Abstract
Background
The purpose of the present study was to evaluate the effect of leptin and leptin receptor (LEPR) expression on the efficacy of neoadjuvant chemotherapy in breast cancer.
Material/Methods
There were 325 breast cancer patients with complete data enrolled in this study. Patients were categorized into 3 groups: pathological complete response group, non-pathological complete response group, and progressive disease group. Immunohistochemistry was performed to determine leptin and its receptor LEPR expression levels that were compared among the 3 groups.
Results
Compared with the non-pathological complete response group, patients in the pathological complete response group had increased leptin and LEPR expression, although the difference was not statistically significant (P=0.194, P=0.110). In addition, the expression of leptin and LEPR in the pathological complete response group was also higher than that in the progressive disease group, and the difference of LEPR expression was statistically significant (P=0.008) while the leptin expression was not (P=0.065). There were more HER2+ breast cancer patients in the pathological complete response group categorized into strong positive, and positive expression of leptin and LEPR compared with the progressive disease group (P<0.05). There were significant differences of leptin and LEPR expression among breast cancer patients under different molecular subtypes HER2+, HR+, and triple negative, in which the triple negative patients had the highest expression of leptin and LEPR. In addition, patients in the progressive disease group had high and low expression of leptin and LEPR: 13.25% versus 11.32% and 13.1% versus 10.42% respectively.
Conclusions
Overexpression of leptin and LEPR improved the therapeutic efficacy of neoadjuvant chemotherapy for patients with breast cancer, especially for those with HER2+ subtype. Overexpression of leptin and LEPR was distinct among the different molecular subtypes of breast cancer, suggesting a certain predictive value for breast cancer prognosis.
MeSH Keywords: Carcinoma, Ductal, Breast; Chemotherapy, Adjuvant; Leptin; Prognosis; Receptors, Leptin; Treatment Outcome
Background
Breast cancer is the most common malignant tumor in women with the highest morbidity and mortality among all malignant tumors in women. Surgery is one of the essential treatment approaches for breast cancer, together with endocrine therapy, adjuvant chemotherapy, radiotherapy, and immunotherapy. Neoadjuvant chemotherapy refers to systemic cytotoxic drug therapy before surgical treatment of patients with locally advanced malignant tumors, which has been an important part of comprehensive treatment of breast cancer, and aims to reduce primary tumors for breast conserving treatments, and control and eliminate systemic micro-metastases and subclinical disseminated lesions [1]. Epidemiology studies have found that obesity, as a global health problem, is one of the important risk factors for breast cancer, especially for postmenopausal breast cancer and is closely related to the increase of breast cancer mortality [2]. There is increasing evidence that obesity significantly contributes to cancer development, progression, and poor prognosis, particularly through the regulator adipokine leptin. Leptin is a small molecular protein encoded by the obese gene with a molecular weight of 16 kD. As an important adipocytokine, it plays an important role in regulating energy expenditure and the occurrence of obesity. Moreover, increased expression of leptin can induce the activation of a series of oncogenic signals, thereby promoting the occurrence and development of cancer [3]. Leptin receptor (LEPR) is a product encoded by the LEPR gene. Leptin binds to LEPR to activate downstream signaling pathways. It has been reported that leptin and LEPR are overexpressed in breast cancer and have received much attention in recent years [4]. Clinical studies have shown that increased leptin levels in obese patients might be a risk factor for breast cancer. With the increase of leptin, women are more likely to suffer highly malignant and poorly differentiated breast cancer. The higher the malignant degree of breast cancer is, the higher the expression of LEPR [5], and the probability of poor prognosis is greatly increased [6,7]. It has also been reported that leptin binding to its receptor can not only promote cell proliferation, inhibit apoptosis, but also induce angiogenesis and promote breast cancer metastasis by regulating multiple signaling pathways including JAK/STAT3, MAPK/ERK1/2, PI3K/AKT, PKC, JNK, and p38 [8]. However, the relationship between leptin and its receptors and the efficacy of breast cancer treatment is unclear. Studies have found that obesity is an independent prognostic factor for the reduction of pathologic complete response (PCR) to neoadjuvant chemotherapy in breast cancer patients. It was reported that a decrease of complete response rate as well as shortening of progression-free survival (PFS) and overall survival was found in obese breast cancer patients compared with patients with normal or light weight after treatment of neoadjuvant chemotherapy, but the effect of leptin on the treatment of breast cancer has not been clarified [9]. This study explored the correlation of leptin as well as its receptor LEPR with the efficacy of neoadjuvant chemotherapy for breast cancer, and evaluated its predictive value for the efficacy and prognosis of neoadjuvant chemotherapy.
