Abstract
Background: Functional electrical stimulation (FES) is the application of electrical pulses to a nerve to achieve a functional muscle contraction. Surface electrical stimulation of the nerves that innervate the abdominal muscles, termed abdominal FES, can cause the abdominal muscles to contract, even when paralysed after spinal cord injury. As the abdominal muscles are the major expiratory muscles, and commonly partially or completely paralysed in tetraplegia, abdominal FES offers a promising method of improving respiratory function for this patient group. Objective: The aim of the article is to provide readers with a better understanding of how abdominal FES can be used to improve the health of the spinal cord–injured population. Methods: A narrative review of the abdominal FES literature was performed. Results: Abdominal FES can achieve an immediate effective cough in patients with tetraplegia, while the repeated application over 6 weeks of abdominal FES can improve unassisted respiratory function. Ventilator duration and tracheostomy cannulation time can also be reduced with repeated abdominal FES. Conclusion: Abdominal FES is a noninvasive method to achieve functional improvements in cough and respiratory function in acute and chronically injured people with tetraplegia. Potential practical outcomes of this include reduced ventilation duration, assisted tracheostomy decannulation, and a reduction in respiratory complications. All of these outcomes can contribute to reduced morbidity and mortality, improved quality of life, and significant potential cost savings for local health care providers.
Keywords: acute, chronic, functional electrical stimulation, respiratory, spinal cord injury
Spinal cord injury (SCI) is a debilitating and life changing event, with an estimated global incidence of 40 to 80 cases per million population per year and a global prevalence of 236 to 4,187 per million population.1 More than half of these injuries will be caused by damage to the cervical (neck) area of the spinal cord, termed tetraplegia.2 Tetraplegia is commonly associated with paralysis of all four limbs, but paralysis also affects the major respiratory muscles, namely the diaphragm, abdominal, and intercostal muscles. This reduces respiratory function, with resultant respiratory complications, such as pneumonia and atelectasis, the leading cause of morbidity and mortality in the first year of tetraplegia.3–5 These respiratory complications are particularly prevalent in the first 6 weeks post injury, regarded as the acute stage of injury,6 with an incidence rate of up to 68%.7 As well as the resultant delay in rehabilitation and reduction in quality of life, the number of these complications is a critical determinant of hospital costs.8
Functional electrical stimulation (FES) is the application of a train of electrical pulses (20–50 Hz) to a nerve to achieve a functional muscle contraction. It has been used in SCI to reduce the effects of immobilization through cycling and rowing-like exercise and to improve gait, pain management, and bowel, bladder, and sexual function, among others.9–12 FES can be delivered transcutaneously, or subcutaneously to stimulate peripheral nerves, and through direct stimulation of the spinal cord.9,13,14 Transcutaneous (surface) electrical stimulation of the abdominal muscles, termed abdominal FES, can cause contraction of the abdominal muscles, even when they are paralysed by SCI.15 Abdominal FES has been used to achieve functional improvements in cough and respiratory function.16 Potential practical outcomes of this include reduced mechanical ventilation duration, assisted tracheostomy decannulation, and a possible reduction in respiratory complications. These outcomes can reduce morbidity and mortality, improve quality of life, and result in a significant cost saving for local health care providers (Figure 1). The aim of the article is to provide readers with a better understanding of how abdominal FES could be used to improve the health of people with tetraplegia within the intensive care, medical ward, and community settings.
Figure 1.
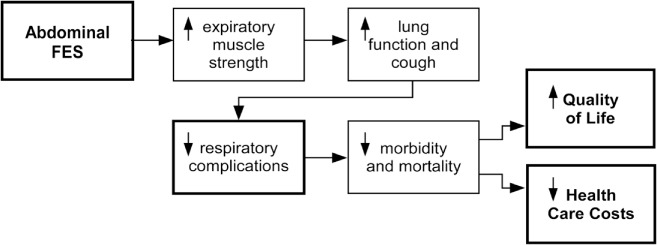
Proposed outcomes of abdominal functional electrical stimulation (FES) training. Decreased health care costs are achieved through a reduction in respiratory complications, decreased incidence and duration of tracheostomy, and reduced hospital readmissions and length of stay.
