Abstract
Improved survival among HIV-1 infected individuals with the advent of antiretroviral therapy has clearly led to a greater prevalence of non-infectious complications. One of the most devastating sequelae in these individuals is the development of pulmonary arterial hypertension (PAH). Various epidemiological studies suggest a worse survival of HIV-PAH patients when compared to other forms of PAH. Given that only a subset and not all HIV-infected individuals develop HIV-PAH, suggests that an additional second hit of genetic or environmental triggers is needed for the development of PAH. In this context, it has been well documented that HIV patients who abuse illicit drugs such as stimulants, opioids etc. are more susceptible to develop PAH. In this review, we highlight the studies that support the significance of a double hit of HIV and drug abuse in the incidence of PAH and focus on the research that has been undertaken to unravel the pathobiology and vascular remodeling mechanisms underlying the deleterious synergy between HIV infection and drugs of abuse in orchestrating the development of PAH.
Introduction
Human immunodeficiency virus (HIV) is an infectious retrovirus that targets host immune cells, primarily CD4+ T-lymphocytes and macrophages, and in its terminal stage manifests as acquired immunodeficiency syndrome (AIDS). Prior to antiretroviral therapy (ART) intervention in the mid-1990s, HIV-infected individuals because of their immunocompromised state had a poor prognosis and mortality was high mainly due to opportunistic pulmonary infections [1].
Now, decades into the ART era, and with improved and combined pharmacological drug combinations leading to highly active antiretroviral therapy (HAART), the clinical attention of HIV-infected individuals has shifted from a once acute terminal diagnosis to a chronic disease with significantly improved life expectancy and better quality of life [2]. According to the Center for Disease Control (CDC) more than 50% of HIV-infected population in the United States were 50 years of age or older in 2015 and this number is estimated to increase to 70% by the year 2030 [3]. However, this improved lifespan in this population has now given rise to an increased incidence of chronic non-infectious co-morbidities [4–9]. With an increasingly older HIV/AIDS population, multitude of prominent non-infectious pathologies of the pulmonary and cardiovascular system have emerged. Among the most prevalent are pulmonary arterial hypertension (PAH), chronic obstructive pulmonary disease (COPD), and non-AIDS-defining cancer (NADC), specifically of the lung [10].
Of these, PAH is among the most devastating and life-threatening non-infectious complication [10]. PAH is a Group 1 sub-classification of pulmonary hypertension (PH) [11] which includes development of PH due to HIV infection, drugs/toxins, congenital heart diseases or idiopathic PAH (IPAH). PH caused due to secondary complications like COPD, sleep apnea, hypoxia is classified as Group 2 and PH associated with cardiovascular complications like cardiomyopathies and left ventricle systolic/diastolic dysfunction is listed under Group 3 class of PH. Pulmonary hypertension involves arteriopathy and occlusion of the pulmonary vasculature leading to right ventricular hypertrophy (RVH), and eventually progression to right heart failure (RHF) and death [12–14]. PH is defined by a hemodynamically assessed mean pulmonary arterial pressure (mPAP) ≥ 25 mmHg at rest with a pulmonary capillary wedge pressure (PCWP) < 15 mmHg [15], and a pulmonary vascular resistance (PVR) of > 3 Wood Units [11, 16]. Symptomatic patients generally present with fatigue, dyspnea upon minimal exertion and edema of the lower extremities, although other symptoms can be present, such as syncope, chest pain, and non-productive cough [2, 14]. However, depending on PH severity, patients can be symptomatic or asymptomatic.
Multiple investigators have established the role of HIV-1 in the pathogenesis of PAH [11, 17–20]. The survival rate among HIV-patients has substantially increased in the ART era compared to pre-ART era [20–23]. Reports suggest that even in well managed HIV-infected patients, PAH can manifest anytime in this population with unfortunate rapid progression and mortality [22]. Many studies report the CD4+ count to be significantly predictive of survival [21, 22] and death [20] of HIV-PAH [24] patients. However, whether the CD4+ cell count is an independent risk factor for PAH, still needs to be established. A recent report by Quezada et al [13] discovered that a detectable viral load is independently associated with HIV-PAH diagnosis upon multivariate analysis (OR 3.3; 95% CI, 1.04–10; p=0.04) [13]. Importantly, an independent relationship between PAH and exposure to drugs of abuse has also been established [25, 26]. Patients who abuse illicit drugs, such as stimulants (cocaine, methamphetamine) and opioids (morphine, heroin) are at increased risk for HIV infection; and substance use is known to potentiate the development of HIV-related complications including HIV-PAH. Taken this prominent use of illicit drugs in the HIV-positive population into account, our laboratory has taken these two independent risk factors into consideration and extensively investigated the potential link between HIV-PAH and intravenous drug use (IVDU), with a particular interest in the mechanistic role in the pathogenesis of PAH [27–34].
Although the second-hit of bacterial or parasitic infections [35, 36] and changes in the immune-genetic profile [37] has also been implicated in the pathogenesis of HIV-PAH, this review will focus on the epidemiology of PAH in HIV-infected individuals who abuse illicit drugs. Furthermore, this review will outline the current mechanistic understanding of the exacerbating effect of HIV-associated viral proteins and common drugs of abuse on the pulmonary vasculature and highlight their molecular role in the development of angio-obliterative endothelium and vascular smooth muscle dysfunction.
Epidemiology of PAH in HIV-Infected and Intravenous Drug Users
Prior to and after the introduction of ART, it had been assessed from cohort studies that an average of 1 in 200 HIV-infected individuals develop HIV-PAH compared to IPAH, which has an incidence of 5.9 patients per million in the general population [2, 14]. However, more recently multiple studies reported the prevalence of HIV-PAH to be higher than originally established with reports ranging from 2.6 −15.5% of HIV-infected patients [18, 38–45] regardless of the route of infection.
More importantly, PAH has emerged as a concerning co-morbidity in the HIV-infected individuals who are illicit drug users (Table 1). In addition to numerous cohort, case series, and case-control studies investigating HIV-PAH, multiple case studies report the use of illicit drugs as a major risk factor of HIV infection in HIV-positive patients diagnosed with pulmonary hypertension [46–64]. Furthermore, systematic reviews of HIV-PAH studies by Mesa et al, Mehta et al, and Janda et al report IVDU to be a risk factor for HIV infection in 42%, 58%, and 49% of PAH diagnosed individuals, respectively [46, 48, 65].
Table 1.
Summary of cohort based studies evaluating the risk and incidence of PAH in HIV-infected patients and IV drug users
| Study | Study Design | Study Criteria | % HIV-PH | % HIV-PH with IVDU Risk Factor, n (%) |
|---|---|---|---|---|
| Himelman et al., 1989[50] | n=1200 HIV infected subjects | - Doppler echocardiography, Right side heart catheterization, Severe right ventricle hypertrophy (RVSP>35mm Hg) | n=6 (0.5%) | n=3 (50%) |
| Speich et al., 1991[66] | - n=1,200 HIV-infected subjects, prospective study OR - n=74 (6.2) HIV-infected subjects with cardiopulmonary complaints |
- Symptomatic HIV-infected patients - Doppler echocardiography (RVSP > 30mmHg) |
n=6, (0.5) OR n=6, (8.1) patients with cardiopulmonary complaint |
n=5, (83.3) |
| Petitpretz et al., 1994[23] | - n=20 HIV-infected patients with PH - n=93 non-HIV-infected patients with PPH |
- Right-Side heart catheterization (mPAP > 25mmHg & normal pulmonary wedge pressure) | n=20, (100) | n=12, (60) |
| Opravil et al., 1997[20] | - n=19 HIV-infected individuals diagnosed with primary pulmonary hypertension (1988 to July 1993) - n=19 matched control subjects without primary pulmonary hypertension |
- Continuous Wave Doppler Echocardiography for PPH diagnosis (RVSP/RAP > 30mmHg) | n=19, (100) | n=16, (84.2) |
| Aguilar & Farber, 2000[69] | - n=6 consecutive patients with severe HIV-associated pulmonary hypertension | - Right Heart Catheterization for Primary Pulmonary Hypertension Diagnosis - Continuous Intravenous Epoprostenol Infusion Therapy in severe HIV-associated PH individuals |
n=6, (100) | n=6, (100) |
| Nunes et al., 2003[21] | - n=82, patients with HIV-associated Pulmonary Arterial Hypertension - n=20, (24.4) of patients previously reported by Petitpretz et al. |
- Retrospective Monocentric Case Series - Right-Side Heart Catheterization (mPAP > 25mmHg) |
n=82, (100) |
Overall: n=48, (59) -NYHA Class I-II (67) -NYHA Class III-IV (55) |
| Ghofrani et al., 2004[67] | - n=8, patients with HIV-associated Pulmonary Hypertension - Safety & efficacy study for inhaled iloprost in patients with severe HIV-related pulmonary hypertension |
- severe PH Diagnosis: Right Heart Catheterization (mPAP > 35mmHg) | n=8, (100) | n=5, (62.5) |
| Zuber et al., 2004[68] | - n=47, HIV-associated PAH patients from Swiss HIV Cohort Study, retrospective analysis (January 1988 to December 2001) OR - n=11,894, entire HIV-infected population in Swiss HIV Cohort Study (January 1988 to December 2001) |
- PAH diagnosis: continuous-wave Doppler echocardiography (RVSP/RAP > 30mmHg) | n=47, (100) OR n=47, (0.4) |
n=33, (70) |
| Reinsch et al., 2008[39] | - n=802 HIV-positive patients; prospective, cross-sectional cohort study [study based on baseline population of HIV-HEART Study (Neumann T. et al., 2007)] | - PAH diagnosis: Continuous-wave Doppler echocardiography (sPAP > 35mmHg & dyspnea) - symptomatic & asymptomatic HIV-infected patients |
Overall: n=38, (4.7) Asymptomatic: n=24, (3.0) Symptomatic (dyspnea): n=14, (1.7) |
n=38, (7.9) |
| Hsue et al., 2008[17] | - n=196 HIV-infected participants - n=52 HIV-uninfected participants |
- patients recruited from ongoing HIV-infected clinical cohort, Study of the Consequences of the Protease Inhibitor Era (SCOPE) - PAH Diagnosis: continuous-wave Doppler echocardiography (PASP > 30mmHg) |
PASP > 30mmHg: (36.1) PASP > 35mmHg: (14.8) PASP > 40mmHg:, (6.7) |
Patients with PASP > 30mmHg: - Stimulant Use Ever (29) - Past IVDU (38) - Current IVDU (42) |
| Sitbon et al., 2008[70] | - n=7,648 consecutive HIV-positive patients, prospective study (March 2004 to March 2005) - n=739, (9.66) presented with dyspnea - n=277, (3.62) per-protocol population |
- PAH Diagnosis: patients with unexplained dyspnea underwent Transthoracic Doppler echocardiography (VTR > 2.5m/s), confirmed by Right Heart Catheterization (mPAP > 25mmHg a rest or mPAP > 30mmHg during exercise & PCWP < 15mmHg) - Asymptomatic patients (without dyspnea) excluded from study |
Overall Population: n=35, (0.46) Dyspneic Population: n=35, (4.74) Per-Protocol Population: n=35, (12.64) |
n=35, (51) |
| Degano et al., 2010[22] | - n=944 consecutive patients admitted for PAH evaluation (October 2000 to January 2008), retrospective study - n=122 (12.9) infected with HIV (45 had associated risk factors for PAH other than HIV infection & were excluded) OR - n=77 (8.2) HIV-infected with infection as only PAH risk factor |
- PAH Diagnosis: right heart catheterization (mPAP > 25mmHg at rest, pulmonary capillary wedge pressure < 15mmHg, & pulmonary vascular resistance > 250 dyn s/cm5) | n=122, (12.9) OR n=77, (8.2) |
Overall: n=28, (36) - NYHA Class II: n=8, (47) - NYHA Class III: n=16, (30) - NYHA Class IV: n=4, (57) |
| Mondy et al., 2011[71] | - n=656, HIV-infected participants, prospective, observational, multi-site cohort (2004 to 2006) - n=322, participants with detectable Tricuspid Regurgitant Flow |
- PAH Diagnosis: Echocardiography (Right Ventricular Pressure > 30mmHg) - Asymptomatic HIV-infected participants |
Overall: n=183, (27.9)OROverall Detectable VTR Population: n=183, (57) - n=109, (34); RVSP 31–35mmHg - n=52, (16); RVSP 36–40mmHg - n=17, (5); 41–50mmHgn=5, (2); RVSP 50mmHg or greater |
Cocaine: n=80, (13) Marijuana: n=200, (31) Inhaled Nitrites: n=123, (10) Heroin or Methamphetamine: n=44, (7) **these values are for the complete cohort (n=656), article did not separate HIV-PH from HIV only participants |
