Abstract
Objective
To study the impact of therapeutic interventions on pain analgesia and endogenous pain modulation in knee osteoarthritis (KOA).
Design
Systematic review and meta-analysis.
Methods
We searched for KOA randomized clinical trials and observational studies with data on therapeutic interventions comparing pain intensity, temporal summation (TS), and conditioned pain modulation (CPM) scores relative to control. These data were pooled as Hedge’s g. To study the relationship between pain intensity and TS/CPM, we performed metaregression with 10,000 Monte-Carlo permutations.
Results
We reviewed 11 studies (559 participants). On studying all the interventions together, we found no significant changes in pain modulation, TS, or CPM. Our findings show that this lack of difference is likely because surgical and nonsurgical interventions resulted in contrary effects. Metaregression significantly correlated pain reduction with normalization of TS and CPM.
Conclusions
We demonstrate an association between pain reduction and TS/CPM normalization. Though we cannot directly compare these interventions, the results allow us to draw hypotheses on potential practice schemas. Recovering defective endogenous pain modulation mechanisms may help establish long-term analgesia. However, to validate these paradigms as robust clinical biomarkers, further investigation into their mechanisms would be necessary. The registration number for this review is CRD42017072066.
Keywords: Conditioned Pain Modulation, Temporal Summation, Osteoarthritis, Chronic Pain, Pain Management
Introduction
Knee osteoarthritis (KOA) is a leading cause of chronic pain, ranked as the 11th greatest contributor to disability worldwide (along with hip osteoarthritis), and significantly impacts the economy [1,2]. Patients with painful, chronic KOA show local and generalized hyperesthesia, attributed to neurophysiological and neuropathological mechanisms that are both peripheral (e.g., tissue nociceptor sensitization) and central (e.g., spinal cord dorsal horn neuron sensitization) [3]. Multiple studies support that chronic pain sufferers share a pattern of increased excitability to pain and limited endogenous pain modulation relative to healthy controls [4–9]. Endogenous pain modulation refers to central nervous system (CNS) mechanisms altering the saliency and experience of pain; it can be assessed by two psychophysical parameters, temporal summation (TS) and conditioned pain modulation (CPM) [10].
There are various observational studies that examine the relationship between different treatment interventions in KOA, examining TS and CPM. For example, in a cohort of KOA patients who received total knee replacement (TKR), patients with greater pain levels before surgery on average developed worse pain outcomes 12 months after surgery and exhibited higher TS (as compared with patients with lower presurgical pain and TS) [11]. Despite surgical removal of the pain-generating mechanism (i.e., mechanical knee degradation), these high-pain patients with aberrant TS scores continued to experience poor treatment response after surgery [11]. Also, the authors found that KOA patients with more TS before surgery experienced less pain relief after surgery [11]. Ensuing studies showed that unlike radiologic severity—which was not a robust predictor of KOA pain—TS correlated better with high preoperative pain levels and predicted postoperative analgesic outcomes more effectively than radiography [12]. Another group also showed that TS predicted sensitivity to physical activity and nonsteroidal anti-inflammatory drug response [13,14]. In contrast, Christensen et al. [15] did not find TS to be prognostically important, although this study was on rheumatoid arthritis. Likewise, negative results were found in diabetic neuropathy [16]. On the other end of the spectrum, dysfunctional CPM predicted chronic pain development in various interventions, and multiple studies allude to decreased CPM as an indicator of pain sensitization and a predictor of analgesic efficacy [14,17–23].
Therefore, TS and CPM may be useful biomarkers to predict a treatment’s analgesic efficacy, as suggested by a considerable number of studies in KOA. Additionally, these paradigms have high good to excellent interclass correlation coefficients [24]. However, to date, no meta-analysis addresses the effects of different therapies on endogenous pain mechanisms and pain intensity in KOA. We consequently performed a systematic review and meta-analysis to assess and quantify the pooled effects of therapeutic interventions on endogenous pain modulation mechanisms and pain intensity in KOA. This approach may advance our understanding of the relationship between endogenous pain modulation, pain sensitivity, and treatment effects in KOA.
Methods
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The International Prospective Register of Systematic Reviews (PROSPERO) registration number is CRD42017072066.
Search Strategy and Selection Criteria
We searched PubMed, EMBASE, ClinicalTrials.gov, Cochrane central register of controlled trials and database of systematic reviews, Google Scholar, LILACS, and PEDRO (without time limits retrospectively) for observational and randomized clinical studies with data on therapeutic interventions in KOA that report pain intensity and TS/CPM scores before and after intervention relative to a control group.
We used the search strategy below and screened additional records from the references of included studies:
PUBMED (inception to 2017) ((knee osteoarthritis) AND (conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain)) NOT (animal*[Title] OR rat*[Title] OR mouse[Title] OR mice[Title])
ClinicalTrials.gov (inception to 2017) Knee osteoarthritis conditioned pain modulation OR CPM OR temporal summation OR TS OR wind up OR WU Cochrane central register of controlled trials and database of systematic reviews (2005-2017) knee osteoarthritis AND (conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain)
EMBASE (inception to 2017) knee osteoarthritis AND (conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain) AND pain NOT (‘animal*’: ti OR ‘rat*’: ti OR ‘mouse’: ti OR ‘mice’: ti)/lim NOT [medline]/lim
Google Scholar (inception to 2017) ((knee osteoarthritis) AND (conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain)) LILACS (inception to 2017) (tw:(knee osteoarthritis)) AND (tw:(conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain) ) AND NOT (tw:((animal OR rat OR mouse)))
PEDRO (inception to 2017) Knee osteoarthritis and conditioned pain modulation Knee osteoarthritis and temporal summation
Cochrane Knee osteoarthritis AND (conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain) (sin terminus relacionados) EBM Reviews – Cochrane Database of Systematic Review <2005 to July 6 2017> EBM Reviews – Cochrane Central Register of Controlled Trials <June 2017>
LILACS (tw:(knee osteoarthritis)) AND (tw:(conditioned pain modulation OR CPM OR descending noxious inhibitory control OR DNIC OR temporal summation OR TS OR wind up OR WU OR second pain) ) AND NOT (tw:((animal OR rat OR mouse)))
As we are evaluating central pain inhibitory mechanisms, we decided to exclude studies that used nonpainful conditioned stimuli, spatial summation protocols, those solely about inhibition–sensitization profiles, and those without a comparison group. As our focus is on therapeutic interventions, we excluded studies that appraised endogenous pain modulation profiles without addressing treatment responses in KOA. We also excluded letters, commentaries, conference reports, case series, case studies, and all studies published in languages other than English, Spanish, or Portuguese.
