Abstract
Purpose: To use a modeled analysis to examine the cost-effectiveness of utilizing fractional exhaled nitric oxide (FeNO) as a biomarker to aid in the identification of omalizumab responders in patients with moderate-to-severe allergic asthma. Omalizumab is a biological drug used to treat asthma in adults and children 12 years and older.
Patients and methods: We conducted a decision analysis in which two alternative strategies for predicting omalizumab response were assessed: 1) testing response via a 12-week trial of omalizumab and 2) using FeNO measurement to screen patients for likely omalizumab response prior to initiating a 12-week trial of omalizumab. In the standard of care arm, trial omalizumab responders continue on to receive 12 months of continuous omalizumab therapy. In the FeNO measurement predictor arm, patients with FeNO measurements >19.5 ppb are started on a trial of omalizumab. Trial omalizumab responders in this arm are then also tracked for 12 months of continuous omalizumab therapy.
Results: Per-patient costs during the trial and initial treatment periods total $10,943 for FeNO + omalizumab and $13,703 for omalizumab only. The expected cost per responder during the trial period is $4,326 for FeNO + omalizumab and $7,786 for omalizumab only.
Conclusion: Use of FeNO measurement to identify omalizumab responders decreases the expected per-patient cost by nearly 50% during the trial period and continues to show cost savings through the initial treatment period of 12 months. Our analysis may serve as a model for policy and clinical practice regarding the use of FeNO to determine omalizumab response and has widespread implications for health care payers, who may choose to require FeNO measurement and prespecify a minimum FeNO value to determine patient eligibility for omalizumab trial.
Keywords: FeNO, omalizumab, cost-effectiveness, asthma
Introduction
Approximately 300 million people worldwide are affected by asthma, a condition characterized by chronic inflammation of the airways.1,2 Common symptoms of asthma can include shortness of breath, tightness in the chest, wheezing, and coughing.1 In 2014 according to the Center of Disease Control (CDC)’s National Center for Health Statistics, there were 17.7 million (7.4%) adults and 6.3 million (8.6%) children living with asthma in the United States (NCHS 2014). It is estimated that between 5% and 10% of asthma patients have difficulty treating the disease (persistent symptoms, frequent asthma attacks, or low lung function despite taking asthma medications) and about 20% of these have severe disease requiring high doses of controller medication and often biologic therapies.1–3
Although difficult-to-treat asthma patients make up about 5% to 10% of all asthmatics, they are responsible for almost 50% of the total cost of asthma therapy.4 Furthermore, these patients experience significant burdens such as decreased quality of life, increased health expenditures, decreased productivity, and higher rates of death and complications.4,5 In addition, severity is also associated with increased recurrence of hospitalization, with 80–85% of all deaths occurring in those with severe asthma.2 A prospective cohort study on patients with severe and “difficult-to-treat” asthma demonstrated that these patients have high rates of health care and medication use and few achieved control over a 2-year period with substantial economic consequences. The study concluded that use of improved management strategies, more effective medications, or both in such patients might significantly reduce the clinical and cost burden of asthma.6
Omalizumab is the first biologic drug to be approved for the treatment of asthma. It is a monoclonal antibody directed against serum IgE, thus improving asthma by reducing circulating concentrations of serum IgE and downregulation of the allergic asthma response. Omalizumab has been used to treat adults and children 12 years and older who suffer from moderate-to-severe allergic asthma not well controlled by medium- to high-dose inhaled steroids in addition to other asthma controller medications. It has been shown in clinical trials and in real-world studies to be effective in reducing exacerbations in such populations.7 However, because of its high cost, health care payers tend to restrict access to it.8,9 Numerous cost-effectiveness analyses of omalizumab use in asthma have already been conducted and demonstrate that it can be cost-saving in a number of populations including allergic asthmatic patients, those with high health care utilization (patients who are hospitalized five or more times or 20 days or longer per year), and those with severe, uncontrolled disease, by reducing exacerbations and improving quality of life.8,10–17 However, incremental cost-effectiveness ratios for omalizumab have been shown to be above conventional National Health Service (NHS) thresholds of cost-effectiveness in broader patient populations.16 One report suggests that omalizumab may not be currently cost-effective for most patients with severe asthma but that projected cost-effectiveness ratios could fall within a favorable range if the cost of omalizumab decreases in the future.18 The collective results of these cost-effectiveness analyses suggest that omalizumab is an appropriate treatment for uncontrolled and severe asthma; however, a more cost-effective predictor model of patient treatment response is warranted due to treatment costs.