| Quezada et al., 2012[13] | - n=392, consecutive HIV-infected individuals, transversal descriptive study (October 2009 to April 2011) | - PAH Diagnosis: Transthoracic Echocardiography (Right Ventricular Pressure > 35mmHg) | Overall: n=39, (9.9) - Mild (< 40mmHg): n=25, (6.4) - Moderate (40–65mmHg): n=11, (2.8) - Severe (> 65mmHg): n=3, (0.8) |
n=17, (58.6) |
| Araujo et al., 2014[72] | - n=18, patients diagnosed with HIV-related PAH, single-center retrospective, observational study utilizing prospectively collected data (June 1998 to June 2012) | - PAH Diagnosis: Right Heart Catheterization (mPAP > 25mmHg at rest & PCWP < 15mmHg) | Overall: n=18, (100) - NYHA Class II: n=8, (44.4) - NYHA Class III: n=9, (50) - NYHA Class IV: n=1, (5.6) |
Overall: n=14, (77.8) - NYHA Class II: n=8, (100) - NYHA Class III: n=5, (55.6) - NYHA Class IV: n=1, (100) |
| Schwarze-Zander et al. 2015[73] | - n=374 HIV-positive patients examined during routine follow-up visits (March 2009 to November 2012), prospective study | - PH Diagnosis: Transthoracic Echocardiography (sPAP >30mmHg) - Symptomatic patients with dyspnea & fatigue, PAH confirmed by right heart catheterization (mPAP > 25mmHg) |
n=23, (6.1) | n=5, (22) |
Among the earliest cases of HIV-1 related PH reported by Himelman et al [50], 3 of the 6 patients that developed RVH and RHF were IVDUs but in the setting of pulmonary infections. Later, a prospective study in Switzerland investigating PAH in HIV-infected individuals reported an overall prevalence of 0.5% in patients presenting with and without cardiopulmonary complications [66]. Of those with cardiopulmonary complaints, a risk factor for HIV infection in 28.3% of infected patients was intravenous (IV) drug abuse [66]. Furthermore, 83.3% of those HIV-infected patients diagnosed with PAH were IVDUs [66]. Prior to the introduction of ART, a study by Petitpretz et al reported that 60% of enrolled patients diagnosed with HIV-associated PAH were IV heroin abusers [23]. Shortly after the introduction of ART, a study by Opravil et al compared HIV-PAH patients to matched HIV-infected controls without PAH from the Swiss HIV Cohort Study and reported a 0.57% incidence of PAH [20]. In addition, this same study reported IVDU as the greatest risk factor for HIV infection in 84.2% of HIV-infected individuals diagnosed with PAH [20]. Again, in a case series by Nunes et al the leading risk factor for HIV infection was IVDU, accounting for 59% of HIV-PAH patients in their cohort [21]. Furthermore, two studies in 2004 by Ghofrani et al [67] and Zuber et al [68] reported IVDU as the most common risk factor for HIV infection with 62.5% and 70% of HIV-PAH patients, respectively to be IVDUs in these studies. In another small cohort of HIV-infected patients diagnosed with PH, 90% were IVDUs, specifically reporting intravenous heroin abuse, with one individual reporting both heroin and cocaine abuse [69]. Furthermore, in the post-HAART era, a large multicenter prospective cohort study in France reported a 0.46% prevalence of HIV-PAH in patients with unexplained dyspnea, and found that 51% of patients diagnosed with HIV-PAH reported IVDU [70].
A San Francisco based prospective observational study [17] had 42% current IVDUs, 38% past IVDUs and 29% stimulant users (cocaine or amphetamine/methamphetamine) among 35.2% of asymptomatic HIV-positive individuals with pulmonary arterial systolic pressure (PASP) > 30mm Hg, however, no significant association was found between drug abuse and an elevated PASP in this patient cohort. Another study investigating HIV-PAH in a retrospective cohort in France reported an 8.2% prevalence of HIV-PAH with IVDU to be the major risk factor for HIV infection in 36% of the overall HIV-PAH patients [22]. More interestingly, in this study, 57% of the HIV-PAH cases diagnosed with New York Heart Association (NYHA) stage IV PAH had IVDU as their major risk factor for HIV infection [22]. Mondy et al surveyed the cross-sectional Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy (SUN Study), for cardiac abnormalities in asymptomatic HIV-infected individuals and found 57% to have a PASP > 30 mmHg with 7% presenting with > 40 mmHg PASP [71]. In this study, among the HIV-infected individuals that underwent echocardiography, 30% reported either cocaine, heroin or methamphetamine use or inhaled nitrite, with an additional 31% of participants reporting marijuana use [71].
A study by Quezada et al investigating both symptomatic and asymptomatic HIV-infected individuals reported an overall PAH prevalence of 9.9%, with nearly 4% of those individuals diagnosed with moderate to severe PAH [13]. This study also reported HIV transmission as a result of IVDU in 58.6% of patients diagnosed with PAH [13]. Additionally, univariate analysis indicated that HIV-infected individuals who were IVDUs were 3.7 times more likely to develop PAH than non-users infected with HIV-1 (OR 3.7, 95% CI 1.71–8.16; p < 0.01) [13]. Although the results of the multivariate analysis were not statistically significant (OR 1.6, 95% CI 0.3–5.5; p = 0.07), the study supports the overwhelming predominance and clinical significance of PAH development in HIV-infected individuals who are IVDUs [13].
Araujo et al in 2014 reported IVDU as a major risk factor (77.8%) for infection in HIV-PAH patients presenting with NYHA functional classes II-IV [72]. Furthermore, the study by Rasoulinejad et al in the same year reported a greater than 35 mmHg PASP in 3% of HIV-infected patients in a cohort that had 50.5% IVDUs [44]. Most recently, a prospective echocardiographic study by Schwarze-Zander et al, reporting a 6.1% prevalence of PAH in asymptomatic HIV-infected individuals, concluded that HIV-infected patients with an IVDU history had an increased prevalence of HIV-PAH compared to HIV-infected individuals without PAH (p < 0.001) [73].
Although not the focus of this review, differences in establishing the PAH diagnosis, for example with the help of transthoracic echocardiogram (TTE) when compared to the gold standard, right heart catheterization (RHC), may potentially contribute to differences in the HIV-PAH prevalence. Additionally, we emphasize that greater attention is needed to detect early PAH in asymptomatic patients.
In the general population, females have an increased incident of IPAH compared to males, with a ratio of nearly 4:1 [74–76]. However, HIV-PAH is more prevalent in males, especially in those who are IVDUs [46, 68]. According to the recent systematic review of HIV-PAH case studies by Janda et al, 59% of all the reported HIV-PAH cases were males [65]. In addition, a recent study by Araujo et al that reported PAH in HIV-infected IVDUs, had 11 males of total 18 HIV-PAH patients [72]. One factor may be that males are more likely to partake in risky behavior such as IVDU, thus increasing their chances for HIV infection and subsequently exposure to known risk factors for PAH. Studies show that males less frequently visit health care providers for health concerns and in HIV-infected patients this results in prolonged elevated viral load levels before intervention and episodes of ART interruption in those already receiving treatment. One recent study highlighted this devastating association and found that HIV-infected individuals who were active IVDUs experience higher mortality rates due to poor viral management and ART interruption [77]. Nevertheless, the analysis of the Cardiovascular Diseases in HIV-infected Subjects (HIV-HEART) study by Reinsch et al [39], the multivariate analysis of an HIV-infected cohort in Spain by Quezada et al [13] and the study by Schwarze-Zander et al [73] report greater predominance of HIV-infected females with PAH.
Antiretroviral drugs and HIV-PAH
Practice guidelines recommend that physicians offer ART to all HIV-positive individuals irrespective of their CD4+ cell count and viral load. HAART regimens combine a minimum of three antiretroviral agents. The most frequent HAART agents being used in viral replication management comprise various combinations of nucleoside and nucleotide reverse transcriptase inhibitors (NRTI), non-nucleoside reverse transcriptase inhibitors (NNRTI), protease inhibitors (PI) and integrase strand transfer inhibitors (INSTI). HAART combination efficacy differs from patient-to-patient and the clinician must consider any other patient co-morbidities, such as PAH, in therapeutic management. However, if manageable viral load is not achieved with initial HAART combinations, other antiretroviral agents, including fusion inhibitors, CCR5 antagonist and post-attachment inhibitors, are available.
The role of HAART on PAH development, severity, and outcome in HIV-infected individuals remains controversial. Follow up of HIV-patients diagnosed with PAH from the Swiss HIV cohort reported both ART (only NRTIs) and HAART to be independent predictors for decreased RVSP-RAP (right atrial pressure) pressure gradient [68]. In addition, they found that those who did not receive HAART had a 25 mmHg median increase in RVSP over RAP [68], suggesting beneficial effects of HAART on HIV-PAH progression. A more recent study reported that HIV-positive individuals receiving NNRTIs had reduced occurrence of HIV-PAH than those not receiving NNRTI therapy but after adjustment for sex, duration of HAART, age, chronic HCV and viral load, no association was found between NNRTIs and PAH [13]. This study also found no association between NRTIs, PIs and integrase inhibitors with PAH development [13]. Furthermore, another HIV-infected cohort analysis did not find HAART to decrease or prevent right heart pressures in HIV-PAH patients, but instead reported HAART to accelerate the development of HIV-PAH in two of the patients [7]. Study by Degano et al. [22] reported no significant association between hemodynamic changes, measured by RHC, in HIV-PAH patients receiving HAART, however an improvement in the 6-minute walk distance (6MWD) was observed in these patients [22]. On the contrary, analysis of the HIV-HEART study suggested HAART to be associated with increased onset of HIV-PAH in their cohort [39].
Conflicting reports on the association between HAART and HIV-PAH are complexed by limitations in study design. Many are retrospective, observational studies in which some use RHC, and others TTE, for PAH screening/diagnosis; TTE based studies generally includes asymptomatic HIV-PAH patients. Furthermore, HIV has a common mode of transmission with other viruses, such as hepatitis B virus (HBV) and hepatitis C virus (HCV) [78], which may present further challenges in comparison between HIV-PAH cohorts and can increase complexity of HAART combinations efficacy, therapeutic management of PAH and potential unfavorable drug-drug interactions. In addition, Quezada et al. [13] reported co-infection of HIV and chronic HCV to be associated with HIV-PAH on multivariate analysis. As highlighted, multiple limitations and complex patient management hinder our current understanding and further studies are needed to see the direct effects of HAART on HIV-PAH. However, ethical concerns limit conduction of a placebo-controlled, long-term, randomized trial to analyze the potential effect of HAART on PAH development, severity and outcomes in the HIV-infected population [79].
Overall, HIV-infected individuals who abuse illicit drugs are at increased risk for developing HIV-PAH and detailed understanding of the underlying complex cellular mechanisms is pivotal. Steady progress has been made regarding identification of the unique role of HIV infection in the multi-factorial changes associated with pulmonary arteriopathy and exacerbation of these changes in the setting of drugs of abuse as detailed in the next section [27–32, 34].
Pathogenesis of HIV-PAH and IVDU
PAH develops as a consequence of chronic occlusion of pulmonary arteries and arterioles [80, 81] which results from increased vasoconstriction, vessel remodeling, and ultimately irreversible narrowing of the vessel lumen [82]. Accumulation of perivascular inflammatory cells [83], specifically macrophages, dendritic cells, B- and T-lymphocytes, and mast cells, are commonly observed in the lung tissue specimens of patients with PAH. Furthermore, accumulation of these cells, as well as hyper-proliferative pulmonary endothelial cells (EC), smooth muscle cell (SMC) hypertrophy, and concentric intimal fibrosis/thickening result in vascular lesion formation, and pre-capillary vasculature occlusion. This vasculature occlusion leads to an increase in PVR and right ventricular systolic pressure (RVSP), culminating in RHF.
