Abstract
Background
Paromomycin-based topical treatments were shown to be effective in curing cutaneous leishmaniasis (CL) lesions caused by Leishmania major in Tunisia. Cure rates of an index lesion were approximately 80%. As a follow on, we conducted a similar Phase 3 trial in Panama to demonstrate the efficacy of these treatments against New World species. The primary objective was to determine if a combination topical cream (paromomycin-gentamicin) resulted in statistically superior final clinical cure rates of an index lesion compared to a paromomycin alone topical cream for the treatment of CL, primarily caused by Leishmania panamensis.
Methods
We conducted a randomized, double blind, Phase 3 trial of topical creams for the treatment of CL caused by Leishmania spp. Three hundred ninety nine patients with one to ten CL lesions were treated by topical application once daily for 20 days. The primary efficacy endpoint was percentage of subjects with clinical cure of an index lesion confirmed to contain Leishmania with no relapse.
Results
The clinical cure of the index lesion for paromomycin-gentamicin was 79% (95% CI; 72 to 84) and for paromomycin alone was 78% (95% CI; 74 to 87) (p = 0.84). The most common adverse events considered related to study cream application were mild to moderate dermatitis, pain, and pruritus.
Conclusions
Superiority of paromomycin-gentamicin was not demonstrated. However, the approximately 80% cure rates for both topical creams were similar to those demonstrated in Tunisia and previously reported with parenteral antimonials.
Author summary
Leishmaniasis, a neglected parasitic infection transmitted by the bite of a female sand fly, is endemic in 98 countries or territories with approximately 0.7 to 1.2 million cutaneous leishmaniasis (CL) cases occurring each year. In Panama, most of the CL cases are caused by L. panamensis and, the first line of treatment is pentavalent antimony, given parenterally for 20 days. These systemic regimen is associated with toxicities that can limit the patient from receiving a full course of treatment. Alternative therapies are needed particularly for patients with mild disease, no mucosal involvement, no immunosuppression, and for patients living in areas with scarce infrastructure. Therefore, less toxic, non-parenteral new therapies against CL are urgently needed. We conducted a comparative clinical study that evaluated Paromomycin topical creams (Paromomycin alone versus Paromomycin+Gentamicin) for the treatment of cutaneous leishmaniasis (n = 399) in three sites of country. Our study demonstrated the efficacy of these preparations against New World leishmanial species (mostly L. panamensis) with a cure rate close to 80%.
Our trial supports Paromomycin as a viable alternative treatment for CL caused for the New World Leishmania species.
Introduction
Leishmaniasis, a neglected parasitic infection transmitted by the bite of a female sand fly, is endemic in 98 countries or territories with approximately 0.7 to 1.2 million cutaneous leishmaniasis (CL) cases occurring each year [1]. CL results from parasitisation of skin macrophages by Leishmania (L) species and generally presents as a papule that enlarges to a nodule that often ulcerates over 1–3 months [2]. The illness has a variety of skin manifestations including small, dry, crusted lesions; ulcerative lesions that are shallow and circular with well-defined borders and a bed of granulated tissue; and large, deep, mutilating ulcers [3]. There are at least five Leishmania species that cause CL in the Old World and 12 species in the New World [4]. Also, in Panama, leishmaniasis is an important parasitic disease with an average estimated 2,200 new cases of CL reported per year, although this number is likely a 4-fold underestimate due to underreporting [1,5]. Among the cases reported in Panama from 2005–2009 the majority were diagnosed as L. panamensis. L. panamensis typically causes CL lesions [1,6]. However, it does have the potential to progress to mucocutaneous leishmaniasis in approximately 5% of cases [3]. CL can create substantial morbidity due to the continued presence of a skin ulcer and the psychological impact of disfigurement [7].
The first line of treatment for CL in Panama is pentavalent antimony, either meglumine antimoniate or sodium stibogluconate, given parenterally for 20 to 28 days [8]. The cure rates for L. panamensis CL in adults treated with systemic antimony have been reported in the range from 25–93% [9,10]. However, these systemic regimens are associated with toxicities that can limit the patient from receiving a full course of treatment [11]. Alternative therapies are needed particularly for patients with mild disease, no mucosal involvement, and who are not immunocompromised, and for patients living in areas with scarce infrastructure (most CL endemic areas) where laboratory monitoring and trained health personnel needed for correct management of pentavalent antimony (SbV) treatments are limited. Children also represent a large part of the affected population in Panama and in other endemic regions and there is evidence that pediatric patients with CL have a significantly lower response rate to pentavalent antimonials [8,12]. In the registry of leishmaniasis cases from the Ministry of Health-Panama in 2014, 77% of those affected were 19 years of age or younger and 60% were under 10 years of age [8].