Biomarkers have been recommended to help identify patients who are candidates for treatment with a biologic and also to monitor treatment response. Fractional exhaled nitric oxide (FeNO) is a validated biomarker of allergic airway inflammation. Airway inflammation is driven by the activation of antigen-specific T-helper cells (Th) type 2 that produce a variety of inflammatory cytokines. Of these inflammatory cytokines, IL- 4 and IL-13 have been shown to induce gene transcription to produce the enzyme inducible nitric oxide synthase (iNOS) in the epithelial cells of the airway which then release nitric oxide (NO) in expired breath.19 Some studies show that FeNO is a predictor of both asthma exacerbation and patient response to omalizumab treatment.20,21 One recent study examining the benefit and persistence of response in long-term omalizumab treatment found that an increase in FeNO values from omalizumab discontinuation (baseline) to week 12 was a predictor of exacerbations.22 Studies have also shown that cost-effectiveness of omalizumab improves when a trial of omalizumab to gauge response is used prior to treatment,8 Many payers require a trial of omalizumab to test for response before approving the prescription.23 The ability of FeNO to identify omalizumab responders aligns with payer interest in cost-effective treatment with omalizumab. We undertook this study to examine the cost-effectiveness of FeNO measurement as an aid in the prediction of response to omalizumab in patients with asthma.
Patients and methods
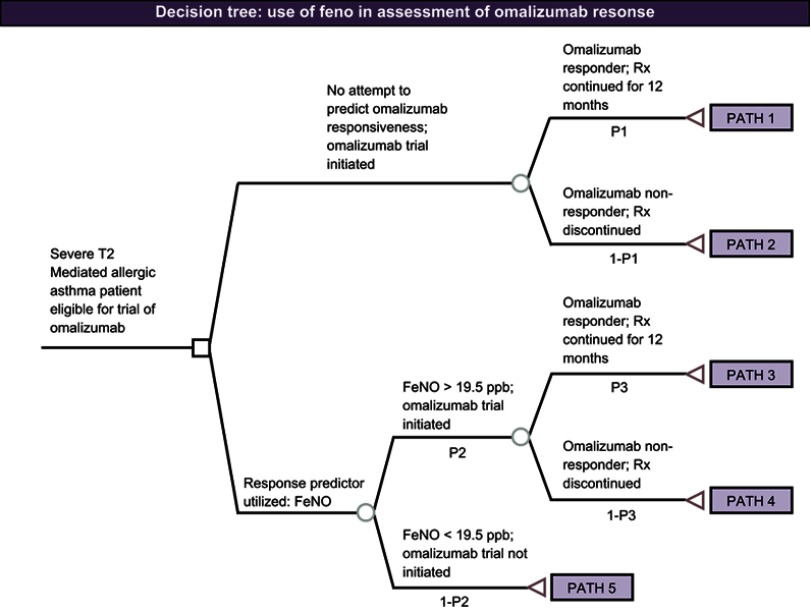
A decision analysis was conducted to examine the cost-effectiveness of utilizing FeNO to identify omalizumab responders. The decision tree model was built and analyzed with TreeAge Pro Healthcare 2017 (Figure 1). Two alternative strategies for predicting omalizumab response were assessed: 1) testing response via a 12-week trial of omalizumab and 2) using FeNO measurement to screen patients for likely omalizumab response prior to initiating a 12-week trial of omalizumab. In the standard of care arm, trial omalizumab responders continue on to receive 12 months of continuous omalizumab therapy. In the FeNO measurement predictor arm, patients with FeNO measurements >19.5 ppb21 are started on a trial of omalizumab. Trial omalizumab responders in this arm are then also tracked for 12 months of continuous omalizumab therapy.
Figure 1.
Decision tree of FeNO use in assessment of omalizumab response.Abbreviation: FeNO, fractional exhaled nitric oxide; Rx, omalizumab therapy.