Dating back to the first AIDS patient, RVH and intimal fibrosis of the pulmonary endothelium were described in HIV-infected patients [84]. Investigators subsequently made great efforts to identify HIV-1 particles, HIV-1 RNA or DNA in the pulmonary vessels of human lung tissues but were not successful [14, 85, 86] therefore ruling out the possibility of productive virus replication in vascular cells [82, 87–89]. Studies in macaques infected with simian immunodeficiency virus (SIV) or chimeric simian-human immunodeficiency virus (SHIV) have also suggested the presence of pulmonary arteriopathy similar to that seen in HIV-infected individuals with HIV-PAH [90–92] but without any evidence of the virus or viral DNA in pulmonary ECs or SMCs [90, 91, 93, 94]. Subsequently, this has led to investigation of the role of HIV-associated viral proteins, rather than the nascent virus, in HIV-PAH pathogenesis. HIV-1 primarily infects macrophages and T cells in the lungs, thus, providing a potential source of localized HIV-viral proteins, such as trans-activator of transcription (Tat), negative regulatory factor (Nef), and glycoprotein 120 (gp120). Chronic exposure to these viral proteins can alter the normal homeostatic mechanism and subsequently propagate proliferative/apoptotic signaling cascades within SMCs and ECs associated with vascular dysfunction.
Lund et al (2011) reported for the first time that HIV-transgenic (Tg) rats containing a gag-pol deleted NL4–3 provirus present with right ventricle (RV) wall thickening, thickened pulmonary arteries (PA), and increased RVSP [8]. These HIV-Tg rats express HIV-viral proteins in circulating monocytes and lymphocytes [95]; therefore, providing a non-infectious model to study HIV-PAH. Later using this model, we suggested the association of enhanced vascular oxidative stress and expression of hypoxia-inducible factor (HIF)-1α and platelet derived growth factor (PDGF)-BB expression with the significant medial thickening of pulmonary vessels and increased RV mass [33]. Porter et al reported elevated RVSP, RVH, increased vascular thickening and up-regulation in lung HIF-1α expression in HIV-Tg rats when exposed to a second hit of hypoxia [96].
Drugs of abuse like morphine/opioids, cocaine and methamphetamine have been described as highly potential second-hit to viral proteins in triggering vascular injury and development of HIV-PAH [97, 98]. Nevertheless, isolated use of stimulants is also known to damage the parenchyma or pulmonary vasculature [26] and a subset of drug-abusing individuals end up developing PAH. In the United States, cocaine is one of the most popular drugs of abuse [99] and is known for its vasoconstrictive properties [100]. The incidence of PAH in former and current cocaine users has been reported in numerous studies [101–105]. Methamphetamine abuse has also been associated as one of the likely risk factors for PAH [106, 107]. The recent study by Zamanian et al demonstrates methamphetamine abuse as one of the definite risk factors in the development of PAH [108], although chronic exposure of rats to methamphetamine did not result in an increase in pulmonary arterial pressures (PAP) or an increase in the Fulton index [109].
More recently, we reported significant augmentation of pulmonary vascular remodeling supported by elevated mPAP and RVSP in HIV-Tg rats exposed to cocaine when compared with HIV-Tg rats or wild-type rats not treated with cocaine [28]. Furthermore, we also published findings utilizing a SIVmacR71/17-infected macaque model investigating the potential effect of viral proteins and opioids in the PAH pathogenesis [32]. SIV-infected macaques exposed chronically to morphine (SIV+M) demonstrated an accentuated pulmonary vascular arteriopathy including the presence of advanced stage intimal and complex plexiform lesions due to enhanced proliferation of ECs as observed in human patients with severe angio-proliferative PAH. Like most of the human studies, no correlation between the CD4/CD8 cell count and the plasma viral load were observed with the severity of pulmonary vascular remodeling in these macaques.
Endothelial Cell Dysfunction and Remodeling
The pathobiology of PAH is highly complex and involves multiple cellular pathways. Animal models demonstrate early endothelial injury and dysfunction to be the initial cellular event in PAH pathogenesis, subsequently leading to abnormal SMC proliferation/migration [80]. The interest of investigators has also been focused on pulmonary EC proliferation which is responsible for the formation of angio-proliferative plexiform lesions, the histological hallmark of severe PAH [110–112].
Multiple studies, including our own, have demonstrated the pulmonary vascular endothelium to be a major target for HIV-viral proteins such as Nef, gp120 and Tat, initiating endothelial injury and early apoptosis [32, 34, 91, 113–118]. Exposure of pulmonary ECs to HIV-Tat and gp120 results in an increased expression of reactive oxygen species (ROS), HIF-1α and PDGF-BB [33], and several studies from our lab illustrate the additive effect of HIV-proteins and illicit drugs in regards to endothelial injury, likely explaining the increased prevalence of HIV-PAH in infected individuals who are illicit drug users [32–34].
We reported enhanced pulmonary arteriopathy in lungs of HIV-infected and IVDUs who were mainly cocaine and/or opioid abusers when compared with lung sections from HIV-infected non-drug users or un-infected IVDUs, therefore suggesting the combined effects of HIV-1 and illicit drugs in potentiating pulmonary vascular remodeling. This correlated with enhanced endothelial injury as suggested by the down regulated expression of endothelial tight junction proteins (TJPs) in HIV and IVDU lung tissues [34]. Additionally, in vitro findings suggest the involvement of oxidative stress mediated activation of the Ras/Raf/Erk1/2 pathway in the augmented disassembly of TJP-1 and endothelial dysfunction in HIV-Tat and cocaine treated human pulmonary microvascular endothelial cells (HPMEC) [30].
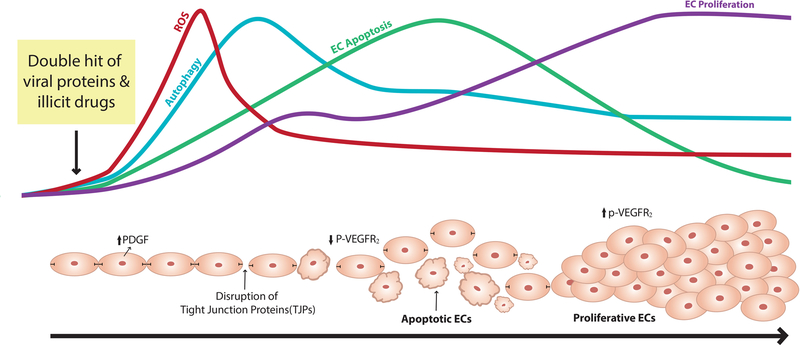
One pathobiological concept is that the trigger of initial endothelial apoptosis is followed by proliferation of apoptosis-resistant ECs that results in the formation of complex plexiform and obliterated angio-proliferative lesions linked to severe form of PAH [119, 120]. While both, HIV-protein Tat and opioids such as morphine are known to promote pro-apoptotic as well as pro-survival signals in ECs [121–123], we demonstrated a significant potentiation of HIV-protein-mediated initial apoptosis and subsequent proliferation of pulmonary ECs after treatment with HIV-proteins: Tat, Nef and gp120 in the presence of morphine compared to mono-treatments [32]. Not only morphine but exposure of HPMECs to cocaine or methamphetamine also potentiated HIV-viral protein Tat (HIV-Tat) mediated early apoptosis followed by proliferation of cells [32] (Figure 1). Similar results were observed in the lung sections from SIV-infected macaques exposed chronically to morphine [32]: there were increased numbers of apoptotic and proliferative ECs in the distal and proximal pulmonary arteries with medial hypertrophy and neointimal lesions; and presence of proliferative ECs within fully occluded vessels [32].
Figure 1:
Outline of the suggested mechanisms involved in the HIV-proteins and drugs of abuse mediated endothelial dysfunction.
This switch of ECs from an early apoptotic phase to the subsequent proliferative phase has been speculated to be due to enhanced autophagy in response to chronic stress of HIV infection and illicit drug use [27]. We reported a significant increase in the expression of autophagy markers as well as an increase in the number of autophagosomes/autolysosomes on simultaneous exposure of viral proteins and morphine/opioids in cultured HPMECs and in the ECs lining the remodeled vessels or within angio-proliferative advanced lesions of SIV-infected macaques and HIV-infected individuals. Using an autophagy gene knockdown/over expression approach as well as autophagy inhibitors/stimulators our findings demonstrate autophagy mediated enhanced proliferation of pulmonary ECs on exposure to Tat and morphine. We suggest that although the synergistic increase in ROS generation during early treatments of viral proteins and opioids induce simultaneous increase in autophagy and apoptosis, continuous activation of controlled autophagy in response to chronic stress of viral proteins and illicit drugs prevent further accumulation of cytotoxic levels of ROS by removal of damaged organelles therefore promoting pro-survival conditions (Figure 1).
Smooth Muscle Cell Dysfunction and Remodeling
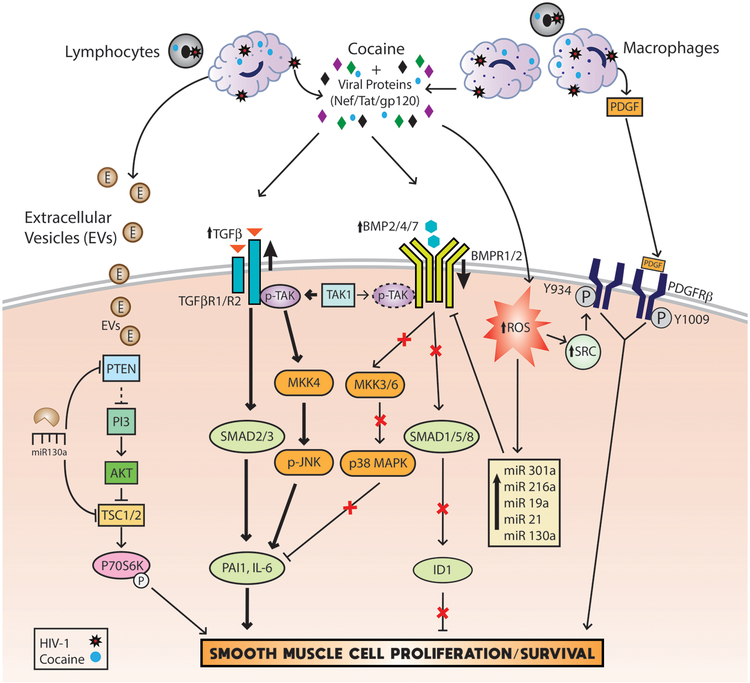
Pulmonary SMCs are the primary cell-type that undergo hyperplasia in PAH development and they are one of the two most studied cell types in the attempt to delineate the mechanism(s) responsible for PAH. In relation to HIV-PAH and IVDU, our group has extensively reported molecular malfunctions in SMCs leading to increased proliferation of pulmonary arterial smooth muscle cells (PASMCs) [28, 29, 31, 34] (Figure 2). The salient finding in all our reports is the synergistic or additive enhancement of damage, either seen in the form of molecular or phenotypic changes in HIV-protein(s) and cocaine exposed PASMCs. In the first related finding, we reported that HIV-Tat and cocaine additively increase human pulmonary arterial smooth muscle cell (HPASMC) proliferation compared to either HIV-Tat or cocaine alone [34]. Later, we also reported an additive increase in the proliferation of HPASMCs after simultaneous treatment with HIV-Tat and morphine compared to mono-treatments [32].
Figure 2:
Schematic representation of signaling mechanisms that mediate the pulmonary smooth muscle proliferation in response to dual hit of HIV-infection/viral proteins and cocaine.
