Abstract
Background: Activity-based therapies aim to improve neuromuscular capacity after spinal cord injury (SCI). Objective: The purpose of this prospective study was to report the impact of Activity-based Locomotor Training (AB-LT) on neuromuscular capacity in pediatric patients with SCI. Methods: Participants were enrolled for their first episode of AB-LT for a minimum of 60 daily, 1.5-hour sessions. The Segmental Assessment of Trunk Control (SATCo) and the Pediatric Neuromuscular Recovery Scale (Pediatric NRS) were assessed initially, every 20 sessions, and post 60 sessions. Results: Twenty-six consecutive patients, mean age 5 years (SD = 3), completed a mean 55 sessions (SD = 4) within 63 weekdays (SD = 9). The Pediatric NRS total score improved significantly, adjusted mean 11.4, from initial to post-60 sessions (p < .05) with an average adjusted evaluation-to-evaluation 3.7 change. SATCo scores improved significantly across 60 sessions, mean change 5.2, an estimated 1.7 change between evaluations (p < .05). Age at enrollment and chronicity had no effect; however, initial neuromuscular capacity scores were negatively correlated with change scores (p < .05). Conclusion: Sixty AB-LT sessions significantly improved trunk and neuromuscular capacity in children with SCI, regardless of age or chronicity at enrollment. Patients with lower initial scores made greater improvements than patients with higher initial neuromuscular capacity. Anecdotal parent reports of their child's functional change in the home and community highlight the synergy between quantitative change in neuromuscular capacity and meaningful, improved quality of life and the need for formal investigation of this relationship.
Keywords: activity-based therapy, acquired spinal cord injury, capacity measures, pediatrics, recovery, rehabilitation
Basic science and clinical research have introduced potential mechanisms for advancing recovery after human spinal cord injury (SCI), resulting in a new era of discovery for neurobiological and neurotherapeutic interventions, some experimental and others clinically applicable.1–7 Activity-based therapies (ABT)8–11 are neurotherapeutic interventions aimed at activating the neuromuscular system below the SCI as well as above and across the lesion. Activation promotes activity-dependent plasticity of the nervous system circuitry resulting in improved neuromuscular capacity underlying performance.12 Capacity is a subdomain of activity in the International Classification of Functioning, Disability and Health (ICF)13 and is a person's ability to execute a task or action. “It [capacity] documents a person's highest level of functioning in a given environment and requires testing in a standardized environment.”12(p114),13 The testing environment may be more or less accommodating than the person's typical environment.
Two pediatric-specific instruments, the Pediatric Neuromuscular Recovery Scale (Pediatric NRS)14 and the Segmental Assessment of Trunk Control (SATCo),15,16 assess, even as proxy measures, a child's neuromuscular capacity within clinical demonstration of functional tasks. Similar to the Graded Redefined Assessment of Strength, Sensation and Prehension (GRASSP),5 the Pediatric NRS was developed to fill a gap in outcome measures sensitive to change based on neurobiological and neurotherapeutic interventions. The aim of activity-based therapy is to promote recovery or restoration of the neuromuscular system to regain function using preinjury motor behaviors, whereas behavioral compensation17 refers to the use of atypical motor patterns, behaviors, body segments, technology, and/or assistive devices to make up for neurologic deficits post injury to accomplish functional tasks.18 Capacity is the demonstrated sensorimotor control in the context of functional tasks for the arm/hand (eg, reach and grasp), trunk (eg, sitting, sit-up, lying down, standing), and lower limb (eg, standing up, standing, walking) without compensation.
Activity-based Locomotor Training (AB-LT) was derived from new scientific knowledge that the spinal cord is not simply a conduit for transmission of neural signals, but like the brain, processes, synthesizes, and integrates sensorimotor information. The “smart” cord below the lesion is also responsive to practice via repetitive delivery of an ensemble of sensory information associated with standing or stepping activities to generate a motor output,19–21 one of the techniques used to modulate the physiological state of the spinal circuitry.7,22,23 AB-LT principles were derived from basic science studies and understanding of activity-dependent plasticity19,20,22,24,25 to promote recovery of postural control, standing, and stepping.26 The training principles include the following: (a) maximize weight-bearing on the legs; (b) optimize sensory cues specific to the tasks, for example, standing, stepping; (c) optimize the kinematics of the trunk, pelvis, and lower and upper extremities associated with specific motor tasks; and (d) maximize recovery strategies and minimize compensation strategies. These principles are applied in both the clinical training environment and the patient's home and community. Standardized AB-LT has been delivered in the Christopher and Dana Reeve Foundation NeuroRecovery Network27 of outpatient rehabilitation facilities serving adults and adolescents with SCI with improvements in balance and locomotion.28,29 A preliminary research report of 45 sessions of AB-LT provided to six nonambulatory children with chronic SCI resulted in three out of six children developing stepping and independent ambulation with a walker. All six, unexpectedly, improved in observed postural control.23,30,31 These findings were the impetus to introduce and translate AB-LT from research and clinical use with adults post SCI to children post SCI in an outpatient therapy clinic. The purpose of this prospective, observational study was to examine the effects of a standardized AB-LT protocol using clinical data and outcomes acquired in a clinical, pediatric outpatient setting in a population with acquired SCI on neuromuscular capacity. The clinical, activity-based program is the usual care therapy in this single clinical setting. In addition, we explored the impact of chronicity (time between injury and initiation of AB-LT) and initial neuromuscular capacity (pretraining) on change in neuromuscular capacity.
Method
Participants
Every candidate for the clinical activity-based therapy, outpatient program was screened by the program's physician to establish medical approval for enrollment. This was a two-step process whereby the physician first reviewed the candidate's medical record relative to program inclusion/exclusion criteria as well as any other relevant medical history. Second, the physician conducted a physical evaluation of the child to confirm that the child was medically ready and appropriate for referral to the activity-based therapy (ABT) program. The patients with a medical history of acquired, traumatic or nontraumatic, nonprogressive SCI and intent to complete 60 daily sessions were eligible for participation in the clinical, outpatient, standardized ABT program. The patients were ineligible for participation in the clinical ABT program if their medical history included a comorbidity impacting participation (eg, unhealed fracture or range of motion limiting weight-bearing and full participation), used Botox within the last 3 months, currently used baclofen and were unwilling to wean from oral baclofen, were unwilling to remove braces during the episode of AB-LT care (eg, thoraco-lumbo-sacral, lower limb, and upper limb orthoses), or were totally ventilator dependent. History of hip subluxation was not an exclusionary factor. In such an instance, the program pediatrician, in consult with the pediatric orthopedist, reviewed the child's medical history and determined eligibility based upon medical, clinical judgment. Injury severity was classified by physical therapists completing standardized training for conducting assessments with pediatric patients and using the International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) and the American Spinal Injury Association Impairment Scale (AIS)32 for children ≥6 years.33 For children <6 years, indication of injury level was documented based on the medical record noting the most cephalad segment. Clinical data were stored in a protected database for consecutively enrolled patients who consented (parent consent and child assent if ≥7 years) to an Institutional Review Board–approved study for clinical data collection, storage, analysis, and dissemination from 2013 to 2018.
Intervention
Standardized AB-LT was delivered five times a week for a minimum of 60 sessions, 1.5 hours total per day.26,30 While we tracked outcomes beyond 60 sessions, we recommended that every child receive a minimum of 60 AB-LT sessions for their first episode of care. For many reasons (eg, insurance limits, family responsibilities), children have variable therapy doses. In order to examine a uniform dose and its impact, we included children who were receiving a minimum of 60 AB-LT sessions.
The process for achieving standardization for clinical use of AB-LT has been reported16,26,27,30 and the therapy is described in detail for standardization purposes, and each will be briefly reported here. Standardization for physical therapists using this therapy includes an approximate 6- to 8-hour online course (initially provided as a live 8-hour lecture) with instructional text26 and a 2.5-day live course with patient volunteers for instruction and practice of manual skills and clinical decision-making. A standardized competency for delivery of the therapy and conduct of outcome measures is achieved by each physical therapist and assisting activity-based technician working in the clinic (ie, Christopher and Dana Reeve Foundation NeuroRecovery Network). The outcome measures have been selected and reported,27 recognizing the limit in availability of pediatric outcome measures.
