Abstract
The study of cognition in Parkinson’s disease (PD) traditionally requires exhaustive recruitment strategies. The current study examines data collected by the Brain Health Registry (BHR) to determine whether ongoing efforts to improve the recruitment base for therapeutic trials in Alzheimer’s disease may be similarly effective for PD research, and whether online cognitive measurements can discriminate between participants who do and do not report a PD diagnosis. Participants enrolled in the BHR (age ≥ 50) with self-reported PD data and online cognitive testing available were included (n=11,813). Associations between baseline cognitive variables and diagnostic group were analyzed using logistic regression. Linear mixed effects models were used to analyze longitudinal data. A total of 634 participants reported PD diagnosis at baseline with no self-reported cognitive impairment and completed cognitive testing. Measures of visual learning and memory, processing speed, attention, and working memory discriminated between self-reported PD and non-PD participants after correcting for multiple comparisons (p values<0.006). Scores on all cognitive tests improved over time in PD and controls with the exception of processing speed, which remained stable in participants with PD while improving in those without. We demonstrate that a novel online approach to recruitment and longitudinal follow-up of study participants is effective for those with self-reported PD, and that significant differences exist between those with and without a reported diagnosis of PD on computerized cognitive measures. These results have important implications for recruitment of participants with PD into targeted therapeutic trials or large-scale genetic and cognitive studies.
Keywords: Aging, Cognition, Neuropsychology, Parkinson disease, Patient selection, Registries
INTRODUCTION
Cognitive symptoms in Parkinson’s disease (PD) are pervasive and are linked to decreased quality of life and impaired functional performance over and above the motor symptoms of the disease [1, 2]. Variation in cognitive presentation and progression presents an especially complex challenge for the identification of effective clinical interventions for this unmet medical need in PD [3]. Adapting an individualized, precision medicine approach to the study of cognitive interventions in PD may represent the most promising avenue for the eventual implementation of effective treatment regimens [4]. This approach, however, requires targeted recruitment strategies that can be difficult to accomplish using traditional methodology. Due to lower lifetime risk of PD as compared to Alzheimer’s disease (AD), large community-based studies of aging typically include low numbers of PD participants [5]. Further, PD-specific cohort studies must engage exhaustive and expensive recruitment strategies to effectively enroll sufficient numbers of participants to address the complex interplay between participant features and cognition in PD [6, 7].
Recognition that targeted enrollment into AD clinical trials is an arduous process plagued by high screen failure rates prompted a novel approach to large-scale screening of potential clinical trials participants, recently implemented by the Brain Health Registry (BHR, brainhealthregistry.org)[8]. Participants in the BHR complete comprehensive online questionnaires as well as cognitive testing, and thus potentially reduce the need for extensive and costly pre-screening efforts. Further, as face-to-face cognitive testing is time consuming and often inconvenient for participants, online cognitive testing offers the potential for detailed assessment of a variety of cognitive domains much more conveniently among large numbers of participants. Such an approach may be particularly effective for both evaluating the impairment and change associated with PD on a larger scale than has been feasible in the past, and for recruiting appropriate participants into targeted therapeutic PD trials.
The major goal of the current study was to determine whether ongoing efforts to improve the recruitment base for AD therapeutic trials by the BHR may be similarly effective for PD research, and specifically to test the hypotheses that a) a large number of people with self-reported PD can be recruited into an online registry b) online self-report may be a useful method to identify large numbers of people with possible PD, and c) it is feasible to use online cognitive testing to evaluate cognition in people with self-reported PD. We further hypothesize that, consistent with clinic-based research, when participants with self-reported PD and without self-reported cognitive impairment take online cognitive tests, they will demonstrate lower scores as compared to their counterparts without PD.
MATERIALS AND METHODS
Participants
BHR methods and recruitment are described in detail elsewhere [9]. Briefly, the BHR is an online public registry that incorporates a variety of recruitment methods, including the BHR website, social media, brochures, online advertising, direct mail, and sponsorships. Online consent involves an information sheet, which the participant may decline or approve; a waiver of signed consent was granted as the study was determined to present no more than minimal risk of harm. Following informed consent procedures, participants may complete online questionnaires and neuropsychological tests, including validated measures of medical and family history, traumatic brain injury/concussion, sleep quality, early childhood history, satisfaction with life scale, Geriatric Depression Scale (GDS), everyday cognition (ECog), and diet.[9] Participants are asked to complete the same procedures at 6 month intervals.
In the Medical History Questionnaire, participants were asked, “Please indicate whether you currently have or have had any of the following conditions in the past,” followed by a list of medical conditions. For the current study, participants were placed into the PD group if they self-reported a PD disease diagnosis at baseline. Non-PD participants were those with no self-reported PD. No information was available concerning timing of symptoms onset, symptom severity, or other clinical data. Exclusions included self-reported motor neuron disease, AD, Lewy body disease (other than PD), dementia or mild cognitive impairment, use of cholinesterase inhibitors or NMDA receptor antagonists, multiple sclerosis, frontotemporal dementia, Huntington’s disease, amyotrophic lateral sclerosis, current or past autism, current or past schizophrenia, or current psychosis. Of the 32,139 participants who provided basic demographic information and self-reported either a “Yes” or “No” PD diagnosis, 12,439 were subsequently excluded due to missing or incomplete data. An additional 7,887 were excluded due to age < 50, missing cognitive data, or the presence of one or more exclusionary diagnoses, for a total of 11,813 available for analysis (see Table 1 for detail).