Screening and Selection of Studies
Two independent researchers (ATO, GR) screened all records from extracted titles and abstracts using Rayyan, a Cochrane-recommended, web-based and mobile application, to maintain blinding [25]. Studies reported some measure of TS/CPM and pain outcomes to pass the initial screening phase, after which the screened studies were partitioned and fully reviewed for eligibility by ATO, MME, RH, PS, and SC. These results were then cross-validated by ATO. We also reviewed the references of accepted articles (ATO, HR, SC).
Assessment of Risk of Bias in Included Studies
Risk of bias was assessed by ATO. Randomized controlled trials were evaluated with the Cochrane Collaboration tool for assessing the risk of bias [26]. The following elements were reviewed: 1) random sequence generation, 2) allocation concealment, 3) blinding of participants and personnel, 4) blinding of outcome assessment, 5) incomplete outcome data, and 6) selective reporting. Observational studies’ risk of bias was assessed using the Newcastle-Ottawa (NOS) checklist; using this scale, each study was judged as low, unclear, or high risk. The NOS scale ranges between 0 and 9 points (stars) and consists of three sections: 1) selection (four points), 2) comparability (two points), and 3) exposure (three points) for case-control studies; higher scores indicate less risk of systematic error [27].
Data Extraction
We extracted the following data: 1) bibliographic details: author, year of publication, and location; 2) demographics: number of participants, age, gender; 3) clinical information: disease duration, clinical pain intensity, medication discontinuation, intervention characteristics, type of stimuli used for respective quantitative sensory testing (QST), site of QST, number of stimuli used for TS, interstimulus interval for TS, use/lack of adjusted thresholds for TS, outcome measures (eg. visual analog scale [VAS], temperature, weight, and pressure), test stimulus, conditioning stimulus for CPM, time of CPM, and respective results per study.
Data Synthesis
First, we synthesized the articles in narrative form. Then we pooled data as Hedge’s g for pain intensity, TS, and CPM paradigms. Interventions were categorized into one of four groups: 1) exercise (e.g., exercise); 2) neuromodulation (e.g., electrical stimulation); 3) pharmacological (e.g., nonsteroidal anti-inflammatory drugs); and 4) surgical (e.g., TKR). Although these are very different therapeutic interventions indicated in different stages of KOA and for different patient profiles, we wanted to compare pain reduction with endogenous pain modulation (TS and CPM) across the spectrum of available KOA therapies. We selected the model for the forest plot based on Cochrane’s Q; when possible, we used pre and post scores of the pain analog scales, or thresholds (e.g., C or kPa), for each outcome to calculate the mean difference between KOA patients and controls. The difference was then converted to a standardized mean difference (i.e., an effect size, Cohen’s d). Given that Cohen’s d has a slight bias to overestimate in small sample sizes, we adjusted Cohen’s d to Hedge’s g by applying a correction factor (Supplementary Data).
For multiple comparisons within the same study, we considered each comparison as one contributor in the meta-analysis. For example, if a TS paradigm was tested separately at 38°C and 40°C, these were each used in the meta-analysis as independent variables. To overcome unit of analysis error, we split the shared group into two or more groups with smaller sample sizes, as described by The Cochrane Handbook, Chapter 16, Section 5.4 (“How to Include Multiple Groups from One Study”) [26]. We studied publication bias of main outcomes via Begg’s funnel plot and Egger’s test. This section of the analysis was completed with RevMan 5.3 software and is available in the Supplementary Data.
Metaregression
We conducted metaregression to identify associations between covariate effects and main outcomes. We followed a method determined a priori and delineated in the PROSPERO registry. Models were constructed based on the entry criterion of P = 0.2 for independent variables. We set a high P value of <0.2 to reduce the risk of omitting variables thought to be potential confounders. A stepdown approach was used to test the variables’ association with each outcome and their contribution to the fit of the model. The maintenance of variables in the final models was determined by statistical significance (P ≤ 0.05), as well as by the best fit of the multiple models, which was evaluated based on Tau2, I2, and adjusted R2. We performed Monte Carlo permutation tests (i.e., repeated random sampling) using 10,000 random permutations to account for the high false-positive rates associated with metaregression. The metaregression was performed using Stata 13 MP.
For the first analysis, which included studies evaluating pain and TS, we used Hedge’s g for pain as the dependent variable (continuous), and the following as independent covariates: Hedge’s g for TS (continuous), group (categorical: 1 = pharmacological, 2 = exercise, 3 = surgical, 4 = neuromodulation), stimuli (binary: 1 = mechanical, 2 = thermal), age (continuous in years), number of females and males (continuous), and medication discontinuation (binary, yes/no). For the second analysis, which included studies evaluating pain and CPM, Hedge’s g for pain was the dependent variable, and the independent variables were Hedge’s g for CPM (continuous), conditioning stimuli (categorical: 1 = mechanical, 2 = thermal, 3 = cold), age, number of females and males, and medication discontinuation. We did not include the CPM test stimuli as a covariate in the second model as all studies used a mechanical noxious test stimulus.
Management of Missing Data
When the main outcome data (i.e., means before and after intervention for pain, TS, and CPM) were missing or unclear, we contacted the authors. We used WebPlotDigitizer v.3.11 to extract data from relevant graphs, and if a study only reported postintervention data, we determined whether to include the data in the analysis by studying baseline comparability on the graphs. If we were unable to contact the authors or extract the data graphically, we excluded the study from the quantitative analysis.
Results
Search Strategy Results
Our electronic search found 2,102 records; we identified one additional record by manually searching the references of included studies. In total, we extracted 11 eligible studies published between 2012 and 2017 based on the search strategy mentioned above. See Figure 1 for a flowchart of the selection process.