Cough
Cough is the body's key airway defense mechanism against respiratory complications. Bach et al17 have shown that patients with a cough peak flow (CPF) of >4.5 L/s are at less risk of developing acute respiratory failure. The use of abdominal FES to enhance cough was first proposed by Linder in 1993.18 A meta-analysis of four subsequent studies showed that the direct application of abdominal FES, without training, led to a statistically significant increase in CPF in patients with an SCI (standardised mean difference 2.43L/s, 95% confidence interval [95% CI], 0.32–4.54).16 This instantaneous improvement in CPF should reduce respiratory complications in tetraplegia. Hence, abdominal FES provides a directly applicable clinical tool that can be used in conjunction with established physiotherapy techniques such as manually assisted cough (MAC), mechanical insufflation-exsufflation (ie, Cough Assist machine),19 and tracheal and bronchial suction and postural drainage.20
While the best clinical indicator for cough efficacy in humans is not clear,21 the gastric (Pga) and esophageal pressure (Pes) generated during a cough have been identified as good laboratory parameters to indicate expiratory muscle strength, which is likely to correlate with cough efficacy in humans.21,22 Butler et al23 and McBain et al24 both found that the application of abdominal FES led to a statistically significant increase in Pga and Pes, using what has been shown to be an optimal electrode position for abdominal FES21 (Figure 2). A meta-analysis suggests further research on the effect of abdominal FES on Pga and Pes is warranted.16 Figure 3 shows an example of the improvements in respiratory function achieved using abdominal FES.
Abdominal FES can directly improve cough.
This may be a useful mechanism to reduce respiratory complications.
Figure 2.
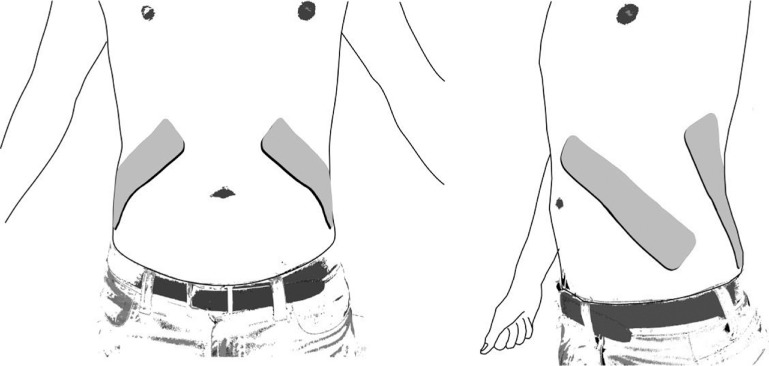
Posterolateral abdominal functional electrical stimulation (FES) electrode placement suggested by Lim et al21 as the optimal abdominal FES electrode position. This position has subsequently been used in clinical studies by Butler et al23 and McBain et al.24 Image shows abdomen viewed front on and at a 45° angle. Note: Depending on the user's body size, a second electrode can be applied to each electrode in series to increase the length of electrode coverage.
Figure 3.
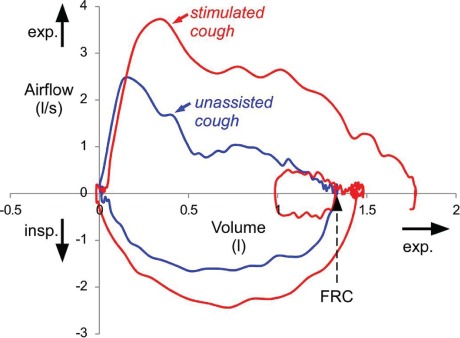
Flow-volume loops during two coughs recorded from a 59-year-old individual with C4/5 tetraplegia (AIS C, time since injury: 43 years). Red line indicates cough during abdominal functional electrical stimulation (FES); blue line indicates a voluntary (unassisted) cough. For the stimulated cough, a normal breath prior to the cough is also shown and functional residual capacity (FRC) is indicated by the dotted arrow. Inspiratory and expiratory flow directions are indicated on the y-axis, and expiratory volume direction is indicated on the x-axis. Note the large increase in cough peak flow and the sustained increase in expiratory flow during the stimulated cough. In contrast to the unassisted cough, the stimulated cough ends below FRC.