AB-LT begins on the treadmill
For 1 hour, the patient trained first in the treadmill environment with partial body weight support (BWS) (Theraped; Innoventor, Earth City, MO [currently manufactured by Power NeuroRecovery, www.powerneurorecovery.com]) while wearing a trunk/pelvis harness (Robertson Harness, Ft. Collins, CO). Stepping was performed for a minimum of 30 minutes with standing activities incorporated to achieve a total of 60 training minutes. Manual cues were provided, facilitating task-specific trunk, leg, and arm kinematics. Trainers maximized weight-bearing during standing and stepping by increasing load-bearing through the legs while continuing to maintain appropriate stand/stepping kinematics. Stepping was performed at age-appropriate speeds reported for children 1–18 years.34–37 Age was not the only factor that determined treadmill speed, but it provided an initial target. Additionally, the child's trunk and limb kinematics were monitored in combination with the amount of body weight load, speed, and therapist/trainer facilitation abilities. The combination should produce a kinematic pattern for standing and stepping consistent with that of a typically developing child of comparable age. Treadmill speed was increased to facilitate a better kinematic stepping pattern and promote a motor response via trunk and lower limbs. In such instances, BWS was initially increased and then the load was gradually restored, if possible, based on ability to maintain typical kinematics. Standing activities focused on attainment and maintenance of appropriate posture during static and dynamic tasks. Such tasks included overhead reaching encouraging trunk extension and upright posture, facilitated marching, squats, and fine motor tasks challenging both trunk and upper extremities.
AB-LT continues over ground
Immediately following the treadmill-based training, AB-LT continued in the over ground environment. In the over ground environment, manual support was provided to aid in achieving sitting, standing, or walking for therapeutic purposes. Additionally, overhead BWS (using a posterior walker with overhead support or a BWS mounted to the ceiling), a walker, and manual cues were used to facilitate posture, limb position, or support. From these positions, manual cues and intent were provided in activities selected to promote use and challenge to the neuromuscular system requiring stability or initiation of movement. For instance, standing may be achieved via manual assistance at the legs and pelvis, whereas a task of stacking blocks – without them falling over – on a table challenges trunk stability. Manual assist at the trunk may be provided at a level as needed determined from the SATCo exam and/or more likely from day-to-day variability in trunk control following AB-LT in the treadmill environment. Therapeutic activities in sitting, standing, or stepping focused on patient-specific items from the Pediatric NRS based on the items with the lowest score. Knowledge of the child's neuromuscular capacity served as the foundation for selecting play items to complement and support the therapeutic goal at a challenging degree during sitting, standing, or stepping.
AB-LT extends into community integration
The third component of AB-LT is integration into daily living and the community. The treating therapist encouraged integration of the training principles into daily activities in the home and community, with the parent and/or caregiver(s) promoting self-training outside of therapy, for example, providing the opportunity to bear weight or to initiate movement. One example of this is using a rocking chair for a child to safely use the trunk, arm, or leg muscles in a naturally, motivating task. These activities are encouraged and discussed with the family and documented in medical notes; however, there is not a time or number of trials expected. Instead, implementation was encouraged for inclusion in a more natural way, such as sitting a child in a parent's lap with appropriate support. Such decisions and ways to promote use of new movement capacity, thus, will become part of everyday life. These activities do not change every day but are consistent with goals to incorporate training principles and challenge the neuromuscular system. Most recently, a parent described this not as exercise, but as a way of life in daily decisions of how a child is seated, whether at school, home, or in the car; whether there is a moment during the day that naturally reinforces voluntary movement below the lesion; whether a device allows a child to advance and use what motor abilities are either emerging or are now available to the child. Although equipment may appear as usual care devices, the strategy, approach, and long-term goals differ.23
Programmatically, neuromuscular electrical stimulation (NMES) was introduced as an ABT, subsequent to AB-LT, for only those with upper extremity (UE) impairment for 1–1.5 hours, five times a week. NMES was added as an ABT in the clinic much later than the initiation of AB-LT. Only several children thus received both therapies. For thoroughness, we present this information. The focus of NMES was UE impairment and neuromuscular recovery of arm/hand capacities with an additional 1.5 hours/day of therapy. With the limited exposure of NMES, based on number of sessions and a report of only four patients, we did not anticipate significant therapeutic impact.
During NMES evaluations, higher pulse width (1000 μS) and frequencies (40–100 Hz) were used to evoke contractions through sensorimotor pathways by stimulating afferents.38,39 Treatment intensities ranged from submotor to motor threshold based on the patient's tolerance to the stimulation. Customized Xcite programs (Xcite multi-channel FES; Restorative Therapies, www.restorative-therapies.com) promoted task-associated muscle activations of targeted muscle groups. Tasks were selected by individual patient's lowest recovery scores on the Pediatric NRS UE items, such as overhead reach and object to mouth.
Capacity outcome measures
Standardized outcome measures were assessed during initial evaluation (Evaluation 1), targeted for reevaluation every 20 sessions according to outcomes protocol (interim evaluations: Evaluation 2 and 3), and at 60 sessions (Evaluation 4) and/or discharge. Measures of neuromuscular capacity included the Pediatric NRS14 and the SATCo.15,16
The Pediatric NRS and SATCo are specifically conducted without compensation during testing of functional tasks and postural control. How the task is performed matters for scoring each assessment and supersedes completion of the task in any manner. Compensation indicates that a task is performed with an assistive device, physical assist, or atypical behavioral strategies (eg, tenodesis to achieve grasp versus finger flexion with a neutral positioned wrist). These instruments compare the current neuromuscular capacity of a patient performing a specific task to a reference of typical movement patterns used to accomplish a task, whether preinjury or development-associated.14,18
The Pediatric NRS was adapted from the adult Neuromuscular Recovery Scale (NRS).18,40–44 Content validity14 and good interrater reliability45 have been determined for children 2–12 years old. It assesses neuromuscular capacity in the context of 10 tasks that may be performed in the clinical environment. Of the 10 items, three items focus on trunk control (Supine to Sit, Sit Inside Base of Support, Sit Outside Base of Support), three on UEs (Object to Mouth, Overhead Reach, and In-Hand Manipulation), and four on lower extremity (Sit to Stand, Static Standing, Dynamic Standing, and Walking). For each task, a hierarchy of capacity for task execution is defined and scaled from 1 (no to low recovery) to 12 (criteria for highest level of recovery). The hierarchy parameters vary by task from amount of time (eg, sitting trunk control) to distance (eg, reach), number of intervening joints controlled (eg, standing), and limb kinematics (eg, grasp). Task difficulty for several items is limited in accordance with typical motor development and thus not all elements of the task hierarchy are expected or scored for patients of a younger age. See eAppendix A (613.5KB, pdf) for scoring cards (available in the online version of this article). Three additional items – Step Retraining, Step Adaptability, and Stand Adaptability – require a treadmill and BWS and are used predominantly for treatment planning and progression in the training environment.
The SATCo was developed for evaluation of trunk control, not as a single unit but to discretely assess segmental levels of control without compensation in children with neuromotor impairments.15,46 The SATCo demonstrates excellent inter- and intrarater reliability, as well as reliability for live to video review for children with cerebral palsy (9 months to 16 years).47 Responsiveness and sensitivity of the SATCo is also excellent in the pediatric population with SCI age 17 months to 11 years.16 Detailed instructions for conducting and scoring the SATCo have been reported.15,16 Scoring is determined for a total of 20 points and indicates the most caudal level at which the patient consecutively is able to achieve and demonstrate trunk control.16
Statistical methods
Data summary
We summarized participant demographics and characteristics and outcome measures using mean and associated standard deviation (SD), median and associated interquartile range, and full range (minimum, maximum) for continuous variables and frequency count and associated percentages for categorical variables. Neuromuscular capacity, assessed using the Pediatric NRS and SATCo, was summarized using mean and SD, median and interquartile range, and minimum and maximum.
Outcome comparisons
The time intervals between evaluations and the number of treatment sessions received by the patient during the same time period are reported in actual weekdays. Under ideal circumstances and according to the standardized clinical protocol, the patient would be seen for 20 weekdays and receive 20 therapy sessions during that period and then be reevaluated. This one-to-one relationship does not occur in the clinical setting and a patient's illness, travel difficulties, or inclement weather, for example, all impact the frequency and dosing for delivery intervention. With the intended frequency (daily) and dose of 60 sessions across 60 weekdays with a 1-day evaluation between the targeted 20 session interval, the fidelity of the protocol for actual attendance, interval periods, and sessions accomplished within the intervals and across time was examined.