Table 1.
Summary of available Brain Health Registry baseline participants
| Total n (PD n) | |
|---|---|
| Total in dataset* | 32,155 |
| …with self-reported PD data (Y/N) collected | 32,139 (1,120) |
| …and fit either “PD” or “Non-PD” criteria | 17,275 (781) |
| … and age at baseline >= 50 years | 13,312 (706) |
| …and with at least one cognitive test completed | 11,813 (634) |
Totals represent number of participants in the BHR database on whom primary covariates (age, education, and gender) were collected
Cognitive measures
The BHR cognitive measures are owned by third party vendors and are self-administered online.
Cogstate Brief Battery (cogstate.com) [10]
a) Detection Test: A simple reaction time test and measure of processing speed; b) Identification Test: A choice reaction time test and measure of attention; lower scores represent better performance; c) One-Card Learning: A measure of visual learning and memory; and d) One-Back: A measure of visual working memory.
MemTrax Memory Test (memtrax.com) [11]
A one-minute online memory test developed as a screening for dementia.
Lumos Labs Neurocognitive Performance Test [12]
a) Go/No Go: A measure of response inhibition and sustained attention; b) Trailmaking Test, Part B: A timed measure of visual divided attention; and c) Forward and Reverse Memory Span: A measure of visual short-term and working memory.
Statistical analyses
The differences in baseline characteristics between PD and non-PD groups were summarized and tested using two-sample t-tests for continuous variables, Wilcoxon rank-sum tests for ordinal variables, or chi-square tests for binary variables. To assess associations between baseline cognitive variables and diagnostic group (PD vs. non-PD), separate multivariate logistic regression models were first performed, controlling for age, education level, sex, and depression (measured by the GDS). Subsequently, all cognitive measures were entered into a single multivariate logistic regression to determine whether any cognitive tests were associated with diagnosis over and above the others. Test scores were converted to a standardized scale to facilitate comparison of the odds ratios. The Bonferroni adjustment was used to control the type 1 error set a priori at 0.05; since there are nine cognitive variables of interested, a significance level of 0.05/9 = 0.006 was used for individual tests. For longitudinal analyses, linear mixed effects models with robust variance were used to determine the association between cognitive variables and diagnostic group (PD, non-PD), time (baseline, month 6, month 12, month 18), and interaction between group and time, controlling for age, education, gender, and GDS score. The patient-specific random intercept is used to account for within-person correlations in the mixed effects regression analysis. All analyses were performed using Stata 14.2.
RESULTS
Baseline characteristics, PD and non-PD groups
Features of participants who self-reported PD or no PD at baseline without cognitive impairment are presented in Table 2. Groups were equivalent in terms of age; however, the PD group had a higher median level of education and a significantly higher proportion of male participants. Significantly more participants in the PD group endorsed chronic pain and a history of concussion, while those in the non-PD group endorsed a higher rate of hypercholesterolemia, diabetes, traumatic brain injury, arthritis, allergies, history of drug or alcohol abuse, past or current smoking, and major depressive disorder. Participants with PD had higher scores on the GDS, poorer quality of life scores, and endorsed more sleep problems than non-PD participants. Dietary differences included fewer servings of vegetables per day, higher red meat consumption, higher rates of soda, milk, and juice consumption, and lower rates of coffee consumption in the PD group. On the ECog, participants in the PD group rated their performance as worse across all cognitive domains.
Table 2.