Therefore, the availability of a topically applied drug, instead of a parenteral therapy, offers a potentially safer and more easily administered treatment.
In the Phase 3 study in Tunisia, 15% paromomycin-0.5% gentamicin (called WR 279,396) topical cream and 15% paromomycin alone topical cream were equally effective but statistically superior to a vehicle control (paromomycin-gentamicin, paromomycin alone, and vehicle-control final clinical cure rates of 81%, 82% versus 58%, P<0.001, respectively) [13]. However, results from a mouse study suggested gentamicin would provide an added benefit in New World Leishmania species [14]. In addition, a Phase 2 study conducted in Panama showed a trend toward superiority of paromomycin-gentamicin over paromomycin alone against L. panamensis [15]. The results of these studies were supportive of testing the superiority of paromomycin-gentamicin over that of paromomycin alone in the New World CL caused by L. panamensis.
Methods
Ethics statement
The protocol was approved by the Gorgas Institutional Bioethics Committee, the National Committee of Bioethics for Research, Panama and by the Human Research Protections Office, U.S. Army Medical Research and Materiel Command. All patients or their legal representatives provided written informed consent, and minors also provided assent.
Study design
This study was a pivotal Phase 3, randomized, double-blind, two-group trial assessing the efficacy and safety of paromomycin-gentamicin and paromomycin alone topical cream in a hydrophilic vehicle (designed to enhance drug penetration while maintaining high tolerability) in subjects with CL in Panama. A vehicle-control group was not included as it was considered unethical to withhold treatment based on the standard of care in Panama and the results of the Phase 3 Tunisian study, which showed the statistical superiority of paromomycin-gentamicin and paromomycin alone compared with the vehicle-control [13].
This study was conducted between May 2013 and March 2016 at three sites in Panama: Penonomé, Panama City, and Changuinola.
Treatments
The same investigational products were used in Tunisia and in this study. Paromomycin-gentamicin and paromomycin alone in a hydrophilic vehicle were manufactured by Teva Pharmaceuticals USA, Sellersville, Pennsylvania in accordance with Good Manufacturing Practices. For each patient, all lesions (i.e., the index lesion, as defined below, plus non-index lesions) were treated topically once daily for 20 days by a member of the study staff who documented treatment application. (For details of the application procedure, see Supporting information, S2 Appendix.)
Study patients
Study subjects were males or non-pregnant non-lactating females, ages 2 and older, and with 10 or fewer lesions. For each subject, an index lesion was selected with the following characteristics: ulcerative, from 1–5cm in diameter, and confirmed to contain Leishmania parasites via culture or microscopic examination of lesion material. Subjects were otherwise healthy and without clinical evidence of mucosal involvement. Whenever possible, infecting species of Leishmania were determined by polymerase chain reaction (PCR) followed by restriction fragment length polymorphism (RFLP) using the heat shock protein 70 for discrimination of Leishmania species [16] and isoenzyme analysis [multilocus enzyme electrophoresis (MLEE)][17]. Eligibility criteria and study procedures are described in the Supporting information, S2 Appendix.
Endpoints
Efficacy
The primary efficacy endpoint was percentage of subjects with final clinical cure. Final clinical cure was defined as: initial clinical cure (100% re-epithelialization of index lesion by nominal.
Day 63) or initial clinical improvement (greater than 50% re-epithelialization of index lesion by nominal Day 63) followed by 100% re-epithelialization of the index lesion on or before nominal Day 100. In addition, no relapse of index lesion by nominal Day 168.
Key secondary efficacy endpoints were: percentage of subjects with all lesions cured, defined as final clinical cure (as defined above) and cure of all other lesions by nominal Day 100 (100% re-epithelialization of all ulcerated lesions and resolution of all other types of lesions); and median time to initial clinical cure (100% re-epithelialization of the index lesion).
The percentage of subjects meeting criteria for final clinical cure of index lesion was also analyzed by age group and infecting species of Leishmania.
Safety
The safety endpoints were adverse events (AE) including application site reactions (pain, erythema, edema, and vesicles) and increased creatinine and transaminases. Examination of the nasal and oral mucosa was performed at baseline and Days 63, 100, and 168 for evidence of mucosal disease. Evidence of mucosal leishmaniasis was also considered an adverse event.
