Abstract
Background:
Limitations regarding the sensitivity and specificity of the systemic inflammatory response (SIRS) criteria prompted the recent revision in consensus definitions of sepsis and septic shock. We evaluated patients with Staphylococcus aureus bacteremia (SAB) who did not meet SIRS criteria for sepsis (SIRS-negative, SIRS-N) to compare host immune response and outcomes with SIRS-positive (P) patients.
Methods:
A prospective observational study of patients hospitalized for SAB during 2012–2015 was conducted. Pro- (TNFα, IL6, IL8) and anti-inflammatory (IL10) cytokine levels (pg/mL) were compared between SIRS-N and SIRS-P patients. Outcome endpoints were day 4 persistence and 30-day mortality.
Results:
Of the 353 study patients, 23% were SIRS-N. A similar proportion of SIRS-N and SIRS-P patients had an infection-related admitting diagnosis (70% vs. 66%, p=0.5946), and both groups received timely antibiotic administration. Less than 1/3 of SIRS-N group had abnormal WBC count, tachycardia, or tachypnea while <15% had fever/hypothermia or hypotension. Initial proand anti-inflammatory cytokine levels were significantly lower and in balance as indicated by IL10/TNF ratio in SIRS-N compared to SIRS-P patients. IL10/TNF ratio increased progressively in patients with increasing sepsis severity and mortality.
Conclusions:
Clinical management of patients with SAB seemed driven largely by clinician assessment rather than SIRS criteria alone, with one in 4 patients not meeting SIRS criteria. Importantly, the severity of presentation and outcomes of SAB correspond well to the magnitude of underlying imbalance in pro- and anti-inflammatory cytokine levels, supporting the updated sepsis definition as “life-threatening organ dysfunction caused by a dysregulated host response to infection”.
Key points:
In a prospective observational study of 353 patients with Staphylococcus aureus bacteremia, 23% did not meet SIRS criteria for sepsis. Severity of sepsis and risk of death is supported by a dysregulated host cytokine response with progressively increasing IL10/TNF ratio.
Keywords: Staphylococcus aureus, Bacteremia, Cytokine, Sepsis
Background
Staphylococcus aureus is a predominant cause of bloodstream infections, with an associated mortality of up to 20% [1]. While S. aureus is one of the leading organisms implicated in sepsis, reports indicate that only 38% to 44% of patients with S. aureus bacteremia (SAB) actually experience severe sepsis or septic shock [2,3]. Previous studies have demonstrated that the presence of severe sepsis/shock is strongly associated with poor outcomes [4], though it is not entirely clear why certain patients progress to this stage of severity in their infection while others do not. The complex host-pathogen interactions and genetic determinants that drive this variable pathophysiologic host response and the associated outcomes have yet to be fully elucidated [5,6]. Furthermore, the degree to which these factors contribute to outcomes among non-septic patients has not been well studied. Kaukonen et al. found that consensus definition of sepsis requiring ≥2 SIRS (systemic inflammatory response syndrome) criteria missed 1 in 8 patients with severe sepsis [7]. Limitations in both the sensitivity and specificity of the SIRS criteria led to revision of the previous sepsis definitions and publication of the updated Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) [8]. The goal of the present study is to apply the previous SIRS-based definition of sepsis to identify the different clinical phenotypes of patients with SAB and relate the varied phenotypes to host cytokine response and outcomes. We hypothesize that patients with SAB who do not meet SIRS criteria (SIRS-N) have overall favorable outcomes compared to SIRS-positive (P) patients, in part due to a less robust but balanced host immune response between pro- and anti-inflammatory cytokines.