Brain Health Registry baseline characteristics, PD vs. Non-PD
| PD (n = 634) | Non-PD (n = 11,179) | p value* | |
|---|---|---|---|
| Demographics | |||
| Age at baseline, years | |||
| mean (sd) | 64.4 (7.8) | 64.0 (7.9) | 0.175 |
| Gender | |||
| n (% male) | 289 (45.6%) | 2861 (25.6%) | <0.001‡ |
| Education, years | |||
| median (range) | 16 (12 – 20) | 16 (<12 – 20) | 0.005‡ |
| Medical history n (%) | |||
| Heart disease | 56 (8.8%) | 866 (7.8%) | 0.321 |
| Hypertension | 217 (34.2%) | 3994 (35.7%) | 0.443 |
| High cholesterol | 239 (37.7%) | 4854 (43.4%) | 0.005† |
| Stroke | 14 (2.2%) | 270 (2.4%) | 0.741 |
| Diabetes | 38 (6.0%) | 939 (8.4%) | 0.032† |
| Cancer | 115 (18.1%) | 1964 (17.6%) | 0.714 |
| Traumatic brain injury | 6 (1.0%) | 234 (2.1%) | 0.046† |
| Concussion | 116 (18.3%) | 1642 (14.7%) | 0.013‡ |
| Seizures | 12 (1.9%) | 260 (2.3%) | 0.453 |
| Asthma | 83 (13.1%) | 1642 (14.7%) | 0.268 |
| Arthritis | 238 (37.5%) | 4870 (43.6%) | 0.003† |
| Lung Disease | 14 (2.2%) | 397 (3.6%) | 0.073 |
| Allergies | 297 (46.1%) | 6030 (53.8%) | <0.001† |
| Alcohol abuse, past or current | 44 (6.9%) | 1282 (11.5%) | <0.001† |
| Drug abuse, past or current | 27 (4.3%) | 704 (6.3%) | 0.038† |
| Tobacco use, past or current | 205 (32.3%) | 4666 (41.7%) | <0.001† |
| Major depressive disorder, past or current | 80 (12.6%) | 1824 (16.3%) | 0.014† |
| Anxiety disorder, past or current | 126 (19.8%) | 2522 (22.6%) | 0.115 |
| Bipolar Disorder, past or current | 15 (2.4%) | 267 (2.4%) | 0.971 |
| Chronic pain | 210 (33.2%) | 3045 (27.2%) | 0.001‡ |
| Mood/ Quality of life | |||
| Geriatric Depression Scale | |||
| mean (sd) | 3.2 (3.1) | 2.4 (2.9) | <0.0001‡ |
| Overall rating of health, 1=excellent, 5=poor | |||
| mean (sd) | 2.9 (0.9) | 2.3 (0.9) | 0.0001‡ |
| Health compared to 1 year ago, 1=better, 5=worse | |||
| mean (sd) | 3.2 (0.9) | 2.8 (0.8) | 0.0001‡ |
| Problems with activities due to physical problems | |||
| n (%) | 391 (65.8%) | 3667 (36.2%) | <0.001‡ |
| Problems with activities due to emotional problems | |||
| n (%) | 216 (36.4%) | 2973 (29.4%) | <0.001‡ |
| Sleep | |||
| Total sleep hours | |||
| median (range) | 7 (<4 – 12) | 7 (<4 – >12) | 0.0001† |
| Nightwaking > 1/week | |||
| n (%) | 442 (74.5%) | 6664 (66.8%) | <0.001‡ |
| Sleep interruption due to… n (%) | |||
| .. Bad dreams > 1/week | 93 (16.0%) | 777 (7.8%) | <0.001‡ |
| .. Pain > 1/week | 199 (34.1%) | 2558 (25.7%) | <0.001‡ |
| .. Difficulty breathing > 1/week | 48 (8.2%) | 695 (7.0%) | 0.255 |
| .. Snoring >1/week | 107 (18.4%) | 1693 (17.0%) | 0.410 |
| Restless legs (partner endorsed >1/week) | |||
| n (%) | 134 (33.0%) | 1257 (19.2%) | <0.001‡ |
| Night confusion (partner endorsed > 1/week) | |||
| n (%) | 79 (17.0%) | 377 (5.4%) | <0.001‡ |
| Diet | |||
| Servings vegetables/day | |||
| mean (sd) | 2.3 (1.3) | 2.5 (1.5) | 0.001† |
| Servings red meat/week | |||
| Mean (sd) | 2.3 (1.7) | 2.1 (1.8) | 0.001‡ |
| Drink… n, % | |||
| ..soda, diet or regular | 215 (33.9%) | 3097 (27.7%) | 0.001‡ |
| ..juice | 265 (41.8%) | 3030 (27.1%) | <0.001‡ |
| ..milk | 341 (53.8%) | 5107, 45.7 | <0.001‡ |
| ..coffee | 384 (60.6%) | 7276, 65.1 | 0.020† |
| Everyday Cognition Scale mean (sd) 1 = better/no change, 4 = much worse | |||
| Memory | 1.8 (0.7) | 1.7 (0.6) | 0.0006‡ |
| Language | 1.6 (0.6) | 1.4 (0.5) | <0.0001‡ |
| Visuospatial | 1.2 (0.4) | 1.1 (0.3) | 0.0325‡ |
| Divided Attention | 1.8 (0.8) | 1.5 (0.6) | <0.0001‡ |
| Planning | 1.3 (0.4) | 1.1 (0.3) | <0.0001‡ |
| Organization | 1.4 (0.6) | 1.3 (0.5) | <0.0001‡ |
p values based on t-tests for continuous variables, chi-square test for categorical variables, and Wilcoxon rank-sum test for ordinal variables
Non-PD > PD
PD > Non-PD
Abbreviations: PD, Parkinson’s disease; sd, standard deviation
Cognitive test outcomes, PD and non-PD groups
Logistic regression analyses yielded poorer performance amongst PD participants across most cognitive tests, with differences on visual learning and memory (One Card Learning), visual attention (Identification), processing speed (Detection), visual recognition memory response time (MemTrax), and spatial working memory/divided attention (Trailmaking B) reaching significance using the criteria set a priori for multiple comparisons (Table 3). Differences were noted in baseline performance across measures of response inhibition (Go/No Go) and spatial attention and working memory (forward and reverse memory span), but these failed to reach significance after adjusting for multiple comparisons. Only the One Back test, which measures attention and reaction time, did not yield any baseline differences between the PD and non-PD groups. There were no gender by cognitive test interactions. When all cognitive tests were entered into a single logistic regression model, the Detection (p=0.002), One Card Learning (p<0.001) and MemTrax response time (p=0.001) were significantly associated with PD diagnostic group at the <0.006 level.
