ABSTRACT.
Allostatic load is defined as the frequent activation of the neuroendocrine, immunological, metabolic and cardiovascular systems, which makes individuals more susceptible to stress-related health problems. According to this model, physiological dysregulations start to emerge decades before diseases manifest. Consequently, stress research has shifted its attention to anticipating the degree of this dysregulation to better understand the impact of stress hormones and other biomarkers on disease progression. In view of the growing number of studies that demonstrate the influence of modifiable risk factors on cognitive decline, in addition to the effects of chronic stress mediators, the objective of the present review was to present an overview of the development of cognitive changes based on studies on stress and its mediators.
Key words: stress, Alzheimer’s disease, allostatic load, cognitive decline, memory
RESUMO.
A carga alostática é definida como a ativação frequente dos sistemas neuroendócrino, imunológico, metabólico e cardiovascular, o que torna os indivíduos mais suscetíveis a problemas de saúde relacionados ao estresse. Segundo este modelo, as desregulações fisiológicas começam a surgir décadas antes das doenças se manifestarem. Consequentemente, a pesquisa de estresse tem desviado sua atenção para antecipar o grau de desregulação para entender melhor o impacto dos hormônios do estresse e outros biomarcadores na progressão da doença. Tendo em vista o crescente número de estudos que demonstram a influência de fatores de risco modificáveis no declínio cognitivo, além dos efeitos dos mediadores crônicos do estresse, o objetivo da presente revisão foi apresentar uma visão geral do desenvolvimento de alterações cognitivas a partir de estudos sobre o estresse e seus mediadores.
Palavras-chave: estresse, doença de Alzheimer, carga alostática, declínio cognitivo, memória
Although various factors are associated with cognitive decline during aging, it is unclear which factors trigger the neurodegenerative process in cases of dementia. Chronic exposure to stress throughout the lifespan has been the focus of many studies because of the similarities between the biological mechanisms involved in chronic stress and the pathophysiology of Alzheimer’s disease (AD), one of the leading causes of dementia worldwide. There is consistent evidence showing that cortisol, the main stress mediator, is associated with both cognitive decline and AD. However, it remains unclear whether the altered cortisol is the cause or consequence of neurodegeneration. Beta-amyloid (Aβ) protein deposition, neuroinflammation, hyperphosphorylation of tau protein, changes in glucose metabolism and insulin signaling are some of the effects of primary mediators of stress that cumulatively can cause decreases in the synaptic network and neuroplasticity, hippocampal and cortical atrophy, and consequently, cognitive decline. This “wear and tear” of chronic stress known as allostatic load is related to the prolonged and sustained exposure to the primary stress mediators that progressively damage brain structures associated with cognitive functions, increasing the risk of disease. This review will explain the similarities between the mechanisms that trigger AD and the effects of stress mediators in the brain to support the hypothesis that, through the allostatic load process, chronic stress during lifespan may seed the vulnerability for developing cognitive impairment later in life.
ALZHEIMER’S DISEASE: NEUROPATHOLOGY AND ASSOCIATED FACTORS
Alzheimer’s disease is the most common form of dementia and is responsible for approximately 60% to 80% of the cases of the disease worldwide.1 The syndrome, which affects approximately 35 million people worldwide, is characterized by gradual and progressive cognitive impairment, including memory decline, impaired reasoning and judgment and slow processing speed allied with executive dysfunction, behavioral and functional decline.2 , 3 AD leads to the death of nerve cells, gradually causing atrophy in different brain regions which, over time, affects almost all of its functions. The exact neuropathological mechanism by which this neurodegenerative process unfolds is still being studied. It has been established that cognitive and behavioral AD symptoms are correlated with the accumulation of amyloid plaques in the extracellular environment and with intracellular neurofibrillary tangles, which destroy synapses essential to learning, memory, planning and decision making.4 - 6 Amyloid plaque formation is due to the accumulation of Aβ peptide, which, in turn, is caused by a change in the amyloid precursor protein (APP) cleavage process.7 APP is a transmembrane protein which normally undergoes either neuroprotective or amyloidogenic cleavage. Through the action of the β-secretase enzyme, APP is transformed into Aβ peptide. In early-onset familial AD, accumulation of Aβ peptide is related to genetic insults in APP and mutations in presenilin genes (PSEN). After age, the presence of the E4 allele of the gene that codes apolipoprotein E (APOE-ε4) represents the largest risk factor for Aβ accumulation in late-onset AD. In addition, epigenetic changes and environmental risks also contribute to the accumulation of toxic species of Aβ in late-onset AD.4
When hyperphosphorylation of tau protein forms neurofibrillary tangles within the cells, cell morphology is altered (microtubule disassembly), disrupting axoplasmic flow and inducing neuronal dysfunction and neurodegeneration.7 , 8 One of the hypotheses for this hyperphosphorylation is that, when combined with neuroinflammation and oxidative stress, Aβ accumulation causes dysregulation in calcium channels and hyperactivation of kinases, leading to tau hyperphosphorylation.4 , 9 , 10
Various studies with animals and humans have shown that neuroinflammation, mediated by microglial activation, plays an important role in the amyloidogenic process (for a review, see Hommet et al., 2014).11 Although microglial activation is beneficial in AD because it facilitates the elimination of Aβ peptide,12 - 15 it may also damage brain tissue due to the release of proinflammatory mediators, such as IL-1β, IL-6, and TNFα.11 Neuroinflammation, in turn, leads to oxidative stress, cell damage and death in structures essential for cognitive abilities and, consequently, to cognitive decline.11 However, the exact factor that contributes to the activation of this neuroinflammation process is still unclear. Although genetic elements such as APOE alleleε4 are significant AD risk factors, other factors represent risk conditions for the disease. Similarities between the effects of chronic stress and the neuropathological mechanisms involved in the development of AD have reinforced the hypothesis that chronic stress is an important risk factor for cognitive decline during the aging process and for the conversion of mild cognitive impairment (MCI) to AD.
The present article reports evidence that supports the hypothesis that chronic stress may be one of these “other factors” involved in the AD neurodegenerative process.