Humbert et al [124] found an increase in platelet derived growth factor (PDGF)-AA expression in HIV-PAH patients but not in HIV patients without PAH, strengthening the role of this growth factor in the PAH pathogenesis. Likewise, we reported a significant increase in the expression of PDGF-BB within and around the remodeled vessels of HIV-infected IVDU lungs. However, although we observed an increase in the levels of PDGF-BB in Tat treated HPMECs and HIV-infected monocyte derived macrophages after exposure to cocaine we observed no further increase in the levels of PDGF-BB in Tat treated HPASMCs on exposure to the second hit of cocaine [34]. However, knockdown of PDGF receptor β (PDGFRβ) significantly reduced HIV-Tat and cocaine mediated HPASMC proliferation, thus suggesting its important role in HIV-PAH pathogenesis [34]. More importantly we discovered ROS triggered Src mediated ligand independent activation of PDGFRβ in Tat and cocaine treated HPASMCs [29] underscoring the importance of alternate pathways as therapeutic targets in addition to inhibition of ligand-dependent PDGFR that has not been promising in the clinical setting so far [29].
The importance of both proliferative and anti-proliferative arms of Transforming Growth Factor-β (TGF-β) superfamily signaling in SMC hyperplasia has been recognized [125–127]. Not only mutations [128, 129] but the reduced expression of anti-proliferative bone morphogenetic protein receptor (BMPR)-2 also predispose vascular SMCs to hyper-proliferation [31]. Likewise exposure of SMCs to HIV-proteins: Tat, Nef or gp-120 in the presence of cocaine results in an additive reduction in the protein levels of bone morphogenetic protein receptor (BMPR)-2, −1A and −1B leading to SMC hyperplasia [31]. Interestingly, this was accompanied by the significant increase in the mRNA expression of BMPRs. These findings highlight the importance of ubiquitination dependent degradation of BMPRs [130] or post-transcriptional regulation of BMPR expression by miRNAs [131]. Significant increase in the levels of miRNAs known to target BMPR-2 expression such as miR-19a, −20a, −21, −130a and miR301 has been observed after combined treatment of HPASMCs with HIV-Tat and cocaine when compared with mono-treatments [131]. Furthermore, a novel miR-216a, that was significantly up-regulated after HIV-Tat and cocaine treatment, was found to directly bind to the 3’UTR of BMPR-2 and inhibit translation without the degradation of BMPR-2 mRNA [131].
Concomitant with this impaired BMPR signaling, exacerbated activation of the proliferative TGFβR axis has been observed in HPASMCs on dual treatment with HIV-Tat and cocaine. In fact, both SMAD2/3 dependent as well as TGFβ-activated kinase 1 (TAK1) mediated, SMAD-independent downstream signaling cascades were noted to be activated and to contribute to the hyper-proliferation of HPASMCs on the dual hit of Tat and cocaine. The TAK1 that is known to compete between BMP and TGF-β signaling was observed to complex more with TGFβR-2 due to the loss of BMPR levels in response to cocaine and HIV-Tat treatment leading to the activation of the pro-proliferative TAK-mitogen activated protein kinase kinase 4 (MKK4)-c Jun N-terminal kinase (JNK) axis [132]. Apart from crosstalk between BMPR and TGFβR signaling, PDGF has been reported to inhibit the expression of SMAD proteins in vascular SMCs [133]. Alternatively, PDGF signaling is enhanced in response to decrease in the activity of BMPR axis in SMCs [134], therefore suggesting the importance of interplay between these signaling pathways (Figure 2).
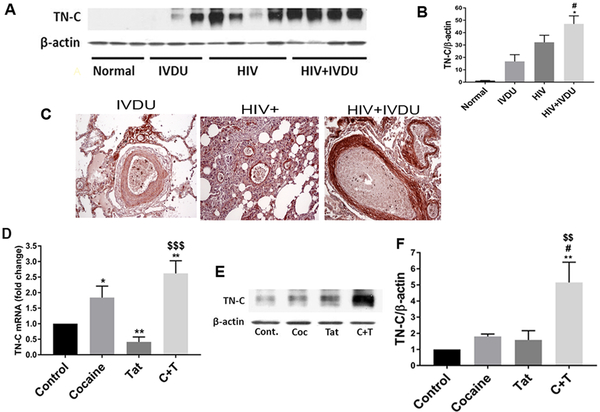
Tenascin-C (TN-C), an important component of the altered extracellular matrix is known to play a pathological role in PAH [135, 136]. TN-C has been observed to be highly up-regulated in the remodeled hypertensive pulmonary arteries [135, 137] and is implicated in potentiation of proliferation and migration of vascular SMCs [138]. Likewise, we also found increased expression of TN-C in lungs from HIV-infected IVDUs compared to HIV-infected non-drug abusers or un-infected IVDUs (Figure 3 A & B). As shown in figure 3C, immunostaining for TN-C resulted in positive staining throughout the lung parenchyma in all the groups however, this staining pattern was more pronounced in the SMC layer of large and small vessels with medial hypertrophy and in the neointima lesions of individuals from HIV+IVDU group; while the SMCs within the vascular lesions from the IVDU group showed identical or less expression of TN-C as in the lung parenchyma. In addition, treatment of HPASMCs with both cocaine and Tat resulted in an increased expression of TN-C (Figure 3 D–F). Given that TN-C is known to promote proliferation of SMCs [139] via modulating the activity of tyrosine kinase receptors[137, 140] including PDGFR [141] and is highly induced specifically in cultured SMCs from PAH patients with BMPR2 mutations [142], underscores the importance of an interplay between BMPR anti-proliferative and PDGFR proliferative signaling in illicit drugs and viral proteins mediated hyper-proliferation of pulmonary SMCs.
Figure 3:
Significant increase in the expression of TN-C in the human pulmonary arterial smooth muscle cells on exposure to HIV-protein(s) and cocaine. Western blot (A) and densitometry analysis (B) of total lung extracts; and immunohistochemical staining of lung sections (C) from HIV-infected and /or IVDUs; TN-C mRNA (D) and protein expression (E-F) in the human primary PASMCs on treatment with cocaine and/or HIV-Tat.
Inflammation and Remodeling
Inflammation is not only gaining wide attention for its significance in the development of PAH but also as a therapeutic target. Humbert et al [143] and recently Groth A et al [144] reviewed the role of inflammation in PAH. While Brock M et al and Parikh et al reported higher serum interleukin (IL)-6 levels [145, 146], Damas et al [147] reported higher levels of IL-8 and MCP-1 in PAH patients [147]. These reports are consistent with our findings regarding a trend in up-regulation of MCP-1 and IL-8 in the plasma of SIV-infected morphine treated macaques demonstrating severe pulmonary arteriopathy [32]. Multiple investigators have reported interstitial infiltration of immune cells within the intima and medial thickening of blood vessels as well as within plexiform lesions in PAH patients [148, 149]. Similarly, significant perivascular inflammation has been observed around the severely remodeled vessels and fully obliterated vessels in SIV-infected macaques exposed to chronic morphine [32] and in the remodeled thickened vessels of HIV-infected opioid and/or cocaine users [34]. Furthermore, the Sprague Dawley (SD) strain of HIV-Tg rats demonstrated significant medial hypertrophy and RVH without any second hit [33] whereas Fischer HIV-Tg rats demonstrated significant changes in hemodynamics and pulmonary vascular remodeling only after exposure to the second hit of cocaine [28]. These differences between strains corresponded to the differences in viral protein positive perivascular inflammatory cells around remodeled vessels with more inflammation observed in SD rats. Additionally, exaggerated proliferation of pulmonary SMCs after exposure to extracellular vesicles (EVs) secreted by HIV-infected and cocaine treated macrophages [150, 151] suggests the role of EVs as important communicators between infected inflammatory cells and not infectable pulmonary vascular cells. These EVs were found to carry higher levels of miR130a in their cargo that when delivered to the recipient SMCs were found to augment the proliferation by inhibiting PTEN expression, consequently stimulating the PI3K/AKT proliferative signaling pathway (Figure 2) [151]. Interestingly, the miR130/301 family has consistently been implicated in the pathogenesis of PAH [152–154].
Therapeutic strategies for HIV-PAH
To date, few PAH therapeutic clinical trials composed of only or predominately HIV-PAH patients exist [155]. Because of this, no specific treatment recommendations for HIV-PAH have been outlined and current PAH management guidelines advise clinicians to follow IPAH therapeutic algorithms, in combination with HAART, for treatment of HIV-PAH [156]. In addition, HIV-PAH patient care should be managed by a clinician with experience caring for this population and must consider significant drug-drug interactions that exist between antiretroviral agents and PAH therapeutics [155]. Currently, four major drug classes are approved for IPAH treatment: prostanoids, endothelin receptor antagonist (ERA) phosphodiesterase type-5 (PDE5) inhibitors and the most recently available, soluble guanylate cyclase (sGC) stimulators that act as vasodilators, reduce pulmonary vascular SMC proliferation and exhibit antithrombotic and immune function modulation effects [157, 158]. These drug regimens can be effectively tried for HIV-PAH as vasoactive mediators like endothelin-1 (ET-1) [159], prostacyclin (PGI2) and nitric oxide (NO) are highly expressed in this condition as well. Studies evaluating specific therapy with prostanoids drugs, epoprostenol (intravenous), treprostinil (subcutaneous) [160], iloprost (inhaled) [67], and beraprost sodium (oral) [161] show potential improvement in 6MWD, hemodynamic pressures, and overall PAH symptoms in HIV-PAH patients. Although the ALPHABET study [161] showed increased 6MWD in PAH patients taking beraprost, follow-up after long-term use did not show preservation of the 6MWD improvement [162]. Furthermore, HIV-PAH patients co-infected with HBV, HCV and HBV/HCV did not demonstrate improvement in NYHA functional classifications on treatment with beraprost [163]. In addition, epoprostenol requires central venous catheter placement for drug administration and long-term use for HIV-PAH presents concern for infection in an already immunocompromised population [164]. Other than these, there are other classes of drugs that are being tried for PAH such as calcium channel blockers (CCBs) or serotonin receptor inhibitors that inhibit vasoconstriction and smooth cell proliferation [158]. A study by Montani et al. [165] reported that only 1.6% of HIV-PAH individuals in their study responded to acute vasodilation testing, a prerequisite for CCB treatment response in PAH [165]. Unlike in patients with severe IPAH, lung transplantation is not advised for HIV-PAH treatment, and guidelines recommend against anti-coagulation therapy in those with increased bleeding risk and potential drug interactions, as well as track record of poor follow-up [166].
In order to develop better and effective therapies for HIV-PAH, it is important to better understand and target novel molecular mechanisms behind EC dysfunction, SMC proliferation, inflammation and role of immune cells in the pathogenesis of PAH [167]. The clinical trials and efforts to develop new therapeutics against PAH are going on that target proliferative signaling mechanisms, growth factors, cytokines, interleukins that promote vascular remodeling as well as fibroblast growth, extracellular matrix remodeling and degradation [157, 167, 168]. These therapies if successful may also be helpful in managing HIV-PAH as mechanisms such as high PDGF expression [34], BMPR2/SMAD/TGF-β modulation [28, 31, 132], ECM remodeling and inflammation [32] have been reported in HIV-PAH including in response to drugs of abuse. Furthermore, recent studies have elucidated the protective role of endothelial progenitor cells (EPCs) and mesenchymal stem cells (MSCs) [169–172] as well as extracellular vesicles /exosomes [173, 174] derived from MSCs in lung inflammation, vascular remodeling and PAH [170, 172, 175, 176]. Modulation of small non-coding miRNAs during the development of PAH including HIV-PAH further creates an opportunity to implement miR-based therapies [151, 167, 168, 174, 177]. Other than these, illicit drugs like cocaine and methamphetamine are known to bind α-adrenergic [178, 179] and sigma receptors [180, 181] to stimulate release of vasoconstrictors and mitogens such as PDGF, VEGF, ET-1 and ILs [182, 183]. Developing therapeutic targets against these receptors [180, 181, 184] can be another promising approach to manage HIV-PAH in IVDUs. Considering that HIV-PAH is worse in survival than other forms of PAH, much investigation is required to test the efficacy and therapeutic potential not only against known but also new and innovative targets involved in the development of HIV-PAH for appropriate management of this complex multi-factorial disease.