As hypothetical patients (adults and children 12 years and older) traverse the model, costs associated with the prediction of omalizumab response (eg, FeNO measurement and/or omalizumab trial) and the continued use of omalizumab through an initial 12-month post-trial treatment cycle accrue as applicable. Model transition probabilities and their data sources are displayed in Table 1. Cost variables and their data sources are displayed in Table 2.
Table 1.
Model transition probabilities and sources
| Variable description | Base-case value | Range | Source |
|---|---|---|---|
| Likelihood that omalizumab candidate will be identified as an omalizumab “responder” following a trial of therapy | 0.550 | 0.468–0.633 | Hanania et al 201321 |
| Likelihood that omalizumab candidate will have FeNO >19.5 ppb | 0.510 | 0.434–0.587 | Hanania et al 201321 |
| Likelihood that patient predicted to be an omalizumab responder (FeNO >19.5 ppb) will be confirmed as an omalizumab responder following a trial of therapy | 1.000 | 0.850–1.000 | This value in actual practice is unknown. To be conservative (with respect to the cost findings), we have assumed that all FeNO predicted responders will be identified as omalizumab responders following the 12-week trial of therapy |
Abbreviation: FeNO, fractional exhaled nitric oxide.
Table 2.
Cost and cost-influencing variables
| Variable description | Payer cost | Notes |
|---|---|---|
| Payer cost of FeNO measurement to predict ICS response | $23.20 | Private payer payment estimated as 120% of 2016 Medicare national average reimbursement for CPT code 95012 (FeNO measurement) ($19.33) |
| Payer cost of one 150 mg vial of omalizumab | $713.70 | Justification: Adams et al, 201026 updated to 2016 US$ |
| Average number of omalizumab vials required per patient per month | 2 | Justification: Wu et al 200718 |
| Number of months of omalizumab trial | 3 | Justification: Typical payer policy |
| Number of months of omalizumab prescription following successful omalizumab trial | 12 | Justification: Typical payer policy |
Abbreviations: FeNO, fractional exhaled nitric oxide; ICS, inhaled corticosteroid; CPT, current procedural terminology.
The cost, effectiveness, and cost-effectiveness measurements of interest are: 1) expected per-patient omalizumab costs during the trial period (short-term cost); 2) expected per-patient omalizumab costs during the trial period plus the initial omalizumab treatment period (longer-term cost); 3) expected rate of responders identified; and 4) the expected cost per omalizumab responder identified during the trial period (inclusive of omalizumab trial costs and FeNO measurement costs, as applicable, but not of longer-term omalizumab treatment).
Model assumptions and parameters
Model assumptions were drawn from recent literature pertaining to probabilities, durations, and treatment costs. In order to be conservative with costs, the likelihood that a predicted omalizumab responder (FeNO >19.5 ppb) will be confirmed as a responder following a trial of omalizumab therapy is 1.000. The model assumes that all FeNO predicted responders will be identified as omalizumab responders following the 12-week trial of therapy solely to capture the maximum cost that could be generated in this arm. Movement through the model is governed by the patient’s predicted response or nonresponse to omalizumab as measured by FeNO testing and/or the patient’s response to omalizumab during the trial period. Table 1 shows the estimates and sources used for the analysis as well as the ranges used for the sensitivity analyses.
Cost estimates and cost-influencing variables were taken from the literature and updated to 2016 US dollars using the consumer price index for medical care services. Medicare 2016 payment rates are used for FeNO measurements and omalizumab costs, and vial assumptions are identified from the literature. Payer policies were reviewed to guide omalizumab therapy assumptions. All costs included in the model were estimated from the perspective of a health care payer. Table 2 shows the cost and cost-influencing variable estimates used for the base-case analysis.
In order to demonstrate the robustness of the preliminary results, a series of one-way sensitivity analyses were performed using TreeAge.
