Abstract
The developing immune system is an adaptive system, primed by antigens, responsive to infectious pathogens, and can be affected by other aspects of the early rearing environment, including deviations from the normal provision of parental care. We investigated whether early rearing in an institutional setting, even when followed by years living in supportive and well-resourced families, would be associated with a persistent shift in T cell profiles. Immunophenotyping was used to enumerate CD4+ CD57+ and CD8+ CD57+ subsets, with gating strategies employed to differentiate naïve, central-memory, effector-memory, and terminally differentiated EM cells expressing CD45RA (TEMRA). Blood samples were collected from 96 adolescents, and PBMC isolated via Ficol gradient, followed by an optimized immunophenotypic characterization. CMV antibody titers were determined via ELISA. Adopted adolescents had lower CD4/CD8 ratios than did the control adolescents. Early rearing had a significant effect on the T cells, especially the CD8+ CD57+ CM, EM, and TEMRA cells and the CD4+ CD57+ EM cells. Adolescents who had spent their infancy in institutions before adoption were more likely to be seropositive for CMV, with higher antibody titers. CMV antibody titers were significantly correlated with the percentages of all CD8+ CD57+ cell subsets. In the statistical modeling, CMV antibody titer also completely mediated the relationship between institutional exposure and the ratio of CD4-to-CD8 cells, as well as the percentages of CD4+ CD57+ and CD8+ CD57+ subsets. These findings demonstrate that persistent immune differences are still evident even years after adoption by supportive American families. The shift in the T cells was associated with being a latent carrier of CMV and may reflect the role of specific T cell subsets in Herpes virus containment. In older adults, sustained CMV antigen persistence and immunoregulatory containment ultimately contributes to an accumulation of differentiated T cells with a decreased proliferative capacity and to immune senescence.
Keywords: Early adversity, Adolescence, Cytomegalovirus, T cells, CD57, CD4/CD8, Herpes virus, TEMRA, Adoption, Institutional care
1. Introduction
Worldwide the prevalence of early life adversity (ELA) during childhood is high (Elwenspoek et al., 2017a). ELA includes many different adverse conditions impacting children and youth in low-, middle-, and high-income countries. These adversities range in severity and, at their most severe, result in stunted growth, reflecting both poor nutrition and chronic stress, marked delays in cognitive and social development, and significant effects on neurodevelopment (Shonkoff and Garner, 2012). ELA significantly increases the risk of poor health throughout the life course and early mortality (Brown et al., 2009; Shonkoff et al., 2009). A number of mechanisms have been investigated to account for this association with morbidity and mortality (Elwenspoek et al., 2017a), including alterations in the functioning of the immune system (Miller et al., 2011).
The initial research on the effects of early experience on immunity extends back over 50 years and provided an important foundation for more recent studies on the interactions among behavioral, neural, endocrine, and immune processes. Experiments in rodent models demonstrated that there were both acute and chronic effects of ELA on immunity, compromising immune competence and altering the regulatory set points for immune responses in adult animals (Ader and Friedman, 1965; Solomon et al., 1968). These findings laid the groundwork for seminal observations that both prenatal events and early rearing might serve to shape the normal development of the immune system (Ader, 1983, 2000). Subsequent research replicated and extended these findings in nonhuman primate models of early experience and immune responses. A number of studies focused on social separation from the mother as an extremely adverse experience. Even a two-week maternal separation during the first year of life in monkeys (i.e., pigtail macaques) was sufficient to result in a sustained suppression of T-lymphocyte proliferation to a mitogen, phytohaemagglutinin (PHA), and led to a small but chronically elevated white blood cell count when measured later in adult animals (Laudenslager et al., 1985). Other researchers demonstrated that infant rhesus monkeys raised by humans in a nursery setting exhibited larger lymphocyte proliferation responses than did mother-reared controls, which was related to an upward skewing of the CD8+ to CD4+ lymphocyte ratio (Coe et al., 1989). In later work, early social deprivation was found to not only alter immune function, but also survival. Rhesus monkeys raised by humans in a nursery setting died at a younger age than did mother-reared animals, and the monkeys that did survive showed a number of immune alterations, including a decrease in the ratio of CD4+ to CD8+ T cells, with males being particularly vulnerable to this age-related thymic alteration (Lewis et al., 2000). Considered together, these studies provided compelling evidence that early rearing conditions can leave a lasting mark on the immune system and provided the rationale to further study the impact of early rearing conditions in humans.
More recently the primary focus of investigations on the ELA-immune relationship in humans shifted to emphasize the increases in inflammation (Slopen et al., 2015). A number of papers documented signs of increased proinflammatory activity, including higher C-reactive protein and interleukin-6, in children from maltreatment backgrounds as well as lower socioeconomic status families. However, some other studies found inconsistent evidence for the connection between ELA and inflammation in children and adolescents (Slopen et al., 2015). Drawing from the literature on animal models of ELA and immune functioning, it is important to consider other aspects of immunity, especially related to the maturation of the thymus and the functioning of T cells. During fetal and infant development, the thymus is particularly vulnerable to insults, and the T cell subsets found in the blood stream can continue to reflect this ontogenetic history. There may be persistent effects on the numbers and types of T cells in circulation, as well as on their state of activation. In addition to the traditional focus on the ratio of CD4+ to CD8+ cells, technical advances have made it possible to conduct more sophisticated analyses of many different subsets. Modern immunophenotyping techniques led to the discovery that ELA may predispose individuals to accumulate of T cells that have reached a state of ‘replicative senescence’, reminiscent of the process of immunosenescence in old age. However, in this case, it would suggest there has been an accelerated type of immune aging that could compromise the ability of the young adult to mount a vigorous response to an infectious pathogen (Elwenspoek et al., 2017a). Specific T cell populations, which are characterized by the glycoprotein CD57+ cell surface marker, can be further differentiated into central memory, effector-memory, and terminally differentiated EM cells expressing CD45RA+ (TEMRA). It has also been shown that the latter cells typically have shorter telomere lengths, reflecting their prior proliferative history and the age of these cells (Verma et al., 2017). Many studies have linked this accumulation of CD57+ T cells with illness and aging, and a reduced ability of cells to proliferate as a reaction to a history of chronic antigen stimulation (Benchly et al., 2003; Strioga et al., 2011). Accumulating evidence points to the association of ELA with a number of other indicators of accelerated aging in multiple systems (Belsky et al., 2017; Felitti et al., 1998; Hayward and Gorman, 2004). However, the mechanisms accounting for this association of ELA with accelerated aging and immune dysfunction have yet to be fully elucidated.
One mechanism through which early experience may influence immune functioning and competence is viral carriage. The burden of repeated, early viral exposure in individuals with a history of ELA could put an additional load on the adaptive immune system, especially in the case of being a carrier of several Herpes viruses. Although not typically viewed as a major pathogen of concern in the healthy adult, there is increasing evidence that the immune containment of cytomegalovirus (CMV) in the quiescent or latent state can take a toll on the immune system over the life course (Chidrawar et al., 2009). This kind of immune containment is hypothesized to be a significant driver of immune senescence in elderly individuals (Pawelec et al., 2005). The seroprevalence of CMV varies across populations, but can range up to 60−95% in adults around the world (Chidrawar et al., 2009). Among American children 6–11 years of age, the seroprevalence rate is 36.3% (Staras et al., 2006). However, the infection rate varies dramatically when sociodemographic factors are taken in consideration. For example, the CMV seroprevalence is much higher at lower levels of household income (Dowd et al., 2009; Dowd et al., 2012; Staras et al., 2008). It has also been associated with family dysfunction in youth (Janicki-Deverts et al., 2014), and has a high prevalence globally (Staras et al., 2006). The age at which infants and children are first cared for in larger group settings, such as an orphanage, is a known early risk factor for CMV exposure because the young child will remain viremic and be infectious for an extended period of time during the primary infection phase (Adler, 1991). Thus, disadvantaged children are likely to be at higher risk to be infected with CMV early in life, possibly with important consequences for their maturing immune system.
