Abstract
Food deserts (FD), low-income areas with low-access to healthful foods, are associated with higher burden of cardiovascular risk factors. Few studies have examined the impact of FD on clinical outcomes in heart failure (HF). FD status was assessed in 457 HF patients (mean age 55.9 ± 12.5 years; 50.3% Black) using the Food Desert Research Atlas. The Andersen-Gill extension of Cox model was used to examine the association of living in a FD with risk of repeat hospitalization (all-cause and HF-specific). Patients living in a FD were younger (P=0.01), more likely to be Black (P<0.0001), less educated (P=0.003), and less likely to have commercial insurance (P=0.003). During a median follow-up of 827 (506, 1379) days, death occurred in 60 (13.1%) subjects, and hospitalizations occurred in 262 (57.3%) subjects. There was no difference in the risk of death based on FD status. The overall frequency of all-cause (94.1 vs. 63.6 per 100 patient-years) and HF-specific (59.6 vs. 30.5 per 100 patient-years) hospitalizations was higher in subjects who lived in a FD. After adjustment for covariates, living in a FD was associated with an increased risk of repeat all-cause (hazard ratio [HR] 1.39, 95% confidence interval [Cl] 1.19 – 1.63; P=0.03) and HF-specific (HR 1.30, 95% Cl 1.02 – 1.65; P=0.03) hospitalizations. In conclusion, patients living in a FD have a higher risk of repeat all-cause and HF-specific hospitalization.
Keywords: food deserts, disparities, heart failure, clinical outcomes
INTRODUCTION
Heart failure (HF) is the primary diagnosis in >1 million hospitalizations annually, at a cost of over $15 billion.1 Recently, increasing attention has been paid to the impact of social and environmental factors as determinants of health and outcomes for patients with cardiovascular disease (CVD).2 Factors such as neighborhood and features of residential environments may impart disadvantage due to low access to particular resources (i.e. medical care, grocery stores, etc), and increased psychosocial stressors due to lack of safety and social cohesion. Since environment can impact lifestyle behaviors3–6, and adherence with medications and HF self-care7, 8, it is important to examine the importance of environment and neighborhood as factors that influence clinical HF outcomes. An estimated 23.5 million people in the US live in a food desert (FD), defined as “parts of the country void of fresh fruit, vegetables, and other healthful whole foods, usually found in impoverished areas”.9 Although the FD designation specifically refers to low access to healthful foods, the impoverished nature of these areas may also designate lack of access to other resources that promote healthy behaviors.9,10. Although prior studies have documented the association between neighborhood disadvantage and risk for HF hospitalization11–13, no studies have specifically examined the risk of death or hospitalization in patients with HF who live in a FD. Thus, the purpose of this analysis was to determine if living in a FD is associated with clinical outcomes including death and hospitalization in patients with HF.
METHODS
We pooled participant-level data from the Atlanta Cardiomyopathy Consortium (PI Butler) and Metabolomics, Oxidative Stress and Vascular Function study (PI Morris) study, 2 prospective cohort studies which recruited patients with prevalent HF from the metropolitan Atlanta area. The Atlanta Cardiomyopathy Consortium enrolled 336 outpatients from the HF clinics at three Emory University-affiliated hospitals from 2007 to 2011, according to inclusion and exclusion criteria previously described.14 The Metabolomics, Oxidative Stress and Vascular Function study is an ongoing follow-up study to the Atlanta Cardiomyopathy Consortium, and has enrolled 165 patients since 2015 according to the same inclusion and exclusion criteria. For the purpose of this analysis, we included subjects with a diagnosis of HF with either reduced (HFrEF) or preserved ejection fraction (HFpEF) who had complete geographic information including home address and zip code (N=457). All participants provided informed consent, and both studies were approved by the Emory Institutional Review Board.