Results
Base-case analysis
Individual patient-level outcomes were assessed for FeNO cost-effectiveness as shown in Table 3. For trial costs alone, the expected per-patient cost is $2,207 for FeNO + omalizumab vs $4,282 for omalizumab only. Per-patient costs during the trial and initial treatment periods total $10,943 for FeNO + omalizumab and $13,703 for omalizumab only. The expected cost per responder during the trial period is $4,326 for FeNO + omalizumab and $7,786 for omalizumab only. Use of FeNO measurement to identify omalizumab responders decreases the expected per-patient cost by nearly 50% during the trial period and continues to show cost savings through the initial treatment period of 12 months in our study (Table 3). Formulas for cost and cost-effectiveness calculations in Table 3 are presented in Appendix A.
Table 3.
Cost-effectiveness of FeNO + omalizumab vs omalizumab only
| Strategy to identify responders | Expected per-patient costs (trial period only) | Expected rate of responders identified | Expected cost per responder (trial period only) |
Expected per-patient costs (trial + initial Tx period) |
|---|---|---|---|---|
| FeNO measurement + omalizumab trial | $2,207 | 0.51 | $4,328 | $10,943 |
| Omalizumab trial only | $4,282 | 0.55 | $7,786 | $13,703 |
Abbreviations: FeNO, fractional exhaled nitric oxide; Tx, treatment.
Sensitivity analyses
The results of one-way (only one parameter at a time is allowed to vary) sensitivity and threshold analyses are presented in Table 4; in these analyses, the variables are increased and decreased by 15% to determine the impact these changes may have upon the cost-effectiveness findings. Note that for the range of values investigated, across all parameters allowed, use of FeNO measurement in addition to omalizumab remains the dominant strategy when compared to use of omalizumab alone.
Table 4.
Sensitivity analyses
| Comparing methods for identifying omalizumab responders | Most cost-effective alternative | Threshold |
|---|---|---|
| Likelihood that omalizumab candidate will be identified as an omalizumab responder via a trial of omalizumab therapy: 0.468 | FeNO measurement + omalizumab trial | No threshold |
| Likelihood that omalizumab candidate will be identified as an omalizumab responder via a trial of omalizumab therapy: 0.633 | No threshold | |
| Likelihood that omalizumab candidate will have FeNO >19.5 ppb: 0.434 | FeNO measurement + omalizumab trial | No threshold |
| Likelihood that omalizumab candidate will have FeNO >19.5 ppb: 0.587 | No threshold | |
| Likelihood that patient predicted to be an omalizumab responder (FeNO >19.5 ppb) will be confirmed as an omalizumab responder following a trial of therapy: 0.850 | FeNO measurement + omalizumab trial | No threshold |
| Likelihood that patient predicted to be an omalizumab responder (FeNO >19.5 ppb) will be confirmed as an omalizumab responder following a trial of therapy: 1.000 | No threshold |
Abbreviation: FeNO, fractional exhaled nitric oxide.
Discussion
Omalizumab is an effective biologic for the treatment of patients with moderate-to-severe allergic asthma who are uncontrolled with standard therapy. However, the cost of a trial of omalizumab is significant, as are the costs of omalizumab use, highlighting the need for the ability to predict response prior to initiation. In a previous study, we have shown that FeNO can predict omalizumab response.21 The current model shows that use of FeNO measurement to identify omalizumab responders prior to initiating a trial of omalizumab therapy is a cost-effective predictor of omalizumab response. In addition, the model demonstrates that use of FeNO decreases the expected per-patient cost by nearly 50% during the trial period and continues to be cost-effective through the initial treatment period of 12 months. Sensitivity analyses showed these results to be robust.
Our analysis only accounts for estimated savings through cost-effectively identifying responders; however, additional cost savings can be expected due to improved clinical outcomes with omalizumab treatment. Hospitalization of asthma patients accounts for half of all asthma-related expenditures, and previous studies have shown that 6 months of therapy with omalizumab reduced the number of hospitalizations, bed days, and use of oral corticosteroids, resulting in overall cost savings with the introduction of omalizumab24,25 and the introduction of FeNO before a trial of omalizumab is expected to further increase these cost savings.
While our economic model has its strengths, it is also subject to restrictions that may limit its generalizability. Due to the nature of this model, some pre-set values or assumptions may not directly transfer to different patient populations, payer networks, or other countries. In addition, the costs of omalizumab may not reflect immediate current market costs due to fluctuations in price.