A number of epidemiological studies across different cultural groups have found that there is a connection between CMV seropositive status and immune dysfunction in elderly individuals (Olsson et al., 2000; Roberts et al., 2010). CMV is associated with changes in T cells associated with aging, especially the CD8+ T cell populations, and has been found to result in a lower CD4+ to CD8+ cell ratio (Pawelec et al., 2005). In CMV-seropositive individuals, the number of naïve CD8+ T cells is reduced and thus CMV may accelerate physiological aging in middle-aged individuals (Chidrawar et al., 2009). Moreover, it has been shown that the T cells in CMV+ adults are more responsive to stress-related neuroendocrine and immune modulators, such as norepinephrine and interleukin-6, which may contribute to the sustained cellular imbalance as the individual matures (Turner et al., 2014).
In order to investigate the unique impact of ELA on immunity, many recent studies have evaluated individuals who experienced a period of adversity during infancy and then were adopted into typically highly-resourced families (Elwenspoek et al., 2017b; Esposito et al., 2016). In one analysis of a small number of young adult participants, CMV appeared to mediate the relationship between ELA and the presence of the CD57 surface protein on T cells, along with other cell surface markers indicative of the CD8+ CD57+ TEMRA subset, but with less clear effects on the CD4+ cell populations (Elwenspoek et al., 2017b). Although it is still unclear whether these cells should be considered immune senescent and replicatively exhausted when assessed in younger individuals (Turner et al., 2014; Verma et al., 2017), their presence does signal that the cells have been repeatedly exposed to cognate antigen, probably of viral origin. Given that these differentiated T-cells have been antigen primed and have a history of prior commitment, they will likely be less flexible in their responses to newly encountered viruses or metastasizing cancer cells. Thus, a differential presence of CD8+ TEMRA as compared to naïve T cells may be indicative of a significant path to reduced immune vigor even in the young adult.
The goal of the present study was to examine the impact of ELA on immune functioning, specifically the CD4+ CD57+ and CDC8+ CD57+ T cell subsets, in youth aged 13 to 21 years. These adolescents had been adopted internationally from orphanages when they were an average 1.5 years of age and had thus spent most of their later childhood living in well-resourced homes with highly educated parents in low risk neighborhoods with good health care. They were compared to controls of similar age and sex who had been reared from birth in families like those of the adopted youth. We examined whether immune effects would be limited to the CD8+ T cells or would also extend to CD4+ T cells. In particular, we examined whether youth adopted internationally as very young children would exhibit a lower ratio of CD4+ to CD8+ T cells, as reported in animal studies of ELA and also as evinced when using cell epigenetic signatures in a previous study by our research group (Esposito et al., 2016). The current study also aimed to replicate and extend the findings of Elwenspoek et al. (2017b), who evaluated young adults (20–34 years of age) adopted at a younger average age of three months from institutional care. We determined whether the presence of CMV might explain observed differences in the adopted youth in either the CD4/CD8 ratio or the TEMRA cell population. Lastly, exploratory analyses investigated whether males and females were similarly affected by ELA, as there is evidence that males reared in institutional care are often more impacted than females (Bos et al., 2011). Findings from rodent and non-human primate models of ELA also suggest that male offspring are more vulnerable to perinatal insults and are more likely to experience the immune dysregulation associated with social deprivation (Lewis et al., 2000).
2. Material and methods
2.1. Participants
The participants were adolescents who had been adopted from institutions in other countries by families in the United States (post-institutionalized, PI) or non-adopted controls (NA) between 13 and 21 years of age (M = 16.3 yrs, SD = 2.01). PI youth were recruited from a registry of families who had adopted children and were interested in participating in research. Each participant had spent at least 70% of their pre-adoption infancy in institutional care (M = 96%, SD = 8%); while the NA comparison youth were born and raised in their families of origin. NA youth were likewise recruited from a registry of birth families interested in being contacted about research. Nine (23.1%) of the PI youth and 11 (24.4%) of the NA youth were drawn from the study reported by Esposito et al. (2016). Exclusion criteria were: major congenital abnormality, regular use of steroid hormone medication or any immunological disorder, Fetal Alcohol Syndrome (FAS)/Fetal Alcohol Effects (FAE) concerns, and a combination of CRP values over 10 mg/L with elevated total white blood cell counts above 15,000 per microliter, which might be indicative of an acute bacterial or viral infection. A total of 4 potential subjects were excluded for one or more of these reasons. We were unable to collect sufficient blood from 3 (1 PI, 2NA), leaving a final sample of 84 of which 45 (22 female) were PI and 39 (25 female) were NA youth. Age at adoption ranged from 5.5 to 45 months (M = 16.1, SD = 9.0 months). These children were adopted from a number of regions: 30 (66.7%) from Eastern Europe; 6 (13.3%) from South Asia; 2 (4.4%) from Latin America; and 7 (15.6%) from Southeast Asia (see Table 1). Preliminary analyses yielded no evidence of significant differences in any key outcome variable by region of adoption. Participants came from well-resourced homes, and the groups did not differ in familial sociodemographic factors. This study was conducted in accordance with Institutional Review Board guidelines at both the Universities of Minnesota and Wisconsin.
Table 1.
Descriptive statistics for PI and NA youth.
| Post-institutionalized (PI)n = 45 | Non-adopted (NA)n = 39 | |
|---|---|---|
| Mean age, years1 | 16.6 (2.2) | 16.0 (1.7) |
| Female, n (%) | 22 (56.4%) | 25 (55.6%) |
| Median Income by Zip code | $77,351 (18,804) | $73,805 (20,162) |
| Race/Ethnicity, n (%)**2,3 | ||
| White, non-Hispanic | 26 (57.8) | 35 (89.7) |
| African American | – | 2 (5.1) |
| Asian | 12 (26.7) | – |
| Hispanic | 2 (4.4) | – |
| Mixed race or other | 2 (4.4) | 2 (5.1) |
| Unknown | 3 (6.7) | – |
| Height, cm**2 | 163.6 (9.22) | 170.55 (10.13) |
| Weight, kg | 60.5 (13.29) | 64.77 (16.72) |
| BMI Percentile | 57.78 (27.87) | 57.5 (28.09) |
| Time of Blood Draw | 10:45 (1:08) | 10:42 (1:04) |
| Early Health Problems, Parent** | 0.76 (0.88) | 0.31 (0.61) |
| Child/Adolescent Health Problems, Parent Report | 2.84 (1.35) | 3.31 (0.92) |
| 30-day Health Symptoms Parent | 1.38 (1.4) | 1.79 (1.78) |
| Temperature at blood draw (F) | 97.9 (0.56) | 97.7 (0.52) |
| CRP (mg/L) | 2.24 (4.07) | 1.26 (2.38) |
| WBC (103/μl) | 6.18 (1.67) | 5.78 (1.52) |
| Lymphocytes (%) | 34.84 (9.5) | 32.18 (6.95) |
| CMV antibody titer*** | 76.87 (40.22) | 33.49 (49.14) |
| CMV Seropositive Status, n (%)*** | 39 (86.7%) | 14 (35.9%) |
Mean and (SD) unless noted.
** = p < 0.01, ***p < 0.001.
Race/Ethnicity operationalized as “white”=1, “non-white”=0 in analyses.