Figure 1.
Flowchart of selection process.
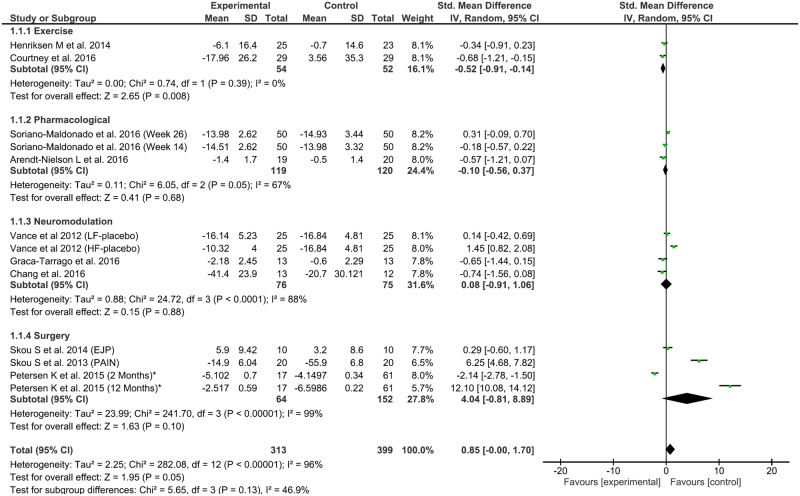
Figure 2.
Forest plot of interventions in pain efficacy by Hedge’s g.
Descriptive Statistics
Across all studies, there were 559 participants (≈201–214 males, 35.9–38.3%); the average age of participants was 62.4 (±36.02) years, and the range of disease duration (±SD) was 64.3 (±11.4) to 181.8 (±6.0) months. Please note that the range in sex is due to Tarragó et al. [28] reporting their sex variable as a total number instead of stratifying by male and female. Eight studies were randomized clinical trials (73%), two were cohorts (18%), and one was a cross-sectional study (9%). Of these 11 studies, two used the same data divided into separate publications (Soriano-Maldonado et al., Henriksen et al.) [29,30]. Seven of the studies were from the same group in Denmark (64%); two studies were conducted in the United States (18%), one in Australia (9%), and one in Brazil (9%). The interventions used were exercise (N = 2), pharmacological (N = 3), neuromodulation (N = 3), and surgical (N = 3). Of these 11 studies, five used only TS, three used only CPM, and three used both paradigms. Mechanical modalities (i.e., pressure or cuff ischemia) were the most commonly used methods for noxious stimuli in both TS and CPM, and for conditioning stimuli in CPM. Six studies controlled for medication use (55%), and all studies were considered low risk for bias.
See Table 1 for the general characteristics of included studies. See the online Supplementary Data for the results of pain intensity, TS, and CPM, for all included studies in this review (including the risk of bias), and for the list of excluded full-text studies.
Table 1.
General characteristics of included studies
| Study | Study Type | Country | Protocol | Group Description | No., Sex | Age, Mean (SD), y | Disease Duration, mo | Discontinued Medication |
|---|---|---|---|---|---|---|---|---|
| Vance et al. 2012 [31] | RCT | USA | TS | Group 1: HF-TENS; Group 2: LF-TENS; Group 3: Placebo | Group 1: 14f, 11m; Group 2: 16f, 9m; Group 3: 16f, 9m | Group 1: 57 (11.8); Group 2: 55 (14.4); Group 3: 57 (10.9) | Group 1: 108.8 (113); Group 2: 121.6 (141.2); Group 3: 83.5 (86.4) | Yes (4 h) |
| Skou et al. 2013 [14] | C | Denmark | TS, CPM | Group 1: Chronic pain; Group 2: No pain | Group 1: 14f, 6m; Group 2: 8f, 12m | Group 1: 61.5 (1.8); Group 2: 65.7 (1.3) | Group 1: 86.6 (14.1); Group 2: 89.1 (13.8); Group 3: 152.2 (24.1); Group 4: 181.8 (6.0) | Yes (24 h) |
| Skou et al. 2014 | CS | Denmark | TS | Group 1: OA & high PPT; Group 2: OA & low PPT; Group 3: RETKA & high PPT; Group 4: RETKA & low PPT | Group 1: 10f, 16m; Group 2: 15f, 12m; Group 3: 7f, 3m; Group 4: 7f, 3m | Group 1: 64.1 (1.5); Group 2: 61.4 (1.6); Group 3: 61.4 (3.1); Group 4: 61.5 (1.8) | Group 1: 167 (22.6); Group 2: 64.3 (11.4) | Yes (24 h) |
| Henriksen et al. 2014 | RCT | Denmark | TS | Group 1: Exercise; Group 2: Control | Group 1: 27f, 4m; Group 2: 21f, 8m | Group 1: 65.9 (8.5); Group 2: 61.3 (7.1) | NA | No |
| Petersen et al. 2015 [11] | C | Denmark | TS, CPM | Group 1: Low pain group; Group 2: High pain group | Group 1: 39f, 22m; Group 2: 7f, 10m | Group 1: 66 (NA); Group 2: 72 (NA) | NA | Yes (24 h) |
| Soriano-Maldonado et al. 2016 [30] & Henriksen et al. 2015 [29] | RCT | Denmark | TS | Group 1: Corticosteroid; Group 2: Placebo | Group 1: 33f, 17m; Group 2: 28f, 22m | Group 1: 65.5 (8.3); Group 2: 61.3 (9.9) | NA | No |
| Arendt-Nielson et al. 2016 | RCT | Denmark | TS, CPM | Group 1: Etoricoxib then placebo; Group 2: Placebo then etoricoxib | Total: 23f, 14m | Total: 63.3 (7.58) | Total: 130.8 (90) | Yes (24 h) |
| Chang et al. 2016 | RCT | Australia | CPM | Group 1: Active tDCS & exercise; Group 2: Sham tDCS & exercise | Group 1: 11f, 4m; Group 2: 9f, 6m | Group 1: 59.8 (9.1); Group 2: 64.1 (11.1) | Group 1: 86.4 (63.6); Group 2: 108 (87.6) | No |
| Courtney et al. 2016 | RCT | USA | CPM | Group 1: Knee joint mobilization; Group 2: Manual cutaneous input | Group 1: 16f, 13m; Group 2: 11f, 0m | Group 1: 59.41 (8.33); Group 2: 58.36 (8.48) | Group 1: 157.8 (107.4); Group 2: 137.16 (103.92) | Yes (24 h) |
| Graca-Tarrago et al. 2016 [32] | RCT | Brazil | CPM | Group 1: Active EIMS; Group 2: Sham | Group 1: Total N = 13; Group 2: Total N = 13 | Group 1: 65.15 (7.44); Group 2: 66.85 (7.53) | >12 mo | No |
C = cohort; CPM = conditioned pain modulation; CS = cross-sectional; EIMS = electrical intramuscular stimulation; HF-TENS = high-frequency transcutaneous electrical nerve stimulation; LF-TENS = low-frequency transcutaneous electrical nerve stimulation; N = sample size; NA = not available; OA = osteoarthritis; PPT = pain pressure threshold; RETKA = revision total knee athroplasty; RCT = randomized controlled trial; tDCS = transcranial direct current stimulation; TS = temporal summation.