Material and Methods
Patients
There were 325 breast cancer patients who were treated with neoadjuvant chemotherapy at the Fourth Hospital of Hebei Medical University (Tumor Hospital of Hebei Province) from January 2014 to December 2017 who were enrolled in this study. There were 67 cases with PCR and 258 cases with non-pathological complete response (NPCR) in which 40 cases were progressive disease (PD). Surgical specimens were used for pathological response evaluation after neoadjuvant chemotherapy. Pathological results were determined by 2 pathologists using double-blind method according to the Miller and Payne scoring system. PCR was defined as no tumor residual in both the breast and the armpit. All patients received radical surgery treatment and the pathological data were complete. The age of the patients ranged from 27 years to 71 years, with an average age of 51 years. The clinicopathological features are shown in Table 1. Patients were categorized into 3 groups in accordance with treatment efficacy: PCR group, NPCR group, and PD group. This study was approved by the ethics committee of the Fourth Hospital of Hebei Medical University (Tumor Hospital of Hebei Province) and written informed consent were obtained from all patients.
Table 1.
Clinic or pathological parameter.
| Mean age at diagnosis (years) | 50.8 (27–71) |
| Menopausal status | N=325 (%) |
| Pre | 170 (52.3) |
| Post | 155 (47.7) |
| Clinical stage | |
| I | 54 (16.6) |
| II | 131 (40.3) |
| III | 140 (43.1) |
| Tumor size at baseline | |
| T1 | 210 (64.6) |
| T2 | 95 (29.2) |
| T3 | 17 (5.2) |
| T4 | 3 (1.0) |
| Node status at baseline | |
| − | 34 (10.5) |
| + | 291 (89.5) |
| Breast cancer subtype | |
| HR+ | 123 (37.8) |
| HER2+ | 136 (41.8) |
| Triple negative | 66 (20.4) |
| Curative effect | |
| PCR | 67 (20.6) |
| NPCR | 258 (79.4) |
| PD | 40 (12.3) |
HR+ – hormone receptor positive; HER2+ – human epidermal growth factor receptor 2 positive; PCR – pathological complete response; NPCR – non-pathological complete response; PD – progressive disease.
Immunohistochemistry
The enrolled 325 cases had a coarse needle biopsy to extract a specimen of tumor tissue. The obtained specimen was embedded in paraffin using routine procedures with immunohistochemistry performed using the SP method (streptavidin peroxidase conjugate method) to stain for leptin and LEPR. Leptin rabbit anti-human polyclonal antibody (ab16227, Abcam, Cambridge, UK) and LEPR rabbit anti-human polyclonal antibody (ab104403, Abcam, Cambridge, UK) were diluted to l: 200. Paraffin sections were cut to about 4 μm slices, dewaxed with conventional xylene, gradient alcohol hydrated and citrate buffer pH 6.0. Then high temperature and high-pressure antigen treatment for 2 minutes. After natural cooling, 10% deionized water solution was as a blocked for 15 minutes. Then sheep serum was added and incubated at 37°C for 20 minutes. The primary antibody was added and incubated at 4°C overnight. After rewarming for 1 hour, we added biotin-labeled secondary antibody and incubated at 37°C for 30 minutes, then added horseradish peroxidase-labeled streptomycin avidin and incubated at 28°C for 30 minutes. The section was washed with 10% PBS 3 times for 5 minutes each time. Freshly prepared DAB solution was added to the section and the coloration result was observed under a microscope. After color development ending, the hematoxylin stain was counterstained for 1–5 minutes, hydrochloric acid was used for differentiation, gradient alcohol was used for dehydration after washing, then xylene was applied, and neutral gum was used to seal the section. Then the section was observed under the microscope. Positively expressed tissue sections were used as the positive controls, and PBS was used instead of primary antibody as the negative control.