ACUTE AND CHRONIC STRESS: PRIMARY MEDIATORS, SIDE EFFECTS, AND ILLNESS
Since its introduction in medical sciences in the 1930s, the concept of stress has evolved, especially because of advances in the field of neuroscience. Stress is a natural and adaptive reaction to challenging or threatening situations and is, therefore, beneficial and necessary for the body to continue functioning. In the short term, various biological, cognitive and behavioral modifications take place so that individuals can adapt to stressful stimuli (acute stress response).16 However, stress responses maintained for prolonged or repetitive periods (chronic stress response) can affect the operation of the organism’s adaptive biological systems, causing illness.16
Stress response starts with the perception that something threatening or challenging is occurring, i.e., when someone is exposed to a stressful event. Stressors can be classified into two types: real or absolute, i.e., those that are undeniably life-threatening (for example: natural catastrophes, or situations of violence, such as a kidnapping or assault) and relative stressors, where the perception of threat or challenge essentially depends on interpretation (for example: new or unpredictable situations, with little control or social judgment). Unlike real stressors, not everyone responds the same way or with the same intensity to relative stressors. New or unpredictable stressors may represent a challenge or threat to some people, whereas for others, stress occurs in situations over which they have little control or in which they are being judged by others. Whether real or relative, exposure to stressors activates the sympathoadrenal system (SAM), which stimulates catecholamine (adrenaline and noradrenaline) secretion by the adrenal medulla and results in cardiovascular changes (increased heart rate and vasoconstriction), metabolic changes (increased oxygen supply and glucose bioavailability), immunological changes (increased anti-inflammatory factors and coagulation) and cognitive changes (activation of attention and memory for decision-making). The hypothalamic-pituitary-adrenal (HPA) axis is then activated, which stimulates the neurons of the paraventricular nucleus of the hypothalamus to secrete corticotropin-releasing hormone (CRH). CRH acts on the anterior pituitary to release adrenocorticotropic hormones (ACTH), responsible for stimulating the cortex of the adrenal gland to synthesize and release glucocorticoids (corticosterone in animals and cortisol in humans).16 - 18 These glucocorticoids then cross the blood-brain barrier, binding to specific receptors - mineralocorticoids (MR) and glucocorticoids (GR) located in brain structures intrinsically related to memory and attention. The interaction of glucocorticoids with these receptors exerts negative feedback, acting on the hypothalamus, which reduces CRH synthesis and ACTH secretion in the anterior pituitary and regulates plasma cortisol concentration, restoring it to concentrations prior to exposure to the stressor.18 - 20
Activation of the SAM and HPA axes modifies the operational parameters of stress response target systems (cardiovascular, immunological, neuroendocrine and metabolic systems), so that, in the short term, energy is mobilized in the form of glucose to prepare the organism for a fight-or-flight response.21 These adaptive modifications, called the allostatic response or allostase, occur through the action of primary mediators (glucocorticoids, dehydroepiandrosterone [DHEA], catecholamines, glucose, and pro- and anti-inflammatory cytokines [IL-6 and TNF-α]) that act on the mitochondrial DNA of target system cells to enhance the ability of the mitochondria to produce energy and, consequently, boost cellular energetic capacity for the fight-or-flight response.21 , 22 Glucocorticoids also modulate the mitochondrial function, increasing membrane potential, Ca2+ signaling, and resistance to apoptosis.23
Overall, in acute situations, these primary mediators (i.e. cortisol, glucose, IL-6, DHEA and catecholamines) seek to ensure the organism’s best possible adaptation to the demands imposed by stressors. However, in chronic situations, the sustained action of these primary mediators through repeated stress responses can dysregulate the target systems in a process called allostatic load characterized by secondary outcomes.24 , 25 The prolonged action of glucocorticoids and glucose on mitochondrial DNA increases oxidative reactions, causing cellular dysfunction, telomere shortening, epigenetic dysregulation with altered gene expression of mitochondrial DNA, and cellular aging.26 , 27 Over time, these combined changes dysregulate the stress response target systems, whose operational parameters are modified to pathophysiological levels (above or below normal limits), as an adaptive mechanism to offset the cumulative effects of the primary mediators. As a result, the different stress response target systems manifest secondary effects such as increased metabolic mediators (insulin, glucose, total cholesterol, high-density lipoprotein, triglycerides, visceral fat deposition), cardiovascular mediators (blood pressure) and immune mediators (fibrinogen and C-reactive protein), in addition to reduced protective mediators, such as high-density lipoproteins (HDL cholesterol) and DHEA hormones.22 , 24 , 28 These mediators reach subclinical concentrations,24 i.e., levels above or below the expected average, indicating a load on the allostatic system (allostatic load), which is a risk condition for disease. If maintained, these effects can progressively accumulate and overload the allostatic system, triggering tertiary effects that are manifested through the emergence of cardiovascular and immunological diseases or mental and cognitive disorders.22 , 24 , 25 , 28
In summary, the effects of the stress response unfold in a structured process through a sequential chain of biological events, which starts with the adaptation of the systems to the demands of stressors, and, if prolonged, leads to multi-systemic dysregulation and culminates in disease (allostasis, allostatic state, allostatic load and allostatic overload). This process can be positively or negatively modulated by genetic, behavioral and environmental factors, biological reserves, lifestyle, and previous experiences that shape individuals’ coping and adaptation ability, i.e. resilience to stress.29
CHRONIC STRESS AND ITS EFFECTS ON THE CENTRAL NERVOUS SYSTEM
The main consequence of chronic exposure to stress response mediators in the central nervous system is cellular and structural damage, primarily in the hypothalamus, cortex and hippocampus.30 - 32 In these areas, a repeated and sustained increase in the concentration of glucocorticoids triggers neurotoxic effects mediated by neuroinflammation and hyperglycemic states. The accumulation of these effects increases the production of reactive specimens and induces oxidative stress. Consequently, the mitochondrial respiratory chain decreases in activity, which curbs potential action of the membrane, impairs cellular ability to produce energy, and sensitizes neuronal apoptosis.23 , 33 In addition, neuroinflammation observed in the hippocampus of rats after exposure to chronic stress (IL-1 Beta, IL-6, TNF-alpha) also appears to result in mental health disorders and cognitive impairment because of increased proinflammatory cytokines, microglial activation and recruitment of monocytes in the caudal hippocampus, associated with temporary working memory loss and neurogenesis impairment. The rat’s immune response was a result of sympathetic activation during exposure to stressors.34
Although there is no direct evidence, these cellular alterations may explain the association noted in other studies between higher glucocorticoid concentration and decreased neurogenesis, dendritic arborization, and neural cell adhesion molecules (NCAM), in addition to reduced synaptic capacity and atrophy in various brain regions, including the hippocampus and cortex.23 , 24 , 30 , 31 , 35 , 36
Another effect of chronically high concentrations of glucocorticoids is hyperglycemia and decreased insulin signaling in the brain.37 - 39 Prolonged hyperglycemia alters mitochondrial morphology37 , 38 causing effects such as excessive fragmentation, oxidative stress and impaired mitochondrial DNA integrity, 22 , 40 , 41 facilitating neuronal apoptosis.42 Moreover, the accumulation of glucose in the blood activates immune cells, such as macrophages, which stimulate the secretion of proinflammatory cytokines (IL-1 and TNF-α). These cytokines, linked to their receptors, promote the activation of a group of kinases, which act on insulin receptors. In these receptors, densely manifested in the hypothalamus, entorhinal cortex and hippocampus,43 these kinases act on insulin receptor substrates, altering metabolism and insulin signaling in the brain, which promotes hyperinsulinemia and insulin resistance.44 , 45 Decreased insulin in the neurons impairs neuroplasticity, learning, and memory formation.46 - 48
From a functional point of view, one of the main effects of the accumulation of these cellular and structural damage in the hypothalamus, prefrontal cortex, and hippocampus is the progressive dysregulation of the HPA axis. Whether by direct action of the glucocorticoids or through its effect on glucose metabolism and insulin signaling, cell damage caused by these structures alters the binding sites of the MR and GR receptors in the hippocampus and prefrontal cortex, besides affecting inhibition of the HPA axis49 and, consequently, the negative feedback process. This maintains glucocorticoid secretion high, which produces a vicious cycle, further impairing HPA axis regulation. Another major side effect produced by the neurotoxic action of glucocorticoids at high concentrations in the hippocampus is poorer learning and memory performance.50 The saturation of GR receptors caused by hypercortisolism reduces long-term potentiation (LTP) - an important neurobiological substrate - and impairs memory formation. Studies have shown that excess circulating glucocorticoid due to chronic stress leads to increased activation of GR receptors. This inhibits LTP in the hippocampus51 - 53 and reduces the brain-derived neurotrophic factor (BDNF) level, which negatively affects memory performance.54 , 55
Whether these cognitive effects are temporary or permanent depends on the period of exposure to the stressor event. The harmful effects of stress are intensified during windows of vulnerability, when there is sustained action of its mediators during periods of insufficient functional biological reserves, i.e., during periods of brain development (in childhood) or during its degeneration (aging).56 The harmful effects of chronic stress on memory may not be permanent in adulthood, i.e., once the stressor has ceased, cortisol concentrations return to baseline levels and memory performance improves. However, the cumulative damage from exposure to stress response mediators over the lifespan produces structural changes in the brain that increase the risk of developing cognitive disorders during aging, such as AD.
CUMULATIVE EFFECTS OF CHRONIC STRESS AND ALZHEIMER’S DISEASE
Prolonged stress conditions and the absence of adaptive coping strategies cause the accumulation of structural and functional changes in the hippocampus and prefrontal cortex, induced by the sustained action of primary mediators of stress response in the central nervous system. This constitutes a risk condition for developing cognitive impairment and dementia. The hypothesis that chronic stress may be a risk factor for AD is primarily based on the similarities between the effects of its primary mediators and the mechanisms that trigger AD (Table 1). As previously explained, chronic exposure to stress mediators induces neuroinflammation, hyperglycemic states associated with changes in glucose metabolism and insulin signaling, Aβ accumulation, and hyperphosphorylation of tau protein (Table 1). Mediated by oxidative stress, these combined changes affect neuronal functioning, altering synaptic transmission and neuroplasticity, and lead to hippocampal atrophy, causing impaired cognitive performance, especially in learning and memory (Table 1).