Methods
This was a prospective observational study of patients hospitalized for SAB between July 2012 and June 2015, at three university-affiliated medical centers. This study was approved by the institutional review board at each site. Informed consent was waived as this was an observational study. Patients were eligible for inclusion if they met all of the following: age 18 years, positive blood culture for S. aureus, saved bacterial and blood specimens, and receipt of at least 48 hours of effective therapy against S. aureus. Those with polymicrobial bacteremia or those who did not receive effective antibiotics within 48 hours of positive blood cultures were excluded from this study. Plasma or serum samples were collected from specimens drawn for routine labs once physician-ordered tests were completed. Samples were collected at onset of SAB and 72 h after start of effective therapy and stored at −80°C until analysis to measure pro-(tissue necrosis factor, TNFα; interleukin-6, IL6; interleukin-8, IL8) and anti-inflammatory (interleukin-10, IL10) cytokine levels (pg/mL) by multiplex Luminex assays. Medical records were reviewed to obtain the following information: demographics, residence prior to admission, comorbid conditions, receipt of immunosuppressive therapy within 30 days of admission which included chemotherapy, monoclonal/polyclonal antibodies (such as rituximab), biologics (such as etanercept), tacrolimus, sirolimus, mycophenolate, or chronic corticosteroids (≥ 20 mg prednisone or equivalent per day); source of bacteremia; clinical presentation (chief complaints on admission, daily vital signs, mental status, need for mechanical ventilation); laboratory values (i.e. WBC with differential, days of positive blood culture, culture and sensitivities); echocardiographic findings; details on antimicrobial treatment and surgical interventions; length of hospital stay and survival at 30-day after onset of SAB. Study data were managed using the Research Electronic Data Capture (REDCap) software hosted at the University of Southern California [9].
Study definitions
SIRS criteria and severity (SIRS-negative, sepsis, severe sepsis, septic shock) were based on vital signs and laboratory values recorded on day 1, in accordance with previous consensus definitions [10]. Sepsis was defined by the presence of at least two or more of the following: temperature >38°C or <36°C, heart rate >90 beats per minute, respiratory rate >20 breaths per minute, and WBC count >12,000/mm3 or <4,000/mm3 [10]. SIRS-N patients were those who did not meet at least 2 of the above criteria at onset of infection. Severe sepsis included those who met sepsis criteria and had at least one of the following: systolic blood pressure <90 mmHg or a drop ≥40 mmHg, lactate >3 mmol/L, total bilirubin>4 mg/dL, platelet count <100,000/mcL, or PaO2/FiO2 <300. Septic shock included those who met sepsis criteria and required vasopressor therapy to maintain a mean arterial pressure (MAP) >65. The source of bacteremia was considered “high-risk” if from an endovascular, lower respiratory, intra-abdominal, or central nervous system source, as previously defined by Soriano et al. [11]. “Effective” therapy was defined on the basis of in vitro activity against S. aureus. Clinical outcome was assessed on day 4 of SAB. Success was defined as either 1) documented or presumed eradication (clinical improvement of signs and symptoms in the absence of repeat blood cultures) or 2) complete resolution or partial improvement of fever, leukocytosis, or signs and symptoms of infection. Failure was defined by any of the following: 1) persistent positive blood culture on day 4, 2) clinical persistence on day 4 (unresolving signs and symptoms of infection or worsening clinical findings), or 3) death.
Data analysis
Patients were grouped as SIRS-N (negative-less than two) vs. SIRS-P (positive - at least two SIRS criteria at infection onset) and by severity of sepsis to compare patient characteristics, host immune response, and clinical outcomes. Host response was analyzed on the basis of cytokine profiles as related to the different clinical phenotypes of patients infected with S. aureus bloodstream infection: SIRS-N, SIRS-P (sepsis, severe sepsis, septic shock). Outcome endpoints were clinical response on day 4 after initiation of effective therapy and 30-day mortality.
Statistical analysis
Categorical variables were compared using Fisher’s exact test. Continuous variables were analyzed using the Mann-Whitney or Kruskall-Wallis test. A p value of <0.05 was considered statistically significant. Graphpad Prism 7 (version 7.0, www.graphpad.com, San Diego, CA) was used for statistical analyses.
