Introduction
Much of the antiviral activity of antibody is mediated by interactions between the Fc segment of immunoglobulin and Fc receptors (FcRs) present on many different cell types. Such interactions could have a beneficial impact on viral infection through, for example, antibody-dependent cellular cytotoxicity (ADCC), phagocytosis, or trogocytosis (wherein plasma membranes are sheared off of one cell by another cell). However, Fc-FcR interactions may also result in antibody-dependent enhancement of infection, a phenomenon best described with respect to dengue virus infections. In this review, we will focus primarily on ADCC in the setting of HIV-1 and related lentiviral infections and also touch briefly on other potentially beneficial functions. For a discussion of antibody-dependent enhancement, the interested reader is directed elsewhere [1,2]. In addition, detailed treatments of FcR biology can be found in recent reviews [3,4].
ADCC occurs when antibody forms a bridge between a target cell bearing foreign antigens on its surface and an effector cell, typically a natural killer (NK) cell expressing FcRs. The cross-linking of the FcRs initiates a cascade of signals leading to the release of lytic compounds from the effector cell that ultimately result in the lysis of the target cell. Thus, as is the case with cytotoxic T cell activity, ADCC has the potential to remove cells producing virus. ADCC is best described for IgG interacting with Fc receptors for IgG (FcγRs). However, IgA-FcαR and IgE-FcεR interactions leading to ADCC also occur [5,6]. Of the IgG subclasses, IgG1 and IgG3 are best at engaging Fc receptors [3,7]. Fc glycosylation can also have a major impact on Fc-FcγR interactions [8]. Finally, polymorphisms in the FcγRs themselves, particularly those in FcγRIIIa and FcγRIIa, can influence the affinity for IgG and the ADCC activity in a subclass-specific manner [7,9].
Antibody-dependent phagocytosis (ADP) occurs when antibody opsonizing a cell or a cell-free pathogen cross-links FcRs on phagocytic cells such as monocytes, macrophages, dendritic cells, or neutrophils. The opsonized cell or pathogen is then internalized and, for the most part, degraded in phagolysosomes. However, FcR-mediated internalization of virus also represents a means of virus entry into susceptible target cells that could potentially result in enhanced infection [10,11]. Using GFP-expressing HIV-1, it has been shown that virions are poor targets for ADP by Env-specific IgG, likely because of the paucity of Env spikes on the virion surface and because of the inability of IgG antibodies to aggregate virus [12]. However, cells decorated with virus on their surface, a state imitating target cells in the process of being infected, may undergo ADP or trogocytosis [12–15]. Whether or not phagocytosis or trogocytosis of such cells, or of infected cells, contributes to virus clearance is unknown.
Apart from functions that result in target cell death, triggering of FcRs by opsonizing antibodies can result in the secretion of various chemokines and cytokines with direct and indirect antiviral and inflammation-modulating effects [16,17]. Finally, the sum total of FcR-triggered effects on virus replication can be measured using the antibody-dependent cell-mediated virus inhibition (ADCVI) assay, in which viral yield is measured after incubating infected lymphocytes with effector cells and antibody [16,18].
Measuring ADCC: concepts and controversies
Env-coated vs. infected target cells.
One of the earliest and most widely used techniques to measure anti-HIV ADCC activity relies on the coating of CD4+ T cells—either cell lines such as CEM-NKr or primary CD4+ T cells—with recombinant Env proteins gp120 or gp140 or with inactivated viral particles [19–32]. Killing of Env-coated target cells is then measured by the release of 51Chromium (chromium release assay) [26,33,34] or by calculating the emergence of pre-labeled coated cells that lose a viability dye (rapid and fluorometric ADCC [RFADCC] assay) [25,32,35]. ADCC activity has also been evaluated by quantifying the cleavage of a fluorogenic peptide substrate that generates a fluorescent signal when cleaved by granzyme B (ADCC GranToxiLux assay) [27,28,36,37], by detecting intracellular IFN-γ or cell-surface expression of CD107a or by measuring the loss of intracellular granzyme B [38,39]. Note that these latter assays do not directly measure ADCC but more precisely measure activities that requires NK effector cell responses similar to those occurring during ADCC.
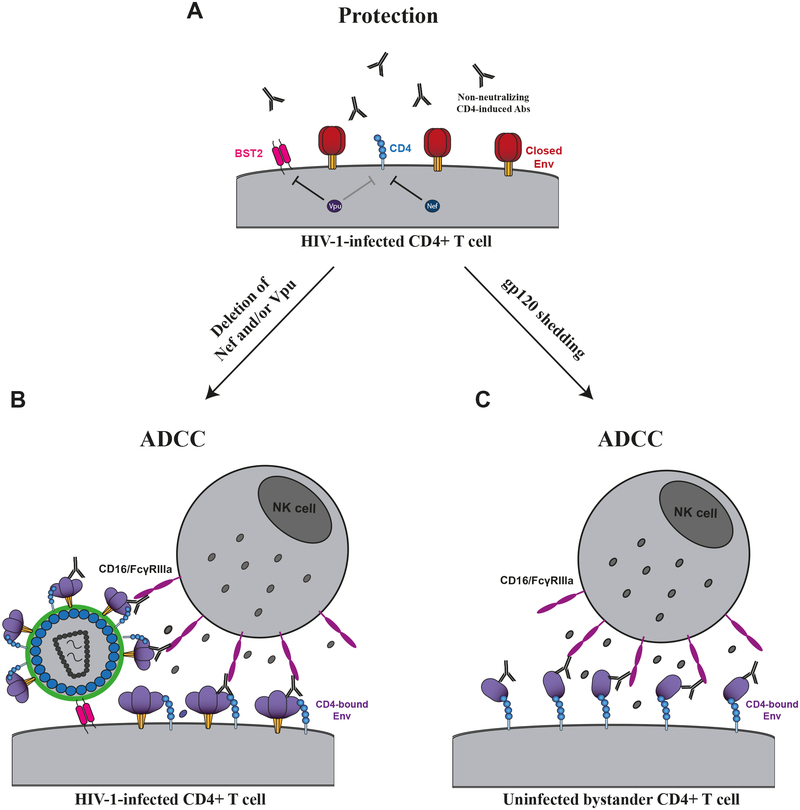
It is important to consider that Env coating of CD4+ T cells is achieved through the interaction of gp120 with the CD4 receptor. Thus, these assays cannot detect activity mediated by antibodies that target the gp120 CD4 binding site (CD4BS) [40–44]. Moreover, gp120-coated cells do not expose quaternary epitopes present in the untriggered Env trimer and therefore are not useful to measure ADCC activity mediated by antibodies recognizing such quaternary epitopes (e.g., PG9, PG16, etc.) [45–48]. These considerations and others have led to several approaches to measure ADCC against infected target cells. Such target cells can be made by infecting CD4+ T cells, mostly CEM-NKr cells or primary CD4+ T cells, with infectious HIV-1 molecular clones (IMCs). However, an overlooked problem of this approach is the intrinsic ability of gp120 to shed from the surface of infected cells. Shed gp120 binds to uninfected, bystander T cells through a CD4 interaction, thus providing another susceptible target cell population in addition to the infected cells (figure 1). This natural phenomenon greatly complicates ADCC measurements. While infected cells express trimeric Env at their surface, uninfected bystander cells are coated with monomeric gp120. It is therefore paramount to discern ADCC responses against infected versus uninfected cells, since anti-Env Abs have different specificities [40]. Indeed, while some preferentially recognize monomeric gp120, others only bind the untriggered “closed” trimer (discussed below).
Figure 1. HIV-1-infected cells are protected from ADCC responses.
To avoid exposing vulnerable CD4-induced epitopes, HIV-1 controls the level of cell surface CD4 through the action of Nef and Vpu and limits Env accumulation through Vpu-mediated BST-2/Tethering downmodulation (A). In the absence of Nef and/or Vpu, Env and CD4 can interact at the cell surface, thus sensitizing infected cells to ADCC mediated by CD4i Abs (B). An intrinsic property of HIV-1 Env is that its gp120 subunits sheds. This is due to the noncovalent association between gp41 and gp120. Shed gp120 interacts with the CD4 receptor present at the surface of uninfected bystander cells, thus resulting in the exposure of vulnerable epitopes leading to the sensitization of these cells to ADCC responses mediated by CD4i Abs (C).
Though NK cell activation and GranToxiLux assays fail to differentiate responses against infected versus uninfected cells, other approaches do. Infected cells can easily be identified by performing intracellular p24+ staining; ADCC activity is then determined by calculating the loss of the percentage of infected (p24+) cells by flow cytometry [36,49–55]. Similar flow cytometry approaches use modified IMCs coding for reporter genes such as green fluorescent protein (GFP) [52,53,56–60]. Another method to measure ADCC activity against the infected cell population is the use of IMCs coding for the luciferase reporter gene (LucR). This approach is amenable to high-throughput assays and has been widely used in the field [37,61–74]. However, great caution should be exerted in selecting these reporter IMCs in order to ensure that insertion of the reporter gene does not affect expression of viral proteins, particularly Nef, that play a critical role in protecting infected cells from ADCC responses (detailed below; figure 1) [75]. Finally, another luciferase-based method uses a T-cell line encoding a Tat-driven luciferase gene, which allows Luciferase to be expressed only upon productive HIV-1 or SIV infection [76]. Since the cell line codes for the reporter gene, unmutated IMCs can be used, and elimination of infected cells is determined by simply calculating the loss of luciferase activity [76]. It is worth noting that this assay, using an unmutated CRF01_AE IMC, was used to identify ADCC as a correlate of protection in the RV144 trial [77].
