Abstract
In order to efficiently process incoming visual information, selective attention acts as a filter that enhances relevant and suppresses irrelevant information. In this study, we used an event-related potential (ERP) approach with systematic lateralization to investigate enhancement and suppression during encoding of information into visual working memory (WM) separately. We used a change detection task in which observers had to memorize some items while ignoring other items. We found that the to-be-ignored items elicited a PD component in the ERP, suggesting that irrelevant information is actively suppressed from WM. The PD amplitude increased with distractor load and decreased with the ability to group distractors according to Gestalt principles. This suggests that the PD can be used as an indicator of how efficiently items can be suppressed from entering WM. Furthermore, while lateral memory-targets elicited a “traditional” CDA (starting ~300 ms), lateral memory-distractors elicited a sustained positivity contralateral to memory-distractors (CDAp, starting ~400 ms). In sum the results suggest that inhibition of irrelevant information is an important factor for efficient WM and is reflected in spontaneous (PD) and sustained suppression (CDAp).
Keywords: working memory, visual attention, suppression, event-related potentials
Introduction
Our ability to maintain information in working memory (WM) is limited to a small amount of information from the environment within a given moment (Cowan 2001; Vogel Woodman and Luck 2001). Because the visual system is often confronted with inputs that greatly exceed these limits, it is critical to selectively regulate access so that only task-relevant information is represented in this finite mnemonic resource. Thus, attentional filtering of task-irrelevant information is crucial way to optimize the operation of this limited capacity by ensuring that few resources are misspent on irrelevant information. Indeed, numerous studies over the past decade have begun to elucidate the critical role that this filtering process plays in the successful completion of many cognitive tasks. In particular, individual differences in the efficiency of filtering distracting information are strongly predictive of an individual’s WM capacity, suggesting that the ability to regulate access to WM is a critical feature in an individual’s overall cognitive and intellectual ability (Vogel et al. 2005; Astle et al. 2008; Fukuda and Vogel 2011; Linke et al. 2011). For example, Vogel et al. (2005) asked subjects to remember only the red items from displays containing a mix of red and blue objects while they measured the contralateral delay activity (CDA)—an event-related potential (ERP) component that is highly sensitive to the number of items that are currently being held in WM. They observed that while individuals with a high WM capacity managed to maintain only the task-relevant items in memory, their low capacity counterparts unnecessarily stored both the irrelevant and relevant items in memory. This general finding has been replicated and extended by several groups that have observed that both neurological patients and healthy elderly adults with low WM are slower and less effective at controlling access to the limited representational space of WM (Fuller et al. 2005; Lee et al. 2010; Jost et al. 2011).
While it is clear that effective attentional filtering is crucial for a well-functioning WM, the precise means by which filtering is achieved still remain unclear. This is due to the fact that attentional filtering could be achieved via the enhancement of the targets, the active suppression of the distractors or some combination of enhancement and suppression. A primary challenge to answering this question is that each of these alternatives would be expected to produce the same outcome: the selective representation of targets in WM, as in the CDA results described above. Indeed, many existing neural measures of attentional selection are ambiguous on this point because they generally reflect the consequences of attention, that could likely be driven by some combination of enhancement and suppression mechanisms. Recently, however, a new ERP component has been reported that appears to specifically reflect an active suppression mechanism that is applied to salient distractors presented within a visual search task. By using systematic stimulus lateralization procedures (Woodman and Luck 2003; Hickey et al. 2006, 2009; Feldmann-Wüstefeld and Schubö 2013a; Gaspar and Mcdonald 2014; Burra et al. 2016), the distractor positivity (PD) component reflecting suppression can be disentangled from the N2pc component which is known to reflect the attentional prioritization of targets. In the present study, we seek to test whether active suppression of task-irrelevant items plays a role in successful filtering of information from WM storage by measuring the PD component within the context of a WM task.
We will test the hypothesis that filtering information in WM requires the active suppression of distracting information prior to reaching WM. We will test this hypothesis in 3 ways. An initial test is simply whether a PD component will be observed while subjects perform a WM filtering task. While we are unaware of any prior studies reporting a PD during a WM task, it is fairly likely we will observe one. This is because the typical visual search task contexts in which the PD is regularly observed have many comparable attentional demands as WM filtering tasks in which one or more targets must be selected amongst a field of task-irrelevant distractors (Luria and Vogel 2011). However, a more thorough test of the contribution of distractor suppression would be whether the varying demands for distractor exclusion modulate the amount of suppression as measured by the PD. To do this, across 3 experiments we will manipulate the amount of information in a display that must be excluded from WM storage and test whether the amplitude and duration of the PD increase with growing demands for distractor exclusion. Finally, we will test whether the amount of distractor suppression observed for an individual subject is related to his or her specific WM capacity. As reviewed earlier, low WM capacity individuals are well known to have difficulty excluding distracting information in many contexts (Vogel et al. 2005; Astle et al. 2008; Fukuda and Vogel 2011; Linke et al. 2011). Consequently, if distractor suppression plays a significant role in successfully executing this exclusion process, we would expect that high WM capacity individuals would show larger PD amplitudes than low capacity individuals. Indeed, there has been a recent report of a positive correlation between the amplitude and duration of the PD during a visual search task and individual differences in a visual change detection WM capacity task (Gaspar et al. 2016). Here, we will test for that specific relationship directly by measuring both WM capacity and PD in a change detection task. To obtain adequate statistical power, we will test for this relationship by pooling all of the subjects across the 3 experiments (N = 90). We also expect to observe an N2pc and CDA component that typically occur in lateralized change detection tasks and reflect selection and maintenance of relevant information. Since we only manipulated distractor, but not target features, we do not have any a priori assumptions about their modulation in the present study.
Methods
Rationale and Overview
The present study seeks to translate the logic of the systematic lateralization approach from visual search to visual WM. Rather than separately lateralizing targets and distractors in a search task, we will separately lateralize them in a change detection task. In half the trials colored squares, serving as targets, were presented laterally while colored circles, serving as distractors, were presented on the vertical midline. These trials allowed us to isolate the pure target-related activity by measuring the lateralized activity (contra minus ipsilateral to distractors) because distractors could not affect the lateralized ERP. In the other half of the trials, targets were presented on the vertical midline and distractors were presented laterally. Analogously, these trials allowed us to isolate the distractor-related activity because targets could not affect the lateralized ERP (Hickey et al. 2009, 2010, 2011). This systematic lateralization approach allowed us to isolate the distractor-related activity and to investigate if a PD component can be observed in a WM task which would suggest that active suppression is involved in maintaining relevant information in WM and reducing unnecessary storage of irrelevant items. If distractors in a WM task elicited a PD, we can furthermore examine how active suppression varies as a function of filtering needs, for example, by using different distractor loads.
In Experiments 1 and 2, the number of targets was kept constant while the number of distractors varied between 2 and 4 (Experiment 1) or between 2, 4, and 6 (Experiment 2). This allowed us to investigate how an increasing distractor load renders suppression of irrelevant information more effortful. Finally in Experiment 3 we kept both the number of targets (2) and the number of distractors (4) stable but varied the color homogeneity of the distractors: the 4 distractors had unique colors (heterogeneous) or had 2 colors, that is, 2 pairs of equally colored distractors were shown. This allowed us to investigate how grouping of similar distractors facilitates suppression of irrelevant information. In all 3 experiments we measured WM capacity (K) in a separate standard change detection task prior to performing the WM filtering task in which we measure the PD.
Experiment 1
Participants
Overall, 31 volunteers naïve to the objective of the experiment participated for payment (~15 USD per hour). The data of 5 participants were excluded from the analysis because fewer than 100 trials per condition remained after exclusion of incorrect trials and trials contaminated with eye-related artifacts (see below for criteria). The remaining 26 participants (13 males) were all but one right-handed, aged 18–28 years (M = 21.2, SD = 2.6) and reported normal or corrected-to-normal visual acuity as well as normal color vision. The experiment was conducted with the written understanding and consent of each participant.
Apparatus
Participants were seated in a comfortable chair in a dimly lit, electrically shielded and sound attenuated chamber. Participants responded with button presses on a standard keyboard that was placed in front of them. Stimulus presentation and response collection were controlled by a Windows PC using PsychToolBox 3 routines in Matlab (version 8.6.0). All stimuli were presented on a LCD-TN screen (BenQ XL2430-B) placed at 110 cm distance from participants.
