure 3. Signaling pathways and molecular players implicated in AVPCs/NEPCs.
Attainment of the aggressive variant/neuroendocrine phenotype is presumably dependent on an extensive network of transcriptional reprogramming events that provide for the expression and maintenance of neuronal/stemness features. Various stem cell self-renewal pathways and molecules such as Wnt, Notch, Hedgehog, Sox2, Oct4, and CD44 have been shown to promote generation of AVPC/NEPC cells. Inhibition of miR200 and increase in EMT-inducing transcriptional factors (e.g., FOXC2, Snail, Twist, Zeb) induces an NEPC-like phenotype in prostate cancer cells. Multiple autocrine and paracrine signaling pathways (such as IL6, EGF, TGFβ, PI3K/AKT/mTOR, and p38 MAPK) facilitate induction and maintenance of the neuroendocrine phenotype. Genetic alterations including genomic amplifications of N-Myc and aurora kinase A, genomic loss of RB1 and PTEN, as well as genomic loss or point mutations of TP53 are also associated with the generation of these aggressive variants. AVPC, aggressive variant prostate carcinoma; NEPC, neuroendocrine prostate cancer.
The human male-specific proto-cadherin PCDH-PC is another factor reported to down-regulate the ligand-dependent activity of AR [108] and stimulate NE trans-differentiation by activating Wnt signaling [109]. Wnt has been directly implicated in facilitating NE differentiation and increased survival of prostate tumor cells both in vitro [110] and in vivo [111]. Overexpression of PCDH-PC drives NE differentiation and resistance to docetaxel. In direct concord, inhibition of PCDH-PC expression in NE cells resensitizes them to this drug [108]. PCDH-PC expression also correlates with NE features in human prostate cancer tissue [108]. Interestingly, one of the proposed downstream effects of the TMPRSS2-ERG chromosomal rearrangement in prostate cancer cells is activation of the Wnt signaling cascade with LEF1 as a critical mediator of its functional effects related to EMT [112].
In addition to androgen deprivation, which results in obvious alterations in normal AR function, interleukin-6 (IL6) is viewed as a critical regulator of NE plasticity of prostate cancer cells. IL6 is a pleiotropic inflammatory cytokine capable of directing multiple cellular responses to stress. Clinical studies have shown that the serum IL6 levels (as well as levels of TGFβ1, a physiological inducer of EMT) are significantly elevated in patients with aggressive, metastatic prostate cancer and are correlate with tumor burden [113]. IL6 actions in prostate tumor cells are mediated via downstream PI3K/AKT, JAK/STAT, or MEK/ERK/MAPK pathways [114]. Treatment of LNCaP cells with IL6 induces PI3K signaling and NE differentiation, whereas addition of androgens blocks this transition. IL6-induced NE differentiation of LNCaP cells involves REST-mediated up-regulation of activated AMPK and a marked down-regulation of phosphorylated mTOR [115].
Functional loss of the tumor suppressors RB1 and TP53 also promotes the transition of AR-dependent luminal epithelial cells to AR-independent basal-like stem cells and confers resistance to anti-androgen therapy [38]. The combined deficiency of both RB1 and TP53 synergistically drives the trans-differentiation to the NE phenotype and the resistance to AR antagonists in prostate cancer. This effect is, at least in part, mediated by the reprogramming factor Sox2. The mitotic deregulation observed in AR− NEPC models is associated with loss of RB1 [116]. In corroboration, a recent study using patient NEPC tissues observed that RB1 loss is indeed a common event and a critical regulator in the development of this disease [117].
The p38 MAPK pathway promotes NE trans-differentiation in prostate cancer cells via up-regulation of the FOXC2-mediated EMT signaling cascade [59]. FOXC2 is a direct target of p38 MAPK [59, 118]. Phosphorylation of this central EMT regulator by p38 MAPK enhances its protein stability and facilitates gain of NE features with concomitant loss of luminal epithelial traits (including AR and PSA expression). FOXC2-mediated NE trans-differentiation is also accompanied by a marked increase in resistance to enzalutamide and docetaxel [59]. Notably, systemic inhibition of p38 MAPK signaling in mice bearing neuroendocrine DU145 xenografts restores AR expression in these tumors as well as sensitivity to enzalutamide and also results in a significant loss in circulating tumor cell count [59]. These data further attest to the reversibility potential of NEPCs.
The oncogenic transcription factor N-Myc and its stabilizing cell cycle-regulatory kinase, aurora kinase A (which are both highly expressed in NEPC) were also shown to be sufficient to drive an AR− NE phenotype in preclinical studies [6, 14]. Of the three most important members of the Myc family implicated in the genesis of multiple tumor types (C-Myc, N-Myc and L-Myc), N-Myc is neural-specific and important for expression of neuroendocrine markers [6]. Inhibition of aurora kinase A (which can induce EMT in other cancer types) using PHA-739358 effectively curbed the growth of NE tumor cells both in vitro and in vivo. In a unique tissue regeneration model of prostate cancer developed from human patient epithelial cells, enforced expression of N-Myc and the activated form of AKT1 (also frequently altered in prostate cancers ) was shown to result in robust induction of NEPC [14]. N-Myc-driven transcriptional reprogramming of prostate tumor cells toward NEPC likely involves EZH2 function [119]. It appears that unregulated expression of N-Myc coupled with either loss of PTEN or gain of AKT1 function results in down-regulation of AR expression and/or activity and development of NE features. These studies have driven the assessment of aurora kinase inhibitors in clinical trials for NEPC [120].
It is clear that a myriad of commonly employed pleiotropic cellular signaling pathways fuel NEPC traits in prostate cancer cells. It is hence not surprising that the phenomenon of neuroendocrine plasticity operates in a variety of tumors (including tumors of the nervous and hematologic systems and neuroendocrine tumors in other organs) [121–123], which may form an instructive basis for exploring potential common features and drivers.
