Abstract
OBJECTIVE
To determine whether the association between overall survival (OS) and response to neoadjuvant chemotherapy (NACT) in breast cancer patients varies with tumor subtype and anatomic extent of pathologic complete response (pCR).
BACKGROUND
pCR after NACT predicts improved OS in breast cancer, but it is unclear whether pCR limited to the breast or axilla is also associated with OS.
METHODS
Women with cT1–3/cN0–1 breast cancer diagnosed 2010–2014 who underwent surgery following NACT were identified in the NCDB and divided into 4 subtypes based on reported hormone receptor (HR) and HER2 status. Kaplan-Meier curves and Cox proportional hazards models were used to estimate OS. Multivariate logistic regression was used to identify factors associated with post-NACT response, defined as upstage (yp stage>clinical stage); no change (clinical stage=yp stage); overall (breast+axilla, ypT0N0), breast-only (ypT0N1/N1mic), or node-only (ypT1–3N0) pCR.
RESULTS
Of 33,162 identified patients, 20,265 experienced overall pCR (n=6,370, 19.2%), breast-only pCR (n=494, 1.5%), node-only pCR (n=1,133, 3.4%), no stage change (n=9,641, 29.1%), or upstage (n=2,627, 7.9%). Compared to no stage change, breast-only pCR was associated with improved OS in triple-negative disease (HR=0.58,95%CI=0.37–0.89), and node-only pCR was associated with improved OS in both triple-negative (HR=0.55,95%CI=0.39–0.76) and HR+/HER2- disease (HR=0.54,95%CI=0.33–0.89). For patients achieving overall (breast+axilla) pCR, unadjusted 5-year OS was 0.94 (95%CI=0.93–0.95), with no difference between patients who were cN0 (0.95,95%CI=0.93–0.96) or cN1 (0.94,95%CI=0.92–0.96) at diagnosis.
CONCLUSIONS
In node-positive patients, pCR limited to either the breast or axilla predicts survival for select receptor subtypes. In patients achieving pCR in both the breast and axilla, survival is driven by response to NACT rather than presenting cN stage.
Among patients undergoing neoadjuvant chemotherapy (NACT) for breast cancer, rates of pathologic complete response (pCR) in the breast, axilla, or both differ according to receptor subtype. pCR is associated with improved overall survival, but the effect of pCR on survival is dependent on both receptor subtype and anatomic extent of pCR.
Introduction
In breast cancer patients, axillary node involvement at diagnosis has long been considered the most critical determinant of long-term prognosis.1 Although this principle has historically been reflected in the anatomic stage/prognostic groupings published by the American Joint Committee on Cancer (AJCC),2 it has become increasingly clear that other clinicopathologic characteristics also play an important role in determining the long-term outcomes of breast cancer patients. Grade, tumor biology, and genomic testing results are now considered in conjunction with tumor size and lymph node involvement to determine breast cancer stage in the most recent AJCC guidelines.3 Furthermore, the development of highly effective HER2-targeted therapy has significantly improved disease-free and overall survival among patients who overexpress HER2, including those presenting with node-positive disease.4–7
Neoadjuvant chemotherapy (NACT) has emerged as a treatment strategy in patients presenting with node-positive breast cancer and a means by which the morbidity of locoregional treatment can be reduced or avoided. Systemic therapy for breast cancer was initially administered in the adjuvant setting, but its potential role in the preoperative treatment of resectable patients started to gain traction in the 1990s.8 NSABP B-18 and EORTC 10902 demonstrated the safety of administering neoadjuvant systemic therapy for non-metastatic breast cancer, allaying concerns that preoperative systemic treatment would be associated with worse survival compared with adjuvant administration.9,10 NACT can downstage the primary breast tumor, potentially facilitating lumpectomy. Furthermore, NACT enables in vivo, pre-surgical observation of treatment efficacy. This observed clinical response can subsequently be confirmed or refuted via pathologic analysis of the surgical specimen. However, the efficacy of neoadjuvant treatment varies significantly between regimens and among different receptor subtypes.
Pathologic complete response (pCR), or the absence of invasive disease in the breast and lymph nodes, is the optimal outcome following NACT and is associated with improved survival.9,11–15 But there has been some evidence that even a partial response to NACT may significantly impact prognosis.16,17 More recently, the role of NACT as a means of downstaging the axilla has been further explored, both as a source of prognostic information and as a way to avert the morbidity of axillary lymph node dissections (ALNDs) and nodal irradiation in clinically node-positive patients.16–21 Yet it remains unclear whether pCR that is anatomically limited to the breast or axilla confers clinically significant prognostic information that could potentially be used to assess the efficacy of novel agents in clinical trials or guide the clinical management and counseling of node-positive patients.
In this study, we sought to (1) establish rates of pCR – whether complete or limited to the breast and/or axilla – by breast cancer subtype and (2) determine whether achieving either full or anatomically limited (breast-only or axilla-only) pCR confers a survival benefit to node-positive patients, rendering their prognosis equivalent to that of patients who present with node-negative disease. We hypothesized that response to NACT, as demonstrated in previous smaller studies, has a greater impact on survival than extent of disease at presentation.
Methods
Patient Cohort
Female patients age ≥ 18 years old diagnosed with clinical tumor stage (cT) 1–3, clinical node stage 0–1 invasive breast cancer between 2010 and 2014 and who received surgery after NACT were identified from the National Cancer Data Base (NCDB). Clinical node (cN) stage is defined in the NCDB according to imaging studies (excluding lymphoscintigraphy), clinical examination demonstrating characteristics highly suspicious for malignancy, and/or pathologic diagnosis obtained via needle biopsy. Patients with noninvasive disease (i.e., Stage 0 or ductal carcinoma in situ [DCIS]) at diagnosis or on post-NACT pathological review (i.e., ypTis); cN2–3 disease; clinical or pathological stage M1 disease; missing stage information; no or unknown number of examined lymph nodes; a surgical procedure coded as “none,” “local tumor destruction only,” “not otherwise specified,” or “unknown”; and/or missing survival information were excluded. Although some definitions of pCR allow for the presence of residual DCIS, we chose to exclude patients with pathological stage ypTis. This strict definition of complete pCR allowed us to minimize heterogeneity in our primary endpoint, given emerging evidence that ypTis may be associated with worse long-term outcomes than ypT0.15
The cohort was divided into 4 subtypes based on combinations of hormone receptor (HR) and HER2 status, with HR-positive (HR+) defined as estrogen receptor-positive (ER+) and/or progesterone receptor-positive (PR+) while HR-negative was defined as estrogen receptor-negative (ER-) and progesterone receptor-negative (PR-): HR+/HER2-, HR+/HER2+, HR-/HER2+, and HR-/HER2- (i.e., triple-negative). pCR was defined as the absence of any residual invasive carcinoma or DCIS on pathologic review of a surgical specimen following NACT. Response to NACT was categorized as: (1) upstage (i.e., a change from lower cT and/or cN stage to higher ypT and/or ypN stage); (2) no stage change (cTN = ypTN); (3) overall pCR (i.e., breast+axilla, ypT0N0), (4) breast-only pCR (ypT0N1/N1mic), or (5) node-only pCR (ypT1–3N0). Breast-only and node-only pCR were only determined for patients who were clinically node-positive at diagnosis; clinically node-negative patients with no residual invasive disease on pathologic review were included in the overall (i.e., breast+axilla) pCR cohort.
