Abstract
Background
The Gustave Roussy Immune Score (GRIm-Score) and the Royal Marsden Hospital prognostic score (RMH score) were recently developed in order to improve a better participant selection for phase I trials. The GRIm-Score is formed by combination of lactate dehydrogenase (LDH), serum albumin concentration, and neutrophil-to-lymphocyte ratio (NLR). The RMH score is calculated by LDH, albumin, and number of metastases. These two scores have been validated only in phase I trials. The purpose of this study was to assess whether these scores are useful for practical treatment of immune-checkpoint inhibitor (ICI) monotherapy in pretreated non-small cell lung cancer (NSCLC).
Methods
This was a retrospective and single-centered study of 76 NSCLC patients treated with ICI monotherapy between December 2015 and October 2018 at our hospital. We divided 76 patients into high and low GRIm-Score and RMH score groups. Comparison of overall survival (OS) and progression free survival (PFS) was performed by Kaplan-Meier curves and log-rank tests. Independent prognostic factors of OS and PFS were analyzed by multivariate Cox proportional hazard analyses.
Results
The OS of the high GRIm-Score group was significantly shorter than that of the low score group (low vs. high; median 19.9 vs. 3.2 months, P < 0.01), while no significant difference was observed in PFS (2.6 vs. 2.1 months, P = 0.13). The PFS of the high RMH score was significantly shorter than that of the low score group (low vs. high; 2.6 vs. 1.8 months, P = 0.01), while there was no significant difference in OS (16.0 vs. 10.4, P = 0.24). Multivariate analyses detected high GRIm-Score (hazard ratio (HR) 3.93, 95% confidence interval (CI) 2.04 - 7.58, P < 0.01), and high RMH score (HR 1.76, 95% CI 1.03 - 3.02, P = 0.04) as poor prognostic factors of OS and PFS, respectively.
Conclusions
Baseline GRIm-Score and RMH score were independent prognostic factors of OS and PFS of ICI monotherapy for pretreated NSCLC patients, respectively. These two scores are not only selection biomarkers for patients in experimental trials, but also useful prognostic biomarkers for NSCLC patients practically treated with ICI therapy.
Keywords: Gustave Roussy Immune Score, Royal Marsden Hospital prognostic score, Immune-checkpoint inhibitor, Nivolumab, Pembrolizumab, Atezolizumab, Pretreated, Non-small cell lung cancer
Introduction
The Royal Marsden Hospital prognostic score (RMH score) [1] and the Gustave Roussy Immune score (GRIm-Score) [2] were recently developed for the purpose of supporting the optimal selection of eligible participants enrolled into early phase I trials of new biologic or cytotoxic-based agents, and mainly of new immune-checkpoint inhibitors (ICIs), respectively. The former scoring system is calculated by the following three objective variables; lactate dehydrogenase (LDH) level (within normal range: 0 vs. > upper limit of normal (ULN): +1 ), serum albumin level (≥ 3.5g/dL: 0 vs. < 3.5g/dL: +1), and number of metastatic sites (< three sites: 0 vs. ≥ three sites: +1) [1], while the latter by LDH, serum albumin, and neutrophil-to-lymphocyte ratio (NLR) (≤ 6:0 vs. > 6: +1) [2]. Thus, the number of metastatic sites in the RMH score is replaced by NLR in the GRIm-Score. This was caused by the result that, in the phase I cohort of ICIs therapies, NLR > 6 was associated with shorter OS, while the number of metastases was not associated with a poorer outcome [2]. The sum of the three variables divided participants into low (total score of 0 or 1) and high (2 or 3) score groups. Patients in low score groups had significantly longer overall survival than those in high score groups [2, 3]. These two scores have been validated only in phase I trials including various solid tumors [2-6]. These scoring systems consist of independent prognostic variables for ICI therapy. Previous retrospective studies indicated that a higher LDH level [7, 8], a decrease in albumin [9], a higher NLR [10-12], and more metastatic sites [13] were associated with shorter PFS and OS in patients with non-small cell lung cancer (NSCLC) treated with ICIs. However, little is known about usefulness of RMH score and GRIm-Score for practical ICI therapy.
The present study investigated whether RMH score and GRIm-Score are independent prognostic markers for pretreated and advanced NSCLC treated with practically available ICI monotherapy.