Table 1. Effects of the primary mediators of chronic stress and their similarity with the mechanisms that trigger Alzheimer's disease.
| Primary mediators | Mechanisms | Effects | Relationship with |
|---|---|---|---|
| Cortisol and glucose | ↑Cortisol induces hyperglycemia and insulin resistance | • Increased Ab peptide formation • Neuroinflammation, oxidative stress and cellular damage • Hyperphosphorylation of tau protein • Decreased neuroplasticity • Hippocampal atrophy and memory loss |
• In individuals with MCI or AD, there is
a change in the HPA axis and cortisol concentration • DM2 is associated with higher risk of AD |
| DHEA-S | ↓DHEA-S is associated with immunological dysfunction | • Lower brain protection against Ab
toxicity • Lower antioxidant defenses and vascular protection • Increased atherogenesis • Memory decline |
• DHEA-S decreased in AD patients |
| Proinflammatory cytokines | ↑IL-6 and IL-1 | • Change in APP metabolism, facilitating
the amyloidogenic pathway • Increased Ab deposition • Demyelination • Synaptic dysregulation and neurodegeneration |
• IL-6 increased in AD patients |
Ab: beta-amyloid; MCI: mild cognitive impairment; AD: Alzheimer's disease; HPA: hypothalamic-pituitary-adrenal; DM2: type 2 diabetes mellitus; DHEA-S: Dehydroepiandrosterone sulfate; IL-6: interleukin 6; IL-1: interleukin 1; APP: amyloid precursor protein.
Animal studies have shown that high concentrations of glucocorticoids (comparable with those observed in stress situations) are associated with increased Aβ peptide formation due to higher APP and β-secretase enzyme concentrations, increased phosphorylation of tau protein in the hippocampus and prefrontal cortex and, consequently, the formation of neurofibrillary tangles.57 , 58 In these studies, poorer performance in learning and memory tasks59 - 61 was also observed in animals with higher corticosterone concentrations. Peptide accumulation and the hyperphosphorylation of tau protein, induced by prolonged exposure to high corticosterone concentrations, were prevented by administering mifepristone, a glucocorticoid receptor antagonist, which strengthens the causal relationship between glucocorticoids and the neuropathology of AD.62 Treatment with mifepristone prevented APP cleavage by β-secretase, blocked Aβ production and, consequently, reversed cognitive deficits.62
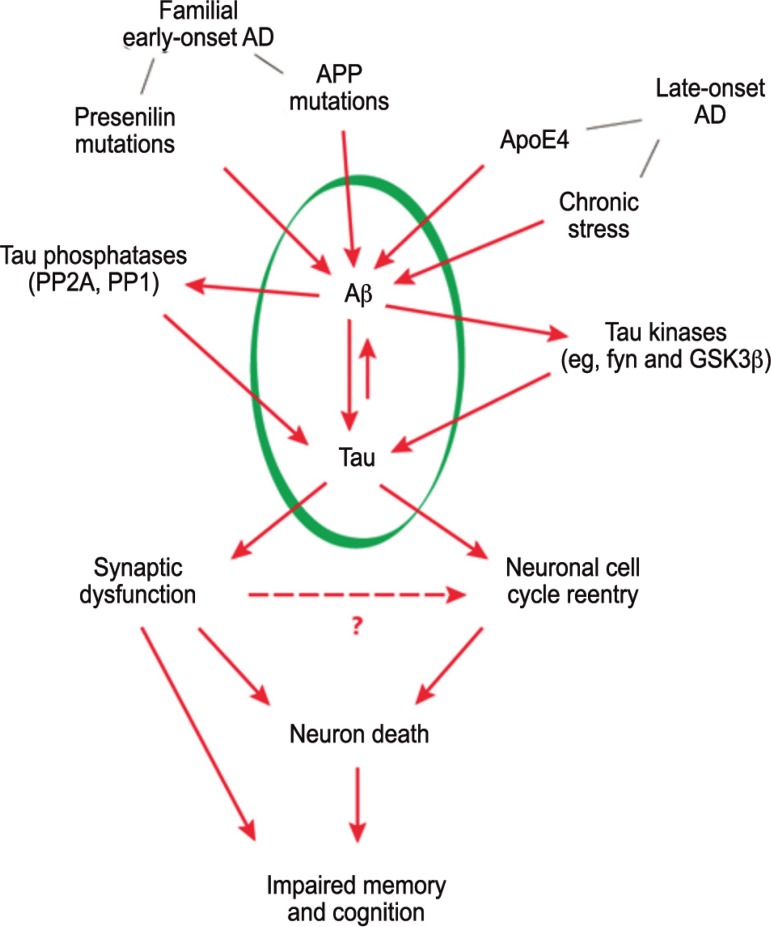
Figure 1. Factors associated with Amyloid-beta peptide accumulation. Chronic stress may constitute another factor that contributes to the accumulation of toxic amyloid beta in late-onset Alzheimer’s disease. Betaamyloid peptide accumulation promotes the formation of pathological tau tangles that lead to synaptic dysfunction and memory-supporting neuron death, impairing cognitive performance.
In humans, considering a continuous health-disease process, changes in cortisol concentration associated with poor cognitive performance have been observed in healthy individuals or with memory complaints in patients with MCI and AD.63 - 78 Older adults without cognitive impairment who had been exposed to intense occupational stress during adulthood had low episodic memory performance.65 , 66 A longitudinal study demonstrated that older adults with high cortisol concentrations over a period of six years had worse declarative memory performance and 14% less hippocampal volume than those with lower cortisol concentrations.64 Individuals with memory complaints - an important prodrome and risk factor for dementia - have higher cortisol concentrations than those not affected by this problem.67 Similarly, workers with high occupational stress levels at 40 years of age have a higher risk of developing MCI and AD 20 years later.68 Various studies have noted higher cortisol concentrations in MCI and AD patients than in cognitively healthy older adults.69 - 76 In older adults with AD77 or MCI,78 the higher the cortisol concentration, the worse the cognitive performance. Furthermore, the conversion of older adults with MCI to AD79 or from mild AD to moderate AD77 is also associated with increased cortisol concentration. As a whole, the relationship between higher cortisol and poorer cognitive performance since adulthood in individuals suffering from memory deficits63 - ranging from individuals with MCI to those who already have AD - strongly suggests that cortisol not only contributes to the evolution of the disease, but is also involved in the pathophysiological mechanisms that cause it.76 , 77 , 79
A large body of evidence has shown that the cumulative effects of chronic stress on AD pathology is based on the concentration of glucocorticoids (corticosterone or cortisol). However, other primary mediators of chronic stress, such as glucose, DHEA-S and proinflammatory cytokines, are also associated with AD (Table 1).
Changes in brain glucose metabolism contribute to cell degeneration and appear to be associated with the onset and progression of AD.42 High glucose concentrations, mediated by hypercortisolism, insulin resistance and altered glucose metabolism in the brain, are associated with Aβ accumulation and increased phosphorylation of tau protein.80 - 85 Various epidemiological studies have demonstrated an independent association between AD and type 2 diabetes mellitus (DM2). A meta-analysis found that DM2 increases the risk of developing AD by 39% regardless of the presence of cardiovascular comorbidities.86 Although there is no conclusive explanation for this association, one hypothesis suggests that it is mediated by moderate hypercortisolism usually present in the early stages of AD.87 The circadian rhythm of cortisol secretion is one of the main determinants of glycemia in humans, and high concentrations of cortisol over a long period of time may be diabetogenic due to adrenal hyperresponsiveness to ACTH.87 Hypercortisolism increases the risk of a pre-diabetic condition and DM2 in older adults with mild AD, decades before dementia manifests.87 See Table 1.