Conclusions
In conclusion, the evidences in support of a potentiation of a pulmonary arteriopathy and PAH in HIV-infected individuals in the presence of a ‘second hit’ of illicit drugs are abundant. It is clearly established that viral proteins and drugs of abuse mediate hyper-proliferation of ECs and SMCs along with inflammation, therefore contributing to the excessive pulmonary vascular remodeling. Given that the mortality is excessive in HIV-PAH characterized by minimal improvement in the hemodynamics in response to currently available therapies when compared to patients with other forms of PAH [12, 14, 185], the in-depth understanding of the mechanisms involved and identification of new specific targets that can be affected with minimal side effects are critical. The role of antiretroviral drugs in pulmonary vascular diseases remains controversial including reports indicating the development of PAH with the use of HAART [2]. Future studies are surely needed. In addition, multiple reports regarding HIV-related COPD have been published [10, 81–83] and suggest PH as a potential complication of COPD in HIV-infected individuals [13, 40] contributing significantly to the mortality associated with the disease [186–191]. In light of these findings and recent report highlighting the association of a mild increase in mPAP (between 17–26mmHg) with poor survival [192] and opioid epidemic on the rise, careful investigation of the potential link between the pulmonary vascular dysfunction and HIV-associated comorbidities in the context of substance abuse is warranted.
Acknowledgements
We acknowledge Bailey Allerd for her help in running western blots.
Sources of funding
This work was supported by NIH grants: R03DA031589, R01DA034542, R01DA042715 and R01HL129875; and American Heart Association’s Scientist Development grant: 11SDG7500016 awarded to N.K.D and by KIDDRC (NIH U54 HD 090216).
Footnotes
Disclosures
None
References
- 1.Brown J, Roy A, Harris R, Filson S, Johnson M, Abubakar I, et al. Respiratory symptoms in people living with HIV and the effect of antiretroviral therapy: a systematic review and meta-analysis. Thorax 2017,72:355–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George MP, Singh V, Gladwin MT. Noninfectious and Nonneoplastic Conditions Associated with Human Immunodeficiency Virus Infection. Semin Respir Crit Care Med 2016,37:289–302. [DOI] [PubMed] [Google Scholar]
- 3.Wing EJ. HIV and aging. Int J Infect Dis 2016,53:61–68. [DOI] [PubMed] [Google Scholar]
- 4.Coplan NL, Shimony RY, Ioachim HL, Wilentz JR, Posner DH, Lipschitz A, et al. Primary pulmonary hypertension associated with human immunodeficiency viral infection. Am J Med 1990,89:96–99. [DOI] [PubMed] [Google Scholar]
- 5.Hebert VY, Crenshaw BL, Romanoff RL, Ekshyyan VP, Dugas TR. Effects of HIV drug combinations on endothelin-1 and vascular cell proliferation. Cardiovasc Toxicol 2004,4:117–131. [DOI] [PubMed] [Google Scholar]
- 6.Pellicelli AM, Barbaro G, Palmieri F, Girardi E, D’Ambrosio C, Rianda A, et al. Primary pulmonary hypertension in HIV patients: a systematic review. Angiology 2001,52:31–41. [DOI] [PubMed] [Google Scholar]
- 7.Pugliese A, Isnardi D, Saini A, Scarabelli T, Raddino R, Torre D. Impact of highly active antiretroviral therapy in HIV-positive patients with cardiac involvement. J Infect 2000,40:282–284. [DOI] [PubMed] [Google Scholar]
- 8.Lund AK, Lucero J, Herbert L, Liu Y, Naik JS. Human immunodeficiency virus transgenic rats exhibit pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 2011,301:L315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuster I, Thoni GJ, Ederhy S, Walther G, Nottin S, Vinet A, et al. Subclinical cardiac abnormalities in human immunodeficiency virus-infected men receiving antiretroviral therapy. Am J Cardiol 2008,101:1213–1217. [DOI] [PubMed] [Google Scholar]
- 10.Triplette M, Crothers K, Attia EF. Non-infectious Pulmonary Diseases and HIV. Curr HIV/AIDS Rep 2016,13:140–148. [DOI] [PubMed] [Google Scholar]
- 11.Simonneau G, Galie N, Rubin LJ, Langleben D, Seeger W, Domenighetti G, et al. Clinical classification of pulmonary hypertension. J Am Coll Cardiol 2004,43:5S–12S. [DOI] [PubMed] [Google Scholar]
- 12.Almodovar S, Cicalini S, Petrosillo N, Flores SC. Pulmonary hypertension associated with HIV infection: pulmonary vascular disease: the global perspective. Chest 2010,137:6S–12S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quezada M, Martin-Carbonero L, Soriano V, Vispo E, Valencia E, Moreno V, et al. Prevalence and risk factors associated with pulmonary hypertension in HIV-infected patients on regular follow-up. AIDS 2012,26:1387–1392. [DOI] [PubMed] [Google Scholar]
- 14.Cicalini S, Almodovar S, Grilli E, Flores S. Pulmonary hypertension and human immunodeficiency virus infection: epidemiology, pathogenesis, and clinical approach. Clin Microbiol Infect 2011,17:25–33. [DOI] [PubMed] [Google Scholar]
- 15.L’Huillier AG, Posfay-Barbe KM, Pictet H, Beghetti M. Pulmonary Arterial Hypertension among HIV-Infected Children: Results of a National Survey and Review of the Literature. Front Pediatr 2015,3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manga P, McCutcheon K, Tsabedze N, Vachiat A, Zachariah D. HIV and Nonischemic Heart Disease. J Am Coll Cardiol 2017,69:83–91. [DOI] [PubMed] [Google Scholar]
- 17.Hsue PY, Deeks SG, Farah HH, Palav S, Ahmed SY, Schnell A, et al. Role of HIV and human herpesvirus-8 infection in pulmonary arterial hypertension. Aids 2008,22:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med 2006,173:1023–1030. [DOI] [PubMed] [Google Scholar]
- 19.Humbert M, Montani D, Perros F, Dorfmuller P, Adnot S, Eddahibi S. Endothelial cell dysfunction and cross talk between endothelium and smooth muscle cells in pulmonary arterial hypertension. Vascul Pharmacol 2008,49:113–118. [DOI] [PubMed] [Google Scholar]
- 20.Opravil M, Pechere M, Speich R, Joller-Jemelka HI, Jenni R, Russi EW, et al. HIV-associated primary pulmonary hypertension. A case control study. Swiss HIV Cohort Study. Am J Respir Crit Care Med 1997,155:990–995. [DOI] [PubMed] [Google Scholar]
- 21.Nunes H, Humbert M, Sitbon O, Morse JH, Deng Z, Knowles JA, et al. Prognostic factors for survival in human immunodeficiency virus-associated pulmonary arterial hypertension. Am J Respir Crit Care Med 2003,167:1433–1439. [DOI] [PubMed] [Google Scholar]
- 22.Degano B, Guillaume M, Savale L, Montani D, Jais X, Yaici A, et al. HIV-associated pulmonary arterial hypertension: survival and prognostic factors in the modern therapeutic era. Aids 2010,24:67–75. [DOI] [PubMed] [Google Scholar]
- 23.Petitpretz P, Brenot F, Azarian R, Parent F, Rain B, Herve P, et al. Pulmonary hypertension in patients with human immunodeficiency virus infection. Comparison with primary pulmonary hypertension. Circulation 1994,89:2722–2727. [DOI] [PubMed] [Google Scholar]
- 24.Graham BB, Kumar R. Schistosomiasis and the pulmonary vasculature (2013 Grover Conference series). Pulm Circ 2014,4:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seferian A, Simonneau G. [Pulmonary hypertension: definition, diagnostic and new classification]. Presse Med 2014,43:935–944. [DOI] [PubMed] [Google Scholar]
- 26.de Jesus Perez V, Kudelko K, Snook S, Zamanian RT. Drugs and toxins-associated pulmonary arterial hypertension: lessons learned and challenges ahead. Int J Clin Pract Suppl 2011:8–10. [DOI] [PubMed] [Google Scholar]
- 27.Dalvi P, Sharma H, Chinnappan M, Sanderson M, Allen J, Zeng R, et al. Enhanced autophagy in pulmonary endothelial cells on exposure to HIV-Tat and morphine: Role in HIV-related pulmonary arterial hypertension. Autophagy 2016:0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dalvi P, Spikes L, Allen J, Gupta VG, Sharma H, Gillcrist M, et al. Effect of Cocaine on Pulmonary Vascular Remodeling and Hemodynamics in Human Immunodeficiency Virus-Transgenic Rats. Am J Respir Cell Mol Biol 2016,55:201–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dalvi PN, Gupta VG, Griffin BR, O’Brien-Ladner A, Dhillon NK. Ligand-Independent Activation of Platelet-Derived Growth Factor Receptor beta during Human Immunodeficiency Virus-Transactivator of Transcription and Cocaine-Mediated Smooth Muscle Hyperplasia. Am J Respir Cell Mol Biol 2015,53:336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dalvi P, Wang K, Mermis J, Zeng R, Sanderson M, Johnson S, et al. HIV-1/cocaine induced oxidative stress disrupts tight junction protein-1 in human pulmonary microvascular endothelial cells: role of Ras/ERK1/2 pathway. PLoS One 2014,9:e85246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dalvi P, O’Brien-Ladner A, Dhillon N. Downregulation of bone morphogenetic protein receptor axis during HIV-1 and cocaine-mediated pulmonary smooth muscle hyperplasia: implications for HIV-related pulmonary arterial hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology 2013,33:2585–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spikes L, Dalvi P, Tawfik O, Gu H, Voelkel NF, Cheney P, et al. Enhanced pulmonary arteriopathy in simian immunodeficiency virus-infected macaques exposed to morphine. Am J Respir Crit Care Med 2012,185:1235–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mermis J, Gu H, Xue B, Li F, Tawfik O, Buch S, et al. Hypoxia-inducible factor-1 alpha/platelet derived growth factor axis in HIV-associated pulmonary vascular remodeling. Respir Res 2011,12:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dhillon NK, Li F, Xue B, Tawfik O, Morgello S, Buch S, et al. Effect of cocaine on human immunodeficiency virus-mediated pulmonary endothelial and smooth muscle dysfunction. Am J Respir Cell Mol Biol 2011,45:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swain SD, Han S, Harmsen A, Shampeny K, Harmsen AG. Pulmonary hypertension can be a sequela of prior Pneumocystis pneumonia. Am J Pathol 2007,171:790–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Swain SD, Siemsen DW, Pullen RR, Han S. CD4+ T cells and IFN-gamma are required for the development of Pneumocystis-associated pulmonary hypertension. Am J Pathol 2014,184:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morse JH, Barst RJ, Itescu S, Flaster ER, Sinha G, Zhang Y, et al. Primary pulmonary hypertension in HIV infection: an outcome determined by particular HLA class II alleles. Am J Respir Crit Care Med 1996,153:1299–1301. [DOI] [PubMed] [Google Scholar]
- 38.Niakara A, Drabo YJ, Kambire Y, Nebie LV, Kabore NJ, Simon F. [Cardiovascular diseases and HIV infection: study of 79 cases at the National Hospital of Ouagadougou (Burkina Faso)]. Bull Soc Pathol Exot 2002,95:23–26. [PubMed] [Google Scholar]
- 39.Reinsch N, Buhr C, Krings P, Kaelsch H, Kahlert P, Konorza T, et al. Effect of gender and highly active antiretroviral therapy on HIV-related pulmonary arterial hypertension: results of the HIV-HEART Study. HIV Med 2008,9:550–556. [DOI] [PubMed] [Google Scholar]
- 40.Morris A, Gingo MR, George MP, Lucht L, Kessinger C, Singh V, et al. Cardiopulmonary function in individuals with HIV infection in the antiretroviral therapy era. AIDS 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isasti G, Perez I, Moreno T, Cabrera F, Palacios R, Santos J. Echocardiographic abnormalities and associated factors in a cohort of asymptomatic HIV-infected patients. AIDS Res Hum Retroviruses 2013,29:20–24. [DOI] [PubMed] [Google Scholar]