Table 3.
Cognitive measures, PD vs. Non-PD
| mean (sd) range | ||||||
|---|---|---|---|---|---|---|
| OR* | 95% CI | p | ||||
| Domain | PD | Non-PD | ||||
| Cogstate Brief Battery | ||||||
| One-Back | Attention & working memory (reaction time, log10 transformed) | 2.88 (0.10) 2.51 – 3.20 | 2.88 (0.09) 2.44 – 3.69 | 1.01 | 0.92 – 1.11 | 0.813 |
| One Card Learning | Visual learning & memory (accuracy, arcsine transformation of the square root) | 0.70 (0.16) 0.18 – 1.18 | 0.76 (0.17) 0.03 – 1.41 | 1.32 | 1.13 – 1.54 | 0.001† |
| Identification | Visual attention (reaction time, log10 transformed) | 2.72 (0.08) 2.52 – 3.05 | 2.71 (0.06) 2.37 – 3.19 | 1.20 | 1.10 – 1.31 | <0.001† |
| Detection | Processing speed (reaction time log10 transformed) | 2.58 (0.10) 2.19 – 3.16 | 2.56 (0.10) 2.01 – 3.49 | 1.30 | 1.19 – 1.42 | <0.001† |
| MemTrax | ||||||
| Visual recognition memory (correct response reaction time, seconds) | 0.97 (0.22) 0.57 – 1.91 | 0.95 (0.21) 0.49 – 2.00 | 1.16 | 1.05 – 1.28 | 0.005† | |
| Neurocognitive Performance Test | ||||||
| Go/No Go | Response inhibition & processing speed (mean reaction time, seconds) | 0.51 (0.10) 0.32 – 1.00 | 0.50 (0.09) 0.26 – 1.28 | 1.09 | 1.00 – 1.19 | 0.062 |
| Trailmaking Part B | Spatial working memory/divided attention (time to complete, seconds) | 62.76 (39.06) 23.34 – 286.11 | 55.88 (33.95) 16.32 – 297.56 | 1.18 | 1.09 – 1.27 | <0.001† |
| Forward Memory Span | Spatial attention (total correct) | 4.86 (1.37) 0 – 8 | 4.98 (1.23) 0 – 9 | 1.12 | 1.03 – 1.22 | 0.010 |
| Reverse Memory Span | Spatial working memory (total correct) | 4.13 (1.62) 0 – 8 | 4.22 (1.56) 0 – 9 | 1.08 | 1.00 – 1.18 | 0.088 |
Controlling for age, education level, sex, and Geriatric Depression Scale score; ORs based on conversion of test scores to a standardized scale (standard deviation=1) to facilitate comparison across measures
Met criteria for significance using the Bonferroni adjustment (0.006)
Abbreviations: CI, confidence interval; OR, odds ratio; PD, Parkinson’s disease; sd, standard deviation
Longitudinal results, PD and non-PD groups
Of the 634 participants with no reported cognitive impairment who completed a baseline visit in the PD group, 443 (69.9%) completed at least one follow up visit, while in the non-PD group, 6795 (60.7%) completed at least one follow up visit. In the PD group that completed a follow up visit, there were no significant differences in age, education, sex or GDS score when comparing those who did and did not complete at least one follow up visit. In the non-PD group, those who completed a follow up visit were older (p<0.0001), had a higher level of education (p<0.0001), and lower GDS score (p<0.0001).
