Abstract
For individuals living with human immunodeficiency virus (HIV), viral suppression positively affects quality and length of life and reduces risks for HIV transmission. Men of color who have sex with men (MoCSM) who have been diagnosed with HIV have disproportionately low rates of viral suppression, with concomitant increases in incidence. We identified specific social, structural, and psychiatric factors associated with viral suppression among a sample of 155 HIV-positive MoCSM. Cigarette smoking and biological markers of recent drug use were significantly associated with detectable viral load. In contrast, individuals reporting a history of psychiatric illness during medical examination were more likely to be virally suppressed. Further analyses demonstrated that psychiatric illness may affect virologic outcomes through increased probability of being prescribed HIV medications. Alternatively, cigarette smoking and drug use appear to negatively affect subsequent HIV Care Continuum milestones such as medication adherence. Findings provide support for comprehensive intervention programs that emphasize prevention and treatment of cigarette, methamphetamine, and other drug use, and promote improved connection to psychiatric care. Continual achievement of this goal may be a crucial step to increase rates of viral suppression and slow HIV incidence in communities of MoCSM in Los Angeles and other urban areas.
Keywords: Minority, MSM, Viral suppression, Psychiatric illness, Cigarette smoking
Introduction
A report published by the Centers for Disease Control in 2014 estimated that 1.2 million persons were living with human immunodeficiency virus (HIV) infection in the US [1]. This same report estimated that, while 86% of HIV- infected individuals had been diagnosed with the disease, only 30% had successfully achieved viral suppression. In the US, viral suppression is both attainable, owing to the availability of effective antiretroviral medications, and critical for management of the disease. Persons living with HIV who maintain viral suppression can expect a nearly normal life expectancy and have low risk of transmitting HIV to others [2, 3]. With these facts in mind, the HIV Care Continuum, which details the progression of individuals through various intermediary steps between diagnosis and attainment of viral suppression, has become the focus of much attention at the national and international levels [4, 5].
The HIV Care Continuum maps a series of consecutive milestones that must be successfully achieved to reduce the burden of HIV disease at the population level: HIV diagnosis, linkage to care, retention in care, receipt of antiretroviral therapy, and attainment of viral suppression [6]. Failure to achieve the linked milestones in the HIV Care Continuum varies across demographic subgroups. A recent study conducted by a large US integrated care provider determined that a statistically greater percentage of White patients living with HIV filled prescriptions for antiretroviral medications and attained viral suppression, relative to patients belonging to all other racial/ethnic subgroups [7]. Among men who have sex with men (MSM) diagnosed with HIV infection, an estimated 28% of African American and 37% of Hispanic men have attained viral suppression compared to 47% of White MSM [8]. Inability to achieve and sustain viral suppression in people living with HIV is an important factor driving continued HIV transmission [3]. As such, the reduced prevalence of suppression among men of color who have sex with men (MoCSM), for whom rates of new diagnoses are already disproportionately high, is a serious public health concern [9].
In addition to variation across demographic subgroups, achievement of HIV Care Continuum milestones also varies across individuals characterized by different social, structural, and psychiatric factors. Factors affecting variability in daily routine, ability to plan and anticipate the future, and ability to maintain financial stability, employment, and stable housing may be important determinants of medication adherence [10]. The accumulation of these factors has been identified as an important barrier to receipt of regular and adequate medical care among individuals living with HIV [11]. Social, structural, and psychiatric factors are often associated with incident stressful experiences such as financial, legal, and relationship stresses, which have been shown to increase the odds of both HIV medication non-adherence and virologic failure [12].
The impact of various social, structural and psychiatric factors on progression through the HIV Care Continuum may also differ between racial/ethnic minority and majority subgroups. Depression, trauma, and stressful events have consistently been associated with increased viral load and other negative disease outcomes among cohorts of predominantly White MSM such as the San Francisco Men’s Health Study and the Multicenter AIDS Cohort Study [13–15]. Alternatively, with a sample of largely minority patients, Himelhoch et al. reported a significantly lower probability of antiretroviral therapy discontinuation during the first 2 years among patients with severe mental illness or depression and identified a significant association between increased count of mental health visits and decreased likelihood of medication discontinuation [16]. In a sample consisting of 60% racial and ethnic minority impoverished persons, Carrico et al. concluded that homelessness and stimulant use disorder decreased, while recent receipt of mental health treatment increased, an individual’s odds of utilizing antiret-roviral therapy [17]. This same study concluded that severe mental illness was significantly associated with increased viral load [17]. Studies such as this highlight the importance of distinguishing between mental health symptoms, diagnosis, and treatment as these factors may impact virologic outcomes differentially or even conversely. To better understand these varied findings, researchers must determine the extent to which specific factors impede or enhance achievement of critical treatment milestones, specifically among MoCSM.
Knowledge of social, structural, and psychiatric factors negatively impacting disease management among persons living with HIV has important programmatic and policy implications. Precise information about how specific factors correlate with failure to achieve HIV Care Continuum milestones can inform policymakers about the type and intensity of interventions that may be helpful in promoting successful virologic outcomes among MoCSM. A major advantage of identifying problematic social, structural, and psychiatric factors is that a wide range of effective, evidence-based approaches to enable behavior change or mitigate personal structural problems currently exist, such as substance abuse treatment and housing stability assistance programs [18, 19]. Mobilization of these and other programs for specific sub-groups such as MoCSM requires allocation of HIV prevention and treatment resources to a relatively small segment of the population. In practice, doing so may coincide with reallocation of resources away from the larger population of individuals living with HIV [20]. Strong evidence to support the association between specific social, structural, and psychiatric factors and disease outcomes is thus necessary to justify policy implementation.
Successful attainment of the HIV Care Continuum mile-stone of viral suppression requires high levels of adherence to antiretroviral treatment, though levels are now believed to be lower than once thought [21, 22]. Although there exists an abundance of literature describing associations between specific social, structural, and psychiatric factors and decreased adherence to antiretroviral therapy, far fewer studies have attempted to examine the association of these factors with the ultimate milestone, viral suppression among MoCSM [23–26]. Adherence is presumed to be the predominant factor determining virologic outcomes. Nevertheless, high levels of adherence measured using self-report or pill count methods do not perfectly correspond with high rates of viral suppression. Even with high self-reported adherence (≥ 90% 1-week adherence), Arnsten et al. observed only 62% of HIV-positive individuals meeting the criteria for viral suppression [27]. High adherence measured using the preferred electronic monitoring approach tends to be more highly correlated with viral suppression, but still imperfect given that only 79% of those individuals with ≥ 90% 1-week adherence were observed to obtain viral suppression [27]. It is thus important to evaluate the impact of social, structural, and psychiatric factors on attainment of viral suppression above and beyond the impact such factors are observed to have on reported or measured adherence.
