Abstract
The Second International Conference on Crimean-Congo Hemorrhagic Fever (CCHF) was held in Thessaloniki, Greece, from September 10–13, 2017, and brought together international public health professionals, clinicians, ecologists, and basic laboratory researchers. Nearly 100 participants, representing 24 countries and the World Health Organization (WHO), were in attendance. Meeting sessions covered the epidemiology of CCHF in humans; ticks and virus-tick interactions; wild and domestic animal hosts; molecular virology; taxonomic classification; pathogenesis and animal models; clinical aspects and diagnosis; clinical management and clinical trials; and disease prevention in humans. The concluding session focused on recent WHO recommendations for public health measures and future research. This report summarizes lectures by the invited speakers and highlights advances in the field
1. Introduction
The Second International Conference on Crimean-Congo Hemorrhagic Fever (CCHF) was held in Thessaloniki, Greece, September 10–13, 2017. The First International Conference was held two years earlier in the same location (Papa et al., 2015). These meetings have brought together researchers from around the world to exchange knowledge and experience on various aspects of CCHF and to identify goals for laboratory and clinical research. Meeting sessions covered the epidemiology of CCHF in humans; ticks and virus-tick interactions; wild and domestic animal hosts; molecular virology; taxonomic classification; pathogenesis and animal models; clinical aspects and diagnosis; clinical management and clinical trials; and prevention in humans. The concluding session focused on recent World Health Organization (WHO) recommendations for public health measures and future research. This report summarizes lectures by the invited speakers and highlights advances in the field presented at the conference.
2. Background
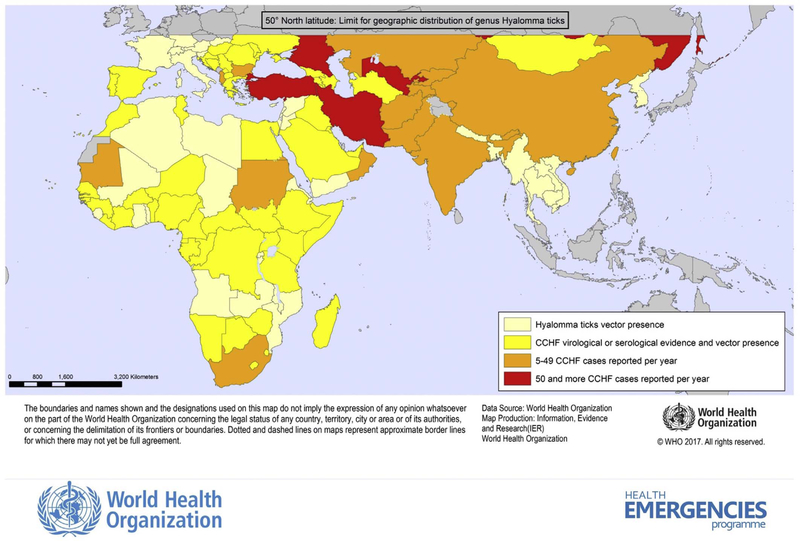
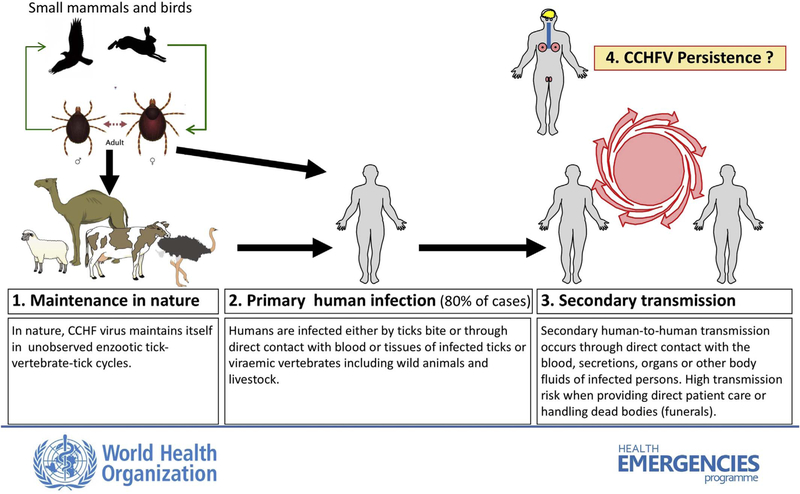
CCHF is a tick-borne zoonotic disease seen exclusively in humans; it can progress from mild fever and nonspecific signs to a severe and occasionally fatal hemorrhagic disease. The causative agent, CCHFV (family Nairoviridae, genus Orthonairovirus) is found across a wide geographic area, circulating in many countries of Asia, Africa, and southern Europe (Fig. 1). The first recognized outbreak of CCHF took place in the summer of 1944, when Soviet troops re-occupying areas of the Crimean peninsula that had been under German occupation developed an acute febrile illness with a high incidence of bleeding and shock (Grashchenkov, 1945). In 1969, it was recognized that the virus isolated from the Crimean peninsula was identical to an agent that had been isolated in the Belgian Congo (present Democratic Republic of the Congo, DRC) in 1956 (Casals, 1969). This discovery prompted a proposed single name of Crimean hemorrhagic fever-Congo virus, which was later simplified to CCHFV. CCHFV is commonly transmitted to humans through bites of infected ticks, usually those belonging to the genus Hyalomma, or through direct contact with blood or tissues of viremic livestock or patients. Ticks function as vectors and as natural reservoirs of CCHFV (Fig. 2). Reported case fatality rates have varied in different countries, ranging from ~5% to as high as 80%. Most infections are either asymptomatic or result in a nonspecific febrile illness that does not require hospitalization or specific therapy. In patients who develop hypotension and hemorrhage, supportive therapy is the mainstay of patient care (Bente et al., 2013). Currently there are no approved therapeutics or vaccines.
Fig. 1.
Geographic distribution of CCHF. Distribution of the principal tick vector of CCHF (pale yellow), of countries where serologic testing shows evidence of endemicity (yellow), and countries reporting from 5 to 49 (orange) and more than 50 cases (red) per year. The northernmost limit of Hyalomma ticks is demonstrated by a grey demarcation at latitude of 50° north. Courtesy of Pierre Formenty, WHO. Updated maps available at: http://www.who.int/csr/disease/crimean_congoHF/en/.
Fig. 2.
Maintenance of CCHF in nature and modes of transmission to and between humans. Courtesy of Pierre Formenty, WHO.
3. Meeting sessions
3.1. Epidemiology (Chairpersons: Anna Papa and Gülay Korukluoğlu)
3.1.1. Turkey
Gülay Korukluoğlu
(Public Health Institution of Turkey, National Virology Reference Laboratory, Ankara, Turkey) reported on current and past occurrences of CCHF in Turkey, where it has been a notifiable disease since December 2003. CCHFV circulating in Turkey belongs to taxonomic group V, the Europe-1 lineage. Since 2002, more than 10,000 confirmed cases have been reported, with a relatively low fatality rate (4.6%) compared to rates reported in other countries. The low case-fatality rate has been attributed to the success of the case management algorithm, which includes rapid laboratory confirmation within 24 h of sample receipt, and extensive experience with clinical management and treatment. Interestingly, the number of suspected cases and the incidence rate have significantly declined in recent years; this pattern will be closely monitored in the coming years to determine whether this decline will persist.
While no cases were reported in Turkey before 2002, seropositivity in persons over 65 years of age indicates that the virus was circulating in some areas before the disease was recognized. The majority of cases were reported from the Middle Anatolia-Black Sea region. Maintenance of virus transmission and increased disease incidence in this region are associated with differences in climate (temperature, humidity, etc.), geography, flora and wildlife, industry (animal husbandry sector), and frequency of human contact with animals and ticks. The main vector is Hyalomma marginatum, and the occupations at risk have primarily been those engaged in animal husbandry and farming, involving close contact with ticks. Nearly two-thirds of the cases are farmers and home-makers in rural areas. In earlier years, cases were reported from April to September, with peak levels in July. More recently, this period has been extended, lasting from March through November.
3.1.2. India
Pragya Yadav
(National Institute of Virology, Pune, India) provided an overview of the CCHF situation in India. Since its first detection, many nosocomial outbreaks and sporadic cases have been investigated and confirmed in Gujarat and the adjoining Rajasthan State, including a nosocomial outbreak in Ahmadabad, Gujarat State, in 2011 (Makwana et al., 2015; Mishra et al., 2011; Mourya et al., 2012; Yadav et al., 2013a; Yadav et al., 2016, 2014). Pragya also summarized the results of various studies, including surveys in ticks and a survey of anti-CCHFV IgG antibodies in livestock (Mourya et al., 2014a,b; Mourya et al., 2015). Animal serosurveys are considered very useful in indicating virus prevalence in an area; tick studies are less indicative, as the appropriate number of ticks that must be collected to be useful and predictive is not clear (Gurav et al., 2014; Mourya et al., 2014a,b; Mourya et al., 2015; Yadav et al., 2013b).