It has already been shown that residual disease is, in general, associated with worse survival as compared to pCR. The focus of our analysis, however, was to determine whether anatomically limited pCR (which could also be thought of as anatomically segregated pCR with residual disease in a different compartment) is associated with survival when, compared with no change in stage and overall pCR. Thus, in order to simplify our statistical analysis and focus on the 3 forms of downstage (pCR in the breast, the axillary nodes, or both) in which we were primarily interested, we excluded cN1 patients who experienced breast or nodal downstaging without achieving pCR as well as cN1 patients who had discordant changes in breast and nodal stage (i.e., breast underwent upstage while axilla was downstaged and vice versa) following NACT from our regression and survival analyses. We also excluded patients with an uncategorized response to NACT.
Statistical Analysis
Chi-square tests and analysis of variance (ANOVA) or Kruskal-Wallis tests, as appropriate, were used to assess differences in categorical and continuous variables, for which we report proportions and median values with interquartile ranges (IQRs), respectively. A generalized logistic regression model was used to estimate the associations between receptor subtype and post-NACT response after adjustment for clinicopathologic characteristics. This model was built in the generalized estimating equations framework and accounted for the correlation of patients treated at the same facility by incorporating an exchangeable correlation structure.
Overall survival (OS) was defined as the time from diagnosis to death or last follow-up; as required by NCDB guidelines, patients diagnosed in 2014 were excluded from survival analyses due to insufficient length of follow-up. Kaplan-Meier (KM) curves were used to visualize unadjusted OS for the entire cohort as well as patients grouped by receptor subtype and clinical N stage, with log-rank p<0.05 defined as significant. Cox proportional hazards modeling was used to estimate the association of receptor subtype and post-NACT response with OS after adjustment for known covariates including clinical T and N stage at presentation after we confirmed weak collinearity with post-NACT outcomes using the rule of Variance Inflation Factor (VIF) <10.22 The supremum test was used to verify the proportional hazards assumption for the fully adjusted OS model. In order to determine if the effect of response on survival differs based on receptor subtype, an adjusted Cox proportional hazards model including a response*subtype interaction term was utilized. A robust sandwich covariance estimator was included in all Cox models to account for the correlation of patients treated at the same facility. We report hazard ratios (HRs) and 95% confidence intervals (CIs) with two-tailed p<0.05 considered significant. All analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC) and R version 3.3.2 (R Foundation for Computing, Vienna, Austria). The Duke University institutional review board granted this study exempt status due to use of de-identified patient data.
Results
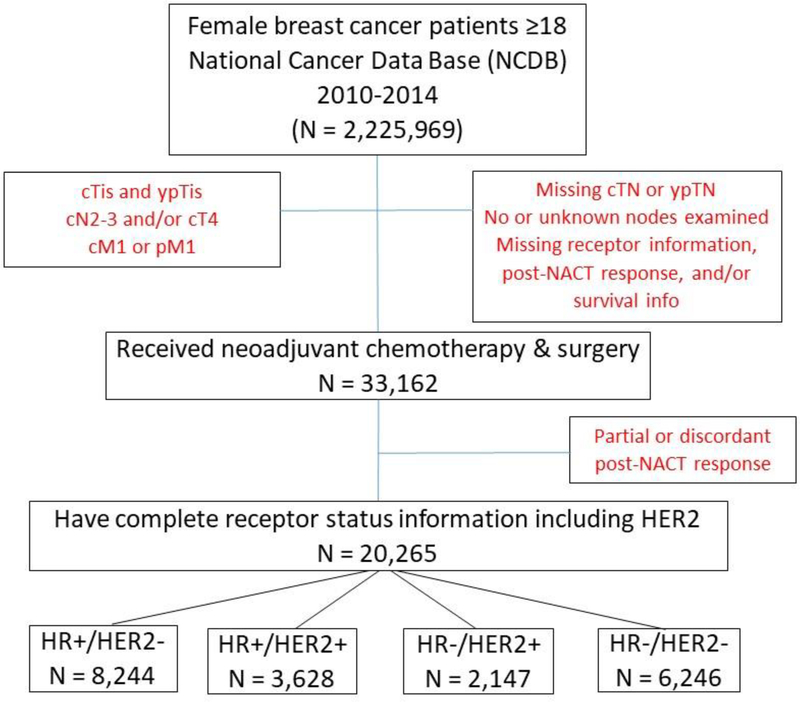
We identified 33,162 female breast cancer patients (cN0 n = 18,804, cN1 n=15,078) who underwent surgery following NACT between 2010 and 2014 (Figure 1, Table 1). Breast+axilla pCR was achieved by 19.2% (n=6,370) of patients, with similar rates among cN0 (18.2%, n=3,284) and cN1 (20.5%, n=3,086) patients. The highest rates of overall pCR occurred among those with the HR-/HER2+ subtype (39.6%, n=1,256) followed by the triple-negative subtype (26.5%, n=2,611, p<0.001, see Table 1). Among cN1 patients, 3.3% (n=494) of patients had breast-only pCR, with the highest rates again seen among HR-/HER2+ (5.1%, n=86) and triple-negative patients (4.2%, n=165, p<0.001). 7.5% (n=1,133) of cN1 patients experienced node-only pCR, but rates were fairly similar across subtypes and ranged from 7.1% (HR+/HER2-) to 8.1% (HR-/HER2-). The HR+/HER2- subtype had the highest proportion of patients without change in stage (33.7%) and with upstage (12.4%, p<0.001). Table 2 summarizes the response to NACT by clinical stage among all patients (see Supplemental Digital Content [SDC], Tables 2a–d for response to NACT by receptor subtype).
Figure 1.
Patient Flow Diagram - Breast Cancer Patients who Underwent Surgery following Neoadjuvant Chemotherapy, National Cancer Data Base (NCDB), 2010–2014 (n=20,265) a
aLimited to patients experiencing no stage change, upstage, and overall or anatomically limited (breast or axilla) pathologic complete response.
Table 1.
