Abstract
The citrullinome cargo in neutrophil extracellular traps varies according to disease condition and stimulation conditions
We read the letter by Shi and Andrade in response to our publication in Science Immunology (1). In their letter, Shi and Andrade focus their comments on the role of NETosis in the generation of citrullinated autoantigens and, thus, the ability of neutrophil extracellular traps (NETs) to “initiate an anticitrullinated protein antibody (ACPA) response.” To clarify, our hypothesis and the conclusions from our manuscript are that citrullinated autoantigens contained and externalized in NETs can be taken up by rheumatoid arthritis (RA) fibroblast-like synoviocytes (FLS) and, in the context of human leukocyte antigen class II (shared epitope), are effectively presented in an antigen-specific manner to the adaptive immune system to generate pathogenic immune responses characteristic of RA.
Although NETosis by itself without the right genetic predisposition may not promote aberrant adaptive immunity, exacerbated NET formation characteristic of RA (2) can lead to the externalization of citrullinated proteins that, because they are bound to nucleic acids, HMGB1 (high mobility group box 1), and other immunostimulatory molecules, display the ability to be internalized by antigen-presenting cells (1). We do not propose that NETs are the only source of citrullinated autoantigens, and several other mechanisms (tobacco and microbes) likely play important roles. Some of these environmental stimuli may also contribute to citrullination through their ability to induce NETs (3, 4). In contrast, the in vivo relevance of leukotoxic “hypercitrullination” in RA with regard to activation of pathogenic immunity and organ damage remains to be determined and is, at this state, primarily an in vitro observation, as recently discussed (5). Whether a threshold of hypercitrullination needs to be achieved to break tolerance and initiate ACPA responses has not been systematically demonstrated to be an important in vivo phenomenon in the context of RA.
We would like to directly respond to several points raised by Shi and Andrade:
-
(1)
They dispute our finding that citrullinated proteins are generated during NETosis. However, the conditions they use differ considerably from those used in our manuscript, making comparisons difficult. We reported the pattern of citrullination in purified NETs that have been induced by rheumatoid factor (RF) (1), whereas Shi and Andrade use whole-cell protein lysates from neutrophils exposed to phorbol 12-myristate 13-acetate (PMA). Compared to many other stimuli, as recently reported, PMA does not induce histone H3 citrullination, and peptidylarginine deiminase (PAD)–dependent pathways do not seem to significantly affect the systematic ability of PMA to induce NETs in human neutrophils (6). In contrast, ionophore and RF induce significant histone citrullination (1, 6). Hence, we consider PMA, although effective at inducing NETs in vitro, to be a somewhat contrived system that does not represent the NETosis induced by many physiologic and/or pathologic stimuli, including the ones used in our studies. Another important point to clarify is that in contrast to the observations that RF induces NET formation, control immunoglobulin M (IgM) does not (2). Therefore, IgM stimulation was not included as a control for the pattern of citrullinated proteins in RF-induced NETosis because NETs are not generated by the control immunoglobulin (2). To further support our findings, we now show additional controls to emphasize that, depending on the type of NETs and the disease state, the pattern of citrullination displayed in NETs varies (Fig. 1A). Spontaneously formed lupus low-density granulocyte NETs have a citrullination profile that differs from NETs induced by RF in control neutrophils. Furthermore, when we compare the spontaneously formed NETs from lupus low-density granulocytes to the spontaneously formed NETs from low-density granulocytes previously exposed to type I interferons (IFNs), the intensity of the citrullination pattern is altered (Fig. 1B). These results indicate that the pattern of citrullination may be specific to the disease state or the stimulus used to induce NETs. In addition, we had previously reported that the NET protein cargo varies depending on the stimulus to induce NETs (2). Using unstimulated neutrophils to compare NETs does not represent, in our view, a good control for extracellular exposure of citrullinated autoantigens because control unstimulated neutrophils do not form NETs (6). The use of neutrophil lysates proposed by Shi and Andrade is also, in our view, not adequate as a NET control because it does not distinguish intracellular from extracellular citrullinated autoantigens.