- 42.Isasti G, Moreno T, Perez I, Cabrera F, Palacios R, Santos J. High prevalence of pulmonary arterial hypertension in a cohort of asymptomatic HIV-infected patients. AIDS Res Hum Retroviruses 2013,29:231–234. [DOI] [PubMed] [Google Scholar]
- 43.ten Freyhaus H, Vogel D, Lehmann C, Kummerle T, Wyen C, Fatkenheuer G, et al. Echocardiographic screening for pulmonary arterial hypertension in HIV-positive patients. Infection 2014,42:737–741. [DOI] [PubMed] [Google Scholar]
- 44.Rasoulinejad M, Moradmand Badie S, Salehi MR, Seyed Alinaghi SA, Dehghan Manshadi SA, Zakerzadeh N, et al. Echocardiographic assessment of systolic pulmonary arterial pressure in HIV-positive patients. Acta Med Iran 2014,52:827–830. [PubMed] [Google Scholar]
- 45.Chillo P, Bakari M, Lwakatare J. Echocardiographic diagnoses in HIV-infected patients presenting with cardiac symptoms at Muhimbili National Hospital in Dar es Salaam, Tanzania. Cardiovasc J Afr 2012,23:90–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta NJ, Khan IA, Mehta RN, Sepkowitz DA. HIV-Related pulmonary hypertension: analytic review of 131 cases. Chest 2000,118:1133–1141. [DOI] [PubMed] [Google Scholar]
- 47.Pellicelli AM, Palmieri F, D’Ambrosio C, Rianda A, Boumis E, Girardi E, et al. Role of human immunodeficiency virus in primary pulmonary hypertension--case reports. Angiology 1998,49:1005–1011. [DOI] [PubMed] [Google Scholar]
- 48.Mesa RA, Edell ES, Dunn WF, Edwards WD. Human immunodeficiency virus infection and pulmonary hypertension: two new cases and a review of 86 reported cases. Mayo Clin Proc 1998,73:37–45. [DOI] [PubMed] [Google Scholar]
- 49.Petureau F, Escamilla R, Hermant C, Mourlanette P, Berjaud J, Krempf M. [Pulmonary artery hypertension in HIV seropositive drug addicts. Apropos of 10 cases]. Rev Mal Respir 1998,15:97–102. [PubMed] [Google Scholar]
- 50.Himelman RB, Dohrmann M, Goodman P, Schiller NB, Starksen NF, Warnock M, et al. Severe pulmonary hypertension and cor pulmonale in the acquired immunodeficiency syndrome. Am J Cardiol 1989,64:1396–1399. [DOI] [PubMed] [Google Scholar]
- 51.Rouveix E, Job C, Delorme G, Jouin H, Franc B, Saveuse H, et al. [Lethal pulmonary arterial hypertension in a heroin and amphetamine addict]. Ann Med Interne (Paris) 1989,140:153. [PubMed] [Google Scholar]
- 52.Legoux B, Piette AM, Bouchet PF, Landau JF, Gepner P, Chapman AM. Pulmonary hypertension and HIV infection. Am J Med 1990,89:122. [DOI] [PubMed] [Google Scholar]
- 53.Saadjian A, Gueunoun M, Philip-Joet F, Magnan A, Ebagosti A, Garbe L, et al. [Pulmonary hypertension secondary to talc microemboli in a HIV seropositive heroin-addict woman]. Arch Mal Coeur Vaiss 1991,84:1369–1373. [PubMed] [Google Scholar]
- 54.Polos PG, Wolfe D, Harley RA, Strange C, Sahn SA. Pulmonary hypertension and human immunodeficiency virus infection. Two reports and a review of the literature. Chest 1992,101:474–478. [DOI] [PubMed] [Google Scholar]
- 55.Piette AM, Legoux B, Gepner P, Chapman A. [“Primary” pulmonary arterial hypertension associated with HIV infection. Two cases]. Presse Med 1992,21:616–618. [PubMed] [Google Scholar]
- 56.Reiser P, Opravil M, Pfaltz M, Speich R, Schneider J. [Primary pulmonary hypertension and mesangioproliferative glomerulonephritis in HIV infection]. Dtsch Med Wochenschr 1992,117:815–818. [DOI] [PubMed] [Google Scholar]
- 57.Arunabh, Edasery B. Human immunodeficiency virus and primary pulmonary hypertension. West J Med 1993,159:708–709. [PMC free article] [PubMed] [Google Scholar]
- 58.Martos A, Carratala J, Cabellos C, Rodriguez P. AIDS and primary pulmonary hypertension. Am Heart J 1993,125:1819. [DOI] [PubMed] [Google Scholar]
- 59.Sala-Blanch X, Fabregas N, Martinez JM, Plaza A. [Primary pulmonary hypertension in an HIV-positive pregnant woman]. Med Clin (Barc) 1994,102:117–118. [PubMed] [Google Scholar]
- 60.Escamilla R, Hermant C, Berjaud J, Mazerolles C, Daussy X. Pulmonary veno-occlusive disease in a HIV-infected intravenous drug abuser. Eur Respir J 1995,8:1982–1984. [DOI] [PubMed] [Google Scholar]
- 61.Speciale A, Ramundi M, Fabiani F, Fiorucci F, Schmid G. Primary pulmonary hypertension in a HIV+ patient. Monaldi Arch Chest Dis 1995,50:451–452. [PubMed] [Google Scholar]
- 62.Muthiah M, Mishriki YY. Respiratory distress in a former drug abuser. Hosp Pract (1995) 1997,32:141–144. [DOI] [PubMed] [Google Scholar]
- 63.Fiorencis R, Zonzin P, Carraro M, Zampieri P, Roncon L, Baracca E, et al. Pulmonary hypertension associated with human immunodeficiency virus infection. Report of two cases and review of the literature. G Ital Cardiol 1998,28:1404–1408. [PubMed] [Google Scholar]
- 64.Weiss JR, Pietra GG, Scharf SM. Primary pulmonary hypertension and the human immunodeficiency virus. Report of two cases and a review of the literature. Arch Intern Med 1995,155:2350–2354. [PubMed] [Google Scholar]
- 65.Janda S, Quon BS, Swiston J. HIV and pulmonary arterial hypertension: a systematic review. HIV Med 2010,11:620–634. [DOI] [PubMed] [Google Scholar]
- 66.Speich R, Jenni R, Opravil M, Pfab M, Russi EW. Primary pulmonary hypertension in HIV infection. Chest 1991,100:1268–1271. [DOI] [PubMed] [Google Scholar]
- 67.Ghofrani HA, Friese G, Discher T, Olschewski H, Schermuly RT, Weissmann N, et al. Inhaled iloprost is a potent acute pulmonary vasodilator in HIV-related severe pulmonary hypertension. Eur Respir J 2004,23:321–326. [DOI] [PubMed] [Google Scholar]
- 68.Zuber J-P, Calmy A, Evison JM, Hasse B, Schiffer V, Wagels T, et al. Pulmonary arterial hypertension related to HIV infection: improved hemodynamics and survival associated with antiretroviral therapy. Clinical infectious diseases 2004,38:1178–1185. [DOI] [PubMed] [Google Scholar]
- 69.Aguilar RV, Farber HW. Epoprostenol (prostacyclin) therapy in HIV-associated pulmonary hypertension. Am J Respir Crit Care Med 2000,162:1846–1850. [DOI] [PubMed] [Google Scholar]
- 70.Sitbon O HIV-related pulmonary arterial hypertension: clinical presentation and management. Aids 2008,22 Suppl 3:S55–62. [DOI] [PubMed] [Google Scholar]
- 71.Mondy KE, Gottdiener J, Overton ET, Henry K, Bush T, Conley L, et al. High Prevalence of Echocardiographic Abnormalities among HIV-infected Persons in the Era of Highly Active Antiretroviral Therapy. Clin Infect Dis 2011,52:378–386. [DOI] [PubMed] [Google Scholar]
- 72.Araujo I, Enjuanes-Grau C, Lopez-Guarch CJ, Narankiewicz D, Ruiz-Cano MJ, Velazquez-Martin T, et al. Pulmonary arterial hypertension related to human immunodeficiency virus infection: A case series. World J Cardiol 2014,6:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwarze-Zander C, Pabst S, Hammerstingl C, Ohlig J, Wasmuth JC, Boesecke C, et al. Pulmonary hypertension in HIV infection: a prospective echocardiographic study. HIV Med 2015,16:578–582. [DOI] [PubMed] [Google Scholar]
- 74.Walker AM, Langleben D, Korelitz JJ, Rich S, Rubin LJ, Strom BL, et al. Temporal trends and drug exposures in pulmonary hypertension: an American experience. Am Heart J 2006,152:521–526. [DOI] [PubMed] [Google Scholar]
- 75.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010,137:376–387. [DOI] [PubMed] [Google Scholar]
- 76.Mair KM, Johansen AK, Wright AF, Wallace E, MacLean MR. Pulmonary arterial hypertension: basis of sex differences in incidence and treatment response. Br J Pharmacol 2014,171:567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhao Y, Zhang M, Shi CX, Huang J, Zhang F, Rou K, et al. Mortality and virological failure among HIV-infected people who inject drugs on antiretroviral treatment in China: An observational cohort study. Drug Alcohol Depend 2017,170:189–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou YH, Liu FL, Yao ZH, Duo L, Li H, Sun Y, et al. Comparison of HIV-, HBV-, HCV- and co-infection prevalence between Chinese and Burmese intravenous drug users of the China-Myanmar border region. PLoS One 2011,6:e16349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sterne JA, Hernan MA, Ledergerber B, Tilling K, Weber R, Sendi P, et al. Long-term effectiveness of potent antiretroviral therapy in preventing AIDS and death: a prospective cohort study. Lancet 2005,366:378–384. [DOI] [PubMed] [Google Scholar]
- 80.de Raaf MA, Schalij I, Gomez-Arroyo J, Rol N, Happe C, de Man FS, et al. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J 2014,44:160–168. [DOI] [PubMed] [Google Scholar]
- 81.Pietra GG, Capron F, Stewart S, Leone O, Humbert M, Robbins IM, et al. Pathologic assessment of vasculopathies in pulmonary hypertension. J Am Coll Cardiol 2004,43:25S–32S. [DOI] [PubMed] [Google Scholar]
- 82.Mette SA, Palevsky HI, Pietra GG, Williams TM, Bruder E, Prestipino AJ, et al. Primary pulmonary hypertension in association with human immunodeficiency virus infection. A possible viral etiology for some forms of hypertensive pulmonary arteriopathy. Am Rev Respir Dis 1992,145:1196–1200. [DOI] [PubMed] [Google Scholar]
- 83.Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011,8:443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Butrous G Human immunodeficiency virus-associated pulmonary arterial hypertension: considerations for pulmonary vascular diseases in the developing world. Circulation 2015,131:1361–1370. [DOI] [PubMed] [Google Scholar]
- 85.George MP, Champion HC, Simon M, Guyach S, Tarantelli R, Kling HM, et al. Physiologic changes in a nonhuman primate model of HIV-associated pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2013,48:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Du L, Sullivan CC, Chu D, Cho AJ, Kido M, Wolf PL, et al. Signaling molecules in nonfamilial pulmonary hypertension. N Engl J Med 2003,348:500–509. [DOI] [PubMed] [Google Scholar]
- 87.Pellicelli AM, D’Ambrosio C, Vizza CD, Borgia MC, Tanzi P, Pino P, et al. HIV-related pulmonary hypertension. From pathogenesis to clinical aspects. Acta Cardiol 2004,59:323–330. [DOI] [PubMed] [Google Scholar]
- 88.Klings ES, Farber HW. The pathogenesis of HIV-associated pulmonary hypertension. Adv Cardiol 2003,40:71–82. [DOI] [PubMed] [Google Scholar]
- 89.Kanmogne GD, Kennedy RC, Grammas P. Analysis of human lung endothelial cells for susceptibility to HIV type 1 infection, coreceptor expression, and cytotoxicity of gp120 protein. AIDS Res Hum Retroviruses 2001,17:45–53. [DOI] [PubMed] [Google Scholar]
- 90.Chalifoux LV, Simon MA, Pauley DR, MacKey JJ, Wyand MS, Ringler DJ. Arteriopathy in macaques infected with simian immunodeficiency virus. Lab Invest 1992,67:338–349. [PubMed] [Google Scholar]
- 91.Marecki JC, Cool CD, Parr JE, Beckey VE, Luciw PA, Tarantal AF, et al. HIV-1 Nef is associated with complex pulmonary vascular lesions in SHIV-nef-infected macaques. Am J Respir Crit Care Med 2006,174:437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.George MP, Brower A, Kling H, Shipley T, Kristoff J, Reinhart TA, et al. Pulmonary vascular lesions are common in SIV- and SHIV-env-infected macaques. AIDS Res Hum Retroviruses 2010,27:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Marecki J, Cool C, Voelkel N, Luciw P, Flores S. Evidence for vascular remodeling in the lungs of macaques infected with simian immunodeficiency virus/HIV NEF recombinant virus. Chest 2005,128:621S–622S. [DOI] [PubMed] [Google Scholar]