6. Role of preclinical models in the study of NEPC plasticity:
As described above, current studies support the model that the NEPC clinical phenotype develops either through evolution of adeno-CRPC due to increased plasticity of epithelial tumor cells and/or through loss of AR expression resulting in resistance to anti-androgen therapy. However, the NEPC/AVPC clinical phenotype is heterogeneous both with respect to histology and genomic alterations. This heterogeneity presents a unique challenge to researchers seeking to recapitulate the clinical phenotype. Preclinical studies have demonstrated that combined loss of tumor suppressors TP53, RB1, and/or PTEN in luminal epithelial cells of the prostate leads to NEPC phenotype in mouse models with tumor cell lineage plasticity and loss of AR/luminal epithelial markers as observed in NEPC and AVPC human tumors selected by clinical criteria [8, 38, 124–126]. These mouse models can therefore be used to dissect underlying mechanisms and to test for reversal of the AR− phenotype. Interestingly, these mice have elevated expression of the pluripotency marker Sox2, indicating the potential association of the AR− phenotype with cellular pluripotency [12].
Defining the role of EMT signaling in these mouse models will provide key mechanistic insights linking tumor suppressor loss with tumor cell plasticity. Although loss of tumor suppressors is highly associated with shorter duration of response to androgen therapy and development of the NEPC phenotype, other genetic alterations such as amplification of N-Myc and the gene encoding aurora kinase A are also associated with a subset of NEPCs. Gene expression analyses of tumors from mouse models with overexpression of N-Myc in epithelial cells of the prostate indicate that activation of EMT signature genes is highly associated with development of the NEPC tumor type [119]. Notably in preclinical models, prostate cancer induced by either loss of tumor suppressors or overexpression of N-Myc demonstrate that alterations in the luminal epithelial cells of the prostate can indeed result in the development of an NEPC phenotype, thereby uniformly supporting a minimal model in which trans-differentiation of luminal epithelial cells bestows NEPC plasticity in the prostate gland.
Patient tumor-derived xenograft (PDX) models such as those described by Tzelepi et al. [116] and Aparicio et al. [127] with supporting clinical annotation serve as extremely important complementary tools for examining the reversal of the AR− phenotype in response to various drugs. Although these models are limited in their capability for the study of metastatic behavior of NEPC cells, the analyses of such PDX models allows us to address the true clinical heterogeneity exemplified in the NEPC phenotype, which is not possible in various mouse models bearing defined genetic alterations such as loss of tumor suppressors or overexpression of N-Myc. An understanding of both mouse genetics and PDX model cellular and genetic phenotypes will be critical for delineating signaling pathways linking AR re-expression to restored anti-androgen therapy response in the clinical NEPC/AVPC setting.
7. Conclusions and perspectives:
NEPC is a critical clinical challenge, particularly relevant with the increasing- and widespread use of next generation AR therapy such as abiraterone and enzalutamide. One feature of non-AR driven NEPC is the expression of NE markers. However, NE differentiation (indicated by the positive scoring of clinical NE markers in typical prostate adenocarcinomas by immunohistochemistry) often appears during the castration-resistant progression of the disease. Although in vitro studies suggested that NE markers have tumor-promoting features, their expression has also been linked to deviant up-regulation of neural and stem cell markers that may indeed be the fundamental drivers of tumor aggressiveness and drug resistance in NEPC. It is, however, important to consider that beyond NEPC, the driving EMT/stem cell-signaling mechanisms promoting tumor plasticity could be implicated in the broader AVPC/androgen-indifferent subset of cells, thus offering opportunities for therapy. Combined alterations in the TP53, RB1, and PTEN marker profile are consistently associated with AVPC and androgen independence. While this indication indeed serves as a potential diagnostic, the signature by itself does not provide for a directly actionable target. The discovery and validation of an underlying core EMT/stem cell signaling pathway, however, could result in an actionable biology that can be translated into therapy. It remains to be determined if combined EZH2/ACK1 with p38 MAPK inhibition will boost the degree or duration of response to platinum-based therapies in this context. Understanding the functional roles of EMT and stem cell signaling in restoring the luminal epithelial program in neuroendocrine prostate cancer cells should expedite the development of biomarkers for prognosis, as well as clinical therapies for these lethal tumors.
Highlights/Key Points.
NEPCs/AVPCs are lethal variants with short responses to therapy, accounting for upto a third of all prostate cancer deaths
These variant tumors often lack expression of AR, and are hence resistant to mainstay androgen-deprivation therapy
Tumor plasticity potentiates neuroendocrine differentiation during cancer evolution, facilitating their origin and/or sustenance
EMT/stem-cell pathways promote cellular plasticity in prostate tumor cells, fueling their therapy-resistance and metastatic competence
Testing and validation of inhibitors of EMT and/or stem-cell pathways may provide targeted-therapy options for this class of patients
Acknowledgements
We thank Drs. Christopher Logothetis, Sue-Hwa Lin, Nupam Mahajan, and Kiran Mahajan for their comments and critiques. This study was supported by P50 CA140388 from the National Cancer Institute [University of Texas MD Anderson Prostate Cancer SPORE Career Enhancement Program Award (RS, SAM)]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institute of Health. We also thank the University of Texas MD Anderson Prostate Cancer Moon Shot Program (AA, SM), Prostate Cancer Foundation Award #17CHAL01 (AA, SM), NIH/NCI-R01CA200970 (SAM), and National Science Foundation-15–597-1605817 (SAM) for financial assistance. We thank Dr. Esmeralda Ramirez-Pena for artwork in figures. We thank Dr. Jacqueline Wyatt for editorial assistance.
Abbreviations:
- ADT
androgen-deprivation therapy
- AR
androgen receptor
- AVPC
aggressive variant prostate carcinoma
- adeno-CRPC
castration-resistant adenocarcinomas
- CRPC
castration-resistant prostate cancer
- CRPC-NE
castration-resistant prostate cancer-neuroendocrine type
- CSC
cancer stem cell
- EMT
epithelial-mesenchymal transition
- NE
neuroendocrine
- NEPC
neuroendocrine prostate cancer
- NSE
neuron-specific enolase
- PDX
patient tumor-derived xenograft
- PSA
prostate-specific antigen
- tNEPC
treatment-induced NEPC
Footnotes
Competing Interests Statement
The authors declare no competing interests.