2.2. Procedure
Following recruitment, parent/youth questionnaire reports were completed at home and brought to the clinical research center. Participants arrived to the university clinical research center for < 25 mL blood draw from antecubital vein by a licensed phlebotomist, with approximately 10 mL drawn into sodium heparin-coated vacutainers and another 14 mL into EDTA-coated vacutainers. Fresh blood samples were shipped overnight with a coolant block by express courier for immune processing; the immunophenotyping was conducted immediately on receipt within 24 h of collection. An assessment of the Complete Blood Counts as well as cell viability on the flow cytometer verified there had not been significant cell loss. All draws were performed between 8:00 – 13:00, and the following information was collected at that time: body temperature, blood pressure, height/ weight, and hip-waist circumferences. See Table 1 for descriptive statistics for each group.
2.3. Immune measures
2.3.1. Complete blood count (CBC)
One mL of blood was sent to a CLIA-certified clinical laboratory (Meriter Labs) to determine a CBC with cell differential. The measures analyzed for this report include the Total White Count, lymphocyte percent, and lymphocyte number per μL of blood.
2.3.2. C-reactive protein (CRP)
CRP levels were quantified in duplicate determinations by single-plex assay using an electrochemiluminescence platform (Meso Scale Discovery, Rockville, MD). The lower limit of detection was 0.1 mg/L, with a high dynamic range up to over 100 mg/L.
2.3.3. Cytomegalovirus (CMV) antibody
CMV status was evaluated by enzyme-linked immunosorbent assay (ELISA, DRG). In addition to determining if the participant was seropositive for CMV, the inclusion of 3 calibrators with known antibody concentrations allowed for a quantification of antibody titer. Values above the second calibrator were considered to be indicative of being a CMV carrier following the manufacturer’s instructions.
2.3.4. Immunophenotyping
Heparinized blood (7 mL) was delivered immediately to the Flow Lab at the UW Carbone Center for immunophenotypic characterization. An optimized 18-color panel of monoclonal antibodies was used to identify the T cell lineages (Moncunill et al., 2014). Some fluorochrome-antibody combinations were modified to accommodate the BD LSR Fortessa configuration and are available upon request. Cell data were first gated for stability of sampling based on time, followed by gating for live, single CD3+ lymphocytes, removing monocytes with a Boolean logic gate defined as “not Classical Monocytes (CD14+ CD16−) and not Intermediate Monocytes (CD14+ CD16+) and not Non-Classical Monocytes (CD14−CD16+) OR HLA-DR+”. CD4+ and CD8+ cells were gated within the CD3+ cells. Memory/Naive populations were gated within both CD4 and CD8 populations by plotting CD45RA against CCR7 using quadrant gates. Naïve and memory populations were defined as follows: Naive as CD45RA+ CCR7+, Central Memory (CM) as CD45RA−CCR7+, Effector Memory (EM) as CD45RA−CCR7−, and Terminally Differentiated Effector Memory (TEMRA) as CD45RA+ CCR7−. CD4 and CD8 populations were also gated for CD57 positivity by plotting each according to whether they expressed CD57. In all cases the gates were set using the corresponding Fluorescent Minus One control. The gating strategy is illustrated in Supplemental Materials (Supplemental Figs. 1–3).
2.4. Health report
Health data were obtained to determine whether the PI and NA participants differed in illness conditions prior to age 5 when PI youth were either in or recovering from institutional care, subsequently from 5 to 18 years when both groups were living under comparable circumstances, or in the last 30 days, which might have acute effects on immune functioning. Health information was obtained from parents and youth.
2.4.1. Parent reported child health
This 45-item questionnaire was completed by parents. It contained a section on preadoption and adoption health conditions completed only by the adoptive parents (Hellerstedt et al., 2008). There was a section on infectious diseases from the Global Health Pediatric Clinic at the University of Minnesota, and questions on asthma and general health adapted from the California Health Interview Survey 2015. Three indices were computed as a total sum score after each item was endorsed as either absent or present (0,1). An Early Life Health Index was created to assess medical diagnoses from birth to 5 years of age. There were 31 items of which 15 were endorsed for one or more of the participants: hepatitis B, anemia, intestinal parasites, cerebral palsy, chronic ear infections, growth delay, dysplasia, heart defects, diabetes, asthma, TB-active, TB-inactive, autoimmune disorder, eczema, sexually transmitted disease, other. A Child and Adolescent Health Index was created to assess medical diagnoses from 5 to 18 years of age as well as other health problems including cold sores, canker sores, and allergies. Certain items (e.g., allergies) were summarized by collapsing or correcting all items subsumed under that condition (e.g., parents who did not endorse their children having allergies but reported allergens or allergy symptoms were recoded as “1” for allergies). Similarly, a correction factor was included to account for parents who did not endorse asthma in the list of medical diagnoses but reported their children having asthma symptoms at any point in the questionnaire. Finally, a 30-Day Health Index was created to assess parent report of youth’s symptoms in the past 30 days. Items on this scale include: fever, chills, cough, sneezing, sore throat, nausea, vomiting and so on. Each symptom was only counted once in the index.
2.4.2. Child reported physical health
All participants completed the Adolescent Health Habits Questionnaire for youth 12–18 years of age, a 79-item measure that included questions on general health, asthma, allergies, recent exposures to infectious diseases, sleep habits and daytime sleepiness, diet and nutrition and alcohol and drug use. The questions were derived and adapted from the (1) General Health, Asthma, Diet, Nutrition and Alcohol/Tobacco/Drug use questions on the California Health Interview Survey 2015 conducted by the UCLA Center for Health Policy Research, (2) Infectious Disease Questionnaire used in the Global Health Pediatric Clinic at the University of Minnesota, (3) Sleep Habit Questions based on work by Wolfson and Carskadon (1998), and the daytime sleepiness from the Cleveland Adolescent Sleepiness Questionnaire (Spilsbury et al., 2007). Two indices were computed from these questions. A Child and Adolescent Health Index reflecting asthma, cold sores, canker sores, allergies, and sexually transmitted conditions (HPV/STI). Responses were coded as Yes (1 = present ever) and No (0 = never present), such that scale responses could range from 0 to 5. Certain items (e.g. allergies) were created by collapsing or correcting all items addressing a relative construct (e.g. youth who did not endorse having allergies but reported allergens or allergy symptoms were recoded as “1” for allergies). A 30-Day Health Index was also computed with the same items as in the parent report measure.
2.5. Data analytic plan
Initial analyses were conducted to determine the comparability of the PI and NA groups with regard to child and adolescent health. Independent group t tests were used for continuous variables and the chi-square test used for discrete variables (Table 1). CRP values were natural log-transformed for analyses. Cell percentages were transformed with the arcsine transformation (2*(asin(sqrt[x]) to stabilize variance. The ratio of CD4-to-CD8 cells was computed by dividing CD4+ by CD8+ cell percentages. Regression analyses predicting group differences in the T cells, CD4+ subsets, CD8+ subsets, and CD4/CD8 ratio controlled for sex, time of blood draw, and age at blood draw. The Benjamini-Hochberg (BH) procedure was used to account for multiple comparisons. Cohen’s Fb was also calculated to aid in interpretation of effect sizes and F2 ≥ 0.02, F2 ≥ 0.15, and F2 ≥ 0.35 represent small, medium, and large effect sizes, respectively.
The final step of the analysis modeled whether CMV antibody levels mediated the relationship between ELA and differences in CD57 + T cell lineages using path analyses. This model also examined whether CMV antibody levels mediated the relationship between ELA and group differences in the CD4/CD8 ratio. CMV antibody levels were used as a continuous measure, and the extent of exposure to ELA was operationalized using time spent in institutional care (in months), with the value for non-adopted youths set to zero.
These analyses focused primarily on the main effect of early rearing. Exploratory analyses were also conducted to examine the possible interaction of Participant Sex with the impact of early rearing. The latter analyses are considered exploratory because of the relatively small number of PI males (see Table 1). Analyses were conducted in R version 3.4.3 with the “lavaan” package (Rosseel, 2012).