Evaluation and judgment of pathological results
Pathological results were determined by 2 pathologists using double-blind method according to the following criteria. When the judgment of the 2 pathologists was severely biased, a third pathologist was consulted. If agreement still could not be reached, the case would be excluded. A 5 high power field was used for the evaluation of leptin and its receptor LEPR. The expression of leptin and its receptor LEPR were localized to the cytoplasm and stained yellow or brownish yellow. The evaluation was assessed by semi-quantitative method. Sections were scored from 2 aspects: proportion of staining cells and staining intensity. There were 4 levels according to the proportion of staining cells which were 0 (negative, 0%), 1 (positive, <10%), 2 (positive, 11–50%), 3 (positive, 51–75%), and 4 (positive, >75%). From staining intensity, sections were scored into 3 levels: 0 (no staining), 1 (light yellow), 2 (dark yellow), and 3 (dark brown). The final score was obtained by multiplying the percentage of positive cells by the staining intensity and was categorized into 4 grades which were <2, 3–5, 6–8, and ≥9 which indicated negative, weak positive (+), positive (++), and strong positive (+++), respectively [10].
Statistical analysis
The different expression levels of leptin and its receptor LEPR which included negative, weak positive, positive, and strong positive, were compared by nonparametric test, Mann-Whitney U and Kruskal-Wallis H test. Comparison of disease progression between PCR and NPCR group and its comparison between leptin and LEPR high expression and low expression group were performed by χ2 test and Fisher’s exact test. The package SPSS 21.0 was used for statistical analysis. P<0.05 was considered statistically significant.
Results
Leptin and LEPR expression between PCR and NPCR group
In the PCR group, the cases with weak positive, positive, and strong positive leptin expression were 12 cases (17.9%), 11 cases (16.4%), and cases 29 (43.3%) respectively, while there were 15 (22.4%) negative cases As for the LEPR, there were 23 cases (34.3%) with strong positive expression, 29 cases (43.3%) with positive expression, 11 cases (16.4%) with weak positive expression, and 4 cases (6.0%) with negative expression. In the NPCR group with 258 cases in total, the expression of leptin was as follows 34 cases (13.2%) were strong positive expression, 92 cases (35.7%) were positive, 66 cases (25.6%) were weak positive, and 66 cases (25.6%) were negative. The LEPR expression was as follows: 73 cases (28.3%) strong positive, 104 cases (40.3%) positive, 43 cases (16.7%) weak positive, and 38 cases (14.7%) negative. The strong and positive strong expression of both leptin and LEPR in PCR group were higher than that in the NPCR group, but the difference was not statistically significant (P=0.194, P=0.110). There was no distinction between the PCR group and the NPCR group in leptin and LEPR expression (Figures 1, 2, Table 2).
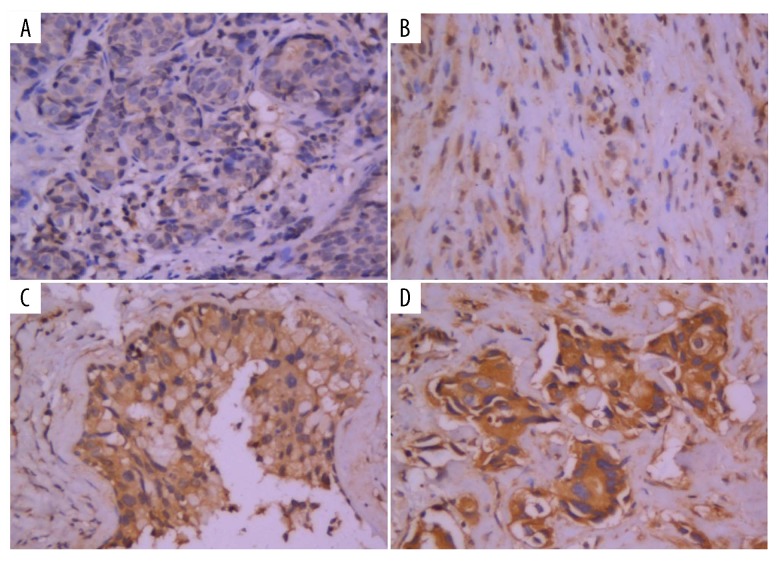
Figure 1.
The expression of leptin detected by immunohistochemistry (400×): (A) negative expression (−), (B) weak positive expression (+), (C) positive expression (++), (D) strong positive expression (+++).