Materials and Methods
Patients and study design
This study was single-centered and retrospective. We collected the pretreated patients with pathologically confirmed diagnosis of NSCLC who had received anti-programmed cell death-1 (PD-1) or anti-programmed cell-death ligand 1 (PD-L1) immuno-monotherapy (nivolumab, pembrolizumab or atezolizumab) between December 2015 and October 2018 at our institution. We excluded the patients treated with the first-line pembrolizumab, which had been approved for patients with tumor proportion score (TPS) ≥ 50%. Epidermal growth factor receptor (EGFR) mutation status was examined by the peptide nucleic acid-locked nucleic acid PCR clamp method or EGFR gene mutation analysis COBAS version 2 by LSI Medience Cooperation (Tokyo, Japan). TPS of PD-L1 expression was examined by an autostainer with PD-L1 immunohistochemistry (IHC) 22C3 pharmDx test at our institution. A pretreatment peripheral venous blood test, performed within 2 weeks prior to the introduction of the immunotherapy, included LDH level, serum albumin concentration, proportion of neutrophils and lymphocytes in leukocytes. In four patients, the missing values of serum albumin were complemented by the mean value of the other patients. From our electrical medical records, we collected the following pretreatment background data; sex, age, smoking history, histology, PD-L1 expression, EGFR mutation status, ALK rearrangement, Eastern Cooperative Oncology Group Performance Status (ECOG-PS), number of metastatic sites, and laboratory data. We counted the number of metastatic sites according to the independent radiologists’ reports of the computed tomography scan and other image examinations taken before the ICI therapy. We also collected the treatment regimen, its efficacy and outcomes. Progression-free and overall survivals (PFS and OS) were calculated from the first day of the immunotherapy administration until progressive disease (PD) or death due to any cause, and until death due to any cause, respectively. Response to immunotherapy was based on Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. Disease control rate (DCR) was defined as complete response (CR) + partial response (PR) + stable disease (SD) per all patients, and overall response rate (ORR) as CR + PR per all patients. The date of data cut-off was the end of December, 2018. The GRIm-Score was developed on the basis of the following three components; NLR (> 6 = 1 vs. ≤ 6 = 0), LDH (> upper limit of normal (ULN) of each center, 225 IU/L in our hospital = 1 vs. ≤ 225 IU/L = 0), and serum albumin (< 3.5 g/dL = 1 vs. ≥ 3.5 g/dL = 0). RMH score was formed by LDH, albumin, and the number of metastatic sites of disease (< three sites = 0 vs. ≥ three sites = 1). The NLR was calculated by dividing neutrophils by lymphocytes. Patients were sorted into a high score group (2 or 3 factors) and a low score group (0 or 1 factors).
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Data analysis
The median value with interquartile range (IQR), frequency, and median time with 95% confidential intervals (CI) express the continuous and categorical variables, and survival times, respectively. For their comparisons, we used Mann-Whitney U test, Fisher’s exact test, Kaplan-Meier curves with log-rank test, respectively. Cox proportional hazards models were used to detect independent factors associated with OS and PFS. Hazard ratios (HR) with 95% CI describe these models’ results. Considering the numbers of events and the previous studies [2, 3, 13], we pre-defined the following explanatory variables in multivariate analyses; age, histology, and ECOG-PS for OS; age, histology, PD-L1 expression, and ECOG-PS for PFS. The number of metastatic sites with GRIm-Score and the NLR with RMH score were mandatory variables in the multivariate analyses. P-value < 0.05 was defined as statistically significant. All statistical analyses were performed with EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria).
Results
This study included 76 patients. We divided 76 patients into high and low GRIm-Score and RMH score groups. At the time of data cut-off, 50, 20, and six patients were dead, alive, and lost to follow-up, respectively. Among 50 dead patients, 35, 11, and four died at our hospital, at other hospitals, and at home, respectively. Six patients still continued ICI therapy. However, one of them continued ICI even after disease had already progressed. In contrast, 51, 10, and nine patients discontinued ICI therapy because of progressive disease (PD), adverse effects, deteriorated general conditions and complications, respectively. One patient changed the regimens without documented PD for some unknown reason. PD was confirmed in 67 patients. Anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) were examined by immunohistochemistry at our institution and by real-time PCR method by SRL, Inc. (Tokyo, Japan) in 53 and nine patients, respectively. As a result, one patient had ALK rearrangement, while none had ROS1 rearrangement. The patient with positive ALK rearrangement had already received crizotinib and alectinib until the introduction of nivolumab, and was classified into RMH score high and GRIm-Score low group. This study included 11 patients with positive EGFR mutation. These 11 patients had received EGFR-tyrosine kinase inhibitors (TKI) until the initiation of ICI therapy. Two patients received erlotinib and osimertinib after PD of pembrolizumab and nivolumab, respectively. Tables 1 and 2 showed patients’ backgrounds, treatment, response, and pretreatment laboratory data according to GRIm-Score and RMH score, respectively.