- 94.Weesner KM, Kaplan K. Hemodynamic and echocardiographic evaluation of the stumptailed macaque: a potential nonhuman primate model for pulmonary vascular disease. J Med Primatol 1987,16:185–202. [PubMed] [Google Scholar]
- 95.Reid W, Sadowska M, Denaro F, Rao S, Foulke J Jr., Hayes N, et al. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. Proc Natl Acad Sci U S A 2001,98:9271–9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porter KM, Walp ER, Elms SC, Raynor R, Mitchell PO, Guidot DM, et al. Human immunodeficiency virus-1 transgene expression increases pulmonary vascular resistance and exacerbates hypoxia-induced pulmonary hypertension development. Pulm Circ 2013,3:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.George MP, Champion HC, Gladwin MT, Norris KA, Morris A. Injection drug use as a “second hit” in the pathogenesis of HIV-associated pulmonary hypertension. Am J Respir Crit Care Med 2012,185:1144–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chin KM, Channick RN, Rubin LJ. Is methamphetamine use associated with idiopathic pulmonary arterial hypertension? Chest 2006,130:1657–1663. [DOI] [PubMed] [Google Scholar]
- 99.Administration SAaMHS. Results from the 2013 National Survey on Drug Use and Health:Summary of National Findings In. Rockville, MD: Center for Behavioral Health Statistics and Quality, U.S. DEPARTMENT OF HEALTH AND HUMAN SERVICES; 2014. [Google Scholar]
- 100.Albertson TE, Walby WF, Derlet RW. Stimulant-induced pulmonary toxicity. Chest 1995,108:1140–1149. [DOI] [PubMed] [Google Scholar]
- 101.Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med 1990,88:325–331. [DOI] [PubMed] [Google Scholar]
- 102.Collazos J, Martinez E, Fernandez A, Mayo J. Acute, reversible pulmonary hypertension associated with cocaine use. Respir Med 1996,90:171–174. [DOI] [PubMed] [Google Scholar]
- 103.Haim DY, Lippmann ML, Goldberg SK, Walkenstein MD. The pulmonary complications of crack cocaine. A comprehensive review. Chest 1995,107:233–240. [DOI] [PubMed] [Google Scholar]
- 104.Itkonen J, Schnoll S, Glassroth J. Pulmonary dysfunction in ‘freebase’ cocaine users. Arch Intern Med 1984,144:2195–2197. [PubMed] [Google Scholar]
- 105.Yakel DL Jr., Eisenberg MJ. Pulmonary artery hypertension in chronic intravenous cocaine users. Am Heart J 1995,130:398–399. [DOI] [PubMed] [Google Scholar]
- 106.Benjelloun H, Sendid M, Bouaouda M, Oukhouya B, Sebti K, Ostowar K. [Textiloma (2 cases)]. Maroc Med 1982,4:71–75. [PubMed] [Google Scholar]
- 107.Schaiberger PH, Kennedy TC, Miller FC, Gal J, Petty TL. Pulmonary hypertension associated with long-term inhalation of “crank” methamphetamine. Chest 1993,104:614–616. [DOI] [PubMed] [Google Scholar]
- 108.Zamanian RT, Hedlin H, Greuenwald P, Wilson DM, Segal JI, Jorden M, et al. Features and Outcomes of Methamphetamine-associated Pulmonary Arterial Hypertension. Am J Respir Crit Care Med 2018,197:788–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Liu M, Wang Y, Wang HM, Bai Y, Zhang XH, Sun YX, et al. Fluoxetine attenuates chronic methamphetamine-induced pulmonary arterial remodelling: possible involvement of serotonin transporter and serotonin 1B receptor. Basic Clin Pharmacol Toxicol 2013,112:77–82. [DOI] [PubMed] [Google Scholar]
- 110.Tuder RM, Groves B, Badesch DB, Voelkel NF. Exuberant endothelial cell growth and elements of inflammation are present in plexiform lesions of pulmonary hypertension. Am J Pathol 1994,144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 111.Budhiraja R, Tuder RM, Hassoun PM. Endothelial dysfunction in pulmonary hypertension. Circulation 2004,109:159–165. [DOI] [PubMed] [Google Scholar]
- 112.Jonigk D, Golpon H, Bockmeyer CL, Maegel L, Hoeper MM, Gottlieb J, et al. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol 2011,179:167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Duffy P, Wang X, Lin PH, Yao Q, Chen C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res 2009,156:257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kanmogne GD, Primeaux C, Grammas P. Induction of apoptosis and endothelin-1 secretion in primary human lung endothelial cells by HIV-1 gp120 proteins. Biochem Biophys Res Commun 2005,333:1107–1115. [DOI] [PubMed] [Google Scholar]
- 115.Hofman FM, Wright AD, Dohadwala MM, Wong-Staal F, Walker SM. Exogenous tat protein activates human endothelial cells. Blood 1993,82:2774–2780. [PubMed] [Google Scholar]
- 116.Peruzzi F The multiple functions of HIV-1 Tat: proliferation versus apoptosis. Front Biosci 2006,11:708–717. [DOI] [PubMed] [Google Scholar]
- 117.Ehrenreich H, Rieckmann P, Sinowatz F, Weih KA, Arthur LO, Goebel FD, et al. Potent stimulation of monocytic endothelin-1 production by HIV-1 glycoprotein 120. J Immunol 1993,150:4601–4609. [PubMed] [Google Scholar]
- 118.Huang MB, Hunter M, Bond VC. Effect of extracellular human immunodeficiency virus type 1 glycoprotein 120 on primary human vascular endothelial cell cultures. AIDS Res Hum Retroviruses 1999,15:1265–1277. [DOI] [PubMed] [Google Scholar]
- 119.Masri FA, Xu W, Comhair SA, Asosingh K, Koo M, Vasanji A, et al. Hyperproliferative apoptosis-resistant endothelial cells in idiopathic pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2007,293:L548–554. [DOI] [PubMed] [Google Scholar]
- 120.Sakao S, Taraseviciene-Stewart L, Lee JD, Wood K, Cool CD, Voelkel NF. Initial apoptosis is followed by increased proliferation of apoptosis-resistant endothelial cells. FASEB J 2005,19:1178–1180. [DOI] [PubMed] [Google Scholar]
- 121.Ding WG, Zhou HC, Cui XG, Li WZ, Guo YP, Zhang B, et al. Anti-apoptotic effect of morphine-induced delayed preconditioning on pulmonary artery endothelial cells with anoxia/reoxygenation injury. Chin Med J (Engl) 2008,121:1313–1318. [PubMed] [Google Scholar]
- 122.Hsiao PN, Chang MC, Cheng WF, Chen CA, Lin HW, Hsieh CY, et al. Morphine induces apoptosis of human endothelial cells through nitric oxide and reactive oxygen species pathways. Toxicology 2009,256:83–91. [DOI] [PubMed] [Google Scholar]
- 123.Chi D, Henry J, Kelley J, Thorpe R, Smith JK, Krishnaswamy G. The effects of HIV infection on endothelial function. Endothelium 2000,7:223–242. [DOI] [PubMed] [Google Scholar]
- 124.Humbert M, Monti G, Fartoukh M, Magnan A, Brenot F, Rain B, et al. Platelet-derived growth factor expression in primary pulmonary hypertension: comparison of HIV seropositive and HIV seronegative patients. Eur.Respir.J. 1998,11:554–559. [PubMed] [Google Scholar]
- 125.Davies RJ, Holmes AM, Deighton J, Long L, Yang X, Barker L, et al. BMP type II receptor deficiency confers resistance to growth inhibition by TGF-beta in pulmonary artery smooth muscle cells: role of proinflammatory cytokines. Am J Physiol Lung Cell Mol Physiol 2012,302:L604–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Upton PD, Davies RJ, Tajsic T, Morrell NW. Transforming growth factor-beta(1) represses bone morphogenetic protein-mediated Smad signaling in pulmonary artery smooth muscle cells via Smad3. Am J Respir Cell Mol Biol 2013,49:1135–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nasim MT, Ogo T, Chowdhury HM, Zhao L, Chen CN, Rhodes C, et al. BMPR-II deficiency elicits pro-proliferative and anti-apoptotic responses through the activation of TGFbeta-TAK1-MAPK pathways in PAH. Hum Mol Genet 2012,21:2548–2558. [DOI] [PubMed] [Google Scholar]
- 128.Lane KB, Machado RD, Pauciulo MW, Thomson JR, Phillips JA 3rd, Loyd JE, et al. Heterozygous germline mutations in BMPR2, encoding a TGF-beta receptor, cause familial primary pulmonary hypertension. The International PPH Consortium. Nat Genet 2000,26:81–84. [DOI] [PubMed] [Google Scholar]
- 129.Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, et al. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet 2000,67:737–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Durrington HJ, Upton PD, Hoer S, Boname J, Dunmore BJ, Yang J, et al. Identification of a lysosomal pathway regulating degradation of the bone morphogenetic protein receptor type II. J Biol Chem 2010,285:37641–37649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chinnappan M, Mohan A, Agarwal S, Dalvi P, Dhillon NK. Network of MicroRNAs Mediate Translational Repression of Bone Morphogenetic Protein Receptor-2: Involvement in HIV-Associated Pulmonary Vascular Remodeling. J Am Heart Assoc 2018,7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dalvi P, Sharma H, Konstantinova T, Sanderson M, Brien-Ladner AO, Dhillon NK. Hyperactive TGF-beta Signaling in Smooth Muscle Cells Exposed to HIV-protein(s) and Cocaine: Role in Pulmonary Vasculopathy. Sci Rep 2017,7:10433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chan MC, Hilyard AC, Wu C, Davis BN, Hill NS, Lal A, et al. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression. EMBO J 2010,29:559–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hansmann G, de Jesus Perez VA, Alastalo TP, Alvira CM, Guignabert C, Bekker JM, et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest 2008,118:1846–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jones PL, Cowan KN, Rabinovitch M. Tenascin-C, proliferation and subendothelial fibronectin in progressive pulmonary vascular disease. Am J Pathol 1997,150:1349–1360. [PMC free article] [PubMed] [Google Scholar]
- 136.Schumann C, Lepper PM, Frank H, Schneiderbauer R, Wibmer T, Kropf C, et al. Circulating biomarkers of tissue remodelling in pulmonary hypertension. Biomarkers 2010,15:523–532. [DOI] [PubMed] [Google Scholar]
- 137.Jones PL, Crack J, Rabinovitch M. Regulation of tenascin-C, a vascular smooth muscle cell survival factor that interacts with the alpha v beta 3 integrin to promote epidermal growth factor receptor phosphorylation and growth. J Cell Biol 1997,139:279–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Golledge J, Clancy P, Maguire J, Lincz L, Koblar S. The role of tenascin C in cardiovascular disease. Cardiovasc Res 2011,92:19–28. [DOI] [PubMed] [Google Scholar]
- 139.Jones PL, Rabinovitch M. Tenascin-C is induced with progressive pulmonary vascular disease in rats and is functionally related to increased smooth muscle cell proliferation. Circ Res 1996,79:1131–1142. [DOI] [PubMed] [Google Scholar]
- 140.Cohen ED, Ihida-Stansbury K, Lu MM, Panettieri RA, Jones PL, Morrisey EE. Wnt signaling regulates smooth muscle precursor development in the mouse lung via a tenascin C/PDGFR pathway. J Clin Invest 2009,119:2538–2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Tanaka R, Seki Y, Saito Y, Kamiya S, Fujita M, Okutsu H, et al. Tenascin-C-derived peptide TNIIIA2 highly enhances cell survival and platelet-derived growth factor (PDGF)-dependent cell proliferation through potentiated and sustained activation of integrin alpha5beta1. J Biol Chem 2014,289:17699–17708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ihida-Stansbury K, McKean DM, Lane KB, Loyd JE, Wheeler LA, Morrell NW, et al. Tenascin-C is induced by mutated BMP type II receptors in familial forms of pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol 2006,291:L694–702. [DOI] [PubMed] [Google Scholar]