-
(2)
Shi and Andrade used calcium ionophores to induce in vitro hypercitrullination; this stimulus is also a very potent inducer of NETs but is nonspecific (6). Hence, differentiating citrullination patterns between NETosis induced by ionophore and leukotoxic hypercitrullination may be complicated when evaluating these experiments.
-
(3)
Similar points are raised with regard to comparing Fig. 1B in our manuscript (1) with Fig. 1B in the technical comment written by Shi and Andrade. Again, we are using purified NETs, whereas Shi and Andrade report whole-cell lysates under PMA-treated conditions, making comparisons difficult. An additional concern here is that Shi and Andrade use whole serum from RA patients with high levels of ACPAs, which may affect specificity and intensity of these signals, whereas we use ACPA antibodies purified from RA patients’ plasma using a previously validated approach (7). Furthermore, in our manuscript, using IgG control does not lead to detection of citrullinated proteins in NETs above background intensity [Fig. 1B in (1)].
-
(4)
We dispute the assertion by Shi and Andrade that NETs are merely redistributors of the steady-state citrullinome. When we compare the proteomic analysis of citrullinated proteins identified in NETs induced by RF in our manuscript [Fig. 1E in (1)] to data published by Romero et al. on the steady-state neutrophil lysates [fig. S2 in (8)], we find a very different composition of citrullinated proteins. Among the proteins we find citrullinated in RF-induced NETs, only three are also citrullinated in steady-state neutrophil lysates, whereas the other 17 proteins are not. Furthermore, previous studies by Romero et al. also showed that myeloperoxidase (MPO) is distinctly citrullinated in RA synovial fluid samples but not in steady-state neutrophils (8), similar to the citrullinated MPO that we detected in NETs and in agreement with our findings of anticitrullinated MPO responses in the synovial fluid of RA patients (1). We consider that a comprehensive analysis of the citrullinated proteome of NETs under several other types of stimulation is warranted to better understand the potential variability among various patients or types of stimulation; this may further our understanding of ACPA response specificities in association with specific sources of NETs.
-
(5)
It is possible that the initial ACPA responses target a limited number of citrullinated proteins present in NETs with further expansion and epitope spreading as disease progresses. This is supported by previous compelling studies in both human RA and animal models of arthritis (9). With regard to ACPA specificity, we have demonstrated both in vitro and in vivo that citrullinated NET peptides, when presented in a major histocompatibility complex class II–dependent manner to antigen-specific T cells, will elicit T cell activation. Furthermore, we have shown significantly enhanced ACPA production in the animals exposed to FLS with internalized NETs by three different methods: (i) results using commercially available anti–cyclic citrullinated peptide 2 (CCP2) test [this assay represents the second generation of anti-CCP tests and is described to be highly specific for RA (95 to 99%) and to provide excellent positive predictive value for RA diagnosis]; (ii) showing by dot blot that the serum from mice exposed to FLS plus NETs recognizes citrullinated proteins at different levels of intensity depending on the protein, whereas minimal detection is observed when using serum from mice that received FLS without NETs, thereby showing specificity to the NET effect; and (iii) finally, by means of the protein array used in our study showing specificity of the antibodies to certain citrullinated epitopes that are considered highly relevant to RA (including histone, vimentin, and fibrinogen) and the target of ACPAs. This is in agreement with the recent work by Corsiero et al. (10) showing that B cells within the RA patients’ synovium target citrullinated proteins generated during NETosis of RA synovial neutrophils, including citrullinated histones, fibrinogen, and vimentin, and that these antibodies selectively recognize NETs from RA neutrophils. To further support the specificity of our findings, we now show in this response (Fig. 1C) that RA serum preferentially recognizes the citrullinated but not the native form of MPO. In contrast, commercial anti-MPO antibody will have the ability to equally recognize both native and citrullinated forms (Fig. 1C). Consistent with this, in our previous work, there is a subset of autoantibodies in ACPA+ RA that bind native epitopes present in citrullinated antigens. Such autoantibodies targeting native epitopes in ACPA+ RA may arise from epitope spreading of the B cell response from citrullinated epitopes, but we do not consider such autoantibodies to be ACPAs (9).