Stimuli
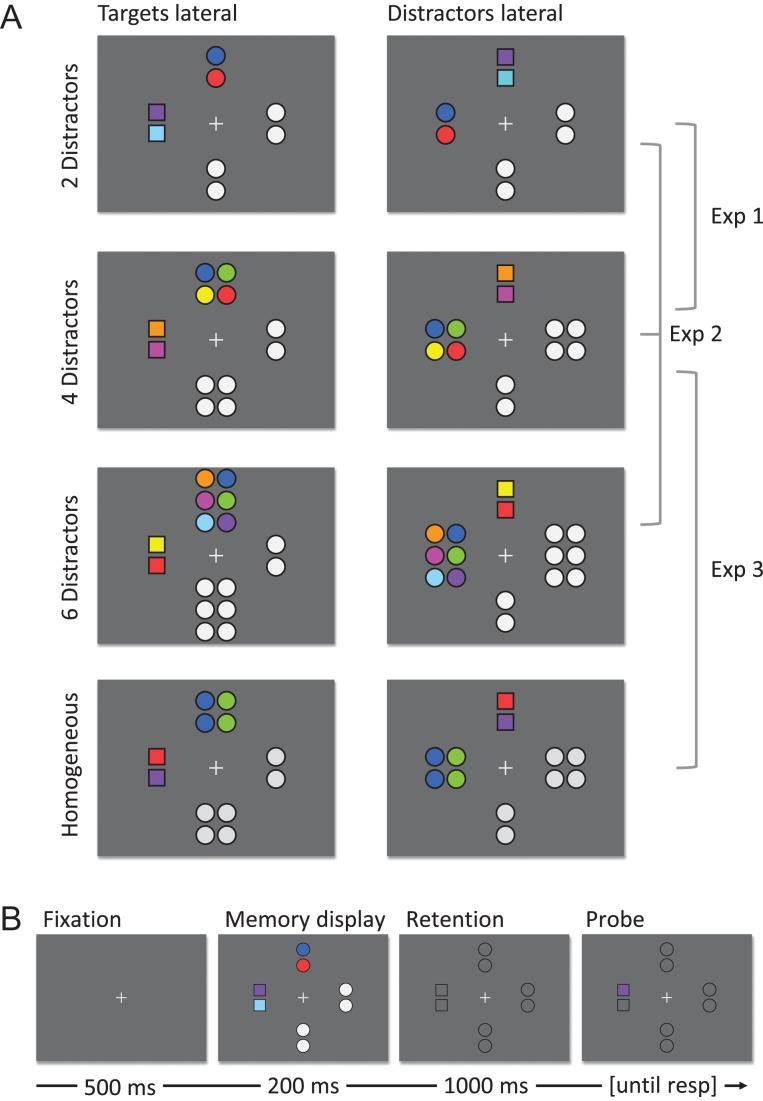
In the WM filtering task, memory displays showed colored squares, serving as targets and colored and grey circles, serving as distractors. In the distractor load 2 condition, 2 colored squares, 2 colored circles, and 4 grey circles (2 groups of 2) were presented. In the distractor load 4 condition, 2 colored squares, 4 colored circles, and 6 grey circles (1 group of 2 and 1 group of 4) were presented. Squares had a side length of 0.9° visual angle and circles had a diameter of 1.0° (squares and circles covered the same area, viz., 3600 pixels). Colors for squares and circles were drawn randomly from a set of 9 colors (RGB values: red [255-0-0], light green [0-255-0], blue [0-0-255], yellow [255-255-0], magenta [255-0-255], cyan [0-255-255], dark green [20-80-20], purple [50-0-100], and orange [255-128-0]). No color was repeated within one memory display. Grey for uncolored circles was 128-128-128 and matched to the average luminance of the colors (175 cd/m2). Items were presented in 4 groups, one groups of colored squares (target group), one group of colored circles (salient distractor group) and 2 groups of grey circles (neutral distractor group), see Figure 1A. Each group was presented in 1 of 4 positions. Two groups were on the vertical midline, 3.4° above and below the center of the screen (measured from the center of the screen to the center of the group). The remaining 2 positions were on the horizontal midline, 3.4° left and right of the center of the screen (center–center). In the target lateral condition (50% of the trials), the target group was presented in the left or right position (equiprobably) and the salient distractor group was presented in the top or bottom position (equiprobably). In the remaining 50% of the trials, this was inversed. The remaining positions were filled with neutral distractor groups. Target groups and salient distractor groups never appeared both on the left/right or both on the top/bottom position. Neutral distractor groups always consisted of an equal number of items as the target group/distractor group on the opposite side. The distance between 2 items within a group was 1.2° (center to center). All items had dark grey (32-32-32) outlines of 2 pixels width. The retention interval display showed a fixation cross and the outlines of all items from the memory display (without any color). The probe display also showed the fixation cross and the outlines, and in addition one of the 2 or 4 squares was filled with color. In 50% of the trials, the color was identical to the color shown in the memory display at this location. In the remaining 50% of the trials, the color was chosen randomly as 1 of the other 8 colors.
Figure 1.
(A) Memory displays used in the WM filtering task in Experiment 1–3. The left column shows memory displays in which targets are presented laterally and distractors are presented on the vertical midline. These trials allowed to isolate target-related processing and extract target-elicited lateralized ERP components (N2pc, CDA). The right column shows memory displays in which distractors are presented laterally and targets are presented on the vertical midline. These trials allowed to isolate distractor-related processing and extract distractor-elicited lateralized ERP components (early PD, PD, CDAp). In Experiment 1, two targets were presented together with two or four distractors. In Experiment 2, in addition, six distractor could be presented. In Experiment 3, two targets and four distractors were presented in each trial; the distractor could be homogeneous or heterogeneous. (B) Shows a trial sequence. Each trial started with a fixation cross that was followed by the memory display. Subsequently an empty screen (with just placeholders) was presented before a probe appeared at one of the previous target locations. Participants had to decide whether the probe had the same color as the target previously presented at the same location.
Prior to the WM filtering task, a standard change detection task (without systematic lateralization and without distractors) was used to measure K as an estimate for participants’ WM capacity. The 4, 6, or 8 squares identical in size and color to the WM filtering task were shown without circles. Squares were randomly distributed across the visual field with the constraint that items had to be at least 1.5 times apart from each other, that the left and right hemifield had the same number of items, and that the upper and lower hemifield had (as close as possible) the same number of items. No outlines were shown during memory display, retention interval or probe display. The memory display showed one of the squares from the memory display, in 50% of the trials in the same and in the remaining 50% of the trials in a different color from the memory display, similar to the WM filtering task. The standard change detection task was used to make results more comparable to previous studies and to have a sufficiently high number of memory items that would allow a better estimate of the WM capacity (larger set sizes allow more reliable estimates of K) (Adam and Vogel 2016).
Procedure
The procedure was identical for the standard change detection task and the WM filtering task. A trial started with a fixation cross (RGB: 60-60-60; 0.3°) that was presented for 500 ms (Fig. 1B). Then, the memory display was presented for 200 ms in addition to the fixation cross. Participants were instructed to remember the color and location of the rectangles. In the WM filtering task, participants were furthermore instructed to ignore all circles and (correctly) informed that circles cannot change color between the memory and probe display. The memory display was followed by a retention interval of 1000 ms; this was a black screen with a fixation cross in the pretest; in the WM filtering task, the outlines of the memory items were shown additionally in order to make it easier for observers to not confuse the 2/4 target items that were in close proximity. The probe display followed and was presented until participants pressed one of the 2 buttons. Participants were instructed to press the left button (“z”) with their left index finger for “same color” (square at probed location did not change color compared with the memory display) and to press the right button (“?”) with their right index finger for “different color” (square at probed location did change color). They were instructed to respond as accurately as possible. The trial ended with the button press and a black screen was presented during an 1000 ms intertrial interval. The onset of the next fixation cross indicated the start of a new trial.
Prior to the pretest, participants performed a couple of practice trials that were identical to the pretest with the exception that immediate feedback (“correct”, “wrong”) was provided after each button press before the next trial started. The practice was aborted when participants indicated they understood the task and felt confident doing the task. No practice for the WM filtering task was provided. The pretest consisted of 144 trials (4 blocks of 36 trials), 48 trials for each set size of 4, 6, or 8 targets. The WM filtering task consisted of 800 trials (25 blocks of 32 trials), 200 trials for each of the condition of the 2 × 2 design matrix: Set size (2 vs. 4) × laterality (lateral targets vs. lateral distractors). Average performance (change detection accuracy) and a minimum break of 30 s were provided after each block for both the pretest and the WM filtering task.
EEG Recording
EEG was recorded with Ag–AgCl active electrodes (BrainProducts actiCap) from 32 scalp sites (according to the International 10/20 System: FP1/2, F7/8, F3/4, Fz, FC5/6, FC1/2, C3/4, Cz, TP9/10, CP5/6, CP1/2, P7/8, P3/4, PO7/8, PO3/4, Pz, O1/2, Oz). Horizontal and vertical EOGs were recorded with passive electrodes bipolarly from the outer canthi of the eyes and from above and below the observers’ right eye, respectively. FPz served as the ground electrode and all electrodes were referenced to TP10 and re-referenced off-line to the average of all electrodes. Impedances for active electrodes were kept below 10 kΩ. Sampling rate was 1000 Hz with a high cutoff filter of 125 Hz and a low cutoff filter of 0.01 Hz (half power cutoff, 24 dB rolloff).
Data Analysis
Behavioral data
To calculate the WM capacity measure K, hit rate (ratio of correctly identified changes) and correct rejection rate (ratio of correctly identified color repetitions) were calculated separately for each set size in the WM filtering task. K for each distractor load was calculated as: KSetSize = Set size × Hit rate × (1-correct rejection rate) and forwarded to a t-test for dependent measures to evaluate the effect of distractor load on WM capacity. Both K and RT was analyzed for trials used in the EEG analysis (see below). For the pretest, K was calculated across Set sizes since this was not a variable of interest.
EEG data
EEG was not analyzed for the pretest as no systematic lateralization of targets/distractors or a lateralized cue was used in that task. For the WM filtering task, EEG was averaged off-line over a 1200-ms epoch including a 200-ms prestimulus baseline with epochs time-locked to memory display onset. Trials with incorrect responses and blinks or saccades between 0 ms and 800 ms were excluded from analysis. Blinks were defined by an absolute amplitude of the vertical EOG exceeding 100 μV. Saccades were defined by an amplitude difference of the horizontal EOG of more than 25 μV for the average ERP in a 50 ms window compared with the average of the succeeding 50 ms window (step criterion). Additionally, segments were excluded from further analysis on an individual-channel basis when the absolute voltage exceeded 100 μV. Data from 5 participants for whom less than 100 trials per condition remained (because of artifacts or incorrect responses) were not used for data analyses. The remaining 26 participants had 15.9 % of unusable trials on average (SD = 8.8%).
Mean contralateral and ipsilateral activity in the ERP was calculated for each participant for the electrode pool PO7/8, separately for each set size, each laterality condition, and separately for sites contra- and ipsilateral to the targets/distractors, resulting in 8 waveforms. To determine the epochs used for statistical analyses of various ERP components, both the lateralized ERP (contra minus ipsi) for trials with lateral targets and for trials with lateral distractors were collapsed across set size conditions (“condition blind”), resulting in 2 waveforms, showing lateralized activity due to targets and lateralized activity due to distractors. The target-N2pc epoch was determined as ± 50 ms from the most negative peak between 200 ms and 300 ms in the lateral-targets grand average waveform. The PD epoch was determined as ± 50 ms from the most positive peak between 250 ms and 350 ms in the lateral-distractors waveform. The mean amplitude for these time windows was calculated separately for laterality (contra versus ipsi), each distractor load (2 vs. 4), and each participant. This resulted in 4 values for each component (N2pc, PD) and each participant. For each component, a 2-way ANOVA with the within-subjects factors laterality (contra, ipsi) and distractor load (2, 4) was calculated.
Unsurprisingly, visual inspection of the lateralized waveforms showed that the CDA did not show a clear peak. We used an analysis epoch of maximally 350–750 ms for the CDA. Within that time window, the starting point of the analysis epoch was determined as the first point in time when (across conditions) the amplitude was negative (CDA). The end point of the analysis epoch was determined as the first point after the starting point when (across conditions) the amplitude was positive (CDA). Mean amplitude within these epochs was calculated and forwarded to a 2-way ANOVA with the within-subjects factors laterality (contra, ipsi) and distractor load (2, 4).
The inter-relation between WM capacity and neural markers of attention and WM was investigated by computing Pearson’s product–moment correlation for mean K (N*(hit rate – false alarm rate)/(1 – false alarm rate)) in the pretest and the mean amplitude of each ERP component in the WM filtering task. Components based on signed area were corrected for noise by subtracting the singed-area baseline activity before correlating them. As measures of effect size, partial eta squared (η2) is reported for ANOVAs. Greenhouse-Geisser correction was applied in all analyses when appropriate.