Conclusion
This cost-effectiveness analysis may serve as a model for the advancement of the health care system and insurer policy, and ultimately, clinical practice regarding the use of FeNO to determine omalizumab response. It also has widespread implications for health care payers, who may choose to require FeNO measurement and prespecify a minimum FeNO value to determine patient eligibility for omalizumab trial. Our model demonstrates that per-patient costs decrease by nearly 50% during the trial period when FeNO measurements are used to identify omalizumab responders. This cost savings also continue through the initial treatment period of 12 months. A future comparative study will be important to validate the findings of the current modeled analysis and also to examine the impact of other biomarkers (eg, blood eosinophil) in addition to FeNO on the cost-effectiveness of identifying omalizumab responders.
Acknowledgments
The research reported and products related to this study were supported by Circassia Pharmaceuticals. This manuscript is partially based on work presented as a poster at the ISPOR 22nd Annual International Meeting (https://www.valueinhealthjournal.com/article/S1098-3015(17)30251-6/pdf).
Appendix A.
Formulas for Cost-effectiveness calculations of FeNO + Omalizumab vs Omalizumab Only
| Strategy to identify responders | Expected per-patient costs (trial period only) | Expected rate of responders identified | Expected cost per responder (trial period only) |
Expected per-patient costs (trial + initial Tx period) |
|---|---|---|---|---|
| FeNO Measurement + omalizumab Trial | 0.51 | |||
| Omalizumab trial only | 0.55 |
Notes: FeNO measurement + omalizumab trials include pathways 1 and 2. Omalizumab trial only includes pathways 3–5. Patients do not continue treatments on pathways 2, 4, or 5, so no treatment costs are incurred for those pathways.
a, payor costs of 1 vial of 150 mg of omalizumab ($713.70); b, number of vials per month (2); c, number of months in omalizumab trials (3); d, payer cost of FeNO measurements to predict ICS response ($23.20); e, number of months of omalizumab prescription following successful omalizumab trial (12).
Abbreviation: FeNO, fractional exhaled nitric oxide.
Disclosure
MM is employed by Circassia Pharmaceuticals. EAB reports personal fees from Circassia Pharmaceuticals, during the conduct of the study. NAH reports personal fees from Circassia Pharmaceuticals as a consultant on other projects non-related to this one. The authors report no other conflicts of interest in this work.
References
- 1.Pakhale S, Mulpuru S, Boyd M. Optimal management of severe/refractory asthma. Clin Med Insights Circ Respir Pulm Med. 2011;5:37–47. doi: 10.4137/CCRPM.S5535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peters SP, Ferguson G, Deniz Y, Reisner C. Uncontrolled asthma: a review of the prevalence, disease burden and options for treatment. Respir Med. 2006;100(7):1139–1151. doi: 10.1016/j.rmed.2006.03.031 [DOI] [PubMed] [Google Scholar]
- 3.Hekking PPW, Wener RR, Amelink M, Zwinderman AH, Bouvy ML, Bel EH. The prevalence of severe refractory asthma. J Allergy Clin Immunol. 2015;135(4):896–902. doi: 10.1016/j.jaci.2014.08.042 [DOI] [PubMed] [Google Scholar]
- 4.Al-Hajjaj MS. Difficult-to-treat asthma, is it really difficult? Annals of thoracic. Medicine (Baltimore). 2011;6(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berry MA, Hargadon B, Shelley M, et al. Evidence of a role of tumor necrosis factor α in refractory asthma. N Engl J Med. 2006;354(7):697–708. doi: 10.1056/NEJMoa050580 [DOI] [PubMed] [Google Scholar]
- 6.Chipps BE. “Key findings and clinical implications from the epidemiology and natural history of asthma: outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012;130(2):332–342. doi: 10.1016/j.jaci.2012.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Humbert M, Busse W, Hanania NA, et al. Omalizumab in asthma: an update on recent developments. J Allergy Clin Immunol Pract. 2014;2(5):525–536.e1. doi: 10.1016/j.jaip.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 8.Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65(9):1141–1148. doi: 10.1111/j.1398-9995.2010.02336.x [DOI] [PubMed] [Google Scholar]