Table 1. Baseline Characteristics, Treatment, and Laboratory Data According to GRIm-Score (N = 76).
| N | GRIm-Score |
P | |
|---|---|---|---|
| Low (N = 55) | High (N = 21) | ||
| Backgrounds | |||
| Sex (N) | |||
| Male/female | 35/20 | 14/7 | 1.00a |
| Age (years) | |||
| Median (IQR) | 69 (62.5 - 73) | 71 (68 - 75) | 0.29b |
| Smoking status (N) | |||
| NS/Ex, CS | 12/43 | 4/17 | 1.00a |
| BMI | |||
| Median (IQR) | 22.7 (21.0 - 25.1) | 22.5 (18.2 - 24.0) | 0.26b |
| ≥ 18.5/< 18.5 (N) | 48/7 | 14/7 | 0.051a |
| Histology (N) | |||
| Non-SQ/SQ | 42/13 | 16/5 | 1.00a |
| EGFR mutation (N) | |||
| (-) or NA/(+) | 46/9 | 19/2 | 0.72a |
| PD-L1 status (N) | |||
| ≥ 50%/1-49%/< 1% /NA | 13/9/5/28 | 1/5/3/11 | 0.25a |
| ECOG-PS (N) | |||
| 0-1/2/3 | 43/11/1 | 11/6/4 | 0.02a |
| Metastatic sites (N) | |||
| < 3/≥ 3 | 25/30 | 10/11 | 1.00a |
| Treatment | |||
| ICI regimen (N) | |||
| Nivolumab | 41 | 17 | |
| Pembrolizumab | 11 | 2 | |
| Atezolizumab | 3 | 2 | 0.08a |
| Previous treatment (N) | |||
| Anti-angiogenic drug | 25 | 11 | 0.62a |
| EGFR-TKIs | 13 | 2 | 0.21a |
| Radiotherapy | 13 | 6 | 0.77a |
| Further line treatment (N) | 31 | 9 | 0.32a |
| ICI efficacy | |||
| ORR (%) (95% CI) | 18.2 (9.1 - 30.9) | 4.8 (0.1 - 23.8) | 0.27a |
| DCR (%) (95% CI) | 41.8 (28.7 - 55.9) | 19.0 (5.4 - 41.9) | 0.11a |
| Laboratory data | |||
| NLR | |||
| Median (IQR) | 2.64 (1.89 - 3.89) | 5.85 (3.57 - 7.18) | < 0.01b |
| > 6 (N) | 1 | 10 | < 0.01a |
| LDH (U/L) | |||
| Median (IQR) | 208 (184.5 - 242.5) | 324 (228 - 387) | < 0.01b |
| > ULN (N) | 19 | 16 | < 0.01a |
| Albumin (g/dL) | |||
| Median (IQR) | 3.8 (3.5 - 4.0) | 3.1 (2.8 - 3.2) | < 0.01b |
| < 3.5 g/dL (N) | 9 | 21 | < 0.01a |
aFisher’s exact test, bMann-Whitney U test. BMI: body mass index; CI: confidence interval; CS: current smoker; DCR: disease control rate; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; EGFR: epidermal growth factor receptor; Ex: ex-smoker; GRIm-Score: Gustave Roussy Immune Score; IQR: interquartile range; ICI: immune-checkpoint inhibitor; LDH: lactate dehydrogenase; NA: not assessed; NLR: neutrophil-to-lymphocyte ratio; NS: non-smoker; ORR: overall response rate; SQ; squamous cell carcinoma; TKI: tyrosine kinase inhibitor; ULN: upper limit of normal.