Results
A total of 353 patients with SAB were included in this analysis. Of those, 23% (n=82) were SIRS-N. Among those who were SIRS-P (n=271), 66% had sepsis, 18% severe sepsis, and 16% shock. Baseline demographics were comparable between SIRS-N and SIRS-P patients, though SIRS-N patients were younger (mean age 54 vs. 59 years; p=0.0314) and less likely to have a comorbid condition (90%, 74/82 vs. 96%, 261/271; p=0.0419) (Table 1).
Table 1:
Comparison of baseline characteristics of SIRS-N vs. SIRS-P patients with SAB.
| Characteristics | SIRS-N (n=82) | SIRS-P (n=271) | P value | ||
|---|---|---|---|---|---|
| Age (years), mean ± standard deviation | 54.07 | ± 16.55 | 58.99 | ± 16.61 | 0.0314 |
| Male | 62 | 75.6% | 181 | 66.8% | 0.1372 |
| Residence prior to admission | |||||
| Home | 63 | 76.8% | 187 | 69.0% | 0.2121 |
| Hospital | 9 | 11.0% | 24 | 8.9% | 0.5241 |
| Healthcare-associated facility | 6 | 7.3% | 34 | 12.5% | 0.2351 |
| Comorbidity | |||||
| None | 8 | 9.8% | 10 | 3.7% | 0.0419 |
| 1 comorbid condition | 22 | 26.8% | 52 | 19.2% | 0.163 |
| ≥ 2 comorbid conditions | 52 | 63.4% | 209 | 77.1% | 0.0152 |
| Hypertension | 37 | 45.1% | 147 | 54.2% | 0.1659 |
| Hyperlipidemia | 13 | 15.9% | 59 | 21.8% | 0.2763 |
| Diabetes | 31 | 37.8% | 111 | 41.0% | 0.7 |
| Heart failure | 8 | 9.8% | 32 | 11.8% | 0.6945 |
| Chronic pulmonary disease | 3 | 3.7% | 34 | 12.5% | 0.0224 |
| Cancer | 6 | 7.3% | 38 | 14.0% | 0.128 |
| Hepatic impairment | 10 | 12.2% | 47 | 17.3% | 0.3075 |
| Renal impairment | 25 | 30.5% | 82 | 30.3% | 1.0000 |
| Immunosuppressive therapy | 18 | 22.0% | 28 | 10.3% | 0.0088 |
| History of S. aureus Infection | 18 | 22.0% | 50 | 18.5% | 0.5231 |
Hypertension was the most common comorbid condition, accounting for about half of all study patients. Chronic pulmonary disease was the single comorbidity that was significantly lower among SIRS-N patients (3.7%, 3/82 vs. 12.5%, 34/271; p=0.0224). Twice as many SIRS-N patients received immunosuppressive therapy at baseline compared to SIRS-P patients (22%, 18/82 vs. 10%, 28/271; p=0.0088) (Table 2).
Table 2:
Clinical presentation and management at infection onset.
| SIRS-N (n = 82) | SIRS-P (n = 271) | P value | |||
|---|---|---|---|---|---|
| Infection-related Admission Diagnosisa | 57 | 69.50% | 179 | 66.10% | 0.5946 |
| SIRS Criteria at Onset (Day 1) | |||||
| Tmax >38 or <36, (%) | 12 | 14.60% | 141 | 52.00% | <0.0001 |
| HR, >90, (%) | 25 | 30.50% | 226 | 83.40% | <0.0001 |
| RR, >20, (%) | 17 | 20.70% | 151 | 55.70% | <0.0001 |
| SBP <90, (%) | 7 | 8.50% | 53 | 19.60% | 0.019 |
| WBC >12 or <4, (%) | 24 | 29.30% | 183 | 67.50% | 0.0009 |
| Effective therapy started before infection onset | 9 | 11.00% | 18 | 6.60% | 0.2341 |
| Effective therapy started day of infection onset | 46 | 56.10% | 175 | 64.60% | 0.1928 |
| Time to start of effective therapy, median hours (IQR) | 2 | (1–5) | 1.5 | (1–4) | 0.2727 |
| Infectious Diseases Consult Obtained | 43 | 52.40% | 150 | 55.40% | 0.7045 |
| Days to ID consult, median days (IQR) | 3 | (1–5) | 2 | (1–3) | 0.0231 |
| Pitt bacteremia score ≥4 | 2 | 2.40% | 51 | 18.80% | <0.0001 |