To determine whether each subject lived in an area with low access, low income, or both (FD), each subjects’ zip code was entered into the USDA Food Desert Research Atlas.9 Low income areas are defined according to criteria developed by the Department of Treasury’s New Markets Tax Credit program as any area where poverty rate is ≥20% or where the median family income is ≤80% of the state-wide median family income. Areas with low access to healthy foods are defined as areas where a significant share of people live more than one mile away in urban areas or ≥10 miles in rural areas from a supermarket, supercenter or large grocery store. To qualify as FD, the area has to be low access and low income.
Information on demographic, socioeconomic, and clinical covariates were collected at the baseline study visit. The primary exposure variable was defined as living in a FD. Covariates of interest included: age, gender, race, history of hypertension, history of diabetes mellitus, chronic kidney disease, history of dyslipidemia, level of education, insurance status, living alone, optimal medical and device therapy, left ventricular ejection fraction, systolic and diastolic blood pressure, body mass index (BMI), serum creatinine, and B-type natriuretic peptide. For those categorical variables with missing data (education N=11 [2.4%], insurance N=6 [1.3%]), an indicator variable was used to allow these patients to contribute their available risk factors in multivariable models.
Data on clinical outcomes including all-cause death and all-cause hospitalizations were prospectively collected at 6-month intervals and adjudicated by an independent review committee. Mortality data were collected through medical record review, information from family members, and Social Security Death Index query. Data on hospitalizations was obtained from electronic health records review, outpatient notes from any specialty encounter for any admission to an outside hospital, and direct patient inquiry during follow-up. The primary endpoint was the analysis of repeat hospitalizations, focusing on all-cause and HF hospitalizations. Censoring was performed at the time of loss to follow-up, receipt of left ventricular assist device or heart transplant, or last date of follow-up on June 1, 2018.
Data are presented as mean ± standard deviation (SD), median (interquartile range [IQR]), or N (%) of patients, as appropriate. Baseline characteristics were compared between patients according to FD status using the Student t-test for normally distributed continuous variables, the Wilcoxon rank-sum test for non-normally distributed continuous variables, and the χ2 test for categorical variables. The average number of hospital admissions per 100 patient-years of follow-up was calculated according to FD status by dividing the total number of hospitalizations (all-cause and HF-specific) in each group by the total follow-up duration of all patients in that group. Modelling the association of FD status with the risk of repeat hospitalization was examined using the Andersen-Gill extension of the Cox proportional hazards model.15 The Andersen–Gill approach is based on unstratified baseline hazards and is closely related to Poisson process theory for handling recurrent failure time data.16 Variables that differed by FD status, and that were associated with the risk of hospitalization were included in the multivariable models. The final model made adjustments for the following risk factors: age, sex, race, education, insurance status, history of hypertension, history of diabetes, BMI, and serum creatinine. All tests of statistical significance were two-tailed, and p values <0.05 were considered significant. Data were analyzed using SAS v9.4 (SAS Institute Inc., Cary, NC).
RESULTS
The baseline characteristics of the cohort are shown in Table 1. The mean age of patients was 55.9 ± 12.5 years; 182 (39.8%) were female and 230 (50.3%) were Black. The majority (407 [89.1%]) had HF with reduced ejection fraction. Patients who lived in a FD were younger, more likely to be of Black race, less educated, and less likely to have commercial insurance. Patients who lived in a FD were more likely to have a history of hypertension, and be on hydralazine therapy. Moreover, patients who lived in a FD had a lower ejection fraction, and were more likely to be New York Heart Association functional class III or IV.
Table 1.
Baseline characteristics of the study cohort according to food desert status.