Table 2. Baseline Characteristics, Treatment, and Laboratory Data According to RMH score (N = 76).
| N | RMH score |
P | |
|---|---|---|---|
| Low (N = 44) | High (N = 32) | ||
| Backgrounds | |||
| Sex (N) | |||
| Male/female | 31/13 | 18/14 | 0.23a |
| Age (years) | |||
| Median (IQR) | 69.5 (63 - 73) | 70.5 (61.25 - 75) | 0.63b |
| Smoking status (N) | |||
| NS/Ex, CS | 7/37 | 9/23 | 0.26a |
| BMI | |||
| Median (IQR) | 22.0 (20.5 - 23.8) | 23.1 (19.3 - 26.5) | 0.20b |
| ≥ 18.5/< 18.5 (N) | 36/8 | 26/6 | 1.00a |
| Histology (N) | |||
| Non-SQ/SQ | 30/14 | 28/4 | 0.06a |
| EGFR mutation (N) | |||
| (-) or NA/(+) | 35/9 | 30/2 | 0.11a |
| PD-L1 status (N) | |||
| ≥ 50%/1-49%/< 1%/NA | 9/10/3/22 | 5/5/5/17 | 0.57a |
| ECOG-PS (N) | |||
| 0-1/2/3 | 33/9/2 | 21/8/3 | 0.58a |
| Metastatic sites (N) | |||
| < 3/≥ 3 | 28/16 | 7/25 | < 0.01a |
| Treatment | |||
| ICI regimen (N) | 0.77a | ||
| Nivolumab | 32 | 26 | |
| Pembrolizumab | 9 | 4 | |
| Atezolizumab | 3 | 2 | |
| Previous treatment (N) | |||
| Anti-angiogenic drug | 18 | 18 | 0.25a |
| EGFR-TKIs | 11 | 4 | 0.25a |
| Radiotherapy | 11 | 8 | 1.00a |
| Further line treatment (N) | 24 | 16 | 0.82a |
| ICI efficacy | |||
| ORR (%) (95% CI) | 20.5 (9.8 - 35.3) | 6.2 (0.8 - 20.8) | 0.11a |
| DCR (%) (95% CI) | 40.9 (26.3 - 56.8) | 25.0 (11.5 - 43.4) | 0.15a |
| Laboratory data | |||
| NLR | |||
| Median (IQR) | 2.92 (2.02 - 3.99) | 3.75 (2.30 - 5.47) | 0.14b |
| > 6 (N) | 4 | 7 | 0.19a |
| LDH (U/L) | |||
| Median (IQR) | 197 (179 - 219) | 304 (233.8 - 344) | < 0.01b |
| > ULN (N) | 9 | 26 | < 0.01a |
| Albumin (g/dL) | |||
| Median (IQR) | 3.8 (3.5 - 4.0) | 3.2 (2.9 - 3.6) | < 0.01b |
| < 3.5 g/dL (N) | 8 | 22 | < 0.01a |
aFisher’s exact test, bMann-Whitney U test. BMI: body mass index; CI: confidence interval; CS: current smoker; DCR: disease control rate; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; EGFR: epidermal growth factor receptor; Ex: ex-smoker; IQR: interquartile range; ICI: immune-checkpoint inhibitor; LDH: lactate dehydrogenase; NA: not assessed; NLR: neutrophil-to-lymphocyte ratio; NS: non-smoker; ORR: overall response rate; RMH score: Royal Marsden Hospital prognostic score; SQ; squamous cell carcinoma; TKI: tyrosine kinase inhibitor; ULN: upper limit of normal.