| High-Risk Source of SABb | 14 | 17.10% | 68 | 25.10% | 0.139 |
| Primary source of infectionc | |||||
| Line infection | 10 | 12.20% | 29 | 10.70% | 0.6905 |
| Endocarditis | 3 | 3.70% | 13 | 4.80% | >0.9999 |
| SSTI | 15 | 18.30% | 42 | 15.50% | 0.6074 |
| Osteomyelitis (total) | 9 | 11.00% | 22 | 8.10% | 0.5034 |
| Osteomyelitis (non-spinal) | 3 | 3.70% | 13 | 4.80% | >0.9999 |
| Osteomyelitis (spinal) | 6 | 7.30% | 9 | 3.30% | 0.1247 |
| Diabetic foot infection | 1 | 1.20% | 2 | 0.70% | 0.5487 |
| Surgical wound | 3 | 3.70% | 10 | 3.70% | >0.9999 |
| Pyomyositis | 1 | 1.20% | 1 | 0.40% | 0.4111 |
| Non-spinal abscess | 3 | 3.70% | 14 | 5.20% | 0.7714 |
| Spinal Abscess | 2 | 2.40% | 10 | 3.70% | 0.74 |
| Pneumonia | 4 | 4.90% | 26 | 9.60% | 0.2576 |
| Urinary tract infection | 4 | 4.90% | 7 | 2.60% | 0.2887 |
| Cardiac device infection | 5 | 6.10% | 11 | 4.10% | 0.5431 |
| Non-cardiac device infection | 2 | 2.40% | 1 | 0.40% | 0.136 |
| Hardware infection | 1 | 1.20% | 10 | 3.70% | 0.4685 |
| Dialysis Catheter infection | 10 | 12.20% | 21 | 7.70% | 0.2637 |
| Decubitus ulcer | 2 | 2.40% | 7 | 2.60% | >0.9999 |
| Unknown | 17 | 20.70% | 42 | 15.50% | 0.3104 |
Infection-related diagnoses included a wide variety of infectious syndromes such as skin and soft tissue infection, pneumonia, urinary tract infection, osteomyelitis, endocarditis, bacteremia, sepsis of unknown etiology, and other (no significant between group differences identified).
“High-risk Source” includes endovascular, lower respiratory tract, or intra-abdominal source [11].
Values do not add to 100% as not all sources reported and patient may have had more than one suspected source.
IQR = interquartile range
Clinical presentation and initial management
A similar proportion of SIRS-N and SIRS-P patients were admitted with an infection-related diagnosis (70% vs. 66%, p=0.5946). A lower proportion of SIRS-N patients had temperature >38C or <36C (14%, 9/64 vs. 55%, 142/257 p<0.0001), heart rate >90 beats/min (29%, 11/38 vs. 84%, 113/135 p<0.0001), and respiratory rate >20 breaths/min (26%, 10/38 vs. 67%, 90/134 p<0.0001) as well as having WBC >12,000 or <4,000/mm3 (30%, 20/67 vs. 65%, 179/262 p<0.0001) compared to SIRS-P patients. Methicillin-resistant S. aureus (MRSA) was the cause of about one third of all SAB cases in this study with similar proportions between SIRS-N and SIRS-P patients (28%, 23/82 vs. 31%, 87/271 p=0.5864). With respect to timing of antibiotic therapy, no significant differences were observed between both groups. The proportion of SIRS-N patients initiated on effective (in vitro) therapy on the day of infection onset was 56% (46/82) compared to 65% (175/271) for SIRS-P patients, p=0.1928. Among those patients, the median time to start of effective therapy was 2 hours (interquartile range [IQR] 1–5 hours) vs. 1.5 hours (IQR 1–4 hours), p=0.2727, for SIRS-N and SIRS-P patients, respectively. A similar proportion of SIRS-N and SIRS-P patients received consultation by an infectious diseases specialist (52%, 43/82 vs. 55%, 150/271). The median time to consultation was delayed by 1 day for SIRS-N compared to SIRS-P patients (3, IQR 1–5 vs. 2, IQR 1–3 days, p=0.0231).