The role of Env conformation and accessory proteins in ADCC.
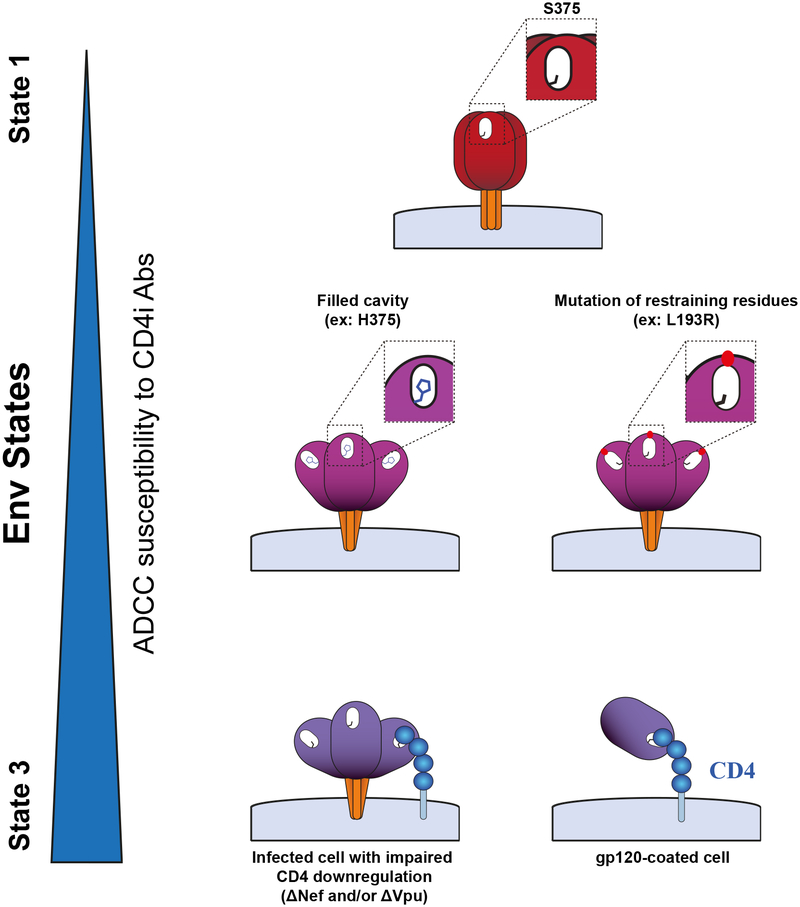
The functional Env trimer is highly flexible and transits from its unliganded “closed” conformation (state 1) to its “open” CD4-bound (state 3) (figure 2). Interaction with CD4 induces changes in Env resulting in some CD4-inducible epitopes (CD4i) exposed by the intermediate “partially open” conformation (state 2). The rest of the CD4i epitopes are unveiled in the open conformation (state 3) [78–80]. Env flexibility and controlled conformational transitions represent a formidable barrier against antibody attack. Envs from primary HIV-1 isolates are relatively resistant to easy-to-elicit CD4i Abs, which are predominant in the sera of HIV-1-infected individuals [58,81,82]. This is mostly due to the “closed” nature of primary Envs which primarily sample state 1 [79], thus effectively occluding epitopes recognized by CD4i Abs. CD4 engagement drives Env into states 2/3 and renders it susceptible to CD4i Ab binding [58,59,78,83–85]. In order to avoid exposure of these vulnerable epitopes, Env tightly controls its transition from state 1 to states 2/3. The V1/V2 and V3 loops play a critical role in preventing spontaneous transitions to downstream conformations [86,87]. A conserved ~150-Å3 pocket where phenylalanine 43 of CD4 engages with the gp120 can predispose Env to spontaneously assume conformations closer to states 2/3 when it is filled with large hydrophobic residues, [84] [88] which in turn sensitizes infected cells to ADCC mediated by CD4i Abs [84] (figure 2). While residue S375 is well-conserved in the majority of group M HIV-1 isolates, CRF01_AE strains have a naturally occurring histidine at this position (H375); such strains predominate in Thailand. A recent report suggests that the presence of H375 in Thai strains might have contributed to the observed vaccine efficacy of RV144 [84]. Other HIV-1 Env strains have a small residue at position 375 (serine or threonine) and therefore predominantly sample closed state 1 conformations [79].
Figure 2. Impact of Env conformation on ADCC.
A small residue at position 375 in the Phe 43 cavity (shown in white) favors a closed (State 1) conformation. Larger hydrophobic residues at this position, such as histidine (H375), or mutations in restraining residues such as L193 shift Env to more open conformations. Env interactions with membrane-anchored CD4, due to Nef and/or Vpu deletion, stabilize State 3. This conformation is also sampled by monomeric gp120 bound to CD4, present at the surface of gp120-coated cells. Env conformational landscape modulates its opening and therefore the exposure of vulnerable epitopes recognized by ADCC-mediating CD4i Abs.
Overall, cells infected with primary viruses and coding for functional Nef and Vpu proteins (see below) are largely resistant to ADCC mediated by CD4i Abs [49,53,56,59,81,89]. Despite the different elements precluding a premature opening of Env, interaction with membrane-bound CD4 results in exposure of vulnerable CD4i epitopes [59]. Consequently, ADCC susceptibility to CD4i Abs is dramatically enhanced when infected cells express Env in its open state 2/3 conformation [58,83,84]. Since CD4i Abs are easily-elicited and achieve high titers during HIV-1 infection [90], the virus evolved to minimize exposure of this vulnerable Env conformation. HIV-1 limits Env-CD4 interaction by downregulating CD4 and preventing accumulation of Env [58,59,91–93]. Nef and Vpu accessory proteins reduce accumulation of CD4 at the cell surface [58,59] in a two-step mechanism. First, during the early phases of the HIV-1 replication cycle, Nef downregulates CD4 from the plasma membrane. Second, Vpu, expressed from a bicistronic mRNA also coding for Env, induces CD4 degradation through an endoplasmic reticulum (ER)-associated protein degradation mechanism [94]. The action of Vpu liberates Env from CD4-dependent retention in the ER [95]. Env is then free to traffic to the plasma membrane in a “closed” conformation effectively occluding CD4i epitopes. Hence, several reports have shown that cells infected with viruses defective for Nef and/or Vpu expression are more susceptible to ADCC responses mediated by HIV+ sera or CD4i antibodies [49,57–59,75,92,93,96–100]. Besides its role in CD4-downregulation, Nef also protects infected cells from ADCC responses by downregulating the expression of NKG2D ligands (MICA, ULBP1, and ULBP2) [97,101,102], which otherwise activate NK cells by interacting with the NKG2D receptor [96,97]. Therefore, Nef and Vpu accessory proteins protect infected cells from ADCC.
Effector cells.
Besides antigen recognition, antibodies through their Fc segment must engage with specific receptors located at the surface of certain immune effector cells. Receptor engagement is required in order to activate effector cells and trigger the release of perforin and granzyme required to kill the target cell. Most assays have relied on peripheral blood mononuclear cells (PBMC) [40,41,50,51,58,59,65,83,84], NK cells isolated from PBMCs [76,81,89,91,96,103,104], or NK cell lines [76,81,103,105,106]. NK cell effector functions have been extensively analyzed and are controlled by an equilibrium established between signals delivered through inhibitory (e.g. KIR, CD94/NKG2A), activating (e.g. FcγRIIIa, NKG2D, DNAM-1, NKp46) or co-activating (e.g. NTB-A, 2B4) receptors [107]. The HIV-1 Nef accessory protein decreases NKG2D ligand expression [49,75,97,101,102] and therefore modulates the activation status of NK cells.
Monocytes, macrophages, neutrophils and NK cells are also reported to mediate ADCC against HIV-1-infected or Env-coated cells [41,108–110] through the FcγRs expressed on those cells [3]. However, it should be noted that monocytes, macrophages and neutrophils function as phagocytes, presenting a problem in interpreting some assays [14]. Discerning ADCC from antibody-mediated phagocytosis in vivo may be particularly difficult [111].
Role of ADCC and other Fc receptor-mediated functions in preventing infection
It is clear from numerous studies using animal models that antibodies capable of neutralizing a lentivirus challenge strain can prevent infection [112–125]. Thus, developing vaccines that elicit neutralizing antibodies against a broad array of virus strains is a major goal of HIV vaccine development. Although some progress has been made, eliciting broadly neutralizing antibodies by vaccination has largely been unsuccessful. An alternative approach would be to depend on non-neutralizing antibodies that nonetheless are potentially antiviral, such as those mediating ADCC or phagocytosis.