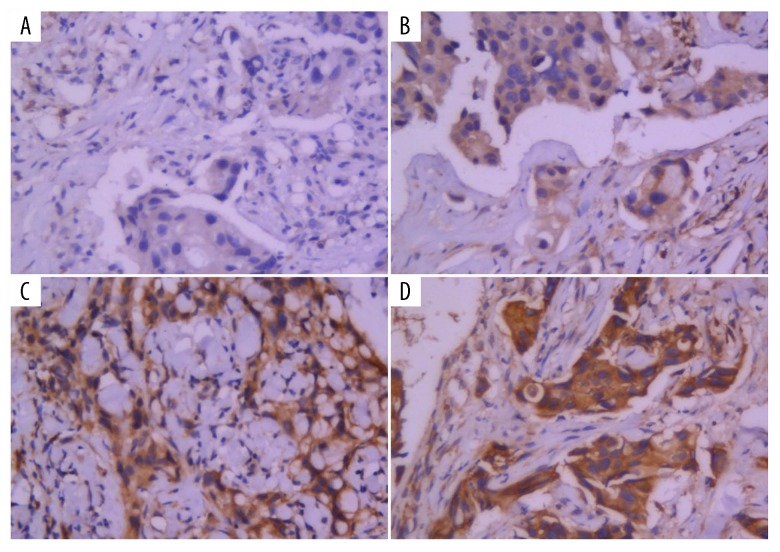
Figure 2.
Leptin receptor expression level detected by immunohistochemistry (400×): (A) negative expression (−), (B) weak positive expression (+), (C) positive expression (++), (D) strong positive expression (+++).
Table 2.
The expression of leptin and leptin receptor in PCR and NPCR group.
| Group | n (%) | Leptin | P value | Leptin receptor | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| PCR | 67 | 15 (22.4) | 12 (17.9) | 29 (43.3) | 11 (16.4) | 0.194 | 4 (6.0) | 11 (16.4) | 29 (43.3) | 23 (34.3) | 0.110 |
| NPCR | 258 | 66 (25.6) | 66 (25.6) | 92 (35.7) | 34 (13.2) | 38 (14.7) | 43 (16.7) | 104 (40.3) | 73 (28.3) | ||
PCR – pathological complete response; NPCR – non-pathological complete response.
Leptin and LEPR expression between the PCR group and the PD group
Among the 40 cases in the PD group, the patients with the strong positive, positive, weak positive, and negative expression of leptin respectively were 4 cases (10.0%), 11 cases (27.5%), 13 cases (32.5%), and 12 cases (30.0%). As for LEPR, there were 7 cases (17.5%) with strong positive expression, 15 cases (37.5%) with positive expression, 11 cases (27.5%) with weak positive expression, and 4 cases (17.5%) with negative expression. The strong positive and positive expression of both leptin and LEPR in the PCR group were higher than that in the PD group, in which the difference of leptin expression was statistically significant (P=0.008) but the expression of LEPR was not (P=0.065). The results indicated patients with high LEPR expression received better therapeutic efficacy (Table 3).
Table 3.
The expression of leptin and leptin receptor in PCR and PD group.
| Group | n (%) | Leptin | P value | Leptin receptor | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| PCR | 67 | 15 (22.4) | 12 (17.9) | 29 (43.3) | 11 (16.4) | 0.065 | 4 (6.0) | 11 (16.4) | 29 (43.3) | 23 (34.3) | 0.008 |
| PD | 40 | 12 (30.0) | 13 (32.5) | 11 (27.5) | 4 (10.0) | 7 (17.5) | 11 (27.5) | 15 (37.5) | 7 (17.5) | ||
PCR – pathological complete response; PD – progressive disease.
Leptin and LEPR expression of patients with different breast cancer molecular subtypes between the PCR group and the PD group
Among the 325 participants, 136 participants were human epidermal growth factor receptor 2 positive (HER2+), 123 participants were hormone receptor positive (HR+), and 66 participants were triple negative. The leptin and LEPR expression of patients with HR+ and triple negative breast cancer in the PCR group were higher than that in the PD group but not statistically different (P>0.05). The patients undergoing HER+ breast cancer in the PCR group with strong positive, positive, weak positive, and negative expressed leptin were 9 patients (25.0%), 15 patients (41.7%), 4 patients (11.1%), and 8 patients (22.2%), respectively. The situation of LEPR expression in the PCR group was: 17 patients (47.2%) were strong positive, 12 patients (33.3%) were positive, 6 patients (16.7%) were weak positive, and 1 patient (2.8%) was negative. Among patients undergoing HER+ breast cancer in the PD group, 1 patient (8.3%), 2 patients (16.7%), 5 patients (41.7%), and 4 patients (33.3%) were strong positive, positive, weak positive, and negative expressed of leptin while 1 patient (8.3%), 7 patients (58.3%), 2 patients (16.7%), and 2 patients (16.7%) were strong positive, positive, weak positive, and negative expression of LEPR, respectively. Positive and strong positive expression of leptin and LEPR was superior in the PCR group to that in the PD group (P=0.049, P=0.025). HER+ breast cancer patients with greater expression of leptin and LEPR received preferred therapeutic outcome (Tables 4–6).