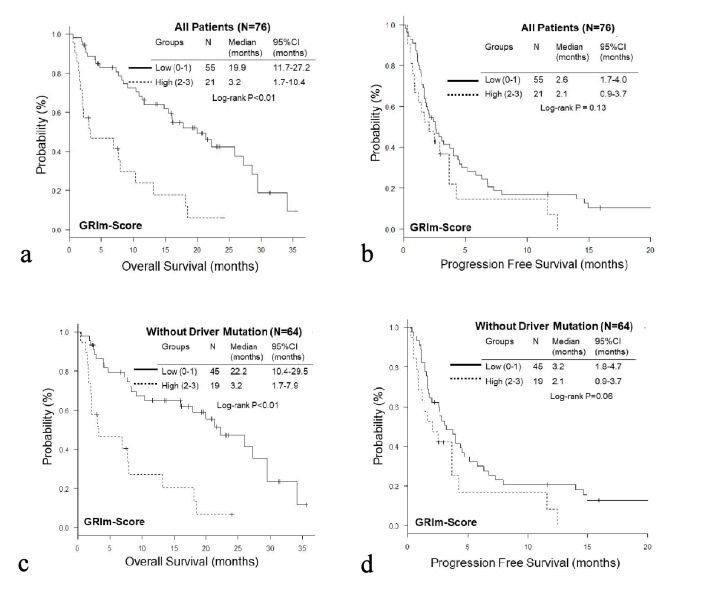
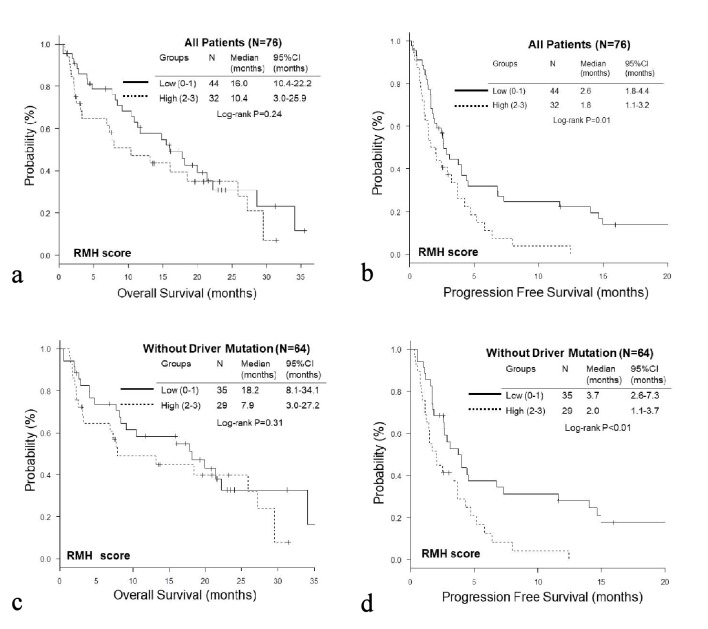
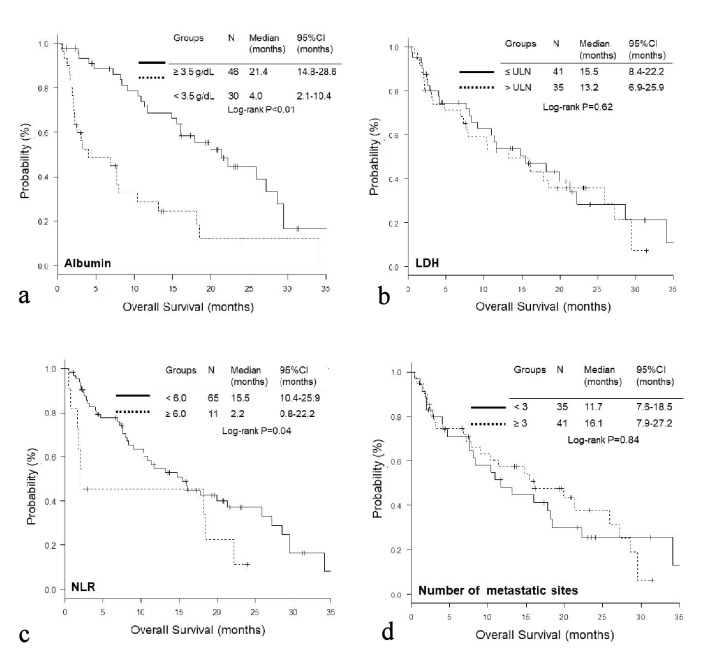
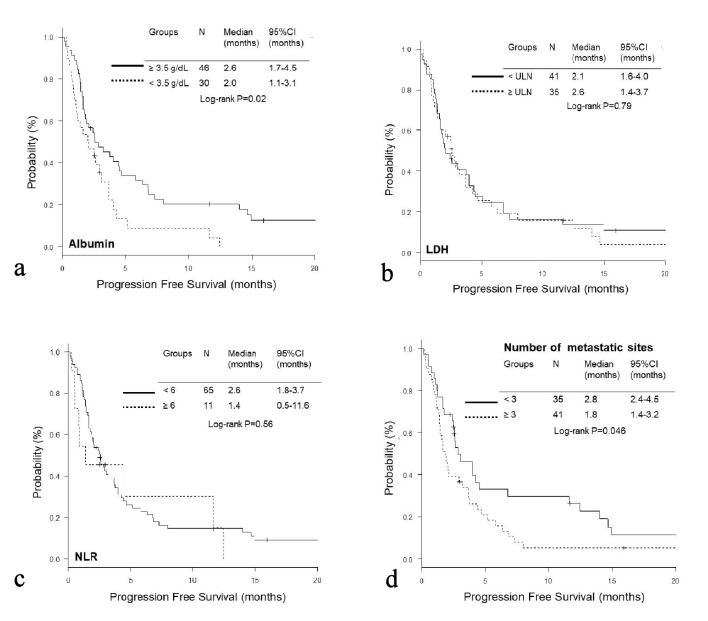
The OS of the high GRIm-Score group was significantly shorter than that of the low score group (low vs. high; median 19.9 vs. 3.2 months, P < 0.01), while no significant difference was observed in PFS (median 2.6 vs. 2.1 months, P = 0.13) (Fig. 1a, b). On the other hand, the PFS of the high RMH score was significantly shorter than that of the low score group (low vs. high; median 2.6 vs. 1.8 months, P = 0.01); while there was no significant difference in OS (median 16.0 vs. 10.4, P = 0.24) (Fig. 2a, b). When we excluded 12 patients with EGFR mutation or ALK rearrangement, similar trends were observed in patients without driver mutations (Fig. 1c, d and Fig. 2c, d). Thereafter, we compared OS and PFS according to albumin, LDH, NLR and number of metastases. Both OS and PFS of patients with high albumin (≥ 3.5g/dL) were longer than those of low albumin (< 3.5g/dL) (Figs. 3a, 4a). Regarding LDH, no difference was detected both in OS and PFS (Fig. 3b, 4b). The OS of patients with high NLR (≥ 6.0) and PFS of patients with more than three metastatic sites were significantly shorter than OS of low NLR (< 6.0) and PFS of number of metastases < 3, respectively, though no significant difference was found in PFS of NLR and OS of number of metastatic sites (Fig. 3c, d and Fig. 4c, d).
Figure 1.
Kaplan-Meier curves of overall survival (a, c) and progression-free survival (b, d) of all patients (a, b) and patients without EGFR mutation or ALK rearrangement (c, d) according to GRIm-Score.
Figure 2.
Kaplan-Meier curves of overall survival (a, c) and progression-free survival (b, d) of all patients (a, b) and patients without EGFR mutation or ALK rearrangement (c, d) according to RMH score.
Figure 3.
Kaplan-Meier curves of overall survival according to albumin (a), LDH (b), NLR (c), and number of metastatic sites (d).
Figure 4.