Breast Cancer Patients who Underwent Surgery following Neoadjuvant Chemotherapy, National Cancer Data Base (NCDB), 2010–2014 (n=20,265)a
| All Patients | Receptor Status | p-value | ||||
|---|---|---|---|---|---|---|
| HR+/HER2− | HR+/HER2+ | HR−/HER2+ | HR−/HER2− | |||
| N=20,265 (100%) | N=8,244 (40.7%) | N=3,628 (17.9%) | N=2,147 (10.6%) | N=6,246 (30.8%) | ||
| Age (yr) – Median (IQR) | 52 (44 – 60) | 52 (44 – 61) | 51 (43 – 59.5) | 53 (45 – 61) | 51 (43 – 59) | <0.001 |
| Follow-up (mo) – Median (IQR) b | 35.9 (24.7 – 48.7) | 36.7 (25.2 – 49.4) | 34.5 (24.1 – 47.4) | 33.5 (23.9 – 46.7) | 36.3 (24.7 – 48.8) | <0.001 |
| Response Typec | N=33,162 | N=14,075 | N=6,076 | N=3,172 | N=9,839 | <0.001 |
| Breast+axilla pCR | 6,370 (19.2%) | 1,134 (8.1%) | 1,369 (22.5%) | 1,256 (39.6%) | 2,611 (26.5%) | |
| Breast-only pCR (% cohort | % cN1) | 494 (1.5% | 3.3%) | 155 (1.1% | 2.4%) | 88 (1.4% | 3.0%) | 86 (2.7% | 5.1%) | 165 (1.7% | 4.2%) | |
| Node-only pCR (% cohort | % cN1) | 1,133 (3.4% | 7.5%) | 465 (3.3% | 7.1%) | 225 (3.7% | 7.6%) | 122 (3.8% | 7.3%) | 321 (3.3% | 8.1%) | |
| No change | 9,641 (29.1%) | 4,740 (33.7%) | 1,593 (26.2%) | 579 (18.2%) | 2,729 (27.7%) | |
| Upstage | 2,627 (7.9%) | 1,750 (12.4%) | 353 (5.8%) | 104 (3.3%) | 420 (4.3%) | |
| Race/Ethnicity | <0.001 | |||||
| Non-Hispanic White | 13,866 (68.4%) | 5,773 (70%) | 2,591 (71.4%) | 1,486 (69.2%) | 4,016 (64.3%) | |
| Non-Hispanic Black | 3,252 (16%) | 1,116 (13.5%) | 458 (12.6%) | 293 (13.6%) | 1,385 (22.2%) | |
| Non-Hispanic Other | 915 (4.5%) | 381 (4.6%) | 189 (5.2%) | 117 (5.4%) | 228 (3.7%) | |
| Hispanic | 1,450 (7.2%) | 628 (7.6%) | 267 (7.4%) | 168 (7.8%) | 387 (6.2%) | |
| Histology | <0.001 | |||||
| Ductal | 17,262 (85.2%) | 6,427 (78%) | 3,249 (89.6%) | 1,975 (92%) | 5,611 (89.8%) | |
| Lobular | 1,086 (5.4%) | 903 (11%) | 124 (3.4%) | 16 (0.7%) | 43 (0.7%) | |
| Other | 272 (1.3%) | 53 (0.6%) | 7 (0.2%) | 18 (0.8%) | 194 (3.1%) | |
| Grade | <0.001 | |||||
| 1 | 1,231 (6.1%) | 1,006 (12.2%) | 160 (4.4%) | 21 (1%) | 44 (0.7%) | <0.001 |
| 2 | 6,001 (29.6%) | 3,479 (42.2%) | 1,378 (38%) | 440 (20.5%) | 704 (11.3%) | |
| 3 | 11,414 (56.3%) | 3,132 (38%) | 1,768 (48.7%) | 1,479 (68.9%) | 5,035 (80.6%) | |
| Tumor Size (mm) – Median (IQR) | 27 (17 – 40) | 27 (17 – 40) | 25 (16 – 38) | 28 (18 – 40) | 28 (18 – 40) | <0.001 |
| Clinical T Stage | <0.001 | |||||
| cT1 | 7,174 (35.4%) | 3,071 (37.3%) | 1,477 (40.7%) | 652 (30.4%) | 1,974 (31.6%) | |
| cT2 | 10,460 (51.6%) | 4,143 (50.3%) | 1,747 (48.2%) | 1,122 (52.3%) | 3,448 (55.2%) | |
| cT3 | 2,631 (13%) | 1,030 (12.5%) | 404 (11.1%) | 373 (17.4%) | 824 (13.2%) | |
| Clinical N Stage | <0.001 | |||||
| cN0 | 11,641 (57.4%) | 4,722 (57.3%) | 1,992 (54.9%) | 1,049 (48.9%) | 3,878 (62.1%) | |
| cN1 | 8,624 (42.6%) | 3,522 (42.7%) | 1,636 (45.1%) | 1,098 (51.1%) | 2,368 (37.9%) | |
| ER Status | <0.001 | |||||
| ER+ | 11,397 (56.2%) | 7,912 (96%) | 3,485 (96.1%) | 0 (0%) | 0 (0%) | |
| ER− | 8,868 (43.8%) | 332 (4%) | 143 (3.9%) | 2,147 (100%) | 6,246 (100%) | |
| PR Status | <0.001 | |||||
| PR+ | 9,301 (45.9%) | 6,613 (80.2%) | 2,688 (74.1%) | 0 (0%) | 0 (0%) | |
| PR− | 10,946 (54%) | 1,621 (19.7%) | 932 (25.7%) | 2,147 (100%) | 6,246 (100%) | |
| HER2 Status | <0.001 | |||||
| HER2+ | 5,775 (28.5%) | 0 (0%) | 3,628 (100%) | 2,147 (100%) | 0 (0%) | |
| HER2− | 14,490 (71.5%) | 8,244 (100%) | 0 (0%) | 0 (0%) | 6,246 (100%) | |
| Breast Surgery Type | <0.001 | |||||
| Lumpectomy | 7,698 (38%) | 2,767 (33.6%) | 1,477 (40.7%) | 915 (42.6%) | 2,539 (40.7%) | |
| Mastectomy | 12,557 (62%) | 5,472 (66.4%) | 2,150 (59.3%) | 1,232 (57.4%) | 3,703 (59.3%) | |
| Radiation | ||||||
| Post-lumpectomyd | 7,131 (92.8%) | 2,565 (92.8%) | 1,364 (92.4%) | 840 (91.4%) | 2,362 (93.2%) | 0.76 |
| Post-mastectomye | 5,713 (45.9%) | 2,797 (51.6%) | 923 (43.1%) | 529 (43.4%) | 1,464 (40.0%) | <0.001 |
| Axillary Surgery | <0.001 | |||||
| [1–5] LNs | 10,338 (51%) | 3,913 (47.5%) | 1,846 (50.9%) | 1,031 (48%) | 3,548 (56.8%) | |
| [6–9] LNs | 2,849 (14.1%) | 1,268 (15.4%) | 505 (13.9%) | 278 (12.9%) | 798 (12.8%) | |
| ≥10 LNs | 6,269 (30.9%) | 2,864 (34.7%) | 1,091 (30.1%) | 679 (31.6%) | 1,635 (26.2%) | |
| LNs Examined – Median (IQR) | 5 (2 – 12) | 6 (3 – 13) | 5 (2 – 12) | 5 (2 – 13) | 4 (2 – 10) | <0.001 |
| Positive LNs – Median (IQR) | 1 (1 – 2) | 2 (1 – 2) | 1 (1 – 3) | 1 (1 – 2) | 1 (1 – 2) | <0.001 |
Limited to patients experiencing no stage change, upstage, and overall or anatomically limited (breast or axilla) pathologic complete response.