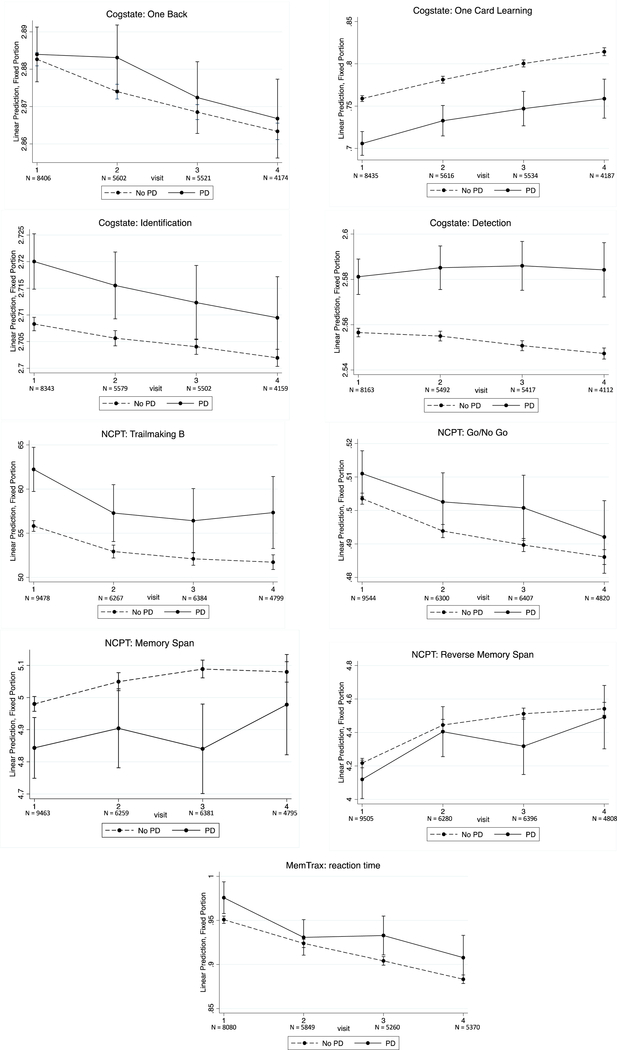
Performance generally improved or remained stable for both PD and non-PD participants across cognitive measures (Figure 1). On the Detection test, there was a statistical trend for a timepoint by diagnosis interaction, with processing speed leveling off at month 12 and month 18 for the PD participants, while it continued to improve for the non-PD participants (month 12 p=0.06, month 18 p=0.05).
Figure 1.
Longitudinal performance on Brain Health Registry cognitive measures for PD and non-PD participants.
DISCUSSION
Our major findings were that it is feasible to enroll a large number of older adults with self-reported PD in an online registry, that self-report may be a practical method of initially identifying those with a possible PD diagnosis in preparation for a trial-ready cohort, that online cognitive testing is practicable in this subgroup of BHR participants, and that the BHR cognitive measures can distinguish between those with and without self-reported PD. Our results thus support that online screening methods developed to improve recruitment into AD clinical trials by the BHR may also help identify, recruit, and evaluate research participants with PD.
Our results show that the BHR is an effective method for recruitment of research participants with self-reported PD, possibly by overcoming known obstacles to participation. One substantial limitation to the identification of large numbers of PD participants is the lower overall population base rate compared to other major diseases. It is estimated that approximately 1% of the US population over age 60 is currently diagnosed with PD, with much lower prevalence at younger ages [13]. Community-based studies thus typically include very small numbers of participants with PD [5, 14]. In contrast, over 5% of BHR participants above age 50 who met research criteria self-identified as having a PD diagnosis. The BHR advertises using messaging related to brain health in older adults [9], which may appeal to older adults with PD seeking information and resources online. Notably, motor impairments associated with PD may impede or dissuade participation in a traditional research setting [15]. Given the high level of engagement in the BHR by participants with self-reported PD, we demonstrate that barriers to participation imposed by the traditional physical research environment may be overcome by engaging an online platform for data collection.
We further demonstrate that self-reported PD may serve as a first step for initial identification of study participants for PD trials. Despite the lack of access to medical records to verify PD diagnosis, associations between clinical and lifestyle factors and those with self-reported PD in the BHR suggest a concordance with clinically diagnosed PD. A formal clinical diagnosis of PD is associated with male sex, chronic pain, depressed mood, specific sleep difficulties (e.g., restless legs, nighttime confusion, sleep interruption), and overall decreased quality of life due to both physical and emotional factors [16, 17]. Each of these factors also separated the self-reported PD and non-PD groups in the BHR. Lifestyle factors that may be protective against PD (e.g., tobacco, coffee consumption, vegetable consumption) are more prevalent in non-PD participants in our sample, while others previously identified as associated with PD diagnosis (red meat consumption, dairy consumption) were also associated with PD in our sample [18] Certainly, a formal clinical diagnosis will be necessary for those recruited into clinical trials or genetic studies, and an important future endeavor will be to validate this method with a subset of participants willing to undergo clinical diagnostic procedures.
Importantly, our results show that participants with self-reported PD are willing and able to complete online cognitive testing. Clinic-based cognitive testing is costly and time-consuming, whereas online measures are designed to be brief, easy to self-administer, and more economical. There is also increasing evidence of the validity of online cognitive testing, including in BHR [19, 20]. Indeed, the PD group completed cognitive testing at similarly high rates to their non-PD counterparts. Further, participants with PD participated in follow up visits at a significantly higher rate than the non-PD sample. We therefore conclude that it is feasible to evaluate cognition longitudinally among people who self-identify with a PD diagnosis using online cognitive measures.