Early studies in monkeys hinted at a role for non-neutralizing antibodies in preventing infection. For example, passive infusion of polyclonal IgG derived from sera of vaccinated and infected monkeys was shown to prevent infection in newborn macaques following oral challenge with SIVmac251 [126]. The IgG was considered to be poorly neutralizing but had substantial ADCVI activity [127]. However, a subsequent similar study found no protection [128]. Moreover, several studies employing poorly neutralizing polyclonal or monoclonal antibodies with ADCVI and/or ADCC activity failed to prevent infection after intravenous, vaginal or rectal SIV or SHIV challenge [129–135]. One of these studies did, however, offer a glimmer of hope in that fewer transmitted/founder (T/F) strains established infection in monkeys challenged rectally with high-dose SHIVBaL after infusion of a non-neutralizing, ADCC-mediating antibody. Another study found two of five animals that received vaginally delivered F240 did not become infected after SHIV162P4 vaginal challenge, but there was no significant difference between the F240-treated and control animals [129]. Moreover, that same study demonstrated an increase in T/F strains after passive parenteral infusion or local application of the non-neutralizing antibody b6 [129]. From a mechanistic standpoint, it is unknown how passively infused antibody could reduce or increase the number of T/F strains without impacting the overall risk of infection; however, it is possible that larger sample sizes could reveal differences in infection risk A study by Hessell, et al. compared infusion of IgG1 and IgG3 versions of a non-neutralizing anti-v2i-specific monoclonal antibody in preventing infection after repeated rectal SHIVBal.P4 challenge. There were no significant differences between the IgG1, IgG3 and control antibodies, but the IgG1 version resulted in reduced plasma and PBMC virus levels and decreased viral DNA in lymphoid tissues compared to control antibody [133]. Finally, in a mouse model, a non-neutralizing antibody directed against influenza hemagglutinin (HA) cleared cells infected with HIV-1 modified to express HA and protected mice against infection; protection was likely mediated through FcγRs [136]. The relevance of this model organism to lentiviral infections in humans and non-human primates is unclear.
Studies of the role of ADCC or other FcR-mediated antibody functions in preventing HIV infection in humans also reveal uncertainty but point toward little, if any benefit. ADCVI antibody activity was demonstrated not to be associated with the risk of HIV-1 superinfection [137]. However, ADCC activity of IgG in breast milk (measured by RF-ADCC assay using gp120-coated CEM.NKr cells) correlated inversely with infant infection risk; however, maternal plasma ADCC activity was not associated with transmission risk [138]. The same group found that ADCC activity in infant plasma was higher in uninfected than in infected infants, although the difference was not significant [74]; infant plasma ADCC activity did, however, correlate with reduced mortality. In a larger study, neither breast milk nor maternal plasma ADCC activity (measured by both gp120-coated and HIV-1 infected CEM.NKr-CCR5 cells) were associated with maternal-to-child transmission [74]. A trial comparing passive immunotherapy with polyclonal IgG (HIVIGLOB) plus nevirapine to nevirapine alone in preventing mother-to-child transmission demonstrated no benefit attributable to HIVIGLOB [139]. Moreover, there was a suggestion, though controversial, that the HIVIGLOB might have increased the rate of infection. HIVIGLOB was able to neutralize some strains of HIV-1, but neither ADCC nor other FcγR-mediated antibody activities in HIVIGLOB were reported [140].
Although studies employing passive infusion do not support the notion that non-neutralizing antibodies, in and of themselves, can prevent infection, vaccine trials in monkeys and humans provide some, albeit non-definitive, evidence to the contrary. For example, adenovirus-based regimens, particularly when boosted with Env glycoprotein, offered protection against intra-rectal challenge with SIVmac251 [141]. Protection correlated with binding antibody titers as well as with a composite measure of FcγR- and complement-mediated antibody functions; ADCC as well as ADP individually correlated with protection, although the magnitude of the correlation was not reported [141]. It should be noted that ADP was measured using 1μM beads coated with recombinant gp140 and ADCC was measured by the RF-ADCC assay using CEM-NKr cells also coated with recombinant gp140; as discussed above, there are questions regarding the relevance of these assays. In another vaccine study using adenovirus- and MVA-based vaccines without Env boosting, protection against acquisition after rectal challenge correlated with V2-specific binding antibodies; there was a trend for ADCC activity that did not meet statistical significance after adjusting for multiple comparisons [142]. Vaccine regimens using DNA and/or MVA expressing SIVmac239 sequences provided about 60% per-challenge protection after intrarectal exposure to SIVsme660 [143]. The vaccines elicited serum ADCC activity, which did not correlate with protection [143]. ADCC, measured using cells that express luciferase upon infection, was associated with complete protection following intravenous SIVmac251NE challenge in animals that had been previously immunized with live-attenuated versions of SIVmac239Δnef [103]. Partial protection from vaginal challenge with a different SIVmac251 variant was also associated with serum ADCC activity [103]. gp120-CD4 chimeric subunit protein vaccines gave protection against low-dose repeated rectal challenges with heterologous SHIV162P3 or SIVmac251, and protection was shown to correlate with ADCC measured using gp120-coated target cells [144]. A recent study using a pentavalent vaccine otherwise similar to the RV144 immunogen resulted in 55% protection from low-dose repeated rectal SHIV challenge; several antibody assays, including a bead-based phagocytosis assay and ADCC using gp120-coated target cells or cells infected with Nef-defective IMCs correlated with protection [65,145]. Finally, immunization with truncated, trimeric HIV-1 gp41 and the P1 peptide (amino acids 649–683 of gp41) inserted into the lipid membrane of virosomes (a virus-like particle derived from influenza virus) resulted in a high degree of protection from low-dose, repeated vaginal challenge of Chinese macaques with SHIVSF162P3. Cervicovaginal-secretion ADCC activity, as well as transcytosis-inhibition activity, correlated with protection. Notably, the vaccine regimen did not elicit any circulating ADCC activity [146].
Vaccine trials in humans also provide correlative evidence of a role for FcγR-mediated antibody functions in preventing HIV-1 infection. The first human phase III HIV-1 vaccine trial (VAX004) utilized bivalent recombinant gp120 [147]. Although no protection was demonstrated, higher serum ADCVI activity against a clinical R5 strain of HIV-1 correlated inversely with infection rate [148]. The only human vaccine trial to register vaccine efficacy was the RV144 trial conducted in Thailand [148]. The approximately 30% efficacy allowed an examination of immune correlates, which revealed an associated between higher V1/V2 binding antibodies and protection [77]. In addition, higher anti-Env IgA antibodies were associated with a higher rate of infection. In a secondary analysis, higher ADCC activity (using HIV-192TH023-infected CEM.NKr-CCR5 target cells) was associated with protection, but only after controlling for IgA levels. It should be noted, however, that neutralizing activity against tier 1 HIV-1 was similarly associated with protection after controlling for IgA levels [77]. A subsequent analysis of antibody responses in the RV144 trial revealed that v1-v2 IgG3 antibodies that correlate with lower infection risk also correlate with ADCC activity using gp120-coated or HIV-192TH023-infected target cells[149].
Whereas animal models have not demonstrated preventative efficacy of non-neutralizing antibodies, there is direct evidence that engagement of FcγRs by IgG is essential for optimal prevention of SHIV infection by neutralizing antibodies. Thus, Hessell, et al., found that mutations in the neutralizing antibody b12 that abrogated FcγR binding resulted in less protection than wild-type b12 following passive infusion and vaginal SHIV challenge of macaques [150,151]. Similar conclusions were reached by Bournazos, et al. using mouse models and HIV-1 [152]. However, a non-fucosylated version of b12, which allowed increased FcγRIIIa binding and greater ADCC (using an NK cell line as effectors) and ADCVI (with human PBMCs as effectors) activities in vitro, did not improve protection over wild-type b12 after a repeated low-dose vaginal challenge with SHIVSF162P3 [153]. ADCVI activity using human monocytes was equivalent between the wild-type and non-fucosylated versions of b12. The lack of improved protection may have been due to already maximum in vivo FcγR-mediated activity of the wild-type antibody. Alternatively, an FcγR-mediated antibody function other than one involving ADCC due to FcγRIIIa on NK cells may be important in the optimal effect of neutralizing antibodies. Finally, a more recent study suggests that FcγR engagement is not always necessary for optimal prevention by a neutralizing antibody: a version of the human mAb PGT121 with the same Fc mutation used in the Hessell study of b12 was equally effective as wild-type PGT121 in preventing intravenous SHIV challenge. Moreover, both versions of the antibody similarly lowered viral loads when used in already infected animals (Stephen Kent, unpublished).
In summary, there is little direct evidence in support of non-neutralizing antibodies with anti-viral activities such as ADCC having a substantial protective effect (table). The correlative evidence is interesting but the intercorrelations between immune functions and the inability to directly pinpoint a given function or functions in prevention makes it difficult to draw conclusions. Fc-FcγR interactions do appear to augment the protective effect of neutralizing antibodies, although the augmented protection may not apply to all antibodies.
Table.