Effects of Interventions on Pain, TS, and CPM for All Interventions
When we studied all interventions together, the effect size for each of the primary outcomes was nonsignificant. To account for the heterogeneity of effects across different treatments, we then performed a sensitivity analysis by type of intervention.
Sensitivity Analysis and Metaregression
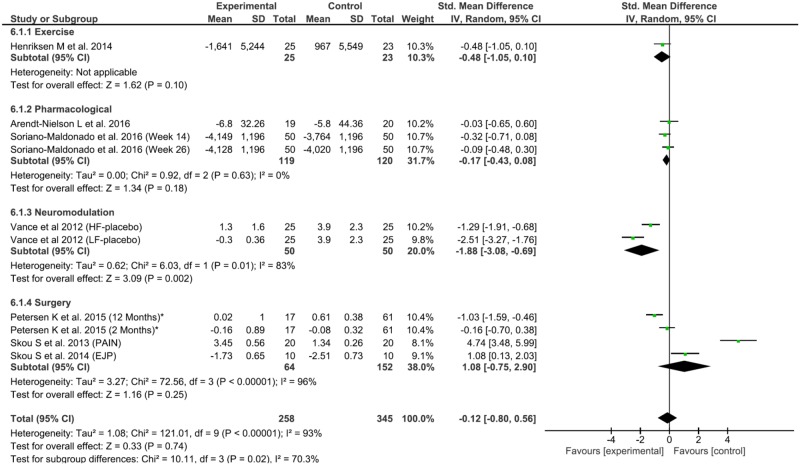
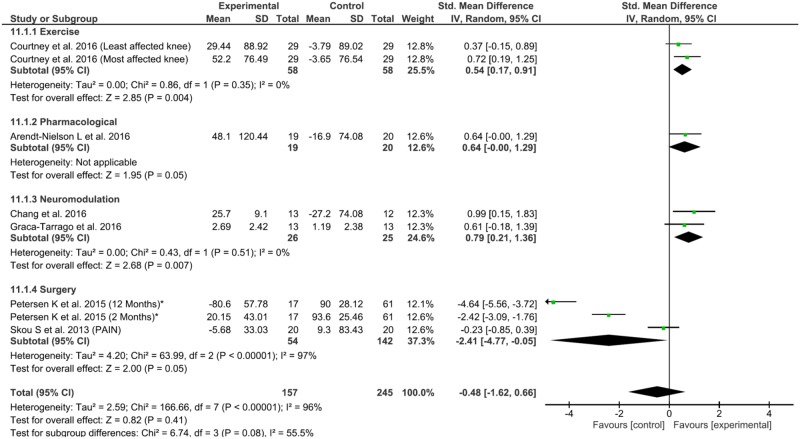
For the exercise interventions, there were significant and homogenous effects on pain reduction (0.52, 95% confidence interval [CI] = –0.91 to –0.41, P = 0.008, Q = 0.74, df = 1, P = 0.39, I2 = 0%) and CPM increase (0.54, 95% CI = 0.17 to 0.91, P = 0.004, Q = 0.86, df = 1, P < 0.35, I2 = 0%), but not TS. The neuromodulation group did not have significant pain reduction, although there were significant improvements in CPM and TS at a respective increase of 0.79 (95% CI = 0.21 to 1.36, P = 0.007, Q = 0.43, df = 1, P < 0.51, I2 = 0%) and decrease of 1.88 (95% CI = –3.08 to –0.69, P = 0.01, Tau2 = 0.62, Q = 6.03, df = 1, P < 0.01, I2= 83%). The pharmacological group had significant effects only on CPM increase (0.64, 95% CI = 0.00 to 1.29, P = 0.05) but no effects on TS or pain reduction. The surgical interventions did not have significant effects on any outcome (Figure 3).
Figure 3.
Forest plot of interventions in temporal summation (TS) by Hedge’s g.
Figure 4.
Forest plot of interventions in conditioned pain modulation (CPM) by Hedge’s g.
Using Monte-Carlo permutations, we found a substantial correlation between pain reduction and a decrease in TS (P = 0.02) and an increase in CPM (P = 0.04). See Table 2 for the results.
Table 2.