3. Results
3.1. Demographics and general health
The adolescents from both rearing conditions were primarily white from European family backgrounds (see Table 1). Nineteen of the adopted participants were from other ethnic/racial backgrounds (42.2%). All were raised by white families in the United States. There were no major differences in current health status based on either self-report or parent-reported health. However, PI youth were on average 3 in. shorter at assessment, t(82) = −3.30, p = 0.001 and, as would be expected, had more early health problems, t(82) = 2.73, p = 0.008. In keeping with the recruitment strategy that excluded potential participants with infectious and chronic illness, the Leukocyte counts and CRP levels were not different between groups. But of particular importance for our immunophenotyping analysis, because of the potential influence on certain T cells, PI youth were significantly more likely to be seropositive for CMV (86.7% vs 35.9%, p < 0.01), and had a significantly higher CMV antibody titer, t(82) = 4.39, p < 0.001.
3.2. Immunophenotyping
3.2.1. T cells
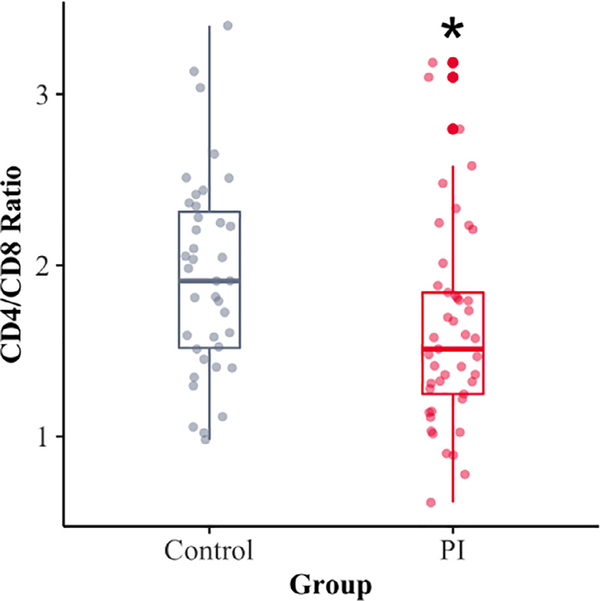
Rearing condition did not have a significant effect on the overall numbers of CD3+ T cells. However, the PI youth had lower percentages of CD4+ cells (54.7%) than did the NA youth (59.9%) (p = 0.007, Cohen’s F2 = 0.01) and a higher percent of CD8+ cells (36%) than the NA youth (32.3%) (p = 0.04, Cohen’s F2 = 0.06) (Table 2). After correcting for multiple comparisons, these differences in the two rearing conditions remained significant. These differences resulted in a significantly lower CD4-to-CD8 ratio in the PI as compared to the NA youth (p = 0.024, BH corrected p = 0.036, Cohen’s F2 = 0.07) (Fig. 1). While statistically significant, the effect size for this difference was small. As displayed in Fig. 1, few youth from either group showed a very low CD4-to-CD8 ratio, or a extremely reduced value that would be indicative of an immunosuppressive disorder.
Table 2.
Group differences in T cell repertoire for PI and NA youth.
| Cell Type | PI1 (n = 45) % | Control1 (n = 39) % | Adj. group difference* | 95% CI | P | Corrected P | Cohen’s F2 |
|---|---|---|---|---|---|---|---|
| CD4+ | 54.7 (8.6) | 59.9 (7.2) | −0.101 | −0.175 to −0.028 | 0.007 | 0.021 | 0.01 |
| CD4+ CD57+ | 2.6 (1.9) | 1.8 (1.7) | 0.060 | 0.013–0.106 | 0.012 | 0.030 | 0.08 |
| CD4+ CD57+ Naïve | 0.5 (0.4) | 0.5 (0.8) | 0.006 | −0.022 to 0.035 | 0.648 | 0.648 | 0.003 |
| CD4+ CD57+ CM | 2.3 (1.3) | 2.1 (1.8) | 0.015 | −0.026 to 0.056 | 0.463 | 0.579 | 0.007 |
| CD4+ CD57+ EM | 8.1 (5.0) | 5.2 (3.6) | 0.116 | 0.039–0.193 | 0.004 | 0.02 | 0.11 |
| CD4+ CD57+ TEMRA | 10.0 (11.8) | 8.4 (10.5) | 0.064 | −0.079 to 0.209 | 0.374 | 0.579 | 0.01 |
| CD8+ | 36.0 (7.4) | 32.3 (7.3) | 0.074 | 0.004–0.144 | 0.040 | 0.040 | 0.06 |
| CD8+ CD57+ | 21.4 (11.4) | 16.1 (10.7) | 0.139 | 0.013–0.265 | 0.032 | 0.040 | 0.06 |
| CD8+ CD57+ Naïve | 2.1 (2.3) | 3.7 (7.7) | −0.046 | −0.132–0.041 | 0.300 | 0.300 | 0.01 |
| CD8+ CD57+ CM | 18.9 (12.3) | 12.5 (11.1) | 0.234 | 0.080–0.389 | 0.003 | 0.005 | 0.12 |
| CD8+ CD57+ EM | 41.6 (17.1) | 28.0 (20.2) | 0.359 | 0.169–0.548 | < 0.001 | 0.002 | 0.18 |
| CD8+ CD57+ TEMRA | 43.6 (20.5) | 29.3 (15.5) | 0.302 | 0.119–0.485 | 0.002 | 0.005 | 0.14 |
Numbers in bold indicate P < .05.
Group difference of arcsine transformation and adjusted for sex, time of blood draw, and age at blood draw, with PI=1 and Control=0. Cohen’s F2 as difference in effect sizes with group and without group as a predictor.
Mean and (SD).
Fig. 1.
The PI adolescents had significantly lower CD4-to-CD8 Ratios than did the NA control adolescents who had been typically-reared from birth (P < 0.01).
3.2.2. CD3+ C57+ cells
It was also more common for the CD4+ and CD8+ cells of the PI youth to express the CD57 glycoprotein surface marker indicative of prior activation and a reduced propensity to proliferate upon exposure to cognate antigen. A history of institutionalization during infancy was associated with a higher percent of CD4+ CD57+ cells (PI: 2.6%, NA: 1.8%, p = 0.012, Cohen’s F2 = 0.08) and a higher percent of CD8+ CD57+ cells (PI: 21.4%, NA: 16.1%, p = 0.032, Cohen’s F2 = 0.06). These associations remained significant after correction for multiple comparisons (Table 2). In an exploratory analysis examining the interaction between rearing and the participant’s sex on CD8+ CD57+ T cells, the PI males evinced a significantly higher percent of CD8+ cells that co-expressed CD57 (PI Male: 28%, PI Female: 15.2%, NA Male: 16.5%, NA Female: 15.9%, p = 0.018, Cohen’s F2 = 0.07, BH corrected p = 0.027).
3.2.3. C57+ T cell lineages
The immunophenotyping gating strategy allowed us to further differentiate the CD4+ C57+ and CD8+ CD57+ cells into 4 subsets by determining the frequency of younger naïve cells (naïve) and the ones co-expressing surface and co-stimulatory markers indicative of a history of activation and replication. Overall, the percentages of CD4+ CD57+ T cells were lower than the CD8+ CD57+ subsets and only the percent of CD4+ CD57+ Effector/Memory (EM) cells was affected by early rearing: significantly higher in the PI adolescents (PI: 8.1%, NA: 5.2%, p = 0.004, Cohen’s F2 = 0.11). After correcting for multiple comparisons, this relationship remained significant (Table 2).