Median follow-up was estimated for patients diagnosed from 2010–2013 only using reverse KM methods; the log-rank p-value is reported.
Rates for “Response Type” are calculated based on all patients receiving surgery after NACT including those with partial or discordant response. Rates calculated using the study cohort are also included in Supplemental Digital Content, Table 1.
Percentages represent rates of radiation receipt among patients receiving lumpectomy.
Percentages represent rates of radiation receipt among patients receiving mastectomy.
Percentages may not add up to 100 due to rounding or missing values. Supplemental Digital Content, Table 1, contains full table with all examined variables.
ER, estrogen receptor. LN, lymph node. Mo, month. PR, progesterone receptor. Yr, year.
Table 2.
Response to Neoadjuvant Chemotherapy by Clinical Stage at Diagnosis among NCDB Breast Cancer Cohort – All tumor subtypes (n=33,162)
| Pathological Stage after Neoadjuvant Chemotherapy | Total | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ypT0 | ypT1 | ypT2 | ypT3 | ||||||||||||
| ypN0 | ypN1mic | ypN1 | ypN0 | ypN1mic | ypN1 | ypN0 | ypN1mic | ypN1 | ypN0 | ypN1mic | ypN1 | ||||
| Clinical Stage at Diagnosis | cT1 | cN0 | 812 | 9 | 23 | 3,017 | 156 | 562 | 330 | 42 | 200 | 28 | 2 | 46 | 5,227 |
| 15.5% | 0.2% | 0.4% | 57.7% | 3.0% | 10.8% | 6.3% | 0.8% | 3.8% | 0.5% | 0.0% | 0.9% | ||||
| cN1 | 561 | 30 | 139 | 413 | 96 | 1,088 | 45 | 7 | 153 | 6 | 2 | 27 | 2,567 | ||
| 22.0% | 1.2% | 5.4% | 16.1% | 3.7% | 42.4% | 1.8% | 0.3% | 6.0% | 0.2% | 0.1% | 1.1% | ||||
| cT2 | cN0 | 2,026 | 20 | 41 | 3,470 | 172 | 449 | 2,644 | 175 | 776 | 168 | 15 | 93 | 10,049 | |
| 20.2% | 0.2% | 0.4% | 34.5% | 1.7% | 4.5% | 26.3% | 1.7% | 7.7% | 1.7% | 0.2% | 0.9% | ||||
| cN1 | 1,856 | 50 | 258 | 1,519 | 224 | 1,784 | 544 | 127 | 1,900 | 43 | 9 | 188 | 8,502 | ||
| 21.8% | 0.6% | 3.0% | 17.9% | 2.6% | 21.0% | 6.4% | 1.5% | 22.4% | 0.5% | 0.1% | 2.2% | ||||
| cT3 | cN0 | 446 | 5 | 10 | 710 | 42 | 122 | 535 | 58 | 192 | 437 | 32 | 219 | 2,808 | |
| 15.9% | 0.2% | 0.4% | 25.3% | 1.5% | 4.3% | 19.1% | 2.1% | 6.8% | 15.6% | 1.1% | 7.8% | ||||
| cN1 | 669 | 31 | 97 | 718 | 121 | 590 | 311 | 57 | 650 | 176 | 34 | 555 | 4,009 | ||
| 16.7% | 0.8% | 2.4% | 17.9% | 3.0% | 14.7% | 7.8% | 1.4% | 16.2% | 4.4% | 0.9% | 13.8% | ||||
| Total | 6,370 19.2% |
145 0.4% |
568 1.7% |
9,847 29.7% |
811 2.4% |
4,595 13.9% |
4,409 13.3% |
466 1.4% |
3,871 13.3% |
858 3.0% |
94 0.3% |
1,128 3.4% |
33,162 | ||
Green – Breast + axilla pCR
Pink – Breast-only pCR
Blue - Node-only pCR
Yellow – No stage change
Orange – Upstage
Gray – EXCLUDED from main analysis: Discordant (i.e., breast underwent upstage while axilla was downstaged or vice versa) or Downstage without pCR
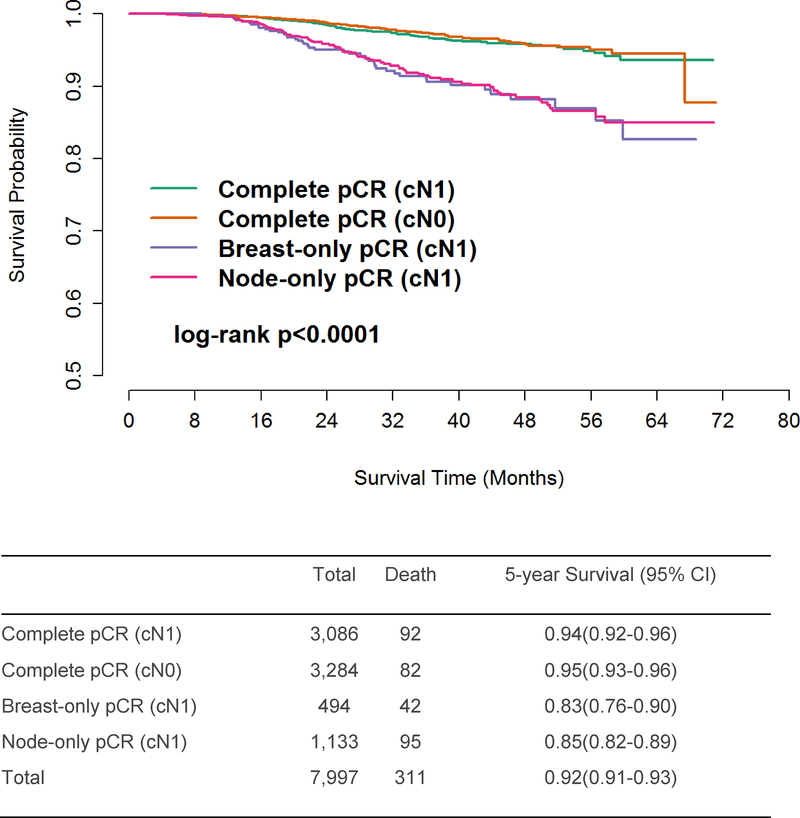
After excluding those with discordant or partial response (n=12,897), 20,265 patients (median follow-up 35.9 months, 95% CI 35.6–36.2 months) were included in our final study cohort. Of these, 11,641 patients had cN0 disease, and 8,624 had cN1 disease (Figure 1, Table 1; see SDC, Table 1 for complete list of covariates). A majority of patients underwent mastectomy (62%). Rates of post-lumpectomy radiation were similar across subtypes, but among mastectomy patients with known radiation status, HR+/HER2- patients had the highest rates of post-mastectomy radiation (PMRT, 51.6%) while triple-negative patients had the lowest rates of PMRT (40.0%, p<0.001). Clinically node-negative patients had less extensive axillary surgery than cN1 patients regardless of receptor subtype, with 70% of cN0 patients having 5 or fewer nodes examined while 51% of cN1 patients had 10 or more nodes examined (p<0.001). Among the 4 subtypes, HR+/HER2- patients had the greatest number of lymph nodes examined at surgery, with 6 or more lymph nodes examined in 50.1%, compared to 39% among triple-negative patients (p<0.001). This finding remained significant when patients were separated by cN stage: 21% of cN0, HR+/HER2- patients and 53.4% of the cN1, HR+/HER2- patients had 10 or more nodes examined while triple-negative patients had the lowest number of nodes retrieved, with only 12.5% of cN0 patients and 48.7% of the cN1 patients having 10 or more nodes examined. Among cN1 patients achieving pCR in the breast (n=3,580), 86.2% (n=3,086) also achieved pCR in the axillary nodes, but among those without breast pCR (n=5,044), only 22.5% (n=1,133) achieved nodal pCR, indicating an expected association between breast and nodal response (p<0.001, Table 2). The unadjusted 5-year OS for the entire cohort was 0.94 (95% CI=0.93–0.95, Figure 2) for patients achieving breast+axilla pCR and did not differ between those who presented with cN0 (0.95, 95% CI=0.93–0.96) vs cN1 disease (0.94, 95% CI=0.92–0.96; see SDC, Figures 1–5 for KM curves by receptor subtype and cN status).