Summary of ADCC (or other Fc receptor-mediated antibody function) effects on infections with HIV-1 and related viruses.
| Impact on Infection | Evidence | Results [refs.] |
|---|---|---|
| Protecting from acquisition | Passive infusion of non-neutralizing antibodies | Largely negative in preventing infection [126–135, 139] |
| Possible impact on number of transmitted/founder variants [129, 132] | ||
| Reduction of viral load in animals becoming infected [133] | ||
| Correlations with vaccine-elicited antibody responses | ADCC associated with vaccine efficacy in some human and animal vaccine trials [77, 141, 103, 144–146, 148] | |
| Modulating infection | Passive infusion of non-neutralizing antibodies |
Infusion of polyclonal antibody can transiently decrease viremia [159] |
| Correlations with vaccine-elicited antibody responses | ADCC activity shown to correlate with reduced viremia levels in some animals studies [155–158] | |
| Correlations with infection-induced responses | ADCC responses associated with disease progression in animals and humans [26, 154, 160–162] | |
| Augmenting in vivo activity of neutralizing antibodies | Passive infusion of neutralizing antibodies | Fc-receptor engagement may improve protective effect of some antibodies and not others [150–152, unpublished] |
| Fc-receptor engagement contributes to reduced viremia in infected animals and humans [165] |
The role of ADCC in modulating infection
There are several studies in both animals and humans suggesting that ADCC plays a role in controlling viremia with HIV, SIV or SHIV. However, most of the studies depend on correlations rather than direct evidence. For example, Banks, et al. demonstrated a correlation between plasma ADCC activity and progression to AIDS in SIV17E-Br-infected macaques [154]. In a vaccine trial using adenovirus type 5 (Ad5)-expressing SIV recombinants followed by gp120 boosting, serum ADCC activity correlated with low viral burden during the acute phase of infection following intrarectal challenge with SIVmac251 [155]. ADCC in this study was measured using the RFADCC assay and chronically SIVmac251-infected H9 target cells. An Ad5-based regimen given intranasally and orally or with the addition of an intratracheal prime, followed by intramuscular Env boosting, resulted in reduced acute viremia following intrarectal challenge with SIVmac251. Reductions in acute viremia correlated with serum ADCC (using gp120-coated CEM-NKr cells) and ADCVI activity (using SIVmac251-infected rhesus PBMCs) [156,157]. Similar findings of reduced viremia following vaccination that correlated with ADCC and ADCVI activity were reported after intravenous challenge with SHIV89.6P [158].
One study did directly show some effect of non-neutralizing antibody on control of viremia, although the effect was weak and transitory. In that study, IgG from SIVmac251-infected animals was infused into other SIVmac251-infected animals deemed to be rapid progressors. The rapid progressors had negligible anti-SIV antibody titers prior to the infusion, but the infusion resulted in a two- to three-fold reduction in viremia that began rising after 12 hours. The kinetics of the viral load reduction suggested that an effector mechanism, such as ADCC, was responsible [159].
In humans, Baum, et al. demonstrated an inverse correlation with serum ADCC antibody responses and disease progression (using a chromium-release assay and HIV-1MN gp120-coated cells) [26]. Another study focused on the function of NK cells to mediate ADCC against cytomegalovirus-infected cells and found an association between higher activity and improved survival [160]. ADCC antibody activities among HIV-infected subjects who control viremia in the absence of anti-retroviral therapy (elite controllers) have been reported to be higher when compared to those with poorer control [161,162]. However, one of these studies found higher ADCC activity in elite controllers only with an assay relying on granzyme B entry into target cells and found no difference when the RF-ADCC assay was used [161]. Although these studies are consistent with a role for FcγR-mediated antibody functions in modulating infection in humans, their correlative nature makes it equally likely that better functions are the result of better viremia control through other mechanisms (table).
Finally, with respect to modulating existing infection, direct evidence in humans indicates that passive infusion of potent bNabs leads to rapid but transient lowering of viral load [163,164]. The fact that clearance of infected cells through an FcγR-mediated process likely contributes to the reduced viremia suggests that neutralizing antibodies with effector functions might be a useful component of strategies aimed to reduce virus reservoirs [165].
Conclusions
The results of the RV144 vaccine trial in Thailand and subsequent evaluations of immune correlates of protection in that trial have generated renewed interest in eliciting antibodies with FcγR-mediated functions to prevent HIV-1 infection. However, studies aimed at directly testing the role for such functions—in the presence of weak neutralizing activity—have been disappointing. HIV-1 appears to have evolved different mechanisms, including Nef and Vpu downregulation of CD4 expression on infected cells and Vpu antagonism of BST/tetherin to evade ADCC. Moreover, many antibodies elicited during infection or vaccination that mediate ADCC are directed against CD4i epitopes, which, related to CD4 downregulation, are generally not expressed on infected cells. Although analyses have revealed correlations between ADCC and protection in human and animal vaccine trials (table), it is difficult to ascertain with any degree of certainty whether or not ADCC is actually a key factor in preventing infection. Stronger evidence indicates that FcγR-mediated antibody functions augment the protective effect of neutralizing antibodies, though the exact function(s) playing such a role is unknown. Finally, the impact of FcγR-mediated activities in augmenting the effect of neutralizing antibodies could be a key factor in immunotherapies designed to reduce viral reservoirs.
Acknowledgements
The authors would like to thank Jonathan Richard for helpful comments. DNF is supported by a grant from the National Institute of Allergy and Infectious Diseases under award number R01AI118581. AF is the recipient of a Canada Research Chair on Retroviral Entry (RCHS0235) and is supported by a CIHR foundation grant (352417). The authors have no conflicts of interest to report.
Contributor Information
Donald N. Forthal, Division of Infectious Diseases, Department of Medicine, School of Medicine and Department of Molecular Biology & Biochemistry, School of Biological Sciences, University of California, Irvine, Irvine, CA USA.
Andrés Finzi, Department of Microbiology, Infectious Diseases and Immunology, Université de Montréal, Centre de Recherche du CHUM and Department of Microbiology and Immunology, McGill University, Montreal, QC, Canada.
References
- 1.Gorlani A, Forthal DN. Antibody-dependent enhancement and the risk of HIV infection. Curr HIV Res 2013; 11:421–426. [DOI] [PubMed] [Google Scholar]
- 2.Taylor A, Foo S-S, Bruzzone R, Dinh LV, King NJC, Mahalingam S. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev 2015; 268:340–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bournazos S, Ravetch JV. Fcγ Receptor Function and the Design of Vaccination Strategies. Immunity 2017; 47:224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaifu T, Nakamura A. Polymorphisms of immunoglobulin receptors and the effects on clinical outcome in cancer immunotherapy and other immune diseases: a general review. Int Immunol 2017; 29:319–325. [DOI] [PubMed] [Google Scholar]
- 5.Lohse S, Loew S, Kretschmer A, Jansen JHM, Meyer S, Ten Broeke T, et al. Effector mechanisms of IgA antibodies against CD20 include recruitment of myeloid cells for antibody-dependent cell-mediated cytotoxicity and complement-dependent cytotoxicity. Br J Haematol 2018; 181:413–417. [DOI] [PubMed] [Google Scholar]
- 6.Karagiannis SN, Josephs DH, Karagiannis P, Gilbert AE, Saul L, Rudman SM, et al. Recombinant IgE antibodies for passive immunotherapy of solid tumours: from concept towards clinical application. Cancer Immunol Immunother CII 2012; 61:1547–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruhns P, Iannascoli B, England P, Mancardi DA, Fernandez N, Jorieux S, et al. Specificity and affinity of human Fcgamma receptors and their polymorphic variants for human IgG subclasses. Blood 2009; 113:3716–3725. [DOI] [PubMed] [Google Scholar]
- 8.Cymer F, Beck H, Rohde A, Reusch D. Therapeutic monoclonal antibody N-glycosylation - Structure, function and therapeutic potential. Biol J Int Assoc Biol Stand 2018; 52:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Bruhns P, Jönsson F. Mouse and human FcR effector functions. Immunol Rev 2015; 268:25–51. [DOI] [PubMed] [Google Scholar]
- 10.Homsy J, Meyer M, Tateno M, Clarkson S, Levy JA. The Fc and not CD4 receptor mediates antibody enhancement of HIV infection in human cells. Science 1989; 244:1357–1360. [DOI] [PubMed] [Google Scholar]
- 11.Halstead SB, Chow JS, Marchette NJ. Immunological enhancement of dengue virus replication. Nature New Biol 1973; 243:24–26. [PubMed] [Google Scholar]
- 12.Gach JS, Bouzin M, Wong MP, Chromikova V, Gorlani A, Yu K-T, et al. Human immunodeficiency virus type-1 (HIV-1) evades antibody-dependent phagocytosis. PLoS Pathog 2017; 13:e1006793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horner H, Frank C, Dechant C, Repp R, Glennie M, Herrmann M, et al. Intimate cell conjugate formation and exchange of membrane lipids precede apoptosis induction in target cells during antibody-dependent, granulocyte-mediated cytotoxicity. J Immunol Baltim Md 1950 2007; 179:337–345. [DOI] [PubMed] [Google Scholar]