Metaregression results
| Independent Variable | Beta Coefficient | Standard Error | P Value | 95% Confidence Interval | Monte-Carlo Unadjusted P Value |
|---|---|---|---|---|---|
| Analysis 1 | |||||
| Hedges g TS | 5.63 | 2.16 | 0.04 | 0.36 to 10.92 | 0.02 |
| Stimuli | 34.70 | 8.79 | 0.00 | 13.20 to 56.20 | 0.00 |
| Age | 3.49 | 0.72 | 0.00 | 1.73 to 5.25 | 0.00 |
| Constant | –255.79 | 54.09 | 0.00 | –388.14 to –123.43 | – |
| N = 10, Tau2 = 15.89, I2 = 92.48%, adjusted R2 = 75.23%, F(4, 3) = 8.09, P = 0.01 | |||||
| Analysis 2 | |||||
| Hedges g CPM | –12.61 | 3.80 | 0.05 | –24.71 to –0.51 | 0.04 |
| Conditioning stimuli | –34.37 | 12.11 | 0.07 | –72.89 to 4.16 | 0.09 |
| No. of females | 1.40 | 0.44 | 0.05 | 0.00 to 2.80 | 0.06 |
| Medication discontinuation | –95.78 | 31.74 | 0.06 | –196.79 to 5.23 | 0.07 |
| Constant | 100.99 | 35.52 | 0.07 | –12.05 to 215.02 | – |
| N = 8, Tau2 = 16.12, I2 = 97.67%, adjusted R2 = 80.05%, F(4, 3) = 6.93, P = 0.07 | |||||
CPM = conditioned pain modulation; TS = temporal summation.
Discussion
Our central hypothesis for this meta-analysis was that the degree to which an intervention impacts a patient’s endogenous pain modulation profile, as measured by TS/CPM, would determine its analgesic efficacy. Due to the heterogeneity and limited availability of data, we are unable to make strong conclusions about the different types of interventions. To note, we were not comparing efficacy between groups of interventions as they are often indicated for different types of patients at different KOA stages. However, we uncovered encouraging relationships between pain, TS, and CPM in our a priori metaregression.
When we pooled the interventions by group, we observed that 1) exercise interventions significantly reduced pain, enhanced CPM, and had a nonsignificant TS reduction; 2) neither neuromodulation nor pharmacological treatment had significant analgesic effects; 3) neuromodulation techniques significantly reduced TS and increased CPM; 4) pharmacological interventions only enhanced CPM; 5) surgical interventions did not favorably modify pain, TS, or CPM. Based on our hypotheses, regulation of TS/CPM should parallel pain reduction (and vice-versa); however, this did not occur for the neuromodulation or pharmacological groups (which did alter TS/CPM in favor of the experimental intervention). There are multiple considerations, such as the limited number of studies, high variability in types of interventions studied within each group, differences in techniques used to measure endogenous pain modulation, and differences in study design.
However, we were able to discern pertinent and significant relationships between pain, TS, and CPM. We found that as participants had higher TS or lower CPM thresholds, they would report higher levels of pain; this suggests that patients with lower TS and higher CPM will conversely experience less pain. It also suggests that patients with better endogenous pain mechanisms would benefit more from therapeutic interventions as opposed to patients in a state of chronic central pain sensitization, a hypothesis that has also been noted by other studies [11–13,21–23].
Endogenous Pain Modulation, Analgesia, and Central Sensitization in KOA
Impaired endogenous pain modulation in the absence of structural pathology is evident in multiple central sensitization conditions, such as fibromyalgia, chronic migraine, and complex regional pain syndrome type I [4,9,33,34]. Patients with KOA and higher levels of pain demonstrated significantly decreased resting motor thresholds and increased intracortical facilitation on transcranial magnetic stimulation (TMS), that is, increased cortical excitability [35]. Other studies of chronic pain populations show that patients with higher intracortical facilitation also report higher pain on VAS scores and that greater disinhibition in the motor cortex was associated with lower CPM scores (i.e., less endogenous pain modulation) [28,32]. Up to 30% of KOA patients continued to experience chronic pain after TKR, potentially also due to defective endogenous pain modulation; although local causes may contribute to the pain, this is unlikely after a 12-month period [18]. Patients with higher pressure or thermal TS experienced greater pain intensity relative to healthy controls and patients with less pain [36,37]. Similarly, dysfunctional CPM predicted chronic pain development after various interventions [14,38]. These mechanisms may bridge discrepancies between high pain intensity and low disease structural severity [19,20]. Various studies allude to TS and CPM as indicators of pain-sensitized patients, and predictors of analgesic efficacy following therapeutic interventions [11,21,22,24].
Animal models of KOA show that following peripheral nerve injury (due to constant joint inflammation), microglia and astrocytes become overly active in the spinal dorsal horn, releasing pro-inflammatory cytokines [39,40]. Nerve growth factor and brain-derived neurotropic factor are also upregulated, maintaining central dorsal horn activation [41]. Additionally, inhibitory gamma–aminobutyric acid immunoreactive cells and fibers are downregulated, which positively correlates with increased sensitivity to pain [42–44]. In humans, there is (an initial) peripheral agent causing pain (e.g., KOA); however, it may not be enough to treat this agent in centrally sensitized patients [18]. Instead, these patients may benefit from interventions focused on adjusting sensorimotor integration and output and on reestablishing aberrant neuronal networks’ integrity and function [45]. Therefore, QST paradigms can aid in identifying patients with aberrant endogenous pain modulation for tailored treatments and modified rehabilitation interventions [46].
Review of Studies Included in Our Meta-analysis
We acknowledge that individual characteristics of included studies are equally as important to the overall quality and utility of our meta-analysis; to this extent, we report on pertinent characteristics and limitations of the individual studies included in our meta-analysis. First, we consider Chang et al. (classified under neuromodulation, N = 30) [47]. In this study, pain scores improved in both the active transcranial direct current stimulation (tDCS) + exercise and the sham tDCS + exercise groups, but there were no significant differences in pain reduction between the groups. It may be that exercise led to a ceiling effect with no additional improvements from active tDCS compared with sham. However, pain reduction in the active tDCS group was double that of sham, and the 95% CI barely overlapped 0; therefore, pain reduction may have been significant with improved power, particularly as CPM significantly improved in the active tDCS group. Also consistent with the low-power interpretation, Henriksen et al. (classified as an exercise intervention, N = 60) [47,48] showed no significant effect size differences in pain reduction between the exercise and no exercise groups, though pain reduction was greater in the exercise group and TS decreased in the exercise group but increased in the control group.