Results Experiment 1
Behavioral Data
The response time (RT) for distractor load 2 (M = 675 ms) and distractor load 4 (M = 669 ms) were only marginally different, t(25) = 1.47, P = 0.077. The WM capacity (K) for distractor load 2 (M = 1.64) and distractor load 4 (M = 1.67) were only marginally different, t(25) = 1.60, P = 0.062. Note that the theoretical maximum K in this Experiment is 2 as there were always 2 targets.
Target-N2pc (239–339 ms)
Targets elicited an N2pc (Fig. 2), that is, a more negative deflection in the ERP at electrodes contralateral (M = 0.19 μV) than at electrodes ipsilateral to targets (1.20 μV), main effect of laterality, F(1,25) = 15.81, P = 0.001, η2 = 0.387. The mean amplitude was more positive for distractor load 4 (M = 0.94 μV) than for distractor load 2 (M = 0.45 μV), main effect of distractor load, F(1,25) = 15.02, P = 0.001, η2 = 0.375. There was no interaction of laterality and distractor load, P = 0.106.
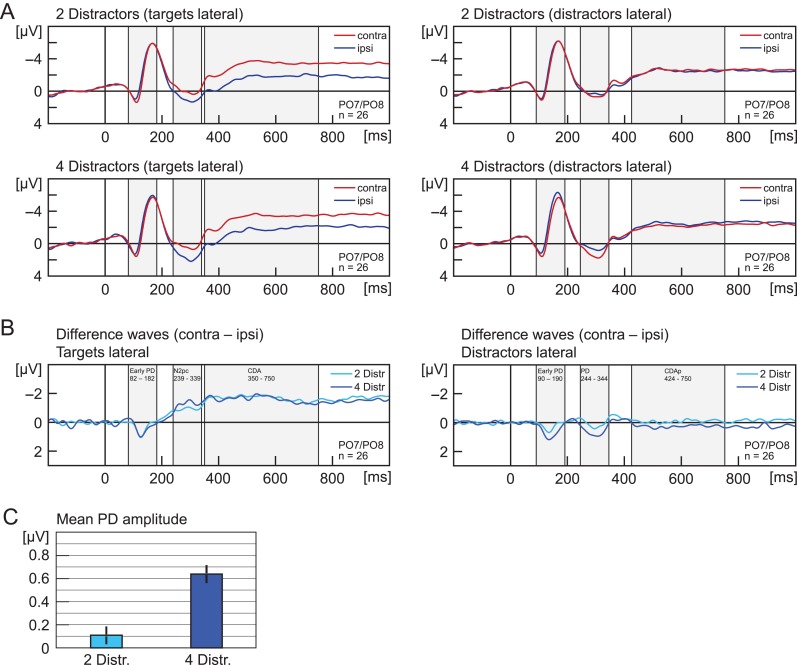
Figure 2.
(A) Experiment 1. Grand average ERPs recorded at PO7/PO8, elicited by memory displays with lateral targets (left panels) and lateral distractors (right panels). Upper panels show activity in trials with 2 distractors, lower panels for 4 distractors. Red lines reflect activity contralateral and blue lines activity ipsilateral to the lateralized items. Grey shades indicate the analysis time window that was used to calculate mean amplitude of ERP components (N2pc, early PD, PD, CDA, CDAp). (B) Shows the same data as (A) but as difference waves (contra minus ipsi). (C) Shows the mean amplitude for the PD. Error bars indicate the standard error of the mean (corrected for individual differences; Cousineau 2005). Light blue indicates lateralized activity for trials with 2 distractors, dark blue for 4 distractors. All waveforms were low-pass filtered at 30 Hz (half power cutoff, 24 dB) using digital filtering.
Distractor Positivity (244–344 ms)
Distractors elicited a PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = 0.72 μV) than at electrodes ipsilateral to distractors (0.35 μV), main effect of laterality, F(1,25) = 11.47, P = 0.002, η2 = 0.315. The mean amplitude was more positive for distractor load 4 (M = 0.77 μV) than for distractor load 2 (M = 0.30 μV), main effect of distractor load, F(1,25) = 9.45, P = 0.005, η2 = 0.274. The PD was larger for distractor load 4 (ΔM = 0.64 μV) than 2 (ΔM = 0.11 μV), interaction of laterality and distractor load, F(1,25) = 14.51, P = 0.001, η2 = 0.367. Follow-up t-tests showed that the PD was reliable for distractor load 4 (t(25) = 4.53, P < 0.001), but not for distractor load 2 (t(25) = 0.92, P = 0.183).
CDA (350–750 ms)
Targets elicited an CDA, that is, a more negative deflection in the ERP at electrodes contralateral (M = −2.88 μV) than at electrodes ipsilateral to targets (M = −1.38 μV), main effect of laterality, F(1,25) = 24.61, P < 0.001, η2 = 0.496. No other effects were significant (all P > 0.633).
Exploratory Analyses
From the literature, there is at least one component that often co-occurs with the PD component in displays with lateralized stimuli, the early PD (also termed Ppc, positivity posterior contralateral). The early PD can be elicited by targets or distractors (Corriveau et al. 2012) and was argued to reflect initial processing of stimuli or to represent physical salience (Fortier-Gauthier et al. 2012; Jannati et al. 2013). A recent study challenged the notion that the early PD merely reflects salience processing (Weaver et al. 2017). The authors found that the amplitude of the distractor-elicited early PD predicted the accuracy for reporting a concurrently presented target, suggesting that the early PD reflects early distractor suppression interpretation. As the reliability of these components as well as the cognitive mechanisms that they reflect still remain unclear, we had no a priori predictions regarding the presence and modulation of the early PD. Visual inspection of the lateralized waveforms showed an early PD along with a late contralateral positivity in a similar time range as the CDA that we will refer to as CDAp (CDA of positive polarity).
Early PD
The early PD epoch was determined similarly to the N2pc and PD, as ±50 ms from the most positive peak between 100 ms and 200 ms in the lateral-targets waveform (82–182 ms) and in the lateral-distractors waveform (90–190 ms). Targets elicited an early PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = −1.96 μV) than at electrodes ipsilateral to targets (M = −2.30 μV), main effect of laterality, F(1,25) = 8.89, P = 0.006, η2 = 0.262. No other effects were significant (all P > 0.519). Distractors also elicited an early PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = −2.38 μV) than at electrodes ipsilateral to distractors (M = −2.80 μV), main effect of laterality, F(1,25) = 13.16, P = 0.001, η2 = 0.345. The distractor-elicited early PD was larger for distractor load 4 (ΔM = 0.61 μV) than 2 (ΔM = 0.22 μV), interaction of laterality and distractor load, F(1,25) = 13.69, P = 0.001, η2 = 0.354. Follow-up t-tests showed that the distractor-elicited early PD was reliable for both distractor load 4 (P < 0.001, t(25) = 4.54), and distractor load 2 (P = 0.036, t(25) = 1.89).
CDAp
The distractor-elicited positive CDA (CDAp) was analyzed similarly to the CDA but with inversed polarity (i.e., first time point within 350–750 ms with positive amplitude until first time point within 350–750 ms with negative amplitude, across conditions). The CDAp time window was determined as 424–750 ms. Results showed that there was no main effect of laterality or distractor load (all P > 0.210), but an interaction of laterality and distractor load revealed that the amplitude of the lateralized ERP was modulated by distractor load, F(1,25) = 4.67, P = 0.040, η2 = 0.157. Follow-up t-tests showed that for distractor load 2, no lateralized ERP was found (ΔM = −0.09 μV), t(25) = 0.64, P = 0.265, whereas for distractor load 4, distractors elicited a CDAp, that is, a more positive deflection in the ERP at electrodes contralateral than at electrodes ipsilateral to distractors (ΔM = 0.24 μV), t(25) = 2.29, P = 0.015.
Intermediate Discussion
Experiment 1 showed that when filtering needs increase due to a larger number of salient distractors, a larger PD component was observed. This is the first direct evidence that active suppression as reflected in the PD component is involved in encoding stimuli into WM. The N2pc and the CDA component were not modulated by the number of distractors, suggesting that attentional prioritization and maintenance of relevant items in WM was not modulated by distractor load. A novel component, the CDAp was identified, suggesting sustained suppression of locations that show irrelevant, potentially distracting information. An open question is how much suppression can be applied to distractors during WM encoding. It is known that the CDA as a marker of WM maintenance increases with the number of items stored in WM up to a point that corresponds to an individual’s WM capacity (Vogel and Machizawa 2004). Experiment 2 will address if there is an upper limit for suppression, that is, a “suppression capacity” as well by further increasing the number of distractor to 6.
Methods Experiment 2
Participants
Overall, 37 volunteers naïve to the objective of the experiment participated for payment (~15 USD per hour). The data of 5 participants were excluded from data analysis because fewer than 100 trials per condition remained after exclusion of incorrect trials and trials contaminated with eye-related artifacts. The remaining 32 participants (16 males) were all but 5 right-handed, aged 18–32 years (M = 25.0, SD = 3.7) and reported normal or corrected-to-normal visual acuity as well as normal color vision. The experiment was conducted with the written understanding and consent of each participant.
Stimuli, Procedure and Data Analysis
Identical to Experiment 1 if not otherwise noted. The number of targets was always 2 and the number of distractors was 2, 4, or 6. The spatial arrangement for 2 and 4 distractors was identical to Experiments 1 (Fig. 1A). When 6 distractors were presented, they appeared in 2 columns of 3 rows, with the identical inter-item distance as in Experiments 1 (Fig. 1). To keep the number of trials per condition identical, the WM filtering task consisted of 1200 trials, presented in 40 blocks of 30 trials. The number of neutral distractors (grey circles) was again matched to the number of salient distractors (colored circles), that is, there were 2, 4, or 6 grey circles in the opposite position from the 2, 4, or 6 colored circles. EEG was recorded with the same system and recording settings and from the dame sites as in Experiments 2 and 3. To increase reliability of the WM capacity measure K, the number of trials in the standard change detection task was increased by 72 to a total number of 216 trials, presented in 6 blocks of 36 trials. Participants had 14.9% of unusable trials on average (SD = 10.0). Analysis epochs were determined as in Experiment 1, see results for each component.
Results Experiment 2
Behavioral Data
RT was not modulated by distractor load, F(2,62) = 2.33, P = 0.105., η2 = 0.070. The WM capacity (K) for trials with 2 (M = 1.73), 4 (M = 1.76), and 6 distractors (M = 1.75) was not statistically different, F(2,62) = 2.01, P = 0.143. Note that the theoretical maximum K in this Experiment is 2 as there were always 2 targets.