Pragya explained that diagnosing high-risk pathogens is a major concern in India, where there are few biosafety level 3 (BSL-3) laboratories and only one BSL-4 laboratory. Guidelines have been prepared for case identification, diagnosis, quarantine policies, and contact tracing. Combining the shared responsibilities of the Indian Council for Medical Research (ICMR), the Integrated Disease Surveillance Program, and the National Vector Borne Disease Control Program, CCHF cases have been traced, diagnosed, quarantined, and further managed in a timely manner. The One Health program requires serious alertness and cohesive work, and thus the ICMR and the Indian Council of Agricultural Research authorities have signed a Memorandum of Understanding to work together.
3.1.3. Pakistan
Bushra Jamil
(Aga Khan University, Karachi, Pakistan) discussed CCHF in Pakistan. Changes in epidemiological features, such as increased temperatures, humidity, expanding habitat of vectors, reservoir population dynamics, and movement of humans and animals have been identified as major contributors to the spread of disease in Pakistan. Thirteen outbreaks were documented between 1960 and 2004, but from 2007 onwards, outbreaks have occurred yearly in different parts of the country (Jamil et al., 2005). As in other regions, the main vectors in Pakistan are Hyalomma spp. ticks; tick-infested, viremic ruminants remain asymptomatic and are the principal source of infection and outbreaks. In view of the high mortality and the potential for nosocomial spread (Hasan et al., 2013), infection must be rapidly recognized and confirmed, but early recognition is challenging due to co-circulating pathogens with similar clinical signs. CCHF occurs in an area which also experiences cases of Dengue fever, and, more recently, Chikungunya (Hasan et al., 2014). Strains from Pakistan, the United Arab Emirates, and Madagascar fall into the Asia-1 lineage, suggesting that the livestock trade, and perhaps bird migration, may provide links between these distant geographic locations.
3.1.4. Bulgaria
Iva Christova
(National Center of Infectious and Parasitic Diseases, Sofia, Bulgaria) stated that CCHF was first detected in Bulgaria in 1952; since then, over 1500 cases have been reported. Most frequently affected are working-age men who are engaged in livestock breeding and reside in southeastern Bulgaria. Molecular analysis has shown that virus of lineage Europe-1 has been predominantly detected in H. marginatum ticks collected from livestock, supporting the leading role of these ticks as vectors. CCHFV detected in Rhipicephalus sanguineus sensu lato ticks clustered in the Europe-2 lineage. Notably, ticks collected from an individual domestic animal were infected either with lineage Europe-1 or with Europe-2 strains. Two seroprevalence studies in healthy individuals were conducted, one in 2011 and another in 2015. Results showed increasing levels of seroprevalence as well as expansion of the virus into new areas.
3.1.5. Spain
María Paz Sánchez-Seco Fariñas
(Instituto de Salud Carlos III, Majadahonda, Spain) presented two human cases that occurred in Spain in 2016, the first in this country (Negredo et al., 2017). The viruses isolated from these patients are included in Africa-3 lineage, the same lineage as virus found in local ticks. Previously, CCHFV genome was detected in ticks collected from red deer in Cáceres, Spain, in 2010, 300 km from the province of Ávila where the index patient acquired the disease through a tick bite. Tick studies confirmed positive identification in 2011, 2013, and 2015 (Cajimat et al., 2017). From September 2016 to March 2017, the presence of CCHFV was detected in 128 (3.2%) of 3959 pools of ticks collected from wild or domestic animals. Further studies are needed to determine the extent of virus circulation in this country and the associated risk for public health.
3.1.6. Mongolia
Randal Schoepp
(United States Army Medical Research Institute of Infectious Diseases (USAMRIID), Fort Detrick, MD, USA) described a collaboration with colleagues from the National Center for Zoonotic Diseases of the Ministry of Health, Ulaanbaatar, Mongolia on the risk of CCHFV infection for humans in Mongolia. The collaborators conducted surveillance for IgG antibody prevalence in healthy persons and virus prevalence in tick vectors in the region. Testing of 1926 human serum samples by enzyme-linked immunosorbent assay (ELISA) demonstrated an overall antibody prevalence of 1.4%. Real-time PCR was used to test 4530 ticks in pools of various numbers; the pools were delineated by species (Hyalomma asiaticum or Dermacentor nuttalli). Sequences of the S and M segments from CCHFV collected from these tick species clustered with other strains from China and South Africa, respectively. This study provides a better understanding of the risk of infection in the human population of Mongolia.
3.1.7. Georgia
Giorgi Chakhunashvili
(National Center for Disease Control and Public Health, Tbilisi, Georgia) presented the CCHF surveillance system in Georgia, operating from September 2009 through May 2017. In total, 54 cases have been reported through 2017. The reported mortality rate (14%) might be higher than in neighboring countries. The majority of cases were male (61%) with a wide age distribution (4–77 years old). Giorgi said that, because the virus was first detected in Georgia in 2009, the epidemiology and burden of disease in this country are not well known. New locations are identified as the surveillance system sensitivity and public awareness increase throughout the country over time.
3.1.8. Russia
Vladimir Dubyanskiy
(Stavropol Research Anti-Plague Institute, Stavropol, Russian Federation) presented a risk-based model for forecasting the epidemiological situation of CCHF in the Russian Federation based on a study of the Stavropol region. This study reported that 545 confirmed cases, 1 of them fatal, were registered in the southern regions of Russia between 2012 and 2016. Effective, specific means of prevention are not currently available, so particular attention must be given to realization of non-specific and preventive measures. Correctly predicting infectious morbidity is the most important prerequisite for planning the implementation of these measures.
Vladimir presented the forecasting model, which was developed based on non-homogeneous sequential pattern recognition procedures used previously for plague prediction (Dubyanskiy and Yeszhanov, 2016; Dubyansky et al., 1992; Gubler, 1978). Monthly values of climatic factors (e.g., average temperature, relative air humidity, rainfall, snow cover depth, atmospheric pressure) and epidemiological data (e.g., number of cases in the past year, numbers of localities with reported CCHF activity) are used as predictors. Forecast results can be considered reliable if error probability is less than 0.1%. The possible prognoses have been highly reliable: only 2 false negative results had been received from 2 of the 32 administrative districts in 2015 (patients might have acquired the disease after travelling to other areas).
Olga Maletskaya
(Stavropol Research Anti-Plague Institute, Stavropol, Russian Federation) reviewed the molecular epidemiology of CCHF in Russia, and stated that 2046 cases, 82 of them fatal (4.1%), were detected in Russia between 1999 and 2016 (Volynkina et al., 2017). Partial S-, M-, and L-segment sequences were obtained from 500 serum samples from patients and from 100 samples of ticks collected in 2007–2016 in the south of Russia. Full-length S-, M-, and L-segment sequencing of 24 isolates was also performed. The investigated viral isolates belonged to lineages Europe-1 (593 samples), Africa-3 (1 sample), and Europe-3 (6 samples). Russian isolates within the Europe 1 genotype formed 4 subgroups (Va–Vd) correlating with the geographic location of virus isolation. The processes of segment reassortment between strains of different subtypes within the Europe-1 genotype were revealed. Analysis of the geographic distribution of genetic variants in the south of Russia showed the presence of local viral populations with partially overlapping boundaries.
3.1.9. Other countries
The final presentation of the session was by Maryam Keshtkar-Jahromi (Johns Hopkins University School of Medicine, Baltimore, MD, USA), who reviewed current knowledge of the status of CCHF to date in the Mediterranean basin and Western Asian countries. Data were collected to better define current disease status and potential for transmission in this area. PubMed, Google Scholar, Scopus, GenBank, GIDEON, ProMED, and the US National Tick Collection were used to review the literature on reported human cases; human and animal seroprevalence; virus detection in ticks; and H. marginatum and Hyalomma rufipes distribution in the Mediterranean basin and Southern and Western Asia; the search terms used were “Crimean,” “Hyalomma,” and each country’s name. Maryam and her colleagues used a One Health approach to evaluate current data and make country-based predictions of endemicity.
Based on these data, countries were categorized into five levels of evidence of CCHF prevalence and potential for disease transmission. Level 1 countries (Iran, Iraq, Pakistan, and Turkey) are those in which human cases are reported annually through surveillance systems. These countries are endemic for CCHF with varying levels of surveillance systems in place. In Level 2 countries (Afghanistan, Egypt, Oman, Saudi Arabia, and UAE), occasional autochthonous human cases are reported. These countries require the most robust active surveillance systems to identify hot spots and increase public awareness. Level 3 countries (Kuwait, Syria) have not reported cases, but available ecologic data point towards the possibility of CCHF cases, and thus efforts to identify undiagnosed human infections are needed. In Level 4 countries (Cyprus, Israel, Jordan, Lebanon, Palestine, Yemen), no human cases have been reported but Hyalomma ticks are present. Given the proximity of Level 4 countries to endemic countries, the chance of CCHFV circulation is high. Future epidemiologic studies should include serosurveys and tick surveys. In Level 5 countries (Bahrain and Qatar), no information is available. In the absence of available information, studies into the presence or absence of Hyalomma ticks are warranted. These data highlight the utility of a One Health approach to accurately evaluate CCHF endemicity, risk of emergence, country level priorities, and resource allocation to control disease.