The primary objective of this study is to identify specific social, structural, and psychiatric factors that are associated with attainment of viral suppression among a sample of urban-dwelling HIV-positive MSM, the majority of whom are MoCSM. Since the primary mechanism by which viral suppression is obtained among HIV-positive individuals is through antiretroviral therapy, this study will also examine the association of social, structural, and psychiatric factors with self-reported achievement of intermediate outcomes such as receipt of a prescription for HIV medications and self-reported recent adherence. To the extent possible, this study will provide a detailed description of the social, structural, and psychiatric factors associated with viral suppression to inform researchers and clinicians working to improve HIV Care Continuum outcomes among this unique population of urban-dwelling MoCSM.
Methods
Study Design and Recruitment
The Men who have sex with Men and Substance Use Cohort at UCLA Linking Infections, Noting Effects (mSTUDY) is an ongoing National Institute on Drug Abuse (NIDA)- sponsored cohort study being conducted at two sites in Los Angeles, California: The Los Angeles LGBT Center, which enrolls HIV-positive MoCSM, and the University of California Los Angeles (UCLA) Vine Street Clinic, which enrolls MoCSM at high risk for HIV infection. The primary objective of the broader study is to explore direct and indirect effects of substance use on the immune system and on behavior and to identify links to HIV transmission dynamics. In this particular study, we used data from HIV-positive members of the cohort and explored associations with viral suppression.
To be eligible for participation, individuals were required to be between 18 and 45 years of age, identify as male, and report having sex with men. During recruitment, efforts were made to enroll African American and Hispanic men with a history of drug use. A comprehensive study recruitment plan involving an extensive collection of materials for dissemination across various social media platforms was developed, implemented, and routinely updated to attract participants from the target population. Recruitment commenced in July 2014. Over time, enrollment steadily increased and diversified in conjunction with outreach efforts undertaken by site and study staff. Through active engagement with community-based organizations serving the Los Angeles LGBT community, substance use support groups, and HIV clinics, study personnel supported the broad dissemination of recruitment materials. Online resources such as Facebook, Twitter, Instagram, Craigslist, and GRINDR were successfully used to recruit from broad demographic and geographically diverse communities.
Participants in The mSTUDY attended a 1–2 h clinic- based enrollment visit. At the visit, participants received a medical examination, provided biological samples of blood, urine, and saliva, and provided information on their sexual behavior and social network by completing a computer-assisted self-interview (CASI). Biological samples were used to test for sexually transmitted infections (STIs), substance use, and for viral load and other basic clinical parameters. Participants were paid for their time ($75 for completing the enrollment visit). STI testing results were made available and study personnel facilitated linkages to care and treatment providers.
Measures
HIV Care Continuum Indicators
Prescription for HIV Medications
In completing the CASI, participants were asked, “Are you currently prescribed HIV medication (ARVs)?” Participants who responded “Yes” were assumed to have a current prescription for HIV medications. Other response options for this item were “No” and “Refuse to answer.”
Recent Adherence
Participants who indicated being currently prescribed HIV medications while completing the CASI were asked, “Did you miss any of your HIV medications this past Saturday or Sunday?” A response of “Yes” was assumed to indicate recent non-adherence. All other responses (including “No,” “Do not know,” and “Refuse to answer”) were assumed to indicate recent adherence.
Viral Suppression
Blood (~ 4 mL) was drawn and sent to LabCorp (Burlington, NC) for quantifying HIV RNA using real-time PCR. Participants with a viral load > 40 copies/mL were considered viremic. Participants with viral load ≤ 40 copies/mL were classified as virally suppressed.
Social, Structural, and Psychiatric Factors
Psychiatric Illness History
During the medical examination, history of psychiatric illness was obtained by the clinician conducting the exam. Clinicians asked participants if they had a history of mental health issues and recorded any details provided. Electronic medical records were not used to confirm diagnoses and any patient who reported psychiatric illness was considered to have a history of psychiatric illness, regardless of note contents. Notes were reviewed by the study team for the presence or absence of text referring specifically to depression, anxiety, posttraumatic stress disorder (PTSD), bipolar disorder, and schizophrenia or schizoaffective disorder. These text extractions were used only to provide a general description of the types of psychiatric disorders experienced by this sample and were not included in any inferential analyses.
Current Depression
Symptoms of depression were measured using the 20-item Center for Epidemiologic Studies Depression Scale (CESD) included as part of the CASI. The CESD assesses depression symptom severity in the general population during the past week [28]. Among individuals who provided responses to at least 16 of the 20 items, total scores were calculated by summing all non-missing items and could range from 0 to 60. A total score of 16 or greater indicated clinically significant depression symptoms [29].
Drug Use
Urine samples were collected from study participants and tested using radio-immune assay strips [Fastect II Drug Screen Dipstick Test, Alere iScreen Dx Single Dip Card, McKesson CONSULT Urinalysis Reagent Strips (for nitrites)] that detected metabolites of common drugs. The following thresholds for detection were used: marijuana (THC 50 ng/mL), methamphetamine (MET 500 ng/mL), amphetamine (AMP 1000 ng/mL), opiates (MOP 300 ng/mL, OXY 100 ng/mL, MTD 300 ng/mL), cocaine (COC 300 ng/mL), nitrites (0.05–0.1 mg/dL), and ecstasy (MDMA 500 ng/mL).
Drug test results for the visit were used to classify participants into one of three mutually exclusive categories: no drug use, use of marijuana only, and other drug use. To be classified under no drug use, results had to be negative for all drug classes. Positive results for marijuana and negative results for all other drugs resulted in classification in the marijuana only category. Positive results for one or more of: methamphetamines, opiates, cocaine, nitrites, ecstasy, and amphetamines resulted in classification in the other drug use category, regardless of marijuana results.
Hazardous Alcohol Use
On the CASI, participants were asked, “During the past 6 months, how often did you have 6 or more drinks on one occasion?” Several examples were then given to clarify the definition of a drink and participants were provided with response options of “Never,” “Less than monthly,” “Monthly,” “Weekly,” and “Daily or almost daily.” Participants who provided a response of “Weekly” or “Daily or almost daily” were considered to exhibit hazardous alcohol use. This item appears on the alcohol use disorders identification test (AUDIT-C) and has demonstrated favorable properties in the detection of heavy drinking and/or active alcohol abuse or dependence [30].
Cigarette Smoking
The CASI also included a question phrased “Do you currently smoke cigarettes?” Participants could choose from response options of “Yes,” “No,” “Occasionally (< 1 cigarette/day),” and “Refuse to answer.” A response of “Yes” was required to indicate cigarette smoking for the purpose of this study.
Unemployment
An item on the CASI asks participants “Which of the following best describes your current work situation:” with response options of “Disabled/on disability,” “Unemployed/not working,” “Working full-time (includes self-employment),” “Working part-time,” “Work at home as a caregiver,” “Full-time student,” and “Refuse to answer.” Participants who provided a response of “Unemployed/not working” were considered to be experiencing unemployment for the purpose of this study.
Housing Instability
On the CASI, participants were also asked, “In the last 6 months, how long have you spent in a place not designed for sleep?” Response options of “Never” or “1 night” were assumed to indicate stable housing and individuals who responded by selecting “Up to 1 week,” “1 week to 1 month,” or “More than 1 month” were considered to be experiencing housing instability. This decision was made by study investigators with the intent of distinguishing between incidental housing difficulties and actual instability.