Table 4.
The expression of leptin and leptin receptor of patients with HR+ in PCR and PD group.
| Group | n (%) | Leptin | P value | Leptin receptor | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| PCR | 12 | 3 (25.0) | 6 (50.0) | 3 (25.0) | 0 (0) | 0.433 | 1 (8.3) | 4 (33.3) | 5 (41.7) | 2 (16.7) | 0.067 |
| PD | 13 | 6 (46.2) | 4 (30.8) | 3 (23.1) | 0 (10.0) | 4 (30.8) | 6 (46.2) | 2 (15.4) | 1 (7.7) | ||
HR+ – hormone receptor positive; PCR – pathological complete response; PD – progressive disease.
Table 5.
The expression of leptin and leptin receptor of patients with HER2+ in PCR and PD group.
| Group | n (%) | Leptin | P value | Leptin receptor | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| PCR | 36 | 8 (22.2) | 4 (11.1) | 15 (41.7) | 9 (25.0) | 0.049 | 1 (2.8) | 6 (16.7) | 12 (33.3) | 17 (47.2) | 0.025 |
| PD | 12 | 4 (33.3) | 5 (41.7) | 2 (16.7) | 1 (8.3) | 2 (16.7) | 2 (16.7) | 7 (58.3) | 1 (8.3) | ||
HER2+ – human epidermal growth factor receptor 2 positive; PCR – pathological complete response; PD – progressive disease.
Table 6.
The expression of leptin and leptin receptor of patients with triple negative in PCR and PD group.
| Group | n (%) | Leptin | P value | Leptin receptor | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| PCR | 19 | 4 (21.1) | 2 (10.5) | 11 (57.9) | 2 (10.5) | 0.866 | 2 (10.5) | 1 (5.3) | 12 (63.2) | 4 (21.1) | 0.849 |
| PD | 15 | 2 (13.3) | 4 (26.7) | 6 (40.0) | 3 (20.0) | 1 (6.7) | 3 (20.0) | 6 (40.0) | 5 (33.3) | ||
PCR – pathological complete response; PD – progressive disease.
Leptin and LEPR expression in breast cancer cases with different molecular subtypes
The positive expression rate (including positive and strong positive) of leptin in patients with HER+, HR+, and triple negative breast cancer were 57.4%, 39.1%, and 60.6%, respectively. The positive expression rate of LEPR of patients with HER+ HR+, and triple negative breast cancer were 73.5%, 61.0%, and 81.8%. In comparison, leptin and LEPR expression of triple negative breast cancer cases were more than that of cases with HER2+ or HR+ breast cancer (P<0.01, Table 7).
Table 7.
The expression of leptin and leptin receptor in patients with different molecular breast cancer subtypes.
| Group | n (%) | Leptin | P value | Leptin receptor | P value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| − | + | ++ | +++ | − | + | ++ | +++ | ||||
| HR+ | 123 | 41 (33.3) | 34 (27.6) | 44 (35.8) | 4 (3.3) | <0.001 | 22 (17.9) | 26 (21.1) | 53 (43.1) | 22 (17.9) | 0.001 |
| HER2+ | 136 | 28 (20.6) | 30 (22.1) | 51 (37.5) | 27 (19.9) | 13 (9.6) | 23 (16.9) | 49 (36.0) | 51 (37.5) | ||
| Triple negative | 66 | 12 (18.2) | 14 (21.2) | 26 (39.4) | 14 (21.2) | 7 (10.6) | 5 (7.6) | 31 (47.0) | 23 (34.8) | ||
HR+ – hormone receptor positive; HER2+ – human epidermal growth factor receptor 2 positive.