Host cytokine response
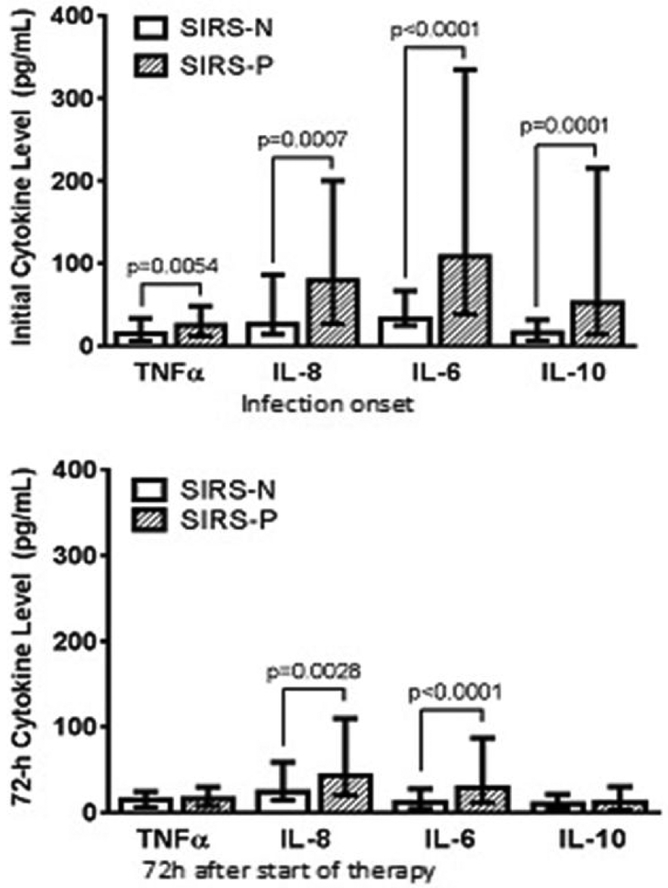
At onset of infection, serum levels of both pro- (TNFα, IL6, IL8) and anti-inflammatory (IL10) cytokines were significantly lower in SIRS-N compared to SIRS-P patients. All cytokine levels decreased following 72 h of effective therapy with similar TNFα and IL10 levels between both groups. However, in SIRS-P patients, IL6 and IL8 levels were significantly more robust at onset and remained significantly elevated at 72 h when compared to SIRS-N patients (Figure 1).
Figure 1:
Comparison of Cytokine Response between SIRS-N and SIRS-P patients.