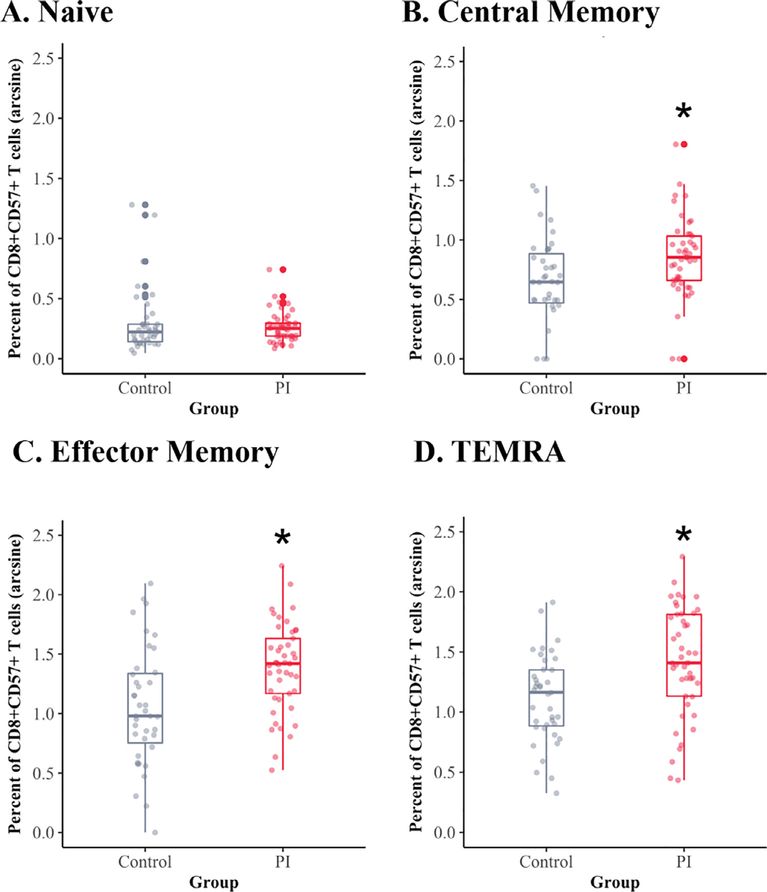
A larger impact of early rearing was evident for the CD8+ CD57+ subsets, especially for the differentiated subsets, because the percent of naïve cells in this cell population was relatively low (Fig. 2A). As shown in Fig. 2A, there were no group differences in the percent of CD8+ CD57+ naïve cells. However, the PI youth had more CD8+ CD57+ Central-Memory cells (PI: 18.9%, NA: 12.5%, p = 0.005, Cohens F2 = 0.12) as compared to the non-adopted adolescents (Fig. 2B). After correcting for multiple comparisons, these relationships remained significant. In an exploratory analysis of the interaction between rearing history and sex of the participant, the increase in CD8+ CD57+ Central-Memory cells was more prominent in the PI males (PI Male: 22.6%, PI Female: 15.9%, NA Male: 9%, NA Female: 15.2%, p = 0.008, Cohen’s F2 = 0.09, BH corrected p = 0.024). As shown in Fig. 2C, the CD8+ CD57+ EM cells were present at higher numbers in adolescents from adoptive backgrounds (PI: 41.6%, NA: 28%, p < 0.001, Cohen’s F2 = 0.18) (Fig. 2C). Finally, significant differences were found in the numbers of the terminally differentiated CD8+ CD57+ cells, with larger numbers of terminally differentiated CD8+ CD57+ cells present in PI youth (PI: 43.6%, NA: 29.3%, p = 0.002, Cohen’s F2 = 0.14) (Fig. 2D). An exploratory investigation of the terminally differentiated CD8+ CD57+ cells again showed that the males from institutionalized backgrounds had higher percentages of these TEMRA cells (PI Male: 52.4%, PI Female: 36.5%, NA Male: 28.6%, NA Female: 30%, p = 0.03, Cohen’s F2 = 0.06, BH corrected p = 0.03). The effect sizes for the rearing differences were largest for the CD8+ CD57+ EM and CD8+ CD57+ terminally differentiated cell subsets.
Fig. 2.
Significant differences were found in the 3 more differentiated subsets of the CD8+ CD57+ cell subsets. The CD8+ CD57+ naïve populations were present at the lowest levels and did not differ across rearing conditions (A. Naive). However, the percent of CD8+ central-memory cells (B. CM), percent of CD8+ CD57+ effector-memory cells (C. EM) and percent of CD8+ CD57+ terminally differentiated cells (D. TEMRA) all were significantly higher in the PI adolescents. The arcsine-transformed percentages of CD8+ CD57+ subsets are portrayed. Raw values are provided in Table 2.
3.2.4. CMV infection
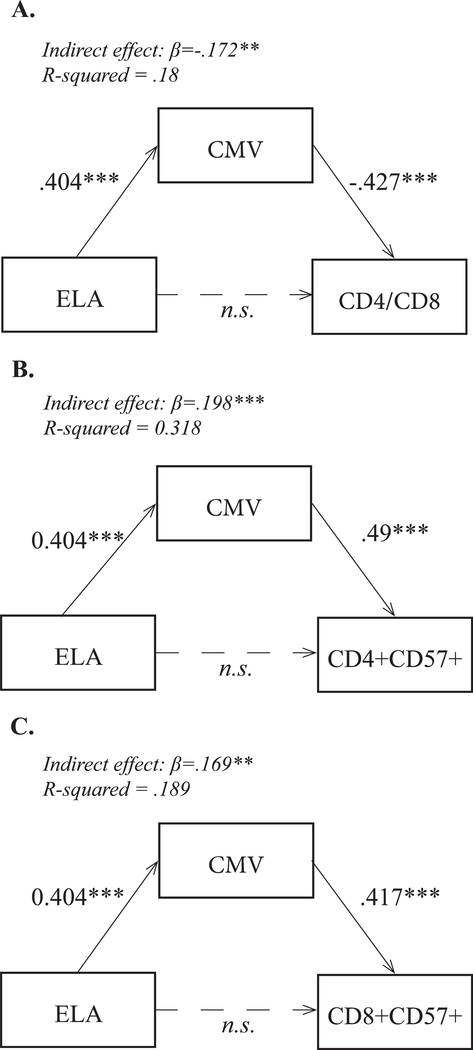
The influence of early rearing (indexed by time spent in institutional care) on the T cell repertoire was significantly associated with the adolescents’ current CMV antibody level. In the statistical model, CMV antibody titer fully mediated the relationship between exposure to ELA and CD4/CD8 ratio (Fig. 3A). A mediation analysis demonstrated a large mediating effect of CMV antibody titer on the CD4+ CD57+ cells (Fig. 3B) and CD8+ CD57+ cells (Fig. 3C). There was also a significant pairwise correlation between the CMV antibody titer and the percentages of CD4+ CD57+ and CD8+ CD57+ cell subsets, which was evident in adolescents from both rearing conditions (Table 3).
Fig. 3.
Mediation analyses or the relationship between ELA, CMV seropositivity, and the CD4/CD8 ratio, CD4+ CD57+ cells, and CD8+ CD57+ cells as outcomes with standardized coefficients. (A) ELA considered as an independent variable, CD4/CD8 ratio as the dependent variable, and CMV titer as mediator variable. (B) ELA as independent variable, arcsine-transformed percentages of CD4+ CD57+ cells as dependent variable, and CMV titer as mediator variable. (C) ELA as independent variable, arcsine-transformed percentage of CD8+ CD57+ as dependent variable, and CMV titer as mediator variable. Statistic modeling: ELA was treated as continuous variable (age at adoption); values for control youth were set to 0. In each path analysis, the dependent outcome controlled for sex and age at blood draw. Significance was set at *p < 0.05, **p < 0.01, ***p < 0.001.
Table 3.