Figure legend 1:
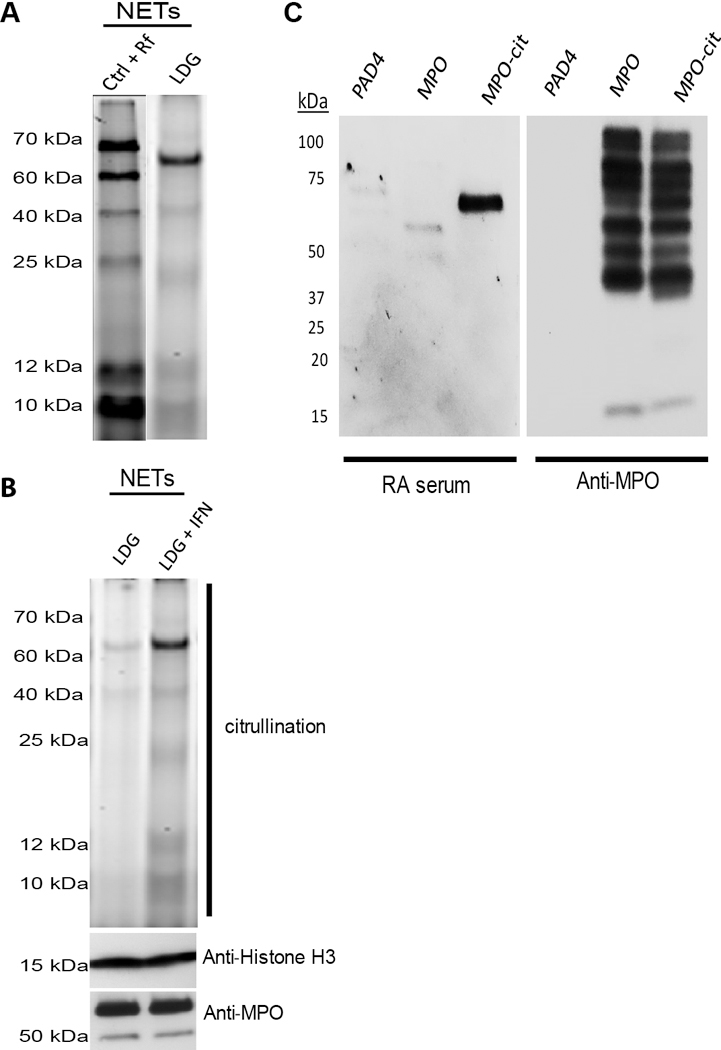
(A and B) Citrullinated protein patterns present in NETs vary depending on the disease state and stimulus. (A) NETs were induced in control (Ctrl) neutrophils by RF or spontaneously generated in lupus low-density granulocytes (LDGs). NETs were purified, and citrullinated proteins were detected using rhodamine-phenylglyoxal probes. The citrullination pattern varies when comparing the two samples. (B) Spontaneously formed NETs were purified from lupus low-density granulocytes isolated from a different patient as in (A), incubated in the presence or absence of 1000 IU of recombinant IFN-α for 2 hours. Citrullinated proteins were detected using rhodamine-phenylglyoxal. Total histone H3 (Abcam) and MPO (Dako) were used as loading controls. Increased citrullination of proteins is evident after IFN treatment. (C) Serum from RA patients preferentially recognizes citrullinated over native MPO. Human recombinant MPO (Lee BioSolutions) was citrullinated in vitro with purified PAD4. Purified PAD4, native MPO, and citrullinated MPO (cit-MPO) were resolved in a gradient 4 to 12% SDS–polyacrylamide gel electrophoresis gel. Proteins were transferred onto a nitrocellulose membrane and probed with high-titer ACPA+ serum from a subject with RA (1:250 dilution). Anti-human IgG coupled to horseradish peroxidase was used to develop the membrane. Same samples were probed against MPO to demonstrate the presence of recombinant MPO. RA serum can differentially recognize the citrullinated version of MPO over the native one. Results are representative of five RA subjects.