| Living in A Food Desert | |||
|---|---|---|---|
| Yes N=106 |
No N=351 |
P | |
| Age (years) | 53.3 ± 13.5 | 56.7 ± 12.0 | 0.01 |
| Women | 52 (49.1%) | 130 (37.0%) | 0.03 |
| Race | <0.0001 | ||
| • White | 17 (16.0%) | 201 (57.3%) | |
| • Black | 89 (84.0%) | 141 (40.2% | |
| • Asian | 0 (0%) | 6 (1.7%) | |
| • Other | 0 (0%) | 3 (0.8%) | |
| Insurance | 0.003 | ||
| • Commercial | 28 (27.2%) | 158 (45.4%) | |
| • Medicare | 55 (53.4%) | 144 (41.4%) | |
| • Medicaid | 13 (12.6%) | 17 (4.9%) | |
| • Veterans Affairs | 1 (1.0%) | 4 (1.1%) | |
| • None | 6 (5.8%) | 25 (7.2%) | |
| Education | 0.0003 | ||
| • College graduate | 15 (14.6%) | 126 (36.7%) | |
| • Some college | 37 (35.9%) | 103 (30.0%) | |
| • High school or less | 51 (49.5%) | 114 (33.2%) | |
| Living alone | 24 (22.6%) | 68 (19.4%) | 0.5 |
| Smoker | 11 (10.4%) | 37 (10.5%) | 0.9 |
| Diabetes mellitus | 44 (41.5%) | 144 (41.0%) | 0.9 |
| Hypertension | 77 (72.6%) | 218 (62.1%) | 0.04 |
| NYHA class | 0.03 | ||
| • 1 | 6 (5.8%) | 31 (9.0%) | |
| • 2 | 50 (48.1%) | 209 (60.6%) | |
| • 3-4 | 48 (46.2%) | 105 (30.4%) | |
| Medical therapy | |||
| • ACEi/ARB | 73 (68.9%) | 262 (74.6%) | 0.1 |
| • Aldosterone antagonists | 51 (48.1%) | 160 (45.6%) | 0.8 |
| • Beta-blocker | 86 (81.1%) | 310 (88.3%) | 0.06 |
| • Diuretics | 91 (85.9%) | 276 (78.6%) | 0.2 |
| • Hydralazine | 44 (42.3%) | 84 (24.5%) | 0.002 |
| • Nitrates | 40 (37.7%) | 102 (29.1%) | 0.2 |
| Device therapy (ICD or CRT/D) | 62 (59.1%) | 222 (63.6%) | 0.4 |
| Ejection fraction (%) | 25.5 ± 14.3 | 28.7 ± 14.2 | 0.04 |
| HFpEF | 9 (8.5%) | 41 (11.7%) | 0.4 |
| Creatinine (mg/dL) | 1.4+ 0.8 | 1.3 ± 0.6 | 0.06 |
| Chronic kidney disease stage | 0.08 | ||
| • 1-2 | 61 (57.6%) | 221 (63.0%) | |
| • 3 | 38 (35.9%) | 122 (34.8%) | |
| • 4-5 | 7 (6.6%) | 8 (2.3%) | |
| Body mass index (kg/m2) | 32.9 ± 8.3 | 31.1 +7.7 | 0.04 |
| B-type natriuretic peptide (pg/mL)* | 283 (67 – 602) | 147 (52–361) | 0.07 |
| Systolic blood pressure (mm Hg) | 109.9 ± 18.2 | 114.5 ± 19.5 | 0.2 |
| Diastolic blood pressure (mm Hg) | 66.3 ± 12.7 | 69.3 ± 17.2 | 0.3 |
Values are mean ± standard deviation, median (interquartile range), or N (%). ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; CRT-D, cardiac resynchronization therapy – defibrillator; HFpEF, heart failure with preserved ejection fraction; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association.
Missing data for N = 179
During a median follow-up of 827 (506, 1379) days, death occurred in 60 (13.1%) subjects. All-cause hospitalizations occurred in 262 (57.3%) subjects, while HF-specific hospitalizations occurred in 108 (23.6%) subjects. There was no difference in the risk of death (11 [10.4%] vs. 49 [14.0%]; P=0.3), or the risk of at least one all-cause hospitalization (64 [60.4%] vs. 198 [56.4%]; P=0.5) between those who lived in a FD compared to those who did not. The risk of at least one HF-specific hospitalization was higher in those who lived in a FD compared to those who did not (36 [34.0%] vs. 72 [20.5%]; P=0.004).