Incarceration
The CASI includes a question regarding life-time history of incarceration. Participants were asked, “How many times have you been incarcerated in a jail, prison or detention facility for more than 24 hours?” Participants who provided responses of 2 or higher were considered to have a history of incarceration. This decision was made by study investigators based in part on the distribution of responses (15% of men provided a response of 1). The aim was to distinguish between individuals struggling with the instability caused as a result of incarceration and individuals who might have been detained temporarily due to a single incident.
Sample
As of September 2016, The mSTUDY cohort consisted of 171 HIV-positive men who were screened, met eligibility criteria, enrolled, and completed the baseline study visit. Given the objectives of this study, we excluded participants who, at baseline: (1) did not have valid toxicity screen results, (2) did not complete the relevant CASI items described previously, (3) did not have valid viral load measurements, or (4) refused to answer the current prescription to HIV medication item. Sixteen individuals were removed following application of these exclusion criteria resulting in a final analytical data set consisting of baseline records from 155 men. Where there existed sufficient sample size, we attempted to assess differences between the 16 excluded and the 155 included individuals. These two samples did not differ significantly with respect to race/ethnicity, age, psychiatric illness history, or drug use. Although we refer generally to this sample as a sample of MoCSM, the analytical sample consists of 88% men who identified as either Hispanic or Black (Non-Hispanic). The remaining 12% likely includes some men of color and at least nine individuals who identify as White (Non-Hispanic).
Statistical Analyses
Frequencies and percentages were calculated to describe the demographic characteristics and prevalence of social, structural, and psychiatric factors among the final sample of 155 men. Frequencies and percentages of men currently prescribed HIV medications, recently adherent, and virally suppressed at baseline were also calculated.
To address the primary objective of this study, we constructed a series of multiple logistic regression models to evaluate how social, structural, and psychiatric factors affect HIV Care Continuum outcomes both directly and indirectly through affecting the probability of being prescribed HIV medications and being recently adherent. To describe the direct association with viral suppression, multiple logistic regression models were constructed (one for each social, structural, and psychiatric factor) by regressing the log odds of viral suppression at baseline on an indicator variable for the presence/absence of the factor, a continuous age variable, and a categorical variable indicating race/ethnicity. Model results were used to calculate adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs).
Among social, structural, and psychiatric factors identified as being significantly associated with viral suppression, cross-tabulations were calculated and Chi-square tests conducted in order to describe the prevalence of co-occurring factors and provide a better understanding of the subgroup of MoCSM at risk for viremia.
To examine the association of social, structural, and psychiatric factors with achievement of intermediate outcomes, we conducted a set of sequential multiple logistic regression analyses. For each factor, we first regressed the log odds of being prescribed HIV medications on an indicator variable for the presence/absence of the factor among the entire sample. We then used the subsample consisting of men who had been prescribed HIV medications to fit a model regressing the log odds of reporting recent adherence on an indicator variable for the presence/absence of the factor. Lastly, we used the subsample consisting of men who had both been prescribed HIV medications and reported recent adherence to fit a model regressing the log odds of viral suppression on an indicator variable for the presence/absence of the factor. Age and race/ethnicity were controlled for in all models.
For each set of models, predicted probabilities were generated for an individual with age equal to the average observed age within the sample (33.9 years) and Hispanic race/ethnicity (the race/ethnicity reported among the highest percentage of participants). Thus, for a Hispanic man aged 33.9 years with the given social, structural, or psychiatric factor, we present (1) the predicted probability of receiving a prescription for HIV medications, (2) the conditional predicted probability of reporting recent adherence to HIV medications given receipt of a prescription, and (3) the conditional predicted probability of attaining viral suppression given receipt of a prescription and reported recent adherence to HIV medications. The predicted probabilities were generated for both the presence and absence of each social, structural, and psychiatric factor. Predicted probabilities were subsequently multiplied to calculate joint event probabilities which were plotted for visual examination of the predicted HIV Care Continuum trajectory in the presence or absence of each factor.
Results
In accordance with the study design and recruitment plan, the final sample consists primarily of relatively young, minority men (Table 1). The mean age in years among the 155 participants is 34. Forty-five percent of these participants identified themselves as Hispanic and 43% identified themselves as Black (Non-Hispanic). Half of the remaining 12% of participants identified themselves as White (Non-Hispanic) and the other nine participants either endorsed multiple races (in addition to Non-Hispanic) or refused to answer. Among the 70 individuals who identified themselves as Hispanic, 49 (70%) indicated that they were of Mexican/ Mexican American/Chicano origin, 1 (1%) indicated that he was of Puerto Rican origin, and 20 (29%) indicated that they were of another Hispanic, Latino or Spanish origin (other than Mexican/Mexican American/Chicano, Puerto Rican, or Cuban). Almost all (97%) of the participants in this study indicated some form of current health insurance with close to half (47%) reporting MediCal, Medicaid, LA Care, or Molina as their current insurance provider. Among this sample of 155 HIV-positive men, approximately half (n = 78) met the criteria for viral suppression at the time of the baseline study visit. The vast majority of participants (87%) reported being currently prescribed HIV medication but only 57% reported recent adherence to HIV medications.
Table 1.
Demographic characteristics, HIV Care Continuum indicators, and social, structural, and psychiatric factors among 155 HIV-positive MoCSM at the time of enrollment in The mSTUDY
| HIV-positive men (N = 155) |
||
|---|---|---|
| n | % | |
| Age (years) | ||
| 18–25 | 20 | 12.9 |
| 26–30 | 31 | 20.0 |
| 31–35 | 39 | 25.2 |
| 36–40 | 35 | 22.6 |
| 41–45 | 30 | 19.4 |
| Mean, SD | 33.9 | 6.7 |
| Race/ethnicity | ||
| Black (Non-Hispanic) | 67 | 43.2 |
| Hispanic | 70 | 45.2 |
| Other/unknown | 18 | 11.6 |
| HIV Care Continuum indicators | ||
| Prescription for HIV medications | 134 | 86.5 |
| Recent adherence | 88 | 56.8 |
| Viral suppression | 78 | 50.3 |
| Social, structural, and psychiatric factors | ||
| Psychiatric illness history | 101 | 65.2 |
| Current depression | 88 | 56.8 |
| Drug use | ||
| No drug use | 73 | 47.1 |
| Marijuana only | 32 | 20.7 |
| Other drug use | 50 | 32.3 |
| Hazardous alcohol use | 25 | 16.1 |
| Cigarette smoking | 63 | 40.7 |
| Unemployment | 49 | 31.6 |
| Housing instability | 42 | 27.1 |
| Incarceration | 21 | 13.6 |
Almost two-thirds of participants indicated a history of psychiatric illness and 57% of men in this sample reported clinically significant depression symptoms (Table 1). Over half of the study participants provided urine samples that tested positive for metabolites of at least one drug including 50 men who screened positive for at least one drug other than marijuana. Sixteen percent of participants indicated hazardous alcohol use and 41% reported current cigarette smoking. Over 30% of men in this sample reported unemployment and 27% reported housing instability in the past 6 months. Fourteen percent of participants reported a history of incarceration.