The reduced concentration of DHEA/DHEA-S inherent to aging is associated with dysfunction and activation of the immune system,88 increased oxidative stress89 and atherogenesis.90 DHEA is an androgen produced by the adrenal glands that acts as an antagonist against the negative effects of increased cortisol by suppressing inflammatory cytokines, improving lipid metabolism, decreasing insulin resistance, and reducing brain damage caused by oxidative stress.91 In a cohort study, patients with AD had lower plasma DHEA-S and DHEA levels than control volunteers.91 - 93 There is evidence that DHEA protects hippocampal cells from the toxicity produced by the accumulation of Aβ protein.94 Decreased circulating DHEA may also contribute to the vascular pathology observed in AD, since DHEA has antioxidant properties.91 By contrast, higher endogenous DHEA-S levels were independently associated with better executive function, concentration, and working memory in healthy older adults.123 Therefore, lower DHEA and DHEA-S concentrations undermine their neuroprotective effect, and represent a risk factor for the cumulative effects of chronic stress and progression or development of AD (Table 1).91 , 94
Regarding proinflammatory cytokines, some authors claim that AD is fundamentally an immunologically driven process, since IL-6 and IL-1 are associated with altered APP metabolism.95 One study used the immunohistochemistry technique on brain tissue and showed that in the early stages of AD, amyloid plaques are colocalized with acute-phase proteins and proinflammatory cytokines.96 Interleukin IL-1 and IL-6 have been associated with neuronal damage because of increased Aβ deposition,97 demyelination and neurodegeneration.98 IL-6 levels were high in the AD population compared to healthy older adults and inversely correlated with Mini-Mental State Examination scores.99 Reducing the concentration of proinflammatory cytokines could have beneficial effects on AD symptomatology.100 A meta-analysis demonstrated that, regardless of age, older adults with AD display higher concentrations of IL-699 (Table 1).
The similarities between chronic stress and AD are not limited to the effects of their primary mediators. Secondary effects of chronic stress, represented by allostatic load biomarkers, are also associated with dementia, reinforcing the hypothesis that prolonged stress constitutes a risk factor for AD.
One of the main allostatic load biomarkers that represents the secondary outcomes of the sustained action of primary stress mediators is altered concentration of the HDL (high-density lipoprotein) fraction of cholesterol. Higher HDL concentrations are positively correlated with cognitive function.101 , 102 Some authors have reported that low HDL concentrations are associated with cognitive impairment, regardless of the presence of atherosclerotic disease.103 Consistent with this finding, a study conducted in middle-aged adults (55 to 61 years old) identified an association between low HDL concentrations (<40 mg/dL) and memory decline over the course of five years, even when controlling for variables known to influence cognitive performance.104 However, individuals with higher HDL concentrations had less cognitive impairment and improved memory performance.105 A meta-analysis showed a positive association between HDL and memory performance during aging.102 The association between low HDL concentrations and cognitive impairment is mainly because of its role in regulating the metabolism and deposition of Aβ protein,106 its influence on atherosclerotic disease, and its anti-inflammatory properties.107 - 109 Low apoA-I concentration (the main protein component of plasma HDL) is the main predictor of cognitive decline over the course of two years in MCI patients.110 In AD patients, the lower the concentration of HDL and apoA-I, the greater the severity of the disease111 and the risk of developing it.112 , 113
Another allostatic load biomarker, also present in AD, is a higher body mass index (BMI).114 - 116 Obesity has been associated with elevated cardiovascular and cortisol responses to acute stress.124 A meta-analysis showed an association between obesity and increased risk of AD.114 Approximately 2% of cases of AD worldwide are potentially related to obesity in middle age.114 A 10% reduction in the prevalence of obesity could prevent more than 66,000 cases of AD worldwide.114 Another study noted a U-shaped association, i.e., the two ends of BMI (low or high) were statistically and significantly associated with cognitive performance and AD risk.116 , 124 Some studies have suggested that being overweight or obese in middle age is a risk for later development of cognitive decline and dementia.116 , 125 One biological pathway hypothesized to link obesity to cognitive impairment is through leptin, a hormone mainly produced by adipocytes which suppresses appetite and regulates energy expenditure. In rodents, leptin receptor disruption is associated with impaired long-term potentiation, synaptic plasticity and spatial learning, while higher levels of leptin in the hippocampus result in decreased neurodegeneration.126 , 127 Moreover, higher plasma leptin is strongly associated with low Aβ levels in the mouse brain, supporting a protective role for the hormone in AD onset.128 Similarly, higher levels of leptin in humans are associated with increased hippocampal and whole brain volume and reduced incidence of AD.129 Conversely, decreased leptin levels in AD patients with inappropriately low weight suggests a malfunction at the hypothalamic level.130 With regard to cognition, one study showed leptin to have a modest protective effect against cognitive decline.131 Interestingly, individuals with greater adiposity and/or higher plasma leptin would be more stress-responsive.132
The immunological side effects of chronic stress also share similarities with AD. One meta-analysis found an association between levels of C-reactive protein (CRP) - a marker of systemic inflammation (acute phase) - and heightened risk of developing AD.117 Another study noted higher concentrations of high-sensitivity CRP in AD patients than in healthy individuals.99 Similarly, high CRP levels are associated with low memory, visuospatial impairment and low global cognitive performance.133 , 134
Finally, the allostatic load index - the estimated risk of illness due to chronic stress, which includes concentrations of primary mediators and secondary effects of stress - is related to poorer cognitive performance.118 - 120 Individuals with higher allostatic load index scores showed greater cognitive decline and mortality risk than those with lower scores.121 , 122 Furthermore, high allostatic load was associated with low total brain volume and white-matter volume and low general cognitive ability, processing speed, and knowledge in older adults.120
Taken together, these studies clearly demonstrate that both cognitive decline and AD are intrinsically linked to primary mediators of stress and its secondary effects manifested by allostatic load biomarkers. Given that chronic stress may be manageable, allostatic load signs allied with subjective cognitive decline may help implement preventive strategies early during adulthood to reduce the prevalence of dementia later in life. As a multisystemic effect, allostatic load may be controlled through diverse pathways including diet interventions whereby the consumption of nutrients exerting antioxidant effects may reduce the oxidative stress produced by sustained action of stress mediators.135 - 137
CONCLUSION
Different elements produce a nonlinear and multisystemic association between the effects of the primary and secondary mediators of chronic stress and the mechanisms that trigger AD. Although acute stress is a natural and necessary response to maintain the human organism, chronic exposure to its biological mediators can cumulatively impair brain structures essential to cognitive functioning, thus representing a risk factor for cerebral aging and vulnerability to cognitive decline. The early identification in adulthood of individuals who report constant psychological stress and have altered allostatic load mediators constitutes a promising target of interventions to reduce the prevalence of dementia and contribute to successful cerebral aging. In this context, diet and nutritional status interventions can play an important preventive role.
Acknowledgements.
The current study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Footnotes
This study was conducted at the School of Nursing, Department of Medical-Surgical Nursing, University of São Paulo, São Paulo, SP, Brazil.