In addition to feasibility, we demonstrate that online cognitive testing differs between participants with and without a self-reported PD diagnosis. Prior studies have shown that even among newly diagnosed patients with PD, a substantial proportion already have at least subtle cognitive impairment [21, 22]. The cognitive measures utilized by the BHR measure domains often found to be impaired in PD, including attention, processing speed, working memory, and learning and memory, and most discriminated between the PD and non-PD groups. The motor symptoms of PD could certainly impact performance on these cognitive tasks, particularly those with a timed component. Interpretation of task performance can thus be difficult given that both cognitive and motor slowing associated with the disease can impair reaction times. Interestingly, we found that participants with self-reported PD also performed worse on tasks where the outcome was total correct rather than reaction time, particularly on a task of visual learning and memory. In contrast, on one task that did measure reaction time, there was no difference by diagnosis. These results suggest a specific pattern of performance, principally characterized by slowed processing speed and poorer visual learning and memory, that are unlikely accounted for solely by motor impairment and that distinguish PD and non-PD groups. Early in the course of PD, dysfunction in dopaminergic pathways between the striatum and the prefrontal cortex lead to deficits in processing speed that are especially insidious [23]. Disruptions in visuospatial function as well as visual learning and memory in PD are also well-established and may occur early in the disease, although the specific underlying pathology may differ across disease subtypes [24–27]. Given the results from the current study, we conclude that the online cognitive tests employed by the BHR, although originally chosen to screen potential AD participants, are useful in distinguishing between those with and without self-reported PD.
In examining longitudinal data, we primarily noted practice effects or stable performance for both groups, with the exception of processing speed, which remained stable in the PD group while improving in the non-PD group, again supporting processing speed as a primary discriminative domain. Often, cognition in patients with PD will remain stable for some time or fluctuate during the course of the disease on cognitive testing [28], thus a longer course of follow up in the BHR with a greater number of participants will provide greater power to understand the course of cognitive change in PD. For the non-PD group, factors such as age, education, and depression differed among those who did and did not complete follow up visits, leading to the possibility that the practice effects seen on testing may be a result of these differences. Such differences were not noted in the PD group who did and did not complete a follow up assessment despite the nature and magnitude of practice effects being similar to those observed in the non-PD group, and yet practice effects were similar. A longer period of follow up will provide better detail about progression of cognitive function in both PD and non-PD groups.
There are limitations to the current study. The specific details of diagnostic methods used to establish a PD diagnosis are unknown and likely vary substantially within the PD group. As such, there are likely undiagnosed participants with PD in the BHR, and well as those who may have misreported or misdiagnosed symptoms. Without more specific information available, we were not able to control for important disease related factors such as severity of disease and time since symptom onset. Cognitive diagnosis, beyond self-report, was unknown and thus BHR participants may have undiagnosed/unreported cognitive impairment. Further, cognitive testing was not performed under standardized conditions, although increasing evidence supports validity of online, unsupervised cognitive testing [20] [19]. Thus, although the BHR methods are likely to be effective in gathering large groups of PD patients for detailed follow up, additional diagnostic methods for both motor and cognitive diagnosis will be necessary for participation in more focused studies of PD. Finally, the use of an online platform presents specific challenges, including a large proportion of participants who begin, but do not complete, the study procedures. Methods to encourage completion of the initial procedures should thus be explored in detail in future endeavors. Further, online platforms can present obstacles with regard to longitudinal follow-up. Interestingly, participants in the current study took part in follow-up examination at a greater rate than BHR participants overall (61% vs. 41%)[29], despite their older age which raises the risk for attrition due to morbidity and mortality. Continued focus on retention procedures, described in Weiner et al.[29], will be key to successful longitudinal outcomes.
Despite these limitations, we confirmed that recruitment of large numbers of participants with PD is possible using methods that are alternative to traditional recruitment strategies. For clinical trials seeking such patients, the BHR could be an effective research pool from which to initially identify potential participants who meet specific study enrollment criteria. To date more than 1798 BHR participants have successfully been enrolled in AD and aging studies, including observational studies and randomized treatment trials; similar methods could be used to recruit to PD trials [9]. Importantly, we confirmed baseline differences between the PD and non-PD groups on computerized cognitive measurements; future endeavors to validate these cognitive measures in a well-characterized PD population and to garner a larger base for greater longitudinal follow up will provide additional valuable information concerning the utility of these methods in identifying and following individuals with PD. These results conceivably change the practice for identification and recruitment of participants with PD into targeted therapeutic trials or large-scale genetic and cognitive studies.
ACKNOWLEDGMENTS
This work was supported by the Larry L. Hillblom Foundation [2015-A-011-NET], Patient-Centered Outcomes Research Institute [PPRN-1501- 26817], California Department of Public Health [16-10054], the Alzheimer’s Drug Discovery Foundation [20150802], and National Institutes of Neurological Disorders and Stroke grant P50 NS0662684.The funding sources did not provide scientific input for the study. We sincerely thank our research subjects for their participation in this study.
Full financial disclosure is provided below:
Dr. Cholerton: Supported by grants from NIH.