Target-N2pc (209–309 ms)
Targets elicited an N2pc (Fig. 3), that is, a more negative deflection in the ERP at electrodes contralateral (M = 0.51 μV) than at electrodes ipsilateral to targets (M = 1.33 μV), main effect of laterality, F(1,31) = 19.60, P < 0.001, η2 = 0.387. The mean amplitude was modulated by distractor load, being smallest for distractor load 2 (M = 0.30 μV), larger for distractor load 4 (M = 1.15 μV) and largest for distractor load 6 (M = 1.30 μV), main effect of distractor load, F(2,62) = 35.28, P < 0.001, η2 = 0.532. The N2pc was not modulated by distractor load, no interaction of laterality and distractor load, F(2,62) = 0.92, P = 0.404, η2 = 0.029.
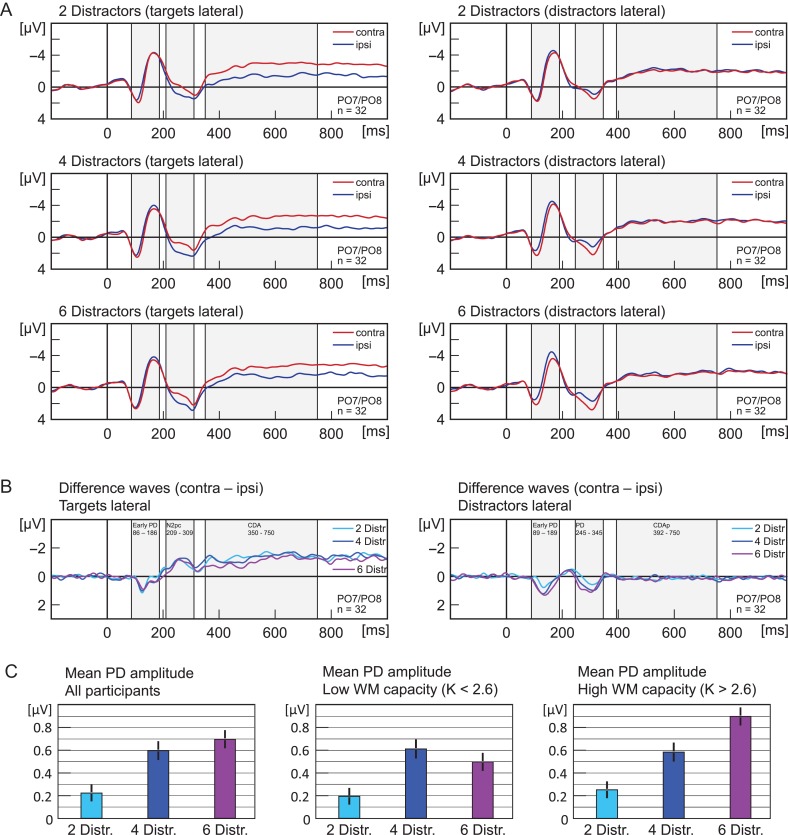
Figure 3.
(A) Experiment 2. Grand average ERPs recorded at PO7/PO8, elicited by memory displays with lateral targets (left panels) and lateral distractors (right panels). Upper panels show activity in trials with 2 distractors, middle panels for 4 distractors, and lower panels for 6 distractors. Red lines reflect activity contralateral and blue lines activity ipsilateral to the lateralized items. Grey shades indicate the analysis time window that was used to calculate mean amplitude of ERP components (N2pc, early PD, PD, CDA, CDAp). (B) Shows the same data as (A) but as difference waves (contra minus ipsi). (C) Shows the mean amplitude for the PD. Error bars indicate the standard error of the mean (corrected for individual differences; Cousineau 2005). Light blue indicates lateralized activity for trials with 2 distractors, dark blue for 4 distractors, and purple for 6 distractors. All waveforms were low-pass filtered at 30 Hz (half power cutoff, 24 dB) using digital filtering.
Distractor Positivity (245–345 ms)
Distractors elicited a PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = 1.19 μV) than at electrodes ipsilateral to distractors (M = 0.69 μV), main effect of laterality, F(1,31) = 23.05, P < 0.001, η2 = 0.426. The mean amplitude was modulated by distractor load, being smallest for distractor load 2 (M = 0.56 μV), larger for distractor load 4 (M = 0.89 μV) and largest for distractor load 6 (M = 1.37 μV), main effect of distractor load, F(2,62) = 21.29, P < 0.001, η2 = 0.407. The PD was modulated by distractor load and was smallest for distractor load 2 (ΔM = 0.22 μV), larger for distractor load 4 (ΔM = 0.60 μV) and largest for distractor load 6 (ΔM = 0.70 μV), interaction of laterality and distractor load, F(2,62) = 8.35, P = 0.001, η2 = 0.212. Follow-up t-tests showed that the PD was reliable for all distractor loads (all P ≤ 0.019). The PD was smaller for distractor load 2 than for distractor load 4 (P = 0.002, t(31) = 3.08) and distractor load 6 (P < 0.001, t(31) = 4.16) but there was no reliable difference in PD amplitude for distractor load 4 and 6 (P = 0.226, t(31) = 0.76).
CDA (350–750 ms)
Targets elicited an CDA, that is, a more negative deflection in the ERP at electrodes contralateral (M = −2.26 μV) than at electrodes ipsilateral to targets (M = −1.10 μV), main effect of laterality, F(1,31) = 42.31, P < 0.001, η2 = 0.577. The CDA was modulated by distractor load and was largest for set size 2 (ΔM = −1.28 μV), smaller for distractor load 4 (ΔM = −1.23 μV) and smallest for distractor load 6 (ΔM = −0.96 μV), interaction of laterality and distractor load, F(2,62) = 4.33, P = 0.017, η2 = 0.122. Follow-up t-tests showed that the CDA was reliable for all distractor loads (all P < 0.001). The CDA was smaller for distractor load 6 than for distractor load 2 (P = 0.007, t(31) = 2.60), and distractor load 4 (P = 0.011, t(31) = 2.42), but distractor loads 2 and 4 showed similar CDA amplitudes (P = 0.350).
Exploratory Analyses
PD for High Versus Low WM Capacity Individuals
Past research showed that the CDA amplitude increases with the number of items maintained in WM up to a point that corresponds to an individual’s WM capacity (Vogel and Machizawa 2004). In order to test if individuals also have a limit of how much suppression they can apply to irrelevant items, that is, a suppression capacity, we split participants into 2 groups, below versus above median K (Fig. 3C). For low K participants, the PD was smaller for distractor load 2 than for distractor load 4 (P = 0.015, t(31) = 2.39) and distractor load 6 (P = 0.041, t(31) = 1.87), but there was no reliable difference in PD amplitude for distractor loads 4 and 6 (P = 0.299, t(31) = 0.54). For high K participants, the PD was smaller for distractor load 2 than for distractor load 4 (P = 0.038, t(31) = 1.90) and smaller than for distractor load 6 (P < 0.001, t(31) = 4.20). Importantly, there was also a reliable difference in PD amplitude for distractor loads 4 and 6 (P = 0.021, t(31) = 2.22).
Early PD
The early PD epoch was determined similarly to the N2pc and PD, as ±50 ms from the most positive peak between 100 ms and 200 ms in the lateral-targets waveform (86–186 ms) and in the lateral-distractors waveform (89–189 ms). Targets elicited an early PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = −0.84 μV) than at electrodes ipsilateral to targets (M = −1.24 μV), main effect of laterality, F(1,31) = 15.12, P < 0.001, η2 = 0.328. The amplitude of the target-elicited early PD was not modulated by distractor load (P = 0.322). Distractors also elicited an early PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = −1.10 μV) than at electrodes ipsilateral to distractors (M = −1.68 μV), main effect of laterality, F(1,31) = 39.14, P < 0.001, η2 = 0.558. The amplitude of the distractor-elicited early PD was modulated by distractor load and was largest for distractor load 6 (ΔM = 0.79 μV), followed by distractor load 4 (ΔM = 0.61 μV) and distractor load 2 (ΔM = 0.32 μV), interaction of laterality and distractor load, F(2,62) = 11.10, P < 0.001, η2 = 0.264. Follow-up t-tests showed that the distractor-elicited early PD was reliable for all distractor loads (all P < 0.001). The distractor-elicited early PD was smaller for distractor load 2 than for distractor load 4 (P = 0.002, t(31) = 3.08) and distractor load 6 (P < 0.001, t(31) = 5.14) but there was no reliable difference for distractor loads 4 and 6 (P = 0.064, t(31) = 1.56).
CDAp
The CDAp time window was determined as 392–750 ms. Distractors elicited a CDAp, that is, a more positive deflection in the ERP at electrodes contralateral (M = −1.62 μV) than at electrodes ipsilateral to targets (M = −1.75 μV), main effect of laterality, F(1,31) = 4.62, P = 0.039, η2 = 0.130. The CDAp was not modulated by distractor load, no interaction of laterality and distractor load, F(2,62) = 0.05, P = 0.922, η2 = 0.002.
Intermediate Discussion
Experiment 2 replicated the finding from Experiment 1 that the PD component was larger for distractor load 4 than distractor load 2. Interestingly, the PD component did not further increase for distractor load 6, suggesting that the average suppression capacity was around 4 distractors. This pattern was however different for individuals with high- versus low-WM capacity. While low-WM capacity participants indeed showed no increase in PD from distractor loads 4 to 6, high-WM capacity participants showed a further increase. Thus, one reason for individuals’ good WM performance might be their ability to apply more suppression in cases with high filtering demands. Apart from the number of distractors, distractor heterogeneity is another factor that affects attention deployment. More heterogeneous distractors are harder to suppress and make attention deployment towards relevant items more difficult (Duncan and Humphreys 1989; Feldmann-Wüstefeld and Schubö 2013b). Experiment 3 will address this and keep the distractor load constant at 4 while these distractors have 4 distinct colors or appear as 2 groups of 2 colors. A larger PD component for 4 different colors compared with 2 different colors would show that suppression of irrelevant items from WM varies as a function of distractor heterogeneity.
Methods Experiment 3
Participants
Overall, 36 volunteers naïve to the objective of the experiment participated for payment (~15 USD per hour). The data of no participant had to be excluded. The participants (18 male) were all but 2 right-handed, aged 18–29 years (M = 22.9, SD = 3.2), and reported normal or corrected-to-normal visual acuity as well as normal color vision. The experiment was conducted with the written understanding and consent of each participant.