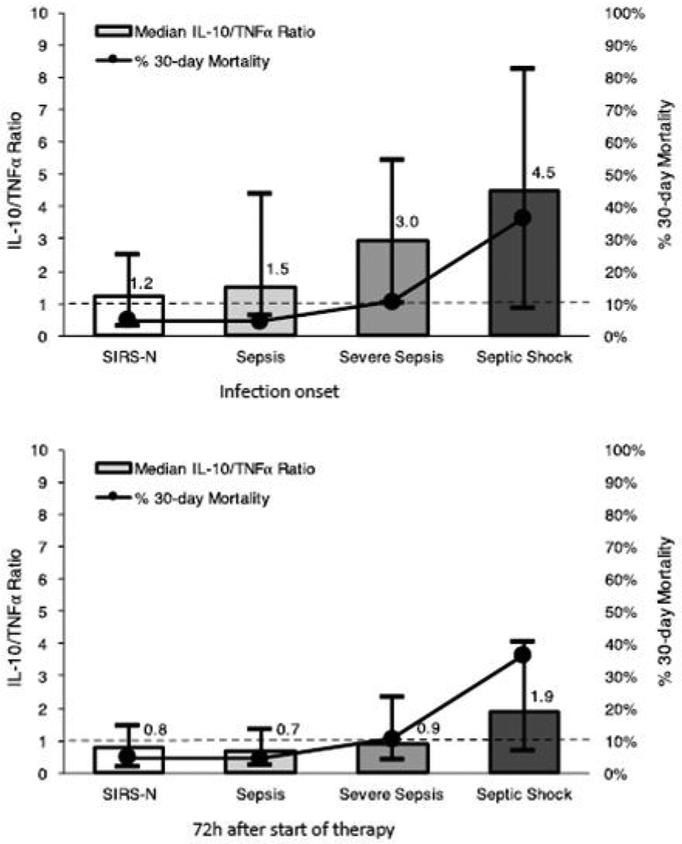
To assess the balance between pro- and anti-inflammatory cytokine responses, the ratio of IL10/TNFα was calculated. At onset of infection, SIRS-N patients had the lowest median IL10/TNF ratio (1.2) with progressively increasing value as severity of sepsis increased, at 1.5, 3.0, and 4.5 for patients with sepsis, severe sepsis, and septic shock, respectively (Figure 2). Following 72 h of effective therapy, these ratios normalized to values less than one for all groups except those presenting with septic shock, which remained significantly elevated at 1.9 (Figure 2).
Figure 2:
IL10/TNF ratio increases with greater degree of sepsis severity.
Outcomes
SIRS-N patients were over 3 times more likely to achieve day 4 clinical success (84% vs. 64%, p=0.0001; OR 3.4, 95% CI 1.7–6.7). Only half as many SIRS-N patients required ICU admission at any given time throughout their hospitalization compared to SIRS-P patients (21%, 17/82 vs. 42%, 113/271, p=0.0006). A trend toward lower 30-day mortality for SIRS-N patients was also observed (5%, 4/82 vs. 11%, 29/271 p=0.1324) (Table 3).
Table 3:
Clinical Course and Outcomes.
| SIRS-N (n = 82) | SIRS-P (n = 271) | P value | |||
|---|---|---|---|---|---|
| ICU Admission During Hospitalization | 17 | 20.7% | 113 | 41.7% | 0.0006 |
| Day 4 success | 71 | 86.6% | 178 | 65.7% | 0.0002 |
| Length of stay, median days (IQR) | 10.5 | (6, 18) | 11 | (7, 22) | 03186 |
| 30-day mortality | 4 | 4.9% | 29 | 10.7% | 0.1324 |
When SIRS-P patients were further grouped by sepsis severity, 30-day mortality rates were 5% (8/179), 10% (5/48), and 36% (16/44), (p<0.0001) for sepsis, severe sepsis, and septic shock patients respectively compared to 5% (4/82) in SIRS-N patients. This is represented graphically in Figure 2 (measured along the secondary Y-axis).
Discussion
The previous consensus definition for sepsis [10] has been frequently applied in clinical trials of sepsis to enroll eligible patients and in clinical practice to identify patients who need prompt initiation of antibiotic therapy. However, the SIRS-based definition of sepsis has previously been criticized for oversensitivity as a number of non-infectious processes can elicit a SIRS response (e.g. trauma, burns) while lacking specificity in some populations since not all patients with an infection experience sepsis [12]. These limitations along with a growing understanding of sepsis pathophysiology eventually led to the updated Sepsis-3 consensus definitions [8]. In our study, we evaluated 353 patients with S. aureus bacteremia and found that 23% did not meet the previous sepsis definition threshold of two SIRS criteria at infection onset. Less than 30% of SIRS-N patients exhibited any abnormal values as defined for each physiologic and laboratory parameter used in SIRS criteria for sepsis; white blood cell count, heart rate or respiratory rate were more frequently abnormal than blood pressure or temperature measurements. Regardless of whether SIRS criteria for sepsis were met, up to two-thirds of all study patients were still admitted with an infection-related diagnosis. Importantly, the failure to meet SIRS criteria did not appear to adversely impact the time to initiation of effective antibiotic therapy, a key factor known to affect outcomes in SAB [13,14]. This seems to suggest that clinical care was largely driven by clinician assessment, which may have included a variety of additional parameters beyond those captured by SIRS criteria alone.