References
- [1].Alanee S, Moore A, Nutt M, Holland B, Dynda D, El-Zawahry A, McVary KT, Contemporary Incidence and Mortality Rates of Neuroendocrine Prostate Cancer, Anticancer Res, 35 (2015) 4145–4150. [PubMed] [Google Scholar]
- [2].Aggarwal R, Zhang T, Small EJ, Armstrong AJ, Neuroendocrine prostate cancer: subtypes, biology, and clinical outcomes, Journal of the National Comprehensive Cancer Network : JNCCN, 12 (2014) 719–726. [DOI] [PubMed] [Google Scholar]
- [3].Hirano D, Okada Y, Minei S, Takimoto Y, Nemoto N, Neuroendocrine differentiation in hormone refractory prostate cancer following androgen deprivation therapy, European urology, 45 (2004) 586–592; discussion 592. [DOI] [PubMed] [Google Scholar]
- [4].Beltran H, Tomlins S, Aparicio A, Arora V, Rickman D, Ayala G, Huang J, True L, Gleave ME, Soule H, Logothetis C, Rubin MA, Aggressive variants of castration-resistant prostate cancer, Clin Cancer Res, 20 (2014) 2846–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, Pagliaro LC, Kim J, Millikan RE, Ryan C, Tannir NM, Zurita AJ, Mathew P, Arap W, Troncoso P, Thall PF, Logothetis CJ, Platinum-based chemotherapy for variant castrate-resistant prostate cancer, Clin Cancer Res, 19 (2013) 3621–3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Wang Y, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA, Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets, Cancer Discov, 1 (2011) 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Aparicio A, Logothetis CJ, Maity SN, Understanding the lethal variant of prostate cancer: power of examining extremes, Cancer Discov, 1 (2011) 466–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, Wu G, Wang X, Troncoso P, Corn P, Thompson TC, Broom B, Baggerly K, Maity SN, Logothetis CJ, Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers, Clin Cancer Res, 22 (2016) 1520–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F, Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer, Nat Med, 22 (2016) 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA, Proposed morphologic classification of prostate cancer with neuroendocrine differentiation, The American journal of surgical pathology, 38 (2014) 756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Humphrey PA, Moch H, Cubilla AL, Ulbright TM, Reuter VE, The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs-Part B: Prostate and Bladder Tumours, European urology, 70 (2016) 106–119. [DOI] [PubMed] [Google Scholar]
- [12].Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, Wongvipat J, Ku SY, Gao D, Cao Z, Shah N, Adams EJ, Abida W, Watson PA, Prandi D, Huang CH, de Stanchina E, Lowe SW, Ellis L, Beltran H, Rubin MA, Goodrich DW, Demichelis F, Sawyers CL, SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer, Science, 355 (2017) 84–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Beltran H, Yelensky R, Frampton GM, Park K, Downing SR, MacDonald TY, Jarosz M, Lipson D, Tagawa ST, Nanus DM, Stephens PJ, Mosquera JM, Cronin MT, Rubin MA, Targeted next-generation sequencing of advanced prostate cancer identifies potential therapeutic targets and disease heterogeneity, European urology, 63 (2013) 920–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee JK, Phillips JW, Smith BA, Park JW, Stoyanova T, McCaffrey EF, Baertsch R, Sokolov A, Meyerowitz JG, Mathis C, Cheng D, Stuart JM, Shokat KM, Gustafson WC, Huang J, Witte ON, N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells, Cancer Cell, 29 (2016) 536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Davies AH, Beltran H, Zoubeidi A, Cellular plasticity and the neuroendocrine phenotype in prostate cancer, Nature reviews. Urology, 15 (2018) 271–286. [DOI] [PubMed] [Google Scholar]
- [16].Beltran H, Antonarakis ES, Morris MJ, Attard G, Emerging Molecular Biomarkers in Advanced Prostate Cancer: Translation to the Clinic, American Society of Clinical Oncology educational book. American Society of Clinical Oncology. Meeting, 35 (2016) 131–141. [DOI] [PubMed] [Google Scholar]
- [17].Papandreou CN, Daliani DD, Thall PF, Tu SM, Wang X, Reyes A, Troncoso P, Logothetis CJ, Results of a phase II study with doxorubicin, etoposide, and cisplatin in patients with fully characterized small-cell carcinoma of the prostate, Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 20 (2002) 3072–3080. [DOI] [PubMed] [Google Scholar]
- [18].Vashchenko N, Abrahamsson PA, Neuroendocrine differentiation in prostate cancer: implications for new treatment modalities, European urology, 47 (2005) 147–155. [DOI] [PubMed] [Google Scholar]
- [19].Alberti C, Neuroendocrine differentiation in prostate carcinoma: focusing on its pathophysiologic mechanisms and pathological features, Il Giornale di chirurgia, 31 (2010) 568–574. [PubMed] [Google Scholar]
- [20].Beltran H, Update on the biology and management of neuroendocrine prostate cancer, Clinical advances in hematology & oncology : H&O, 14 (2016) 513–515. [PubMed] [Google Scholar]
- [21].Akamatsu S, Inoue T, Ogawa O, Gleave ME, Clinical and molecular features of treatment-related neuroendocrine prostate cancer, International journal of urology : official journal of the Japanese Urological Association, 25 (2018) 345–351. [DOI] [PubMed] [Google Scholar]
- [22].Santoni M, Conti A, Burattini L, Berardi R, Scarpelli M, Cheng L, Lopez-Beltran A, Cascinu S, Montironi R, Neuroendocrine differentiation in prostate cancer: novel morphological insights and future therapeutic perspectives, Biochimica et biophysica acta, 1846 (2014) 630–637. [DOI] [PubMed] [Google Scholar]