Initial, 20-, 40-, and 60-session outcomes: Comparisons for Pediatric NRS and SATCo scores
Change in Pediatric NRS and SATCo scores was evaluated using linear mixed models including (a) age at time of injury and (b) time between injury and start of AB-LT as covariates and a random intercept for each participant to account for in-person variability resulting from multiple measurements for each participant. The two-by-two evaluation comparisons, for each interval of evaluations after 20 sessions, were obtained from constructed linear contrasts to obtain the adjusted mean differences. To obtain the overall adjusted estimated mean range from one evaluation to the next, we included the evaluation variable as a continuous variable in the model. All statistical tests were two-sided with a significance level of .05. Data processing and analysis were performed in SAS 9.4.
Effect of chronicity and initial scores on change scores following 60 sessions of AB-LT
First, we analyzed the association of years since injury to onset of AB-LT (ie, chronicity) and changes from Evaluation 1 (initial evaluation) to Evaluation 4 (post 60 sessions) using Spearman correlation. We also evaluated whether the outcome changes observed in participants treated in the acute rehabilitation phase (ie, <1 year since injury) were different from those observed in participants treated in the chronic phase (ie, ≥1 year post injury). For this analysis, we stratified the participants into two groups by chronicity and, within each one, we analyzed whether the change was statistically significant using the Wilcoxon signed-rank test. Then, we compared the changes between the two groups using the Wilcoxon signed-rank test. We chose the nonparametric tests because the data were not normally distributed. Second, we performed a Spearman correlation to evaluate the association between the initial scores and the change score.
Results
Participant demographics
Twenty-six patients, ages 1–13 years with an average age of 5 years (SD = 3), were enrolled in the AB-LT program. There was an approximate equal representation of male to female (respectively, 48% and 52%), nontraumatic to traumatic etiology (respectively, 54% and 46%), and enrolled during acute rehabilitation (< 1 year post SCI, 50%) to enrolled during the chronic period post-injury (≥ 1 year post SCI, 50%). The average time since injury to enrollment in the AB-LT program was 1 year (SD = 1) (see Table 1). At the initial evaluation, 100% of patients demonstrated a positive Babinski and clonus.
Table 1.
Demographics
| Variables | Total (N = 26) | Acute (n=13) | Chronic (n=13) | p | |
|---|---|---|---|---|---|
| Age at initial eval, years | Mean (SD) | 5 (3) | 6 (4) | 5 (2) | .9795 |
| Median (Q1-Q3) | 5 (3–6) | 5 (3–7) | 6 (4–6) | ||
| Range, min–max | 1 to 13 | 1 to 13 | 2 to 9 | ||
| Age at injury, years | Mean (SD) | 4 (3) | 5 (4) | 3 (2) | .0577 |
| Median (Q1-Q3) | 4 (2–5) | 5 (2–6) | 3 (1–4) | ||
| Range, min–max | 0 to 13 | 1 to 13 | 0 to 4 | ||
| Years between injury and AB-LT | Mean (SD) | 1.4 (1.3) | 0.4 (0.3) | 2.3 (1.2) | <.0001 |
| Median (Q1-Q3) | 1.0 (0.3–2) | 0.3 (0.2–0.7) | 2.0 (1.4–3.3) | ||
| Range, min–max | 0.1 to 5.3 | 0.1 to 0.9 | 1 to 5.3 | ||
| Level of injury, n (%) | Cervical | 9 (35%) | 4 (31%) | 5 (38%) | .3366 |
| Thoracic | 15 (58%) | 7 (54%) | 8 (62%) | ||
| Lumbar | 2 (7%) | 2 (15%) | 0 (0%) | ||
| AIS, n (%) | A | 6 (23%) | 1 (8%) | 5 (38%) | .0916 |
| B | 4 (15%) | 1 (8%) | 3 (23%) | ||
| C | 3 (12%) | 1 (8%) | 2 (15%) | ||
| D | 1 (4%) | 1 (8%) | 0 (0%) | ||
| Missinga | 12 (46%) | 9 (69%) | 3 (23%) | ||
| Gender, n (%) | Female | 11 (42%) | 5 (38%) | 6 (46%) | .6914 |
| Male | 15 (58%) | 8 (62%) | 7 (54%) | ||
| Nontraumatic/Traumatic, n (%) | Nontraumatic | 14 (54%) | 8 (62%) | 6 (46%) | .4314 |
| Traumatic | 12 (46%) | 5 (38%) | 7 (54%) | ||
| Chronicity, n (%) | Acute | 13 (50%) | 13 (100%) | 0 (0%) | <.0001 |
| Chronic | 13 (50%) | 0 (0%) | 13 (100%) | ||
| No. of sessions from Eval 1 to 4 | Mean (SD) | 55 (4) | 55 (3) | 55 (5) | .5167 |
| Median (Q1-Q3) | 56 (53–57) | 56 (55–56) | 55 (53–57) | ||
| Range, min–max | 49 to 68 | 50 to 59 | 49 to 68 | ||
| Enrollment weekdays from Eval 1 to 4 | Mean (SD) | 63 (9) | 62 (8) | 64 (11) | .9180 |
| Median (Q1-Q3) | 60 (57–65) | 60 (58–62) | 60 (57–70) | ||
| Range, min–max | 53 to 89 | 57 to 86 | 53 to 89 | ||
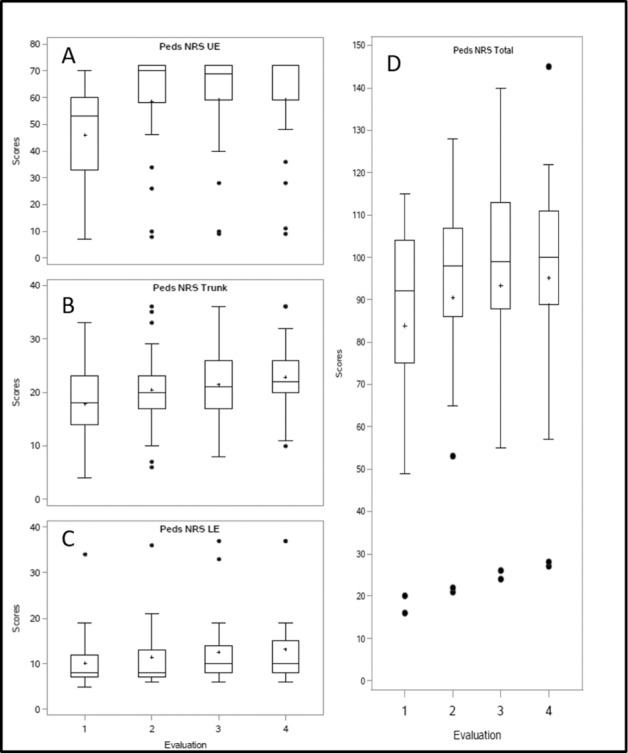
| Intensity = no. of sessions/5 weekdays | Mean (SD) | 5 (1) | 5 (1) | 4 (1) | .7254 |
| Median (Q1-Q3) | 5 (4–5) | 5 (4–5) | 5 (4–5) | ||
| Range, min–max | 3 to 5 | 3 to 5 | 3 to 5 | ||
Note: AB-LT = Activity-based Locomotor Training; AIS = American Spinal Injury Association Impairment Scale; eval = evaluation.
aNote missing data due to age <6 years and AIS not valid.