Progressive disease (PD) and overall survival
As of December 1, 2018, there were 45 patients among 325 with PD and 23 deaths. In the PCR group, there were 5 patients (7.46%) with PD, while 40 patients (15.5%) in the NPCR group. The PD rate of patients with high and low expressed of leptin and LEPR were 13.25% versus 11.32% and 13.1% versus 10.42%, indicating the prognosis with high expression of leptin and LEPR might be worse, but the difference was not statistically significant (P>0.05, Tables 8, 9).
Table 8.
Progression disease in PCR and NPCR group.
| Group | n (%) | Progression disease | P value | |
|---|---|---|---|---|
| Yes | No | |||
| PCR | 67 | 5 (7.5) | 62 (92.5) | 0.090 |
| NPCR | 258 | 40 (15.5) | 218 (84.5) | |
PCR – pathological complete response; NPCR – non-pathological complete response.
Table 9.
Progression disease at different expression levels of leptin and leptin receptor.
| Group | n (%) | Progression disease | P value | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| Leptin | Low expression (−~+) | 159 | 18 (11.3) | 141 (88.7) | 0.596 |
| High expression (++~+++) | 166 | 22 (13.3) | 144 (86.7) | ||
| Leptin receptor | Low expression (−~+) | 96 | 10 (10.4) | 86 (89.6) | 0.502 |
| High expression (++~+++) | 229 | 30 (13.1) | 199 (86.9) | ||
Discussion
Leptin is a protein product encoded by the obese gene, containing 167 amino acids and as an important adipose cell cytokine, it is closely related to obesity. In addition to regulating food intake and energy balance, leptin plays a significant role in promoting tumor invasion, metastasis, neovascularization, and epithelial-mesenchymal transition by binding to LEPR [11,12]. It has been reported in many studies to be crucial for the development of various malignant tumors. The effect of leptin and LEPR on the treatment of breast cancer has received many concerns due to its high correlation with obesity and that obesity is closely associated with breast cancer. Some in vivo and in vitro experiments suggested that leptin and LEPR were contributing factors to the development of breast cancer [13–15], while other studies found no significant correlation between leptin and breast cancer [16]. But the leptin and LEPR acting in the onset and therapeutics of breast cancer has not been thoroughly defined for inadequate direct evidence to favor the causality between leptin and LEPR expression.
Del Fabbro et al. reported overweight patients receiving neoadjuvant therapy had lower PCR rates and shorter PFS times [9,17]. Karatas et al. [9] suggested leptin was an independent prognostic factor for the reduction of PCR to neoadjuvant chemotherapy in breast cancer patients. But the effect of leptin on the therapeutic efficacy has not consistently reported. Slomian et al. [18] found that serum adiponectin and leptin levels were not associated with chemotherapy response to ovarian cancer. Bain et al. [19] presented a study analyzing the neoadjuvant chemotherapy treatment of gastric cancer and found a higher TRG4-5 expression compared with TRG1-3 and showed a close correlation of high expression of leptin with poor PCR to neoadjuvant chemotherapy. Méndez-Hernández et al. [20] found that the polymorphisms of leptin rs7799039 and ADIPOQ rs1501299 were associated with overweight/obesity, and obese patients were more likely to have no response to chemotherapy. In this study, leptin and LEPR expression of patients with PCR was higher than that with NPCR after neoadjuvant chemotherapy treatment, and the difference of leptin and LEPR expression between patients with PCR and PD inclined greater. The difference in expression of LEPR was statistically significant. It might be because patients with high leptin and LEPR expression were more likely to experience tumor invasion and metastasis and thus these patients were more susceptible to chemotherapy.
Further comparison among patients with different molecular types revealed that HER2+ breast cancer patients with high leptin and LEPR expression had better therapeutic effects, and the difference was statistically significant. Ray et al. [21] found that leptin could activate HER/neu receptor in breast cancer cells. Suimacz et al. [22] reported co-expression of HER2 with leptin and LEPR might enhance the activity of HER2 in a study of multiple cell lines and 59 cases of tumor tissues of breast cancer. In addition, Wang et al. [23] found LEPR were associated with HER2 expression and might be a leading cause of the poor prognosis in breast cancer. This might explain why neoadjuvant chemotherapy received a better efficacy in HER+ patients with high leptin and LEPR expression.
The current study also initiated the expression distinction of leptin and LEPR among breast cancer patients with different subtypes. The expression of triple negative and HER2+ patients was greater than that with HR+, in which leptin and LEPR expression in triple negative patients was the highest. Previous studies have found that persistent obesity increases the risk of triple negative and HR+ breast cancer, and the effect on the triple negative type is more obvious [24].