3.2. Ticks and virus-tick interactions (Chairpersons: Agustin Estrada-Peña, Dennis Bente)
Agustin Estrada-Peña
(University of Zaragoza, Zaragoza, Spain) focused on the tick vector and reservoir, and provided a detailed overview of the tick life-cycle, including the concept of co-feeding that may significantly contribute to populations of Hyalomma spp. adults with high infection levels. Hyalomma spp. ticks are considered “wanderers,” and must be collected from the ground. To accomplish this, traditional flagging techniques (sweeping cloth attached to a pole through leaf litter or vegetation) cannot be used. Rather, the researcher must walk over the area and stimulate the ticks; this collection technique is very different from those used to capture other species. Importantly, Agustin detailed proper interpretation of data from the field. Detection of CCHFV in a questing tick indicates the presence of the virus in the area, but does not provide information about transstadial or transovarial transmission in the tick species, and does not prove that this species can transmit the virus. A positive tick collected while feeding on a host also indicates the presence of the virus in the area, but not whether the tick was infected prior to attachment, or became infected from the viremic host.
Agustin also noted that a trend towards a warmer climate creates more areas suitable for colonization by infected ticks. New permanent populations have been found in France, and birds infested with these ticks have been collected as far north as the United Kingdom. The effect of a warming climate can only be addressed with reliable field surveys in European countries. Future CCHFV research should be based on understanding the epidemiological and pathogenic properties of strains circulating in the areas of distribution in the Mediterranean region, as well as ascertaining the role of birds in dispersing infected ticks. The development of active surveys at critical sites of the Mediterranean region would be a crucial step to ascertain if the virus is already present. Laboratory work should ideally be complemented by field data. Modeling strategies may help understand the trends, but fail when trying to capture local patterns.
Dennis Bente
(University of Texas Medical Branch, Galveston, Texas) reviewed current gaps in understanding of tick-virus interactions and the tick-virus-host interface, and emphasized the need to conduct tick-host transmission studies in the laboratory. These present a unique challenge, as they require high biocontainment and tick colony maintenance, and typically last years, making funding difficult. Using a H. marginatum transmission model, Dennis’s team found a substantial number of consensus-level mutations in CCHFV recovered from ticks after only a single transstadial transmission event, whereas none were detected in virus obtained from the mammalian host. Furthermore, greater viral intra-host diversity was detected in the tick compared to the vertebrate host (Xia et al., 2016). Additionally, it was demonstrated that components of H. marginatum salivary gland extract may mask infection of dermal antigen-presenting cells, which might be an important virulence mechanism in the early pathogenesis of the disease.
Anna Papa
(Aristotle University of Thessaloniki, Greece) reported on a strain of lineage Europe-2 (or clade VI) isolated from Rhipicephalus bursa ticks collected in 2015 from an anemic goat in northern Greece. Next-generation sequencing and phylogenetic analysis showed that the strain differed in amino acid sequence from the prototype Greek AP92 strain in S, M, and L segments by 1%, 11.1%, and 1.3%, respectively. Anna emphasized the need for in vitro and in vivo studies on this lineage, to investigate whether it is less virulent for humans than other lineages, as has been previously suggested.
Sabri Hacıoğlu
(Ankara University, Ankara, Turkey) presented preliminary findings of a tick survey performed in Mersin and Van provinces in southern and eastern Anatolia (Dinçer et al., 2017). Pools of Rhipicephalus spp. and Hyalomma spp. ticks were tested by PCR for nairoviruses. Five R. bursa pools were positive for CCHFV, and sequences of these isolates differed by 2.3–2.5% from the prototype Greek AP92 strain. These isolates form a separate monophyletic lineage with the AP92 strain (Europe-2 or clade VI), distinct from other lineages in the maximum likelihood analysis. This report constitutes the initial detection of AP92-related viruses in the screened locations, and indicates a larger zone of activity for this lineage in Turkey, including southern (Mediterranean) and eastern Anatolia.
3.3. Wild and domestic animal hosts (Chairpersons: Martin Groschup, Aykut Ozkul)
Many studies have been performed investigating seroprevalence in animals (Spengler et al., 2016a), but most of these data are based on pre-modern assays with unknown sensitivity and specificity. Furthermore, currently no validated commercial tests are available for testing animal sera. Martin Groschup (Friedrich Loeffler Institute, Insel Riems, Germany) discussed the use of animal assays to reveal CCHFV infection risks. He emphasized the value of animals as indicators of risk to permit timely introduction of public health protection measures, and stated that testing should focus on wildlife, followed by small ruminants and then cattle.
ELISA is the preferred method for serological assays in animal samples. However, antibody presence says nothing about the virulence of the virus. Because use of these assays requires proper validation, a reference serum panel (cattle, sheep, and goats), along with a species-matched negative serum panel, has been established for crucial assay development, validation and control. Several new assays based on recombinant N-protein were described, including a competition ELISA (Schuster et al., 2016) and a double-antigen-sandwich ELISA, which offer excellent specificity and sensitivity without species restrictions.
These ELISAs have been performed on animal samples collected in Europe and in Africa. In Europe, high seroprevalence was detected in Turkey, FYR Macedonia, Bulgaria, Romania, Greece, Kosovo, and Albania, while no positive results were obtained in samples from Germany and central France. Future studies should concentrate on CCHFV presence in Spain, southern France, Italy, Hungary, and Slovakia. Recently, several studies have been performed in Africa through partnerships developed under the German Partnership Program for Excellence in Biological Health Security. As a result, successful capacity building of both personnel and laboratories was achieved in Mauritania, Sierra Leone, Cameroon, Democratic Republic of the Congo, and Egypt. These studies indicated low seroprevalence in Sierra Leone, Democratic Republic of the Congo (Katanga Province), and Egypt. These studies emphasize the need to consider regional effects and ecological factors (e.g., tick habitats). Far higher seroprevalence rates than expected were detected in Mauritania, Mali, and Cameroon, given the absence of case reports, highlighting the need for additional research on the true incidence of infection and disease in these countries.
Sabri Hacıoğlu
(Ankara University, Ankara, Turkey) examined the role of wild birds and domestic ruminants in local CCHFV epidemiology in Turkey, focusing on virological and serological findings obtained from wild migratory birds and ruminants slaughtered in an endemic area. A total of 1337 serum and plasma samples were taken from slaughtered animals in three endemic cities, and organ samples were collected from 20 wild birds (18 migratory and 2 non-migratory) in Kizilirmak Delta, Northern Turkey. ELISA performed on serum samples detected a seroprevalence rate of around 36% (340/939) in cattle, 6% (24/383) in sheep, and 7% (1/15) in goats. To detect viremia, plasma samples were tested by quantitative RT-PCR. Viral RNA was found in 9 of the 1337 plasma samples (0.67%): 0.74% (7/939) of cattle and 0.52% (2/383) of sheep samples. The viral RNA load in these animals was calculated between × 101−2.40 × 103 copies/mL. To elucidate CCHFV infection in migratory wild birds, tissue samples from 20 birds were screened via single-step generic nairovirus RT-PCR. The tissues were collected and sent directly to the laboratory in Ankara University, where analysis is currently ongoing.
3.4. Molecular virology (Chairpersons: Friedemann Weber, Stuart Nichol)
Eric Bergeron
(Centers for Disease Control and Prevention (CDC), Atlanta, GA, USA) presented recent progress in the field of CCHFV reverse genetics. Using the plasmid-based rescue system (Bergeron et al., 2015), his group has generated recombinant viruses of three strains belonging to distinct phylogenetic lineages (Africa-3, Asia-1, and Europe-2), and created S, M, and L reassortants between them. These recombinant viruses will be used to address potential differences in the biology and pathogenicity of various strains. Moreover, the fact that reassortants of all the attempted combinations could be successfully rescued suggests a surprisingly high degree of freedom in the capacity of the virus to exchange genome segments, even among highly divergent strains.
Using virus-like particles (VLPs), it was demonstrated that the glycoproteins (GPCs) from CCHFV strains of the Europe-2 lineage failed to produce VLP reporter activity efficiently in human cells (Zivcec et al., 2015). As representative GPCs from all the other lineages did produce VLPs, GPC from the Europe-2 lineage appears to be fundamentally different from other lineages.
Subtilisin kexin isozyme-1/site-1 protease (SKI-1/S1P) cleaves both Gn and Gc precursors (PreGn and PreGc). Mutating the PreGc cleavage site completely abrogated virus rescue and VLP reporter activity. In contrast, mutating the PreGn cleavage site still led to low yields of recombinant virus and VLPs. However, virus expressing this protease-resistant form of PreGn was lost after few passages. These results suggest that both PreGn and PreGc are important for CCHFV replication, highlighting SKI-1/S1P as an interesting target for the development of antivirals.