Statistical analysis
The original sample size was 300 subjects. During the conduct of this trial, new Leishmania species were identified that were not found in the Phase 2 trial previously performed in Panama. Of 149 subjects randomized to the trial for which speciation data were available, 74% of subjects had L. panamensis and the others were split between L. guyanensis and L. braziliensis. Since it was not clear what impact various species may have had on overall treatment effect, it was felt that a more conservative approach to trial power was needed. The sample size of the study was adjusted to 400 total subjects, adding 50 subjects to each study arm to maintain at least 90% power with a two-sided alpha of 0.05 to detect statistically significant superiority of paromomycin-gentamicin over paromomycin alone for L. panamensis patients.
The modified intention-to-treat (mITT) population (N = 399) and the safety population consisted of all subjects who received any administration of investigational product and was used as the primary analytic population for efficacy and safety analyses. The evaluable population (N = 387) included all subjects who received daily doses of investigational product for at least 18 of the total 20 days and did not have missing lesion measurements at Day 63 and 168.
Final clinical cure rates of the index lesion and all lesions (proportions) were compared between the two treatment groups by uncorrected chi-square test using the mITT group.
The Kaplan Meier product-limit method was used to determine the median time to initial clinical cure. The time to initial clinical cure curves were compared using the log-rank test. Within subgroup level two treatment groups were compared by uncorrected chi-square test using the mITT group. A Cochran-Mantel-Haenszel was used to test if there were treatment differences across the subgroup levels.
A two-sided alpha of 0.05 was used to demonstrate statistical significance. No adjustments were made to correct for multiplicity of comparisons of secondary efficacy endpoints. (The complete Statistical Analysis Plan is provided in Supporting information, S3 Appendix).
Results
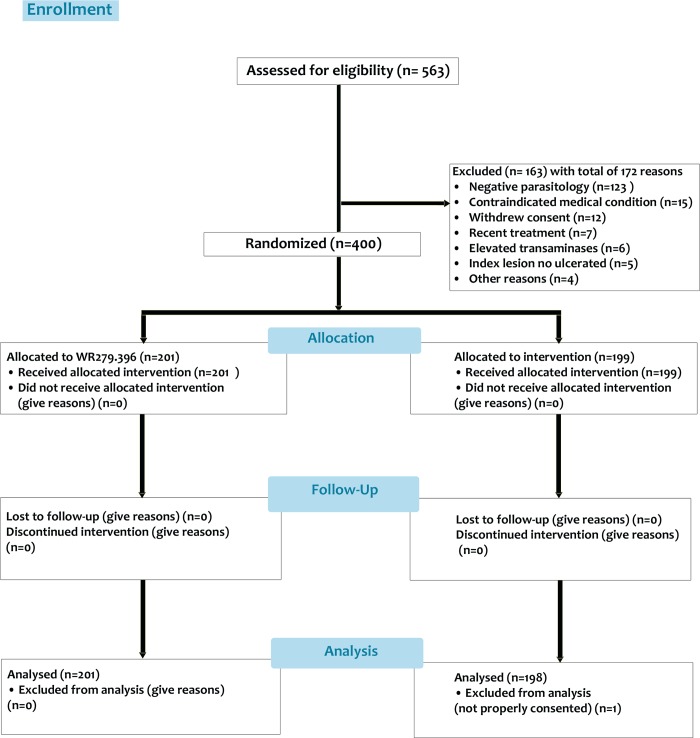
Of 563 subjects screened, 400 were randomized and received an investigational product (Fig 1).
Fig 1. Patient disposition.
A total of 563 patients were consented and screened, 400 were randomized and, 163 were not eligible. One randomized patient (a minor) was later determined to not be properly consented (legal guardian could not provide documentation) and was not included in the analysis.
Patient characteristics are shown in Table 1. There was a slightly higher proportion of males (62.7%) than females and adults constituted 53.9% of patients studied. The average number of lesions per patient was 2.2 with areas ranging from 0.2–1158 mm2. Age of lesions ranged from 10 to 559 days prior to start of treatment. Baseline characteristics were not significantly different between groups (Table 1).