Respiratory Training
The repeated application of abdominal FES, termed abdominal FES training, has been shown to lead to a significant increase in unassisted forced vital capacity (FVC, p = .043), vital capacity (Vc, p = .013), and peak expiratory flow (PEF, p = .026)16 and a very small increase in unassisted Pga produced in a cough.24 Respiratory complications, such as pneumonia and atelectasis, are the leading cause of morbidity and mortality in the first year of tetraplegia and remain a significant source of morbidity and mortality throughout the lives of these patients.3–5 As respiratory function is a predictor of respiratory complications in tetraplegia,8 improvements in these outcomes should reduce respiratory complications in this vulnerable patient group. More research is required to determine for how long these improvements persist if training is ceased.
Abdominal FES training can improve overall unassisted respiratory function.
These improvements should facilitate better airway clearance in tetraplegia.
Abdominal FES training may reduce respiratory complications in tetraplegia.
Tracheostomy Decannulation
A study by Lee et al25 describes one 65-year-old patient with tetraplegia who, due to repeated respiratory complications, remained on noninvasive ventilation via a tracheostomy 8 months post injury. The patient then underwent an abdominal FES training program. This abdominal FES training led to a 27% and 29% increase in unassisted PEF and FVC, respectively. This improvement enabled the patient to cough unaided after 2 weeks of training and have the tracheostomy removed a week later. Subsequently, the patient had no respiratory complications in the following 11 months. The patient continued to use abdominal FES in the community for the next 15 years and remained tracheostomy-free. This suggests that abdominal FES can be used to reduce tracheostomy cannulation times in tetraplegia, with this study also being the first to suggest that abdominal FES may reduce ventilation duration. As the need for tracheostomy is associated with a number of related complications,26 a reduction in tracheostomy cannulation time contributes to reduced morbidity and mortality, improved quality of life, and a reduction in costs for the health care provider.
Abdominal FES should be considered to reduce tracheostomy cannulation times and should be considered at an early time point after injury.
Ventilator Weaning
In a pilot study by McCaughey et al,15 abdominal FES training in 10 participants with acute tetraplegia led to an 11-day reduction in mechanical ventilation duration compared to 10 age-, sex-, and injury level-matched controls. This supplements other pilot research that has shown abdominal FES can be used to reduce ventilation duration in critical illness (without SCI).27 With the need for mechanical ventilation increasing morbidity and mortality and costing the health care provider an additional $2,000/day,28 abdominal FES has the potential to improve the lives of a large and diverse cohort of patients who require mechanical ventilation to support respiration.
Mechanical ventilation duration may be reduced by an effective abdominal FES training regime.
Abdominal FES is also applicable in non-SCI patients (see Future Directions section).
Future Directions
The major cause of mortality in the first year after SCI is respiratory complications, and this is amplified with higher mortality in low income clinical settings.29 The repeated application of abdominal FES improves the respiratory function of people with tetraplegia.16 However, while respiratory function is a predictor of respiratory complications in tetraplegia,8 evidence that abdominal FES reduces respiratory complications is only anecdotal. As such, we are undertaking the first prospective, multicentre, randomised placebo-controlled trial to determine whether abdominal FES reduces respiratory complications in acute tetraplegia (Australia New Zealand Clinical Trial Registry: ACTRN12618000214235). Definitive evidence of the effectiveness of abdominal FES to reduce respiratory complications in tetraplegia, in both low- and high-income settings, will assist the rapid worldwide translation of this low cost and easily applied technology for this vulnerable patient group.
While abdominal FES has most commonly been applied in SCI, its application is universal and it has the potential to benefit all patients with reduced respiratory function. This includes critically ill ventilator-dependent patients, with a pilot study suggesting that abdominal FES maintains expiratory muscle thickness and reduces intensive care length of stay.27 To build on this evidence, we are investigating the effectiveness of abdominal FES to reduce ventilation duration in critical illness (Australia New Zealand Clinical Trial Registry: ACTRN12618000209291). Demonstration of the effectiveness of abdominal FES to reduce ventilation duration in all critically ill patients (particularly those at risk of prolonged ventilation) will greatly increase the applicability of abdominal FES in nonspecialist spinal centres. We believe this will facilitate faster clinical translation of this technology for people with tetraplegia.