Stimuli, Procedure, and Data Analysis
Identical to Experiment 1 with the following exceptions. The number of targets was always 2 and the number of distractors was always 4. In 50% of the trials, however, only 2 colors were used for the 4 distractors, that is, there were 2 pairs of the same color. In the remaining 50% of the trials, 4 different colors were used for the distractors (as in Experiment 1, see Fig. 1, lower panels). The number of neutral distractors (grey circles) was again matched to the number of salient distractors (colored circles), that is, there were always 4 grey circles in the opposite position from the 4 colored circles. EEG was recorded with the same system and recording settings and from the dame sites as in Experiment 1. Participants had 19.2 % of unusable trials on average (SD = 10.4). Analysis epochs were determined as in Experiment 1, see results for each component.
Results Experiment 3
Behavioral Data
The RT for trials with homogeneous and heterogeneous distractors was identical (M = 643 ms), t(35) = 0.04, P = 0.484. The WM capacity (K) for trials with homogeneous (M = 1.65) and heterogeneous distractors (M = 1.63) showed no reliable difference, t(35) = 1.27, P = 0.107. Note that the theoretical maximum K in this Experiment is 2 as there were always 2 targets.
Target-N2pc (209–309 ms)
Targets elicited an N2pc (Fig. 4), that is, a more negative deflection in the ERP at electrodes contralateral (M = 0.18 μV) than at electrodes ipsilateral to targets (1.14 μV), main effect of laterality, F(1,35) = 19.77, P < 0.001, η2 = 0.361. No other effects were significant (all P > 0.102).
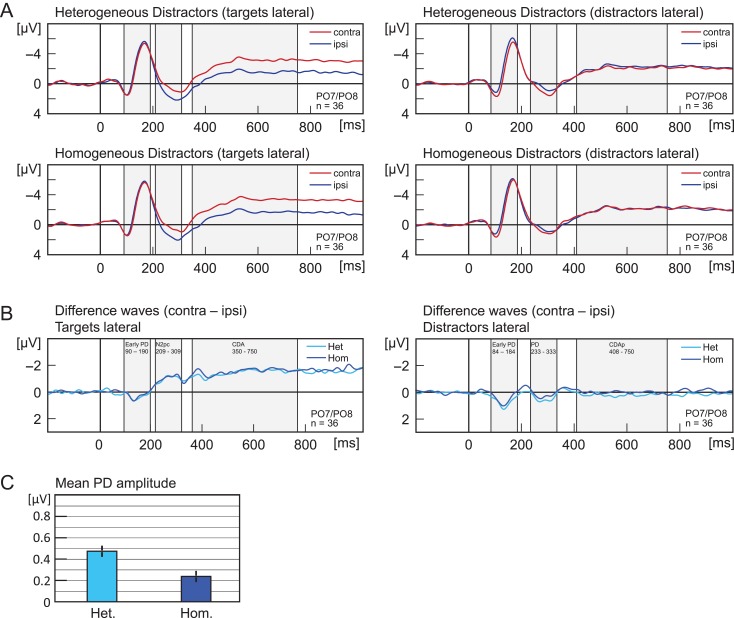
Figure 4.
(A) Experiment 3. Grand average ERPs recorded at PO7/PO8, elicited by memory displays with lateral targets (left panels) and lateral distractors (right panels). Upper panels show activity in trials with heterogeneous distractors (4 colors), lower panels for homogeneous distractors (2 colors). Red lines reflect activity contralateral and blue lines activity ipsilateral to the lateralized items. Grey shades indicate the analysis time window that was used to calculate mean amplitude of ERP components (N2pc, early PD, PD, CDA, CDAp). (B) Shows the same data as (A) but as difference waves (contra minus ipsi). (C) Shows the mean amplitude for the PD. Error bars indicate the standard error of the mean (corrected for individual differences; Cousineau 2005). Light blue indicates lateralized activity for trials with heterogeneous distractors, dark blue for homogeneous distractors. All waveforms were low-pass filtered at 30 Hz (half power cutoff, 24 dB) using digital filtering.
Distractor Positivity (233–333 ms)
Distractors elicited a PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = 0.81 μV) than at electrodes ipsilateral to distractors (0.45 μV), main effect of laterality, F(1,35) = 16.64, P < 0.001, η2 = 0.322. The PD was larger for heterogeneous (ΔM = 0.48 μV) than homogeneous distractors (ΔM = 0.24 μV), interaction of laterality and distractor heterogeneity, F(1,35) = 7.10, P = 0.012, η2 = 0.169. Follow-up t-tests showed that the PD was reliable for homogeneous (P < 0.001, t(35) = 4.59) and heterogeneous distractors (P = 0.007, t(35) = 2.58).
CDA (350–750 ms)
Targets elicited an CDA, that is, a more negative deflection in the ERP at electrodes contralateral (M = −2.62 μV) than at electrodes ipsilateral to targets (M = −1.24 μV), main effect of laterality, F(1,35) = 43.67, P < 0.001, η2 = 0.555. No other effects were significant (all P > 0.211).
Exploratory Analyses
Early PD
The early PD epoch was determined similarly to the N2pc and PD, as ±50 ms from the most positive peak between 100 ms and 200 ms in the lateral-targets waveform (90–190 ms) and in the lateral-distractors waveform (84–184 ms). Targets elicited an early PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = −2.20 μV) than at electrodes ipsilateral to targets (M = −2.49 μV), main effect of laterality, F(1,35) = 15.22, P < 0.001, η2 = 0.303. No other effects were significant (all P > 0.244). Distractors also elicited an early PD, that is, a more positive deflection in the ERP at electrodes contralateral (M = −1.77 μV) than at electrodes ipsilateral to distractors (M = −2.37 μV), main effect of laterality, F(1,35) = 91.97, P < 0.001, η2 = 0.724. The distractor-elicited early PD was larger for heterogeneous (ΔM = 0.70 μV) than homogeneous distractors (ΔM = 0.50 μV), interaction of laterality and distractor load, F(1,35) = 5.09, P = 0.030, η2 = 0.127. Follow-up t-tests showed that the distractor-elicited early PD was reliable for homogeneous (t(35) = 7.96, P < 0.001,) and heterogeneous distractors (P < 0.001, t(35) = 7.77).
CDAp
The CDAp time window was determined as 408–750 ms. Distractors elicited a CDAp, that is, a more positive deflection in the ERP at electrodes contralateral (M = −1.87 μV) than at electrodes ipsilateral to targets (M = −1.96 μV), main effect of laterality, F(1,35) = 4.28, P = 0.046, η2 = 0.109. The CDAp was marginally larger for heterogeneous (ΔM = 0.18 μV) than homogeneous distractors (ΔM = 0.01 μV), but this interaction of laterality and distractor was only marginally significant, F(1,35) = 3.65, P = 0.064, η2 = 0.095. Follow-up t-tests showed that the CDAp was reliable for heterogeneous (t(35) = 2.86, P1-tailed = 0.004) but not for homogeneous distractors (t(35) = 0.18, P1-tailed = 0.430).
Individual Differences and PD
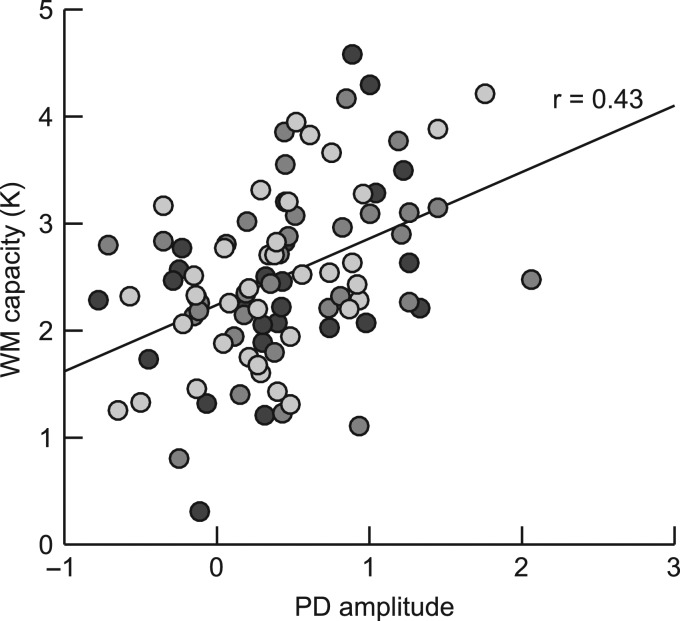
Previous studies suggest that attention markers in the ERP can predict WM capacity (Drew and Vogel 2008; Gaspar et al. 2016). For example, Gaspar et al. found that for participants with a high-WM capacity, K, measured in a change detection task, salient distractors in a visual search task elicited larger PD components. To reveal any such correlation in the present study we aggregated the mean signed area of the PD across conditions and experiments and calculated Pearson’s product–moment correlation with K (2-tailed tests). We replicated Gaspar et al.’s (2016) findings that the PD was correlated positively with K (r = 0.43; P < 0.001; 95% confidence interval: 0.25 < r < 0.58) (Fig. 5). Although a correlation between WM capacity and other target-related components (CDA, N2pc) were found in previous studies, we had no a priori assumptions about such correlations in the present study since we did not vary target features or number of targets. Nevertheless, we calculated the correlation between all reported components and K in an exploratory manner. We found that the distractor-elicited early PD (r = 0.24; P2 t = 0.019; 0.04 < r < 0.42) and CDAp (r = 0.30; P = 0.003; 0.10 < r < 0.47) were also correlated positively with K, that is, participants with higher WM capacity showed a larger early PD and CDAp component. The CDA was negatively correlated with K (r = −0.21; P = 0.044; 0.01 > r > −0.40), that is, participants with higher WM capacity showed a larger CDA (note that the CDA has a negative polarity). The target-elicited early PD and N2pc were not correlated with K (all P > 0.158).
Figure 5.
Scatter plot illustrating the correlation between WM capacity measure K and mean PD amplitude across 3 experiments and across all conditions. Each dot represents one participant. Bright grey indicates participation in Experiment 1, dark grey Experiment 2, and black Experiment 3. The black thick line indicates the linear trend of the correlation and r indicates Pearson’s product–moment correlation.
A linear regression analysis showed that the aforementioned components combined can account for 24.9% of the variance of K, which is just slightly more than PD can account for alone (18.3%). N2pc: 2.1%, target-elicited early PD: 2.2%, distractor-elicited early PD: 5.9%, CDA: 4.4%, CDAp: 9.0%.