- [23].Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, Wu R, Brahmbhatt S, Mo F, Jong L, Bell RH, Anderson S, Hurtado-Coll A, Fazli L, Sharma M, Beltran H, Rubin M, Cox M, Gout PW, Morris J, Goldenberg L, Volik SV, Gleave ME, Collins CC, Wang Y, High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development, Cancer Res, 74 (2014) 1272–1283. [DOI] [PubMed] [Google Scholar]
- [24].Mucci NR, Akdas G, Manely S, Rubin MA, Neuroendocrine expression in metastatic prostate cancer: evaluation of high throughput tissue microarrays to detect heterogeneous protein expression, Hum Pathol, 31 (2000) 406–414. [DOI] [PubMed] [Google Scholar]
- [25].Hansel DE, Nakayama M, Luo J, Abukhdeir AM, Park BH, Bieberich CJ, Hicks JL, Eisenberger M, Nelson WG, Mostwin JL, De Marzo AM, Shared TP53 gene mutation in morphologically and phenotypically distinct concurrent primary small cell neuroendocrine carcinoma and adenocarcinoma of the prostate, Prostate, 69 (2009) 603–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Williamson SR, Zhang S, Yao JL, Huang J, Lopez-Beltran A, Shen S, Osunkoya AO, MacLennan GT, Montironi R, Cheng L, ERG-TMPRSS2 rearrangement is shared by concurrent prostatic adenocarcinoma and prostatic small cell carcinoma and absent in small cell carcinoma of the urinary bladder: evidence supporting monoclonal origin, Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 24 (2011) 1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Segal NH, Cohen RJ, Haffejee Z, Savage N, BCL-2 proto-oncogene expression in prostate cancer and its relationship to the prostatic neuroendocrine cell, Archives of pathology & laboratory medicine, 118 (1994) 616–618. [PubMed] [Google Scholar]
- [28].Gong J, Lee J, Akio H, Schlegel PN, Shen R, Attenuation of apoptosis by chromogranin A-induced Akt and survivin pathways in prostate cancer cells, Endocrinology, 148 (2007) 4489–4499. [DOI] [PubMed] [Google Scholar]
- [29].Cox ME, Deeble PD, Lakhani S, Parsons SJ, Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression, Cancer Res, 59 (1999) 3821–3830. [PubMed] [Google Scholar]
- [30].Pal SK, He M, Chen L, Yang L, Pillai R, Twardowski P, Hsu J, Kortylewski M, Jones JO, Synaptophysin expression on circulating tumor cells in patients with castration resistant prostate cancer undergoing treatment with abiraterone acetate or enzalutamide, Urologic oncology, 36 (2018) 162 e161–162 e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].De Marzo AM, Meeker AK, Epstein JI, Coffey DS, Prostate stem cell compartments: expression of the cell cycle inhibitor p27Kip1 in normal, hyperplastic, and neoplastic cells, The American journal of pathology, 153 (1998) 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Aggarwal RR, Youngren J, Sokolov A, Huang J, Thomas GV, True LD, Foye A, Alumkal JJ, Ryan CJ, Beer TM, Evans CP, Gleave M, Rettig M, Stuart JM, Lara P, Goldstein TC, Zhang L, Reiter RE, Chi KN, Small EJ, Persistence of AR signaling in small cell neuroendocrine prostate cancer (SCNC) and intermediate atypical carcinoma (IAC): Results from the SU2C/PCF/AACR West Coast Prostate Cancer Dream Team (WCDT), Journal of Clinical Oncology, 34 (2016) 5045–5045. [Google Scholar]
- [33].Wright ME, Tsai MJ, Aebersold R, Androgen receptor represses the neuroendocrine transdifferentiation process in prostate cancer cells, Mol Endocrinol, 17 (2003) 1726–1737. [DOI] [PubMed] [Google Scholar]
- [34].Huss WJ, Gregory CW, Smith GJ, Neuroendocrine cell differentiation in the CWR22 human prostate cancer xenograft: association with tumor cell proliferation prior to recurrence, Prostate, 60 (2004) 91–97. [DOI] [PubMed] [Google Scholar]
- [35].Nouri M, Caradec J, Lubik AA, Li N, Hollier BG, Takhar M, Altimirano-Dimas M, Chen M, Roshan-Moniri M, Butler M, Lehman M, Bishop J, Truong S, Huang SC, Cochrane D, Cox M, Collins C, Gleave M, Erho N, Alshalafa M, Davicioni E, Nelson C, Gregory-Evans S, Karnes RJ, Jenkins RB, Klein EA, Buttyan R, Therapy-induced developmental reprogramming of prostate cancer cells and acquired therapy resistance, Oncotarget, 8 (2017) 18949–18967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Farach A, Ding Y, Lee M, Creighton C, Delk NA, Ittmann M, Miles B, Rowley D, Farach-Carson MC, Ayala GE, Neuronal Trans-Differentiation in Prostate Cancer Cells, Prostate, 76 (2016) 1312–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, Ghosh D, Pienta KJ, Sewalt RG, Otte AP, Rubin MA, Chinnaiyan AM, The polycomb group protein EZH2 is involved in progression of prostate cancer, Nature, 419 (2002) 624–629. [DOI] [PubMed] [Google Scholar]
- [38].Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, Goodrich MM, Labbe DP, Gomez EC, Wang J, Long HW, Xu B, Brown M, Loda M, Sawyers CL, Ellis L, Goodrich DW, Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance, Science, 355 (2017) 78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Li K, Liu C, Zhou B, Bi L, Huang H, Lin T, Xu K, Role of EZH2 in the growth of prostate cancer stem cells isolated from LNCaP cells, Int J Mol Sci, 14 (2013) 11981–11993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Crea F, Hurt EM, Mathews LA, Cabarcas SM, Sun L, Marquez VE, Danesi R, Farrar WL, Pharmacologic disruption of Polycomb Repressive Complex 2 inhibits tumorigenicity and tumor progression in prostate cancer, Mol Cancer, 10 (2011) 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kleb B, Estecio MR, Zhang J, Tzelepi V, Chung W, Jelinek J, Navone NM, Tahir S, Marquez VE, Issa JP, Maity S, Aparicio A, Differentially methylated genes and androgen receptor re-expression in small cell prostate carcinomas, Epigenetics, 11 (2016) 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lu J, Van der Steen T, Tindall DJ, Are androgen receptor variants a substitute for the full-length receptor?, Nature reviews. Urology, 12 (2015) 137–144. [DOI] [PubMed] [Google Scholar]