REFERENCES
- 1.Alzheimer's Association 2013 Alzheimer's disease facts and figures. Alzheimer's & dementia. 2013;9(2):208–245. doi: 10.1016/j.jalz.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010;9(11):1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 3.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia:a systematic review and metaanalysis. Alzheimers Dement. 2013;9(1):63–75. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Bloom GS. Amyloid-β and tau:the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. J Neurosci. 1996;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, et al. The Alzheimer's Disease Neuroimaging Initiative. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132(4):1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe DJ. The molecular pathology of Alzheimer's disease. Neuron. 1991;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 8.Busciglio J, Lorenzo A, Yeh J, Yankner BA. β-Amyloid fibrils induce tau phosphorylation and loss of microtubule binding. Neuron. 1995;14(4):879–888. doi: 10.1016/0896-6273(95)90232-5. [DOI] [PubMed] [Google Scholar]
- 9.Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8(1):57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Liu L, Barger SW, Griffin WST. Interleukin-1 mediates pathological effects of microglia on tau phosphorylation and on synaptophysin synthesis in cortical neurons through a p38-MAPK pathway. J Neurosci. 2003;23(5):1605–1611. doi: 10.1523/JNEUROSCI.23-05-01605.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hommet C, Mondon K, Camus V, Ribeiro MJ, Beaufils E, Arlicot N, et al. Neuroinflammation and β amyloid deposition in Alzheimer's disease:in vivo quantification with molecular imaging. Dement Geriatr Cogn Disord. 2014;37(1-2):1–18. doi: 10.1159/000354363. [DOI] [PubMed] [Google Scholar]
- 12.Meda L, Baron P, Scarlato G. Glial activation in Alzheimer's disease: the role of Abeta and its associated proteins. Neurobiol Aging. 2001;22(6):885–893. doi: 10.1016/s0197-4580(01)00307-4. [DOI] [PubMed] [Google Scholar]
- 13.Morgan D, Gordon MN, Tan J, Wilcock D, Rojiani AM. Dynamic complexity of the microglial activation response in transgenic models of amyloid deposition: implications for Alzheimer therapeutics. J Neuropathol Exp Neurol. 2005;64(9):743–753. doi: 10.1097/01.jnen.0000178444.33972.e0. [DOI] [PubMed] [Google Scholar]
- 14.Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int J Biochem Cell Biol. 2005;37(2):289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 15.Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28(33):8354–8360. doi: 10.1523/JNEUROSCI.0616-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities:building a new framework for health promotion and disease prevention. JAMA. 2009;301(21):2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- 17.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21(1):55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 18.Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition:Implications for the Field of brain and cognition. Brain and Cognition. 2007;65(3):209–237. doi: 10.1016/j.bandc.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Joels M, de Kloet ER. Effects of glucocorticoids and norepinephrine on the excitability in the hippocampus. Science. 1989;245(4925):1502–1505. doi: 10.1126/science.2781292. [DOI] [PubMed] [Google Scholar]
- 20.de Kloet ER, Oitzl MS, Joels M. Stress and cognition:are corticosteroids good or bad guys? Trends in Neurosci. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 21.Sterling P, Eyer J. Allostasis a new paradigma to explain arousal pathology. Philadelphia (Pennsyvania): John Wiley & Sons; 1988. Allostasis: Stress, cognition and health; pp. 629–640. [Google Scholar]
- 22.Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the "gluc" back in glucocorticoids. Nat Rev Endocr. 2014;10(5):1–8. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- 23.Du J, Wang Y, Hunter R, Wei Y, Blumenthal R, Falke C, et al. Dynamic regulation of mitochondrial function by glucocorticoids. Proc Natl Acad Sci USA. 2009;106(9):3543–3548. doi: 10.1073/pnas.0812671106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. doi: 10.1016/j.neubiorev.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 25.McEwen BS. Interacting mediators of allostasis and allostatic load: towards an understanding of resilience in aging. Metabolism. 2003;52:10–16. doi: 10.1016/s0026-0495(03)00295-6. [DOI] [PubMed] [Google Scholar]
- 26.Sapolsky RM. The physiological relevance of glucocorticoid endangerment of the hippocampus. Ann N Y Acad Sci. 1994;746(1):294–304. doi: 10.1111/j.1749-6632.1994.tb39247.x. [DOI] [PubMed] [Google Scholar]
- 27.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 28.McEwen BS. Proctetive and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 29.McEwen BS. Stress, adaptation, and disease:Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840(1):33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 30.Madrigal JL, Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Rodrigo J, et al. Glutathione depletion, lipid peroxidation and mitochondrial dysfunction are induced by chronic stress in rat brain. Neuropsychopharmacology. 2001;24(4):420–429. doi: 10.1016/S0893-133X(00)00208-6. [DOI] [PubMed] [Google Scholar]
- 31.Gong Y, Chai Y, Ding JH, Sun XL, Hu G. Chronic mild stress damages mitochondrial ultrastructure and function in mouse brain. Neurosci Lett. 2011;488(1):76–80. doi: 10.1016/j.neulet.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Rezin GT, Cardoso MR, Gonçalves CL, Scaini G, Fraga DB, Riegel RE, et al. Inhibition of mitochondrial respiratory chain in brain of rats subjected to an experimental model of depression. Neurochem Int. 2008;53(6-8):395–400. doi: 10.1016/j.neuint.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 33.Tang VM, Young AH, Tan H, Beasley C, Wang JF. Glucocorticoids increase protein carbonylation and mitochondrial dysfunction. Horm Metab Res. 2013;45(10):709–715. doi: 10.1055/s-0033-1345119. [DOI] [PubMed] [Google Scholar]
- 34.McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci. 2016;36(9):2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nature Rev Neurosci. 2004;5(12):917–917. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 36.Landfield P, Baskin RK, Pitler TA. Brain-aging correlates: retardation by hormonal-pharmacological treatments. Science. 1981;214(4520):581–583. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- 37.Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology:a lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, Marra M, et al. Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabetic Med. 2011;28(11):1388–1394. doi: 10.1111/j.1464-5491.2011.03370.x. [DOI] [PubMed] [Google Scholar]
- 39.Stein LJ, Dorsa DM, Baskin DG, Figlewicz DP, Porte JR, Woods SC. Reduced effect of experimental peripheral hyperinsulinemia to elevate cerebrospinal fluid insulin concentrations of obese Zucker rats. Endocrinology. 1987;121(5):1611–1615. doi: 10.1210/endo-121-5-1611. [DOI] [PubMed] [Google Scholar]
- 40.Medikayala S, Piteo B, Zhao X, Edwards JG. Chronically elevated glucose compromises myocardial mitochondrial DNA integrity by alteration of mitochondrial topoisomerase function. Am J Physiol Cell. 2011;300(2):C338–C348. doi: 10.1152/ajpcell.00248.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki S, Hinokio Y, Komatu K, Ohtomo M, Onoda M, Hirai S, et al. Oxidative damage to mitochondrial DNA and its relationship to diabetic complications. Diabetes Res Clin Pract. 1999;45(2-3):161–168. doi: 10.1016/s0168-8227(99)00046-7. [DOI] [PubMed] [Google Scholar]