The median pediatric incidence rate of SCI in North America is approximately 18 per million population, per year (unpublished data, publication under review). As in the adult population, respiratory complications from SCI are significant causes of morbidity and mortality in children.30 FES cycling has been shown as a safe treatment modality that may reduce the effects of immobilisation in childhood SCI.31 Thus, abdominal FES may offer a safe and useful pathway to reduce respiratory complication in pediatric tetraplegia and critical illness, further increasing the scope of this technique.
Mortality after tetraplegia is higher in low-income settings.
Studies are currently underway to evaluate the effectiveness of abdominal FES to prevent respiratory complications and reduce ventilation duration in low- and high-income settings.
Abdominal FES may also be a useful tool in pediatric tetraplegia and critical illness.
Alternatives
A number of techniques have been used to activate the abdominal muscles to support exhalation, including noninvasive magnetic stimulation applied over the T10 spinous process to activate the motor nerve roots that innervate the abdominal muscles (T8-12)21,22 and low (50 Hz) and high (500 Hz) frequency electrical stimulation of the spinal cord (epidural spinal stimulation).13,14,32 Both of these techniques have been shown to achieve high Pga and Pes,14,21,22,32 with spinal cord stimulation generating some of the highest respiratory pressures achieved with electrical stimulation to date.32 However, there is currently no low-cost, portable, magnetic stimulation system available. Epidural stimulation of the spinal cord at high intensities can result in significant co-contraction of the muscles in the back and leg and equipment failure rates as high as 25%; implantation of the device requires a minimally invasive surgical procedure, which has its own complications.33 Transcutaneous abdominal FES offers an effective noninvasive alternative to activate the abdominal muscles to assist respiration.
Limitations
As intact lower motor neurons are necessary for FES to be successful, abdominal FES will not be suitable for every patient with an SCI. Patients with lower motor neuron damage will have flaccid paralysis, meaning that atrophy occurs quickly after injury. As a result, respiratory function may be more severely compromised in these patients. As these patients will not be candidates for abdominal FES, they require alternative interventions to manage respiratory health.
As with any form of electrical stimulation, abdominal FES carries a risk of autonomic dysreflexia for people with an injury at T6 and above. However, there are no reports of abdominal FES causing autonomic dysreflexia in the literature to date.34 If a person does experience autonomic dysreflexia as result of abdominal FES, immediate cessation of the stimulation should resolve the symptoms. Finally, it should also be acknowledged that the proportion of people suffering incomplete SCIs is increasing.2 As these people will be able to feel the stimulation, stimulation intensity may need to be lower than in those with a complete injury.
Conclusion
Abdominal FES is a noninvasive method to achieve functional improvements in cough and respiratory function in the spinal cord–injured population. Potential and realistic outcomes of this include reduced ventilation duration, assisted tracheostomy decannulation, and a reduction in respiratory complications. All of these outcomes will reduce morbidity and mortality, improve quality of life, and result in a significant cost saving for local health care providers.
Footnotes
Conflicts of Interest
Dr. McCaughey reports grants and personal fees from Liberate Medical LLC, outside the submitted work. Dr. Butler reports grants from National Health and Medical Research Council during the conduct of the study and grants from National Health and Medical Research Council and Wings for Life outside the submitted work. Dr. Hudson reports grants from the Lung Foundation Australia, grants from Boehringer Ingelheim, during the conduct of the study and grants from Rebecca L. Cooper Foundation, the Lung Foundation Australia, Boehringer Ingelheim, National Health and Medical Research Council (Australia), and University of New South Wales outside the submitted work.
The other authors report no conflicts of interest.