- 14.Kramski M, Parsons MS, Stratov I, Kent SJ. HIV-specific antibody immunity mediated through NK cells and monocytes. Curr HIV Res 2013; 11:388–406. [DOI] [PubMed] [Google Scholar]
- 15.Daubeuf S, Lindorfer MA, Taylor RP, Joly E, Hudrisier D. The direction of plasma membrane exchange between lymphocytes and accessory cells by trogocytosis is influenced by the nature of the accessory cell. J Immunol Baltim Md 1950 2010; 184:1897–1908. [DOI] [PubMed] [Google Scholar]
- 16.Forthal DN, Landucci G, Daar ES. Antibody from patients with acute human immunodeficiency virus (HIV) infection inhibits primary strains of HIV type 1 in the presence of natural-killer effector cells. J Virol 2001; 75:6953–6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oliva A, Kinter AL, Vaccarezza M, Rubbert A, Catanzaro A, Moir S, et al. Natural killer cells from human immunodeficiency virus (HIV)-infected individuals are an important source of CC-chemokines and suppress HIV-1 entry and replication in vitro. J Clin Invest 1998; 102:223–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Forthal DN, Landucci G. In vitro reduction of virus infectivity by antibody-dependent cell-mediated immunity. J Immunol Methods 1998; 220:129–138. [DOI] [PubMed] [Google Scholar]
- 19.Rook AH, Lane HC, Folks T, McCoy S, Alter H, Fauci AS. Sera from HTLV-III/LAV antibody-positive individuals mediate antibody-dependent cellular cytotoxicity against HTLV-III/LAV-infected T cells. J Immunol Baltim Md 1950 1987; 138:1064–1067. [PubMed] [Google Scholar]
- 20.Ackerman ME, Mikhailova A, Brown EP, Dowell KG, Walker BD, Bailey-Kellogg C, et al. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog 2016; 12:e1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung AW, Ghebremichael M, Robinson H, Brown E, Choi I, Lane S, et al. Polyfunctional Fc-effector profiles mediated by IgG subclass selection distinguish RV144 and VAX003 vaccines. Sci Transl Med 2014; 6:228ra38. [DOI] [PubMed] [Google Scholar]
- 22.Parker SJ, Sadlon TA, Gordon DL. Enhancement of NK cell-mediated antibody-dependent lysis of recombinant gp120-coated CD4 cells by complement. J Infect Dis 1995; 171:186–189. [DOI] [PubMed] [Google Scholar]
- 23.Karnasuta C, Paris RM, Cox JH, Nitayaphan S, Pitisuttithum P, Thongcharoen P, et al. Antibody-dependent cell-mediated cytotoxic responses in participants enrolled in a phase I/II ALVAC-HIV/AIDSVAX B/E prime-boost HIV-1 vaccine trial in Thailand. Vaccine 2005; 23:2522–2529. [DOI] [PubMed] [Google Scholar]
- 24.Gómez-Román VR, Florese RH, Peng B, Montefiori DC, Kalyanaraman VS, Venzon D, et al. An adenovirus-based HIV subtype B prime/boost vaccine regimen elicits antibodies mediating broad antibody-dependent cellular cytotoxicity against non-subtype B HIV strains. J Acquir Immune Defic Syndr 1999 2006; 43:270–277. [DOI] [PubMed] [Google Scholar]
- 25.Gómez-Román VR, Florese RH, Patterson LJ, Peng B, Venzon D, Aldrich K, et al. A simplified method for the rapid fluorometric assessment of antibody-dependent cell-mediated cytotoxicity. J Immunol Methods 2006; 308:53–67. [DOI] [PubMed] [Google Scholar]
- 26.Baum LL, Cassutt KJ, Knigge K, Khattri R, Margolick J, Rinaldo C, et al. HIV-1 gp120-specific antibody-dependent cell-mediated cytotoxicity correlates with rate of disease progression. J Immunol Baltim Md 1950 1996; 157:2168–2173. [PubMed] [Google Scholar]
- 27.Bonsignori M, Pollara J, Moody MA, Alpert MD, Chen X, Hwang K-K, et al. Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 2012; 86:11521–11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pollara J, Hart L, Brewer F, Pickeral J, Packard BZ, Hoxie JA, et al. High-throughput quantitative analysis of HIV-1 and SIV-specific ADCC-mediating antibody responses. Cytom Part J Int Soc Anal Cytol 2011; 79:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Madhavi V, Wren LH, Center RJ, Gonelli C, Winnall WR, Parsons MS, et al. Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: relevance to global HIV vaccine design. AIDS Lond Engl 2014; 28:1859–1870. [DOI] [PubMed] [Google Scholar]
- 30.Karlsson I, Borggren M, Jensen SS, Heyndrickx L, Stewart-Jones G, Scarlatti G, et al. Immunization with Clinical HIV-1 Env Proteins Induces Broad Antibody Dependent Cellular Cytotoxicity-Mediating Antibodies in a Rabbit Vaccination Model. AIDS Res Hum Retroviruses Published Online First: 17 November 2017. doi: 10.1089/AID.2017.0140 [DOI] [PubMed] [Google Scholar]
- 31.Tolbert WD, Gohain N, Alsahafi N, Van V, Orlandi C, Ding S, et al. Targeting the Late Stage of HIV-1 Entry for Antibody-Dependent Cellular Cytotoxicity: Structural Basis for Env Epitopes in the C11 Region. Struct Lond Engl 1993 2017; 25:1719–1731.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan Y, Pazgier M, Sajadi MM, Kamin-Lewis R, Al-Darmarki S, Flinko R, et al. Diverse specificity and effector function among human antibodies to HIV-1 envelope glycoprotein epitopes exposed by CD4 binding. Proc Natl Acad Sci U S A 2013; 110:E69–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tomescu C, Mavilio D, Montaner LJ. Lysis of HIV-1-infected autologous CD4+ primary T cells by interferon-alpha-activated NK cells requires NKp46 and NKG2D. AIDS Lond Engl 2015; 29:1767–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tyler DS, Stanley SD, Nastala CA, Austin AA, Bartlett JA, Stine KC, et al. Alterations in antibody-dependent cellular cytotoxicity during the course of HIV-1 infection. Humoral and cellular defects. J Immunol Baltim Md 1950 1990; 144:3375–3384. [PubMed] [Google Scholar]
- 35.Orlandi C, Flinko R, Lewis GK. A new cell line for high throughput HIV-specific antibody-dependent cellular cytotoxicity (ADCC) and cell-to-cell virus transmission studies. J Immunol Methods 2016; 433:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smalls-Mantey A, Doria-Rose N, Klein R, Patamawenu A, Migueles SA, Ko S-Y, et al. Antibody-dependent cellular cytotoxicity against primary HIV-infected CD4+ T cells is directly associated with the magnitude of surface IgG binding. J Virol 2012; 86:8672–8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrari G, Pollara J, Kozink D, Harms T, Drinker M, Freel S, et al. An HIV-1 gp120 envelope human monoclonal antibody that recognizes a C1 conformational epitope mediates potent antibody-dependent cellular cytotoxicity (ADCC) activity and defines a common ADCC epitope in human HIV-1 serum. J Virol 2011; 85:7029–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun Y, Asmal M, Lane S, Permar SR, Schmidt SD, Mascola JR, et al. Antibody-dependent cell-mediated cytotoxicity in simian immunodeficiency virus-infected rhesus monkeys. J Virol 2011; 85:6906–6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratov I, Chung A, Kent SJ. Robust NK cell-mediated human immunodeficiency virus (HIV)-specific antibody-dependent responses in HIV-infected subjects. J Virol 2008; 82:5450–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard J, Prévost J, Baxter AE, von Bredow B, Ding S, Medjahed H, et al. Uninfected Bystander Cells Impact the Measurement of HIV-Specific Antibody-Dependent Cellular Cytotoxicity Responses. mBio 2018; 9. doi: 10.1128/mBio.00358-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richard J, Veillette M, Batraville L-A, Coutu M, Chapleau J-P, Bonsignori M, et al. Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J Virol Methods 2014; 208:107–114. [DOI] [PubMed] [Google Scholar]
- 42.Julien J-P, Sok D, Khayat R, Lee JH, Doores KJ, Walker LM, et al. Broadly neutralizing antibody PGT121 allosterically modulates CD4 binding via recognition of the HIV-1 gp120 V3 base and multiple surrounding glycans. PLoS Pathog 2013; 9:e1003342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thali M, Furman C, Ho DD, Robinson J, Tilley S, Pinter A, et al. Discontinuous, conserved neutralization epitopes overlapping the CD4-binding region of human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol 1992; 66:5635–5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu X, Yang Z-Y, Li Y, Hogerkorp C-M, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science 2010; 329:856–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blattner C, Lee JH, Sliepen K, Derking R, Falkowska E, de la Peña AT, et al. Structural delineation of a quaternary, cleavage-dependent epitope at the gp41-gp120 interface on intact HIV-1 Env trimers. Immunity 2014; 40:669–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature 2014; 509:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang J, Kang BH, Pancera M, Lee JH, Tong T, Feng Y, et al. Broad and potent HIV-1 neutralization by a human antibody that binds the gp41-gp120 interface. Nature 2014; 515:138–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 2009; 326:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alsahafi N, Ding S, Richard J, Markle T, Brassard N, Walker B, et al. Nef Proteins from HIV-1 Elite Controllers Are Inefficient at Preventing Antibody-Dependent Cellular Cytotoxicity. J Virol 2015; 90:2993–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richard J, Pacheco B, Gohain N, Veillette M, Ding S, Alsahafi N, et al. Co-receptor Binding Site Antibodies Enable CD4-Mimetics to Expose Conserved Anti-cluster A ADCC Epitopes on HIV-1 Envelope Glycoproteins. EBioMedicine 2016; 12:208–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richard J, Prévost J, von Bredow B, Ding S, Brassard N, Medjahed H, et al. BST-2 Expression Modulates Small CD4-Mimetic Sensitization of HIV-1-Infected Cells to Antibody-Dependent Cellular Cytotoxicity. J Virol 2017; 91. doi: 10.1128/JVI.00219-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richard J, Veillette M, Brassard N, Iyer SS, Roger M, Martin L, et al. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc Natl Acad Sci U S A 2015; 112:E2687–2694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Richard J, Veillette M, Ding S, Zoubchenok D, Alsahafi N, Coutu M, et al. Small CD4 Mimetics Prevent HIV-1 Uninfected Bystander CD4 + T Cell Killing Mediated by Antibody-dependent Cell-mediated Cytotoxicity. EBioMedicine 2016; 3:122–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mujib S, Liu J, Rahman AKMN-U, Schwartz JA, Bonner P, Yue FY, et al. Comprehensive Cross-Clade Characterization of Antibody-Mediated Recognition, Complement-Mediated Lysis, and Cell-Mediated Cytotoxicity of HIV-1 Envelope-Specific Antibodies toward Eradication of the HIV-1 Reservoir. J Virol 2017; 91. doi: 10.1128/JVI.00634-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruel T, Guivel-Benhassine F, Amraoui S, Malbec M, Richard L, Bourdic K, et al. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun 2016; 7:10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ding S, Veillette M, Coutu M, Prévost J, Scharf L, Bjorkman PJ, et al. A Highly Conserved Residue of the HIV-1 gp120 Inner Domain Is Important for Antibody-Dependent Cellular Cytotoxicity Responses Mediated by Anti-cluster A Antibodies. J Virol 2016; 90:2127–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pham TNQ, Lukhele S, Hajjar F, Routy J-P, Cohen ÉA. HIV Nef and Vpu protect HIV-infected CD4+ T cells from antibody-mediated cell lysis through down-modulation of CD4 and BST2. Retrovirology 2014; 11:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veillette M, Coutu M, Richard J, Batraville L-A, Dagher O, Bernard N, et al. The HIV-1 gp120 CD4-bound conformation is preferentially targeted by antibody-dependent cellular cytotoxicity-mediating antibodies in sera from HIV-1-infected individuals. J Virol 2015; 89:545–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veillette M, Désormeaux A, Medjahed H, Gharsallah N-E, Coutu M, Baalwa J, et al. Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 2014; 88:2633–2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pham TNQ, Lukhele S, Dallaire F, Perron G, Cohen ÉA. Enhancing Virion Tethering by BST2 Sensitizes Productively and Latently HIV-infected T cells to ADCC Mediated by Broadly Neutralizing Antibodies. Sci Rep 2016; 6:37225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomaras GD, Ferrari G, Shen X, Alam SM, Liao H-X, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A 2013; 110:9019–9024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pollara J, Bonsignori M, Moody MA, Liu P, Alam SM, Hwang K-K, et al. HIV-1 vaccine-induced C1 and V2 Env-specific antibodies synergize for increased antiviral activities. J Virol 2014; 88:7715–7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Permar SR, Fong Y, Vandergrift N, Fouda GG, Gilbert P, Parks R, et al. Maternal HIV-1 envelope-specific antibody responses and reduced risk of perinatal transmission. J Clin Invest 2015; 125:2702–2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sung JAM, Pickeral J, Liu L, Stanfield-Oakley SA, Lam C-YK, Garrido C, et al. Dual-Affinity Re-Targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Invest 2015; 125:4077–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradley T, Pollara J, Santra S, Vandergrift N, Pittala S, Bailey-Kellogg C, et al. Pentavalent HIV-1 vaccine protects against simian-human immunodeficiency virus challenge. Nat Commun 2017; 8:15711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, Ferrari G, Alter G, Forthal DN, Kappes JC, Lewis GK, et al. Diversity of Antiviral IgG Effector Activities Observed in HIV-Infected and Vaccinated Subjects. J Immunol Baltim Md 1950 2016; 197:4603–4612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Viegas EO, Tembe N, Nilsson C, Meggi B, Maueia C, Augusto O, et al. Intradermal HIV-1 DNA Immunization Using Needle-Free Zetajet Injection Followed by HIV-Modified Vaccinia Virus Ankara Vaccination Is Safe and Immunogenic in Mozambican Young Adults: A Phase I Randomized Controlled Trial. AIDS Res Hum Retroviruses Published Online First: 27 November 2017. doi: 10.1089/AID.2017.0121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meyerhoff RR, Scearce RM, Ogburn DF, Lockwood B, Pickeral J, Kuraoka M, et al. HIV-1 Consensus Envelope-Induced Broadly Binding Antibodies. AIDS Res Hum Retroviruses 2017; 33:859–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Joachim A, Munseri PJ, Nilsson C, Bakari M, Aboud S, Lyamuya EF, et al. Three-Year Durability of Immune Responses Induced by HIV-DNA and HIV-Modified Vaccinia Virus Ankara and Effect of a Late HIV-Modified Vaccinia Virus Ankara Boost in Tanzanian Volunteers. AIDS Res Hum Retroviruses 2017; 33:880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Costa MR, Pollara J, Edwards RW, Seaman MS, Gorny MK, Montefiori DC, et al. Fc Receptor-Mediated Activities of Env-Specific Human Monoclonal Antibodies Generated from Volunteers Receiving the DNA Prime-Protein Boost HIV Vaccine DP6–001. J Virol 2016; 90:10362–10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Joachim A, Bauer A, Joseph S, Geldmacher C, Munseri PJ, Aboud S, et al. Boosting with Subtype C CN54rgp140 Protein Adjuvanted with Glucopyranosyl Lipid Adjuvant after Priming with HIV-DNA and HIV-MVA Is Safe and Enhances Immune Responses: A Phase I Trial. PloS One 2016; 11:e0155702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wise MC, Hutnick NA, Pollara J, Myles DJF, Williams C, Yan J, et al. An Enhanced Synthetic Multiclade DNA Prime Induces Improved Cross-Clade-Reactive Functional Antibodies when Combined with an Adjuvanted Protein Boost in Nonhuman Primates. J Virol 2015; 89:9154–9166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dennison SM, Anasti KM, Jaeger FH, Stewart SM, Pollara J, Liu P, et al. Vaccine-induced HIV-1 envelope gp120 constant region 1-specific antibodies expose a CD4-inducible epitope and block the interaction of HIV-1 gp140 with galactosylceramide. J Virol 2014; 88:9406–9417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pollara J, McGuire E, Fouda GG, Rountree W, Eudailey J, Overman RG, et al. Association of HIV-1 Envelope-Specific Breast Milk IgA Responses with Reduced Risk of Postnatal Mother-to-Child Transmission of HIV-1. J Virol 2015; 89:9952–9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Prévost J, Richard J, Medjahed H, Alexander A, Jones J, Kappes JC, et al. Incomplete Downregulation of CD4 Expression Affects HIV-1 Env Conformation and Antibody-Dependent Cellular Cytotoxicity Responses. J Virol 2018; 92. doi: 10.1128/JVI.00484-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alpert MD, Heyer LN, Williams DEJ, Harvey JD, Greenough T, Allhorn M, et al. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 2012; 86:12039–12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Herschhorn A, Ma X, Gu C, Ventura JD, Castillo-Menendez L, Melillo B, et al. Release of gp120 Restraints Leads to an Entry-Competent Intermediate State of the HIV-1 Envelope Glycoproteins. mBio 2016; 7. doi: 10.1128/mBio.01598-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Munro JB, Gorman J, Ma X, Zhou Z, Arthos J, Burton DR, et al. Conformational dynamics of single HIV-1 envelope trimers on the surface of native virions. Science 2014; 346:759–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ma X, Lu M, Gorman J, Terry DS, Hong X, Zhou Z, et al. HIV-1 Env trimer opens through an asymmetric intermediate in which individual protomers adopt distinct conformations. eLife 2018; 7. doi: 10.7554/eLife.34271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.von Bredow B, Arias JF, Heyer LN, Moldt B, Le K, Robinson JE, et al. Comparison of Antibody-Dependent Cell-Mediated Cytotoxicity and Virus Neutralization by HIV-1 Env-Specific Monoclonal Antibodies. J Virol 2016; 90:6127–6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 2005; 201:1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Prévost J, Richard J, Ding S, Pacheco B, Charlebois R, Hahn BH, et al. Envelope glycoproteins sampling states 2/3 are susceptible to ADCC by sera from HIV-1-infected individuals. Virology 2018; 515:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Prévost J, Zoubchenok D, Richard J, Veillette M, Pacheco B, Coutu M, et al. Influence of the Envelope gp120 Phe 43 Cavity on HIV-1 Sensitivity to Antibody-Dependent Cell-Mediated Cytotoxicity Responses. J Virol 2017; 91. doi: 10.1128/JVI.02452-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Madani N, Princiotto AM, Zhao C, Jahanbakhshsefidi F, Mertens M, Herschhorn A, et al. Activation and Inactivation of Primary Human Immunodeficiency Virus Envelope Glycoprotein Trimers by CD4-Mimetic Compounds. J Virol 2017; 91. doi: 10.1128/JVI.01880-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwon YD, Finzi A, Wu X, Dogo-Isonagie C, Lee LK, Moore LR, et al. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc Natl Acad Sci U S A 2012; 109:5663–5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Bartesaghi A, Borgnia MJ, Sapiro G, Subramaniam S. Molecular architecture of native HIV-1 gp120 trimers. Nature 2008; 455:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xiang S-H, Kwong PD, Gupta R, Rizzuto CD, Casper DJ, Wyatt R, et al. Mutagenic stabilization and/or disruption of a CD4-bound state reveals distinct conformations of the human immunodeficiency virus type 1 gp120 envelope glycoprotein. J Virol 2002; 76:9888–9899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bruel T, Guivel-Benhassine F, Lorin V, Lortat-Jacob H, Baleux F, Bourdic K, et al. Lack of ADCC Breadth of Human Nonneutralizing Anti-HIV-1 Antibodies. J Virol 2017; 91. doi: 10.1128/JVI.02440-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Decker JM, Bibollet-Ruche F, Wei X, Wang S, Levy DN, Wang W, et al. Antigenic conservation and immunogenicity of the HIV coreceptor binding site. J Exp Med 2005; 201:1407–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.von Bredow B, Arias JF, Heyer LN, Gardner MR, Farzan M, Rakasz EG, et al. Envelope Glycoprotein Internalization Protects Human and Simian Immunodeficiency Virus-Infected Cells from Antibody-Dependent Cell-Mediated Cytotoxicity. J Virol 2015; 89:10648–10655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alvarez RA, Hamlin RE, Monroe A, Moldt B, Hotta MT, Rodriguez Caprio G, et al. HIV-1 Vpu antagonism of tetherin inhibits antibody-dependent cellular cytotoxic responses by natural killer cells. J Virol 2014; 88:6031–6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Arias JF, Heyer LN, von Bredow B, Weisgrau KL, Moldt B, Burton DR, et al. Tetherin antagonism by Vpu protects HIV-infected cells from antibody-dependent cell-mediated cytotoxicity. Proc Natl Acad Sci U S A 2014; 111:6425–6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Magadán JG, Pérez-Victoria FJ, Sougrat R, Ye Y, Strebel K, Bonifacino JS. Multilayered mechanism of CD4 downregulation by HIV-1 Vpu involving distinct ER retention and ERAD targeting steps. PLoS Pathog 2010; 6:e1000869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kimura T, Nishikawa M, Ohyama A. Intracellular membrane traffic of human immunodeficiency virus type 1 envelope glycoproteins: vpu liberates Golgi-targeted gp160 from CD4-dependent retention in the endoplasmic reticulum. J Biochem (Tokyo) 1994; 115:1010–1020. [DOI] [PubMed] [Google Scholar]
- 96.Parsons MS, Richard J, Lee WS, Vanderven H, Grant MD, Finzi A, et al. NKG2D Acts as a Co-Receptor for Natural Killer Cell-Mediated Anti-HIV-1 Antibody-Dependent Cellular Cytotoxicity. AIDS Res Hum Retroviruses 2016; 32:1089–1096. [DOI] [PubMed] [Google Scholar]
- 97.Alsahafi N, Richard J, Prévost J, Coutu M, Brassard N, Parsons MS, et al. Impaired Downregulation of NKG2D Ligands by Nef Proteins from Elite Controllers Sensitizes HIV-1-Infected Cells to Antibody-Dependent Cellular Cytotoxicity. J Virol 2017; 91. doi: 10.1128/JVI.00109-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Batraville L-A, Richard J, Veillette M, Labbé A-C, Alary M, Guédou F, et al. Short communication: Anti-HIV-1 envelope immunoglobulin Gs in blood and cervicovaginal samples of Beninese commercial sex workers. AIDS Res Hum Retroviruses 2014; 30:1145–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Finzi A, Pacheco B, Zeng X, Kwon YD, Kwong PD, Sodroski J. Conformational characterization of aberrant disulfide-linked HIV-1 gp120 dimers secreted from overexpressing cells. J Virol Methods 2010; 168:155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tokarev A, Stoneham C, Lewinski MK, Mukim A, Deshmukh S, Vollbrecht T, et al. Pharmacologic Inhibition of Nedd8 Activation Enzyme Exposes CD4-Induced Epitopes within Env on Cells Expressing HIV-1. J Virol 2015; 90:2486–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cerboni C, Neri F, Casartelli N, Zingoni A, Cosman D, Rossi P, et al. Human immunodeficiency virus 1 Nef protein downmodulates the ligands of the activating receptor NKG2D and inhibits natural killer cell-mediated cytotoxicity. J Gen Virol 2007; 88:242–250. [DOI] [PubMed] [Google Scholar]
- 102.Norman JM, Mashiba M, McNamara LA, Onafuwa-Nuga A, Chiari-Fort E, Shen W, et al. The antiviral factor APOBEC3G enhances the recognition of HIV-infected primary T cells by natural killer cells. Nat Immunol 2011; 12:975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M, Carville A, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog 2012; 8:e1002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Parsons MS, Loh L, Gooneratne S, Center RJ, Kent SJ. Role of education and differentiation in determining the potential of natural killer cells to respond to antibody-dependent stimulation. AIDS Lond Engl 2014; 28:2781–2786. [DOI] [PubMed] [Google Scholar]
- 105.Parsons MS, Wren L, Isitman G, Navis M, Stratov I, Bernard NF, et al. HIV infection abrogates the functional advantage of natural killer cells educated through KIR3DL1/HLA-Bw4 interactions to mediate anti-HIV antibody-dependent cellular cytotoxicity. J Virol 2012; 86:4488–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wren L, Parsons MS, Isitman G, Center RJ, Kelleher AD, Stratov I, et al. Influence of cytokines on HIV-specific antibody-dependent cellular cytotoxicity activation profile of natural killer cells. PloS One 2012; 7:e38580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat Immunol 2008; 9:495–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Smalls-Mantey A, Connors M, Sattentau QJ. Comparative efficiency of HIV-1-infected T cell killing by NK cells, monocytes and neutrophils. PloS One 2013; 8:e74858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Orlikowsky TW, Wang ZQ, Dudhane A, Dannecker GE, Niethammer D, Wormser GP, et al. Dexamethasone inhibits CD4 T cell deletion mediated by macrophages from human immunodeficiency virus-infected persons. J Infect Dis 2001; 184:1328–1330. [DOI] [PubMed] [Google Scholar]
- 110.Worley MJ, Fei K, Lopez-Denman AJ, Kelleher AD, Kent SJ, Chung AW. Neutrophils mediate HIV-specific antibody-dependent phagocytosis and ADCC. J Immunol Methods 2018; 457:41–52. [DOI] [PubMed] [Google Scholar]
- 111.Gordan S, Biburger M, Nimmerjahn F. bIgG time for large eaters: monocytes and macrophages as effector and target cells of antibody-mediated immune activation and repression. Immunol Rev 2015; 268:52–65. [DOI] [PubMed] [Google Scholar]
- 112.Safrit JT, Fung MS, Andrews CA, Braun DG, Sun WN, Chang TW, et al. hu-PBL-SCID mice can be protected from HIV-1 infection by passive transfer of monoclonal antibody to the principal neutralizing determinant of envelope gp120. AIDS Lond Engl 1993; 7:15–21. [DOI] [PubMed] [Google Scholar]
- 113.Parren PW, Ditzel HJ, Gulizia RJ, Binley JM, Barbas CF, Burton DR, et al. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS Lond Engl 1995; 9:F1–6. [DOI] [PubMed] [Google Scholar]
- 114.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, et al. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med 2000; 6:200–206. [DOI] [PubMed] [Google Scholar]
- 115.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, et al. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med 2000; 6:207–210. [DOI] [PubMed] [Google Scholar]
- 116.Gautam R, Nishimura Y, Pegu A, Nason MC, Klein F, Gazumyan A, et al. A single injection of anti-HIV-1 antibodies protects against repeated SHIV challenges. Nature 2016; 533:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, et al. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol 2001; 75:8340–8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hessell AJ, Rakasz EG, Poignard P, Hangartner L, Landucci G, Forthal DN, et al. Broadly neutralizing human anti-HIV antibody 2G12 is effective in protection against mucosal SHIV challenge even at low serum neutralizing titers. PLoS Pathog 2009; 5:e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol 2010; 84:1302–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moldt B, Rakasz EG, Schultz N, Chan-Hui P-Y, Swiderek K, Weisgrau KL, et al. Highly potent HIV-specific antibody neutralization in vitro translates into effective protection against mucosal SHIV challenge in vivo. Proc Natl Acad Sci U S A 2012; 109:18921–18925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pegu A, Yang Z, Boyington JC, Wu L, Ko S-Y, Schmidt SD, et al. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med 2014; 6:243ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rudicell RS, Kwon YD, Ko S-Y, Pegu A, Louder MK, Georgiev IS, et al. Enhanced potency of a broadly neutralizing HIV-1 antibody in vitro improves protection against lentiviral infection in vivo. J Virol 2014; 88:12669–12682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, et al. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med 1999; 5:204–210. [DOI] [PubMed] [Google Scholar]
- 124.Nishimura Y, Igarashi T, Haigwood NL, Sadjadpour R, Donau OK, Buckler C, et al. Transfer of neutralizing IgG to macaques 6 h but not 24 h after SHIV infection confers sterilizing protection: implications for HIV-1 vaccine development. Proc Natl Acad Sci U S A 2003; 100:15131–15136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ferrantelli F, Rasmussen RA, Buckley KA, Li P-L, Wang T, Montefiori DC, et al. Complete protection of neonatal rhesus macaques against oral exposure to pathogenic simian-human immunodeficiency virus by human anti-HIV monoclonal antibodies. J Infect Dis 2004; 189:2167–2173. [DOI] [PubMed] [Google Scholar]
- 126.Van Rompay KK, Berardi CJ, Dillard-Telm S, Tarara RP, Canfield DR, et al. Passive Immunization of Newborn Rhesus Macaques Prevents Oral Simian Immunodeficiency Virus Infection. J Infect Dis 1998; 177:1247–1259. [DOI] [PubMed] [Google Scholar]