Correlations between CMV antibody titers and CD4/CD8 ratio, CD4+ T cell percentages, CD8+ T cell percentages, and CD3+ CD4+ CD57+ and CD3+ CD8+ CD57+ subsets.
| Cell type | CMV antibody titers correlation coefficient | Body Temperature (Degrees F) | Body Mass Index Percentile |
|---|---|---|---|
| CD4/CD8 Ratio | −0.426** | −0.093 | −0.124 |
| Arcsine % CD4 T cells | −0.458** | −0.035 | −0.079 |
| Arcsine % CD4+ CD57+ cells | 0.501** | 0.201 | 0.122 |
| Arcsine % CD4+ CD57+ Naive cells | 0.157 | −0.082 | −0.233* |
| Arcsine % CD4+ CD57+ CM cells | 0.151 | 0.033 | −0.077 |
| Arcsine % CD4+ CD57+ EM cells | 0.585** | 0.148 | 0.176 |
| Arcsine % CD4+ CD57+ TEMRA cells | 0.443** | 0.129 | −0.086 |
| Arcsine % CD8+ T cells | 0.429** | 0.100 | 0.186 |
| Arcsine % CD8+ CD57+ cells | 0.501** | 0.062 | 0.169 |
| Arcsine % CD8+ CD57+ Naïve cells | 0.319** | −0.073 | 0.008 |
| Arcsine % CD8+ CD57+ CM cells | 0.263* | 0.174 | 0.046 |
| Arcsine % CD8+ CD57+ EM cells | 0.281** | 0.131 | 0.185 |
| Arcsine % CD8+ CD57+ TEMRA cells | 0.527** | 0.085 | 0.210 |
Correlation is significant at the 0.05 level (2-tailed).
Correlation is significant at the 0.01 level (2-tailed).
4. Discussion
This study has confirmed that early rearing in an institutional setting is associated with different T cell profiles. Not only did we replicate our earlier finding of a decrease in the CD4 to CD8 cell ratio that had been obtained using epigenetic markers (Esposito et al., 2016), we also demonstrated a significant increase in both CD4+ and CD8+ cells expressing the CD57+ surface marker indicative of a history of prior activation and proliferation. The current finding replicates the conclusion from earlier identification of cell types via methylation patterns now using standard flow cytometry methods and only a partially overlapping (23.8%) sample of participants. In our statistical modeling of this effect of early rearing on the T cell profiles, the findings were shown to be mediated by being a latent carrier of CMV and were more specifically associated with current CMV antibody titer. These results extend prior research with animal models to humans and convey the need to broaden our examination of the effects of ELA on immunity beyond just the recent focus on inflammatory physiology to also include the maturation of the thymus and T cell regulation. The findings also suggest that the post-institutionalized individuals may be less able to respond effectively to an infectious pathogen and could be predisposed to an accelerated pace of immune aging later in adulthood. Similar results on T cell populations were obtained in a study of young adults, 20–34 years of age, who had been adopted by 3 months of age (Elwenspoek et al., 2017b), but our research indicates the T cell differences are already evident by early to mid-adolescence. The biological processes that may drive the findings are yet unclear. However, it is likely that they arise in part from the PI children’s increased exposure to CMV in the institutional setting as group care is a known risk factor for CMV exposure in young children. Therefore, it is most parsimonious to conclude that the findings are driven by this early viral exposure and the sustained exposure of their cells to viral antigen. Less likely but plausible is that the absence of parenting and the severity of adversity engendered a greater susceptibility to acquiring a prolonged CMV infection with recurrent reactivation.
The prevalence of CMV infection in the PI adolescents was remarkably high. Of the 45 PI adolescents studied, 86.7% were seropositive. This level of Herpes infection is much higher than would be expected at the same age in the U.S. population. A more typical prevalence of 35.9% CMV seropositivity was found among the control adolescents who were reared by their biological parents. Our adopted participants had been exposed to institutional care in a large group setting for an average of 16 months. We also observed this high prevalence of CMV infection in both children adopted from Eastern European institutions as well as from other countries (N = 30 and 15, respectively), suggesting that early exposure to a group setting is the mostly likely cause. It is known that there is an increased incidence of CMV in children attending childcare centers at a young age, as described previously by others (Adler, 1991). Our adopted participants were exposed to a longer period of ELA than those evaluated by El-wenspoek (2017b). We were also able to quantify CMV-specific antibody titers and thus could demonstrate the statistical magnitude of how virus exposure mediated the influence of ELA on the T cell repertoires.
We also replicated the finding that ELA is associated with a lower CD4/CD8 ratio (Esposito et al., 2016). In the statistical models, current CMV antibody levels fully mediated this relationship between ELA and the CD4/CD8 ratio. An association between being CMV seropositive and having lower CD4/CD8 ratios had also been reported in two studies of elderly Swedish adults (Olsson et al., 2001; Wikby et al., 2002). In addition, a similar relationship was reported for a cohort of healthy young adults (Turner et al., 2014). These findings have led a number of investigators to conclude that the immune containment of CMV contributes to the accelerated aging like that described for the immune systems of older adults (Hadrup et al., 2006; Olsson et al., 2001; Roberts et al., 2010). It may drive a progressive accumulation of cells with a decreased proliferative capacity and reduced responsiveness in the young adult (Elwenspoek et al., 2017b; Turner et al., 2014). Our study provides an important demonstration of CMV effects on CD4+ and CD8+ subsets that is already evident by adolescence and highlights its prominent role in mediating the relationship between ELA and T cell biology.
The association was especially evident for the CD8+ CD57+ TEMRA cells, which replicates findings of several other laboratories on both typical family-raised participants and adopted individuals (Elwenspoek et al., 2017b). In general, TEMRA are thought to exhibit a ‘replicative senescence’ and be less functional for mounting an immune response to a new infection. As mentioned earlier, they have also been found to have shorter telomere lengths, reflecting their history of prior replication and activation. Their presence in higher numbers in adopted adolescents, especially in the CMV carriers, probably also reflects their role in keeping Herpes viruses in the quiescent latent state, although it could partially be a reflection of chronic, asymptomatic viral reactivation. This shift in the percentages of T cell subsets is unlikely to be due to early selection in the thymus or the apoptotic death of certain emigrants, because we did not detect a general lymphocytopenia or decreases in the number or percent of naïve T cell populations. Further, the PI adolescents actually evinced a higher overall percent of CD8+ cells than did the control teens.
The age of CMV exposure in our participants is not known. Prenatal infections to CMV or maternal transmission during delivery can be lethal, and are a known risk factor for neurodevelopmental disorders. We believe it is more likely that the adopted participants were exposed as young infants in the orphanage settings. A study of international adoptees found a 45% seroprevalence of CMV, and most cases of CMV were determined to be postnatal rather than via congenital exposure (Hostetter et al., 1989; Murray et al., 2005). The seroprevalence in that study was more comparable to the national seroprevalence for American children under 6 years (~36%) (Staras et al., 2006); however, the majority of children in that survey were likely adopted after foster care rather than institutional settings (Hostetter et al., 1989). Dowd et al (2012) reported that the seroprevalence in a nationally representative sample of children aged 6–16 in the US was 43% for children above the poverty line. But it should be highlighted that our participants had a much higher seroprevalence (PI = 86%, control = 34.8%). Determining the role of CMV and other Herpes virus infections in accounting for the impact of ELA on immunity is critical not only for PI youth, but also for other children experiencing ELA in the form of poverty and maltreatment around the world.