Three other presentations focused on the ovarian tumor (OTU) domain present in the N terminus of the L RNA-dependent RNA-polymerase of nairoviruses. The OTU domain of CCHFV is a deubiquitinase that also functions as a deISGylase, cleaving the ubiquitin-like ISG15; studies have shown that over-expression of the isolated OTU domain interferes with innate immunity, especially the type I interferon (IFN) system. Stephanie Devignot (Justus Liebig University, Giessen, Germany) used her previously published system to generate transcriptionally competent VLPs (tc-VLPs) (Devignot et al., 2015) to investigate anti-innate activities of the OTU domain in the context of a full-length, biologically active L polymerase. tc-VLPs with a mutated, catalytically inactive OTU domain (C40A) exhibited much lower polymerase activity than VLPs with a wild-type L. Surprisingly, the attenuated phenotype could not be rescued by inactivating the IFN system, nor could the L-resident OTU activity confer a detectable increase in IFN resistance or inhibition of IFN signaling. However, the attenuated phenotype of the C40A OTU mutant could be relieved by co-expressing ISG15, whereas co-expression of ubiquitin or the isolated wild-type OTU domain did not result in transcomplementation. This indicates that OTU activity in the context of CCHFV may be different from what was deduced from experiments with plasmid expression of the isolated OTU domain.
Florine Scholte
(CDC, Atlanta, GA, USA) attempted to generate a recombinant virus with the catalytically inactive C40A OTU mutation, but was unable to obtain a live virus. Combining the C40A OTU mutation with a mutation disrupting ubiquitin binding (Q16R) allowed virus rescue. The difference in rescue ability was interpreted to mean that a catalytically inactive mutant OTU that retains ubiquitin binding activity (C40A alone) may be sequestered by ubiquitinated substrates, which possibly block virus replication. The viable double-mutant OTU (carrying both Q16R and C40A mutations) exhibited growth kinetics similar to wild-type virus in cells incapable of activating an IFN response to infection. In contrast, growth of the OTU double mutant was severely impaired in cells producing IFN in response to CCHFV. Moreover, another recombinant virus with reduced OTU deubiquitinase activity elicited increased IFN responses, suggesting that innate immune responses are countered by OTU activity (Scholte et al., 2017).
The OTU domain in the L protein is one of the defining elements distinguishing nairoviruses from other bunyaviruses. As mentioned, these proteases are not only potent deubiquitinases, but in the case of CCHFV also function as deISGylase. Unlike ubiquitin, ISG15 shows marked sequence diversity across species. Building on his previous work (Capodagli et al., 2013), Scott Pegan (University of Georgia, Athens, GA, USA) revealed the impact of ISG15 sequence diversity on its ability to be engaged by recombinant OTUs of CCHFV and other nairoviruses. Additionally, the group characterized atom level species-specific ISG15 recognition mechanisms of OTUs along with structural differences in ISG15 of various virus species (Daczkowski et al., 2017; Deaton et al., 2016). This study provides the first insights into the influence of ISG15 diversity on CCHFV and nairovirus OTU effectiveness.
3.5. Panel discussion on taxonomic classification (Chairperson: Gustavo Palacios)
Traditional approaches to classification of CCHFV have focused on phylogenetic analysis of the individual genome segments, which can result in conflicting taxonomic breakdown due in part to frequent reassortment events. Gustavo Palacios (USAMRIID, Fort Detrick, MD, USA) discussed potential approaches to improve classification of CCHFV below the species level. Discussion focused on developing standardized terminology, data structure, sequence submission, and taxonomic classification strategies within the CCHF scientific community to make cross-study comparisons more robust and accurate. He emphasized that short reads from one segment (i.e., a PCR product) are not sufficient for phylogenetic analysis. Ideally, analyses should be based on sequencing of significant portions of all three segments.
Gustavo encouraged a standardized approach, with several suggestions presented. These included the approach to naming used in the influenza field, and centralized management of sequences. Gustavo recognized that not all institutes have the resources to perform comprehensive sequence analyses, and suggested that larger institutes aid these efforts and share results with the individual groups that have provided samples. The newly formed International CCHF Society was encouraged to make recommendations on future approaches to improved taxonomy.
3.6. Pathogenesis and animal models (Chairpersons: Ali Mirazimi, Jessica Spengler)
Ali Mirazimi
(Karolinska Institute, Public Health Agency of Sweden, and National Veterinary Institute) opened the session by highlighting that research on CCHF pathogenesis faces two principal challenges: handling the virus requires high-containment laboratories, of which limited numbers exist, and no good animal models mimic the human disease. Ali proceeded with an overview of publications on CCHF molecular pathogenesis. The first part of the presentation focused mainly on interactions with host cells, and the second concerned the implications of the pro-inflammatory response in pathogenesis. Lastly, Ali put the in vitro data in the context of available in vivo data. He concluded by stressing that CCHF pathogenesis depends on a complex interplay of a multitude of viral and host factors.
Jessica Spengler
(CDC, Atlanta, GA, USA) reviewed efforts to develop laboratory animal models of the virus cycle in nature and of the human disease. In nature, vertebrates serve principally as hosts to the tick vector. They are susceptible to infection, but show no signs of illness (Spengler et al., 2016b). They develop a short-term viremia, contributing to maintenance and transmission of the virus in the tick population. Several aspects of virus ecology can be characterized by experimental studies in animals, including persistence of virus in host species; the public health risk (e.g., for abattoir workers, veterinarians); and the level of viremia required for transmission to feeding ticks. However, these studies have several caveats that must be considered in study design, such as host-tick matching, virus strain, route of inoculation, and inoculation dose.
The limited number of animal models for pathogenesis studies and screening of therapies continues to restrict progress in the field. Attempts to recapitulate human disease in laboratory animals have been largely unsuccessful. Historical studies report the general absence of clinical signs in animals, with the exception of newborn rodents (Smirnova, 1979). These studies were important in identifying newborn mice as tools for CCHFV isolation and early in vivo studies.
Despite the challenges in animal modeling, there have been notable advances in the field. These include the production of disease with features resembling those in humans in IFN-deficient mice (Stat-1−/−and IFNAR−/−) and a recently described humanized mouse strain (hu-NSG-SGM3) (Bente et al., 2010; Bereczky et al., 2010; Spengler et al., 2017; Zivcec et al., 2013). The presentation concluded with several general considerations for animal model study design and data interpretation: inoculation route and dose, viral strain, and passage history of inoculum. Advances in the field will require consideration of these factors along with animal species. Future attempts to develop models must include a variety of approaches, detailing the aforementioned factors in order to avoid unnecessary duplication, promote informed study design, and facilitate data interpretation and comparison.
In experimental studies, nonhuman primates (NHPs) have shown few to no signs of illness when inoculated with CCHFV (Alimonti et al., 2014; Butenko et al., 1970; Fagbami et al., 1975; Smirnova, 1979). However, Elaine Haddock (Rocky Mountain Laboratories, NIH, Hamilton, MT, USA) described recent success in developing an NHP model of CCHF using the 2001 Kosova Hoti strain in cynomolgus macaques. High-dose inoculation of rhesus macaques with the 1966 Nigerian IbAr10200 strain via various routes produced no observable clinical signs or pathological changes. Subcutaneous/intravenous inoculations of rhesus macaques or African green monkeys with the Kosova Hoti virus were equally unremarkable. However, high dose inoculation of Kosova Hoti in cynomolgus macaques resulted in varying degrees of clinical disease. Intravenous (25% survival) and subcutaneous/intravenous (75% survival) routes produced more consistent and severe disease than subcutaneous inoculation alone (100% survival). Clinical signs included reduced food intake, piloerection and hunched posture, oliguria, facial and body edema, and various hemorrhagic signs. Virus was detected in a variety of tissues and was isolated most consistently from the liver. Altogether, these data represent significant progress in the use of nonhuman primates in CCHF research, and highlight the importance of viral strain and experimental approach in attempts to develop new models of disease.
Vanessa Monteil
(Karolinska Institute & Public Health Agency of Sweden) presented preliminary data from a new transwell assay developed to study the site of entry and release of CCHFV in polarized epithelial cells. Previously the same team developed an in vitro assay to investigate the interactions between CCHFV and virus-polarized Madin-Darby canine kidney 1 (MDCK-1) epithelial cells (Connolly-Andersen et al., 2007). To further investigate the site of entry and release in cells of human origin, a transwell system based on human epithelial colorectal adenocarcinoma cells (CaCo-2) was developed. This model was used to compare the site of entry and release of CCHFV and the non-pathogenic Hazara virus; Hazara virus and CCHFV, although closely related, differed in their preferred site of entry, suggesting that preferred cellular entry receptors may contribute to disease pathogenesis. Furthermore, the preferred side of viral entry changed in the presence of monocyte derived dendritic cells, demonstrating cell-type specific preferences in entry mechanism.
Cristiano Salata
(University of Padova, Padova, Italy, and Public Health Agency of Sweden) highlighted the importance of investigating interactions between CCHFV and tick cells. Using wild-type (WT) IbAr10200 and a recombinant virus containing an AEVA sequence to replace the DEVD domain, Cristiano evaluated the effects of mutating the highly conserved DEVD domain on CCHFV infection in tick cell lines. The AEVA mutant virus replicated less efficiently than WT-CCHFV in tick cell lines. Virus titration and PCR data demonstrated that WT-CCHFV was able to establish persistent infection in tick cells, whereas levels of the mutant virus decreased over time and eventually the cells cleared infection.