Figure 2.
Unadjusted Overall Survival in NCDB Breast Cancer Patients following Neoadjuvant Chemotherapy and Surgery – Patients with overall and anatomically limited pCR (n=7,997)
Those variables with VIFs<10, i.e., without collinearity, were included in the final multivariate model; cN and cT stage were not found to be collinear with treatment response and thus were included in the overall analyses but excluded from the subset analyses to avoid overfitting. Achieving overall pCR and node-only pCR (compared with no stage change) and having the HR+/HER2+ subtype (compared with the HR+/HER2- subtype) were associated with improved OS for the entire cohort. Having upstage, triple-negative subtype, high-grade disease, cT2 or cT3 stage, cN1 stage, more extensive axillary surgery (i.e., greater number of lymph nodes removed and examined), and mastectomy were all associated with worse OS; black race and not having private insurance were also associated with worse OS (Table 3). When the analysis was stratified by cN status, improved survival continued to be associated with the HR+/HER2+ (vs HR+/HER2-) subtype and with overall pCR (vs no stage change) for both cN0 and cN1 patients; cN1 patients also saw improved survival with breast-only and node-only pCR, though the effect size was smaller for both than for breast+axilla pCR (see SDC, Tables 3a and 3b). There was a significant interaction between response and receptor subtype, indicating that the effect of anatomically limited response on survival differs based on receptor subtype (interaction p<0.001, (see SDC, Table 3c).
Table 3.
Cox Proportional Hazards Model for Adjusted Overall Survival in NCDB Breast Cancer Patients following Neoadjuvant Chemotherapy and Surgery – All Patients (n=16,748)a,b
| HR (95% CI) | p-value | Overall p-value | |
|---|---|---|---|
| Receptor Subtype | <0.001 | ||
| HR+/HER2− | -REF- | ||
| HR+/HER2+ | 0.67 (0.53 – 0.82) | <0.001 | |
| HR−/HER2+ | 0.87 (0.66 – 1.15) | 0.342 | |
| HR−/HER2− | 1.97 (1.70 – 2.28) | <0.001 | |
| Response Type | <0.001 | ||
| No change | -REF- | ||
| Breast+axilla pCR | 0.23 (0.19– 0.28) | <0.001 | |
| Breast-only pCR | 0.71 (0.50 – 1.01) | 0.056 | |
| Node-only pCR | 0.62 (0.48 – 0.81) | <0.001 | |
| Upstage | 1.49 (1.25 – 1.78) | <0.001 | |
| Age (years) | 1.01 (1.01 – 1.02) | <0.001 | <0.001 |
| Race/Ethnicity | 0.005 | ||
| Non-Hispanic White | -REF- | ||
| Non-Hispanic Black | 1.19 (1.02 – 1.40) | 0.03 | |
| Non-Hispanic Other | 0.63 (0.43 – 0.90) | 0.012 | |
| Hispanic | 0.93 (0.74 – 1.17) | 0.536 | |
| Grade | <0.001 | ||
| 1 | -REF- | ||
| 2 | 1.08 (0.81 – 1.46) | 0.587 | |
| 3 | 1.89 (1.41 – 2.55) | <0.001 | |
| Histology | 0.117 | ||
| Ductal | -REF- | ||
| Lobular | 0.74 (0.54 – 1.01) | 0.061 | |
| Other | 0.89 (0.72 – 1.09) | 0.257 | |
| Breast Surgery Type | 0.012 | ||
| Lumpectomy | -REF- | ||
| Mastectomy | 1.22 (1.05 – 1.43) | 0.012 | |
| Axillary Surgery | 0.435 | ||
| [1–5] LNs | -REF- | ||
| [6–9] LNs | 1.07 (0.90 – 1.28) | 0.449 | |
| ≥10 LNs | 1.11 (0.95 – 1.29) | 0.201 | |
| Clinical T Stage | |||
| cT1 | -REF- | <0.001 | |
| cT2 | 1.43 (1.25 – 1.64) | <0.001 | |
| cT3 | 1.98 (1.63 – 2.41) | <0.001 | |
| Clinical N Stage | <0.001 | ||
| cN0 | -REF- | ||
| cN1 | 1.54 (1.31 – 1.81) | <0.001 | |
| Receptor Subtype | <0.001 | ||
| HR+/HER2− | -REF- | ||
| HR+/HER2+ | 0.67 (0.53 – 0.82) | <0.001 | |
| HR−/HER2+ | 0.87 (0.66 – 1.15) | 0.342 | |
| HR−/HER2− | 1.97 (1.70 – 2.28) | <0.001 | |
| Response Type | <0.001 | ||
| No change | -REF- | ||
| Breast+axilla pCR | 0.23 (0.19– 0.28) | <0.001 | |
| Breast-only pCR | 0.71 (0.50 – 1.01) | 0.056 | |
| Node-only pCR | 0.62 (0.48 – 0.81) | <0.001 | |
| Upstage | 1.49 (1.25 – 1.78) | <0.001 | |
| Age (years) | 1.01 (1.01 – 1.02) | <0.001 | <0.001 |
| Race/Ethnicity | 0.005 | ||
| Non-Hispanic White | -REF- | ||
| Non-Hispanic Black | 1.19 (1.02 – 1.40) | 0.03 | |
| Non-Hispanic Other | 0.63 (0.43 – 0.90) | 0.012 | |
| Hispanic | 0.93 (0.74 – 1.17) | 0.536 | |
| Grade | <0.001 | ||
| 1 | -REF- | ||
| 2 | 1.08 (0.81 – 1.46) | 0.587 | |
| 3 | 1.89 (1.41 – 2.55) | <0.001 | |
| Histology | 0.117 | ||
| Ductal | -REF- | ||
| Lobular | 0.74 (0.54 – 1.01) | 0.061 | |
| Other | 0.89 (0.72 – 1.09) | 0.257 | |
| Breast Surgery Type | 0.012 | ||
| Lumpectomy | -REF- | ||
| Mastectomy | 1.22 (1.05 – 1.43) | 0.012 | |
| Axillary Surgery | 0.435 | ||
| [1–5] LNs | -REF- | ||
| [6–9] LNs | 1.07 (0.90 – 1.28) | 0.449 | |
| ≥10 LNs | 1.11 (0.95 – 1.29) | 0.201 | |
| Clinical N Stage | <0.001 | ||
| cN0 | -REF- | ||
| cN1 | 1.54 (1.31 – 1.81) | <0.001 | |
| Clinical T Stage | |||
| cT1 | -REF- | <0.001 | |
| cT2 | 1.43 (1.25 – 1.64) | <0.001 | |
| cT3 | 1.98 (1.63 – 2.41) | <0.001 |
HR, hazard ratio. HR+, hormone receptor-positive. HR-, hormone receptor-negative.