Dr. Weiner: Supported by grants from the NIH, DOD, CA Dept. of Public Health, Alzheimer’s Disease Discovery Foundation; ADDF, Larry L. Hillblom Foundation, PCORI, Alzheimer’s Association, Biogen, as well as funding from the Global Alzheimer’s Platform Foundation (GAP), European Brain Health Registry/NL, Johnson & Johnson, and Monell Chemical Senses Center. Dr. Weiner has served on the Scientific Advisory Boards for Alzheon, Inc., Accera, Merck, Nestle (Nolan), PCORI (PPRN), Eli Lilly, Delfino Logic Ltd. (for Merck), Dolby Ventures, Brain Health Registry, and ADNI. He served on the Editorial Boards for Alzheimer’s & Dementia and MRI. He has provided consulting and/or acted as a speaker/lecturer to Synarc, Pfizer, Accera, Inc., Alzheimer’s Drug Discovery Foundation (ADDF), Merck, BioClinica, Eli Lilly, Howard University, Guidepoint, Denali Therapeutics, Nestle/Nestec, GLG Research, Atheneum Partners, BIONEST Partners, American Academy of Neurology (AAN), and Society for Nuclear Medicine and Molecular Imaging (SNMMI). He holds stock options with Alzheon, Inc. The following entities have provided funding for academic travel; Kenes, Intl., Merck, ADCS, ATRI, Eli Lilly, The Alzheimer’s Association, Merck, Tokyo University, Kyoto University, Rose Li & Associates, AAN, and SNMMI.
Dr. Nosheny: Supported by grants from the NIH, Alzheimer’s Association, Alzheimer’s Drug Discovery Foundation (ADDF), Patient Centered Outcomes Research Institute (PCORI), California Dept. Public Health, and Larry L. Hillblom Foundation.
Dr. Poston: Supported by grants from NIH and Michael J. Fox Foundation.
Dr. Tian: Supported by grants from NIH.
Dr. Mackin: Dr. Mackin receives research funding from the following grants from the National Institute of Mental Health: R01101472, R01098062 and funding for research projects from the Larry L. Hillblom Foundation, AVID Radiopharmaceuticals, Janssen Pharmaceuticals, & J&J Innovations
Dr. Ashford: Supported by the Department of Veterans Affairs.
Dr. Montine: Supported by grants from NIH.
Abbreviations
- AD
Alzheimer’s disease
- BHR
Brain Health Registry
- ECog
Everyday Cognition
- GDS
Geriatric Depression Scale
- PD
Parkinson’s disease
Footnotes
CONFLICT OF INTEREST/ DISCLOSURE STATEMENT
All authors have materially participated in the research and/or article preparation (see roles, below). All authors have approved the final submitted article. This article represents original work by the authors, has not been published elsewhere, and is not under consideration for publication elsewhere.
Dr. Ashford is an unpaid consultant for MemTrax, LLC. All other authors report no direct conflict of interest related to the work on this manuscript.
REFERENCES
- [1].Biundo R, Fiorenzato E, Antonini A (2017) Nonmotor Symptoms and Natural History of Parkinson’s Disease: Evidence From Cognitive Dysfunction and Role of Noninvasive Interventions. Int Rev Neurobiol 133, 389–415. [DOI] [PubMed] [Google Scholar]
- [2].Macleod AD, Counsell CE (2016) Predictors of functional dependency in Parkinson’s disease. Mov Disord 31, 1482–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].LaBelle DR, Walsh RR, Banks SJ (2017) Latent Cognitive Phenotypes in De Novo Parkinson’s Disease: A Person-Centered Approach. J Int Neuropsychol Soc 23, 551–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cholerton B, Larson EB, Quinn JF, Zabetian CP, Mata IF, Keene CD, Flanagan M, Crane PK, Grabowski TJ, Montine KS, Montine TJ (2016) Precision Medicine: Clarity for the Complexity of Dementia. Am J Pathol 186, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Crane PK, Gibbons LE, Dams-O’Connor K, Trittschuh E, Leverenz JB, Keene CD, Sonnen J, Montine TJ, Bennett DA, Leurgans S, Schneider JA, Larson EB (2016) Association of Traumatic Brain Injury With Late-Life Neurodegenerative Conditions and Neuropathologic Findings. JAMA Neurol 73, 1062–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G, Norwegian ParkWest Study G (2009) Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 72, 1121–1126. [DOI] [PubMed] [Google Scholar]
- [7].Cholerton BA, Zabetian CP, Quinn JF, Chung KA, Peterson A, Espay AJ, Revilla FJ, Devoto J, Watson GS, Hu SC, Edwards KL, Montine TJ, Leverenz JB (2013) Pacific Northwest Udall Center of excellence clinical consortium: study design and baseline cohort characteristics. J Parkinsons Dis 3, 205–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, Harvey D, Jack CR Jr., Jagust W, Morris JC, Petersen RC, Salazar J, Saykin AJ, Shaw LM, Toga AW, Trojanowski JQ, Alzheimer’s Disease Neuroimaging I (2017) The Alzheimer’s Disease Neuroimaging Initiative 3: Continued innovation for clinical trial improvement. Alzheimers Dement 13, 561–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Weiner MW, Nosheny R, Camacho M, Truran-Sacrey D, Mackin RS, Flenniken D, Ulbricht A, Insel P, Finley S, Fockler J, Veitch D (2018) The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lim YY, Ellis KA, Harrington K, Ames D, Martins RN, Masters CL, Rowe C, Savage G, Szoeke C, Darby D, Maruff P, The Aibl Research G (2012) Use of the CogState Brief Battery in the assessment of Alzheimer’s disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol 34, 345–358. [DOI] [PubMed] [Google Scholar]