Aura Garrison
(USAMRIID, Fort Detrick, MD, USA) presented, on behalf of Joseph Golden, a novel murine model of CCHF, in which IFN-I signaling was transiently blocked within two days of infection by a monoclonal antibody (mAb) 5A3, conferring susceptibility to disease. Using this model, the group found that CCHFV-mediated liver injury results in activation of death signaling molecules TNF-α, TRAIL, and Fas starting 48 h before death, and causes cell death in both infected and uninfected cells. The transient immunosuppression approach allows studies in other background strains; Rag2−/− mice injected with the 5A3 mAb also succumbed to lethal disease, indicating that cytotoxic T-cells may be dispensable for pathogenesis. Furthermore, unlike wild-type mice, Rag2−/− mice could be lethally infected if IFN-I signaling was blocked 2 weeks post infection, suggesting that adaptive immunity is required to fully control CCHFV.
3.7. Clinical aspects and diagnostics (Chairpersons: Bushra Jamil Ali, Felicity Burt)
Meltem Arzu Yetkin
(Giresun University School of Medicine, Giresun, Turkey) opened the session with an extensive overview of CCHFV, describing its distribution and transmission, and focused on the clinical manifestations of CCHF, including observations of experiences in Turkey. Four distinct phases of incubation, pre-hemorrhagic, hemorrhagic, and convalescence have previously been reported. Most patients (90%) present with fever and hemorrhage ranging from petechia to widespread hematomas. Frequently described symptoms include fatigue, nausea, dizziness, and myalgia. Abnormal clinical pathology results include prolonged prothrombin and activated partial thromboplastin times; thrombocytopenia; leukopenia; and elevated aminotransaminase, alanine transaminase, creatine phosphokinase, and lactatate dehydrogenase levels. Predictors of clinical course and outcome, including early onset of thrombocytopenia and abnormal clinical pathology values, which are both associated with a poor prognosis, aid in appropriate management of the disease.
The second talk, by Kashif Aziz (Aga Khan University, Karachi, Pakistan), described an outbreak that started with a nurse who was likely exposed through contact with infected cattle at her home, but was diagnosed retrospectively. The patient presented at Bahawal Victoria Hospital with abdominal pain and thrombocytopenia, and an exploratory laparotomy was performed. When the patient died, CCHF had not been considered as a cause of the illness. The surgeon who performed the laparotomy presented one week later with fever, myalgia and bruising, but in the absence of any suspicion of CCHF, he was not isolated. As his condition deteriorated, he was transferred to Aga Khan University Hospital in Karachi, where he was isolated and CCHF was diagnosed by RT-PCR. Following his death, close contacts were screened and treated prophylactically with ribavirin. A trainee resident who assisted with the laparotomy and was present during transport of the second patient presented with symptoms a month later and was diagnosed by RT-PCR, but investigations could not identify any other possible source of infection. Illness in this patient was mild with decreased platelets, and recovery was uneventful.
Kashif commented on the public response to the outbreak and the media frenzy, highlighting the need for increased awareness of CCHF and for education of health care workers and the public, to help reduce fear of an unknown disease and paranoia. An isolation ward with required personal protective equipment was established in the hospital where the first case was treated. Raising awareness in the form of educational billboards erected in the hospital and lectures to healthcare workers in the hospital contributed to stabilizing the situation.
Xhevat Jakupi
(National Institute of Public Health, Kosova), speaking on behalf of Salih Ahmeti (University of Prishtina, Kosova), presented an overview of CCHF in Kosova from 1989 to 2016, when a total of 296 cases with a mean mortality rate of 24.66% were detected. Natural foci of disease have been identified in rural areas at altitudes ranging between 300 and 878 m. Cases occur from March to September, with the highest numbers recorded in June. Annually, the outbreaks occur sporadically; no cases are identified some years, while in other years as many as 46 are identified, as in 1995. The main route of transmission in these cases was through tick bites (53.37%), with a smaller proportion infected via human-to-human contact (16.55%) or intra-hospital infections in medical personnel (2.36%). In utero transmission from mother to child was recorded for one case, and the source of infection was unknown in 27.36% of cases. Xhevat presented clinical and laboratory features of the disease as seen in Kosova and noted that laboratory parameters particularly associated with a fatal outcome included high viral loads in serum samples (108.5 viral copies/mL), lactate dehydrogenase values above 2700 U/mL, leukocyte counts above 7700 per μL, and platelet counts below 50,000 per μL. No differences in the disease course were noted between patients treated with ribavirin and patients who did not receive ribavirin.
Robert Watson
(Public Health England, Porton Down, Salisbury, UK) discussed novel molecular diagnostics, focusing on a recombinase polymerase amplification assay for CCHFV (Bonney et al., 2017). Briefly, the isothermal assay was able to detect viral RNA or synthetic viral RNA representing the 7 clades based on the nucleotide sequence data of the S-segment. The assay was applied to crude preparations of mock field samples and was shown to tolerate the presence of potential inhibitors, suggesting an application of the assay for field work with minimal sample preparation. In addition, the assay was used to screen and detect CCHFV RNA in a panel of clinical samples from Tajikistan. The assay is a rapid, isothermal, simple molecular diagnostic that can be performed using a portable real-time detection device, facilitating application in the field and in low resource regions.
3.8. Clinical management and clinical trials (Chairpersons: Tom Fletcher, Bertrand Renaud)
Tom Fletcher
(Liverpool School of Tropical Medicine, Liverpool, UK) opened the session with a review of current knowledge and practice in CCHF treatment. After describing the typical features of the disease seen in hospitals, he noted that almost 90% of infections are estimated to be subclinical. Viral load is the most reliable predictor of outcome, with genome copies/mL at admission being up 1000-fold higher in fatal than non-fatal cases (Cevik et al., 2007; Duh et al., 2007; Saksida et al., 2010). When available, supportive therapy should include blood component therapy; monitoring of blood pressure, oxygenation and fluid balance; and the appropriate use of critical care interventions including vasopressors and renal replacement therapy (Leblebicioglu et al., 2012) Secondary bacteremia has been shown to be a rare complication of CCHF in Turkey, and predominantly healthcare associated (Sunbul et al., 2015). In a study of 51 high-risk CCHF nosocomial exposures, the majority were as result of needle stick injury, and a benefit of post-exposure prophylaxis with ribavirin was reported (Leblebicioglu et al., 2016).
In the concluding portion of his lecture, Tom discussed two recent studies. In the first, he and a large number of co-investigators surveyed specialist CCHF units in hospitals in 10 CCHF-endemic countries to assess infection prevention and control practices (Fletcher et al., 2017). The 23 responding hospitals all reported having at least one high-risk exposure of a staff member in the preceding 5 years. All hospitals had isolation rooms, but a third reported cohorting suspected and confirmed cases in the same rooms, and nearly a half reported adequate staffing to perform CCHF patient care. The second study, recently completed, examines the use of thromboelastometry in evaluating CCHF cases. The method (ROTEM®) has been used extensively in the fields of trauma care, cardiac surgery, and liver transplantation, but is only now being applied to the study of viral hemorrhagic fevers. To date, 65 patients have been tested and if successful, ROTEM may permit the early detection of coagulopathy and guide blood product replacement.
Bertrand Renaud, speaking on behalf of Nikki Shindo (WHO Health Emergencies Programme), described current efforts to provide evidence-based guidance for the clinical management of CCHF, including some issues not previously covered in the care of patients with viral hemorrhagic fevers. The interim guidelines for front-line health workers can be downloaded at http://apps.who.int/medicinedocs/en/d/Js22501en/. Topics include the effectiveness of antivirals and specific immunotherapies, hematological management, and issues related to exposure risk (efficacy of post-exposure prophylaxis, appropriate personal protective equipment for care of suspected and confirmed cases, and sexual and mother-to-child transmission). A final set of guidelines is now being developed by a team of experts representing a broad range of areas of expertise and geographic regions. Drafting of the text, external review, submission to the WHO Guideline Review Committee, and publication should be completed before the end of this year.
María Paz Sánchez-Seco Fariñas
(Instituto de Salud Carlos III, Majadahonda, Spain) speaking on behalf of Marta Mora-Rillo (Hospital Universitario La Paz, Madrid, Spain), presented two cases of CCHF in Spain (Negredo et al., 2017). The virus was first detected in Spain in ticks in 2010, but human infection was not observed until 2016, when a 60-year-old man who noted a tick bite while hiking was hospitalized with fever, thrombocytopenia, and coagulopathy, leading to fatal hepatic failure. An autopsy was performed, but the diagnosis was not made until a nurse in the internal care unit who had been in close contact with the patient developed a similar syndrome. She received intensive supportive case, and ribavirin was administered 4 days after illness onset. Although some 400 hospital workers were potentially exposed to virus-containing material, no other cases were observed.