- 143.Price LC, Wort SJ, Perros F, Dorfmuller P, Huertas A, Montani D, et al. Inflammation in pulmonary arterial hypertension. Chest 2012,141:210–221. [DOI] [PubMed] [Google Scholar]
- 144.Groth A, Vrugt B, Brock M, Speich R, Ulrich S, Huber LC. Inflammatory cytokines in pulmonary hypertension. Respir Res 2014,15:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Brock M, Trenkmann M, Gay RE, Michel BA, Gay S, Fischler M, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 2009,104:1184–1191. [DOI] [PubMed] [Google Scholar]
- 146.Parikh RV, Scherzer R, Nitta EM, Leone A, Hur S, Mistry V, et al. Increased levels of asymmetric dimethylarginine are associated with pulmonary arterial hypertension in HIV infection. AIDS 2014,28:511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Damas JK, Otterdal K, Yndestad A, Aass H, Solum NO, Froland SS, et al. Soluble CD40 ligand in pulmonary arterial hypertension: possible pathogenic role of the interaction between platelets and endothelial cells. Circulation 2004,110:999–1005. [DOI] [PubMed] [Google Scholar]
- 148.Stacher E, Graham BB, Hunt JM, Gandjeva A, Groshong SD, McLaughlin VV, et al. Modern age pathology of pulmonary arterial hypertension. Am J Respir Crit Care Med 2012,186:261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Savai R, Pullamsetti SS, Kolbe J, Bieniek E, Voswinckel R, Fink L, et al. Immune and inflammatory cell involvement in the pathology of idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med 2012,186:897–908. [DOI] [PubMed] [Google Scholar]
- 150.Dalvi Pranjali N. C L, Upadhya Samarth, O’Brien-Ladner Amy, Dhillon Navneet K.. Involvement of HIV Infected and Cocaine Exposed Macrophage Derived Extracellular Vesicles in Pulmonary Smooth Muscle Hyperplasia. Am J Respir Crit Care Med 2016,193:A7289. [Google Scholar]
- 151.Sharma H, Chinnappan M, Agarwal S, Dalvi P, Gunewardena S, O’Brien-Ladner A, et al. Macrophage-derived extracellular vesicles mediates smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV-infection and substance abuse. FASEB J 2018:fj201701558R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bertero T, Lu Y, Annis S, Hale A, Bhat B, Saggar R, et al. Systems-level regulation of microRNA networks by miR-130/301 promotes pulmonary hypertension. The Journal of clinical investigation 2014,124:3514–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bertero T, Cottrill K, Krauszman A, Lu Y, Annis S, Hale A, et al. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. Journal of Biological Chemistry 2015,290:2069–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bertero T, Cottrill KA, Lu Y, Haeger CM, Dieffenbach P, Annis S, et al. Matrix remodeling promotes pulmonary hypertension through feedback mechanoactivation of the YAP/TAZ-miR-130/301 circuit. Cell reports 2015,13:1016–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jarrett H, Barnett C. HIV-associated pulmonary hypertension. Curr Opin HIV AIDS 2017,12:566–571. [DOI] [PubMed] [Google Scholar]
- 156.Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016,37:67–119. [DOI] [PubMed] [Google Scholar]
- 157.Humbert M, Ghofrani HA. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016,71:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Seferian A, Simonneau G. Therapies for pulmonary arterial hypertension: where are we today, where do we go tomorrow? Eur Respir Rev 2013,22:217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Parikh RV, Ma Y, Scherzer R, Heringer AS, Macgregor JS, Martin JN, et al. Endothelin-1 Predicts Hemodynamically Assessed Pulmonary Arterial Hypertension in HIV Infection. PLoS One 2016,11:e0146355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Cea-Calvo L, Escribano Subias P, Tello de Menesses R, Lazaro Salvador M, Gomez Sanchez MA, Delgado Jimenez JF, et al. [Treatment of HIV-associated pulmonary hypertension with treprostinil]. Rev Esp Cardiol 2003,56:421–425. [DOI] [PubMed] [Google Scholar]
- 161.Galie N, Humbert M, Vachiery JL, Vizza CD, Kneussl M, Manes A, et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: a randomized, double-blind, placebo-controlled trial. J Am Coll Cardiol 2002,39:1496–1502. [DOI] [PubMed] [Google Scholar]
- 162.Barst RJ, McGoon M, McLaughlin V, Tapson V, Rich S, Rubin L, et al. Beraprost therapy for pulmonary arterial hypertension. J Am Coll Cardiol 2003,41:2119–2125. [DOI] [PubMed] [Google Scholar]
- 163.Dai HL, Lu YB, Guang XF, Xiao ZC, Zhang M, Zhang WH. Bosentan and antiretroviral therapy in an HIV/HBV/HCV coinfected Chinese patient with HIV-related pulmonary arterial hypertension. Chin Med J (Engl) 2013,126:1197–1198. [PubMed] [Google Scholar]
- 164.Correale M, Palmiotti GA, Lo Storto MM, Montrone D, Foschino Barbaro MP, Di Biase M, et al. HIV-associated pulmonary arterial hypertension: from bedside to the future. Eur J Clin Invest 2015,45:515–528. [DOI] [PubMed] [Google Scholar]
- 165.Montani D, Savale L, Natali D, Jais X, Herve P, Garcia G, et al. Long-term response to calcium-channel blockers in non-idiopathic pulmonary arterial hypertension. Eur Heart J 2010,31:1898–1907. [DOI] [PubMed] [Google Scholar]
- 166.Galie N, Negro L, Simonneau G. The use of combination therapy in pulmonary arterial hypertension: new developments. Eur Respir Rev 2009,18:148–153. [DOI] [PubMed] [Google Scholar]
- 167.Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D, et al. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: importance of endothelial communication. Chest 2015,147:529–537. [DOI] [PubMed] [Google Scholar]
- 168.Thompson AAR, Lawrie A. Targeting Vascular Remodeling to Treat Pulmonary Arterial Hypertension. Trends Mol Med 2017,23:31–45. [DOI] [PubMed] [Google Scholar]
- 169.Kourembanas S Expanding the pool of stem cell therapy for lung growth and repair. Circulation 2014,129:2091–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.van der Laarse A, Cobbaert CM, Umar S. Stem and progenitor cell therapy for pulmonary arterial hypertension: effects on the right ventricle (2013 Grover Conference Series). Pulm Circ 2015,5:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sdrimas K, Kourembanas S. MSC microvesicles for the treatment of lung disease: a new paradigm for cell-free therapy. Antioxid Redox Signal 2014,21:1905–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suen C, Mei SHJ, Stewart DJ. Cell Therapy for PAH: Potential Efficacy of Endothelial Progenitor Cells and Mesenchymal Stem Cells. Advances in Pulmonary Hypertension 2012,1:33–38. [Google Scholar]
- 173.Willis GR, Fernandez-Gonzalez A, Anastas J, Vitali SH, Liu X, Ericsson M, et al. Mesenchymal Stromal Cell Exosomes Ameliorate Experimental Bronchopulmonary Dysplasia and Restore Lung Function through Macrophage Immunomodulation. Am J Respir Crit Care Med 2018,197:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Aliotta JM, Pereira M, Wen S, Dooner MS, Del Tatto M, Papa E, et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc Res 2016,110:319–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Loisel F, Provost B, Haddad F, Guihaire J, Amsallem M, Vrtovec B, et al. Stem cell therapy targeting the right ventricle in pulmonary arterial hypertension: is it a potential avenue of therapy? Pulm Circ 2018,8:2045893218755979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hansmann G, Fernandez-Gonzalez A, Aslam M, Vitali SH, Martin T, Mitsialis SA, et al. Mesenchymal stem cell-mediated reversal of bronchopulmonary dysplasia and associated pulmonary hypertension. Pulm Circ 2012,2:170–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Austin ED, West J, Loyd JE, Hemnes AR. Translational Advances in the Field of Pulmonary Hypertension Molecular Medicine of Pulmonary Arterial Hypertension. From Population Genetics to Precision Medicine and Gene Editing. Am J Respir Crit Care Med 2017,195:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Perry AN, Westenbroek C, Jagannathan L, Becker JB. The Roles of Dopamine and alpha1-Adrenergic Receptors in Cocaine Preferences in Female and Male Rats. Neuropsychopharmacology 2015,40:2696–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wieczerzak K, Witarski T, Kowalska M, Nawrat D, Roman A, Bielawski A, et al. Effect of cocaine on responsiveness of alpha(1)-adrenergic receptors in rat cerebral cortex: modulation by GABA-mimetic drugs. Pharmacol Rep 2008,60:980–984. [PubMed] [Google Scholar]
- 180.Bolshakova Anastasia V. EO K, Gainullina Anastasia N., Zhemkov Vladimir A., Korban Svetlana A., Bezprozvanny Ilya B.. Sigma-1 receptor as a potential pharmacological target for the treatment of neuropathology. St. Petersburg Polytechnical University Journal: Physics and Mathematics 2016,2:31–40. [Google Scholar]
- 181.Matsumoto RR. Targeting sigma receptors: novel medication development for drug abuse and addiction. Expert Rev Clin Pharmacol 2009,2:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen L, Xin X, Eckhart AD, Yang N, Faber JE. Regulation of vascular smooth muscle growth by alpha 1-adrenoreceptor subtypes in vitro and in situ. J Biol Chem 1995,270:30980–30988. [DOI] [PubMed] [Google Scholar]
- 183.Dumas SJ, Bru-Mercier G, Courboulin A, Quatredeniers M, Rucker-Martin C, Antigny F, et al. NMDA-Type Glutamate Receptor Activation Promotes Vascular Remodeling and Pulmonary Arterial Hypertension. Circulation 2018,137:2371–2389. [DOI] [PubMed] [Google Scholar]
- 184.Billman GE. The effect of adrenergic receptor antagonists on cocaine-induced ventricular fibrillation: alpha but not beta adrenergic receptor antagonists prevent malignant arrhythmias independent of heart rate. J Pharmacol Exp Ther 1994,269:409–416. [PubMed] [Google Scholar]
- 185.Almodovar S, Hsue PY, Morelli J, Huang L, Flores SC, Lung HIVS. Pathogenesis of HIV-associated pulmonary hypertension: potential role of HIV-1 Nef. Proc Am Thorac Soc 2011,8:308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Delgado JF, Conde E, Sanchez V, Lopez-Rios F, Gomez-Sanchez MA, Escribano P, et al. Pulmonary vascular remodeling in pulmonary hypertension due to chronic heart failure. Eur J Heart Fail 2005,7:1011–1016. [DOI] [PubMed] [Google Scholar]
- 187.Hoeper MM, Barbera JA, Channick RN, Hassoun PM, Lang IM, Manes A, et al. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol 2009,54:S85–96. [DOI] [PubMed] [Google Scholar]
- 188.Moraes DL, Colucci WS, Givertz MM. Secondary pulmonary hypertension in chronic heart failure: the role of the endothelium in pathophysiology and management. Circulation 2000,102:1718–1723. [DOI] [PubMed] [Google Scholar]
- 189.Farkas L, Gauldie J, Voelkel NF, Kolb M. Pulmonary hypertension and idiopathic pulmonary fibrosis: a tale of angiogenesis, apoptosis, and growth factors. Am J Respir Cell Mol Biol 2011,45:1–15. [DOI] [PubMed] [Google Scholar]
- 190.Naeije R Pulmonary hypertension and right heart failure in chronic obstructive pulmonary disease. Proc Am Thorac Soc 2005,2:20–22. [DOI] [PubMed] [Google Scholar]
- 191.Crothers K, Huang L, Goulet JL, Goetz MB, Brown ST, Rodriguez-Barradas MC, et al. HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011,183:388–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Douschan P, Kovacs G, Avian A, Foris V, Gruber F, Olschewski A, et al. Mild Elevation of Pulmonary Arterial Pressure as a Predictor of Mortality. Am J Respir Crit Care Med 2018,197:509–516. [DOI] [PubMed] [Google Scholar]