To provide a general description of the types of psychiatric disorders affecting our sample of MoCSM, we extracted information from clinician notes taken at the time of the medical exam and made a rough determination of the numbers of participants with a reported history of depression, anxiety, PTSD, bipolar disorder, and schizophrenia or schizoaffective disorder. Of the 101 participants with a history of psychiatric illness, 69% reported depression, 47% reported anxiety, 15% reported PTSD, 21% reported bipolar disorder, and 12% reported schizophrenia or schizoaffective disorder. Over one third (36%) of participants with a history of psychiatric illness reported both anxiety and depression. Approximately half of the participants (n = 50) reported more than one of the disorders considered.
Results from the set of models regressing the log odds of viral suppression on each social, structural, and psychiatric factor while controlling for age and race/ethnicity are presented in Table 2. Supplemental Table 1 contains the age and race/ethnicity parameter estimates for these adjusted models. History of psychiatric illness was significantly associated with increased odds of viral suppression (adjusted OR = 2.16, 95% CI = 1.08–4.32). Cigarette smoking (adjusted OR = 0.48, 95% CI = 0.24–0.93) and other drug use (adjusted OR = 0.29, 95% CI = 0.13–0.64) were significantly associated with decreased odds of viral suppression. As expected, viral suppression was significantly associated with being prescribed HIV medications (adjusted OR = 6.85, 95% CI = 1.90–24.66), and reporting recent adherence (adjusted OR = 2.56, 95% CI = 1.21–5.41) after controlling for age and race/ethnicity (results not shown).
Table 2.
Associations between social, structural, and psychiatric factors and viral suppression among 155 HIV-positive MoCSM enrolled in The mSTUDY
| B | SE | p | OR | 95% CI | |
|---|---|---|---|---|---|
| Social, structural, and psychiatric factors | |||||
| Psychiatric illness history | 0.77 | 0.35 | 0.029 | 2.16 | 1.08, 4.32 |
| Current depression | − 0.08 | 0.33 | 0.813 | 0.93 | 0.48, 1.77 |
| Drug usea | |||||
| Marijuana only | − 0.08 | 0.45 | 0.849 | 0.92 | 0.38, 2.21 |
| Other drug use | − 1.24 | 0.40 | 0.002 | 0.29 | 0.13, 0.64 |
| Hazardous alcohol use | 0.34 | 0.45 | 0.449 | 1.41 | 0.58, 3.39 |
| Cigarette smoking | − 0.74 | 0.34 | 0.030 | 0.48 | 0.24, 0.93 |
| Unemployment | − 0.25 | 0.35 | 0.473 | 0.78 | 0.39, 1.55 |
| Housing instability | 0.41 | 0.37 | 0.274 | 1.50 | 0.73, 3.11 |
| Incarceration | − 0.50 | 0.37 | 0.182 | 0.61 | 0.29, 1.26 |
All models adjusted for age and race/ethnicity. Reference is the absence of the factor unless otherwise noted
Reference is no drug use
Given the significant associations of psychiatric illness, cigarette smoking, and other drug use with viral suppression, we examined the prevalence of co-occurring factors and the specific drugs used by other drug users in this sample. Participants with a history of psychiatric illness were not significantly more likely to be classified as other drug users (33 vs. 32%, p = 0.88) or cigarette smokers (45 vs. 33%, p = 0.18), relative to participants with no history of psychiatric illness. Other drug users were significantly more likely to smoke cigarettes (58%) relative to participants who did not use other drugs (32%, p < 0.01). Twenty-one men were classified as having a history of psychiatric illness, being cigarette smokers, and other drug users (Fig. 1). A high percentage of other drug users tested positive for methamphetamine use (82%). Sixty-eight percent of other drug users tested positive for amphetamine use, the vast majority of whom also used methamphetamine. When used, opiates and ecstasy tended to be used alongside methamphetamines and amphetamines, whereas the majority of the cocaine users did not test positive for any other drug except marijuana.
Fig. 1.
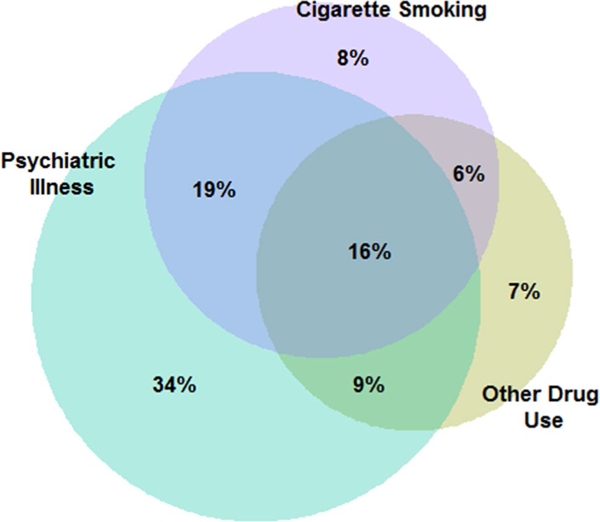
Diagram depicting the distribution and overlap of psychiatric illness, cigarette smoking, and other drug use among the 128 HIV- positive MSM participating in The mSTUDY who indicated at least one of these three factors. Circles are scaled such that the area is proportional to the number of individuals with the particular factor
Results from the set of multiple logistic regression models examining the sequential achievement of HIV Care Continuum milestones are presented in Table 3. Across all social, structural, and psychiatric factors and regardless of factor presence or absence, predicted probabilities of receiving a prescription were high and ranged from 0.84 to 0.95. The only significant difference with respect to factor presence versus absence was for psychiatric illness history. Men with a history of psychiatric illness had a predicted 0.94 probability of receiving a prescription relative to a 0.84 probability among men with no history of illness (p = 0.0167). Among the subsample of men who received a prescription, predicted probabilities of reporting recent adherence to medications were moderate and ranged from 0.53 to 0.80. Although results were not significant, the conditional predicted probability of reporting recent adherence to medications was noticeably lower (0.53) among other drug users relative to men using marijuana only (0.74) and using no drugs (0.69). Among the subsample of men who received a prescription and reported recent adherence, predicted probabilities of attaining viral suppression were low to moderate and ranged from 0.37 to 0.72 with the majority of probabilities falling between 0.55 and 0.70. The conditional predicted probability of viral suppression was significantly lower among men using other drugs (0.37) relative to men using no drugs (0.72, p = 0.0138). Cigarette smoking was also associated with a significantly reduced conditional predicted probability of viral suppression (0.39 vs. 0.71, p = 0.0076).
Table 3.