Limited to patients experiencing no stage change, upstage, and overall or anatomically limited (breast or axilla) pathologic complete response.
Additional covariates included in the model were Charlson/Deyo Comorbidity Score, Facility Type, Facility Location, Histology, Insurance Type, and receipt of Radiation Therapy; of these, only Comorbidity and Insurance Type were significant predictors of survival (both p<0.001). Other covariates such as tumor size were excluded to avoid overfitting and collinearity. The model has accounted for correlation of patients treated at the same hospital.
When survival was examined within cohorts defined by both cN stage and receptor subtype, pCR was associated with improved survival for cN0 patients with all subtypes except HR+/HER2+, while upstaging was associated with worse survival for the HR-/HER2+ (HR 2.31, 95% CI 1.13–4.74, p=0.02) and triple-negative subtypes (HR 2.19, 95% CI 1.59–3.02, p<0.001, Table 4). Among cN1 patients, breast-only pCR was associated with improved OS only in triple-negative disease (HR 0.58, 95% CI 0.37–0.89, p=0.01), while node-only pCR was associated with improved OS in both triple-negative (HR 0.55, 95% CI 0.39–0.76, p<0.001) and HR+/HER2- disease (HR 0.54, 95% CI 0.33–0.89, p=0.02). In the fully adjusted OS model, the proportional hazards assumption held for pCR but did not hold for HR/HER2 receptor subtypes. As such, we have presented both the fully adjusted model with pCR and receptor subtypes as well as the adjusted models stratified by receptor subtype and cN stage (see SDC, Tables 4a–d, 5a–d, and 6a–d).
Table 4.
Association between Response to Neoadjuvant Chemotherapy and Adjusted Overall Survival by Receptor Subtype and cN Stage among NCDB Breast Cancer Patients*
| HR+/HER2− | HR+/HER2+ | HR−/HER2+ | HR−/HER2− | |||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI)p-value | HR (95% CI)p-value | HR(95% CI)p-value | HR (95% CI)p-value | |||||
| cN0 (n=4,030) | cN1 (n=2,877) | cN0 (n=1,926) | cN1 (n=1,478) | cN0 (n=1,049) | cN1 (n=1,098) | cN0 (n= 3,283) | cN1 (n=1,895) | |
| Response to NACT | ||||||||
| No change | Reference | |||||||
| Breast+axilla pCR | 0.58 (0.36–0.96)p=0.03 | 0.31 (0.17–0.55)p<0.001 | NS | 0.34(0.19–0.63)p<0.001 | 0.29(0.13–0.64)p=0.002 | 0.25 (0.12–0.54)p<0.001 | 0.29 (0.20–0.43)p<0.001 | 0.16 (0.11–0.22)p<0.001 |
| Breast-only pCR | - | NS | - | NS | - | NS | - | 0.58 (0.37–0.89)p=0.01 |
| Node-only pCR | - | 0.54 (0.33–0.89)p=0.02 | - | NS | - | NS | - | 0.55 (0.39–0.76)p<0.001 |
| Upstage | NS | NS | NS | NS | 2.31 (1.13–4.74)p=0.02 | NS | 2.19 (1.59–3.02)p<0.001 | NS |
| Overall p-value | 0.03 | <0.001 | 0.11 | 0.005 | <0.001 | 0.002 | <0.001 | <0.001 |
| Adjustment Covariates* | a,b,c,d,e | a,b,d,e | a,b | a | a | - | a,b,c,d,e | a,b,c,e |
NS, not significant.
Limited to patients experiencing no stage change, upstage, and overall or anatomically limited (breast or axilla) pathologic complete response. Models are adjusted for different covariates as determined by limited events and differing sample sizes.
Age
Race/Ethnicity
Facility Location and Histology
Grade
Charlson/Deyo Comorbidity Score, Axillary Surgery, Facility Type, Insurance Type, Radiation Therapy, Breast Surgery Type
Discussion
In this large, population-based study of patients undergoing NACT for breast cancer, rates of pCR in the breast, axilla, or both differ according to receptor subtype. We confirmed that pCR is associated with improved overall survival but found that the magnitude of its effect on survival is dependent on both receptor subtype and anatomic extent of pCR. Specifically, a subset of node-positive patients with breast-only or node-only pCR had improved survival compared to those experiencing no change in stage but had worse survival compared to those experiencing pCR in both the breast and axilla.
In keeping with our findings, in two institutional retrospective analyses16,17 and a pooled analysis of trial participants,12 patients with persistently positive nodes following NACT had worse OS. Accordingly, breast-only pCR should be considered as distinct from pCR in both the breast and the lymph nodes, as they appear to represent different disease trajectories. Nevertheless, our findings confirm that anatomically limited pCR can also provide important, subtype-specific prognostic information.