- [43].Mahajan K, Malla P, Lawrence HR, Chen Z, Kumar-Sinha C, Malik R, Shukla S, Kim J, Coppola D, Lawrence NJ, Mahajan NP, ACK1/TNK2 Regulates Histone H4 Tyr88-phosphorylation and AR Gene Expression in Castration-Resistant Prostate Cancer, Cancer Cell, 31 (2017) 790–803 e798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Mahajan NP, Coppola D, Kim J, Lawrence HR, Lawrence NJ, Mahajan K, Blockade of ACK1/TNK2 To Squelch the Survival of Prostate Cancer Stem-like Cells, Sci Rep, 8 (2018) 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mahajan K, Coppola D, Rawal B, Chen YA, Lawrence HR, Engelman RW, Lawrence NJ, Mahajan NP, Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer, The Journal of biological chemistry, 287 (2012) 22112–22122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bishop JL, Thaper D, Vahid S, Davies A, Ketola K, Kuruma H, Jama R, Nip KM, Angeles A, Johnson F, Wyatt AW, Fazli L, Gleave ME, Lin D, Rubin MA, Collins CC, Wang Y, Beltran H, Zoubeidi A, The Master Neural Transcription Factor BRN2 Is an Androgen Receptor-Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer, Cancer Discov, 7 (2017) 54–71. [DOI] [PubMed] [Google Scholar]
- [47].Diss JK, Faulkes DJ, Walker MM, Patel A, Foster CS, Budhram-Mahadeo V, Djamgoz MB, Latchman DS, Brn-3a neuronal transcription factor functional expression in human prostate cancer, Prostate Cancer Prostatic Dis, 9 (2006) 83–91. [DOI] [PubMed] [Google Scholar]
- [48].Diss JK, Calissano M, Gascoyne D, Djamgoz MB, Latchman DS, Identification and characterization of the promoter region of the Nav1.7 voltage-gated sodium channel gene (SCN9A), Molecular and cellular neurosciences, 37 (2008) 537–547. [DOI] [PubMed] [Google Scholar]
- [49].Berwick DC, Diss JK, Budhram-Mahadeo VS, Latchman DS, A simple technique for the prediction of interacting proteins reveals a direct Brn-3a-androgen receptor interaction, The Journal of biological chemistry, 285 (2010) 15286–15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kregel S, Kiriluk KJ, Rosen AM, Cai Y, Reyes EE, Otto KB, Tom W, Paner GP, Szmulewitz RZ, Vander Griend DJ, Sox2 is an androgen receptor-repressed gene that promotes castration-resistant prostate cancer, PLoS One, 8 (2013) e53701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Berenguer C, Boudouresque F, Dussert C, Daniel L, Muracciole X, Grino M, Rossi D, Mabrouk K, Figarella-Branger D, Martin PM, Ouafik L, Adrenomedullin, an autocrine/paracrine factor induced by androgen withdrawal, stimulates ‘neuroendocrine phenotype’ in LNCaP prostate tumor cells, Oncogene, 27 (2008) 506–518. [DOI] [PubMed] [Google Scholar]
- [52].Deng X, Liu H, Huang J, Cheng L, Keller ET, Parsons SJ, Hu CD, Ionizing radiation induces prostate cancer neuroendocrine differentiation through interplay of CREB and ATF2: implications for disease progression, Cancer Res, 68 (2008) 9663–9670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Deng X, Elzey BD, Poulson JM, Morrison WB, Ko SC, Hahn NM, Ratliff TL, Hu CD, Ionizing radiation induces neuroendocrine differentiation of prostate cancer cells in vitro, in vivo and in prostate cancer patients, American journal of cancer research, 1 (2011) 834–844. [PMC free article] [PubMed] [Google Scholar]
- [54].Krauss DJ, Hayek S, Amin M, Ye H, Kestin LL, Zadora S, Vicini FA, Cotant M, Brabbins DS, Ghilezan MI, Gustafson GS, Martinez AA, Prognostic significance of neuroendocrine differentiation in patients with Gleason score 8–10 prostate cancer treated with primary radiotherapy, Int J Radiat Oncol Biol Phys, 81 (2011) e119–125. [DOI] [PubMed] [Google Scholar]
- [55].Lehmann W, Mossmann D, Kleemann J, Mock K, Meisinger C, Brummer T, Herr R, Brabletz S, Stemmler MP, Brabletz T, ZEB1 turns into a transcriptional activator by interacting with YAP1 in aggressive cancer types, Nature communications, 7 (2016) 10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, Campbell LL, Polyak K, Brisken C, Yang J, Weinberg RA, The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells, Cell, 133 (2008) 704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, Wang RY, Brisken C, Guerra R, Andreeff M, Mani SA, Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells, Stem Cells, 28 (2010) 1435–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, Herschkowitz JI, Guerra R, Chang JT, Miura N, Rosen JM, Mani SA, FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer, Cancer Res, 73 (2013) 1981–1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Paranjape AN, Soundararajan R, Werden SJ, Joseph R, Taube JH, Liu H, Rodriguez-Canales J, Sphyris N, Wistuba I, Miura N, Dhillon J, Mahajan N, Mahajan K, Chang JT, Ittmann M, Maity SN, Logothetis C, Tang DG, Mani SA, Inhibition of FOXC2 restores epithelial phenotype and drug sensitivity in prostate cancer cells with stem-cell properties, Oncogene, 35 (2016) 5963–5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhau HE, Odero-Marah V, Lue HW, Nomura T, Wang R, Chu G, Liu ZR, Zhou BP, Huang WC, Chung LWK, Epithelial to mesenchymal transition (EMT) in human prostate cancer: Lessons learned from ARCaP model, Clinical and Experimental Metastasis, 25 (2008) 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Zhu ML, Kyprianou N, Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells, FASEB Journal, 24 (2010) 769–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li P, Yang R, Gao WQ, Contributions of epithelial-mesenchymal transition and cancer stem cells to the development of castration resistance of prostate cancer, Mol Cancer, 13 (2014) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Li P, Wang J, Chu M, Zhang K, Yang R, Gao WQ, Zeb1 promotes androgen independence of prostate cancer via induction of stem cell-like properties, Exp Biol Med (Maywood), 239 (2014) 813–822. [DOI] [PubMed] [Google Scholar]