Data were missing for three patients for the Pediatric NRS as the instrument had not yet been introduced into the clinic, thus 23 patients were analyzed. Eight patients demonstrated maximum scores on the Pediatric NRS UE items, thus 15 were included in this analysis. The maximum score for the Pediatric NRS suggests full recovery as defined by the instrument's specific tasks. If UE items and maximum scores were demonstrated at baseline, such individuals likely have a lesion below T1. Four out of 15 patients with UE impairment received NMES (2 males, 2 females), with all four receiving NMES to the UEs and two receiving NMES also to the trunk. The other patients with UE impairment did not receive NMES as it had not been introduced to the clinic at the time of their enrollment into outpatient therapy. For thoroughness of reporting, we included the information concerning NMES that was provided to four patients during this period. Two of 26 patients undergoing the SATCo assessment achieved the maximum score of 20 at the initial evaluation and thus their SATCo data were not included in this analysis (n = 24). Prior study of the responsiveness of the SATCo in children with SCI indicated that there was not a floor or ceiling effect.16
Participation compliance and response to interventions
All patients (n = 26) with the intent of completing 60 AB-LT sessions completed a mean of 55 (SD = 4) AB-LT sessions within an average of 63 (SD = 9) weekdays. Intensity was maintained with a mean of 5 (SD = 1) therapy sessions/week (see Tables 1 and 2). Number of NMES sessions received by the sample during this period varied (ie, 7, 12, 20, and 40). Fidelity of the protocol for AB-LT intervention frequency (5x/week on weekdays) and dose (60 therapy sessions) is reported in Table 2. The planned (protocol designated) weekdays between each evaluation (1 to 2, 2 to 3, and 3 to 4) was 20, and thus the planned sessions between each evaluation was also 20. A comparison of actual weekdays and actual sessions is presented in concert with deviations from the protocol. For instance, the mean actual weekdays between Evaluation 1 and 2 was 22 (SD = 6) and the actual mean sessions within the 22 weekdays was 19 (SD = 1). Fidelity for the protocol was demonstrably high with a mean of 63 (SD = 9) weekdays to complete a mean of 55 (SD = 4) therapy sessions. Absences from therapy were due to patient illness, travel difficulties, inclement weather, or other family circumstances. Limitation in provider resources may also have inhibited the achievement of 60 sessions as evaluations are counted in the number of approved visits.
Table 2.
Comparison of observed vs planned training frequency and dose by evaluation periods
| Evaluation periods | Weekdays (excluding eval days) | No. of sessions | 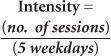 |
|||||
|---|---|---|---|---|---|---|---|---|
| Actual days it took | Deviation from planned | Actual completed | Deviation from planned | Actual intensity | Deviation from planned | |||
| Between two consecutive evals (planned days=20) (planned sessions=20) | Eval 1 to 2 | Mean (SD) | 22 (6) | 2 (6) | 19 (1) | −1 (1) | 5 (1) | −1 (1) |
| Median (IQR) | 21 (19, 24) | 1 (−1, 4) | 19 (18, 19) | −1 (−2, −1) | 5 (4, 5) | 0 (−1, 0) | ||
| Range, min–max | 16 to 42 | −4 to 22 | 16 to 21 | −4 to 1 | 2 to 5 | −3 to 0 | ||
| Eval 2 to 3 | Mean (SD) | 21 (3) | 1 (3) | 19 (2) | −1 (2) | 5 (1) | 0 (1) | |
| Median (IQR) | 21 (19, 22) | 1 (−1, 2) | 19 (18, 20) | −1 (−2, 0) | 5 (4, 5) | 0 (−1, 0) | ||
| Range, min–max | 17 to 30 | −3 to 10 | 15 to 22 | −5 to 2 | 3 to 5 | −2 to 0 | ||
| Eval 3 to 4 | Mean (SD) | 20 (5) | 0 (5) | 18 (3) | −2 (3) | 5 (1) | 0 (1) | |
| Median (IQR) | 19 (18, 21) | −1 (−2, 1) | 18 (17, 19) | −2 (−3, −1) | 5 (4, 5) | 0 (−1, 0) | ||
| Range, min–max | 10 to 37 | −10 to 17 | 10 to 28 | −10 to 8 | 3 to 5 | −2 to 0 | ||
| Between three evals (planned days=40) (planned sessions=40) | Eval 1 to 3 | Mean (SD) | 43 (6) | 3 (6) | 37 (2) | −3 (2) | 5 (1) | −1 (1) |
| Median (IQR) | 42 (40, 45) | 2 (0, 5) | 38 (36, 39) | −3 (−4, −1) | 5 (4, 5) | 0 (−1, 0) | ||
| Range, min–max | 35 to 59 | −5 to 19 | 34 to 43 | −6 to 3 | 3 to 5 | −2 to 0 | ||
| Eval 2 to 4 | Mean (SD) | 41 (7) | 1 (7) | 37 (3) | −4 (3) | 5 (1) | 0 (1) | |
| Median (IQR) | 39 (37, 43) | −2 (−3, 3) | 37 (35, 38) | −4 (−5, −2) | 5 (4, 5) | 0 (−1, 0) | ||
| Range, min–max | 33 to 62 | −7 to 22 | 30 to 48 | −10 to 8 | 3 to 5 | −2 to 0 | ||
| Between four evals (planned days=60) (planned sessions=60) | Eval 1 to 4 | Mean (SD) | 63 (9) | 3 (9) | 55 (4) | −5 (4) | 5 (1) | −1 (1) |
| Median (IQR) | 60 (57, 65) | 0 (−3, 5) | 56 (53, 57) | −4 (−7, −3) | 5 (4, 5) | 0 (−1, 0) | ||
| Range, min–max | 53 to 89 | −7 to 29 | 49 to 68 | −11 to 8 | 3 to 5 | −2 to 0 | ||
Note: Eval(s) = evaluation(s).
Patients participating in AB-LT infrequently experienced skin irritation from the harness or from repetitive manual contact from the trainer's contact with the skin. After altering harness fit and manual contacts or adding second skin, such irritation readily dissipated within a day. We observed signs and symptoms of autonomic dysreflexia in two out of 26 children (eg, splotchy red skin above the lesion, reported headache), one with cervical and one with high thoracic injury. In each child, the signs were recognized, and bladder/bowel fullness and harness wear were checked as possible mediating factors. We monitored patients for potential hip subluxation, and three out of 26 patients were referred for orthopedic evaluation. Continued participation in AB-LT occurred in each case. To date, no child has presented at the initial evaluation using oral baclofen. NMES was tolerated by the four patients using ramp times of approximately 1 second to promote comfort and intensity increased by 1 mA to allow patient adjustment. Responses were monitored through verbal response and facial expression.
Score change across evaluation time points for the Pediatric NRS
The Pediatric NRS total score varied from 84/154 (initial) to 95/154 (Evaluation 4) for 23 patients. The greatest improvement was observed from the initial to fourth evaluation (adjusted mean of 11.4, p < .05). The effect size (Cohen's d)48 for the Pediatric NRS total change from Evaluation 1 to 4 was calculated and determined to be very large, 1.71.49 Considering evaluation-to-evaluation changes, a significant change occurred from initial to 20 sessions (6.6, p < .05) followed by small changes between Evaluations 2 and 3 (3.1, p < .05), then 3 and 4 (1.7, p > .05). On average, the inter-evaluation adjusted change was 3.7 (p < .05). See Table 3 and Figure 1.
Table 3.
Pediatric Neuromuscular Recovery Scale (Ped NRS) and Segmental Assessment of Trunk Control (SATCo) outcomes
| Evaluation | Raw scores at each evaluation | Adjusted estimated mean changes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 1 to 2 | 2 to 3 | 3 to 4 | 1 to 3 | 2 to 4 | 1 to 4 | Each eval to the next | ||
| Expected no. of sessions | Initial | 20 | 40 | 60 | ||||||||
| Ped NRS | ||||||||||||
| Upper extremity (n=15) | Mean (SD) | 46 (19) | 51 (22) | 52 (21) | 52 (22) | 5.5* | 1.0 | 0.1 | 6.5* | 1.1 | 6.5* | 2.1* |
| Median (Q1-Q3) | 53 (33–60) | 60 (34–70) | 60 (40–69) | 60 (36–72) | ||||||||
| Range, min–max | 7–70 | 8–72 | 9–72 | 9–72 | ||||||||
| Trunk (n=23) | Mean (SD) | 19 (8) | 20 (8) | 22 (8) | 23 (7) | 1.8* | 1.1 | 1.3* | 2.9* | 2.4* | 4.2* | 1.4* |
| Median (Q1-Q3) | 18 (14–23) | 20 (17–23) | 21 (17–26) | 22 (20–26) | ||||||||
| Range, min–max | 4 to 35 | 6 to 36 | 8 to 36 | 10 to 36 | ||||||||
| Lower extremity (n=23) | Mean (SD) | 10 (6) | 11 (7) | 13 (8) | 13 (8) | 1.3 | 1.1 | 0.7 | 2.3* | 1.7* | 3.0* | 1.0* |
| Median (Q1-Q3) | 8 (7–12) | 8 (7–13) | 10 (8–14) | 10 (8–15) | ||||||||
| Range, min–max | 5 to 34 | 6 to 36 | 6 to 37 | 6 to 37 | ||||||||
| Total (n=23) | Mean (SD) | 84 (27) | 90 (27) | 94 (28) | 95 (28) | 6.6* | 3.1* | 1.7 | 9.7* | 4.8* | 11.4* | 3.7* |
| Median (Q1-Q3) | 92 (75–104) | 98 (86–107) | 100 (88–113) | 100 (89–111) | ||||||||
| Range, min–max | 16 to 115 | 21 to 128 | 24 to 140 | 27 to 145 | ||||||||
| SATCo (n=24) | Mean (SD) | 8 (5) | 11 (4) | 12 (4) | 13 (3) | 2.6* | 1.6* | 0.9 | 4.3* | 2.5* | 5.2* | 1.7* |
| Median (Q1-Q3) | 8 (5–11) | 10 (8–13) | 11 (10–16) | 13 (11–15) | ||||||||
| Range, min–max | 0–19 | 2–20 | 6–20 | 8–20 | ||||||||
* Significant, p < .05.