Impact of Tumor Phenotype
Notably, anatomically limited pCR confers no significant survival advantage in HER2+ breast cancers. The improvement in survival achieved by the efficacy of HER2-targeted agents such as trastuzumab and pertuzumab has resulted in HER2+ patients’ having the highest rates of pCR of any breast cancer subtype.12,13,23,24 Accordingly, any response to HER2-targeted therapy short of pCR in both the breast and lymph nodes signals poor prognosis, with OS comparable to that seen in patients with no response to NACT. Furthermore, upstage in HR-/HER2+ patients was observed in our study to be a significant negative prognosticator (Table 4) because it represents an uncommon and untoward response to what is typically highly effective treatment. In contrast, upstage following NACT was not associated with reduced OS in patients with HR+/HER2- cancer, supporting the previously observed finding that long-term outcomes in these patients rely more on long-term response to endocrine treatment than to chemotherapy.25
The HR+/HER2+ subtype emerged as the group with the best survival, likely as a result of the many effective targeted treatment options – including endocrine therapy and HER2-directed therapy – available to patients with this phenotype (Table 3). As a result, however, pCR is less critical to these patients’ long-term outcome, and, indeed, pCR was not associated with survival among cN0, HR+/HER2+ patients. This finding can be attributed to the limited number of events observed among these patients during the period of observation (Table 4) as well as the generally favorable prognosis of this group. In contrast, triple-negative and HR+/HER2- patients have fewer, less effective systemic options for locally advanced disease. Accordingly, among HER2- patients, those experiencing any form of favorable response such as overall pCR or even breast- or node-only pCR have improved survival as compared to those who do not. These partial and complete responders represent a singular group, notable for having overcome relative chemoresistance (on the part of HR+/HER2- disease) and inherently unfavorable biology (on the part of triple-negative disease) to demonstrate a favorable response to systemic therapy.
Impact of Surgery Extent
We also evaluated the impact of surgery on survival in our study cohort. We found that mastectomy was associated with worse adjusted OS, a finding observed in other population-based analyses.26,27 This association may, in part, be due to unmeasured variables that could not be adjusted for in the multivariate analysis: specifically, patients who receive mastectomy after systemic therapy may have worse disease at baseline as well as less evidence of a clinical response to neoadjuvant treatment, hence the decision to ultimately pursue mastectomy. Unfortunately, registries such as the NCDB do not capture the clinical assessment – including imaging and physical exam as well as patient preference and reconstructive concerns – that occurs after NACT but immediately before surgery. Although the focus of our study was to examine anatomically limited and overall pCR, our findings about the relationship between breast surgery type and overall survival highlight the extent to which the path from diagnosis to treatment completion is marked by milestones and decision nodes that are difficult to document and quantify but may nonetheless have as great an impact on survival as more concrete assessments such as whether and to what extent pCR is achieved.
Implications for Future Research
Our findings come at a time of increased interest with regards to how pCR might be used to inform both research and clinical practice. In 2014, the results of the Collaborative Trials in Neoadjuvant Breast Cancer (CTNeoBC) pooled analysis were published.12 This study was an international collaboration led by the United States Food and Drug Administration (FDA) and included 12 clinical trials and 11,955 patients. As with our study, the investigators reported that overall pCR in both the breast and nodes -- but not when limited to the breast – was associated with better long-term outcomes and holds more promise as a potential surrogate for survival. Likewise, they concluded that pCR is strongly associated with survival and that rates of pCR were highest in triple-negative and HER2+ disease. Following this report, pCR has been used by the FDA as an endpoint to facilitate accelerated approval for agents such as pertuzumab, which was expeditiously approved after producing an 18% improvement in pCR as part of the NeoSphere trial.5,28,29
Our study did not include trial-level data, but we were able to evaluate pooled, subtype-specific outcomes in over 20,000 patients. Importantly, our cohort was limited to an era in which trastuzumab had widespread use, thus avoiding the potential attenuation of effect size observed in studies such as CTNeoBC that included unidentified HER2+ patients who were not treated with HER2-targeted therapies. Our analysis confirms and further extends the findings of CTNeoBC by demonstrating that pCR in both the breast and axilla has the potential to be an effective clinical trial endpoint for all tumor subtypes, while the prognostic value of pCR in either the breast or nodes alone must be considered in the context of intrinsic tumor biology.
Limitations
The limitations of our study are the same as those pertaining to other retrospective analyses of the NCDB. Neither disease-specific mortality nor recurrence data are captured, so our assessments of long-term outcome were limited to overall survival. Pathologic response to NACT is reported by member institutions, but these assessments are not subjected to central pathologic review that might otherwise identify and reclassify cases for which the pathologic stage was incorrect. Prior to 2009, there was sparse coding of HER2 status in the NCDB, hence we limited our analysis to patients diagnosed in 2010 and beyond; we did not include use of HER2-targeted therapy in our multivariate models given the high uptake of anti-HER2 treatment by 2010 and the collinearity of this covariate with HER2 status. However, we note that the data did not allow for identification of those patients treated with single- versus dual-agent HER2-targeted therapy, and limited chemotherapy data precluded any additional regimen-specific analyses. The HR+/HER2- subtype was the reference group in our regression analyses, but we recognize that there is significant heterogeneity within this group and that associations with pCR and survival may align with other characteristics such as luminal type and genomic make-up that are not captured in our analysis.
We limited our analysis to cN0/1 patients because of the heterogeneity of anatomical nodal involvement in cN2/3 patients and our inability to assess achievement of pCR in non-axillary nodal basins. Accordingly, caution should be used in applying our findings to patients with large-volume and/or non-axillary nodal disease. In using the cutoffs of 1–5, 6–9, and ≥10 LNs to divide the cohort according to extent of axillary surgery, we are using a strategy employed by others given the coding limitations in the NCDB.30,31 We know that nodal yield is often lower after NACT and that the aforementioned category cutoffs represent yield rather than intent. Accordingly, it is possible that a higher number of ALNDs (for which ≥10 LNs has been used as a proxy) were performed than our data reflect but that <10 LNs were sometimes obtained even when axillary dissection was intended.
Finally, as previously described in the methods section, in order to simplify our statistical analysis and focus on the 3 forms of downstage in which we were primarily interested, we excluded cN1 patients who experienced breast or nodal downstaging without achieving nodal pCR as well as cN1 patients who had discordant changes in breast and nodal stage (i.e., breast underwent upstage while axilla was downstaged and vice versa) following NACT. Patients who did not fall into the 5 post-NACT responses we defined represent a heterogeneous group for whom we did not feel we could provide definitive conclusions. The results of a sensitivity analysis confirmed this suspicion when we calculated hazards ratios for the patients who experienced discordant response or downstage without pCR (data not shown). As suspected, due to the heterogeneity of these groups, most of the hazards ratios failed to reach statistical significance and the cohort size within each stage-specific response was relatively small; thus, eliminating those two groups allowed for more streamlined analysis and reporting. While we recognize that some of the upstaged patients may be patients who received incomplete or inaccurate staging at diagnosis rather than patients who progressed through treatment, we chose to include upstage because progression in both the breast and axilla is relatively unambiguous, whereas the definition of a mixed, partial, or discordant response to NACT is difficult to ascertain. We recognize that downstage that falls short of overall, breast-only, and node-only pCR may also provide important prognostic information that merits further investigation, but this topic falls outside the scope of our study.