Multiple logistic regression modeling results presented in the form of predicted probabilities and conditional predicted probabilities for sequentially achieving HIV Care Continuum milestones among 155 HIV-positive MoCSM enrolled in The mSTUDY
| Predicted probability of: | Receiving a prescription (N = 155) |
Recent adherence to medicationa (N = 134) |
Viral suppressionb
(N = 88) |
|||
|---|---|---|---|---|---|---|
| Predictor of viremia: | PP | 95% CI | CPP | 95% CI | CPP | 95% CI |
| Social, structural, and psychiatric factorsc | ||||||
| Psychiatric illness history | ||||||
| No | 0.84 | 0.68, 0.93 | 0.61 | 0.43, 0.76 | 0.53 | 0.32, 0.74 |
| Yes | 0.94* | 0.86, 0.98 | 0.67 | 0.53, 0.79 | 0.67 | 0.49, 0.81 |
| Current depression | ||||||
| No | 0.89 | 0.76, 0.95 | 0.71 | 0.55, 0.83 | 0.65 | 0.46, 0.80 |
| Yes | 0.92 | 0.81, 0.97 | 0.60 | 0.45, 0.73 | 0.60 | 0.40, 0.76 |
| Drug use* | ||||||
| No drug use | 0.91 | 0.80, 0.96 | 0.69 | 0.55, 0.81 | 0.72 | 0.54, 0.85 |
| Marijuana only | 0.92 | 0.76, 0.98 | 0.74 | 0.50, 0.89 | 0.59 | 0.29, 0.83 |
| Other drug use | 0.88 | 0.73, 0.95 | 0.53 | 0.35, 0.70 | 0.37* | 0.17, 0.63 |
| Hazardous alcohol use | ||||||
| No | 0.91 | 0.81, 0.96 | 0.62 | 0.49, 0.74 | 0.62 | 0.45, 0.76 |
| Yes | 0.89 | 0.69, 0.97 | 0.80 | 0.55, 0.92 | 0.64 | 0.37, 0.84 |
| Cigarette smoking | ||||||
| No | 0.92 | 0.82, 0.97 | 0.67 | 0.53, 0.78 | 0.71 | 0.54, 0.84 |
| Yes | 0.87 | 0.72, 0.95 | 0.61 | 0.43, 0.76 | 0.39* | 0.20, 0.63 |
| Unemployment | ||||||
| No | 0.87 | 0.75, 0.94 | 0.66 | 0.52, 0.78 | 0.67 | 0.49, 0.81 |
| Yes | 0.95 | 0.85, 0.99 | 0.63 | 0.46, 0.78 | 0.55 | 0.34, 0.74 |
| Housing instability | ||||||
| No | 0.90 | 0.80, 0.96 | 0.65 | 0.51, 0.76 | 0.62 | 0.45, 0.76 |
| Yes | 0.90 | 0.75, 0.96 | 0.66 | 0.46, 0.81 | 0.65 | 0.40, 0.84 |
| Incarceration | ||||||
| No | 0.90 | 0.79, 0.95 | 0.65 | 0.52, 0.77 | 0.63 | 0.46, 0.77 |
| Yes | 0.92 | 0.78, 0.97 | 0.64 | 0.45, 0.79 | 0.60 | 0.36, 0.80 |
All models are adjusted for age and race/ethnicity and all probabilities are calculated for an individual at mean age (33.9 years) and Hispanic race/ethnicity
PP predicted probability, CPP conditional predicted probability
Indicates p < 0.05 for the model parameter estimate associated with the social, structural, or psychiatric factor
Conditional on having received a prescription
Conditional on having received a prescription and reporting adherence to medication
Three separate models were constructed for each factor (receiving a prescription, recent adherence to medication, and viral suppression
Figure 2 displays predicted probabilities for three sequential events indicating progression along the HIV Care Continuum. Plots allow for comparisons across men with and without a history of psychiatric illness, current depression, drug use (no drug use, marijuana only, and other drug use), and cigarette smoking. Across the three sequential events, we identify events at which the difference in predicted probabilities between an individual with and without a given factor appears to increase in magnitude relative to the previous event. A difference in probabilities between men with and without a history of psychiatric illness appears at the first event, corresponding to receipt of a prescription for medications, and this difference remains fairly constant across the next two events. Alternatively, the difference between probabilities for individuals with no drug use versus other drug use and between cigarette smokers and non-smokers appears to increase when moving from receipt of a prescription to adherence and suppression events. This is particularly true among cigarette smokers when moving from reported recent adherence to attainment of viral suppression.
Fig. 2.
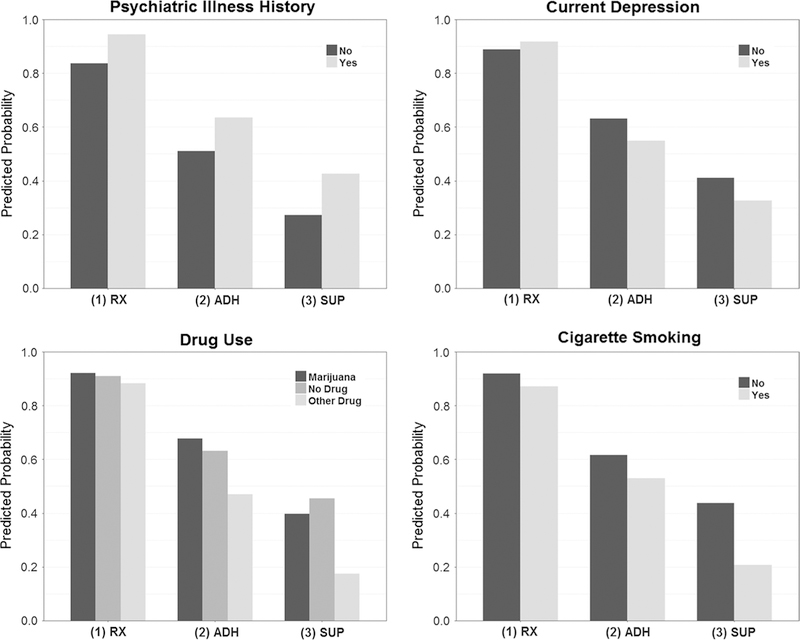
Model-based predicted probabilities for the event of receiving a prescription for HIV medications (RX) and joint event predicted probabilities for the events of receiving a prescription and reporting recent adherence (ADH), and the events of receiving a prescription, reporting recent adherence, and attaining viral suppression (SUP) among HIV-positive MoCSM enrolled in The mSTUDY
Discussion
We evaluated a number of social, structural, and psychiatric factors prevalent within a largely minority sample of urban-dwelling HIV-positive MSM and identified several factors significantly associated with viral suppression and intermediate HIV Care Continuum outcomes. Cigarette smoking and other drug use were significantly associated with a decreased likelihood of viral suppression among participants in this sample. In contrast, a self-reported history of psychiatric illness was significantly associated with an increased likelihood of viral suppression. Men who reported a history of psychiatric illness had an increased likelihood of attaining a prescription for HIV medications which was the intermediate outcome corresponding most strongly with ultimate differences in viral suppression. On the contrary, differences in the probability of receiving a prescription were minimal when comparing other drug users to marijuana users and non-users. Instead, differences across these groups arose when comparing intermediate outcomes related to self-reported recent adherence.