A tick-borne disease similar to CCHF, severe fever with thrombocytopenia syndrome (SFTS), was first reported in northeast China in 2008, and is now known to occur in other countries of East Asia. Masayuki Saijo (National Institute of Infectious Diseases, Tokyo, Japan) presented the clinical history of the first recognized case in Japan, and described studies performed with the antiviral drug favipiravir (T-705), which is approved for the treatment of severe influenza in Japan. He and colleagues found that favipiravir was more active than ribavirin against SFTS virus in vitro and in a mouse model of the disease (Tani et al., 2016). A clinical trial of favipiravir treatment of SFTS is under way, with 10 patients enrolled to date.
The session concluded with presentations by two researchers from US CDC. Stephen Welch described the construction of a recombinant CCHFV with a gene encoding the fluorescent protein ZsGreen1 inserted into the viral S segment just upstream of the NP gene (Welch et al., 2017). The recombinant virus replicates as well as wild-type, and is now being used in a 96-or 384-well format for high-throughput screening of candidate antivirals in Huh7 or SW13 cells. Stephen and colleagues used this virus to demonstrate the antiviral activity of 2′-deoxy-2′-fluorocytidine and its synergistic activity with favipiravir.
Marko Zivcec then described the use of virus-like particles to identify mAbs capable of blocking the entry of CCHFV into cells (Zivcec et al., 2017). He noted that, because surface glycoproteins sequenced to date are only ~70% conserved at the amino acid level, a therapeutically useful mAb should neutralize a range of virus strains. Using a VLP system that delivers a luciferase gene into cells, he and colleagues identified 3 mAbs that neutralized viruses isolated in Africa, the Middle East, and the Balkans, and that bound to different sites on the viral glycoprotein. Sequential passaging in the presence of these mAbs did not lead to the selection of escape mutants.
3.9. Prevention (Chairpersons: Connie Schmaljohn, Roger Hewson)
In an introductory lecture, Roger Hewson (Public Health England, Porton Down, Salisbury, United Kingdom) described the growing threat of CCHF and the increased potential of zoonotic transmission in relation to the timing of the Eid-al-Adha festival in the coming years. He then described the rationale for vaccine development and the regulatory requirements of licensing authorities, such as the Medicines and Healthcare Products Regulatory Agency, the Food and Drug Administration, and the European Medicines Agency, including key steps in modern vaccine development, within the context of vaccination strategies. His lecture closed with an update on Public Health England’s modified vaccinia Ankara (MVA)-GP vaccine (Buttigieg et al., 2014; Dowall et al., 2016), which is being developed for a phase I clinical trial.
Marko Zivcec
(CDC, Atlanta, GA, USA) summarized work carried out at the University of Manitoba, Winnipeg, and the NIH Rocky Mountain Laboratories on recombinant adenovirus vaccines, which found that antibody responses are correlated with protection against lethal disease in the IFNAR−/− mouse model. Interestingly, and in contrast what has been observed with other vaccine platforms, adenoviral vectors expressing NP alone provide non-sterilizing protection. The data suggest that adenovirus confers protection by mechanisms different from other platforms. Marko also discussed the potential to combine the adenovirus vectors with other glycoprotein-based vaccine platforms.
In the third presentation, Aura Garrison (USAMRIID, Fort Detrick, MD, USA) presented data showing that a DNA-based vaccine targeting the viral M segment protects against CCHF in two lethal mouse models. She detailed an alternative vaccination approach using a naked DNA, which conferred significant protection against disease in IFNAR−/− mice (Garrison et al., 2017). The vaccine was generated using a codon-optimized GPC gene with demonstrated enhanced expression in vitro, and delivered intramuscularly by electroporation. Interestingly, the vaccine produced similar levels of protection in a novel murine system in which the IFN-α/β signaling response of immunocompetent mice was transiently suppressed. The advantage of the transient blockade model is that vaccines can be evaluated in mice with intact IFN-α/β signaling at the time of vaccination, and then during challenge IFN-α/β can be blocked to test protective efficacy.
The DNA vaccination theme was continued in the final presentation by Ergin Sahin (Ankara University, Turkey) on the in vitro expression of CCHFV surface glycoprotein Gc via a plasmid vector, generated as a DNA-based vaccine. Ergin summarized the details of vector construction using a Turkish strain of CCHFV. Gc expression was successfully evaluated in cell culture, and a series of quality control steps culminated in a vector that will be used in subsequent DNA vaccination work using mouse models of disease.
Overall, several vaccine platforms are showing promising results, and the continued development of animal models of disease aids these efforts to develop and screen candidates. In the future, the utility of the newly developed nonhuman primate model discussed in an earlier session should catalyze further advances and support clinical trials in humans.
3.10. Concluding session (Chairpersons: Pierre Formenty, Mike Bray)
Pierre Formenty
(Health Emergencies Programme, WHO) gave the concluding presentation. He noted that the WHO is taking an increasing interest in CCHF, as demonstrated by a meeting sponsored by the WHO Eastern Mediterranean Region in 2016.
Following consultation with the World Health Assembly, the WHO secretariat is implementing the Research and Development (R&D) Blueprint for action against epidemics (www.who.int/csr/research-and-development/en/). Under the R&D Blueprint, WHO has recently published a report on the revised list of priority diseases that need urgent R&D in order to prevent public health emergencies in the near future. The list includes CCHF among 9 diseases for which few or no medical countermeasures exist due to market failures or lack of scientific knowledge, and provides the basis for work on the WHO R&D Blueprint for emergency preparedness and response.
With partner support, WHO has developed a Baseline Situation Analysis (BSA) document that will serve to build a CCHF R&D Roadmap. The BSA document has identified a number of urgent needs for research and public health intervention. Among the most important were:
Epidemiology: A standard case definition should be created for use in all endemic countries. Careful studies are required to identify instances of aerosol transmission in hospital settings.
Pathogenesis: Research is needed on the presence of CCHFV in body fluids during illness, virus persistence in semen and other fluids during convalescence, and the sequelae of infection in survivors.
Diagnostics: a commercially available, bio-safe, rapid, point-of-care test to quantify viremia must be developed, with the operational characteristics suited to field use or low-resource or low capacity settings, together with validated serological assays to support clinical trials.
Therapeutics: Favipiravir in synergy with ribavirin and mAbs seems to be a good therapeutic candidate. Successful research into CCHF therapeutics will rely on collaboration among endemic countries.
Vaccines: A CCHF vaccine for humans may not be a cost-effective control measure, but animal anti-tick vaccines are potentially valuable, and may also be beneficial for other animal and human diseases.
Pierre presented WHO’s proposed timelines for action on CCHF, including production of a R&D Roadmap, completion of target product profiles, prequalification review of diagnostic tests, and the organization of clinical trials, extending through the end of 2020. A meeting to develop the Roadmap is planned for early February 2018. Additional information on current and upcoming projects is available at: http://www.who.int/csr/disease/crimean_congoHF/en/.
4. Formation of an International Society on CCHF
A proposal to form an International Society on CCHF was put forward on the second day of the meeting, and was approved by acclamation. Anna Papa, Ali Mirazimi, and Roger Hewson were elected president, vice-president, and secretary, respectively. An advisory council, consisting of researchers from 10 countries where CCHF is endemic, plus representatives from the USA and Germany, has since been created. For more information on the 2017 meeting and the new International Society, go to http://www.med.auth.gr/cc-conference-2017.
Acknowledgements
We thank all speakers for their contributions and for sharing unpublished information.
Funding
AM and FW are supported by the CCHVaccine consortium that has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement no. 732732. AP is supported by the European projects COMPARE (grant agreement no. 643476) and EMERGE (grant agreement no. 677066).