Méndez-Hernández et al. suggested the higher levels of leptin were found in HR+ specimens [20]. Wang et al. [23] indicated that LEPR expression was not associated with estrogen receptor and progesterone receptor expression but related to the expression of HER2. Whereas, Wang et al. [25] found that LEPR expression was no significantly difference among breast cancer subtypes. Hence, the expression of them in different molecular subtypes remains arguable and commitment to be confirmed by further studies.
Previous clinical studies have found that patients with an increase of leptin often receive poor prognosis [26–28]. There is no uniform conclusion about the effect of LEPR on the prognosis of early breast cancer. Some studies suggested the overall survival and disease-free survival were significantly shortened in breast cancer patients with high expression of LEPR [13]. Conversely, another study showed that LEPR expression levels were not associated with disease-free survival and overall survival [25]. In our study, there were 45 cases with PD and 23 deaths. The PD rate of patients with leptin and LEPR overexpression was higher than that with low expression, suggesting that overexpression of leptin and LEPR might be the sign of poor prognosis. The follow-up time was short in this study, and it could not exclude the possibility of the incidence of significant difference between the 2 groups with the extension of follow-up time.
Conclusions
High expression of leptin and its receptor LEPR might lead to better therapeutic efficacy of neoadjuvant chemotherapy for breast cancer patients, especially for patients with HER2+ breast cancer. High expression of leptin and LEPR were different among patients with different molecular subtypes of breast cancer, which might have a certain predictive value for the prognosis.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Wu JH, He JS, Ni Y, Wang XM. Evaluation methods for the efficacy of neoadjuvant chemotherapy for breast cancer. Chin J Cancer. 2010;29:223–28. doi: 10.5732/cjc.009.10257. [DOI] [PubMed] [Google Scholar]
- 2.Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Res Treat. 2012;134:769–81. doi: 10.1007/s10549-012-2073-x. [DOI] [PubMed] [Google Scholar]
- 3.Shao Z, Wang H, Li X, et al. Morphological distribution and internal enhancement architecture of contrast-enhanced magnetic resonance imaging in the diagnosis of non-mass-like breast lesions: A meta-analysis. Breast J. 2013;19:259–68. doi: 10.1111/tbj.12101. [DOI] [PubMed] [Google Scholar]
- 4.Grossmann ME, Ray A, Nkhata KJ, et al. Obesity and breast cancer: Status of leptin and adiponectin in pathological processes. Cancer Metastasis Rev. 2010;29:641–53. doi: 10.1007/s10555-010-9252-1. [DOI] [PubMed] [Google Scholar]
- 5.Riolfi M, Ferla R, Del Valle L, et al. Leptin and its receptor are overexpressed in brain tumors and correlate with the degree of malignancy. Brain Pathol. 2010;20:481–89. doi: 10.1111/j.1750-3639.2009.00323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santillan-Benitez JG, Mendieta-Zeron H, Gomez-Olivan LM, et al. The tetrad BMI, leptin, leptin/adiponectin (L/A) ratio and CA 15-3 are reliable biomarkers of breast cancer. J Clin Lab Anal. 2013;27:12–20. doi: 10.1002/jcla.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ressel L, Finotello R, Innocenti VM, et al. Preliminary report on the expression of leptin and leptin receptor (ObR) in normal, hyperplastic and neoplastic canine mammary tissues. Res Vet Sci. 2012;93:343–49. doi: 10.1016/j.rvsc.2011.07.020. [DOI] [PubMed] [Google Scholar]
- 8.Lin W, Wei W, Man Z, Yuan H. [The research progress of Leptin signaling pathways in breast cancer]. Journal of Modern Oncology. 2017 [in Chinese] [Google Scholar]
- 9.Karatas F, Erdem GU, Sahin S, et al. Obesity is an independent prognostic factor of decreased pathological complete response to neoadjuvant chemotherapy in breast cancer patients. Breast. 2016;32:237–44. doi: 10.1016/j.breast.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 10.Yuan L, Li X, Wei F, et al. Expression and clinical significance of Leptin and NF-κBp65 in cervical intraepithelial neoplasia. Chinese Journal of Oncology Prevention & Treatment. 2015 [in Chinese] [Google Scholar]