Previous studies have concluded that HIV-positive individuals experiencing mental health symptoms are at an increased risk of medication non-adherence [31–33]. Each of these studies evaluated the association between recent or current mental health symptom experiences and adherence at the present time. By examining history of psychiatric illness, our study extends this work by describing the association of mental health problems with viro- logic outcomes. Despite the fact that a high percentage of men in our sample reported depression as one of the psychiatric illnesses they had experienced, current depression assessed using the CESD was not associated with improved HIV outcomes and predicted probabilities actually indicated a slight decrease in the likelihood of recent adherence and viral suppression among men experiencing current depression (Fig. 2). While experiencing mental health symptoms such as depression may negatively impact medication adherence, reporting a history of psychiatric illness could indicate improved connection to care, which is a strong predictor of viral suppression achievement [34, 35]. Having a psychiatric illness will lead many to seek treatment for their symptomatology and frequently this will involve taking psychotropic medications consistently. It is possible that the skills required in successfully managing a psychiatric diagnosis directly transfer to the effective management of HIV disease and regular engagement in HIV-related care. Lastly, individuals who have been successfully treated for psychiatric illness may have been both more likely to indicate a history of psychiatric illness, relative to individuals with untreated or subclinical psychiatric symptoms, and may also be more likely to have an avenue for receipt of medical care for HIV.
There are several potential measurement issues that should be noted as limitations of this study. The biomarker assessment used to identify drug use captured only recent/ current drug use for many substances and may not accurately represent drug use as a persistent factor capable of impacting viral suppression. Determination of hazardous alcohol use was made based on responses provided to a single item from the three-item AUDIT-C. While this item has been used previously to detect active alcohol abuse or dependence [30], it captures only frequency of binge drinking. The full AUDIT-C inquires about drinking frequency and quantity in even non-binge settings which could impact an individual’s ability to maintain viral suppression. The criteria used to determine history of incarceration and housing instability based on single-item responses provided by participants in this study were developed by study investigators rather than being previously established. The properties and performance of these measures remains to be evaluated and could have limited our ability to accurately measure the association between these factors and viral suppression.
History of psychiatric illness was self-disclosed to a clinician in this study. Information regarding the specific diagnosis and chronological progression of the psychiatric illness was limited and collected inconsistently across patients and providers. Relative to the general population, persons living with HIV are known to experience heightened levels of mental health symptoms with even higher levels observed among minority samples and MSM [36, 37]. It is thus possible that the reference group used in this study consisting of participants with no reported history of psychiatric illness actually includes individuals with undiagnosed and therefore untreated psychiatric illness. In that case, our findings may reflect the benefit of diagnosis and treatment of existing mental health disorders in improving virologic outcomes relative to the alternative in which existing symptoms remain undiagnosed and untreated. Further, since the timing of mental health symptom presentation was not ascertained among men reporting a history of psychiatric illness, those individuals who had received treatment could potentially have reduced current symptom levels relative to symptomatic individuals with no identified history of psychiatric illness.
While results suggest that the association of psychiatric illness with viral suppression is partly attributable to increased likelihood of being prescribed medication, other attributions should be considered. In 2004, Ingersoll reported that among individuals living with HIV, those who screened positive for an anxiety disorder were at reduced risk of failing to take HIV medications as directed [24]. Ingersoll attributed this finding to increased self-protective vigilance about following directions among individuals endorsing symptoms of anxiety. Based on provider notes, almost half of the men with a history of psychiatric illness in the present sample reported experiencing anxiety, three quarters of whom also reported experiencing depression. Future studies are needed to determine whether the association between history of psychiatric illness and viral suppression is driven primarily by the presence or absence of anxiety symptoms. Since psychiatric symptoms such as symptoms of depression and anxiety are highly correlated, future studies should use more detailed information obtained from rigorous diagnostic tools administered in a structured clinical interview setting, for instance the Structured Clinical Interview (SCID) for DSM-IV or the Mini-International Neuropsychiatric Interview (MINI) [38, 39]. These tools would also allow for the reliable categorization of numerous other specific psychiatric diagnoses which may be co-morbid with HIV.
Previous studies attempting to describe the impact of drug use on adherence to HIV medications have published discordant findings. Studies which demonstrated no relationship between drug use and adherence often used self-report as the method to measure medication adherence. Other studies relied on pill counts or electronic monitoring devices, which have also demonstrated serious limitations [40]. In the present study, we chose to examine the association of drug use with both a crude indicator of recent adherence and the final milestone in the HIV Care Continuum, viral suppression. Although there are definite limitations associated with the recent adherence measure, we used an unbiased and accurate objective measure to ascertain recent drug use (urine drug screens), as opposed to drug use over the past several months, and the most relevant biomarker of HIV disease status, RNA quantification using real-time PCR. Analogously, Qian et al. [41] recently used urine drug screen information to demonstrate a significant association between a negative screen and viral suppression. In doing so, they advocated for the use of urine drug screens for rapid point-of-care screening at routine clinic visits to identify patients at risk for treatment failure and to drive patient management and referral to services such as directed adherence counseling and drug use treatment programs [41]. Results presented here further support the consideration of real-time drug screen results to optimize HIV disease management.
A high proportion of other drug using participants in this study reported use of methamphetamines (82%). Several previous studies have found that stimulant use, particularly use of cocaine or methamphetamines, is associated with decreased overall medication adherence [25, 26]. Hinkin et al. posit that, relative to other non-stimulant drugs, meth-amphetamines use may exert greater disruptions to sleep and eating patterns and increased levels of environmental instability leading to decreased adherence [25]. These findings are in line with the results presented here, in that drug use was shown to impact viral suppression but did not significantly influence the likelihood of receiving a prescription, implying that viremia may have resulted instead from factors other than access to medications, such as inconsistent adherence or methamphetamine effects on immune system functioning [42].
In the present sample, cigarette smoking was associated with an increased likelihood of detectable viral load. A recently published paper by O’Cleirigh et al. parallels these findings with clinical evidence gathered from the electronic health records of a sample of over 300 individuals living with HIV [43]. O’Cleirigh et al. observed that compared to non-smokers, cigarette smokers reported significantly lower adherence to HIV medications, attended fewer routine medical visits, were more likely to use cocaine and other drugs, and were more likely to have a detectable viral load. The authors also noted that smokers endorsed lower importance of health to their overall quality of life and hypothesized that this lower endorsement may correspond to decreased engagement in health protective behaviors such as attending preventive medical visits and adhering to medications. Since smoking was not associated with a decrease in the likelihood of being prescribed HIV medications within our sample, it is reasonable to attribute the elevated risk of detectable viral load among cigarette smokers to issues of adherence. Interestingly, cigarette smokers did not report dramatically lower frequencies of recent adherence compared to their non-smoking counterparts. Nevertheless, smokers experienced significantly lower probabilities of attaining suppression which may indicate poor reliability of this adherence measure for use with cigarette smoking populations or that suppression is being compromised via mechanisms other than recent adherence among this population. Although future effectiveness research is necessary, interventions aimed at reducing cigarette smoking as a means of improving HIV disease management among MoCSM may be impactful given that 41% of men in the present sample reported cigarette smoking compared to only 17% in the general population [44].