Intermediate Discussion
Just like Experiments 1 and 2, Experiment 3 showed that irrelevant items in a change detection task elicit a PD component, indicating the importance of attentional suppression for WM maintenance. While the distractor load was kept constant in Experiment 3, the number of colors the distractors had was varied, which induced heterogeneous (4 colors) and homogeneous (2 pairs of 2 colors) distractors. More suppression was applied for heterogeneous distractors than for homogeneous distractors, as suggested by the larger PD component in the former condition. Thus, the present study identified 2 factors that affect how much suppression is applied during WM encoding: The number of irrelevant items (Experiments 1 and 2) and the heterogeneity of distractors (Experiment 3). Correlational analyses across all experiments showed that a high-WM capacity goes hand in hand with high amounts of suppression. In fact the PD explained almost as much variance in WM capacity as all attentional ERP components combined, demonstrating the crucial rule suppression plays for WM.
General Discussion
The key findings of the present study were that irrelevant items in a WM filtering task elicit a PD component and that the amplitude of the PD scales with the number of irrelevant items. In addition, the PD amplitude correlated with overall WM capacity K; participants with high-WM capacity showed larger PD amplitudes. This is in line with findings that individuals with high-WM capacity show better filtering mechanisms (Vogel et al. 2005; Gaspar et al. 2016). Our results thus suggest that in addition to the common notion that good WM performance depends on prioritization of relevant items in WM, active suppression of irrelevant items prior to reaching WM also contribute to efficiently controlling of access to this limited memory system. We found that the PD increased with the number of distractors and decreased with how well distractors can be grouped. Thus, the PD can be used as a marker of how effortful suppression from WM is.
PD as a Measure of Early Item-Based Attentional Suppression
The PD is typically found in visual search tasks as a component in the EEG elicited contralaterally to a single salient distractor with the target presented nonlaterally (Hickey et al. 2009; Kiss et al. 2012; Sawaki and Luck 2013; Gaspar and Mcdonald 2014; Feldmann-Wüstefeld et al. 2015). As a result of systematic lateralization the PD can be attributed solely to processing of the distractor and was argued to reflect active suppression that contributes to efficient selective attention (Hickey et al. 2009; Sawaki and Luck 2013; Feldmann-Wüstefeld and Schubö 2013a; Burra and Kerzel 2014; Gaspar and Mcdonald 2014). To our knowledge, this is the first example to show that a PD component can also be elicited by distractors in a WM task; which is not surprising given the highly overlapping demands the 2 tasks, and the common mechanisms of attention and WM in general (Awh et al. 2006; Soto et al. 2008; Olivers et al. 2011; Gazzaley & Nobre 2012; for a review see Kiyonaga and Egner 2013). In line with these studies, our results provide further evidence that visual attention and WM may also be closely related in terms of active suppression. The PD amplitude increased when the number of distractors (distractor load) increased from 2 to 4 (Experiments 1 and 2). This suggests that it was more effortful for individuals to suppress 4 rather than 2 items from entering WM. Similarly, the PD was larger when 4 distractors were presented in 4 different colors rather than in pairs of 2 colors (Experiment 3), showing that participants can more easily suppress homogeneous than heterogeneous distractors from entering WM. The PD amplitude did not, however, increase when distractor load increased from 4 to 6 items (Experiment 2), suggesting that this form of suppression may be resource-limited.
At first glance it may seem surprising that the PD varied as a function of distractor load because it may have been possible that the PD merely reflects the suppression of a region within the visual field which should be irrespective of the number or properties of the items presented within that region. Previous studies suggest that the N2pc reflects individuation (Mazza and Caramazza 2011; Pagano and Mazza 2012). This raises the question whether the modulation of the PD by distractor load may indicate that the distractors were individuated in the present study. The number of distractors is typically not varied in studies that apply the systematic lateralization account. One study that varied both target and distractor load in a visual search task found that the N2pc amplitude increased with the number of targets, but PD did not increase with the number of distractors (Munneke et al. 2013). However, an important novelty in the present study is that the number of distractors exceeded 2 which was not the case in Munneke et al.’s study. For relatively small suppression demands (up to 2 items), PD might not differ much. Secondly, as Munneke et al. (2013) admit, targets and distractors were very dissimilar (green diamonds vs. red lines) which means that fewer attentional resources were presumably necessary to discard distractors. In addition, distractor similarity was high (in fact they were identical), which means that distractors were more likely rejected as a group. In the present study, however, targets and distractors were highly similar: colors from the same pool were used for targets and distractors which means that no color could be discarded prior to the presentation of the memory display. Furthermore distractors were highly dissimilar; except for the homogeneous condition in Experiment 3 (see below) distractor colors were distinct. High target–distractor similarity and low distractor–distractor similarity result in high filtering demands (Duncan and Humphreys 1989; Feldmann-Wüstefeld and Schubö 2013a, 2013c). This may have led to the distractors being processed up to the individual level (to identify which are targets and which are distractors) and thus the PD was affected by numerosity because more or fewer individual distractors had to be suppressed. Alternatively, the differential PD may reflect that a suppression resource is tapped into to a different degree, depending on how much of a “threat” they are to a processing free of interference. That is, if the visual system is confronted with 4 distractors, there is a higher probability that any of these irrelevant items is encoded into WM than when it is confronted with only 2 distractors. Thus, the visual system may apply more suppression so that the probability for such erroneous encoding is minimized.
When distractors were homogeneous (2 pairs of identically colored distractors), PD was smaller than when distractors were heterogeneous (4 distinctly colored distractors), which is in line with earlier findings that 2 identical distractors elicit a relatively small PD (Munneke et al. 2013), and that grouping of distractors according to gestalt principles is very fast and allows rejection of distractors efficiently (Nothdurft 1992; Feldmann-Wüstefeld et al. 2013). These results from Experiment 3 also indicate that it is not merely the larger area that 4 compared with 2 distractors cover in the visual field that modulate the PD amplitude. Instead the larger PD for heterogeneous than for grouped distractors suggests that the PD reflects how effortful filtering is—more suppression was required for heterogeneous than for grouped distractors. Our results indicate that visual WM can benefit from advantageous properties of the stimuli—an effect that has already been found for implicit long-term memory (Feldmann-Wüstefeld and Schubö 2014).
It is noteworthy that the distractor load did not affect behavioral performance. This may have been due to the low number of targets that was used in the present set of experiments, namely 2. In this context, the differential PD amplitude may also indicate that different cognitive processes (viz., more suppression) were required to put up with the task demands. In other words, participants’ performance was equally good after a little (2 distractors) or a lot (4 or 6 distractors) suppression was applied. Future studies varying the number of targets will be required to examine how suppression is applied when the WM load is close or exceeds the WM capacity.
PD as an Indicator of Negative Attentional Weights on Salience Maps
Enhancing relevant information over short periods of time while it is not present to the senses anymore is a crucial feature of visual WM. Visual WM defined as “active maintenance of visual information to serve the needs of ongoing tasks” (Luck and Vogel 2014) refers to active maintenance as a transient modulation in energy-requiring neural activity (as opposed to synaptic changes or passively stored long-term memories). While there is quite some agreement that certain stimuli can get priority over others, there are some dissents what qualitative status a stimulus representations can obtain. For example, there is a current debate whether certain stimuli in WM are granted extra priority in terms of higher accessibility; while some studies suggest that items in WM are equally accessible (Beck et al. 2012; Hollingworth and Beck 2016), other studies suggest that only one item at a time can get extra priority in terms of higher accessibility (Olivers et al. 2011; van Moorselaar et al. 2014). Similarly, items may receive a “negative” priority status to enhance maintenance of relevant items. How can such a negative weight be conceptualized?
Selection of relevant stimuli is often modeled based upon activation patterns on a “priority map” that codes items in the visual field in a topographical manner (Itti and Koch 2000; Fecteau and Munoz 2006). The activation pattern coded on the priority map reflects salience (based on contrast, Theeuwes 2010) weighted by top-down processes such as an observer’s current goals (Folk et al. 1992, 2008) or selection history (Kristjánsson and Driver 2008; Awh et al. 2012). While peaks on the salience map will increase the probability that the according location will receive more elaborated, attentive processing, it has also been suggested that suppression of certain locations is realized in terms of deprioritized locations on the priority map (Sawaki et al. 2012; Jannati et al. 2013; Burra and Kerzel 2014; Mirpour et al. 2016). It was suggested that the PD component reflects a salient-signal suppression within attentional control (Sawaki and Luck 2010; Jannati et al. 2013). Salient items generate an “attend-to-me signal” and thus compete for attentional resources prior to visual selection. Attention is then in a second step deployed toward the most salient stimulus for further processing or that stimulus’ location is actively suppressed to avoid the potential waste of attentional resources and make selection of other locations more likely.
Suppression from visual WM may work in a similar way (Zelinsky and Bisley 2015). Stimuli that are likely probed in the end of a change detection task trial will receive high weights on a priority map so that they are more likely encoded into WM. Generally irrelevant stimuli (e.g., distractors in the present task) and stimuli that need to be discarded because WM slots are full may receive low weights so that they are less likely encoded into WM where they would potentially interfere with relevant content. Recent studies even provide evidence for suppression of irrelevant items below baseline (Gaspelin et al. 2015, 2016). Analogously, the PD in the present study distractors may reflect negative weights in a salience map for items identified as irrelevant that makes them less likely to be encoded into WM and thus promotes an efficient maintenance of the relevant items.
Early PD and CDAp and the Salience Map
Similar to previous studies that employed the systematic lateralization approach we found an early lateralized positivity contralateral to both distractors and targets, in the time range 100–200 ms. Since only few studies systematically analyzed the early PD (Sawaki and Luck 2010; Corriveau et al. 2012; Fortier-Gauthier et al. 2013; Feldmann-Wüstefeld et al. 2016; Jannati et al. 2013; Gokce et al. 2014) and its meaning remains unclear, we only analyzed the early PD in an explorative manner. Previous results suggest that the early PD reflects early processing of physical discontinuities and/or an associated fleeting spatial suppression (Corriveau et al. 2012; Jannati et al. 2013; Feldmann-Wüstefeld et al. 2016). The early PD does not seem to solely reflect physical imbalance (Fortier-Gauthier et al. 2012, 2013; Gokce et al. 2014) but rather initial activation on salience maps (Fortier-Gauthier et al. 2012; Jannati et al. 2013). In line with this, the amplitude of the early PD was found to predict target accuracy (Weaver et al. 2017) and intertrial prioritization of locations affects the amplitude of the early PD (Gokce et al. 2014), suggesting that the early PD may reflect negative weights or presets on a salience map. Also in line with the salience map notion, orientation deviants (but not color deviants) elicit a larger early PD in contexts of homogeneously than contexts of heterogeneously oriented lines (Feldmann-Wüstefeld et al. 2016). Finally, the early PD follows a similar result pattern than the subsequent N2pc (Pomerleau et al. 2014), suggesting a close link to attentional selection processes based on a salience map.