Footnotes
Publisher's Disclaimer: Disclaimer
Publisher's Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
References
- Alimonti J, Leung A, Jones S, Gren J, Qiu X, Fernando L, Balcewich B, Wong G, Ströher U, Grolla A, Strong J, Kobinger G, 2014. Evaluation of transmission risks associated with in vivo replication of several high containment pathogens in a bio-safety level 4 laboratory. Sci. Rep 4, 5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Alimonti JB, Shieh W, Camus G, Ströher U, Zaki S, Jones SM, Stroher U, Wung-Shu S, 2010. Pathogenesis and immune response of Crimean-Congo hemorrhagic fever virus in a STAT-1 knockout mouse model. J. Virol 84, 11089–11100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bente DA, Forrester NL, Watts DM, McAuley AJ, Whitehouse CA, Bray M, 2013. Crimean-Congo hemorrhagic fever: history, epidemiology, pathogenesis, clinical syndrome and genetic diversity. Antivir. Res 100, 159–189. [DOI] [PubMed] [Google Scholar]
- Bereczky S, Lindegren G, Karlberg H, Akerström S, Klingström J, Mirazimi A, 2010. Crimean-Congo hemorrhagic fever virus infection is lethal for adult type I interferon receptor-knockout mice. J. Gen. Virol 91, 1473–1477. [DOI] [PubMed] [Google Scholar]
- Bergeron É, Zivcec M, Chakrabarti AK, Nichol ST, Albariño CG, Spiropoulou CF, 2015. Recovery of recombinant Crimean Congo Hemorrhagic fever virus reveals a function for non-structural glycoproteins cleavage by Furin. PLoS Pathog 11, e1004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonney LC, Watson RJ, Afrough B, Mullojonova M, Dzhuraeva V, Tishkova F, Hewson R, 2017. A recombinase polymerase amplification assay for rapid detection of Crimean-Congo Haemorrhagic fever Virus infection. PLoS Neglected Trop. Dis 1–16. [DOI] [PMC free article] [PubMed]
- Butenko A, Chumakov M, Smirnova S, Vasilenko S, Zavodova T, Tkachenko E, Zarubina L, Bashkirtsev V, Zgurskaya G, Vishnivetskaya L, 1970. Isolation of CHF virus from patient blood and autopsy material (1968–1969 investigation data) in Rostov and Astrakhan Oblasts, and Bulgaria (in English: NAMRU3-T650). In: Chumakov M (Ed.), Third Regional Workshop at Rostov-on-Don in May 1970
- Buttigieg KR, Dowall SD, Findlay-Wilson S, Miloszewska A, Rayner E, Hewson R, Carroll MW, 2014. A novel vaccine against Crimean-Congo Haemorrhagic Fever protects 100% of animals against lethal challenge in a mouse model. PLos One 9, e91516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cajimat MNB, Rodriguez SE, Schuster IUE, Swetnam DM, Ksiazek TG, Habela MA, Negredo AI, Estrada-Peña A, Barrett ADT, Bente DA, 2017. Genomic characterization of Crimean–Congo hemorrhagic fever virus in Hyalomma tick from Spain, 2014. Vector Borne Zoonotic Dis XX vbz.2017.2190. [DOI] [PMC free article] [PubMed]
- Capodagli GC, Deaton MK, Baker E.a, Lumpkin RJ, Pegan SD, 2013. Diversity of ubiquitin and ISG15 specificity amongst nairoviruses’ viral ovarian tumor domain proteases. J. Virol [DOI] [PMC free article] [PubMed]
- Casals J, 1969. Antigenic similarity between the virus causing crimean hemorrhagic fever and Congo virus. Proc. Soc. Exp. Biol. Med 131, 233–236. [DOI] [PubMed] [Google Scholar]
- Cevik MA, Erbay A, Bodur H, Eren SS, Akinci E, Sener K, Ongürü P, Kubar A, 2007. Viral load as a predictor of outcome in Crimean-Congo hemorrhagic fever. Clin. Infect. Dis 45, e96–e100. [DOI] [PubMed] [Google Scholar]
- Connolly-Andersen A-M, Magnusson K-E, Mirazimi A, 2007. Basolateral entry and release of Crimean-Congo hemorrhagic fever virus in polarized MDCK-1 cells. J. Virol 81, 2158–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daczkowski CM, Dzimianski JV, Clasman JR, Goodwin O, Mesecar AD, Pegan SD, 2017. Structural insights into the interaction of coronavirus papain-like proteases and interferon-stimulated gene product 15 from different species. J. Mol. Biol 429, 1661–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deaton MK, Dzimianski JV, Daczkowski CM, Whitney GK, 2016. Biochemical and structural insights into the preference of nairoviral DeISGylases for interferon-stimulated gene product 15 Originating from certain species. J. Virol 90, 8314–8327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devignot S, Bergeron E, Nichol S, Mirazimi A, Weber F, 2015. A virus-like particle system identifies the Endonuclease domain of Crimean-Congo hemorrhagic fever virus. J. Virol 89, 5957–5967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinçer E, Brinkmann A, Hekimoğlu O, Hacıoğlu S, Földes K, Karapınar Z, Polat PF, Oğuz B, Orunç Kılınç Ö, Hagedorn P, Özer N, Özkul A, Nitsche A, Ergünay K, 2017. Generic amplification and next generation sequencing reveal Crimean-Congo hemorrhagic fever virus AP92-like strain and distinct tick phleboviruses in Anatolia, Turkey. Parasit. Vectors 10, 335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowall SD, Graham VA, Rayner E, Hunter L, Watson R, Taylor I, Rule A, Carroll MW, 2016. Protective effects of a modified Vaccinia Ankara-based vaccine candidate against Crimean-Congo Haemorrhagic Fever virus require both cellular and humoral responses. PLos One 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubyanskiy VM, Yeszhanov AB, 2016. Ecology of Yersinia pestis and epidemiology of plague. In: In: Yang R, Anisimov A (Eds.), Yersinia Pestis: Retrospective and Perspective. Advances in Experimental Medicine and Biology, vol. 918 Springer, Dordrecht, pp. 101–170. [DOI] [PubMed] [Google Scholar]
- Dubyansky MA, Kenzhebayev A, Stepanov VM, Asenov GA, Dubyanskaya L, 1992. Prediction of plague epizootic activity in the Pre-Aral and Kyzylkum (in Russian). Nukus of Karakalpakstan 240. [Google Scholar]
- Duh D, Saksida A, Petrovec M, Ahmeti S, Dedushaj I, Panning M, Drosten C, Avsic-Zupanc T, 2007. Viral load as predictor of Crimean-Congo hemorrhagic fever outcome. Emerg. Infect. Dis 13, 1769–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagbami AH, Tomori O, Fabiyi A, Isoun T, 1975. Experimental Congo virus (Ib-AN 7620) infection in primates. Virologie 26, 33–37. [PubMed] [Google Scholar]
- Fletcher TE, Gulzhan A, Ahmeti S, Al-Abri SS, Asik Z, Atilla A, Beeching NJ, Bilek H, Bozkurt I, Christova I, Duygu F, Esen S, Khanna A, Kader Ç, Mardani M, Mahmood F, Mamuchishvili N, Pshenichnaya N, Sunbul M, Yalcin TY, Leblebicioglu H, 2017. September 8 Infection prevention and control practice for Crimean-Congo hemorrhagic fever-A multi-center cross-sectional survey in Eurasia. PLos One 12 (9), e0182315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison AR, Shoemaker CJ, Golden JW, Fitzpatrick CJ, Suschak JJ, Richards MJ, Badger CV, Six CM, Martin JD, Hannaman D, Zivcec M, Bergeron E, Koehler JW, Schmaljohn CS, 2017. A DNA vaccine for Crimean-Congo hemorrhagic fever protects against disease and death in two lethal mouse models. PLoS Neglected Trop. Dis 11, e0005908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grashchenkov N, 1945. Investigations of etiology, pathogenesis, and clinical symptomatology of Crimean hemorrhagic fever (In English: NAMRU3–T1189). In: Reports 1944 Sci. Investig. Inst. Neurol. Akad. Med Nauk SSSR; Moskva, pp. 100–107. [Google Scholar]
- Gubler EV, 1978. Computational Methods of Analysis and Detection of Pathological Processes, vol. 296 (in Russian: ). Medical, Leningr. branch. [Google Scholar]
- Gurav Y, Yadav P, Deoshatwar A, Dhruwey V, Shete A, Chaubal G, Tandale B, Raval D, Mourya D, 2014. Serosurvey of Crimean Congo Hemorrhagic fever (CCHF) among high-risk group during 2011 and 2013 CCHF outbreak in Gujarat, India. Indian J. Appl. Res 4, 430–434. [Google Scholar]
- Hasan Z, Atkinson B, Jamil B, Samreen A, Altaf L, Hewson R, 2014. Short report: diagnostic testing for hemorrhagic fevers in Pakistan: 2007–2013. Am. J. Trop. Med. Hyg 91, 1243–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan Z, Mahmood F, Jamil B, Atkinson B, Mohammed M, Samreen A, Altaf L, Moatter T, Hewson R, 2013. Crimean-Congo hemorrhagic fever nosocomial infection in a immunosuppressed patient, Pakistan: case report and virological investigation. J. Med. Virol 85, 501–504. [DOI] [PubMed] [Google Scholar]
- Jamil B, Hasan RS, Sarwari AR, Burton J, Hewson R, Clegg C, 2005. Crimean-Congo hemorrhagic fever: experience at a tertiary care hospital in Karachi, Pakistan. Trans. Roy. Soc. Trop. Med. Hyg 99, 577–584. [DOI] [PubMed] [Google Scholar]