- [64].Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, Chen D, Seo K, Modrusan Z, Gao WQ, Settleman J, Johnson L, Androgen deprivation causes epithelial-mesenchymal transition in the prostate: Implications for androgen- deprivation therapy, Cancer Research, 72 (2012) 527–536. [DOI] [PubMed] [Google Scholar]
- [65].Shang Z, Cai Q, Zhang M, Zhu S, Ma Y, Sun L, Jiang N, Tian J, Niu X, Chen J, Sun Y, Niu Y, A switch from CD44(+) cell to EMT cell drives the metastasis of prostate cancer, Oncotarget, 6 (2015) 1202–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Kahn B, Collazo J, Kyprianou N, Androgen receptor as a driver of therapeutic resistance in advanced prostate cancer, Int J Biol Sci, 10 (2014) 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].McKeithen D, Graham T, Chung LW, Odero-Marah V, Snail transcription factor regulates neuroendocrine differentiation in LNCaP prostate cancer cells, Prostate, 70 (2010) 982–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Esposito S, Russo MV, Airoldi I, Tupone MG, Sorrentino C, Barbarito G, Di Meo S, Di Carlo E, SNAI2/Slug gene is silenced in prostate cancer and regulates neuroendocrine differentiation, metastasis-suppressor and pluripotency gene expression, Oncotarget, 6 (2015) 17121–17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Emadi Baygi M., Soheili ZS, Essmann F, Deezagi A, Engers R, Goering W, Schulz WA, Slug/SNAI2 regulates cell proliferation and invasiveness of metastatic prostate cancer cell lines, Tumour Biol, 31 (2010) 297–307. [DOI] [PubMed] [Google Scholar]
- [70].Pinto F, Pertega-Gomes N, Vizcaino JR, Andrade RP, Carcano FM, Reis RM, Brachyury as a potential modulator of androgen receptor activity and a key player in therapy resistance in prostate cancer, Oncotarget, 7 (2016) 28891–28902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Leshem O, Madar S, Kogan-Sakin I, Kamer I, Goldstein I, Brosh R, Cohen Y, Jacob-Hirsch J, Ehrlich M, Ben-Sasson S, Goldfinger N, Loewenthal R, Gazit E, Rotter V, Berger R, TMPRSS2/ERG promotes epithelial to mesenchymal transition through the ZEB1/ZEB2 axis in a prostate cancer model, PLoS One, 6 (2011) e21650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O, FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells, Cancer Res, 70 (2010) 6735–6745. [DOI] [PubMed] [Google Scholar]
- [73].Lotan TL, Gupta NS, Wang W, Toubaji A, Haffner MC, Chaux A, Hicks JL, Meeker AK, Bieberich CJ, De Marzo AM, Epstein JI, Netto GJ, ERG gene rearrangements are common in prostatic small cell carcinomas, Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc, 24 (2011) 820–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Svensson C, Ceder J, Iglesias-Gato D, Chuan YC, Pang ST, Bjartell A, Martinez RM, Bott L, Helczynski L, Ulmert D, Wang Y, Niu Y, Collins C, Flores-Morales A, REST mediates androgen receptor actions on gene repression and predicts early recurrence of prostate cancer, Nucleic Acids Res, 42 (2014) 999–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Lin TP, Chang YT, Lee SY, Campbell M, Wang TC, Shen SH, Chung HJ, Chang YH, Chiu AW, Pan CC, Lin CH, Chu CY, Kung HJ, Cheng CY, Chang PC, REST reduction is essential for hypoxia-induced neuroendocrine differentiation of prostate cancer cells by activating autophagy signaling, Oncotarget, 7 (2016) 26137–26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, Lam HM, Dumpit R, Corey E, Chery L, Lakely B, Higano CS, Montgomery B, Roudier M, Lange PH, Nelson PS, Vessella RL, Morrissey C, SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer, Clin Cancer Res, 21 (2015) 4698–4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chang YT, Lin TP, Campbell M, Pan CC, Lee SH, Lee HC, Yang MH, Kung HJ, Chang PC, REST is a crucial regulator for acquiring EMT-like and stemness phenotypes in hormone-refractory prostate cancer, Sci Rep, 7 (2017) 42795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Goldstein AS, Stoyanova T, Witte ON, Primitive origins of prostate cancer: in vivo evidence for prostate-regenerating cells and prostate cancer-initiating cells, Mol Oncol, 4 (2010) 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Goldstein AS, Huang J, Guo C, Garraway IP, Witte ON, Identification of a cell of origin for human prostate cancer, Science, 329 (2010) 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Szczyrba J, Niesen A, Wagner M, Wandernoth PM, Aumuller G, Wennemuth G, Neuroendocrine Cells of the Prostate Derive from the Neural Crest, The Journal of biological chemistry, 292 (2017) 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].English HF, Santen RJ, Isaacs JT, Response of glandular versus basal rat ventral prostatic epithelial cells to androgen withdrawal and replacement, Prostate, 11 (1987) 229–242. [DOI] [PubMed] [Google Scholar]
- [82].Isaacs JT, New principles in the management of metastatic prostatic cancer, Progress in clinical and biological research, 185A (1985) 383–405. [PubMed] [Google Scholar]
- [83].Kyprianou N, Isaacs JT, Identification of a cellular receptor for transforming growth factor-beta in rat ventral prostate and its negative regulation by androgens, Endocrinology, 123 (1988) 2124–2131. [DOI] [PubMed] [Google Scholar]
- [84].Ousset M, Van Keymeulen A, Bouvencourt G, Sharma N, Achouri Y, Simons BD, Blanpain C, Multipotent and unipotent progenitors contribute to prostate postnatal development, Nat Cell Biol, 14 (2012) 1131–1138. [DOI] [PubMed] [Google Scholar]