In the present study, the early PD followed the PD pattern closely in all 3 experiments and also showed a correlation with WM capacity K. At the same time, the early PD and PD combined explain only marginally more variance of K than the PD alone, suggesting that the PD reflects more elaborate processing of the distractors. The early PD does seem to reflect color discontinuities (Barras and Kerzel 2016) or orientation discontinuities (Weaver et al. 2017). Moreover, in Weaver et al.’s (2017) study, the early Pd predicted overt saccadic behavior; an early PD was only observed when individuals deploy their eyes away from the distractor. Thus, while the PD may reflect attentional suppression on the overall map of activations (Fecteau and Munoz 2006), the early PD may reflect pre-attentive suppression of discontinuities on feature maps.
In addition to the early PD, we also found a sustained positivity contralateral to distractors, that is, a CDA of inverse polarity. To our knowledge this is the first time such a CDAp was found which is why we had no a priori expectations about it. Although we first analyzed the CDAp in an exploratory manner in Experiment 1, we could replicate it in Experiments 2 and 3 and thus showed that the CDAp may be an appropriate tool to investigate sustained suppression and/or suppression from WM. Although the exact functional role of the CDAp needs further clarification in future experiments, the current data suggest that it reflects sustained suppression at previous distractor locations, possibly a tagging of such location as “irrelevant” avoiding any post-presentation encoding from there. Analogously to the PD and NT adding up to the N2pc (Hickey et al. 2009), the classical CDA may actually consist of a negative component reflecting prioritizing of memory items (CDAn) and a positive component reflecting active suppression from WM. As the CDAp was not modulated by distractor load, the sustained suppression may not be subject to individuation but rather reflect suppression of an entire region. In terms of salience maps, the CDAp may reflect lingering negative weights at such suppressed locations.
One may argue that the differences in both the early PD and PD component may be due to a purely sensory laterality rather than attentional lateralization. According to that argumentation, for example, the PD for 4 distractors would be larger than for 2 distractors, not because more suppression is required for 4 distractors, but because more salient items are presented in one hemifield. However, several reasons support the notion that the early PD and PD differences reflect attentional differences. First, the distractors were never the only lateral items presented—there were always neutral (grey) distractors on the opposite side of the display. As these neutral distractors were equiluminant to the salient distractors, there was no physical imbalance between the left and right hemifield in terms of how many pixels were illuminated. Second, any sensory laterality should create negative potentials, as has been observed in earlier work (Hickey et al. 2006), whereas PD and early PD are both of positive polarity. Third, in the present study the PD and early PD are correlated with K, suggesting that the differences PD and early PD show reflect meaningful cognitive differences between conditions. Fourth, Experiment 3 shows that even when the number of salient distractors is identical, the filtering demands induced by the heterogeneity of distractors modulate the size of the PD and early PD.
Links Between PD and Task Performance
It is known that individuals can vary a lot in their attentional control, that is, how well they can filter relevant from irrelevant information (Vogel et al. 2005; Astle et al. 2008; Fukuda and Vogel 2011; Linke et al. 2011). There is a growing body of evidence linking the PD to successful attentional control performance. The design of the current study allowed us to use the PD as a measure of active suppression and thus investigate how individuals vary in their ability to filter out irrelevant items from WM.
Within-Subject Variability
Previous studies found that N2pc subcomponents (PD = distractor positivity as suppression contralateral to distractors; NT = target negativity as selection contralateral to targets) seem to be closely related to actual search performance. RT can vary as a function of attentional capture as indicated by the distractor-N2pc (Hickey et al. 2010; McDonald et al. 2013; Feldmann-Wüstefeld et al. 2016). For example, McDonald et al. (2013) found a distractor-N2pc toward an additional singleton only in slow trials and a more pronounced NT toward targets in fast trials. Analogously, a larger PD was found for fast responses compared with slow responses (Sawaki et al. 2012; Qi et al. 2013). Similarly, a PD in fast trials can even be inversed in slow trials (i.e., a distractor-N2pc emerges instead of a PD), indicating that when active suppression of irrelevant information fails, involuntary attention capture can occur, deteriorating search performance (Gaspar and Mcdonald 2014). In line with this notion, it was found that distractors in homogenous context, that allow parallel, efficient visual search, elicit an early PD and no distractor-N2pc whereas distractors in heterogeneous context that require more effortful search, elicit a distractor-N2pc first and then a later PD (Feldmann-Wüstefeld and Schubö 2013a). In sum, these results suggest that the PD component is crucial to prevent initial attentional capture by irrelevant items and may also help to terminate the deployment of attention to relevant items (Sawaki et al. 2012).
Between-Subjects Variability
ERPs may also reflect more general individual differences that become evident in behavioral measures. Previous studies found that participants with high-WM capacity show larger N2pc amplitudes in multiple object tracking (Drew and Vogel 2008) and larger PD amplitudes in additional singleton search tasks (Gaspar et al. 2016). Gaspar et al. found that the signed area of the PD elicited by salient distractors (additional color singletons) in a visual search task correlated with the WM capacity (K) measured in a standard change detection task. In the present study we used a standard change detection task very similar to Gaspar et al. (2016) and also measured the signed area of the PD. The main difference to Gaspar et al. was that we measured the PD elicited by distractors in a WM filtering task, allowing a direct link between active suppression from WM and WM capacity. We replicated their findings and show that individuals with high-WM capacity had a generally larger PD. This means converging evidence for the notion that a functional WM relies on active early suppression. The major part of variance explained by various components (PD, N2pc, CDA, early PD, CDAp) however was contributed by the PD (18.5% out of 25.4%). Thus, our study demonstrates the importance of active suppression from WM for efficient maintenance of relevant items in WM. A common mechanism for visual attention and WM may help suppressing irrelevant items both from capturing attention and prevent encoding of irrelevant items into WM.
The present study can also shed new light on how a high-WM capacity can help to filter efficiently (Vogel et al. 2005). As we found highWM capacity individuals to show a larger PD, but not a larger N2pc, it may be that in Vogel et al.’s study high capacity individuals were more successful in suppressing distractors from entering WM rather than less likely encoding distractors accidentally into WM. Our study may also help to interpret previous findings about the time course of filtering from WM. For example, it was found that observers with high-WM capacity show less attention capture in the beginning of a trial and store less irrelevant information (Fukuda and Vogel 2009), while they disengage more rapidly from irrelevant information (Fukuda and Vogel 2011). Both the less pronounced attentional capture by and the more rapid disengagement from distractors may be due to the more pronounced active suppression that high capacity participants apply. Note that suppression applied in the present study was presumably first-order feature suppression (Gaspelin and Luck 2017; or top-down suppression, Noonan et al. 2017), that is, suppression was set to ignore a specific feature values (here: circles). Future research will have to show if suppression from WM based on second-order features (suppression of an entire dimension, e.g., shape) or global salience (suppression of the item with the strongest bottom-up signal) is predictive of WM capacity as well.
Conclusions
The present study shows that the PD, a subcomponent of the N2pc that indicates active suppression of irrelevant items in the visual field, can be observed in WM tasks. This shows that suppression is a crucial process not only for attention deployment, but also for encoding of visual stimuli that are expected to not be present to the senses anymore but may become relevant in the future. The PD increased with increasing distractor load and distractor heterogeneity, showing that suppression from WM is more effortful the more items need to be discarded and the more difficult it is to group them according to gestalt principles. Among all attention and WM components measured (early PD, N2pc, PD, CDA, CDAp) the PD can account for the major share of the variance of individuals’ WM capacity. In sum our results show that suppression of potentially distracting information is a crucial factor in the maintenance of WM and efficient suppression can contribute largely to a good WM performance.
Funding
Funded by National Institute of Mental Health grant 5R01 MH087214-08 and Office of Naval Research grant N00014-12-1-0972.