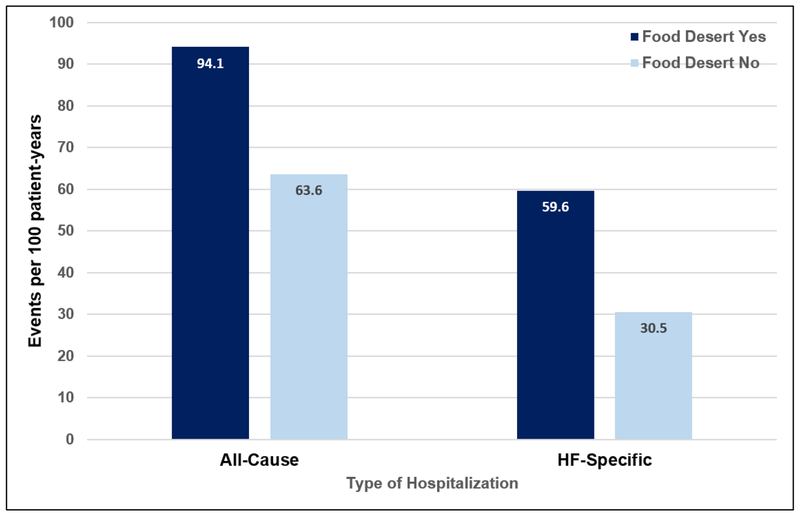
The overall frequency of all-cause hospitalizations was higher in patients who lived in a FD. Including repeat episodes, there were 94.1 (82.4 – 107.4) all-cause hospitalizations per 100 patient-years in the group who lived in a FD, compared with 63.6 (58.5 – 69.1) in the group who did not live in a FD (Figure 1). After adjustment for covariates, living in a FD was associated with a 21% higher risk of repeat all-cause hospitalizations (Table 2). Other variables associated with an increased risk of repeat all-cause hospitalizations included age, male sex, education, history of hypertension, insurance status, and serum creatinine (Supplemental Table).
Figure 1.
Rates of all-cause and heart-failure (HF) specific hospitalizations in subjects according to food desert status.
Table 2.
Unadjusted and adjusted estimates for all-cause and heart failure (HF) specific hospitalizations using the Cox proportional hazard ratios (Andersen-Gill).
| All-cause hospitalization | HF-specific hospitalization | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | p | |
| • Univariate | 1.39 (1.19 – 1.63) | <0.0001 | 1.68 (1.35 – 2.09) | <0.0001 |
| • Multivariable | 1.21 (1.02 – 1.44) | 0.03 | 1.30 (1.02 – 1.65) | 0.03 |
The estimates shown are adjusted forage, gender, race, insurance, education, hypertension, diabetes, body mass index, serum creatinine, and type of heart failure. CI, confidence interval; HR, hazard ratio; RR, rate ratio.
The overall frequency of HF-specific hospitalizations was also higher in patients who lived in a FD, as shown in Figure 1. Including repeat episodes, there were 59.6 (50.4 – 70.4) HF-specific hospitalizations per 100 patient-years in the group who lived in a FD, compared with 30.5 (27.1 – 34.4) in the group who did not live in a FD. After adjustment for covariates, living in a FD was associated with a 30% higher risk of repeat all-cause hospitalizations (Table 2). Other variables that were associated with an increased risk of repeat HF-specific hospitalizations included age, male sex, black race, education, history of hypertension, insurance status, and serum creatinine (Supplemental Table).
DISCUSSION
In this analysis of patients with HF, we have shown the association of FD status with the risk of repeat hospitalizations. Specifically, patients who lived in a FD had a 21% increased risk of repeat all-cause hospitalization and a 30% increased risk of repeat HF-specific hospitalization, even after adjustment for differences in baseline demographic and clinical characteristics. These data confirm that for patients living with HF, hospital admission is common and repeat admissions are frequent. However, for patients with HF who also live in a FD, this risk is amplified despite adjustment for conventional socioeconomic and clinical risk factors, suggesting that other unmeasured confounders present in FD may account for some of the increased risk observed in our cohort.