- 11.Gonzalez-Perez RR, Lanier V, Newman G. Leptin’s pro-angiogenic signature in breast cancer. Cancers. 2013;5:1140–62. doi: 10.3390/cancers5031140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barone I, Giordano C, Bonofiglio D, et al. Leptin, obesity and breast cancer: Progress to understanding the molecular connections. Curr Opin Pharmacol. 2016;31:83–89. doi: 10.1016/j.coph.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Giordano C, Chemi F, Panza S, et al. Leptin as a mediator of tumor-stromal interactions promotes breast cancer stem cell activity. Oncotarget. 2016;7:1262–75. doi: 10.18632/oncotarget.6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Lili D, Han C, et al. Association of expressions and gene polymorphisms of leptin receptor with breast cancer. Cancer Res Clin. 2015;27:740–44. [Google Scholar]
- 15.Gui Y, Pan Q, Chen X, et al. The association between obesity related adipokines and risk of breast cancer: A meta-analysis. Oncotarget. 2017;8:75389. doi: 10.18632/oncotarget.17853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi F, Diao S, Yuan X, Li J. [Association of plasma leptin levels and soluble leptin receptor with breast cancer]. Zhonghua Yu Fang Yi Xue Za Zhi. 2018;52:253–59. doi: 10.3760/cma.j.issn.0253-9624.2018.03.007. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 17.Egidio DF, Henrique P, Warneke CL, et al. The relationship between body composition and response to neoadjuvant chemotherapy in women with operable breast cancer. Oncologist. 2012;17:1240–45. doi: 10.1634/theoncologist.2012-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Słomian GJ, Nowak D, Buczkowska M, et al. The role of adiponectin and leptin in the treatment of ovarian cancer patients. Endokrynol Pol. 2019;70(1):57–63. doi: 10.5603/EP.a2018.0081. [DOI] [PubMed] [Google Scholar]
- 19.Bain GH, Collie-Duguid E, Murray GI, et al. Tumour expression of leptin is associated with chemotherapy resistance and therapy-independent prognosis in gastro-oesophageal adenocarcinomas. Br J Cancer. 2014;110:1525–34. doi: 10.1038/bjc.2014.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Méndez-Hernández A, Gallegos-Arreola MP, Moreno-Macías H, et al. LEP rs7799039, LEPR rs1137101, and ADIPOQ rs2241766 and 1501299 polymorphisms are associated with obesity and chemotherapy response in Mexican women with breast cancer. Clin Breast Cancer. 2017;17:453–62. doi: 10.1016/j.clbc.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 21.Ray A, Nkhata KJ, Cleary MP. Effects of leptin on human breast cancer cell lines in relationship to estrogen receptor and HER2 status. Int J Oncol. 2007;30:1499–509. [PubMed] [Google Scholar]
- 22.Fiorio E, Mercanti A, Terrasi M, et al. Leptin/HER2 crosstalk in breast cancer: In vitro study and preliminary in vivo analysis. BMC Cancer. 2008;8:305. doi: 10.1186/1471-2407-8-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Cheng X, Xiu-Juan LV. The expression of leptin receptor in breast cancer and the relationship with clinical prognosis. China Practical Medicine. 2013 [in Chinese] [Google Scholar]
- 24.Yuan XL, Xu ZP, Liu CR, et al. [Study of the association between polymorphism of persistent obesity, human leptin gene/leptin receptor gene and molecular subtypes of breast cancer]. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51:533–38. doi: 10.3760/cma.j.issn.0253-9624.2017.06.015. [in Chinese] [DOI] [PubMed] [Google Scholar]
- 25.Wang CJ, Li P, Pan B, et al. Prognostic value of leptin receptor in early breast cancer. Basic & Clinical Medicine. 2016 [in Chinese] [Google Scholar]
- 26.Anneli E, Meri U, Pirkko KL, et al. Obesity and physical inactivity are related to impaired physical health of breast cancer survivors. Anticancer Res. 2013;33:1595–602. [PubMed] [Google Scholar]
- 27.Virginie D, Laetitia D, Hermine B, et al. Breast cancer and obesity: In vitro interferences between adipokines and proangiogenic features and/or antitumor therapies? PLoS One. 2013;8:e58541. doi: 10.1371/journal.pone.0058541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orecchioni S, Reggiani F, Talarico G, Bertolini F. Mechanisms of obesity in the development of breast cancer. Discov Med. 2015;20:121–28. [PubMed] [Google Scholar]