- 42.Lee YJ, Jeong SY, Karbowski M, Smith CL, Youle RJ. Roles of the mammalian mitochondrial fission and fusion mediators Fis1, Drp1, and Opa1 in apoptosis. Mol Biol Cell. 2004;15(11):5001–5011. doi: 10.1091/mbc.E04-04-0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marks JL, King MG, Baskin DG. Localization of insulin and type 1 IGF receptors in rat brain by in vitro autoradiography and in situ hybridization. Adv Exp Med Biol. 1991;293:459–470. doi: 10.1007/978-1-4684-5949-4_41. [DOI] [PubMed] [Google Scholar]
- 44.Rehman K, Akash MS. Mechanisms of inflammatory responses and development of insulin resistance:how are they interlinked? J Biomed Sci. 2016;23(1):87–87. doi: 10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roman-Pintos LM, Villegas-Rivera G, Rodriguez-Carrizalez AD, Miranda-Diaz AG, Cardona-Muñoz EG. Diabetic Polyneuropathy in Type 2 Diabetes Mellitus: Inflammation, Oxidative Stress, and Mitochondrial Function. J Diabetes Res. 2016 doi: 10.1155/2016/3425617. Article ID 3425617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cardona-Gómez GP, Mendez P, DonCarlos LL, Azcoitia I, Garcia-Segura LM. Interactions of estrogens and insulin-like growth factor-I in the brain:implications for neuroprotection. Brain Res Rev. 2001;37(1-3):320–334. doi: 10.1016/s0165-0173(01)00137-0. [DOI] [PubMed] [Google Scholar]
- 47.Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275(5300):661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 48.Martin ED, Sanchez-Perez A, Trejo JL, Martin-Aldana JA, Cano Jaimez M, Pons S, et al. IRS-2 Deficiency impairs NMDA receptor-dependent long-term potentiation. Cereb Cortex. 2012;22(8):1717–1727. doi: 10.1093/cercor/bhr216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sapolsky R, Krey L, McEwen BS. The adrenocortical stress response in the aged male rat:impairment of recovery from stress. Exp Gerontol. 1983;18(1):55–64. doi: 10.1016/0531-5565(83)90051-7. [DOI] [PubMed] [Google Scholar]
- 50.Lupien SJ, de Leon M, De Santi S, Convit A, Tarshish C, Nair NPV, et al. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neurosci. 1998;1(1):69–69. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 51.Artola A, Von Frijtag JC, Fermont PC, Gispen WH, Schrama LH, Kamal A, et al. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. Eur J Neurosci. 2006;23(1):261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- 52.Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nature Rev Neurosci. 2002;3(6):453–453. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- 53.Kim JJ, Song EY, Kim JJ, Song EY, Kosten TA. Stress effects in the hippocampus:synaptic plasticity and memory. Stress. 2006;9(1):1–11. doi: 10.1080/10253890600678004. [DOI] [PubMed] [Google Scholar]
- 54.Park HJ, Lee S, Jung JW, Kim BC, Ryu JH, Kim DH. Glucocorticoid-and long-term stress-induced aberrant synaptic plasticity are mediated by activation of the glucocorticoid receptor. Arch Pharm Res. 2015;38(6):1204–1212. doi: 10.1007/s12272-015-0548-0. [DOI] [PubMed] [Google Scholar]
- 55.Wosiski-Kuhn M, Erion JR, Gomez-Sanchez EP, Gomez-Sanchez CE, Stranahan AM. Glucocorticoid receptor activation impairs hippocampal plasticity by suppressing BDNF expression in obese mice. Psychoneuroendocrinology. 2014;42:165–177. doi: 10.1016/j.psyneuen.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Rev Neurosci. 2009;10(6):434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 57.Green KN, Billings LM, Roozendaal B, McGaugh J, LaFerla FM. Glucocorticoids increase amyloid-β and tau pathology in a mouse model of Alzheimer´s disease. Neurobiol Dis. 2006;26(35):9047–9056. doi: 10.1523/JNEUROSCI.2797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulstad JJ, McMillan PJ, Leverenz JB, Cook DG, Green PS, Peskind ER, et al. Effects of chronic glucocorticoidadministration on insulin-degrading enzyme and amyloid-β peptide in the aged macaque. J. Neuropathol. Exp Neurol. 2005;64(2):139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- 59.Sotiropoulos I, Catania C, Pinto LG, Silva R, Pollerberg GE, Takashima A, et al. Stress acts cumulatively to precipitate Alzheimer's disease-like tau pathology and cognitive deficits. J Neurosci. 2011;31(21):7840–7847. doi: 10.1523/JNEUROSCI.0730-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, et al. The amyloidogenic potential and behavioral correlates of stress. Mol psych. 2009;14(1):95–95. doi: 10.1038/sj.mp.4002101. [DOI] [PubMed] [Google Scholar]
- 61.Dong H, Yuede CM, Yoo HS, Martin MV, Deal C, Mace AG, et al. Corticosterone and related receptor expression are associated with increased β-amyloid plaques in isolated Tg2576 mice. Neuroscience. 2008;155(1):154–163. doi: 10.1016/j.neuroscience.2008.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol psych. 2013;74(5):357–366. doi: 10.1016/j.biopsych.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peavy GM, Santiago DP, Edland SD. Subjective memory complaints are associated with diurnal measures of salivary cortisol in cognitively intact older adults. Am J Geriatr Psychiatry. 2013;21(9):925–928. doi: 10.1016/j.jagp.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. Pt. 1J Neurosci. 1994;14(5):2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Andel R, Crowe M, Kåreholt I, Wastesson J, Parker MG. Indicators of job strain at midlife and cognitive functioning in advanced old age. J Gerontol B Psychol Sci Soc Sci. 2011;66(3):287–291. doi: 10.1093/geronb/gbq105. [DOI] [PubMed] [Google Scholar]
- 66.Andel R, Infurna FJ, Hahn Rickenbach EA, Crowe M, Marchiondo L, Fisher GG. Job strain and trajectories of change in episodic memory before and after retirement. J Epidemiol Community Health. 2015;69(5):442–446. doi: 10.1136/jech-2014-204754. [DOI] [PubMed] [Google Scholar]
- 67.Fiocco AJ, Wan N, Weekes N, Pim H, Lupien SJ. Diurnal cycle of salivary cortisol in older adult men and women with subjective complaints of memory deficits and or depressive symptoms:Relation to cognitive functioning. Stress. 2006;9(3):143–152. doi: 10.1080/10253890600965674. [DOI] [PubMed] [Google Scholar]
- 68.Sindi S, Hagman G, Håkansson K, Kulmala J, Nilsen C, Kåreholt I, Soininen H, Solomon A, Kivipelto M. Midlife Work-Related Stress Increases Dementia Risk in Later Life:The CAIDE 30-Year Study. J Gerontol B Psychol Sci Soc Sci. 2016;72(6):1044–1053. doi: 10.1093/geronb/gbw043. [DOI] [PubMed] [Google Scholar]
- 69.Davis KL, Davis BM, Greenwald BS, Mohs RC, Mathe AA, Johns CA, et al. Cortisol and Alzheimer's disease, I:Basal studies. Am J Psychiatry. 1986;143:300–305. doi: 10.1176/ajp.143.3.300. [DOI] [PubMed] [Google Scholar]
- 70.Maeda K, Tanimoto K, Terada T, Shintani T, Kakigi T. Elevated urinary free cortisol in patients with dementia. Neurobiol Aging. 1991;12(2):161–163. doi: 10.1016/0197-4580(91)90055-o. [DOI] [PubMed] [Google Scholar]
- 71.O'Brien JT, Ames D, Schweitzer I, Colman P, Desmond P, Tress B. Clinical and magnetic resonance imaging correlates of hypothalamic-pituitary-adrenal axis function in depression and Alzheimer's disease. Br J Psychiatry. 1996;168(6):679–687. doi: 10.1192/bjp.168.6.679. [DOI] [PubMed] [Google Scholar]
- 72.Hartmann A, Veldhuis JD, Deuschle M, Standhardt H, Heuser I. Twenty-four-hour cortisol release profiles in patients with Alzheimer's and Parkinson's disease compared to normal controls:ultradian secretory pulsatility and diurnal variation. Neurobiol Aging. 1997;18(3):285–289. doi: 10.1016/s0197-4580(97)80309-0. [DOI] [PubMed] [Google Scholar]
- 73.Swanwick GR, Kirby M, Bruce I, Buggy F, Coen RF, Coakley D, et al. Hypothalamic-pituitary-adrenal axis dysfunction in Alzheimer's disease:lack of association between longitudinal and cross-sectional findings. Am J Psychiatry. 1988;155(2):286–289. doi: 10.1176/ajp.155.2.286. [DOI] [PubMed] [Google Scholar]
- 74.Umegaki H, Ikari H, Nakahata H, Endo H, Suzuki Y, Ogawa O, et al. Plasma cortisol levels in elderly female subjects with Alzheimer's disease:a crosssectional and longitudinal study. Brain Res. 2000;881(2):241–243. doi: 10.1016/s0006-8993(00)02847-x. [DOI] [PubMed] [Google Scholar]