It is well known that environmental conditions are a contributing factor in promoting health disparities, particularly because neighborhoods with predominantly low-income and/or racial/ethnic minority residents are disproportionately affected by adverse health outcomes.17 Kelli et al. recently examined a comprehensive set of CVD risk factors and subclinical measures of vascular disease in 1421 subjects free of prevalent HF.18 Subjects living in a FD had increased systemic oxidative stress, inflammation, and arterial stiffness, processes which are all predictive of increased risk of adverse outcomes in HF patients in other cohorts.
In addition to affecting subclinical biomarkers and vascular processes, neighborhood characteristics may also influence patients’ propensity for optimal HF self-care, such as maintaining recommendations for healthy diet, physical activity, sodium reduction, and other behaviors. For example, Powell et al. documented that commercial physical activity–related facilities were less likely to be present in lower-income neighborhoods and in neighborhoods with higher proportions of race/ethnic minorities.19 Morland et al. confirmed that low-income and predominantly black neighborhoods have fewer supermarkets or specialty food stores than high-income or predominantly white neighborhoods.20 Prior studies have confirmed that food insecurity is associated with medication nonadherence as well as inadequate transportation.21,22 These factors may be general markers of poverty that impact health behaviors, and ultimately have an adverse effect on health outcomes. However, it is unclear how to best incorporate social determinants of health into current CV risk prediction models. Krumholz et al. used data from the Telemonitoring to Improve HF Outcomes study to determine whether a hospital readmission risk model that incorporated SES, health status, and psychosocial characteristics could improve risk prediction compared with a model that incorporated only clinical and demographic factors.23 The authors concluded that inclusion of the SES and psychosocial variables led to only a minor improvement in the discrimination of the risk model.
Since patients may experience multiple admissions during the course of their illness, traditional methods that analyze time to first event may not measure the true burden of disease due to worsening HF either for the individual or for health care systems.24 Recurrent hospitalizations are an important contributor to lower patient quality of life, as well as the economic burden of HF, with hospitalization accounting for 80% of the total cost of this condition to health care systems.1 This may be particularly important for patients living in a FD, where environmental and neighborhood factors may have a greater impact on factors that affect HF outcomes such as availability of nutritious foods, and access to supermarkets or retail pharmacy outlets that could impact medication adherence. Our analysis adjusted for most standard clinical, demographic, and socioeconomic confounders. However, there may be additional unmeasured confounders that may influence the frequency of hospitalization, including individual income, inflammatory biomarkers, walking environments, and other variables that were not measured in this study.
Currently, there is significant enthusiasm for developing US governmental programs and regulations designed to eliminate FD, in an effort to improve population health and clinical outcomes, and reduce health disparities.25 Additional analyses have shown that FD exist in parts of Africa, Australia, and Europe, suggesting that food insecurity in both urban and rural environments is a global concern.26–28 Since FD may negatively affect risk factors and health behaviors, more research is needed to determine how access to healthy foods influences the types of foods consumers purchase and eat, as well as other healthy behaviors. While public health advocates would hope that increased access would also increase the intake of affordable and nutritious foods, studies have shown that consumers often continue to make unhealthy choices based on personal preferences even after healthier food options are more widely available.29 Although low sodium food choices and dietary compliance are important for self-care in patients with HF, other unmeasured confounders present in FD may also impact CV risk.
There are limitations to our analysis that are worth addressing. First, the number of patients living in a FD in our cohort is relatively small. However, national data suggests that only a small fraction of Americans (23.5 million, or approximately 7% of the population) live in FD, and even fewer (2.3 million) live in FD without access to a privately owned car.10 Moreover, our cohort represents patients from specialty HF clinics at an urban tertiary care facility, and may not be representative of patients in community or rural settings. Although the definition of a FD incorporates information on neighborhood income, we lacked information on individual income, which may also be an important determinant of clinical outcomes. Finally, there are multiple methods available to analyze recurrent events. We used the Andersen-Gill method because it incorporates inter-event times in addition to event rates. Other statistical methods to analyze recurrent events are available, however there is controversy as to which are most appropriate.30
In conclusion, our analysis has confirmed that living in a FD is associated with an increased risk of repeat all-cause and HF-specific hospitalization in patients with HF. This association may be mediated through decreased access to healthy food, or other socioeconomic variables that were not accounted for in this analysis. In order to further improve clinical HF outcomes, more research is needed that addresses social determinants of health, as well as strategies to improve HF management that is sensitive to unique aspects of patients’ neighborhood and environment.