The transcutaneous electrical nerve stimulation (TENS) study is more difficult to interpret. In Vance et al. (N = 75), the three groups saw reduced raw pain scores compared with baseline, but pain in the placebo and low-frequency TENS (LF-TENS) groups was reduced almost to the same degree (overlapping, nonsignificant), whereas high-frequency TENS (HF-TENS) had less pain reduction than placebo (worse results, significant). Both HF-TENS and LF-TENS had significantly improved (reduced) TS effect sizes compared with placebo, yet when compared with baseline, the raw data showed a worsening of TS in the HF-TENS group and a minor improvement in the LF-TENS group (the placebo group did three times worse than HF-TENS in TS when compared with baseline). It seems that TS had little relation to pain reduction in this study despite the significantly improved TS effect size in HF-TENS and LF-TENS when compared with placebo, which may relate to study heterogeneity [31].
Regarding pharmacological groups, the two treatments studied were an injected corticosteroid and a nonsteroidal anti-inflammatory drug. The corticosteroid study included as many subjects as the combined exercise interventions, and neither study showed significant effects on pain reduction, TS, or CPM, so the null results are unlikely to be due to type II error.
Finally, the surgical studies in this meta-analysis were derived from observational study data, and therefore lack some of the rigor possessed by randomized clinical trials. Additionally, the data were analyzed in a retrospective manner, specifically between patients with abnormal TS and CPM vs patients with functional endogenous pain modulation. Therefore, it is understandable why the interventions (revision total knee athroplasty or total knee replacement) were less effective in modulating pain up to 12 months in these groups. Perhaps earlier surgery (before entrenched central sensitization) may have led to better results.
Aside from particulars, we draw the reader’s attention to the common element (and from the metaregression), which suggests that patients with more pronounced aberrant endogenous pain modulation were likely to experience higher pain levels before the interventions, and even after them.
Strengths and Weaknesses of Our Study
The strengths of our study include the registration of our meta-analysis before commencement; the thorough nature of our literature search, study selection, and data abstraction process; and our predefined data analysis plan. However, there are limitations that merit discussion. Despite having a moderately large sample size of individual participants, we only included 11 studies, which were further stratified into their respective interventions. Therefore, there were limited data, and due to the diversity of the interventions, it is difficult to completely appreciate their pooled relevance. However, this problem reflects the current state of the literature. Therefore, we focused on the metaregression results, which highlight the relationship between pain intensity, TS, and CPM. Furthermore, although we are unable to confirm our initial hypothesis, we were able to model a relationship between pain and TS/CPM in KOA patients. Another limitation is that QST requires participant cooperation and may not be standardized across studies. Participants’ expectancy management was not explicitly described in most studies, which may bias outcomes, given that participants’ expectancy significantly affects TS/CPM results [9,49–52]. KOA severity also varied between the different intervention categories, a fact worth mentioning even though radiologic severity and chronic pain intensity does not seem to correlate [19]. Most of the sample was derived from Denmark, which limits the generalizability of the results; we encourage replication of these methods in other settings. Likewise, further research into covariate effects and protocols is still needed to optimize the applicability of QST paradigms in clinical scenarios for KOA.
Concerning other sources of systematic error, we found that the largest source of potential bias was derived from improper reporting of the “random sequence generation” in two studies (Courtney et al. and Graca-Tarrago et al. [32]), blinding of participants and personnel in four studies (Chang et al., Courtney et al., Henriksen et al., and Vance et al. [31]), and blinding of outcome assessment in one study (Courtney et al.). Although unclear reporting is not akin to their being actual methodological limitations, it is suggestive. Improper randomization sequence generation affects baseline exchangeability between groups, and thereby the distribution of unknown bias. Likewise, improper blinding of participants, personnel, and assessors can lead to selection and measurement bias.
Strengths and Weakness of Our Study in Relation to Other Meta-analyses
There are three published systematic reviews and meta-analyses on CPM and TS in KOA. In one, CPM was reviewed by Lewis et al. [53] across multiple pain conditions (two observational studies on KOA were included). The overall conclusion was that descending pain modulation was impaired in KOA patients >40 years of age (though this conclusion is limited considering the number of studies aggregated). In another meta-analysis, Fingleton et al. [3] reviewed various QST approaches in 15 KOA publications, some of which included TS and CPM. Like Lewis et al. [53], Fingleton et al. [3] found that most studies on CPM and KOA demonstrated a dysfunctional pain inhibitory response (with the exception of one study comparing different symptom and disease severity groups).
For TS, Fingleton et al. [3] found increased TS in KOA both around the knee and in remote areas (areas such as the arms, back, and abdomen are used to test if the pain is localized or generalized) when compared with healthy controls. Also, TS and pain severity were positively and significantly correlated, whereas TS dysfunction—but not disease severity—was associated with the development of chronic pain postoperatively. Finally, O’Leary et al. [54] reviewed 13 publications on different musculoskeletal conditions (eight of which were on KOA) and different QST paradigms. Unlike the other two studies, which were more focused on phenotyping the KOA patients’ endogenous pain response, the study by O’Leary and colleagues used these QST paradigms to predict treatment response. Only one of the included studies in the review examined CPM and TS in KOA; this study demonstrated higher TS in their “high-pain” group postoperatively when compared with the “low-pain group” at 12 months. Additionally, there was a positive and significant correlation between preoperative TS and postoperative pain at 12 months.
Two of the aforementioned meta-analyses were designed to phenotype KOA patients, whereas the study by O’Leary used these QST paradigms to predict treatment response. These prior works establish the theoretical foundations for our hypothesis: that reduction of pain should parallel beneficial changes in TS and CPM (i.e., reduced hyperexcitability and enhanced pain inhibition). Indeed, through the metaregressions, we show that such a relationship is conceivable.
Conclusions
By aggregating multiple studies, we studied the effects of therapeutic interventions on endogenous pain modulation mechanisms relative to pain intensity in KOA. Though we cannot directly compare these interventions side by side, the results allow us to hypothesize that phenotyping KOA patients with TS/CPM may be useful for understanding therapeutic approaches. To validate TS and CPM as robust clinical biomarkers, it will be necessary to further investigate their mechanisms—and any patient factors driving responses—in a strict methodological manner. We established preliminary evidence of a relationship between pain reduction and TS/CPM normalization, which is encouraging and should motivate clinicians and researchers to further study these paradigms.
Supplementary Data
Supplementary data are available at Pain Medicine online.