As expected, the subtle clinical signs of inflammatory response correlated with a relatively blunted pro- and anti-inflammatory cytokine response at onset in the SIRS-N compared to SIRS-P patients. The pro-inflammatory cytokines selectively measured in our patients (TNF, IL-8, and IL-6) represent some of the key pro-inflammatory mediators involved in what has been referred to as the “cytokine storm”, responsible for the clinical features that characterize sepsis [15]. The difference between SIRS-N and SIRS-P patients was particularly pronounced for IL6 (32.7 vs. 108.7 pg/mL, p<0.0001), consistent with previous studies which have highlighted the role of IL6 as an early marker of infection and severity particularly in complicated S. aureus bloodstream infection [16,17]. Similarly, the anti-inflammatory cytokine (IL-10) response in SIRS-N patients was also significantly less robust at onset compared to SIRS-P patients (16.3 vs. 52.5, p=0.0001). IL10 is responsible for potentiating the compensatory anti-inflammatory environment that has been termed the “immune-paralysis” phase of sepsis which can occur early and simultaneously during the pro-inflammatory cytokine storm [18]. Importantly, we have previously published our findings that an early dysregulated balance between proand anti-inflammatory cytokine response as indicated by an elevated IL10/TNF ratio at 72 h after the start of effective therapy is the strongest predictor for persistence and mortality in SAB [19]. This finding was also supported by others who demonstrated that an elevated IL10/TNFα ratio was predictive of mortality in sepsis [20,21]. As shown in this study cohort, the median IL10/TNF ratio was lowest among SIRS-N patients at 1.2 and increased progressively with increasing severity of sepsis to 4.5 among those with septic shock. The initially elevated IL10/TNF ratio normalized to <1 for both SIRS-N and SIRS-P (sepsis and severe sepsis) patients following 72 h of effective therapy while those with septic shock remained elevated at 1.9. The latter corresponded to a 30-day mortality rate of 36% compared to 5–10% for those with ratio of <1.
Conclusion
In conclusion, previous SIRS-based definition of sepsis failed to identify 1 in 5 patients with SAB. SIRS-N patients with SAB present with subtle signs of altered physiologic response indicative of a systemic infection, however this does not appear to adversely impact initial clinical assessment and antibiotic management. This altered clinical presentation of SIRS-N patients corresponds to a relatively blunted but balanced pro- and anti-inflammatory cytokine response reflective of immune competence compared to SIRS-P patients whose cytokine profiles were increasingly predominated by anti-inflammatory response suggestive of immunoparalysis as sepsis severity increased. Accordingly, SIRS-N patients experienced more favorable clinical outcomes compared to SIRS-P patients, which was likely due in part to their balanced immune response. Taken together, our findings support the updated Sepsis-3 definition as life-threatening organ dysfunction caused by a dysregulated host response to infection. Furthermore, these findings highlight the potential role for biomarker-directed immune activating therapy as adjunctive treatment for patients with severe/complicated SAB.