Kaplan-Meier curves of progression-free survival according to albumin (a), LDH (b), NLR (c), and number of metastatic sites (d).
Multivariate Cox proportional hazard analyses of all patients detected high GRIm-Score (HR 3.93, 95% CI 2.04 - 7.58, P < 0.01) (Table 3), and high RMH score (HR 1.76, 95% CI 1.03 - 3.02, P = 0.04) (Table 4) as poor prognostic factors of OS and PFS, respectively.
Table 3. Multivariate Cox Hazard Proportional Analyses of Overall Survival of All Patients (N = 76).
| Variable | GRIm-Score |
RMH score |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||
| < 70 | 1 (reference) | 1 (reference) | ||
| ≥ 70 | 0.87 (0.48 - 1.59) | 0.66 | 1.13 (0.61 - 2.10) | 0.71 |
| Histology | ||||
| Non-SQ | 1 (reference) | 1 (reference) | ||
| SQ | 0.62 (0.28 - 1.36) | 0.23 | 0.77 (0.35 - 1.71) | 0.53 |
| ECOG-PS | ||||
| 0 or 1 | 1 (reference) | 1 (reference) | ||
| ≥ 2 | 1.80 (0.98 - 3.30) | 0.056 | 1.93 (1.04 - 3.59) | 0.04* |
| No. of metastases | ||||
| < 3 | 1 (reference) | |||
| ≥ 3 | 1.05 (0.57 - 1.94) | 0.88 | ||
| GRIm-Score | ||||
| Low (0 - 1) | 1 (reference) | |||
| High (2 - 3) | 3.93 (2.04 - 7.58) | < 0.01** | ||
| NLR | ||||
| < 6 | 1 (reference) | |||
| ≥ 6 | 1.92 (0.85 - 4.34) | 0.12 | ||
| RMH score | ||||
| Low (0 - 1) | 1 (reference) | |||
| High (2 - 3) | 1.42 (0.78 - 2.57) | 0.25 | ||
CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; GRIm-Score: Gustave Roussy Immune Score; HR: hazard ratio; NLR: neutrophil-to-lymphocyte ratio; RMH score: Royal Marsden Hospital prognostic score; SQ: squamous cell carcinoma. *P < 0.05, **P < 0.01.
Table 4. Multivariate Cox Hazard Proportional Analyses of Progression-Free Survival of All Patients (N = 76).
| Variable | GRIm-Score |
RMH score |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Age (years) | ||||
| < 70 | 1 (reference) | 1 (reference) | ||
| ≥ 70 | 0.60 (0.34 - 1.06) | 0.08 | 0.67 (0.38 - 1.19) | 0.17 |
| Histology | ||||
| Non-SQ | 1 (reference) | 1 (reference) | ||
| SQ | 0.92 (0.47 - 1.81) | 0.81 | 0.87 (0.45 - 1.69) | 0.68 |
| PD-L1 expression | ||||
| < 1% or NA | 1 (reference) | 1 (reference) | ||
| ≥ 1% | 0.57 (0.33 - 0.97) | 0.04* | 0.67 (0.39 - 1.16) | 0.15 |
| ECOG-PS | ||||
| 0 or 1 | 1 (reference) | 1 (reference) | ||
| ≥ 2 | 2.32 (1.28 - 4.20) | < 0.01** | 2.45 (1.35 - 4.41) | < 0.01** |
| No. of metastases | ||||
| < 3 | 1 (reference) | |||
| ≥ 3 | 1.92 (1.10 - 3.33) | 0.02* | ||
| GRIm-Score | ||||
| Low (0 - 1) | 1 (reference) | |||
| High (2 - 3) | 1.36 (0.75 - 2.44) | 0.31 | ||
| NLR | ||||
| < 6 | 1 (reference) | |||
| ≥ 6 | 0.90 (0.42 - 1.90) | 0.78 | ||
| RMH score | ||||
| Low (0 - 1) | 1 (reference) | |||
| High (2 - 3) | 1.76 (1.03 - 3.02) | 0.04* | ||
CI: confidence interval; ECOG-PS: Eastern Cooperative Oncology Group Performance Status; GRIm-Score: Gustave Roussy Immune Score; HR: hazard ratio; NA: not assessed; NLR: neutrophil-to-lymphocyte ratio; RMH score: Royal Marsden Hospital prognostic score; SQ: squamous cell carcinoma. *P < 0.05, **P < 0.01.
Discussion
This was the first study evaluating GRIm-Score and RMH score in NSCLC patients treated with post-marketing and practical ICI monotherapy. As a result, this study showed that pretreatment GRIm-Score and RMH score were independent prognostic factors of OS and PFS of ICI monotherapy for pretreated NSCLC patients, respectively. Thus, these two scores are not only selection biomarkers for patients enrolled in early phase I trials, but also useful prognostic biomarkers for NSCLC patients practically treated with ICI therapy.