- [85].Pignon JC, Grisanzio C, Geng Y, Song J, Shivdasani RA, Signoretti S, p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia, Proc Natl Acad Sci U S A, 110 (2013) 8105–8110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Xin L, Lawson DA, Witte ON, The Sca-1 cell surface marker enriches for a prostate-regenerating cell subpopulation that can initiate prostate tumorigenesis, Proc Natl Acad Sci U S A, 102 (2005) 6942–6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Burger PE, Xiong X, Coetzee S, Salm SN, Moscatelli D, Goto K, Wilson EL, Sca-1 expression identifies stem cells in the proximal region of prostatic ducts with high capacity to reconstitute prostatic tissue, Proc Natl Acad Sci U S A, 102 (2005) 7180–7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Goldstein AS, Lawson DA, Cheng D, Sun W, Garraway IP, Witte ON, Trop2 identifies a subpopulation of murine and human prostate basal cells with stem cell characteristics, Proc Natl Acad Sci U S A, 105 (2008) 20882–20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Leong KG, Wang BE, Johnson L, Gao WQ, Generation of a prostate from a single adult stem cell, Nature, 456 (2008) 804–808. [DOI] [PubMed] [Google Scholar]
- [90].Tsujimura A, Koikawa Y, Salm S, Takao T, Coetzee S, Moscatelli D, Shapiro E, Lepor H, Sun TT, Wilson EL, Proximal location of mouse prostate epithelial stem cells: a model of prostatic homeostasis, The Journal of cell biology, 157 (2002) 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Germann M, Wetterwald A, Guzman-Ramirez N, van der Pluijm G, Culig Z, Cecchini MG, Williams ED, Thalmann GN, Stem-like cells with luminal progenitor phenotype survive castration in human prostate cancer, Stem Cells, 30 (2012) 1076–1086. [DOI] [PubMed] [Google Scholar]
- [92].Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, Badani KK, McKiernan JM, Benson MC, Hibshoosh H, Shen MM, Single luminal epithelial progenitors can generate prostate organoids in culture, Nat Cell Biol, 16 (2014) 951–961, 951–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, Dowling CM, Gao D, Begthel H, Sachs N, Vries RG, Cuppen E, Chen Y, Sawyers CL, Clevers HC, Identification of multipotent luminal progenitor cells in human prostate organoid cultures, Cell, 159 (2014) 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Hu Y, Ippolito JE, Garabedian EM, Humphrey PA, Gordon JI, Molecular characterization of a metastatic neuroendocrine cell cancer arising in the prostates of transgenic mice, The Journal of biological chemistry, 277 (2002) 44462–44474. [DOI] [PubMed] [Google Scholar]
- [95].Rybak AP, Tang D, SOX2 plays a critical role in EGFR-mediated self-renewal of human prostate cancer stem-like cells, Cell Signal, 25 (2013) 2734–2742. [DOI] [PubMed] [Google Scholar]
- [96].Russo MV, Esposito S, Tupone MG, Manzoli L, Airoldi I, Pompa P, Cindolo L, Schips L, Sorrentino C, Di Carlo E, SOX2 boosts major tumor progression genes in prostate cancer and is a functional biomarker of lymph node metastasis, Oncotarget, 7 (2016) 12372–12385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Palapattu GS, Wu C, Silvers CR, Martin HB, Williams K, Salamone L, Bushnell T, Huang LS, Yang Q, Huang J, Selective expression of CD44, a putative prostate cancer stem cell marker, in neuroendocrine tumor cells of human prostate cancer, Prostate, 69 (2009) 787–798. [DOI] [PubMed] [Google Scholar]
- [98].Simon RA, di Sant’Agnese PA, Huang LS, Xu H, Yao JL, Yang Q, Liang S, Liu J, Yu R, Cheng L, Oh WK, Palapattu GS, Wei J, Huang J, CD44 expression is a feature of prostatic small cell carcinoma and distinguishes it from its mimickers, Hum Pathol, 40 (2009) 252–258. [DOI] [PubMed] [Google Scholar]
- [99].Qin J, Liu X, Laffin B, Chen X, Choy G, Jeter CR, Calhoun-Davis T, Li H, Palapattu GS, Pang S, Lin K, Huang J, Ivanov I, Li W, Suraneni MV, Tang DG, The PSA(-/lo) prostate cancer cell population harbors self-renewing long-term tumor-propagating cells that resist castration, Cell Stem Cell, 10 (2012) 556–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Sotomayor P, Godoy A, Smith GJ, Huss WJ, Oct4A is expressed by a subpopulation of prostate neuroendocrine cells, Prostate, 69 (2009) 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Mani SA, Yang J, Brooks M, Schwaninger G, Zhou A, Miura N, Kutok JL, Hartwell K, Richardson AL, Weinberg RA, Mesenchyme Forkhead 1 (FOXC2) plays a key role in metastasis and is associated with aggressive basal-like breast cancers, Proceedings of the National Academy of Sciences of the United States of America, 104 (2007) 10069–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H, Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells, Cancer Res, 72 (2012) 1878–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Kong D, Banerjee S, Ahmad A, Li Y, Wang Z, Sethi S, Sarkar FH, Epithelial to mesenchymal transition is mechanistically linked with stem cell signatures in prostate cancer cells, PLoS One, 5 (2010) e12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Lee E, Wang J, Yumoto K, Jung Y, Cackowski FC, Decker AM, Li Y, Franceschi RT, Pienta KJ, Taichman RS, DNMT1 Regulates Epithelial-Mesenchymal Transition and Cancer Stem Cells, Which Promotes Prostate Cancer Metastasis, Neoplasia, 18 (2016) 553–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Bang YJ, Pirnia F, Fang WG, Kang WK, Sartor O, Whitesell L, Ha MJ, Tsokos M, Sheahan MD, Nguyen P, Niklinski WT, Myers CE, Trepel JB, Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP, Proc Natl Acad Sci U S A, 91 (1994) 5330–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Shen R, Dorai T, Szaboles M, Katz AE, Olsson CA, Buttyan R, Transdifferentiation of cultured human prostate cancer cells to a neuroendocrine cell phenotype in a hormone-depleted medium, Urologic oncology, 3 (1997) 67–75. [DOI] [PubMed] [Google Scholar]