References
- Adam KC, Vogel EK. 2016. Reducing failures of working memory with performance feedback. Psychon Bull Rev. 23(5):1520–1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astle DE, Harvey H, Stokes M, Mohseni H, Nobre AC, Scerif G. 2008. Distinct neural mechanisms of individual and developmental differences in VSTM capacity. Dev Psychobiol. 56(4):601–610. [DOI] [PubMed] [Google Scholar]
- Awh E, Belopolsky AAV, Theeuwes J. 2012. Top-down versus bottom-up attentional control: a failed theoretical dichotomy. Trends Cogn Sci. 16:437–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh S-H. 2006. Interactions between attention and working memory. Neuroscience. 139:201–208. [DOI] [PubMed] [Google Scholar]
- Barras C, Kerzel D. 2016. Active suppression of salient-but-irrelevant stimuli does not underlie resistance to visual interference. Biol Psychol. 121:74–83. [DOI] [PubMed] [Google Scholar]
- Beck VM, Hollingworth A, Luck SJ. 2012. Simultaneous control of attention by multiple working memory representations. Psychol Sci. 23(8):887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burra N, Barras C, Coll SY, Kerzel D. 2016. Electrophysiological evidence for attentional capture by irrelevant angry facial expressions. Biol Psychol. 120:69–80. [DOI] [PubMed] [Google Scholar]
- Burra N, Kerzel D. 2014. The distractor positivity (Pd) signals lowering of attentional priority: evidence from event-related potentials and individual differences. Psychophysiology. 51:685–696. [DOI] [PubMed] [Google Scholar]
- Corriveau I, Fortier-Gauthier U, Pomerleau VJ, McDonald J, Dell’Acqua R, Jolicoeur P. 2012. Electrophysiological evidence of multitasking impairment of attentional deployment reflects target-specific processing, not distractor inhibition. Int J Psychophysiol. 86:152–159. [DOI] [PubMed] [Google Scholar]
- Cousineau D. 2005. Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutor Quant Methods Psychol. 1:42–45. [Google Scholar]
- Cowan N. 2001. Metatheory of storage capacity limits. Behav Brain Sci. 24(1):154–176. [DOI] [PubMed] [Google Scholar]
- Drew T, Vogel EK. 2008. Neural measures of individual differences in selecting and tracking multiple moving objects. J Neurosci. 28:4183–4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan J, Humphreys GW. 1989. Visual search and stimulus similarity. Psychol Rev. 96:433–458. [DOI] [PubMed] [Google Scholar]
- Fecteau JJH, Munoz DP. 2006. Salience, relevance, and firing: a priority map for target selection. Trends Cogn Sci. 10:382–390. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Brandhofer R, Schubö A. 2016. Rewarded visual items capture attention only in heterogeneous contexts. Psychophysiology. 53:1063–1073. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Schubö A. 2013. a. Context homogeneity facilitates both distractor inhibition and target enhancement. J Vis. 13:1–12. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Schubö A. 2013. b. Context homogeneity facilitates both distractor inhibition and target enhancement Anna Schub o. J Vis. 13(11):1–12. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Schubö A. 2013. c. Textures shape the attentional focus: evidence from exogenous and endogenous cueing. Attention Percept Psychophys. 75:1644–1666. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Schubö A. 2014. Stimulus homogeneity enhances implicit learning: evidence from contextual cueing. Vision Res. 97:108–116. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Uengoer M, Schubö A. 2015. You see what you have learned. Evidence for an interrelation of associative learning and visual selective attention. Psychophysiology. 52:1483–1497. [DOI] [PubMed] [Google Scholar]
- Feldmann-Wüstefeld T, Wykowska A, Schubö A. 2013. Context heterogeneity has a sustained impact on attention deployment: behavioral and electrophysiological evidence. Psychophysiology. 50:722–733. [DOI] [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. 2008. Top-down control settings and the attentional blink: evidence for nonspatial contingent capture. Vis cogn. 16:616–642. [Google Scholar]
- Folk CL, Remington RW, Johnston JC. 1992. Involuntary covert orienting is contingent on attentional control settings. J Exp Psychol Hum Percept Perform. 18:1030–1044. [PubMed] [Google Scholar]
- Fortier-Gauthier U, Dell’Acqua R, Jolicœur P. 2013. The “red-alert” effect in visual search: evidence from human electrophysiology. Psychophysiology. 50:671–679. [DOI] [PubMed] [Google Scholar]
- Fortier-Gauthier U, Moffat N, Dell’Acqua R, McDonald JJ, Jolicœur P. 2012. Contralateral cortical organisation of information in visual short-term memory: evidence from lateralized brain activity during retrieval. Neuropsychologia. 50:1748–1758. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. 2009. Human variation in overriding attentional capture. J Neurosci. 29(27):8726–8733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda K, Vogel EK. 2011. Individual differences in recovery time from attentional capture. Psychol Sci. 22:361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, McMahon RP, Gold JM. 2005. Working memory consolidation is abnormally slow in schizophrenia. J Abnorm Psychol. 114:279–290. [DOI] [PubMed] [Google Scholar]
- Gaspar JM, Christie GJ, Prime DJ, Jolicœur P, McDonald JJ. 2016. Inability to suppress salient distractors predicts low visual working memory capacity. Proc Natl Acad Sci. 113:3696–3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspar JM, Mcdonald JJ. 2014. Suppression of salient objects prevents distraction in visual search. 34:5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, Luck SJ. 2016. Suppression of overt attentional capture by salient-but-irrelevant color singletons. Atten Percept Psychophys. 79(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Leonard CJ, Luck SJ, Gaspelin N, Leonard CJ, Luck SJ. 2015. Direct evidence for active suppression of salient-but-irrelevant sensory inputs. Psychol Sci. 26(11):1740–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaspelin N, Luck SJ. 2017. Distinguishing among potential mechanisms of suppression. J Exp Psychol Hum Percept Perform. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. 2012. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 16:129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokce A, Geyer T, Finke K, Müller HJ, Töllner T. 2014. What pops out in positional priming of pop-out: insights from event-related EEG lateralizations. Front Psychol. 5:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey C, Di Lollo V, McDonald JJ. 2009. Electrophysiological indices of target and distractor processing in visual search. J Cogn Neurosci. 21:760–775. [DOI] [PubMed] [Google Scholar]
- Hickey C, McDonald J, Theeuwes J. 2006. Electrophysiological evidence of the capture of visual attention. J Cogn Neurosci. 18:604–613. [DOI] [PubMed] [Google Scholar]
- Hickey C, Olivers C, Meeter M, Theeuwes J. 2011. Feature priming and the capture of visual attention: linking two ambiguity resolution hypotheses. Brain Res. 1370:175–184. [DOI] [PubMed] [Google Scholar]
- Hickey C, van Zoest W, Theeuwes J. 2010. The time course of exogenous and endogenous control of covert attention. Exp Brain Res. 201:789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingworth A, Beck VM. 2016. Memory-based attention capture when multiple items are maintained in visual working memory. J Exp Psychol Hum Percept Perform. 42:911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti L, Koch C. 2000. A saliency-based search mechanism for overt and covert shifts of visual attention. Vision Res. 40:1489–1506. [DOI] [PubMed] [Google Scholar]
- Jannati A, Gaspar JM, McDonald JJ. 2013. Tracking target and distractor processing in fixed-feature visual search: evidence from human electrophysiology. J Exp Psychol Hum Percept Perform. 39:1713–1730. [DOI] [PubMed] [Google Scholar]
- Jost K, Bryck RL, Vogel EK, Mayr U. 2011. Are old adults just like low working memory young adults? Filtering efficiency and age differences in visual working memory. Cereb Cortex. 21:1147–1154. [DOI] [PubMed] [Google Scholar]
- Kiss M, Grubert A, Petersen A, Eimer M. 2012. Attentional capture by salient distractors during visual search is determined by temporal task demands. J Cogn Neurosci. 24:749–759. [DOI] [PubMed] [Google Scholar]
- Kiyonaga A, Egner T. 2013. Working memory as internal attention: toward an integrative account of internal and external selection processes. Psychon Bull Rev. 20:228–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristjánsson Á, Driver J. 2008. Priming in visual search: separating the effects of target repetition, distractor repetition and role-reversal. Vision Res. 48:1217–1232. [DOI] [PubMed] [Google Scholar]
- Lee EY, Cowan N, Vogel EK, Rolan T, Fernando VI, Hackley SA. 2010. Visual working memory deficits in patients with Parkinson’s disease are due to both reduced storage capacity and impaired ability to filter out irrelevant information. Brain. 133:2677–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke AC, Mitchell DJ, Cusack R. 2011. Neuropsychologia encoding strategy accounts for individual differences in change detection measures of VSTM. Neuropsychologia. 49:1476–1486. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Vogel EK. 2014. Visual working memory capacity: from psychophysics and neurobiology to individual differences. Trends Cogn Sci. 17:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria R, Vogel EK. 2011. Visual search demands dictate reliance on working memory storage. J Neurosci. 31:6199–6207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza V, Caramazza A. 2011. Temporal brain dynamics of multiple object processing: the flexibility of individuation. PLoS One. 6(2):e17453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JJ, Green JJ, Jannati A, Di Lollo V. 2013. On the electrophysiological evidence for the capture of visual attention. J Exp Psychol Hum Percept Perform. 39:849–860. [DOI] [PubMed] [Google Scholar]
- Mirpour K, Arcizet F, Ong WS, Bisley JW, Mirpour K, Arcizet F, Ong WS, Bisley JW. 2016. Been there, seen that: a neural mechanism for performing efficient visual search been there, seen that: a neural mechanism for performing efficient visual search. J Neurophysiol. 102:3481–3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munneke J, Fait E, Mazza V. 2013. Attentional processing of multiple targets and distractors. Psychophysiology. 50:1104–1108. [DOI] [PubMed] [Google Scholar]
- Noonan MP, Crittenden BM, Jensen O, Stokes MG. 2017. Selective inhibition of distracting input. Behav Brain Res. [DOI] [PubMed] [Google Scholar]
- Nothdurft HC. 1992. Feature analysis and the role of similarity in preattentive vision. Percept Psychophys. 52:355–375. [DOI] [PubMed] [Google Scholar]
- Olivers CNL, Peters J, Houtkamp R, Roelfsema PR. 2011. Different states in visual working memory: when it guides attention and when it does not. Trends Cogn Sci. 15:327–334. [DOI] [PubMed] [Google Scholar]
- Pagano S, Mazza V. 2012. Neuropsychologia Individuation of multiple targets during visual enumeration: new insights from electrophysiology. Neuropsychologia. 50:754–761. [DOI] [PubMed] [Google Scholar]
- Pomerleau VJ, Fortier-Gauthier U, Corriveau I, Dell’Acqua R, Jolicœur P. 2014. Colour-specific differences in attentional deployment for equiluminant pop-out colours: evidence from lateralised potentials. Int J Psychophysiol. 91:194–205. [DOI] [PubMed] [Google Scholar]
- Qi S, Zeng Q, Ding C, Li H. 2013. Neural correlates of reward-driven attentional capture in visual search. Brain Res. 1532:32–43. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Geng JJ, Luck SJ. 2012. A common neural mechanism for preventing and terminating the allocation of attention. J Neurosci. 32:10725–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. 2010. Capture versus suppression of attention by salient singletons: electrophysiological evidence for an automatic attend-to-me signal. Atten Percept Psychophys. 72:1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawaki R, Luck SJ. 2013. Active suppression after involuntary capture of attention. Psychon Bull Rev. 20:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto D, Hodsoll J, Rotshtein P, Humphreys GW. 2008. Automatic guidance of attention from working memory. Trends Cogn Sci. 12:342–348. [DOI] [PubMed] [Google Scholar]
- Theeuwes J. 2010. Top-down and bottom-up control of visual selection. Acta Psychol (Amst). 135:77–99. [DOI] [PubMed] [Google Scholar]
- van Moorselaar D, Theeuwes J, Olivers CNL. 2014. In competition for the attentional template: can multiple items within visual working memory guide attention? J Exp Psychol Hum Percept Perform. 40:1450–1464. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Woodman GF, Luck SJ. 2001. Storage of features, conjunctions, and objects in visual working memory. J Exp Psychol Hum Percept Perform. 27(1):92. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Machizawa MG. 2004. Neural activity predicts individual differences in visual working memory capacity. Nature. 428:748–751. [DOI] [PubMed] [Google Scholar]
- Vogel EK, Mccollough AW, Machizawa MG. 2005. Neural measures reveal individual differences in controlling access to working memory. Nature. 438:500–503. [DOI] [PubMed] [Google Scholar]
- Weaver MD, Zoest W Van, Hickey C. 2017. NeuroImage A temporal dependency account of attentional inhibition in oculomotor control. Neuroimage. 147:880–894. [DOI] [PubMed] [Google Scholar]
- Woodman GF, Luck SJ. 2003. Serial deployment of attention during visual search. J Exp Psychol Hum Percept Perform. 29:121–138. [DOI] [PubMed] [Google Scholar]
- Zelinsky GJ, Bisley JW. 2015. The what, where, and why of priority maps and their interactions with visual working memory. Ann N Y Acad Sci. 1339:154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]