Supplementary Material
Acknowledgments
SOURCES OF FUNDING
A.A.M is supported by funding from the NIH/NHLBI (K23 HL124287) and the Robert Wood Johnson Foundation (Harold Amos Medical Faculty Development Program).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Piña IL, Trogdon JG. Forecasting the Impact of Heart Failure in the United States. Circ Heart Fail 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Havranek EP, Mujahid MS, Barr DA, Blair IV, Cohen MS, Cruz-Flores S, Davey-Smith G, Dennison-Himmelfarb CR, Lauer MS, Lockwood DW, Rosal M, Yancy CW. Social Determinants of Risk and Outcomes for Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2015;132:873–898. [DOI] [PubMed] [Google Scholar]
- 3.Anderson RT, Sorlie P, Backlund E, Johnson N, Kaplan GA. Mortality effects of community socioeconomic status. Epidemiology 1997;8:42–47. [DOI] [PubMed] [Google Scholar]
- 4.Robert SA. Community-level socioeconomic status effects on adult health. J Health Soc Behav 1998;39:18–37. [PubMed] [Google Scholar]
- 5.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, Sorlie P, Szklo M, Tyroler HA, Watson RL. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 2001;345:99–106. [DOI] [PubMed] [Google Scholar]
- 6.O’Toole TE, Conklin DJ, Bhatnagar A. Environmental risk factors for heart disease. Rev Environ Health 2008;23:167–202. [DOI] [PubMed] [Google Scholar]
- 7.Tsuyuki RT, McKelvie RS, Arnold JM, Avezum A Jr, Barretto AC, Carvalho AC, Isaac DL, Kitching AD, Piegas LS, Teo KK, Yusuf S. Acute precipitants of congestive heart failure exacerbations. Arch Intern Med 2001;161:2337–2342. [DOI] [PubMed] [Google Scholar]
- 8.Michalsen A, Konig G, Thimme W. Preventable causative factors leading to hospital admission with decompensated heart failure. Heart 1998;80:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine RS, Foster JE, Fullilove RE, Fullilove MT, Briggs NC, Hull PC, Husaini BA, Hennekens CH. Black-white inequalities in mortality and life expectancy, 1933–1999: implications for healthy people 2010. Public Health Rep 2001;116:474–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hummer RA, Chinn JJ. Race/ethnicity and U.S. Adult Mortality: Progress, Prospects, and New Analyses. Du Bois Rev 2011;8:5–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kind AJ, Jencks S, Brock J, Yu M, Bartels C, Ehlenbach W, Greenberg C, Smith M. Neighborhood Socioeconomic Disadvantage and 30 Day Rehospitalizations: An Analysis of Medicare Data. Ann Intern Med 2014;161:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleming LM, Gavin M, Piatkowski G, Chang JD, Mukamal KJ. Derivation and Validation of a 30-Day Heart Failure Readmission Model. Am J Cardiol 2014;114:1379–1382. [DOI] [PubMed] [Google Scholar]
- 13.Foraker RE, Rose KM, Suchindran CM, Chang PP, McNeill AM, Rosamond WD. Socioeconomic Status, Medicaid Coverage, Clinical Comorbidity, and Rehospitalization or Death After an Incident Heart Failure Hospitalization: Atherosclerosis Risk in Communities Cohort (1987 to 2004). Circ Heart Fail 2011;4:308–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marti CN, Georgiopoulou VV, Giamouzis G, Cole RT, Deka A, Tang WH, Dunbar SB, Kaloeropoulos AP, Butler J. Patient-Reported Selective Adherence to Heart Failure Self-Care Recommendations: A Prospective Cohort Study: The Atlanta Cardiomyopathy Consortium. Congest Heart Fail 2013;19:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersen PK, Gill RD. Cox’s Regression Model for Counting Processes: A Large Sample Study. Ann Stat 1982;10:1100–1120. [Google Scholar]