Figure 1.
Median, interquartile, and mean scores for Pediatric Neuromuscular Recovery Scale (Peds NRS). Subscores (A) upper extremity (UE), (B) trunk, and (C) lower extremity (LE) across evaluation time points (Evaluation 1, initial; Evaluation 2, after 20 sessions; Evaluation 3, after 40 sessions; and Evaluation 4, after 60 sessions of Activity-based Locomotor Training) and (D) total scores. ± = mean score.
We observed similar changes in the Pediatric NRS subscores, especially in the Pediatric NRS UE (n = 15, participants included with UE impairment) and trunk categories. Only four out of 15 participants with UE impairment also received NMES. There was a significant change from the initial evaluation to the second evaluation followed by small changes adding to a significant change between the initial and fourth evaluation (UE and trunk, 6.5 and 4.2, respectively; both ps < .05), resulting in an evaluation-to-evaluation change of 2.1 and 1.4 (p < .05). For the Pediatric NRS lower extremity (LE) category, the 20-session changes were not significant (ie, initial to Evaluation 2, Evaluation 2 to 3, Evaluation 3 to 4). However, the 40-session changes (Evaluation 1 to 3, Evaluation 2 to 4) were significant (2.3 and 1.7, respectively; p < .05), therefore there was a significant 3-point improvement for 60 sessions. Refer to Table 3 and Figure 1.
Score change across evaluation time points for the SATCo
We observed significant improvements for comparisons of the SATCo scores from initial to second evaluation and from second to third evaluation, but not for the third to fourth evaluation. The 60-session change from the initial evaluation was 5.2 with an estimated evaluation-to-evaluation change of 1.7 (both ps < .05). Refer to Table 3 and Figure 2. The effect size for the SATCo change from Evaluation 1 to 4 was 2.21 and notably large.48,49
Figure 2.
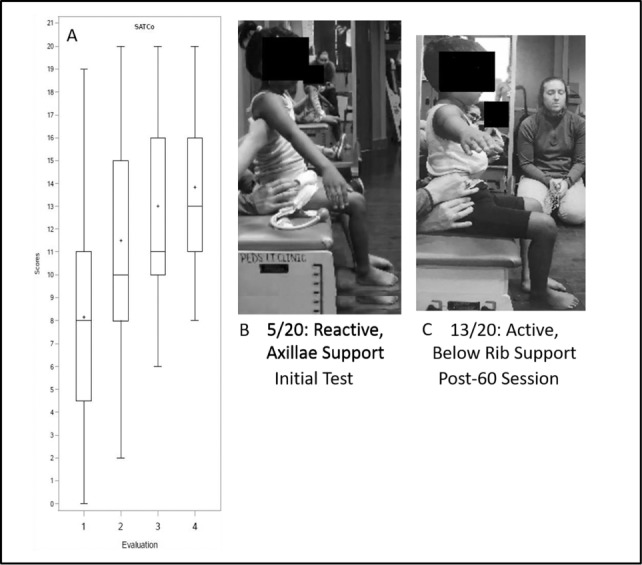
Segmental Assessment of Trunk Control (SATCo) median, interquartile, and mean scores across evaluation time points (A). (B, C) Patient example of SATCo scores pre-post 60-session Activity-Based Locomotor Training. ± = mean.
Effect of chronicity on Pediatric NRS and SATCo changes
When the study population was divided into two chronicity groups (acute and chronic), we found that within each group, changes in the Pediatric NRS and the SATCo were significant and similar to the combined population (p < .05). There were no significant differences when comparing outcomes for the acute group to the chronic group. The correlations were weak and nonsignificant.
Effect of initial scores on Pediatric NRS and SATCo changes
We found that the initial score was negatively correlated with both the Pediatric NRS and SATCo score changes (p < .05). Participants with initial scores that are relatively high do not improve as much as participants with relatively low initial scores. These correlations were significant for the Pediatric NRS UE and trunk categories and SATCo.
Ambulatory status at enrollment and post AB-LT program
Seventy-seven percent of the patients were nonambulatory (n = 20/26) at enrollment with a wheelchair being their only means of mobility, and they remained nonambulatory after 60 sessions of AB-LT. Of the six remaining patients, four at enrollment used a wheelchair as the primary means of self-mobility in the community, one was transported by a stroller, and one was a full-time ambulator without a device. Following 60 AB-LT sessions, gait speed improved for these six patients from 0.52 m/sec (SD = 0.57) to 1.03 m/sec (SD = 1.01). Three patients had progressed to ambulation being the primary means of mobility with only one using an assistive device (wheeled walker). Two continued to use a posterior walker for part-time mobility. While functional changes occurred, improved gait pattern and efficiency resulted (eg, reducing or eliminating leg circumduction and hip hiking, promoting knee flexion during swing phase).
Discussion
This is the first report of neuromuscular capacity as a primary outcome measure and indicates statistically significant score changes, large effect sizes, and meaningful impact in children with acquired SCI after 60 sessions (5x/week) of AB-LT, as measured with the SATCo and Pediatric NRS total scores. Incremental progress was demonstrated across the 60 sessions and was similar for the SATCo (average change of 1.7) and Pediatric NRS components (UE and trunk average change, 2.1 and 1.4), though less for the Pediatric NRS LE component (average change of 1.0). The initial 20 sessions of AB-LT resulted in larger significant change scores for the SATCo and Pediatric NRS UE and trunk components compared to the latter two 20 session sets. The LE components of the Pediatric NRS, however, made significant changes following 40 sessions (Evaluation 1 to 3, Evaluation 2 to 4).
Children benefited from ABTs, resulting in significant score changes regardless of chronicity. Whether children were treated during the acute phase or the chronic phase post SCI, positive changes in the SATCo and Pediatric NRS were deemed significant and similar. This is an important finding for health care professionals and physicians justifying services and expectations of benefit, particularly for children in the chronic phase post SCI. Health care providers typically indicate to parents that after a year post SCI, there will not be significant gains or changes in their child's recovery or functional status.50,51 Our findings challenge this dogma and support a prior finding of responsiveness of the SATCo in children with either acute or chronic SCIs.16 Initial severity of impairments below the lesion (eg, ISNCSCI, AIS motor scores, and incomplete vs complete) has also been viewed as negatively impacting the potential for recovery or functional gains.50,52 In this study, children who initially demonstrated severe limited neuromuscular capacity (ie, low scores on the SATCo and/or Pediatric NRS) were associated with higher gains in neuromuscular capacity after receiving AB-LT compared to children with higher initial scores. Certainly, the children with lower initial scores have more room to improve, however current assumptions and clinical experience would not predict significant gains by those with severely limited capacity post SCI. Together, these two aspects offer additional evidence for a recalibration of expectations for gains and improvements when children with severe injury and/or those with chronic injuries (>1 year and <6 years post-SCI) receive AB-LT.16,23,30,31,53
No patient (77% of study population; 20/26) with nonambulatory status at enrollment became a full-time or part-time ambulator across the 60 AB-LT sessions. The patients with part-time ambulatory status did demonstrate functional gains, walking speed gains, device changes, and improved gait pattern. While we expected changes in ambulatory status based on preliminary work,30 this did not occur in this report of clinical outcomes. Ongoing research points to the benefit of neuromodulatory techniques (eg, transcutaneous electrical spinal stimulation and epidural stimulation) as potential effective tools to enhance neural drive and central state of excitability.3,54,55 Such stimulation in combination with AB-LT has provided the catalyst for achievement of over ground walking in adults with complete SCI56 and demonstrated potential for other motor improvements.57 The accessibility of transcutaneous spinal stimulation makes it appealing for use with the pediatric population; specific study of its safety, feasibility, and efficacy in children post SCI is needed.