Supplementary Material
Acknowledgments
Funding sources: FF has received funding support from National Institutes of Health (NIH) RO1 grants 1R01HD082302-01A1 and 1R01AT009491-01A1. ATO is a recipient of the Harvard Chan Central Grant. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest: The authors state that they have no significant competing financial, professional, or personal interests that might have influenced the performance or presentation of the work described in this manuscript.
References
- 1. Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: Estimates from the Global Burden of Disease 2010 Study. Ann Rheum Dis 2014;737:1323–30. [DOI] [PubMed] [Google Scholar]
- 2. March LM, Bachmeier CJM.. Economics of osteoarthritis: A global perspective. Bailliere’s Clin Rheumatol 1997;114:817–34. [DOI] [PubMed] [Google Scholar]
- 3. Fingleton C, Smart K, Moloney N, Fullen B.. Pain sensitization in people with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr 2015;237:1043–56. [DOI] [PubMed] [Google Scholar]
- 4. Staud R, Bovee CE, Robinson ME, Price DD.. Cutaneous C-fiber pain abnormalities of fibromyalgia patients are specifically related to temporal summation. Pain 2008;1392:315–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Potvin S, Marchand S.. Pain facilitation and pain inhibition during conditioned pain modulation in fibromyalgia. Pain 2016;1578:1704–10. [DOI] [PubMed] [Google Scholar]
- 6. Fingleton C, Smart K, Doody C.. Exercise-induced hypoalgesia in people with knee osteoarthritis with normal and abnormal conditioned pain modulation. Clin J Pain 2017;335:395–404. [DOI] [PubMed] [Google Scholar]
- 7. Kosek E, Ordeberg G.. Lack of pressure pain modulation by heterotopic noxious conditioning stimulation in patients with painful osteoarthritis before, but not following, surgical pain relief. Pain 2000;881:69–78. [DOI] [PubMed] [Google Scholar]
- 8. Staud R, Vierck CJ, Cannon RL, Mauderli AP, Price DD.. Abnormal sensitization and temporal summation of second pain (wind up) in patients with fibromyalgia syndrome. Pain 2001;91(1–2):165–75. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien AT, Deitos A, Triñanes Pego Y, Fregni F., Carrillo-de-la-Peña MT.. Defective endogenous pain modulation in fibromyalgia: A meta-analysis of temporal summation and conditioned pain modulation paradigms. J Pain 2018. doi:10.1016/j.jpain.2018.01.010. [DOI] [PubMed] [Google Scholar]
- 10. Yarnitsky D. Role of endogenous pain modulation in chronic pain mechanisms and treatment. Pain 2015;156(suppl 1):S24–31. [DOI] [PubMed] [Google Scholar]
- 11. Petersen K, Arendt-Nielsen L, Simonsen O.. Presurgical assessment of temporal summation of pain predicts the development of chronic postoperative pain 12 months after total knee replacement. Pain 2015;1561:55–61. [DOI] [PubMed] [Google Scholar]
- 12. Petersen KK, Simonsen O, Laursen MB, Arendt-Nielsen L.. The role of preoperative radiological severity, sensory testing, and temporal summation on chronic postoperative pain following total knee arthroplasty. Clin J Pain 2018;343:193–7. [DOI] [PubMed] [Google Scholar]
- 13. Wideman TH, Finan PH, Edwards RR, et al. Increased sensitivity to physical activity among individuals with knee osteoarthritis: Relation to pain outcomes, psychological factors, and responses to quantitative sensory testing. Pain 2014;1554:703–11. [DOI] [PubMed] [Google Scholar]
- 14. Katz NP, Paillard FC, Edwards RR.. Review of the performance of quantitative sensory testing methods to detect hyperalgesia in chronic pain patients on long-term opioids. Anesthesiology 2015;1223:677–85. [DOI] [PubMed] [Google Scholar]
- 15. Christensen AW, Rifbjerg-Madsen S, Christensen R, et al. Ultrasound Doppler but not temporal summation of pain predicts DAS28 response in rheumatoid arthritis: A prospective cohort study. Rheumatology 2016;556:1091–8. [DOI] [PubMed] [Google Scholar]
- 16. Yarnitsky D, Granot M, Nahman-Averbuch H.. Conditioned pain modulation predicts duloxetine efficacy in painful diabetic neuropathy. Pain 2012;1536:1193–8. [DOI] [PubMed] [Google Scholar]
- 17. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;1381:22–8. [DOI] [PubMed] [Google Scholar]
- 18. Bourne RB, Chesworth BM, Davis AM, Mahomed NN, Charron KDJ.. Patient satisfaction after total knee arthroplasty: Who is satisfied and who is not? Clin Orthop Relat Res 2010;4681:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finan P, Hussain S, Haque U, et al. Discordance between radiographic and clinical osteoarthritis symptoms is associated with altered pain processing. J Pain 2012;13:S27. [Google Scholar]
- 20. Neogi T, Frey-Law L, Scholz J, et al. Sensitivity and sensitisation in relation to pain severity in knee osteoarthritis: Trait or state? Ann Rheum Dis 2015;744:682–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Petersen K, Graven-Nielsen T, Simonsen O.. Preoperative pain mechanisms assessed by cuff algometry are associated with chronic postoperative pain relief after total knee replacement. Pain 2016;1577:1400–6. [DOI] [PubMed] [Google Scholar]
- 22. Arendt-Nielsen L, Jiang GL, DeGryse R, Turkel CC.. Intra-articular onabotulinumtoxinA in osteoarthritis knee pain: Effect on human mechanistic pain biomarkers and clinical pain. Scand J Rheumatol 2017;464:303–16. [DOI] [PubMed] [Google Scholar]
- 23. Petersen KK, Arendt-Nielsen L, Finocchietti S, et al. Age interactions on pain sensitization in patients with severe knee osteoarthritis and controls. Clin J Pain 2017;3312:1081–7. [DOI] [PubMed] [Google Scholar]
- 24. Kjær Petersen K, Bjarke Vaegter H, Arendt-Nielsen L.. An updated view on the reliability of different protocols for the assessment of conditioned pain modulation. Pain 2017;1585:988. [DOI] [PubMed] [Google Scholar]
- 25. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A.. Rayyan—A web and mobile app for systematic reviews. Syst Rev 2016;51:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higgins JPT, Green S. Table 7.7.a: Formulae for combining groups. In: Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; 2011.