REFERENCES
- 1.Lee B, Cripps RA, Fitzharris M, Wing P. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord. 2014;52:110–116. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 2.McCaughey EJ, Purcell M, McLean AN et al. Changing demographics of spinal cord injury over a 20-year period: A longitudinal population-based study in Scotland. Spinal Cord. 2016;54(4):270–276. doi: 10.1038/sc.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berlly M, Shem K. Respiratory management during the first five days after spinal cord injury. J Spinal Cord Med. 2007;30(4):309–318. doi: 10.1080/10790268.2007.11753946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devivo MJ, Krause SJ, Lammertse DP. Recent trends in mortality and causes of death among persons with spinal cord injury. Arch Phys Med Rehabil. 1999;80:1411–1419. doi: 10.1016/s0003-9993(99)90252-6. [DOI] [PubMed] [Google Scholar]
- 5.Chamberlain JD, Meier S, Mader L, von Groote PM, Brinkhof MWG. Mortality and longevity after a spinal cord injury: Systematic review and meta-analysis. Neuroepidemiology. 2015;44(3):182–198. doi: 10.1159/000382079. [DOI] [PubMed] [Google Scholar]
- 6.Berney S, Bragge P, Granger C, Opdam H, Denehy L. The acute respiratory management of cervical spinal cord injury in the first 6 weeks after injury: A systematic review. Spinal Cord. 2011;49(1):17–29. doi: 10.1038/sc.2010.39. [DOI] [PubMed] [Google Scholar]
- 7.Jackson AB, Groomes TE. Incidence of respiratory complications following spinal cord injury. Arch Phys Med Rehabil. 1994;75(3):270–275. doi: 10.1016/0003-9993(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 8.Winslow C, Bode RK, Felton D, Chen D, Meyer PR., Jr Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest. 2002;121(5):1548–1554. doi: 10.1378/chest.121.5.1548. [DOI] [PubMed] [Google Scholar]
- 9.Sheffler LR, Chae J. Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve. 2007;35(5):562–590. doi: 10.1002/mus.20758. [DOI] [PubMed] [Google Scholar]
- 10.Hamzaid NA, Pithon KR, Smith RM, Davis GM. Functional electrical stimulation elliptical stepping versus cycling in spinal cord-injured individuals. Clin Biomech. 2012;27(7):731–737. doi: 10.1016/j.clinbiomech.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Valles M, Rodriguez A, Borau A, Mearin F. Effect of sacral anterior root stimulator on bowel dysfunction in patients with spinal cord injury. Dis Colon Rectum. 2009;52(5):986–992. doi: 10.1007/DCR.0b013e31819ed459. [DOI] [PubMed] [Google Scholar]
- 12.Hascakova-Bartova R, Dinant JF, Parent A, Ventura M. Neuromuscular electrical stimulation of completely paralyzed abdominal muscles in spinal cord-injured patients: A pilot study. Spinal Cord. 2008;46(6):445–450. doi: 10.1038/sj.sc.3102166. [DOI] [PubMed] [Google Scholar]
- 13.Kowalski KE, Romaniuk JR, Kowalski T, DiMarco AF. Effects of expiratory muscle activation via high-frequency spinal cord stimulation. J Appl Physiol (1985) 2017;123(6):1525–1531. doi: 10.1152/japplphysiol.00402.2017. [DOI] [PubMed] [Google Scholar]
- 14.DiMarco AF, Kowalski KE, Hromyak DR, Geertman RT. Long-term follow-up of spinal cord stimulation to restore cough in subjects with spinal cord injury. J Spinal Cord Med. 2014;37(4):380–388. doi: 10.1179/2045772313Y.0000000152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCaughey EJ, Berry HR, McLean AN, Allan DB, Gollee H. Abdominal functional electrical stimulation to assist ventilator weaning in acute tetraplegia: A cohort study. PLoS One. 2015;10(6):e0128589. doi: 10.1371/journal.pone.0128589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCaughey EJ, Borotkanics RJ, Gollee H, Folz RJ, McLachlan AJ. Abdominal functional electrical stimulation to improve respiratory function after spinal cord injury: A systematic review and meta-analysis. Spinal Cord. 2016;54(9):628–639. doi: 10.1038/sc.2016.31. [DOI] [PubMed] [Google Scholar]
- 17.Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest. 1997;112(4):1024–1028. doi: 10.1378/chest.112.4.1024. [DOI] [PubMed] [Google Scholar]
- 18.Linder SH. Functional electrical stimulation to enhance cough in quadriplegia. Chest. 1993;103(1):166–169. doi: 10.1378/chest.103.1.166. [DOI] [PubMed] [Google Scholar]