Instrumental to delivery of AB-LT to the pediatric population, in contrast to adults with SCI, is the knowledge and integration of developmental considerations. Such considerations are at the core of successful therapeutic interventions delivered to children who are 2 or 10 or 16 years old, while also taking into account individual differences. The fundamental delivery of AB-LT is protocol driven, applying principles and promoting progression across each of the three environments: treadmill, over ground, and community integration. Furthermore, the “play” activities during standing on or off the treadmill, for example, are selected based on therapeutic goal and capacity challenges (eg, active trunk extension). The activities are then tailored to age-appropriateness according to the standardized protocol; meaning and relevance to cognitive, behavioral, and developmental phase; and the individual motivational will and interests of a child. Thus, reaching overhead for a ball and shooting baskets is appropriate for one child, whereas standing with trunk upright and carefully removing blocks from a tower without it falling is appropriate for another. Both activities address the goal of maintaining active trunk extension. The therapy is, thus, standardized while taking into consideration the stage of development and the child's needs and motivation. Furthermore, the amount of practice experienced when a toddler learns to walk has been documented (ie, 7.7 times the length of an American football field per hour).58 This is a window into the scaling of practice and necessary repetition for a child with SCI for retraining standing, stepping, or trunk control.
The primary pediatric population with the potential for benefit from AB-LT are children with upper motor neuron spinal cord lesions, presenting with hyperreflexia, clonus, passive resistance to stretch and Babinski due to the presence of intact afferent-efferent neural connections below the lesion. As an apparent lower motor neuron lesion may truly be a mixed upper and lower motor neuron injury, we have included children with such injuries and determine benefit during a reevaluation after every 20 therapy sessions. If they show improvement, we continue AB-LT. Children with acute injuries (<1 year post SCI) or in the chronic phase (>1 year post SCI) appear, from the data, to benefit from AB-LT.
This is an intense program and there are several barriers to participation. One is economic resources. Funding can be through private insurance, self-pay, government-based Medicaid, or private charities. Many families do not have insurance coverage or have coverage for only up to 20 visits a year. The restriction of Medicaid funding to within one's home state also limits access for families seeking services. There are a dearth of specialized centers providing standardized AB-LT. The establishment of specialized pediatric centers, as was developed with the adult NeuroRecovery Network,27 would make standardized, trained, and competent, activity-based therapy programs more accessible.
Continued advances in our understanding of neurobiological principles of activity-dependent plasticity and neuromodulation will improve accessibility and accelerate the development and accessibility of neurotherapeutic interventions, changing the future delivery platform for children.
Limitations
The treatments were provided by staff physical therapists who had achieved competency standards in delivering AB-LT and the outcome measures. The outcome measures were assessed in routine clinical care and documented with the aim of reevaluation every 20 sessions, consistent with our clinical protocol (Manual of Pediatric Outcome Measures) by the treating physical therapist. Seven therapists provided AB-LT and one provided NMES. Blinding was not used as all of the outcome measures and session data were collected from the medical records and from usual care.
One potential critique of this data is that neuromuscular capacity does not necessarily correspond with functional changes in the real world that are relevant to a child or family's quality of life. A major thread of our current work seeks to substantiate these potential connections between neuromuscular capacity and real-world utility. Improved capacity to sit upright is critical for a child to engage in play, to perform activities of daily living, or to achieve increased independence. We have substantial informal data that improved neuromuscular capacity does, in fact, correspond with changes in a child's capabilities and performance that are important to families. For example, parents frequently make specific and detailed comments to therapists about the behavioral “firsts” they have observed as a result of ABT. We are currently performing a qualitative analysis of formative/quality improvement-focused interviews with parents that include spontaneous, but unexpectedly frequent, comments about improved quality of life. These observations give real-world context and meaning to the significant quantitative neuromuscular improvements measured by the SATCo and Pediatric NRS as actualized in children's daily life and activities with their family in their home and community. For instance, a mother reported being able to sit her child in a grocery cart for the first time, as opposed to pushing both a grocery cart and her child in an upright cart that provided trunk support. This is a meaningful change in the quality of life for both the child and the caregiver. Documented gains in upright trunk control paralleled a parent report and clinical observations (eg, the ability of child to use the upper extremities to stack blocks as opposed to weight-bearing through arms for sitting support). A halo effect in the context of a child receiving therapy may be a reasonable perspective for interpreting parent report of positive changes. The specific, targeted comments of a parent's initial report of a child's capacity (eg, “She's not going anywhere that I don't take her or put her”) and their description of specific changes in their child's behavior and ability in context (eg, “I now need to child-proof my home as she is getting into things that she shouldn't and I guess that is a good problem to have”) in concert with therapist observation provides face validity to parent reporting.
With the addition of NMES to the clinic, four patients received both NMES and AB-LT. For these four patients, any changes in neuromuscular capacity may be attributed to or confounded by the presence of two therapies. These patients received between 7 and 40 sessions of NMES during the episode of care and likely began with lower scores on the Pediatric NRS UE and the overall score. In this instance of two ABTs, we cannot be certain of an association of a specific therapy with the outcomes.
The Pediatric NRS and SATCo are proxy measures of spinal cord, activity-dependent plasticity. Certainly, more direct measures of nervous system response and plasticity59 are needed to identify mechanisms of behavioral change and recovery.
The inability to designate the level of injury or AIS classification for children <6 years of age is a limit in all pediatric SCI documentation, whether in the clinic or in research. We reported level of injury for children <6 years of age based on a review of the medical record by the therapist, though we recognize certain variability in its determination.
While responsiveness of the SATCo for the pediatric population post SCI has been established and shown to have a significantly larger effect size16 compared to other typical trunk control and balance measures (eg, modified functional reach, Berg Balance Test), content validity14 and interrater reliability45 have been determined for the Pediatric NRS. The adult NRS, also applied to adolescents older than 13 years old, has been tested and determined to have good test-retest reliability,40 good interrater reliability,41 responsiveness in the outpatient setting,43 and validity.42 Testing of psychometric properties (eg, test-retest reliability, responsiveness) for the Pediatric NRS has yet to be conducted and is forthcoming. The National Institute of Neurological Disorders and Stroke Common Data Element Sets (CDES)60 has designated both of these instruments, Pediatric NRS and SATCo, as exploratory for children with SCI. As published works further delineating the utility of each instrument14,16,45 are published, we anticipate that the exploratory status will be reexamined.
The Pediatric NRS and SATCo offer new measurement tools with which to examine the effect of neurotherapeutic interventions in children with SCI targeting improved neuromuscular capacity below the lesion. Significant improvements in a child's neuromuscular capacity, promoted by AB-LT, provide the foundation for physical exploration, play, and engagement guided by a child's curiosity, interests, and motivation. While we focused on immediate outcomes for neurocapacity following AB-LT, the long-term benefits of improved capacity, engagement, and health, as well as potential cost-benefit, merit future investigation.
Supplementary Material
Acknowledgments
The authors acknowledge Molly King, Lead Research Activity-based Technician; Colleen Logue, Lead Clinic Activity-based Technician; Lisa Clayton, Research Manager; Kate McNamara, DPT; and Natalie Goodrich, DPT, for their contributions to data management, coordination of services, and patient care. We thank the many Activity-Based Technicians who worked as a team with the therapists to deliver successfully Activity-based Locomotor Training to children. We also recognize Laura Westberg, Program Manager, for her thorough assistance in manuscript preparation and submission. We thank Dr. Kyle Brothers and Mary Schmidt Read, PT, DPT, MS, for their insightful reviews of the manuscript and suggestions. Our colleague and friend, Shelley Trimble, PT, passed away from metastatic breast cancer on November 15, 2016. She was integral to the development of the Pediatric Neurorecovery Program, its mission and vision. She has left a lasting imprint on our program, hearts, and the lives of many children and their families. She contributed specifically to the development of the Pediatric Neuromuscular Recovery Scale, the integration of the Segmental Assessment of Trunk Control into the program's standardized outcomes, direct patient care, and mentored the next generation of therapists to apply Activity-Based Therapies to advance recovery in children with spinal cord injury. For that and more, we remember Shelley Trimble, PT, and honor her as senior author of this article.