Nevertheless, the high rate of co-occurring drug use among the cigarette smoking subgroup may indicate that adherence is not compromised by cigarette smoking alone and points to the importance of encouraging smoking cessation at all points of care, including drug use treatment, in order to reach the goal of viral suppression. Although we observed higher rates of psychiatric illness and elevated depressive symptoms among cigarette smokers relative to non-smokers, these differences did not meet the traditional threshold for statistical significance (p = 0.18 and 0.08, respectively). However, our sample size is limited and previous research has suggested that smoking may be associated with negative HIV disease outcomes in part due to increased levels of depressive symptoms among HIV-positive smokers [45]. The mSTUDY focused recruitment efforts on men with a history of substance use and future studies are needed to describe the unique contribution of cigarette smoking on viral suppression in the absence of drug use and psychiatric illness.
The results presented herein provide valuable insights into potential mechanisms by which the prevalence of viral suppression may be increased among the subgroup of urban-dwelling, MoCSM. Although our sample of 155 HIV-positive men is limited in size, it is valuable in that it was drawn from this specific target population in which intervention efforts could greatly impact disease progression at the individual-and community-levels. Results presented in this paper are also strengthened by our use of reliable and objective tools for the measurement of drug use and viral load. Although self-reported, the medical history of psychiatric illness indication was obtained during the clinical exam component of the study visit in the presence of a health care professional responsible for delivering patient care.
Although the sequential analyses provide support for the pathway through which a history of psychiatric illness impacts the likelihood of attaining a prescription for HIV medications, thereby impacting virologic outcomes, the chronological ordering of these events is unverified. For example, a study participant could have obtained his prescription for HIV medications prior to experiencing psychiatric illness. Viral suppression could also have occurred prior to any psychiatric illness. Due to our limited sample size and specific research objective, we constructed models controlling only for participant age and race/ethnicity and did not include multiple social, structural, and psychiatric factors in the same model. Given the high rate of co-occurring factors, the MoCSM participants in this study with detectable viral load are likely to have a complex assortment of social, structural, and psychiatric factors which hinder optimal disease management. A limitation of the results presented herein is that we did not attempt to answer questions regarding the impact of co-occurring risk factors through a syndemic score or other approach. Due to sample constraints and the exploratory nature of this research we also did not correct for multiple comparisons. Future studies would benefit from exploring the associations presented here in greater detail with a larger sample and a data collection effort designed to facilitate understanding of how the interaction of various social, structural, and psychiatric factors and other demographic characteristics contributes to the likelihood of attaining viral suppression.
At the national level, increasing the rates at which MoCSM progress through the HIV Care Continuum has important implications for reducing morbidity and mortality, decreasing incidence, and eliminating racial/ethnic disparities in HIV disease burden. Efforts aimed at accomplishing this goal need to be multifaceted and designed to target barriers to viral suppression that are specific to the MoCSM subgroup. The results of this study support the development of comprehensive programs aimed at reducing cigarette smoking and substance use and increasing rates of treatment and connection to care among individuals experiencing psychiatric illness.
Supplementary Material
Acknowledgements
The mSTUDY was funded by the National Institute of Drug Abuse (NIDA) under Project Number 5U01DA036267 awarded to Dr. Pamina Gorbach and Dr. Steve Shoptaw at the University of California Los Angeles (UCLA). Additional support for this research was provided by National Institutes of Health (NIH) Grant Number P30 MH058107 awarded to Principal Investigator Dr. Steve Shoptaw at the UCLA Center for HIV Identification, Prevention and Treatment Services (CHIPTS).
Footnotes
Compliance with Ethical Standards
Conflict of interest Dr. Hilary J. Aralis, Dr. Ron Brookmeyer, Amy Ragsdale, Dr. Robert Bolan and Dr. Pamina M. Gorbach declare that they have no conflict of interest. Dr. Steve Shoptaw receives clinical supplies for his research from Medicinova, Inc.
Ethical Approval All procedures performed in this study were in accordance with the Ethical Standards of the Institutional Research Committee and with the 1964 Helsinki Declaration and its later amendments. This article does not contain any studies with animals performed by any of the authors.
Informed Consent Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material The online version of this article (https://doi.org/10.1007/s10461–018-2055-z) contains supplementary material, which is available to authorized users.
References
- 1.Centers for Disease Control and Prevention. HIV in the United States: the stages of care. https://www.cdc.gov/nchhstp/newsroom/docs/HIV-Stages-of-Care-Factsheet-508.pdf. Accessed 13 Jan 2017.
- 2.Nakagawa F, May M, Phillips A. Life expectancy living with HIV: recent estimates and future implications. Curr Opin Infect Dis. 2013;25(1):17–25. [DOI] [PubMed] [Google Scholar]
- 3.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375:830–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardner EM, McLees MP, Steiner JF, Rio CD, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risher K, Mayer K, Beyrer C. The HIV treatment cascade in men who have sex with men, people who inject drugs and sex workers. Curr Opin HIV AIDS. 2015;10(6):420–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mugavero MJ, Amico KR, Horn T, Thompson MA. The state of engagement in HIV care in the United States: from cascade to continuum to control. Clin Infect Dis. 2013;57(8):1164–71. [DOI] [PubMed] [Google Scholar]
- 7.Horberg MA, Hurley LB, Klein DB, et al. The HIV Care Cacade measured over time and by age, sex, and race in a large national integrated care system. AIDS Patient Care STDs. 2015;29(11):582–90. [DOI] [PubMed] [Google Scholar]
- 8.Hall HI, Holtgrave DR, Tang T, Rhodes P. HIV transmission in the United States: considerations of viral load, risk behavior, and health disparities. AIDS Behav. 2013;17:1632–6. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. HIV surveillance report. Diagnoses of HIV infection in the United States and dependent areas, vol 6 2014. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-us.pdf. Accessed 3 Feb 2017. [Google Scholar]
- 10.Zullig LL, Shaw RJ, Crowley MJ, Lindquist J, Grambow SC, Peterson E, Shah BR, Bosworth HB. Association between perceived life chaos and medication adherence in a postmyocar- dial infarction population. Circ Cardiovasc Qual Outcomes. 2013;6:619–25. [DOI] [PubMed] [Google Scholar]