Conclusion
We believe ours to be the largest population-based study to evaluate the prognostic significance of overall pCR as well as anatomically limited pCR in breast cancer patients treated with NACT. We found that patients who are node-positive at presentation and achieve pCR have a comparable prognosis to those who are clinically node-negative at presentation. Furthermore, survival is improved even if pCR is limited to the breast or axilla, but the extent of this survival benefit is subtype-specific. These findings are especially relevant in the context of ongoing attempts to identify patients in whom pCR has been achieved and surgical resection can potentially be safely avoided.32–35 Finally, the results of our study can help inform the conversations and shared decision-making of clinicians caring for patients undergoing neoadjuvant therapy and can provide researchers with pragmatic, short-term endpoints for assessing the efficacy of treatments being evaluated as part of clinical trials.
Supplementary Material
Acknowledgements
Portions of this manuscript were presented at the Annual Meeting of the American Surgical Association on April 20, 2018. The National Cancer Data Base (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and its participating hospitals are the source of the de-identified data used herein; the CoC has not verified these data and are not responsible for the statistical validity of the data analysis or the conclusions derived by the authors.
Funding
Dr. O. Fayanju is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) under Award Number 5KL2TR001115 (PI: Boulware). Dr. R. Greenup is supported by the NIH BIRCWH K12HD043446 (PI: Andrews). This work is also supported by the Duke Cancer Institute through NIH grant P30CA014236 (PI: Kastan). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflicts of Interest to Disclose: None
References
- 1.Giuliano AE, Dale PS, Turner RR, Morton DL, Evans SW, Krasne DL. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222(3):394–399; discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Joint Commission on Cancer (AJCC) Staging Manual 2010; 7th:https://cancerstaging.org/references-tools/deskreferences/Pages/default.aspx. Accessed 1 March 2018.
- 3.Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA: a cancer journal for clinicians. 2017;67(4):290–303. [DOI] [PubMed] [Google Scholar]
- 4.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375(9712):377–384. [DOI] [PubMed] [Google Scholar]
- 5.Gianni L, Pienkowski T, Im Y-H, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17(6):791–800. [DOI] [PubMed] [Google Scholar]
- 6.Piccart-Gebhart M, Holmes E, Baselga J, et al. Adjuvant Lapatinib and Trastuzumab for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: Results From the Randomized Phase III Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization Trial. J Clin Oncol. 2016;34(10):1034–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Untch M, Fasching PA, Konecny GE, et al. Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups. J Clin Oncol. 2011;29(25):3351–3357. [DOI] [PubMed] [Google Scholar]
- 8.DeVita VT Jr., Chu E. A history of cancer chemotherapy. Cancer research. 2008;68(21):8643–8653. [DOI] [PubMed] [Google Scholar]
- 9.Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16(8):2672–2685. [DOI] [PubMed] [Google Scholar]
- 10.van Nes JG, Putter H, Julien JP, et al. Preoperative chemotherapy is safe in early breast cancer, even after 10 years of follow-up; clinical and translational results from the EORTC trial 10902. Breast Cancer Res Treat. 2009;115(1):101–113. [DOI] [PubMed] [Google Scholar]
- 11.Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. [DOI] [PubMed] [Google Scholar]
- 12.Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. [DOI] [PubMed] [Google Scholar]
- 13.Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012;30(26):3242–3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999;17(2):460–469. [DOI] [PubMed] [Google Scholar]
- 15.von Minckwitz G, Untch M, Blohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–1804. [DOI] [PubMed] [Google Scholar]
- 16.Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230(1):72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rouzier R, Extra J-M, Klijanienko J, et al. Incidence and Prognostic Significance of Complete Axillary Downstaging After Primary Chemotherapy in Breast Cancer Patients With T1 to T3 Tumors and Cytologically Proven Axillary Metastatic Lymph Nodes. J Clin Oncol. 2002;20:1304–1310. [DOI] [PubMed] [Google Scholar]
- 18.Al-Hilli Z, Hoskin TL, Day CN, Habermann EB, Boughey JC. Impact of Neoadjuvant Chemotherapy on Nodal Disease and Nodal Surgery by Tumor Subtype. Ann Surg Oncol. 2018;25(2):482–493. [DOI] [PubMed] [Google Scholar]
- 19.Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. Jama. 2013;310(14):1455–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Diego EJ, McAuliffe PF, Soran A, et al. Axillary Staging After Neoadjuvant Chemotherapy for Breast Cancer: A Pilot Study Combining Sentinel Lymph Node Biopsy with Radioactive Seed Localization of Pre-treatment Positive Axillary Lymph Nodes. Ann Surg Oncol. 2016;23(5):1549–1553. [DOI] [PubMed] [Google Scholar]
- 21.Mamtani A, Barrio AV, King TA, et al. How Often Does Neoadjuvant Chemotherapy Avoid Axillary Dissection in Patients With Histologically Confirmed Nodal Metastases? Results of a Prospective Study. Ann Surg Oncol. 2016;23(11):3467–3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neter J, Kutner M, Wasserman W, Nachtsheim C. Applied linear statistical models. Vol 4 Chicago: Irwin; 1996. [Google Scholar]
- 23.Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. European journal of cancer (Oxford, England : 1990). 2012;48(18):3342–3354. [DOI] [PubMed] [Google Scholar]
- 24.von Minckwitz G, Untch M, Nüesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011;125(1):145–156. [DOI] [PubMed] [Google Scholar]
- 25.Ellis MJ, Tao Y, Luo J, et al. Outcome Prediction for Estrogen Receptor–Positive Breast Cancer Based on Postneoadjuvant Endocrine Therapy Tumor Characteristics. J Natl Cancer Inst. 2008;100(19):1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurian AW, Lichtensztajn DY, Keegan THM, Nelson DO, Clarke CA, Gomez SL. Use of and Mortality After Bilateral Mastectomy Compared With Other Surgical Treatments for Breast Cancer in California, 1998–2011. Jama. 2014;312(9):902–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status. Cancer. 2013;119(7):1402–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cortazar P, Geyer CE, Jr. Pathological complete response in neoadjuvant treatment of breast cancer. Ann Surg Oncol. 2015;22(5):1441–1446. [DOI] [PubMed] [Google Scholar]
- 29.F.D.A. Guidance for Industry Pathologic Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. 2012; http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM305501.pdf. Accessed 1 March 2018.
- 30.Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–2953. [DOI] [PubMed] [Google Scholar]
- 31.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432; discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van la Parra RFD, Kuerer HM. Selective elimination of breast cancer surgery in exceptional responders: historical perspective and current trials. Breast Cancer Res. 2016;18(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cancer Researchers Report Progress in Studying Exceptional Responders. 2017; https://www.cancer.gov/news-events/cancer-currents-blog/2017/exceptional-responders-progress.
- 34.Tadros AB, Yang WT, Krishnamurthy S, et al. Identification of Patients With Documented Pathologic Complete Response in the Breast After Neoadjuvant Chemotherapy for Omission of Axillary Surgery. JAMA surgery. 2017;152(7):665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuerer HM, Rauch GM, Krishnamurthy S, et al. A Clinical Feasibility Trial for Identification of Exceptional Responders in Whom Breast Cancer Surgery Can Be Eliminated Following Neoadjuvant Systemic Therapy. Ann Surg. 2018;267(5):946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.