- 16.Jahn-Eimermacher A Comparison of the Andersen–Gill model with poisson and negative binomial regression on recurrent event data. Comput Stat Data Anal 2008;52:4989–4997. [Google Scholar]
- 17.Walker RE, Keane CR, Burke JG. Disparities and access to healthy food in the United States: A review of food deserts literature. Health Place 2010;16:876–884. [DOI] [PubMed] [Google Scholar]
- 18.Kelli HM, Hammadah M, Ahmed H, Ko Y-A, Topel M, Samman-Tahhan A, Awad M, Patel K, Mohammed K, Sperling LS, Pemu P, Vaccarino V, Lewis T, Taylor H, Martin G, Gibbons GH, Quyyumi AA. Association Between Living in Food Deserts and Cardiovascular Risk. Circ Cardiovasc Qual Outcomes 2017;10(9). doi: 10.1161/CIRCOUTCOMES.116.003532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Powell LM, Slater S, Chaloupka FJ, Harper D. Availability of Physical Activity–Related Facilities and Neighborhood Demographic and Socioeconomic Characteristics: A National Study. Am J Public Health 2006;96:1676–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morland K, Wing S, Diez Roux A, Poole C. Neighborhood characteristics associated with the location of food stores and food service places. Am J Prev Med 2002;22:23–29. [DOI] [PubMed] [Google Scholar]
- 21.Cornelius T, Jones M, Merly C, Welles B, Kalichman MO, Kalichman SC. Impact of food, housing, and transportation insecurity on ART adherence: a hierarchical resources approach. AIDS Care 2017;29:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalichman SC, Hernandez D, Cherry C, Kalichman MO, Grebler T. Food Insecurity and Other Poverty Indicators among People Living with HIV/AIDS: Effects on Treatment and Health Outcomes. J Community Health 2014;39:1133–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krumholz HM, Chaudhry SI, Spertus JA, Mattera JA, Hodshon B, Herrin J. Do Non-Clinical Factors Improve Prediction of Readmission Risk?: Results From the Tele-HF Study. JACC Heart Fail 2016;4:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rogers JK, McMurray JJV, Pocock SJ, Zannad F, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, Vincent J, Pitt B. Eplerenone in Patients with Systolic Heart Failure and Mild Symptoms: Analysis of Repeat Hospitalizations. Circulation 2012;126:2317–2323. [DOI] [PubMed] [Google Scholar]
- 25.Block JP, Subramanian SV. Moving Beyond “Food Deserts”: Reorienting United States Policies to Reduce Disparities in Diet Quality. PLoS Med 2015;12:e1001914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crush J, Frayne B, Pendleton W. The Crisis of Food Insecurity in African Cities. J Hunger Environ Nutr 2012;7:271–292. [Google Scholar]
- 27.Pollard C, Landrigan T, Ellies P, Kerr D, Lester M, Goodchild S. Geographic factors as determinants of food security: a Western Australian food pricing and quality study. Asia Pac J Clin Nutr 2014;23:703–713. [DOI] [PubMed] [Google Scholar]
- 28.Furey S, Strugnell C, McIlveen MH. An investigation of the potential existence of “food deserts” in rural and urban areas of Northern Ireland. Agric Human Values 2001;18:447–457. [Google Scholar]
- 29.Dubowitz T, Ghosh-Dastidar M, Cohen DA, Beckman R, Steiner ED, Hunter GP, Florez KR, Huang C, Vaughan CA, Sloan JC, Zenk SN, Cummins S, Collins RL. Diet And Perceptions Change With Supermarket Introduction In A Food Desert, But Not Because Of Supermarket Use. Health Aff 2015;34:1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Claggett B, Pocock S, Wei LJ, Pfeffer MA, McMurray JJV, Solomon SD. Comparison of Time-to-First Event and Recurrent Event Methods in Randomized Clinical Trials. Circulation 2018. March 27 doi: 10.1161/CIRCULATIONAHA.117.033065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.