- [11].Ashford JW, Gere E, Bayley PJ (2011) Measuring memory in large group settings using a continuous recognition test. J Alzheimers Dis 27, 885–895. [DOI] [PubMed] [Google Scholar]
- [12].Morrison GE, Simone CM, Ng NF, Hardy JL (2015) Reliability and validity of the NeuroCognitive Performance Test, a web-based neuropsychological assessment. Front Psychol 6, 1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reeve A, Simcox E, Turnbull D (2014) Ageing and Parkinson’s disease: why is advancing age the biggest risk factor? Ageing Res Rev 14, 19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR (2012) Pre-motor features of Parkinson’s disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord 18 Suppl 1, S199–202. [DOI] [PubMed] [Google Scholar]
- [15].Nilsson MH, Iwarsson S, Thordardottir B, Haak M (2015) Barriers and Facilitators for Participation in People with Parkinson’s Disease. J Parkinsons Dis 5, 983–992. [DOI] [PubMed] [Google Scholar]
- [16].Lee SJ, Kim SR, Chung SJ, Kang HC, Kim MS, Cho SJ, Kwon HK, Kim J, Jung SY (2017) Predictive model for health-related quality of life in patients with Parkinson’s disease. Geriatr Nurs. [DOI] [PubMed] [Google Scholar]
- [17].Pfeiffer RF (2016) Non-motor symptoms in Parkinson’s disease. Parkinsonism Relat Disord 22 Suppl 1, S119–122. [DOI] [PubMed] [Google Scholar]
- [18].Seidl SE, Santiago JA, Bilyk H, Potashkin JA (2014) The emerging role of nutrition in Parkinson’s disease. Front Aging Neurosci 6, 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Cromer JA, Harel BT, Yu K, Valadka JS, Brunwin JW, Crawford CD, Mayes LC, Maruff P (2015) Comparison of Cognitive Performance on the Cogstate Brief Battery When Taken In-Clinic, In-Group, and Unsupervised. Clin Neuropsychol 29, 542–558. [DOI] [PubMed] [Google Scholar]
- [20].Mackin RS, Insel P, Truran D, Finley S, Flenniken D, Nosheny R, Ulbricht A, Camacho M, Bickford D, Harel BT, Maruff P, Weiner MW (In press) Unsupervised online neuropsychological test performance for individuals with MCI and Dementia: Results form the Brain Health Registry. Alzheimer’s & Dementia: Diagnosis, Assessment, & Disease Monitoring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Fengler S, Liepelt-Scarfone I, Brockmann K, Schaffer E, Berg D, Kalbe E (2017) Cognitive changes in prodromal Parkinson’s disease: A review. Mov Disord. [DOI] [PubMed] [Google Scholar]
- [22].Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23, 837–844. [DOI] [PubMed] [Google Scholar]
- [23].Jokinen P, Karrasch M, Bruck A, Johansson J, Bergman J, Rinne JO (2013) Cognitive slowing in Parkinson’s disease is related to frontostriatal dopaminergic dysfunction. J Neurol Sci 329, 23–28. [DOI] [PubMed] [Google Scholar]
- [24].Ellfolk U, Joutsa J, Rinne JO, Parkkola R, Jokinen P, Karrasch M (2013) Brain volumetric correlates of memory in early Parkinson’s disease. J Parkinsons Dis 3, 593–601. [DOI] [PubMed] [Google Scholar]
- [25].Ibarretxe-Bilbao N, Junque C, Marti MJ, Tolosa E (2011) Brain structural MRI correlates of cognitive dysfunctions in Parkinson’s disease. J Neurol Sci 310, 70–74. [DOI] [PubMed] [Google Scholar]
- [26].Karadi K, Lucza T, Aschermann Z, Komoly S, Deli G, Bosnyak E, Acs P, Horvath R, Janszky J, Kovacs N (2015) Visuospatial impairment in Parkinson’s disease: the role of laterality. Laterality 20, 112–127. [DOI] [PubMed] [Google Scholar]
- [27].Santangelo G, Vitale C, Picillo M, Moccia M, Cuoco S, Longo K, Pezzella D, di Grazia A, Erro R, Pellecchia MT, Amboni M, Trojano L, Barone P (2015) Mild Cognitive Impairment in newly diagnosed Parkinson’s disease: A longitudinal prospective study. Parkinsonism Relat Disord 21, 1219–1226. [DOI] [PubMed] [Google Scholar]
- [28].Pigott K, Rick J, Xie SX, Hurtig H, Chen-Plotkin A, Duda JE, Morley JF, Chahine LM, Dahodwala N, Akhtar RS, Siderowf A, Trojanowski JQ, Weintraub D (2015) Longitudinal study of normal cognition in Parkinson disease. Neurology 85, 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Weiner MW, Nosheny R, Camacho M, Truran-Sacrey D, Mackin RS, Flenniken D, Ulbricht A, Insel P, Finley S, Fockler J, Veitch D (2018) The Brain Health Registry: An internet-based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement 14, 1063–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]