- 9.Cohen J, Wilson AW. New challenges to medicare beneficiary access to mAbs. MAbs. 2009;1:56–66. doi: 10.4161/mabs.1.1.7246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114(2):265–269. doi: 10.1016/j.jaci.2004.05.049 [DOI] [PubMed] [Google Scholar]
- 11.Dewilde S, Turk F, Tambour M, Sandström T. The economic value of anti-IgE in severe persistent, IgE-mediated (allergic) asthma patients: adaptationof INNOVATE to Sweden. Curr Med Res Opin. 2006;22(9):1765–1776. doi: 10.1185/030079906X132389 [DOI] [PubMed] [Google Scholar]
- 12.Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007;62(2):149–153. doi: 10.1111/j.1398-9995.2006.01310.x [DOI] [PubMed] [Google Scholar]
- 13.Sullivan SD, Turk F. An evaluation of the cost-effectiveness of omalizumab for the treatment of severe allergic asthma. Allergy. 2008;63(6):670–684. doi: 10.1111/j.1398-9995.2008.01723.x [DOI] [PubMed] [Google Scholar]
- 14.Del Vennera M, Valero A, Uría E, Forné C, Picado C. Cost-effectiveness analysis of omalizumab for the treatment of severe persistent asthma in real clinical practice in Spain. Clin Drug Investig. 2016;36(7):567–578. doi: 10.1007/s40261-016-0402-2 [DOI] [PubMed] [Google Scholar]
- 15.Levy AN, García ARJ, García-Agua NS, Hidalgo MVS. Cost-effectiveness of omalizumab in severe persistent asthma in Spain: A real-life perspective. ResearchGate. 2014;52(2):1–26. [DOI] [PubMed] [Google Scholar]
- 16.Norman G, Faria R, Paton F, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. NIHR J Lib. 2013;17(52):1–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjermer L, Alving K, Diamant Z, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014;108(6):830–841. doi: 10.1016/j.rmed.2014.02.005 [DOI] [PubMed] [Google Scholar]
- 18.Wu AC, Paltiel AD, Kuntz KM, Weiss ST, Fuhlbrigge AL. Cost-effectiveness of omalizumab in adults with severe asthma: results from the asthma policy model. J Allergy Clin Immunol. 2007;120(5):1146–1152. doi: 10.1016/j.jaci.2007.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chibana K, Trudeau JB, Mustovitch AT, et al. IL-13 induced increases in nitrite levels are primarily driven by increases in inducible nitric oxide synthase as compared with effects on arginases in human primary bronchial epithelial cells. Clin Exp Allergy. 2008;38:936–946. doi: 10.1111/j.1365-2222.2008.02969.x [DOI] [PubMed] [Google Scholar]
- 20.Hoch HE. Can we predict fall asthma exacerbations? Validation of the seasonal asthma exacerbation index. J Allergy Clin Immunol. 2017. doi: 10.1016/j.jaci.2017.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013;187(8):804–811. doi: 10.1164/rccm.201208-1414OC [DOI] [PubMed] [Google Scholar]
- 22.Ledford D. A randomized multicenter study evaluating Xolair persistence of response after long-term therapy. J Allergy Clin Immunol. 2017;140(1):162–169. doi: 10.1016/j.jaci.2016.08.054 [DOI] [PubMed] [Google Scholar]
- 23.Barnes N, Menzies-Gow A, Mansur AH, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013;50(5):529–536. doi: 10.3109/02770903.2013.790416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith DH, Malone DC, Lawson KA, Okamoto LJ, Battista C, Saunders WB. A national estimate of the economic costs of asthma. Am J Respir Crit Care Med. 1997;156(3):787–793. doi: 10.1164/ajrccm.156.3.9611072 [DOI] [PubMed] [Google Scholar]
- 25.Costello RW, Long DA, Gaine S, Donnell TM, Gilmartin JJ, Lane SJ. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci. 2011;180(3):637–641. doi: 10.1007/s11845-011-0716-2 [DOI] [PubMed] [Google Scholar]
- 26.Adams EN, Marks A. Asthma maintenance: an update on new medications. US Pharm. 2010;35(7):1–5. [Google Scholar]