- [107].Burchardt T, Burchardt M, Chen MW, Cao Y, de la Taille A, Shabsigh A, Hayek O, Dorai T, Buttyan R, Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo, The Journal of urology, 162 (1999) 1800–1805. [PubMed] [Google Scholar]
- [108].Terry S, Maille P, Baaddi H, Kheuang L, Soyeux P, Nicolaiew N, Ceraline J, Firlej V, Beltran H, Allory Y, de la Taille A, Vacherot F, Cross modulation between the androgen receptor axis and protocadherin-PC in mediating neuroendocrine transdifferentiation and therapeutic resistance of prostate cancer, Neoplasia, 15 (2013) 761–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Yang X, Chen MW, Terry S, Vacherot F, Chopin DK, Bemis DL, Kitajewski J, Benson MC, Guo Y, Buttyan R, A human- and male-specific protocadherin that acts through the wnt signaling pathway to induce neuroendocrine transdifferentiation of prostate cancer cells, Cancer Res, 65 (2005) 5263–5271. [DOI] [PubMed] [Google Scholar]
- [110].Uysal-Onganer P, Kawano Y, Caro M, Walker MM, Diez S, Darrington RS, Waxman J, Kypta RM, Wnt-11 promotes neuroendocrine-like differentiation, survival and migration of prostate cancer cells, Mol Cancer, 9 (2010) 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Yu X, Wang Y, DeGraff DJ, Wills ML, Matusik RJ, Wnt/beta-catenin activation promotes prostate tumor progression in a mouse model, Oncogene, 30 (2011) 1868–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Wu L, Zhao JC, Kim J, Jin HJ, Wang CY, Yu J, ERG is a critical regulator of Wnt/LEF1 signaling in prostate cancer, Cancer Res, 73 (2013) 6068–6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Adler HL, McCurdy MA, Kattan MW, Timme TL, Scardino PT, Thompson TC, Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma, The Journal of urology, 161 (1999) 182–187. [PubMed] [Google Scholar]
- [114].Ge D, Gao AC, Zhang Q, Liu S, Xue Y, You Z, LNCaP prostate cancer cells with autocrine interleukin-6 expression are resistant to IL-6-induced neuroendocrine differentiation due to increased expression of suppressors of cytokine signaling, Prostate, 72 (2012) 1306–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Zhu Y, Liu C, Cui Y, Nadiminty N, Lou W, Gao AC, Interleukin-6 induces neuroendocrine differentiation (NED) through suppression of RE-1 silencing transcription factor (REST), Prostate, 74 (2014) 1086–1094. [DOI] [PubMed] [Google Scholar]
- [116].Tzelepi V, Zhang J, Lu JF, Kleb B, Wu G, Wan X, Hoang A, Efstathiou E, Sircar K, Navone NM, Troncoso P, Liang S, Logothetis CJ, Maity SN, Aparicio AM, Modeling a lethal prostate cancer variant with small-cell carcinoma features, Clin Cancer Res, 18 (2012) 666–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, Mosier S, Gocke CD, Epstein JI, Netto GJ, Liu W, Isaacs WB, De Marzo AM, Lotan TL, Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma, Clin Cancer Res, 20 (2014) 890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Werden SJ, Sphyris N, Sarkar TR, Paranjape AN, LaBaff AM, Taube JH, Hollier BG, Ramirez-Pena EQ, Soundararajan R, den Hollander P., Powell E, Echeverria GV, Miura N, Chang JT, Piwnica-Worms H, Rosen JM, Mani SA, Phosphorylation of serine 367 of FOXC2 by p38 regulates ZEB1 and breast cancer metastasis, without impacting primary tumor growth, Oncogene, 35 (2016) 5977–5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, Puca L, Cyrta J, Sboner A, Noorzad Z, MacDonald T, Cheung C, Yuen KS, Gao D, Chen Y, Eilers M, Mosquera JM, Robinson BD, Elemento O, Rubin MA, Demichelis F, Rickman DS, N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer, Cancer Cell, 30 (2016) 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, Wang Y, Sheikh KL, Terry S, Tagawa ST, Dhir R, Nelson JB, de la Taille A, Allory Y, Gerstein MB, Perner S, Pienta KJ, Chinnaiyan AM, Collins CC, Gleave ME, Demichelis F, Nanus DM, Rubin MA, Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets, Cancer Discov, 1 (2011) 487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Rickman DS, Schulte JH, Eilers M, The Expanding World of N-MYC-Driven Tumors, Cancer Discov, 8 (2018) 150–163. [DOI] [PubMed] [Google Scholar]
- [122].Rickman DS, Beltran H, Demichelis F, Rubin MA, Biology and evolution of poorly differentiated neuroendocrine tumors, Nat Med, 23 (2017) 1–10. [DOI] [PubMed] [Google Scholar]
- [123].Oser MG, Janne PA, Small-Cell Neuroendocrine Tumors: Cell State Trumps the Oncogenic Driver, Clin Cancer Res, 24 (2018) 1775–1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Garg R, Blando JM, Perez CJ, Abba MC, Benavides F, Kazanietz MG, Protein Kinase C Epsilon Cooperates with PTEN Loss for Prostate Tumorigenesis through the CXCL13-CXCR5 Pathway, Cell Rep, 19 (2017) 375–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Zhou Z, Flesken-Nikitin A, Corney DC, Wang W, Goodrich DW, Roy-Burman P, Nikitin AY, Synergy of p53 and Rb deficiency in a conditional mouse model for metastatic prostate cancer, Cancer Res, 66 (2006) 7889–7898. [DOI] [PubMed] [Google Scholar]
- [126].Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, Le Magnen C, Chester D, Mostaghel EA, Califano A, Rubin MA, Shen MM, Abate-Shen C, Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer, Cancer Discov, 7 (2017) 736–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Aparicio A, Tzelepi V, Araujo JC, Guo CC, Liang S, Troncoso P, Logothetis CJ, Navone NM, Maity SN, Neuroendocrine prostate cancer xenografts with large-cell and small-cell features derived from a single patient’s tumor: morphological, immunohistochemical, and gene expression profiles, Prostate, 71 (2011) 846–856. [DOI] [PMC free article] [PubMed] [Google Scholar]