Overall, we consider that on the basis of the extensive work from various groups including ours, there is now ample and compelling evidence, both in vitro and in vivo in human and animal systems, that NETs represent one of the sources of citrullinated autoantigens and that these structures have the ability to activate innate and adaptive immune responses in RA in a pathogenic manner.
Acknowledgments
Funding: This work was supported by the Intramural Research Program at National Institute of Arthritis and Musculoskeletal and Skin/NIH (ZIAAR041199). This was also supported by NIH through Public Health Service grants GM079357 (P.R.T.) and GM110394 (P.R.T.), 5 U01 AI101981–04 (J.H.B. is sub-investigator), W81XWH-15–1-003 (J.H.B.), R01AR06367603 (to W.H.R.), and 5UM1AI 10055703 (D.A.F.) and by the Rheumatology Research Foundation (M.J.K.).
References
- 1.Carmona-Rivera C, Carlucci PM, Moore E, Lingampalli N, Uchtenhagen H, James E, Liu Y, Bicker KL, Wahamaa H, Hoffmann V, Catrina AI, Thompson PR, Buckner JH, Robinson WH, Fox DA, Kaplan MJ, Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2, eaag3358 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khandpur R, Carmona-Rivera C, Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S, Li S, Patel RM, Subramanian V, Thompson P, Chen P, Fox DA, Pennathur S, Kaplan MJ, NETs are a source of citrullinated autoantigens and stimulate inflammatory responses in rheumatoid arthritis. Sci. Transl. Med. 5, 178ra40 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee J, Luria A, Rhodes C, Raghu H, Lingampalli N, Sharpe O, Rada B, Sohn DH, Robinson WH, Sokolove J, Nicotine drives neutrophil extracellular traps formation and accelerates collagen-induced arthritis. Rheumatology 56, 644–653 (2017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jayaprakash K, Demirel I, Khalaf H, Bengtsson T, The role of phagocytosis, oxidative burst and neutrophil extracellular traps in the interaction between neutrophils and the periodontal pathogen Porphyromonas gingivalis. Mol. Oral Microbiol. 30, 361–375 (2015) [DOI] [PubMed] [Google Scholar]
- 5.Gazitt T, Lood C, Elkon KB, Citrullination in rheumatoid arthritis—A process promoted by neutrophil lysis? Rambam Maimonides Med. J. 7, e0027 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kenny EF, Herzig A, Krüger R, Muth A, Mondal S, Thompson PR, Brinkmann V, von Bernuth H, Zychlinsky A, Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 6, e24437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ossipova E, Cerqueira CF, Reed E, Kharlamova N, Israelsson L, Holmdahl R, Nandakumar KS, Engström M, Harre U, Schett G, Catrina AI, Malmström V, Sommarin Y, Klareskog L, Jakobsson P-J, Lundberg K, Affinity purified anti-citrullinated protein/peptide antibodies target antigens expressed in the rheumatoid joint. Arthritis Res. Ther. 16, R167 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romero V, Fert-Bober J, Nigrovic PA, Darrah E, Haque UJ, Lee DM, van Eyk J, Rosen A, Andrade F, Immune-mediated pore-forming pathways induce cellular hypercitrullination and generate citrullinated autoantigens in rheumatoid arthritis. Sci. Transl. Med. 5, 209ra150 (2013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan Y-C, Kongpachith S, Blum LK, Ju C-H, Lahey LJ, Lu DR, Cai X, Wagner CA, Lindstrom TM, Sokolove J, Robinson WH, Barcode-enabled sequencing of plasmablast antibody repertoires in rheumatoid arthritis. Arthritis Rheumatol. 66, 2706–2715 (2014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corsiero E, Bombardieri M, Carlotti E, Pratesi F, Robinson W, Migliorini P, Pitzalis C, Single cell cloning and recombinant monoclonal antibodies generation from RA synovial B cells reveal frequent targeting of citrullinated histones of NETs. Ann. Rheum. Dis. 75, 1866–1875 (2016) [DOI] [PMC free article] [PubMed] [Google Scholar]