- 127.Forthal DN, Landucci G, Cole KS, Marthas M, Becerra JC, Van Rompay K. Rhesus macaque polyclonal and monoclonal antibodies inhibit simian immunodeficiency virus in the presence of human or autologous rhesus effector cells. J Virol 2006; 80:9217–9225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Florese RH, Van Rompay KKAV, Aldrich K, Forthal DN, Landucci G, Mahalanabis M, et al. Evaluation of Passively Transferred, Nonneutralizing Antibody-Dependent Cellular Cytotoxicity-Mediating IgG in Protection of Neonatal Rhesus Macaques against Oral SIVmac251 Challenge. J Immunol 2006; 177:4028–4036. [DOI] [PubMed] [Google Scholar]
- 129.Burton DR, Hessell AJ, Keele BF, Klasse PJ, Ketas TA, Moldt B, et al. Limited or no protection by weakly or nonneutralizing antibodies against vaginal SHIV challenge of macaques compared with a strongly neutralizing antibody. Proc Natl Acad Sci U S A 2011; 108:11181–11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dugast A-S, Chan Y, Hoffner M, Licht A, Nkolola J, Li H, et al. Lack of protection following passive transfer of polyclonal highly functional low-dose non-neutralizing antibodies. PloS One 2014; 9:e97229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Moog C, Dereuddre-Bosquet N, Teillaud J-L, Biedma ME, Holl V, Van Ham G, et al. Protective effect of vaginal application of neutralizing and nonneutralizing inhibitory antibodies against vaginal SHIV challenge in macaques. Mucosal Immunol 2014; 7:46–56. [DOI] [PubMed] [Google Scholar]
- 132.Santra S, Tomaras GD, Warrier R, Nicely NI, Liao H-X, Pollara J, et al. Human Non-neutralizing HIV-1 Envelope Monoclonal Antibodies Limit the Number of Founder Viruses during SHIV Mucosal Infection in Rhesus Macaques. PLOS Pathog 2015; 11:e1005042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hessell AJ, Shapiro MB, Powell R, Malherbe DC, McBurney SP, Pandey S, et al. Reduced Cell-Associated DNA and Improved Viral Control in Macaques following Passive Transfer of a Single Anti-V2 Monoclonal Antibody and Repeated Simian/Human Immunodeficiency Virus Challenges. J Virol 2018; 92:e02198–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Siegel F, Kurth R, Norley S. Neither whole inactivated virus immunogen nor passive immunoglobulin transfer protects against SIVagm infection in the African green monkey natural host. J Acquir Immune Defic Syndr Hum Retrovirology Off Publ Int Retrovirology Assoc 1995; 8:217–226. [DOI] [PubMed] [Google Scholar]
- 135.Astronomo RD, Santra S, Ballweber-Fleming L, Westerberg KG, Mach L, Hensley-McBain T, et al. Neutralization Takes Precedence Over IgG or IgA Isotype-related Functions in Mucosal HIV-1 Antibody-mediated Protection. EBioMedicine 2016; 14:97–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Horwitz JA, Bar-On Y, Lu C-L, Fera D, Lockhart AAK, Lorenzi JCC, et al. Non-neutralizing Antibodies Alter the Course of HIV-1 Infection In Vivo. Cell 2017; 170:637–648.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.FORTHAL DN, LANDUCCI G, CHOHAN B, RICHARDSON BA, MCCLELLAND RS, JAOKO W, et al. Antibody-dependent cell-mediated virus inhibition (ADCVI) antibody activity does not correlate with risk of HIV-1 superinfection. J Acquir Immune Defic Syndr 1999 2013; 63:31–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-Specific Antibodies Capable of ADCC Are Common in Breastmilk and Are Associated with Reduced Risk of Transmission in Women with High Viral Loads. PLOS Pathog 2012; 8:e1002739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Onyango-Makumbi C, Omer SB, Mubiru M, Moulton LH, Nakabiito C, Musoke P, et al. Safety and efficacy of HIV hyperimmune globulin for prevention of mother-to-child HIV transmission in HIV-1-infected pregnant women and their infants in Kampala, Uganda (HIVIGLOB/NVP STUDY). J Acquir Immune Defic Syndr 1999 2011; 58:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Guay LA, Musoke P, Hom DL, Nakabiito C, Bagenda D, Fletcher CV, et al. Phase I/II trial of HIV-1 hyperimmune globulin for the prevention of HIV-1 vertical transmission in Uganda. AIDS Lond Engl 2002; 16:1391–1400. [DOI] [PubMed] [Google Scholar]
- 141.Barouch DH, Alter G, Broge T, Linde C, Ackerman ME, Brown EP, et al. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 2015; 349:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature 2012; 482:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lai L, Kwa S-F, Kozlowski PA, Montefiori DC, Nolen TL, Hudgens MG, et al. SIVmac239 MVA vaccine with and without a DNA prime, similar prevention of infection by a repeated dose SIVsmE660 challenge despite different immune responses. Vaccine 2012; 30:1737–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fouts TR, Bagley K, Prado IJ, Bobb KL, Schwartz JA, Xu R, et al. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A 2015; 112:E992–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–2220. [DOI] [PubMed] [Google Scholar]
- 146.Bomsel M, Tudor D, Drillet A-S, Alfsen A, Ganor Y, Roger M-G, et al. Immunization with HIV-1 gp41 subunit virosomes induces mucosal antibodies protecting nonhuman primates against vaginal SHIV challenges. Immunity 2011; 34:269–280. [DOI] [PubMed] [Google Scholar]
- 147.Flynn NM, Forthal DN, Harro CD, Judson FN, Mayer KH, Para MF, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005; 191:654–665. [DOI] [PubMed] [Google Scholar]
- 148.Forthal DN, Gilbert PB, Landucci G, Phan T. Recombinant gp120 vaccine-induced antibodies inhibit clinical strains of HIV-1 in the presence of Fc receptor-bearing effector cells and correlate inversely with HIV infection rate. J Immunol Baltim Md 1950 2007; 178:6596–6603. [DOI] [PubMed] [Google Scholar]
- 149.Yates NL, Liao H-X, Fong Y, deCamp A, Vandergrift NA, Williams WT, et al. Vaccine-induced Env V1-V2 IgG3 correlates with lower HIV-1 infection risk and declines soon after vaccination. Sci Transl Med 2014; 6:228ra39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hessell AJ, Hangartner L, Hunter M, Havenith CEG, Beurskens FJ, Bakker JM, et al. Fc receptor but not complement binding is important in antibody protection against HIV. Nature 2007; 449:101–104. [DOI] [PubMed] [Google Scholar]
- 151.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, et al. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med 2009; 15:951–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Bournazos S, Klein F, Pietzsch J, Seaman MS, Nussenzweig MC, Ravetch JV. Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity. Cell 2014; 158:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moldt B, Shibata-Koyama M, Rakasz EG, Schultz N, Kanda Y, Dunlop DC, et al. A nonfucosylated variant of the anti-HIV-1 monoclonal antibody b12 has enhanced FcγRIIIa-mediated antiviral activity in vitro but does not improve protection against mucosal SHIV challenge in macaques. J Virol 2012; 86:6189–6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Banks ND, Kinsey N, Clements J, Hildreth JEK. Sustained antibody-dependent cell-mediated cytotoxicity (ADCC) in SIV-infected macaques correlates with delayed progression to AIDS. AIDS Res Hum Retroviruses 2002; 18:1197–1205. [DOI] [PubMed] [Google Scholar]
- 155.Gómez-Román VR, Patterson LJ, Venzon D, Liewehr D, Aldrich K, Florese R, et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with SIVmac251. J Immunol Baltim Md 1950 2005; 174:2185–2189. [DOI] [PubMed] [Google Scholar]
- 156.Hidajat R, Xiao P, Zhou Q, Venzon D, Summers LE, Kalyanaraman VS, et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol 2009; 83:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xiao P, Patterson LJ, Kuate S, Brocca-Cofano E, Thomas MA, Venzon D, et al. Replicating adenovirus-simian immunodeficiency virus (SIV) recombinant priming and envelope protein boosting elicits localized, mucosal IgA immunity in rhesus macaques correlated with delayed acquisition following a repeated low-dose rectal SIV(mac251) challenge. J Virol 2012; 86:4644–4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xiao P, Zhao J, Patterson LJ, Brocca-Cofano E, Venzon D, Kozlowski PA, et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 2010; 84:7161–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Binley JM, Clas B, Gettie A, Vesanen M, Montefiori DC, Sawyer L, et al. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 2000; 270:237–249. [DOI] [PubMed] [Google Scholar]
- 160.Forthal DN, Landucci G, Haubrich R, Keenan B, Kuppermann BD, Tilles JG, et al. Antibody-dependent cellular cytotoxicity independently predicts survival in severely immunocompromised human immunodeficiency virus-infected patients. J Infect Dis 1999; 180:1338–1341. [DOI] [PubMed] [Google Scholar]
- 161.Madhavi V, Wines BD, Amin J, Emery S, Lopez E, Kelleher A, et al. HIV-1 Env- and Vpu-Specific Antibody-Dependent Cellular Cytotoxicity Responses Associated with Elite Control of HIV. J Virol 2017; 91. doi: 10.1128/JVI.00700-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lambotte O, Ferrari G, Moog C, Yates NL, Liao H-X, Parks RJ, et al. Heterogeneous neutralizing antibody and antibody-dependent cell cytotoxicity responses in HIV-1 elite controllers. AIDS Lond Engl 2009; 23:897–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Caskey M, Schoofs T, Gruell H, Settler A, Karagounis T, Kreider EF, et al. Antibody 10–1074 suppresses viremia in HIV-1-infected individuals. Nat Med 2017; 23:185–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Caskey M, Klein F, Lorenzi JCC, Seaman MS, West AP, Buckley N, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature 2015; 522:487–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lu C-L, Murakowski DK, Bournazos S, Schoofs T, Sarkar D, Halper-Stromberg A, et al. Enhanced clearance of HIV-1-infected cells by broadly neutralizing antibodies against HIV-1 in vivo. Science 2016; 352:1001–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]