The most interesting finding was difference in usefulness of these two scores between OS and PFS. GRIm-Score was an independent prognostic marker for OS, but not for PFS. In contrast, RMH score was an independent predictive marker for PFS, but not for OS. The difference between these two scores is only NLR and number of metastatic sites. Comparisons of survival times and subsequent multivariate analyses indicated that NLR was associated with OS, but not with PFS, while number of metastases showed the opposite response to NLR. During the development process of GRIm-Score, neither response to ICI therapy nor PFS was evaluated. The aim of these two scores was to identify patients who was likely to die early in phase I trials. Thus, these two scores were originally developed based on OS. As a prognostic biomarker of OS and PFS, many studies demonstrated baseline NLR, but some studies failed. A Japanese study of 101 patients treated with nivolumab [10] and a Korean study of 54 patients treated with anti-PD-1 antibody [14] showed that pretreatment high NLR (≥ 3 and ≥ 5) was not associated with PFS, but post-treatment high NLR (≥ 3) at 4 weeks or (≥ 5) at 6 week was associated with inferior PFS. Thus, as a prognostic marker of PFS, baseline NLR may be still controversial. On the other hand, a little has been reported on number of metastatic sites as a prognostic factor. A univariate analysis of 201 Japanese patients treated with nivolumab found a significant association between the number of metastatic organ sites and shorter PFS [15]. Multivariate analyses of 175 Spanish patients treated with nivolumab indicated that more than one metastatic location was independently associated with shorter PFS and OS [16]. In a multivariate analysis of European patients treated with various immunotherapies, involvement of two or more metastatic sites was selected as an independent prognostic factor of OS [13]. Organ-specific response to ICI was recently suggested [17, 18]. It is not clear which is more important as a prognostic marker of ICI treatment, number or location of metastatic sites.
The unexpected finding was that Kaplan-Meier curves of OS and PFS did not differ by baseline LDH value. Owing to small number of events, we could not choose LDH as an explanatory variable in multivariate analyses. Some studies of ICI for NSCLC have selected LDH as a prognostic factor of OS [13, 19] and PFS [7, 8, 19, 20]. A prognostic factor of LDH has been investigated mainly in melanoma and NSCLC. In melanoma patients treated with ICI, association of normal baseline LDH with improved response and OS has been shown [21]. We have to evaluate LDH again when we collect more cases in the future.
There were some limitations in this study. First, this was a retrospective, single-institutional study with a small sample size. Our sample size made it impossible to individually evaluate albumin, LDH, number of metastases, and NLR in the multivariate analyses. Second, the first ICI in the second or further line will be an old-fashioned regimen in the near future. Currently, the new regimens of combination of ICI with cytotoxic chemotherapy or another ICI is becoming a standard first-line regimen for advanced and metastatic NSCLC. Thus, our forthcoming challenge may be whether these biomarkers are useful in these new regimens in the first-line setting.
Conclusions
Baseline GRIm-Score and RMH score were independent prognostic factors of OS and PFS of ICI monotherapy for pretreated NSCLC patients, respectively. These two scoring systems are not only selection biomarkers for patients in experimental trials, but also useful prognostic biomarkers for NSCLC patients practically treated with ICI therapy.
Acknowledgments
We are grateful to Tsunehiro Tanaka, Kanako Nishimatsu, Saori Ikebe, Hideyasu Okada and Kazuki Hashimoto at the Department of Respiratory Medicine, Osaka Police Hospital for their medical records, diagnosis, treatment and care of their patients.
Financial Disclosure or Funding
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interests.
Informed Consent
The Osaka Police Hospital Ethics Committee approved the study protocol and waiver of the written informed consents from each patient, considering the retrospective design and anonymous clinical data.
Author Contributions
SM designed, performed the statistical analysis of the data, and drafted the manuscript. All authors were involved in the conceptual design, review of the draft, and approved the final manuscript. KK supervised all aspects of the study.