- 27. Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. The Ottawa Hospital Research Institute, Ottawa, Ontario K1J 8M5, Canada; 2013. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm; accessed December 9, 2017. [Google Scholar]
- 28. Graca LM da, Deitos A, Brietzke A, Vercelino R.. Descending control of nociceptive processing in knee osteoarthritis is associated with intracortical disinhibition: An exploratory study. Medicine (Baltimore) 2016;9517:e3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Henriksen M, Christensen R, Klokker L, et al. Evaluation of the benefit of corticosteroid injection before exercise therapy in patients with osteoarthritis of the knee a randomized clinical trial. Jama Intern Med 2015;1756:923–30. [DOI] [PubMed] [Google Scholar]
- 30. Soriano-Maldonado A, Bartholdy C, Bandak E, et al. Intra-articular corticosteroids in addition to exercise for reducing pain sensitivity in knee osteoarthritis: Exploratory outcome from a randomized controlled trial. PLoS One 2016;112:e0149168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vance CGT, Rakel BA, Blodgett NP, et al. Effects of transcutaneous electrical nerve stimulation on pain, pain sensitivity, and function in people with knee osteoarthritis: A randomized controlled trial. Phys Ther 2012;927:898–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Da Graca-Tarrago M, Deitos A, Brietzke AP, et al. Electrical intramuscular stimulation in osteoarthritis enhances the inhibitory systems in pain processing at cortical and cortical spinal system. Pain Med 2016;175:877–91. [DOI] [PubMed] [Google Scholar]
- 33. Mathew NT, Kailasam J, Seifert T.. Clinical recognition of allodynia in migraine. Neurology 2004;635:848–52. [DOI] [PubMed] [Google Scholar]
- 34. Huge V, Lauchart M, Förderreuther S, et al. Interaction of hyperalgesia and sensory loss in complex regional pain syndrome type I (CRPS I). PLoS One 2008;37:e2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kittelson AJ, Thomas AC, Kluger BM, Stevens-Lapsley JE.. Corticospinal and intracortical excitability of the quadriceps in patients with knee osteoarthritis. Exp Brain Res 2014;23212:3991–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain 2010;1493:573–81. [DOI] [PubMed] [Google Scholar]
- 37. King C, Sibille K, Goodin B, Cruz-Almeida Y.. Experimental pain sensitivity differs as a function of clinical pain severity in symptomatic knee osteoarthritis. Osteoarthr 2013;219:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yarnitsky D, Crispel Y, Eisenberg E, et al. Prediction of chronic post-operative pain: Pre-operative DNIC testing identifies patients at risk. Pain 2008;1381:22–8. [DOI] [PubMed] [Google Scholar]
- 39. Calvo M, Bennett DLH.. The mechanisms of microgliosis and pain following peripheral nerve injury. Exp Neurol 2012;2342:271–82. [DOI] [PubMed] [Google Scholar]
- 40. Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B.. Importance of glial activation in neuropathic pain. Eur J Pharmacol 2013;716(1–3):106–19. [DOI] [PubMed] [Google Scholar]
- 41. Lopez-Alvarez VM, Modol L, Navarro X, Cobianchi S.. Early increasing-intensity treadmill exercise reduces neuropathic pain by preventing nociceptor collateral sprouting and disruption of chloride cotransporters homeostasis after peripheral nerve injury. Pain 2015;1569:1812–25. [DOI] [PubMed] [Google Scholar]
- 42. Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J.. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience 1997;763:845–58. [DOI] [PubMed] [Google Scholar]
- 43. Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K.. Changes in GAD- and GABA- immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat 1998;161:57–72. [DOI] [PubMed] [Google Scholar]
- 44. Kami K, Taguchi Ms S, Tajima F, Senba E.. Improvements in impaired GABA and GAD65/67 production in the spinal dorsal horn contribute to exercise-induced hypoalgesia in a mouse model of neuropathic pain. Mol Pain 2016;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pelletier R, Higgins J, Bourbonnais D.. Addressing neuroplastic changes in distributed areas of the nervous system associated with chronic musculoskeletal disorders. Phys Ther 2015;9511:1582–91. [DOI] [PubMed] [Google Scholar]
- 46. Pelletier R, Higgins J.. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC 2015;16:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang WJ, Bennell KL, Hodges PW, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial. PLoS One 2017;126:e0180328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Henriksen M, Graven-Nielsen T, Joergensen TS, et al. Exercise therapy reduces pain sensitivity in patients with knee osteoarthritis: A randomized controlled trial. Arthritis Rheum 2013;6612:1836–43. [DOI] [PubMed] [Google Scholar]
- 49. Kennedy DL, Kemp HI, Ridout D, Yarnitsky D, Rice ASC.. Reliability of conditioned pain modulation: A systematic review. Pain 2016;15711:2410–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cormier S, Piché M, Rainville P.. Expectations modulate heterotopic noxious counter-stimulation analgesia. J Pain 2013;142:114–25. [DOI] [PubMed] [Google Scholar]
- 51. Goffaux P, de Souza JB, Potvin S, Marchand S.. Pain relief through expectation supersedes descending inhibitory deficits in fibromyalgia patients. Pain 2009;1451:18–23. [DOI] [PubMed] [Google Scholar]
- 52. Robinson ME, Wise EA, Gagnon C, Fillingim RB, Price DD.. Influences of gender role and anxiety on sex differences in temporal summation of pain. J Pain 2004;5:77–82. [DOI] [PubMed] [Google Scholar]
- 53. Lewis G, Rice D, McNair P.. Conditioned pain modulation in populations with chronic pain: A systematic review and meta-analysis. J Pain 2012;1310:936–44. [DOI] [PubMed] [Google Scholar]
- 54. O’Leary H, Smart KM, Moloney NA, Doody CM.. Nervous system sensitization as a predictor of outcome in the treatment of peripheral musculoskeletal conditions: A systematic review. Pain Pract 2017;172:249–66. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.