Footnotes
Conflicts of Interest
Dr. Behrman reports grants from the Craig H. Neilsen Foundation, Helmsley Charitable Trust, and Kosair Charities during the conduct of the study and personal fees from NeuroRecovery Training Institute, other from NeuroRecovery Learning, Inc., and personal fees from Shriners Research Foundation outside the submitted work.
Dr. Thompson, Dr. Ugiliweneza, Dr. Argetsinger, Dr. Roberts, and Ms. Stout have nothing to disclose.
Funding Support
This work was supported by Kosair Charities (Award No. OMGB141540), the Leona M. and Harry B. Helmsley Charitable Trust (Award No. 2016PG-MED004), and the NeuroRecovery Network funded by the Christopher and Dana Reeve Foundation through Grant/Cooperative Agreement Number U10/CCU220379 between CRF and Centers for Disease Control and Prevention (CDC). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC. This project was supported, in part, by grant number 90PR3002, from the US Administration for Community Living, Department of Health and Human Services, Washington, DC. Grantees undertaking projects under government sponsorship are encouraged to express freely their findings and conclusions. Points of view or opinions do not, therefore, necessarily represent official Administration for Community Living policy.
REFERENCES
- 1.Edgerton VR, Tillakaratne NJ, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Ann Rev Neurosci. 2004;27:145–167. doi: 10.1146/annurev.neuro.27.070203.144308. [DOI] [PubMed] [Google Scholar]
- 2.Kapadia N, Zivanovic V, Popovic MR. Restoring voluntary grasping function in individuals with incomplete chronic spinal cord injury: Pilot study. Top Spinal Cord Inj Rehabil. 2013;19(4):279–287. doi: 10.1310/sci1904-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mayr W, Krenn M, Dimitrijevic MR. Epidural and transcutaneous spinal electrical stimulation for restoration of movement after incomplete and complete spinal cord injury. Curr Opin Neurol. 2016;29(6):721–726. doi: 10.1097/WCO.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 4.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 5.Kalsi-Ryan S, Beaton D, Curt A et al. The Graded Redefined Assessment of Strength, Sensibility and Prehension: Reliability and validity. J Neurotrauma. 2012;29(5):905–914. doi: 10.1089/neu.2010.1504. [DOI] [PubMed] [Google Scholar]
- 6.Cote MP, Murray LM, Knikou M. Spinal control of locomotion: Individual neurons, their circuits and functions. Front Physiol. 2018;9:784. doi: 10.3389/fphys.2018.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edgerton VR, Courtine G, Gerasimenko YP et al. Training locomotor networks. Brain Res Rev. 2008;57(1):241–254. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadowsky CL, McDonald JW. Activity-based restorative therapies: Concepts and applications in spinal cord injury-related neurorehabilitation. Dev Disabil Res Rev. 2009;15:112–116. doi: 10.1002/ddrr.61. [DOI] [PubMed] [Google Scholar]
- 9.Dolbow DR, Gorgey AS, Recio AC et al. Activity-based restorative therapies after spinal cord injury: Inter-institutional conceptions and perceptions. Aging Dis. 2015;6(4):254–261. doi: 10.14336/AD.2014.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrman AL, Ardolino EM, Harkema SJ. Activity-based therapy: From basic science to clinical application for recovery after spinal cord injury. J Neurol Phys Ther. 2017;41(Suppl 3):S39–S45. doi: 10.1097/NPT.0000000000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behrman AL, Harkema SJ. Physical rehabilitation as an agent for recovery after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18(2):183–202. v. doi: 10.1016/j.pmr.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Marino RJ. Domains of outcomes in spinal cord injury for clinical trials to improve neurological function. J Rehabil Res Dev. 2007;44(1):113–122. doi: 10.1682/jrrd.2005.08.0138. [DOI] [PubMed] [Google Scholar]
- 13.Wood P. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 14.Ardolino EM, Mulcahey MJ, Trimble S et al. Development and initial validation of the Pediatric Neuromuscular Recovery Scale. Pediatr Phys Ther. 2016;28(4):416–426. doi: 10.1097/PEP.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 15.Butler P, Saavedra S, Sofranac M, Jarvis S, Woollacott M. Refinement, reliability and validity of the Segmental Assessment of Trunk Control (SATCo) Pediatr Phys Ther. 2010;22(3):246–257. doi: 10.1097/PEP.0b013e3181e69490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Argetsinger LC, Trimble SA, Roberts MT, Thompson JE, Ugiliweneza B, Behrman AL. Sensitivity to change and responsiveness of the Segmental Assessment of Trunk Control (SATCo) in children with spinal cord injury. Dev Neurorehabil. 2018;22:1–12. doi: 10.1080/17518423.2018.1475429. [DOI] [PubMed] [Google Scholar]
- 17.Levin MF, Kleim JA, Wolf SL. What do motor “recovery” and “compensation” mean in patients following stroke? Neurorehabil Neural Repair. 2009;23(4):313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 18.Behrman AL, Ardolino E, Vanhiel LR et al. Assessment of functional improvement without compensation reduces variability of outcome measures after human spinal cord injury. Arch Phys Med Rehabil. 2012;93(9):1518–1529. doi: 10.1016/j.apmr.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 19.Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. J Neurophysiol. 1997;77(2):797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- 20.Beres-Jones JA, Harkema SJ. The human spinal cord interprets velocity-dependent afferent input during stepping. Brain. 2004;127(Pt 10):2232–2246. doi: 10.1093/brain/awh252. [DOI] [PubMed] [Google Scholar]
- 21.Rejc E, Angeli CA, Harkema S. Effects of lumbosacral spinal cord epidural stimulation for standing after chronic complete paralysis in humans. PLoS One. 2015;10(7):e0133998. doi: 10.1371/journal.pone.0133998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy RR, Harkema SJ, Edgerton VR. Basic concepts of activity-based interventions for improved recovery of motor function after spinal cord injury. Arch Phys Med Rehabil. 2012;93(9):1487–1497. doi: 10.1016/j.apmr.2012.04.034. [DOI] [PubMed] [Google Scholar]
- 23.Howland DR, Trimble SA, Behrman AL. Neurologic recovery and restorative rehabilitation. In: Vogel LC, Zebracki K, Betz RR, Mulcahey MJ, editors. Spinal Cord Injury in the Child and Young Adult. London: Mac Keith Press; 2014. pp. 399–410. [Google Scholar]
- 24.Behrman AL, Bowden MG, Nair PM. Neuroplasticity after spinal cord injury and training: An emerging paradigm shift in rehabilitation and walking recovery. Phys Ther. 2006;86(10):1406–1425. doi: 10.2522/ptj.20050212. [DOI] [PubMed] [Google Scholar]
- 25.Harkema SJ, Hillyer J, Schmidt-Read M, Ardolino E, Sisto SA, Behrman AL. Locomotor training: As a treatment of spinal cord injury and in the progression of neurologic rehabilitation. Arch Phys Med Rehabil. 2012;93(9):1588–1597. doi: 10.1016/j.apmr.2012.04.032. [DOI] [PubMed] [Google Scholar]
- 26.Harkema SJ, Behrman AL, Barbeau H. Locomotor Training: Principles and Practice. New York: Oxford University Press, Inc.; 2011. [Google Scholar]