- Leblebicioglu H, Bodur H, Dokuzoguz B, Elaldi N, Guner R, Koksal I, Kurt H, Senturk GC, 2012. September Case management and supportive treatment for patients with Crimean-Congo hemorrhagic fever. Vector Borne Zoonotic Dis 12 (9), 805–811. [DOI] [PubMed] [Google Scholar]
- Leblebicioglu H, Sunbul M, Guner R, Bodur H, Bulut C, Duygu F, Elaldi N, Cicek Senturk G, Ozkurt Z, Yilmaz G, Fletcher TE, Beeching NJ, 2016. April Healthcare-associated Crimean-Congo haemorrhagic fever in Turkey, 2002–2014: a multicentre retrospective cross-sectional study. Clin. Microbiol. Infect 22 (4), 387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makwana D, Yadav P, Kelaiya A, Mourya DT, 2015. First confirmed case of Crimean-Congo haemorrhagic fever from Sirohi district in Rajasthan State, India. Indian J. Med. Res 142, 489–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra AC, Mehta M, Mourya DT, Gandhi S, 2011. Crimean-Congo haemorrhagic fever in India. Lancet 378, 372. [DOI] [PubMed] [Google Scholar]
- Mourya DT, Yadav PD, Patil DY, 2014a. Highly infectious tick borne viral diseases: kyasanur Forest Disease and Crimean-Congo hemorrhagic fever in India. WHO Southeast Asia J. Pub. Heal 3, 8–21. [DOI] [PubMed] [Google Scholar]
- Mourya DT, Yadav PD, Shete A, Majumdar TD, Kanani A, Kapadia D, Chandra V, Kachhiapatel AJ, Joshi PT, Upadhyay KJ, Dave P, Raval D, 2014b. Serosurvey of Crimean-Congo hemorrhagic fever virus in domestic animals, Gujarat, India, 2013. Vector Borne Zoonotic Dis 14, 690–692. [DOI] [PubMed] [Google Scholar]
- Mourya DT, Yadav PD, Shete AM, Gurav YK, Raut CG, Jadi RS, Pawar SD, Nichol ST, Mishra AC, 2012. Detection, isolation and confirmation of Crimean-Congo hemorrhagic fever virus in human, ticks and animals in Ahmadabad, India, 2010–2011. PLoS Neglected Trop. Dis 6, e1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourya DT, Yadav PD, Shete AM, Sathe PS, Sarkale PC, Pattnaik B, Sharma G, Upadhyay KJ, Gosavi S, Patil DY, Chaubal GY, Majumdar TD, Katoch VM, 2015. Cross-sectional serosurvey of Crimean-Congo hemorrhagic fever virus IgG in livestock, India, 2013–2014. Emerg. Infect. Dis 21, 1837–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negredo A, de la Calle-Prieto F, Palencia-Herrejón E, Mora-Rillo M, Astray-Mochales J, Sánchez-Seco MP, Bermejo Lopez E, Menárguez J, Fernández-Cruz A, Sánchez-Artola B, Keough-Delgado E, Ramírez de Arellano E, Lasala F, Milla J, Fraile JL, Ordobás Gavín M, Martinez de la Gándara A, López Perez L, Diaz-Diaz D, López-García MA, Delgado-Jimenez P, Martín-Quirós A, Trigo E, Figueira JC, Manzanares J, Rodriguez-Baena E, Garcia-Comas L, Rodríguez-Fraga O, García-Arenzana N, Fernández-Díaz MV, Cornejo VM, Emmerich P, Schmidt-Chanasit J, Arribas JR, 2017. Autochthonous Crimean–Congo hemorrhagic fever in Spain. N. Engl. J. Med 377, 154–161. [DOI] [PubMed] [Google Scholar]
- Papa A, Weber F, Hewson R, Weidmann M, Koksal I, Korukluoglu G, Mirazimi A, 2015. Meeting report: first international conference on Crimean-Congo hemorrhagic fever. Antivir. Res 120, 57–65. [DOI] [PubMed] [Google Scholar]
- Saksida A, Duh D, Wraber B, Dedushaj I, Ahmeti S, Avsic-Zupanc T, Fever CH, 2010. Interacting roles of immune mechanisms and viral load in the pathogenesis of crimean-Congo hemorrhagic fever. Clin. Vaccine Immunol 17, 1086–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholte FEM, Zivcec M, Dzimianski JV, Deaton MK, Spengler JR, Welch SR, Nichol ST, Pegan SD, Spiropoulou CF, Bergeron É, 2017. Crimean-Congo hemorrhagic fever virus suppresses innate immune responses via a ubiquitin and ISG15 specific protease. Cell Rep 20, 2396–2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster I, Mertens M, Köllner B, Korytár T, Keller M, Hammerschmidt B, Müller T, Tordo N, Marianneau P, Mroz C, Rissmann M, Stroh E, Dähnert L, Hammerschmidt F, Ulrich RG, Groschup MH, 2016. A competitive ELISA for species-independent detection of Crimean-Congo hemorrhagic fever virus specific antibodies. Antivir. Res 134, 161–166. [DOI] [PubMed] [Google Scholar]
- Smirnova SE, 1979. A comparative study of the crimean hemorrhagic Fever-Congo group of viruses. Arch. Virol 62, 137–143. [DOI] [PubMed] [Google Scholar]
- Spengler JR, Bergeron É, Rollin PE, 2016a. Seroepidemiological studies of Crimean-Congo hemorrhagic fever virus in domestic and wild animals. PLoS Neglected Trop. Dis 10, e0004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron E, Bente D, 2016b. A chronological review of experimental infection studies on the role of wild animals and livestock in maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antivir. Res 135, 31–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spengler JR, Keating MK, Mcelroy AK, Zivcec M, Coleman JD, Zaki SR, Nichol ST, Spiropoulou CF, 2017. Crimean-Congo hemorrhagic fever in humanized mice reveals glial cells as primary targets of neurological infection. J. Infect. Dis 1–11. [DOI] [PMC free article] [PubMed]
- Sunbul M, Leblebicioglu H, Fletcher TE, Elaldi N, Ozkurt Z, Bastug A, Yilmaz G, Guner R, Duygu F, Beeching NJ, 2015. November Crimean-Congo haemorrhagic fever and secondary bacteraemia in Turkey. J. Infect 71 (5), 597–599. [DOI] [PubMed] [Google Scholar]
- Tani H, Fukuma A, Fukushi S, Taniguchi S, Yoshikawa T, Iwata-Yoshikawa N, Sato Y, Suzuki T, Nagata N, Hasegawa H, Kawai Y, Uda A, Morikawa S, Shimojima M, Watanabe H, Saijo M, 2016. Efficacy of T-705 (Favipiravir) in the treatment of infections with lethal severe fever with thrombocytopenia syndrome virus. mSphere 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volynkina AS, Kotenev ES, Lisickaya YV, Maleckaya OV, Shaposhnikova LI, Kulichenko AN, 2017. Epidemiological situation on crimean hemorrhagic fever in the Russian Federation in 2016, and prognosis for 2017. Пробл. особо опасных инф 1, 24–28. [Google Scholar]
- Welch SR, Scholte FEM, Flint M, Chatterjee P, Nichol ST, Bergeron É, Spiropoulou CF, 2017. Identification of 2′-deoxy-2′-fluorocytidine as a potent inhibitor of Crimean-Congo hemorrhagic fever virus replication using a recombinant fluorescent reporter virus. Antivir. Res 147, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H, Beck AS, Gargili A, Forrester N, Barrett ADT, Bente DA, 2016. Transstadial transmission and long-term association of Crimean-Congo hemorrhagic fever virus in ticks shapes genome plasticity. Sci. Rep 6, 35819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PD, Raut CG, Mourya DT, 2013a. Re-occurrence of Crimean-Congo haemorrhagic fever in Ahmedabad, Gujarat, India (2012): a fatal case report. Indian J. Med. Res 138, 1027–1028. [PMC free article] [PubMed] [Google Scholar]
- Yadav P, Raut C, Patil D, Majumdar T, Mourya D, 2013b. Crimean Congo hemorrhagic fever virus: current scenario in India. Proc NatlAcadSci, India. Sect. B Biol. Sci June 26, 1–10 ([E pub ahead of print]). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav PD, Gurav YK, Mistry M, Shete AM, Sarkale P, Deoshatwar AR, Unadkat VB, Kokate P, Patil DY, Raval DK, Mourya DT, 2014. Emergence of Crimean-Congo hemorrhagic fever in Amreli district of Gujarat state, India, June to July 2013. Int. J. Infect. Dis 18, 97–100. [DOI] [PubMed] [Google Scholar]
- Yadav PD, Patil DY, Shete AM, Kokate P, Goyal P, Jadhav S, Sinha S, Zawar D, Sharma SK, Kapil A, Sharma DK, Upadhyay KJ, Mourya DT, 2016. Nosocomial infection of CCHF among health care workers in Rajasthan, India. BMC Infect. Dis 16, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcec M, Guerrero LIW, Albariño CG, Bergeron É, Nichol ST, Spiropoulou CF, 2017. Identification of broadly neutralizing monoclonal antibodies against Crimean-Congo hemorrhagic fever virus. Antivir. Res 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcec M, Metcalfe MG, Albariño CG, Guerrero LW, Pegan SD, Spiropoulou CF, Bergeron É, 2015. Assessment of inhibitors of pathogenic Crimean-Congo hemorrhagic fever virus strains using virus-like particles. PLoS Neglected Trop. Dis 9, e0004259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcec M, Safronetz D, Scott D, Robertson S, Ebihara H, Feldmann H, 2013. Lethal Crimean-Congo hemorrhagic fever virus infection in interferon α/β receptor knockout mice is associated with high viral loads, pro-inflammatory responses and coagulopathy. J. Infect. Dis 207, 1909–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]