- 75.Lind K, Edman A, Nordlund A, Olsson T, Wallin A. Increased saliva cortisol awakening response in patients with mild cognitive impairment. Dement Geriatr Cogn Dis. 2007;24(5):389–395. doi: 10.1159/000109938. [DOI] [PubMed] [Google Scholar]
- 76.Popp J, Schaper K, Kolsch H, Cvetanovska G, Rommel F, Klingmüller D, et al. CSF cortisol in Alzheimer´s disease and mild cognitive impairment. Neurobiol Aging. 2007;21(3):1–3. doi: 10.1016/j.neurobiolaging.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 77.Csernansky JG, Dong H, Fagan AM, Wang L, Chengjie X, Holtzman DM, et al. Plasma Cortisol and Progression of Dementia in Subjects with Alzheimer-Type Dementia. Am J Psychiatry; 2006;163(12):2164–2169. doi: 10.1176/appi.ajp.163.12.2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Souza-Talarico JN, Chaves EC, Lupien SJ, Nitrini R, Caramelli P. Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment:evidence from healthy elderly, mild cognitive impairment and Alzheimer's disease subjects. J Alzheimers Dis. 2010;19(3):839–848. doi: 10.3233/JAD-2010-1282. [DOI] [PubMed] [Google Scholar]
- 79.Popp J, Wolfsgruber S, Heuser I, Peters O, Hüll M, Schröder J, et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer's type. Neurobiol Aging. 2015;36(2):601–607. doi: 10.1016/j.neurobiolaging.2014.10.031. [DOI] [PubMed] [Google Scholar]
- 80.Gasparini L, Netzer WJ, Greengard P, Xu H. Does insulin dysfunction play a role in Alzheimer's disease? Trends in Pharmacol Sci. 2002;23(6):288–283. doi: 10.1016/s0165-6147(02)02037-0. [DOI] [PubMed] [Google Scholar]
- 81.Salkovic-Petrisic M, Hoyer S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology:an experimental approach. J Neural Transm. 2007;72:217–233. doi: 10.1007/978-3-211-73574-9_28. [DOI] [PubMed] [Google Scholar]
- 82.Correia SC, Santos RX, Perry G, Zhu X, Moreira PL, Smith MA. Insulin-resistant brain state:The culprit in sporadic Alzheimer's disease? Aging Res Rev. 2011;10(2):264–273. doi: 10.1016/j.arr.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Correia SC, Santos RX, Carvalho C, Cardoso S, Candeia E, Santos MS, et al. Insulin signaling, glucose metabolism and mitochondria: Majorplayers in Alzheimer's disease and diabetes interrelation. Brain Res. 2012;1441:64–78. doi: 10.1016/j.brainres.2011.12.063. [DOI] [PubMed] [Google Scholar]
- 84.Santos TO, Mazucanti CHY, Xavier GF, Torrão AS. Early and late neurodegeneration and memory disruption after intracerebroventricular streptozotocin. Physiol Behav. 2012;107(3):401–413. doi: 10.1016/j.physbeh.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 85.de la Monte SM, Wands JR. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: relevance to Alzheimer's disease. J Alzheimers Dis. 2005;7(1):45–61. doi: 10.3233/jad-2005-7106. [DOI] [PubMed] [Google Scholar]
- 86.Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes:a systematic review and meta-analysis. PloS one. 2009;4(1):e4144. doi: 10.1371/journal.pone.0004144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Notarianni E. Cortisol. Mediator of association between Alzheimer's disease and diabetes mellitus? Psychoneuroendocrinology. 2017;81:129–137. doi: 10.1016/j.psyneuen.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 88.Ledochowski M, Murr C, Jäger M, Fuchs D. Dehydroepiandrosterone, ageing and immune activation. Exp Gerontol. 2001;36(10):1739–1747. doi: 10.1016/s0531-5565(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 89.Bastianetto S, Ramassamy C, Poirier J, Quirion R. Dehydroepiandrosterone (DHEA) protects hippocampal cells from oxidative stress-induced damage. Mol Brain Res. 1999;66(1):35–41. doi: 10.1016/s0169-328x(99)00002-9. [DOI] [PubMed] [Google Scholar]
- 90.Khalil A, Lehoux JG, Wagner RJ, Lesur O, Cruz S, Dupont É, et al. Dehydroepiandrosterone protects low density lipoproteins against peroxidation by free radicals produced by γ-radiolysis of ethanol-water mixtures. Atherosclerosis. 1998;136(1):99–107. doi: 10.1016/s0021-9150(97)00194-9. [DOI] [PubMed] [Google Scholar]
- 91.Aldred S, Mecocci P. Decreased dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) concentrations in plasma of Alzheimer's disease (AD) patients. Arch Gerontol Geriatr. 2010;51(1):e16–e18. doi: 10.1016/j.archger.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 92.Genedani S, Rasio G, Cortelli P, Antonelli F, Guidolin D, Galantucci M, et al. Studies on homocysteine and dehydroepiandrosterone sulphate plasma levels in Alzheimer's disease patients and in Parkinson's disease patients. Neurotox Res. 2004;6(4):327–332. doi: 10.1007/BF03033443. [DOI] [PubMed] [Google Scholar]
- 93.Cho SH, Jung BH, Lee WY, Chung BC. Rapid column-switching liquid chromatography/mass spectrometric assay for DHEA-sulfate in the plasma of patients with Alzheimer's disease. Biomed Chromatogr. 2006;20(10):1093–1097. doi: 10.1002/bmc.647. [DOI] [PubMed] [Google Scholar]
- 94.Cardounel A, Regelson W, Kalimi M. Dehydroepiandrosterone Protects Hippocampal Neurons Against Neurotoxin-Induced Cell Death: Mechanism of Action 2 (44437) Proc Soc Exp Biol Med. 1999;222(2):145–149. doi: 10.1046/j.1525-1373.1999.d01-124.x. [DOI] [PubMed] [Google Scholar]
- 95.Cojocaru IM, Cojocaru M, Miu GA, Sapira V. Study of interleukin-6 production in Alzheimer's disease. Rom J Intern Med. 2011;49(1):55–58. [PubMed] [Google Scholar]
- 96.Eikelenboom P, Veerhuis R, Scheper W, Rozemuller AJM, Van Gool WA, Hoozemans JJ. The significance of neuroinflammation in understanding Alzheimer's disease. J Neural Transm. 2006;113(11):1685–1685. doi: 10.1007/s00702-006-0575-6. [DOI] [PubMed] [Google Scholar]
- 97.Fogal B, Hewett SJ. Interleukin-1β: a bridge between inflammation and excitotoxicity? J Neuroch. 2008;106(1):1–23. doi: 10.1111/j.1471-4159.2008.05315.x. [DOI] [PubMed] [Google Scholar]
- 98.Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, et al. Inflammation and Alzheimer's disease. Neurobiol Aging. 2000;21(3):383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lai KSP, Liu CS, Rau A, Lanctôt KL, Köhler CA, Pakosh M, et al. Peripheral inflammatory markers in Alzheimer's disease:a systematic review and meta-analysis of 175 studies. J Neurol Neurosurg Psychiatry. 2017;88(10):876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- 100.Grimaldi LME, Zappalà G, Iemolo F, Castellano AE, Ruggieri S, Bruno G, et al. A pilot study on the use of interferon beta-1a in early Alzheimer's disease subjects. J Neuroinfl. 2014;11(1):30–30. doi: 10.1186/1742-2094-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Atzmon G, Gabriely I, Greiner W, Davidson D, Schechter C, Barzilai N. Plasma HDL levels highly correlate with cognitive function in exceptional longevity. J Gerontol Biol Sci Med Sci. 2002;57(11):M712–M715. doi: 10.1093/gerona/57.11.m712. [DOI] [PubMed] [Google Scholar]
- 102.Hottman DA, Chernick D, Cheng S, Wang Z, Li L. HDL and cognition in neurodegenerative disorders. Neurobiol Dis. 2014;72:22–36. doi: 10.1016/j.nbd.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Van Exel E, de Craen AJ, Gussekloo J, Houx P, Bootsma-van der Wiel A, Macfarlane PW, et al. Association between high-density lipoprotein and cognitive impairment in the oldest old. Annals Neurol. 2002;51(6):716–721. doi: 10.1002/ana.10220. [DOI] [PubMed] [Google Scholar]
- 104.Singh-Manoux A, Gimeno D, Kivimaki M, Brunner E, Marmot MG. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife. Arterioscler, thromb vasc biol. 2008;28(8):1556–1562. doi: 10.1161/ATVBAHA.108.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van den Kommer TN, Dik MG, Comijs HC, Jonker C, Deeg DJ. The role of lipoproteins and inflammation in cognitive decline:Do they interact? Neurobiol Aging. 2012;33(1):196–1e1. doi: 10.1016/j.neurobiolaging.2010.05.024. [DOI] [PubMed] [Google Scholar]