- 11.Wong MD, Sarkisian CA, Davis C, Kinsler J, Cunningham WE. The association between life chaos, health care use, and health status among HIV-infected persons. J Gen Intern Med. 2007;22(9):1286–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mugavero MJ, Raper JL, Reif S, Whetton K, Leserman J, Thielman NM, Pence BW. Overload: the impact of incident stressful events on antiretroviral medication adherence and virologic failure in a longitudinal, multi-site HIV cohort study. Psychosom Med. 2009;71(9):920–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leserman J Role of depression, stress, and trauma in HIV disease progression. Psychosom Med. 2008;70:539–45. [DOI] [PubMed] [Google Scholar]
- 14.Mayne TJ, Vittinghoff E, Chesney MA, Barrett DC, Coates TJ. Depressive affect and survival among gay and bisexual men infected with HIV. Arch Intern Med. 1996;156:2233–8. [PubMed] [Google Scholar]
- 15.Farinpour R, Miller EN, Satz P, et al. Psychosocial risk factors of HIV morbidity and mortality: findings from the Multi center AIDS Cohort Study (MACS). J Clin Exp Neuropsychol. 2003;25(5):654–70. [DOI] [PubMed] [Google Scholar]
- 16.Himelhoch S, Brown CH, Walkup J, et al. HIV patients with psychiatric disorders are less likely to discontinue HAART. AIDS. 2009;23(13):1735–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carrico AW, Bangsberg DR, Weiser SD, Chartier M, Dilworth SE, Riley ED. Psychiatric correlates of HAART utilization and viral load among HIV-positive impoverished persons. AIDS. 2011;25(8):1113–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garner BR. Research on the diffusion of evidence-based treatments within substance abuse treatment: a systematic review. J Subst Abuse Treat. 2009;36:376–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson G, Aubrey T, Lafrance A. A review of the literature on the effectiveness of housing and support, assertive community treatment, and intensive case management interventions for persons with mental illness who have been homeless. Am J Orthopsychiatry. 2007;77(3):350–61. [DOI] [PubMed] [Google Scholar]
- 20.Forsythe S, Stover J, Bollinger L. The past, present and future of HIV, AIDS and resource allocation. BMC Public Health. 2009;9(Suppl 1):S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bangsberg DR, Deeks SG. Is average adherence to HIV antiretroviral therapy enough? J Gen Intern Med. 2002;17(10):812–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalichman SC, Kalichman MO, Cherry C, Hoyt G, Washington C, Grebler T, Merely C, Welles B. Intentional medication non-adherence due to interactive toxicity beliefs among HIV positive active drug users. J Acquir Immune Defic Syndr. 2015;70(5):503–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Halkitis PN, Kutnick AH, Slater S. The social realities of adherence to protease inhibitor regimens: substance use, health care and psychological states. J Health Psychol. 2005;10(4):545–58. [DOI] [PubMed] [Google Scholar]
- 24.Ingersoll K The impact of psychiatric symptoms, drug use, and medication regimen on non-adherence to HIV treatment. AIDS Care. 2004;16(2):199–211. [DOI] [PubMed] [Google Scholar]
- 25.Hinkin CH, Barclay TR, Castellon SA, Levine AJ, Durvasula RS, Marion SD, Myers HF, Longshore D. Drug use and medication adherence among HIV-1 infected individuals. AIDS Behav. 2007;11:185–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arnsten JH, Demas PA, Grant RW, Gourevitch MN, Farzadegan H, Howard AA, Schoenbaum EE. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV- infected drug users. J Gen Intern Med. 2002;17:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Cheng CJ, Buono D, Eckholdt H, Howard AA, Schoen-baum EE. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: comparison of self-report and electronic monitoring. Clin Infect Dis. 2001;33:1417–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 29.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging. 1997;12(2):277–87. [DOI] [PubMed] [Google Scholar]
- 30.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Arch Intern Med. 1998;158:1789–95. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: a review and meta-analysis. J Acquir Immune Defic Syndr. 2011. 10.1097/qai.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tucker JS, Burnam MA, Sherbourne CD, Kung FY, Gifford AL. Substance use and mental health correlates of nonad herence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–80. [DOI] [PubMed] [Google Scholar]
- 33.Holzemer WL, Corless IB, Nokes KM, Turner JG, Brown MA, Powell-Cope GM, Inouye J, Henry SB, Nicholas PK, Portillo CJ. Predictors of self-reported adherence in persons living with HIV disease. AIDS Patient Care STDs. 1999;13(3):185–97. [DOI] [PubMed] [Google Scholar]
- 34.Valdez H, Lederman MM, Woolley I, Walker CJ, Vernon LT, Hise A, Gripshover BM. Human Immunodeficiency Virus 1 protease inhibitors in clinical practice: predictors of virologic outcome. Arch Intern Med. 1999;159:1771–6. [DOI] [PubMed] [Google Scholar]
- 35.Lucas GM, Chaisson RE, Moore RD. Highly active antiretroviral therapy in a large urban clinic: risk factors for virologic failure and adverse drug reactions. Ann Intern Med. 1999;131:81–7. [DOI] [PubMed] [Google Scholar]
- 36.Bogart LM, Wagner GJ, Galvan FH, Landrine H, Klein DJ, Stick-lor LA. Perceived discrimination and mental health symptoms among Black men with HIV. Cult Divers Ethn Minor Psychol. 2011;17(3):295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kamen C, Flores S, Taniguchi S, Khaylis A, Lee S, Koopman C, Gore-Felton C. Sexual minority status and trauma symptom severity in men living with HIV/AIDS. J Behav Med. 2012;35:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sheevan DV, Lecrubier Y, Sheevan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(S20):22–33. [PubMed] [Google Scholar]
- 39.First M, Spitzer R, Gibon M, Williams J. Structured clinical interview for DSM-IV-TR Axis I, Research Version, Non-patient Edition (SCID-I/NP). Biometrics Research, New York State Psychiatric Institute. 2002. [Google Scholar]
- 40.Turner BJ. Adherence to antiretroviral therapy by human immunodeficiency virus-infected patients. J Infect Dis. 2002;185(S2):S143–51. [DOI] [PubMed] [Google Scholar]
- 41.Qian H, Mitchell VJ, Bebawy S, Cassell H, Perez G, McGowan CC, Sterling TR, Vermund SH, D’Aquila R, Hulgan T. Current drug use and lack of HIV virologic suppression: point- of-case urine drug screen versus self-report. BMC Infect Dis. 2014;14:508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shoptaw S, Stall R, Bordon J, Kao U, Cox C, Li X, Ostrow DG, Plankey MW. Cumulative exposure to stimulants and immune function outcomes among HIV-positive and HIV-negative men in the Multicenter AIDS Cohort Study. Int J STD AIDS. 2012;23:576–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Cleirigh C, Valentine SE, Pinkston M, Herman D, Bedoya CA, Gordon JR, Safren SA. The unique challenges facing HIV-positive patients who smoke cigarettes: HIV viremia, ART adherence, engagement in HIV care, and concurrent substance use. AIDS Behav. 2015;19:178–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention. Current cigarette smoking among adults—United States, 2005–2014. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6444a2.htm. Accessed 26 Jan 2017.
- 45.Webb MS, Vanable PA, Carey MP, Blair DC. Medication adherence in HIV-infected smokers: the mediating role of depressive symptoms. AIDS Educ Prev. 2009;21(Supplement A):94–150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