- 19.McCaughey EJ, McLean AN, Allan DB, Gollee H. Abdominal functional electrical stimulation to enhance mechanical insufflation-exsufflation. J Spinal Cord Med. 2016;39(6):720–725. doi: 10.1080/10790268.2015.1114226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray N, McKenzie DK. Respiratory function in motor neurone disease. In: Kiernan M, editor. The Motor Neurone Disease Handbook. Sydney, Australia: Australasian Medical Publishing Company Ltd; 2007. pp. 125–143. [Google Scholar]
- 21.Lim J, Gorman RB, Saboisky JP, Gandevia SC, Butler JE. Optimal electrode placement for noninvasive electrical stimulation of human abdominal muscles. J Appl Physiol (1985) 2007;102(4):1612–1617. doi: 10.1152/japplphysiol.00865.2006. [DOI] [PubMed] [Google Scholar]
- 22.Polkey MI, Luo Y, Guleria R, Hamnegard CH, Green M, Moxham J. Functional magnetic stimulation of the abdominal muscles in humans. Am J Respir Crit Care Med. 1999;160(2):513–522. doi: 10.1164/ajrccm.160.2.9808067. [DOI] [PubMed] [Google Scholar]
- 23.Butler JE, Lim J, Gorman RB et al. Posterolateral surface electrical stimulation of abdominal expiratory muscles to enhance cough in spinal cord injury. Neurorehabil Neural Repair. 2011;25(2):158–167. doi: 10.1177/1545968310378509. [DOI] [PubMed] [Google Scholar]
- 24.McBain RA, Boswell-Ruys CL, Lee BB, Gandevia SC, Butler JE. Abdominal muscle training can enhance cough after spinal cord injury. Neurorehabil Neural Repair. 2013;27(9):834–843. doi: 10.1177/1545968313496324. [DOI] [PubMed] [Google Scholar]
- 25.Lee BB, Boswell-Ruys C, Butler JE, Gandevia SC. Surface functional electrical stimulation of the abdominal muscles to enhance cough and assist tracheostomy decannulation after high-level spinal cord injury. J Spinal Cord Med. 2008;31(1):78–82. doi: 10.1080/10790268.2008.11753985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Leyn P, Bedert L, Delcroix M et al. Tracheotomy: Clinical review and guidelines. Eur J Cardio-Thorac. 2007;32(3):412–421. doi: 10.1016/j.ejcts.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 27.Dall'Acqua AM, Sachetti A, Santos LJ et al. Use of neuromuscular electrical stimulation to preserve the thickness of the abdominal and chest muscles of critically ill patients: A randomized clinical trial. J Rehabil Med. 2017;49:40–48. doi: 10.2340/16501977-2168. [DOI] [PubMed] [Google Scholar]
- 28.Dasta JF, McLaughlin TP, Mody SH, Piech CT. Daily cost of an intensive care unit day: the contribution of mechanical ventilation. Crit Care Med. 2005;33(6):1266–1271. doi: 10.1097/01.ccm.0000164543.14619.00. [DOI] [PubMed] [Google Scholar]
- 29.Lee BB, Cripps R, New P . Demographic profile of spinal cord injury. In: Chhabra HS, editor. ISCoS Textbook of Comprehensive Management of Spinal Cord Injuries. India: Wolters Kluwer; 2015. pp. 36–52. [Google Scholar]
- 30.Padman R, Alexander M, Thorogood C, Porth S. Respiratory management of pediatric patients with spinal cord injuries: Retrospective review of the duPont experience. Neurorehab Neural Rehabil. 2003;17(1):32–36. doi: 10.1177/0888439003251751. [DOI] [PubMed] [Google Scholar]
- 31.Mayson TA, Harris SR. Functional electrical stimulation cycling in youth with spinal cord injury: A review of intervention studies. J Spinal Cord Med. 2014;37(3):266–277. doi: 10.1179/2045772313Y.0000000183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiMarco AF, Geertman RT, Tabbaa K, Polito RR, Kowalski KE. Minimally invasive method to activate the expiratory muscles to restore cough. J Spinal Cord Med. 2017:1–7. doi: 10.1080/10790268.2017.1357916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiMarco AF, Kowalski KE, Geertman RT, Hromyak DR. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: Results of a National Institutes of Health-sponsored clinical trial. Part I: Methodology and effectiveness of expiratory muscle activation. Arch Phys Med Rehabil. 2009;90(5):717–725. doi: 10.1016/j.apmr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton AR, Brown R, Macefield VG. Selective activation of muscle and skin nociceptors does not trigger exaggerated sympathetic responses in spinal-injured subjects. Spinal Cord. 2008;46(10):660–665. doi: 10.1038/sc.2008.33. [DOI] [PubMed] [Google Scholar]