- 106.Reiss AB, Siller KA, Rahman MM, Chan ES, Ghiso J, de Leon MJ. Cholesterol in neurologic disorders of the elderly:stroke and Alzheimer's disease. Neurobiol Aging. 2004;25(8):977–989. doi: 10.1016/j.neurobiolaging.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Libby P, Ridker PM. Novel inflammatory markers of coronary risk. Circulation. 1999;100(11):1148–1150. doi: 10.1161/01.cir.100.11.1148. [DOI] [PubMed] [Google Scholar]
- 108.Sacco RL, Benson RT, Kargman DE, Boden-Albala B, Tuck C, Lin IF, et al. High-density lipoprotein cholesterol and ischemic stroke in the elderly: the Northern Manhattan Stroke Study. JAMA, 2001;285(21):2729–2735. doi: 10.1001/jama.285.21.2729. [DOI] [PubMed] [Google Scholar]
- 109.Cockerill GW, Huehns TY, Weerasinghe A, Stocker C, Lerch PG, Miller NE, et al. Elevation of plasma high-density lipoprotein concentration reduces interleukin-1-induced expression of E-selectin in an in vivo model of acute inflammation. Circulation. 2001;103(1):108–112. doi: 10.1161/01.cir.103.1.108. [DOI] [PubMed] [Google Scholar]
- 110.Song F, Poljak A, Crawford J, Kochan NA, Wen W, Cameron B, et al. Plasma apolipoprotein levels are associated with cognitive status and decline in a community cohort of older individuals. PloS One. 2012;7(6):e34078. doi: 10.1371/journal.pone.0034078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Merched A, Xia Y, Visvikis S, Serot JM, Siest G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer's disease? Neurobiol Aging. 2000;21(1):27–30. doi: 10.1016/s0197-4580(99)00103-7. [DOI] [PubMed] [Google Scholar]
- 112.Reitz C, Tang MX, Schupf N, Manly JJ, Mayeux R, Luchsinger JA. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch Neurol. 2010;67(12):1491–1497. doi: 10.1001/archneurol.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonarek M, Barberger-Gateau P, Letenneur L, Deschamps V, Iron A, Dubroca B, et al. Relationships between cholesterol, apolipoprotein E polymorphism and dementia:a cross-sectional analysis from the PAQUID study. Neuroepidemiology. 2000;19(3):141–148. doi: 10.1159/000026249. [DOI] [PubMed] [Google Scholar]
- 114.Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10(9):819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Profenno LA, Porsteinsson AP, Faraone SV. Meta-analysis of Alzheimer's disease risk with obesity, diabetes, and related disorders. Biol Psychiatry. 2010;67(6):505–512. doi: 10.1016/j.biopsych.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 116.Beydoun MA, Beydoun HA, Wang Y. Obesity and central obesity as risk factors for incident dementia and its subtypes:a systematic review and meta-analysis. Obes Rev. 2008;9(3):204–218. doi: 10.1111/j.1467-789X.2008.00473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Koyama A, O'Brien J, Weuve J, Blacker D, Metti AL, Yaffe K. The role of peripheral inflammatory markers in dementia and Alzheimer's disease:a meta-analysis. J Gerontol Biom Sci Med Sci. 2012;68(4):433–440. doi: 10.1093/gerona/gls187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Seeman TE, McEwen BS, Rowe JW, Singer BH. Allostatic load as a marker of cumulative biological risk:MacArthur studies of successful aging. Proc Nat Acad Sci. 2001;98(8):4770–4775. doi: 10.1073/pnas.081072698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Karlamangla AS, Miller-Martinez D, Lachman ME, Tun PA, Koretz BK, Seeman TE. Biological correlates of adult cognition:Midlife in the United States (MIDUS) Neurobiol Aging. 2014;35(2):387–394. doi: 10.1016/j.neurobiolaging.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Booth T, Royle NA, Corley J, Gow AJ, Hernández MDCV, Maniega SM, et al. Association of allostatic load with brain structure and cognitive ability in later life. Neurobiol Aging. 2015;36(3):1390–1399. doi: 10.1016/j.neurobiolaging.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Goldman N, Turra CM, Glei DA, Seplaki CL, Lin YH, Weinstein M. Predicting mortality from clinical and nonclinical biomarkers. J Gerontol Biol Sci Med Sci. 2006;61(10):1070–1074. doi: 10.1093/gerona/61.10.1070. [DOI] [PubMed] [Google Scholar]
- 122.Karlamangla AS, Singer BH, Seeman TE. Reduction in allostatic load in older adults is associated with lower all-cause mortality risk:MacArthur studies of successful aging. Psychosom Med. 2006;68(3):500–507. doi: 10.1097/01.psy.0000221270.93985.82. [DOI] [PubMed] [Google Scholar]
- 123.Davis SR, Shah S, McKenzie D, Kulkarni J, Davison S, Bell R. Dehydroepiandrosterone Sulfate Levels Are Associated with More Favorable Cognitive Function in Women. Clin Endocrinol Metabol. 2008;93(3):801–808. doi: 10.1210/jc.2007-2128. [DOI] [PubMed] [Google Scholar]
- 124.Takeda JRT, Matos TM, Souza-Talarico JN. Cardiovascular risk factors and cognitive performance in aging. Dement Neuropsychol, 2017;11:442–448. doi: 10.1590/1980-57642016dn11-040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hassing LB, Dahl AK, Pedersen NL, Johansson B. Overweight in midlife is related to lower cognitive function 30 years later:A prospective study with longitudinal assessments. Dement Geriatr Cogn Disord. 2010;29:543–552. doi: 10.1159/000314874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Harvey J, Solovyova N, Irving A. Leptin and its role in hippocampal synaptic plasticity. Prog Lipid Res. 2006;45:369–378. doi: 10.1016/j.plipres.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Perez-Gonzalez R, Antequera D, Vargas T, Spuch C, Bolos M, Carro E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of alzheimer's disease. J Alzheimers Dis. 2011;24(Suppl 2):17–25. doi: 10.3233/JAD-2011-102070. [DOI] [PubMed] [Google Scholar]
- 128.Niedowicz DN, Studzinski CM, Weidner AM, Platt TL, Kingry KN, Beckett TL, Bruce-Keller AJ, Keller JN, Murphy MP. Leptin Regulates Amyloid β Production Via the γ-Secretase Complex. Biochim Biophys Acta. 2013;1832(3):439–444. doi: 10.1016/j.bbadis.2012.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lieb W, Beiser AS, Vasan RS, Tan ZS, Au R, Harris TB, et al. Association of plasma leptin levels with incident alzheimer disease and mri measures of brain aging. JAMA. 2009;302:2565–2572. doi: 10.1001/jama.2009.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Power DA, Noel J, Collins R, O'Neill D. Circulating leptin levels and weight loss in alzheimer's disease patients. Dement Geriatr Cogn Disord. 2001;12:167–170. doi: 10.1159/000051252. [DOI] [PubMed] [Google Scholar]
- 131.Holden KF, Lindquist K, Tylavsky FA, Rosano C, Harris TB, Yaffe K. Serum leptin level and cognition in the elderly:Findings from the health abc study. Neurobiol Aging. 2009;30:1483–1489. doi: 10.1016/j.neurobiolaging.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, et al. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34:181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 133.Watanabe Y, Kitamura K, Nakamura K, Sanpei K, Wakasugi M, Yokoseki A, et al. Elevated C-Reactive Protein Is Associated with Cognitive Decline in Outpatients of a General Hospital:The Project in Sado for Total Health (PROST) Dement Geriatr Cogn Dis Extra. 2016;6(1):10–19. doi: 10.1159/000442585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Noble JM, Manly JJ, Schupf N, Tang MX, Mayeux R, Luchsinger JA. Association of C-Reactive Protein With Cognitive Impairment. Arch Neurol. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Otaegui-Arrazola A, Amiano P, Elbusto A, Urdaneta E, Martinez-Lage P. Diet, cognition, and Alzheimer's disease:food for thought. Eur J Nutr. 2014;53:1–23. doi: 10.1007/s00394-013-0561-3. [DOI] [PubMed] [Google Scholar]
- 136.Santos JR, Gois AM, Mendonça DM, Freire MAM. Nutritional status, oxidative stress and dementia:the role of selenium in Alzheimer's disease. Front Aging Neurosci. 2014;6:206–206. doi: 10.3389/fnagi.2014.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Spaccavento S, DelPrete M, Craca A, Fiore P. Influence of nutritional status on cognitive, functional and neuropsychiatric deficits in Alzheimer's disease. Arch Gerontol Geriatr. 2009;48:356–360. doi: 10.1016/j.archger.2008.03.002. [DOI] [PubMed] [Google Scholar]