Although the current health status of PI youth did not differ from the comparison group, these findings could have multiple implications for the health of youth with a history of ELA as they become older adults. Their lower CD4/CD8 ratios already is suggestive of an immune dysregulation and with age it may be associated with a reduced production of T cell cells by the thymus. In addition, several of the current immune findings were more evident in the male adolescents from adoptive backgrounds, suggesting they are more impacted by ELA. This conclusion would be in keeping with the greater vulnerability of males to several neurodevelopmental disorders and also concurs with the many animal studies that reported a sex difference in the effects of exposure to ELA. However, the potential interaction of ELA with sex needs to be viewed cautiously because of the relatively small number of PI males in our study.
4.1. Limitations
Notwithstanding the significance and clarity of our findings, several limitations should be acknowledged. First, while all adoptees experienced a similar period of postnatal ELA, it was not possible to control for differences in prenatal conditions. It is known that the maturation of the thymus is very sensitive to prenatal insults, including exposure to alcohol, which can result in thymic atrophy and involution (Di Naro et al., 2006). While it is known that the fetal thymus will undergo a marked reduction in size and structure in response to maternal illness, stress and teratogenic exposure, it is not known if the cell populations produced by the thymus after recovery and birth will continue to be impacted. However, if the thymus is entirely removed from a human infant in order to gain access to the heart for cardiac surgery, then there will continue to be differences in T cell subsets that persist (Schadenberg et al., 2014). With regard to this type of extreme explanation for our findings it should be reiterated that we excluded for phenotypic features indicative of fetal alcohol syndrome. Future studies should attempt to consider the summative influence of both prenatal and postnatal conditions, given that maternal stress and poor nutrition during pregnancy as well as ELA after birth can affect neurological and immunological development (Coe and Lubach, 2005; Veru et al., 2015, Veru et al., 2014). A second limitation is that we did not consider a functional measure of immune competence in this analysis, such as the capacity to respond to a viral infection or effectively to immunization. However, in future analyses, we will be addressing the proliferative capacity of their cells in response to antigenic stimulation during in vitro culturing. With regard to interpreting the changes in the CD8+ TEMRA cells as an early indicator of replicative senescence, it would also have been helpful to include measures of telomere length and telomerase activity. Telomere length has been associated with ELA, and meta-analyses suggest that early adversity may have a long-lasting influence on biological aging (Ridout et al., 2018). The Elwenspoek study of young adults from PI backgrounds had already reported that it was not possible to demonstrate a general association between ELA and leukocyte telomere length (2017b). To do so in a sensitive manner would likely have required cell sorting to more specifically focus just on the CD8+ CD57+ TEMRA cells. In addition, to carefully examine the senescent state of specific cell populations can sometimes require a focused study of their proliferative capacity to respond to unique and specific antigens (Verma et al., 2017).
Additionally, there are a few procedural limitations to acknowledge. First, participant blood was drawn between 08:00 and 13:00, which is a large enough range to potentially introduce an influence of the diurnal rhythm in CD4+ and CD8+ cells. Though we addressed this concern by controlling for time at blood draw in all analyses, future work should carefully consider the potential influence of biological rhythms. Another issue is the contemporaneous measurement of CMV antibody and the immunophenotyping of T cells at one age point, making it more difficult to verify causality and establish the antecedence of the CMV infection. Though a large literature supports our positing CMV infection as primary and the likely vector especially given influence of CMV on immune senescence in the elderly individual, our mediation model should be interpreted with caution, as it was not possible to definitively establish temporality between these two measures. Finally, as already discussed, there were suggestive indications that the PI males were more impacted, but this finding of a gender difference in vulnerability should be replicated with a larger cohort.
4.2. Conclusion
In summary, ELA in the form of institutional rearing during infancy was associated with persistent immune differences that were still evident in adolescents, years after the adversity had ended. Specifically, there were significant differences in the T cell profile that were statistically associated with exposure to CMV. Early infections with CMV are thus likely to contribute to the connection between ELA and immune dysregulation. In older adults, this type of sustained antigen persistence and immunoregulatory activity leads to immunological declines and ultimately to immune senescence. Future research still needs to determine whether the length of time in the orphanage setting is a factor of critical concern, highlighting the policy implications of striving for an early age for adoption.
Supplementary Material
Acknowledgements
We would like to express our gratitude to the families who make this research possible. Thanks are also due to the recruiting and collection team: Lea Neumann, Heather Taylor, Tori Simenec, Aurora Wiseman, Melissa Engel, and Anna Parenteau, and the phlebotomy team at the UM Clinical and Translational Science Institute. This research was supported by a grant from the National Institute of Child Health and Human Development [R21 HD086312] to MRG and CLC. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional support for the immunophenotyping was provided by the Special BD LSR Fortessa Shared Instrumentation Grant 1S100OD018202–01 and the University of Wisconsin Carbone Cancer Center Support Grant P30 CA014520. This material is based upon work also supported by the National Science Foundation Graduate Research Fellowship (NSF Grant No. 00039202, awarded to BMR and NSF Grant No. 2016199960, awarded to CMD). All opinion, findings, conclusions or recommendations expressed are those of the authors and do not necessarily reflect the views of the NSF.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbi.2019.01.001.
References
- Ader R, 1983. Developmental psychoneuroimmunology. Dev. Psychobiol 16, 251–267. [DOI] [PubMed] [Google Scholar]
- Ader R, 2000. On the development of psychoneuroimmunology. Eur. J. Pharmacol 405, 167–176. [DOI] [PubMed] [Google Scholar]
- Ader R, Friedman SB, 1965. Differential early experiences and susceptibility to transplanted tumor in the rat. J. Comp. Physiol. Psychol 59, 361–364. [DOI] [PubMed] [Google Scholar]
- Adler SP, 1991. Molecular epidemiology of cytomegalovirus: a study of factors affecting transmission among children at three day-care centers. Pediatr. Infect. Dis. J 10, 584–590. [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Cohen HJ, Kraus WE, Ramrakha S, Poulton R, Moffitt TE, 2017. Impact of early personal-history characteristics on the pace of aging: implications for clinical trials of therapies to slow aging and extend healthspan. Aging Cell 16, 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchly JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BU, Cortty LE, Casazza JP, Kuroppus J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA, 2003. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood 101, 2711–2720. [DOI] [PubMed] [Google Scholar]
- Bos K, Zeanah CH, Fox NA, Drury SS, McLaughlin KA, Nelson CA, 2011. Psychiatric outcomes in young children with a history of institutionalization. Harv. Rev. Psychiatry 19, 15–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH, 2009. Adverse childhood experiences and the risk of premature mortality. Am. J. Prev. Med 37, 389–396. [DOI] [PubMed] [Google Scholar]