- 27.Harkema SJ, Schmidt-Read M, Behrman AL, Bratta A, Sisto SA, Edgerton VR. Establishing the NeuroRecovery Network: Multisite rehabilitation centers that provide activity-based therapies and assessments for neurologic disorders. Arch Phys Med Rehabil. 2012;93(9):1498–1507. doi: 10.1016/j.apmr.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 28.Harkema SJ, Schmidt-Read M, Lorenz DJ, Edgerton VR, Behrman AL. Balance and ambulation improvements in individuals with chronic incomplete spinal cord injury using locomotor training-based rehabilitation. Arch Phys Med Rehabil. 2012;93(9):1508–1517. doi: 10.1016/j.apmr.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 29.Behrman AL, Watson E, Fried G et al. Restorative rehabilitation entails a paradigm shift in pediatric incomplete spinal cord injury in adolescence: An illustrative case series. J Pediatr Rehabil Med. 2012;5(4):245–259. doi: 10.3233/PRM-2012-00225. [DOI] [PubMed] [Google Scholar]
- 30.Behrman AL, Nair PM, Bowden MG et al. Locomotor training restores walking in a nonambulatory child with chronic, severe, incomplete cervical spinal cord injury. Phys Ther. 2008;88(5):580–590. doi: 10.2522/ptj.20070315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fox EJ, Tester NJ, Phadke CP et al. Ongoing walking recovery 2 years after locomotor training in a child with severe incomplete spinal cord injury. Phys Ther. 2010;90(5):793–802. doi: 10.2522/ptj.20090171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirshblum SC, Waring W, Biering-Soerensen F et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med. 2011;34(6):547–554. doi: 10.1179/107902611X13186000420242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulcahey M, Gaughan JP, Chafetz RS, Vogel LC, Samdani AF, Betz RR. Interrater reliability of the International Standards for Neurological Classification of Spinal Cord Injury in youths with chronic spinal cord injury. Arch Phys Med Rehabil. 2011;92(8):1264–1269. doi: 10.1016/j.apmr.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 34.Sutherland D, Olshen R, Biden E, Wyatt M. The Development of Mature Walking. London: Mac Keith Press; 1988. [Google Scholar]
- 35.Waters RL, Lunsford BR, Perry J, Byrd R. Energy-speed relationship of walking: standard tables. J Orthop Res. 1988;6:215–222. doi: 10.1002/jor.1100060208. [DOI] [PubMed] [Google Scholar]
- 36.Schuyler J, Miller F, Herzog R, Catagno P, Lennon N, Richards J. Predicting changes in kinematic of gait relating to age and velocity. Paper presented at: 5th Annual Meeting of the Gait and Clinical Movement Analysis Society; 2000; Rochester, Minnesota. [Google Scholar]
- 37.Sutherland DH, Olshen R, Cooper L, Woo SL. The development of mature gait. J Bone Joint Surg Am. 1980;62(3):336–353. [PubMed] [Google Scholar]
- 38.Collins DF. Central contributions to contractions evoked by tetanic neuromuscular electrical stimulation. Exerc Sport Sci Rev. 2007;35(3):102–109. doi: 10.1097/jes.0b013e3180a0321b. [DOI] [PubMed] [Google Scholar]
- 39.Dean JC, Yates LM, Collins DF. Contribution to contractions evoked by neuromuscular electrical stimulation. J Appl Physiol. 2007;103(1):170–176. doi: 10.1152/japplphysiol.01361.2006. [DOI] [PubMed] [Google Scholar]
- 40.Behrman AL, Velozo C, Suter S, Lorenz D, Basso DM. Test-retest reliability of the Neuromuscular Recovery Scale. Arch Phys Med Rehabil. 2015;96(8):1375–1384. doi: 10.1016/j.apmr.2015.03.022. [DOI] [PubMed] [Google Scholar]
- 41.Basso DM, Velozo C, Lorenz D, Suter S, Behrman AL. Interrater reliability of the Neuromuscular Recovery Scale for spinal cord injury. Arch Phys Med Rehabil. 2015;96(8):1397–1403. doi: 10.1016/j.apmr.2014.11.026. [DOI] [PubMed] [Google Scholar]
- 42.Velozo C, Moorhouse M, Ardolino E et al. Validity of the Neuromuscular Recovery Scale: A measurement model approach. Arch Phys Med Rehabil. 2015;96(8):1385–1396. doi: 10.1016/j.apmr.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Tester NJ, Lorenz DJ, Suter SP et al. Responsiveness of the neuromuscular recovery scale during outpatient activity-dependent rehabilitation for spinal cord injury. Neurorehabil Neural Repair. 2016;30(6):528–538. doi: 10.1177/1545968315605181. [DOI] [PubMed] [Google Scholar]
- 44.Harkema SJ, Shogren C, Ardolino E, Lorenz DJ. Assessment of functional improvement without compensation for human spinal cord injury: Extending the neuromuscular recovery scale to the upper extremities. J Neurotrauma. 2016;33(24):2181–2190. doi: 10.1089/neu.2015.4213. [DOI] [PubMed] [Google Scholar]
- 45.Behrman AL, Trimble SA, Argetsinger LC et al. Interrater reliability of the pediatric neuromuscular recovery scale for spinal cord injury. Top Spinal Cord Inj Rehabil. 2019;25(2):120–129. doi: 10.1310/sci2502-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saavedra SL, Woollacott MH. Segmental contributions to trunk control in children with moderate-to-severe cerebral palsy. Arch Phys Med Rehabil. 2015;96(6):1088–1097. doi: 10.1016/j.apmr.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hansen L, Erhardsen KT, Bencke J, Magnusson SP, Curtis DJ. The reliability of the Segmental Assessment of Trunk Control (SATCo) in children with cerebral palsy. Phys Occup Ther Pediatr. 2018;38(3):291–304. doi: 10.1080/01942638.2017.1337662. [DOI] [PubMed] [Google Scholar]
- 48.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum Associates; 1988. [Google Scholar]
- 49.Sawilowski SS. New effect size rules of thumb. J Mod Appl Stat Methods. 2009;8(2):467–474. [Google Scholar]
- 50.Burns AS, Marino RJ, Flanders AE, Flett H. Clinical diagnosis and prognosis following spinal cord injury. Handb Clin Neurol. 2012;109:47–62. doi: 10.1016/B978-0-444-52137-8.00003-6. [DOI] [PubMed] [Google Scholar]
- 51.Zariffa J, Kramer JL, Fawcett JW et al. Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord. 2011;49(3):463–471. doi: 10.1038/sc.2010.140. [DOI] [PubMed] [Google Scholar]
- 52.Marino RJ, Ditunno JFJ, Donovan WH, Maynard FJ. Neurologic recovery after traumatic spinal cord injury: Data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil. 1999;80:1391–1396. doi: 10.1016/s0003-9993(99)90249-6. [DOI] [PubMed] [Google Scholar]
- 53.Gorski K, Harbold K, Haverstick K, Schultz E, Shealy SE, Krisa L. Locomotor training in the pediatric spinal cord injury population: A systematic review of the literature. Top Spinal Cord Inj Rehabil. 2016;22(2):135–148. doi: 10.1310/sci2202-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain. 2014;137(Pt 5):1394–1409. doi: 10.1093/brain/awu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayr W, Kreen M, Dimitrijevic MR. Motor control of human spinal cord disconnected from the brain and under external movement. Ady Exp Med Biol. 2016;957:159–171. doi: 10.1007/978-3-319-47313-0_9. [DOI] [PubMed] [Google Scholar]
- 56.Angeli CA, Boakye M, Morton RA et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244–1250. doi: 10.1056/NEJMoa1803588. [DOI] [PubMed] [Google Scholar]
- 57.Rath M, Vette AH, Ramasubramaniam S et al. Trunk stability enabled by noninvasive spinal electrical stimulation after spinal cord injury. J Neurotrauma. 2018;35(21):2540–2553. doi: 10.1089/neu.2017.5584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adolph KE, Cole WG, Komati M et al. How do you learn to walk? Thousands of steps and dozens of falls per day. Psychol Sci. 2012;23(11):1387–1394. doi: 10.1177/0956797612446346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saksena S, Mohamed FB, Middleton DM et al. Diffusion tensor imaging assessment of regional white matter changes in the cervical and thoracic spinal cord in pediatric subjects. J Neurotrama. 2019;36(6):853–861. doi: 10.1089/neu.2018.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mulcahey M, Vogel LC, Sheikh M et al. Recommendations for the National Institute for Neurologic Disorders and Stroke spinal cord injury common data elements for children and youth with SCI. Spinal Cord. 2017;55(4):331–340. doi: 10.1038/sc.2016.139. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