Acknowledgements
We thank Anne Au, Fatima Vasquez-Beltran, Consuelo Hernandez, and Joan Hsu from Clinical Laboratory at Huntington Hospital for retrieving blood specimens; the Clinical Laboratory and Microbiology staff at Los Angeles County-University of Southern California Medical Center, Huntington Hospital, and Keck Hospital for assistance in collection of blood and bacterial specimens; and Multiplex assays were performed in the USC Immune Monitoring Core Facility that is supported in part by the National Cancer Institute Cancer Center Shared Grant award P30CA014089. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
References
- 1.Van Hal SJ, Jensen SO, Vaska VL, Espedido BA, Paterson DL, et al. (2012) Predictors of mortality in Staphylococcus aureus Bacteremia. Clin Microbiol Rev. 25: 362–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ammerlaan H, Seifert H, Harbarth S, Brun-Buisson C, Torres A, et al. (2009) European Practices of Infections with Staphylococcus aureus (SEPIA) Study Group. Adequacy of antimicrobial treatment and outcome of Staphylococcus aureus bacteremia in 9 Western European countries. Clin Infect Dis. 49: 997–1005. [DOI] [PubMed] [Google Scholar]
- 3.Guilarde AO, Turchi MD, Martelli CM, Primo MG (2006) Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. J Hosp Infect. 63: 330–336. [DOI] [PubMed] [Google Scholar]
- 4.Angus DC, Van der Poll T (2013) Severe sepsis and septic shock. N Engl J Med. 369: 2063. [DOI] [PubMed] [Google Scholar]
- 5.Kreisel KM, Stine OC, Johnson JK, Perencevich EN, Shardell MD, et al. (2011) USA300 methicillin-resistant Staphylococcus aureus bacteremia and the risk of severe sepsis: is USA300 methicillin-resistant Staphylococcus aureus associated with more severe infections? Diagn Microbiol Infect Dis. 70: 285–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers ME, Bubeck Wardenburg J (2014) Igniting the fire: Staphylococcus aureus virulence factors in the pathogenesis of sepsis. PLoS Pathog 10: e1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaukonen KM, Bailey M, Pilcher D, Cooper DJ, Bellomo R (2015) Systemic inflammatory response syndrome criteria in defining severe sepsis. N Engl J Med. 372: 1629–1638. [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW (2016) The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harris PA, Taylor R, Thielke R (2009) Research electronic data capture (REDCap)–A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D (2001) SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31: 1250–1256. [DOI] [PubMed] [Google Scholar]
- 11.Soriano A, Martínez JA, Mensa J, Marco F, Almela M, et al. (2000) Pathogenic significance of methicillin resistance for patients with Staphylococcus aureus bacteremia. Clin Infect Dis. 30: 368–373. [DOI] [PubMed] [Google Scholar]
- 12.Vincent JL, Opal SM, Marshall JC, Tracey KJ (2013) Sepsis definitions: time for change. Lancet 381: 774–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lodise TP, McKinnon PS, Swiderski L, Rybak MJ (2003) Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis. 36: 1418–1423. [DOI] [PubMed] [Google Scholar]
- 14.Paul M, Kariv G, Goldberg E, Raskin M, Shaked H (2010) Importance of appropriate empirical antibiotic therapy for methicillin-resistant Staphylococcus aureus bacteraemia. J Antimicrob Chemother 65: 2658–2665. [DOI] [PubMed] [Google Scholar]
- 15.Boomer JS, Green JM, Hotchkiss RS (2014) The changing immune system in sepsis: is individualized immuno-modulatory therapy the answer? Virulence 5: 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damas P, Ledoux D, Nys M, Vrindts Y, De Groote D, (1992) Cytokine serum level during severe sepsis in human IL-6 as a marker of severity. Ann Surg. 215: 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNicholas S, Talento AF, O’Gorman J, Hannan MM, Lynch M (2014) Cytokine responses to Staphylococcus aureus bloodstream infection differ between patient cohorts that have different clinical courses of infection. BMC Infect Dis. 14: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotchkiss RS, Monneret G, Payen D (2013) Sepsis-induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 13: 862–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minejima E, Bensman J, She RC, Mack WJ, Tuan Tran M, et al. (2016) A Dysregulated Balance of Pro-inflammatory and Anti-Inflammatory Host Cytokine Response Early During Therapy Predicts Persistence and Mortality in Staphylococcus aureus Bacteremia. Crit Care Med 44: 671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gogos CA, Drosou E, Bassaris HP, Skoutelis A (2000) Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis 181: 176–180. [DOI] [PubMed] [Google Scholar]
- 21.Van Dissel JT, Van Langevelde P, Westendorp RG, Kwappenberg K, Frölich M (1998) Anti-inflammatory cytokine profile and mortality in febrile patients. Lancet 351: 950–953. [DOI] [PubMed] [Google Scholar]