- Chidrawar S, Khan N, Wei W, McLarnon A, Smith N, Nayak L, Moss P, 2009. Cytomegalovirus-seropositivity has a profound influence on the magnitude of major lymphoid subsets within healthy individuals. Clin. Exp. Immunol 155, 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, 2005. Prenatal origins of individual variation in behavior and immunity. Neurosci. Biobehav. Rev 29, 39–49. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Ershler WB, Klopp RG, 1989. Influence of early rearing on lymphocyte proliferation responses in juvenile rhesus monkeys. Brain Behav. Immun 3, 47–60. [DOI] [PubMed] [Google Scholar]
- Di Naro E, Cromi A, Ghezzi F, Raio L, Uccella S, D’Addario V, Loverro G, 2006. Fetal thymic involution A sonographic marker of the fetal inflammatory response syndrome. Am. J. Obset. Gynecol 194 (1), 153–159. [DOI] [PubMed] [Google Scholar]
- Dowd JB, Aiello AE, Alley DE, 2009. Socioeconomic disparities in the seroprevalence of cytomegalovirus infection in the US population: NHANES III. Epidemiol. Infect 137, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowd JB, Palermo TM, Aiello AE, 2012. Family poverty is associated with cytomegalovirus antibody titers in U.S. children. Health Psychol. 31, 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwenspoek MMC, Kuehn A, Muller CP, Turner JD, 2017a. The effects of early life adversity on the immune system. Psychoneuroendocrinology 82, 140–154. [DOI] [PubMed] [Google Scholar]
- Elwenspoek MMC, Sias K, Hengesch X, Schaan VK, Leenen FAD, Adams P, Meriaux SB, Schmitz S, Bonnemberger F, Ewen A, Schachinger H, Vogele C, Muller CP, Turner JD, 2017b. T cell immunosenescence after early life adversity: association with cytomegalovirus infection. Front. Immunol 8, 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito EA, Jones MJ, Doom JR, MacIsaac JL, Gunnar MR, Kobor MS, 2016. Differential DNA methylation in peripheral blood mononuclear cells in adolescents exposed to significant early but not later childhood adversity. Dev. Psychopathol 28, 1385–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS, 1998. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am. J. Prev. Med 14, 245–258. [DOI] [PubMed] [Google Scholar]
- Hadrup SR, Strindhall J, Kollgaard T, Seremet T, Johansson B, Pawelec G, thor Straten P, Wikby A, 2006. Longitudinal studies of clonally expanded CD8 T cells reveal a repertoire shrinkage predicting mortality and an increased number of dysfunctional cytomegalovirus-specific T cells in the very elderly. J. Immunol 176, 2645–2653. [DOI] [PubMed] [Google Scholar]
- Hayward MD, Gorman BK, 2004. The long arm of childhood: the influence of early-life social conditions on men’s mortality. Demography 41, 87–107. [DOI] [PubMed] [Google Scholar]
- Hellerstedt WL, Madsen NJ, Gunnar MR, Grotevant HD, Lee RM, Johnson DE, 2008. The International Adoption Project: population-based surveillance of Minnesota parents who adopted children internationally. Matern. Child Health J 12, 162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostetter MK, Iverson S, Dole K, Johnson D, 1989. Unsuspected infectious diseases and other medical diagnoses in the evaluation of internationally adopted children. Pediatrics 83, 559–564. [PubMed] [Google Scholar]
- Janicki-Deverts D, Cohen S, Doyle WJ, Marsland AL, Bosch J, 2014. Childhood environments and cytomegalovirus serostatus and reactivation in adults. Brain Behav. Immun 40, 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudenslager M, Capitanio JP, Reite M, 1985. Possible effects of early separation experiences on subsequent immune function in adult macaque monkeys. Am. J. Psychiatry 142, 862–864. [DOI] [PubMed] [Google Scholar]
- Lewis MH, Gluck JP, Petitto JM, Hensley LL, Ozer H, 2000. Early social deprivation in nonhuman primates: long-term effects on survival and cell-mediated immunity. Biol. Psychiatry 47, 119–126. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Parker KJ, 2011. Psychological stress in childhood and susceptibility to the chronic diseases of aging: moving toward a model of behavioral and biological mechanisms. Psychol. Bull 137, 959–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncunill G, Campo JJ, Dobaño C, 2014. Quantification of multiple cytokines and chemokines using cytometric bead arrays In: Cytokine Bioassays. Humana Press, New York, NY, pp. 65–86. [DOI] [PubMed] [Google Scholar]
- Murray TS, Groth ME, Weitzman C, Cappello M, 2005. Epidemiology and management of infectious diseases in international adoptees. Clin. Microbiol. Rev 18, 510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson B-O, Ferguson FG, 2001. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev 121, 187–201. [DOI] [PubMed] [Google Scholar]
- Olsson J, Wikby A, Johansson B, Löfgren S, Nilsson BO, Ferguson FG, 2000. Age-related change in peripheral blood T-lymphocyte subpopulations and cytomegalovirus infection in the very old: the Swedish longitudinal OCTO immune study. Mech. Ageing Dev 121, 187–201. [DOI] [PubMed] [Google Scholar]
- Pawelec G, Akbar A, Caruso C, Solana R, Grubeck-Loebenstein B, Wikby A, 2005. Human immunosenescence: is it infectious? Immunol. Rev 205, 257–268. [DOI] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, Price LH, Tyrka AR, 2018. Early life adversity and telomere length: a meta-analysis. Mol. Psychiatry 23, 858–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE, 2010. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am. J. Epidemiol 172, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosseel Y, 2012. Lavaan: an R package for structural equation modeling and more. Version 0.5–12 (BETA). J. Stat. Softw 48. [Google Scholar]
- Schadenberg AL, van den Broek T, Siemelink MA, Algra SO, de Jon PR, Jansen NJG, Prakken BJ, van Wijk F, 2014. Differential homeostatic dynamics of human regulatory T cell subsets frollowing neonatal thymectomy. J. Allerg. Clin. Immunol 133 (1), 277–280. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS, 2009. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA 301, 2252–2259. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Garner AS, 2012. The lifelong effects of early childhood adversity and toxic stress. Pediatrics 129, e232–246. [DOI] [PubMed] [Google Scholar]
- Slopen N, Loucks EB, Appleton AA, Kawachi I, Kubzansky LD, Non AL, Buka S, Gilman SE, 2015. Early origins of inflammation: an examination of prenatal and childhood social adversity in a prospective cohort study. Psychoneuroendocrinology 51, 403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon GF, Levine S, Kraft JK, 1968. Early experience and immunity. Nature 220, 821–822. [DOI] [PubMed] [Google Scholar]
- Spilsbury JC, Drotar D, Rosen CL, Redline S, 2007. The Cleveland adolescent sleepiness questionnaire: a new measure to assess excessive daytime sleepiness in adolescents. J. Clin. Sleep Med 3, 603–612. [PMC free article] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ, 2006. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin. Infect. Dis 43, 1143–1151. [DOI] [PubMed] [Google Scholar]
- Strioga M, Pasukoniene V, Characiejus D, 2011. CD8+CD28+ and CD8+CD57+ T cells and their role in health and disease. Immunol 134 (1), 17–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras SA, Flanders WD, Dollard SC, Pass RF, McGowan JE Jr., Cannon MJ, 2008. Cytomegalovirus seroprevalence and childhood sources of infection: a population-based study among pre-adolescents in the United States. J. Clin. Virol 43, 266–271. [DOI] [PubMed] [Google Scholar]
- Turner JE, Campbell JP, Edwards KM, Howarth LJ, Pawelec G, Aldred S, Moss P, Drayson MT, Burns VE, Bosch JA, 2014. Rudimentary signs of immunosenescence in Cytomegalovirus-seropositive healthy young adults. Age 36, 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K, Ogonek J, Varanasi PR, Luther S, Bunting I, Thomay K, Behrens YL, Mischak-Weissinger E, Hambach L, 2017. Human CD8+ CD57− TEMRA cells: too young to be called “old”. PLoS One 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veru F, Dancause K, Laplante DP, King S, Luheshi G, 2015. Prenatal maternal stress predicts reductions in CD4+ lymphocytes, increases in innate-derived cytokines, and a Th2 shift in adolescents: project ice storm. Physiol. Behav 144, 137–145. [DOI] [PubMed] [Google Scholar]
- Veru F, Laplante DP, Luheshi G, King S, 2014. Prenatal maternal stress exposure and immune function in the offspring. Stress 17, 133–148. [DOI] [PubMed] [Google Scholar]
- Wikby A, Johansson B, Olsson J, Lofgren S, Nilsson BO, Ferguson F, 2002. Expansions of peripheral blood CD8 T-lymphocyte subpopulations and an association with cytomegalovirus seropositivity in the elderly: the Swedish NONA immune study. Exp. Gerontol 37, 445–453. [DOI] [PubMed] [Google Scholar]
- Wolfson AR, Carskadon MA, 1998. Sleep schedules and daytime functioning in adolescents. Child Dev. 69, 875–887. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.