References
- 1.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. Br J Cancer. 2008;98(6):1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bigot F, Castanon E, Baldini C, Hollebecque A, Carmona A, Postel-Vinay S, Angevin E. et al. Prospective validation of a prognostic score for patients in immunotherapy phase I trials: the Gustave Roussy Immune Score (GRIm-Score) Eur J Cancer. 2017;84:212–218. doi: 10.1016/j.ejca.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Arkenau HT, Barriuso J, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27(16):2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 4.Wheler J, Tsimberidou AM, Hong D, Naing A, Falchook G, Piha-Paul S, Fu S. et al. Survival of 1,181 patients in a phase I clinic: the MD Anderson Clinical Center for targeted therapy experience. Clin Cancer Res. 2012;18(10):2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Livingston JA, Hess KR, Naing A, Hong DS, Patel S, Benjamin RS, Ludwig JA. et al. Validation of prognostic scoring and assessment of clinical benefit for patients with bone sarcomas enrolled in phase I clinical trials. Oncotarget. 2016;7(39):64421–64430. doi: 10.18632/oncotarget.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garrido-Laguna I, Janku F, Vaklavas C, Falchook GS, Fu S, Hong DS, Naing A. et al. Validation of the Royal Marsden Hospital prognostic score in patients treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer. 2012;118(5):1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 7.Taniguchi Y, Tamiya A, Isa SI, Nakahama K, Okishio K, Shiroyama T, Suzuki H. et al. Predictive factors for poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. Anticancer Res. 2017;37(10):5857–5862. doi: 10.21873/anticanres.12030. [DOI] [PubMed] [Google Scholar]
- 8.Oya Y, Yoshida T, Kuroda H, Mikubo M, Kondo C, Shimizu J, Horio Y. et al. Predictive clinical parameters for the response of nivolumab in pretreated advanced non-small-cell lung cancer. Oncotarget. 2017;8(61):103117–103128. doi: 10.18632/oncotarget.21602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Svaton M, Zemanova M, Skrickova J, Jakubikova L, Kolek V, Kultan J, Koubkova L. et al. Chronic inflammation as a potential predictive factor of nivolumab therapy in non-small cell lung cancer. Anticancer Res. 2018;38(12):6771–6782. doi: 10.21873/anticanres.13048. [DOI] [PubMed] [Google Scholar]
- 10.Nakaya A, Kurata T, Yoshioka H, Takeyasu Y, Niki M, Kibata K, Satsutani N. et al. Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634–640. doi: 10.1007/s10147-018-1250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, Templeton AJ. et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–181. doi: 10.1016/j.lungcan.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 12.Bagley SJ, Kothari S, Aggarwal C, Bauml JM, Alley EW, Evans TL, Kosteva JA. et al. Pretreatment neutrophil-to-lymphocyte ratio as a marker of outcomes in nivolumab-treated patients with advanced non-small-cell lung cancer. Lung Cancer. 2017;106:1–7. doi: 10.1016/j.lungcan.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 13.Mezquita L, Auclin E, Ferrara R, Charrier M, Remon J, Planchard D, Ponce S. et al. Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non-small cell lung cancer. JAMA Oncol. 2018;4(3):351–357. doi: 10.1001/jamaoncol.2017.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suh KJ, Kim SH, Kim YJ, Kim M, Keam B, Kim TM, Kim DW. et al. Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459–470. doi: 10.1007/s00262-017-2092-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tamiya M, Tamiya A, Inoue T, Kimura M, Kunimasa K, Nakahama K, Taniguchi Y. et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: A retrospective multicenter trial. PLoS One. 2018;13(2):e0192227. doi: 10.1371/journal.pone.0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garde-Noguera J, Martin-Martorell P, De Julian M, Perez-Altozano J, Salvador-Coloma C, Garcia-Sanchez J, Insa-Molla A. et al. Predictive and prognostic clinical and pathological factors of nivolumab efficacy in non-small-cell lung cancer patients. Clin Transl Oncol. 2018;20(8):1072–1079. doi: 10.1007/s12094-017-1829-5. [DOI] [PubMed] [Google Scholar]
- 17.Shiroyama T, Suzuki H, Tamiya M, Tamiya A, Tanaka A, Okamoto N, Nakahama K. et al. Clinical characteristics of liver metastasis in nivolumab-treated patients with non-small cell lung cancer. Anticancer Res. 2018;38(8):4723–4729. doi: 10.21873/anticanres.12779. [DOI] [PubMed] [Google Scholar]
- 18.Schmid S, Diem S, Li Q, Krapf M, Flatz L, Leschka S, Desbiolles L. et al. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC) Cancer Immunol Immunother. 2018;67(12):1825–1832. doi: 10.1007/s00262-018-2239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capone M, Giannarelli D, Mallardo D, Madonna G, Festino L, Grimaldi AM, Vanella V. et al. Baseline neutrophil-to-lymphocyte ratio (NLR) and derived NLR could predict overall survival in patients with advanced melanoma treated with nivolumab. J Immunother Cancer. 2018;6(1):74. doi: 10.1186/s40425-018-0383-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kataoka Y, Hirano K, Narabayashi T, Hara S, Fujimoto D, Tanaka T, Ebi N. et al. Carcinoembryonic antigen as a predictive biomarker of response to nivolumab in non-small cell lung cancer. Anticancer Res. 2018;38(1):559–563. doi: 10.21873/anticanres.12259. [DOI] [PubMed] [Google Scholar]
- 21.Hopkins AM, Rowland A, Kichenadasse G, Wiese MD, Gurney H, McKinnon RA, Karapetis CS. et al. Predicting response and toxicity to immune checkpoint inhibitors using routinely available blood and clinical markers. Br J Cancer. 2017;117(7):913–920. doi: 10.1038/bjc.2017.274. [DOI] [PMC free article] [PubMed] [Google Scholar]