Table 1. Patient and disease characteristics at baseline.
| Characteristic | Paromomycin-Gentamicin N = 201 |
Paromomycin N = 198 |
All Subjects N = 399 |
|---|---|---|---|
| Male Gender: no. (%) | 125 (62) | 125 (63) | 250 (63) |
| Age (years): mean ± SD (range) | 23 ± 17 (2–78) | 24 ± 15 (2–73) | 23 ± 16 (2–78) |
| >17 yrs: no. (%) | 105 (52) | 110 (56) | 215 (54) |
| Total Number of lesions | 417 | 396 | 813 |
| Area of all lesion ulcers (mm2): mean ± SD (range) | 120 ± 146 (0.2–1053) | 121 ± 152 (5.2–1158) | 120 ± 149 (0.2–1158) |
| Lesions per subject: mean ± SD (range) | 2.3 ± 1.7 (1–10) | 2.1 ± 1.6 (1–9) | 2.2 ± 1.7 (1–10) |
| Lesion age (days): mean ± SD (range) | 59.7 ± 53.6 (15–374) | 62.1 ± 57.7 (10–559) | 60.9 ± 55.6 (10–559) |
| Infecting species: no. (%) L. panamensis L. guyanensis L. braziliensis L. naiffi Not identified | 155 (77.1) 36 (17.9) 5 (2.5) 1 (0.5) 4 (2.0) | 146 (73.7) 42 (21.2) 3 (1.5) 0 (0.0) 7 (3.5) | 301 (75.4) 78 (19.5) 8 (2.0) 1 (0.3) 11 (2.8) |
Treatment compliance
Of the 399 patients who received treatment, a total of 16 subjects, 9 in the paromomycin-gentamicin group and 7 in the paromomycin alone Group missed at least 1 day of application of investigational product. Two patients missed treatment due to adverse events of mild and transient hypoacusia or vomiting, neither of which were considered to be related to study cream.
Efficacy
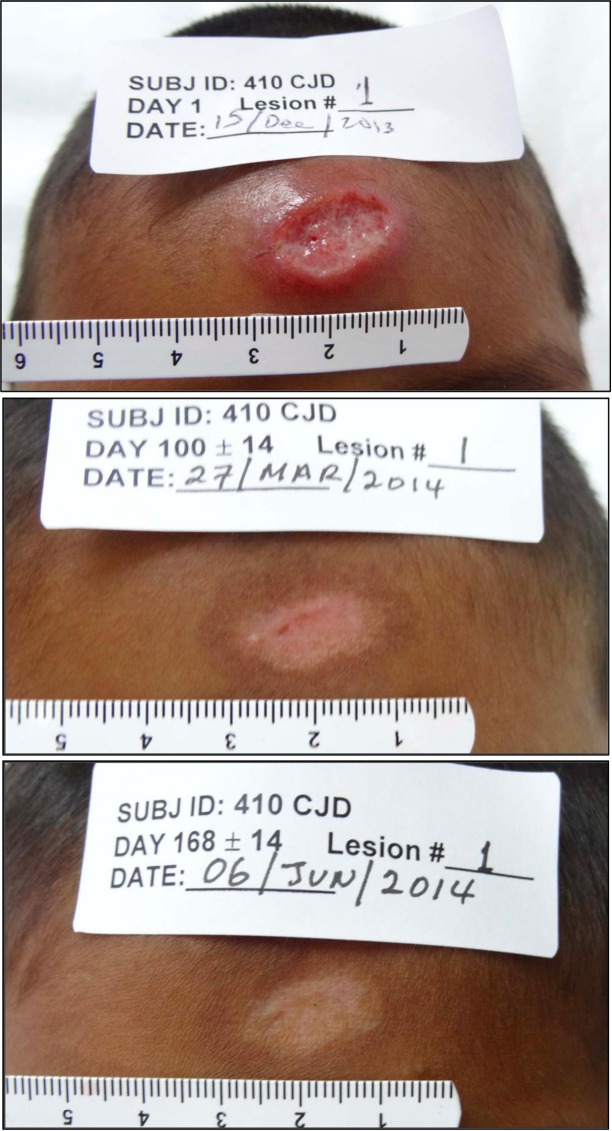
There was no significant difference in the primary efficacy endpoint, final clinical cure rate of an index lesion, between groups (79% vs. 78% of subjects; paromomycin-gentamicin vs. paromomycin alone; p = 0.84, a difference of -0.83% (95% CI -8.93 to 7.27) (Table 2). The evaluable population showed similar results (81% vs. 80% of subjects; paromomycin-gentamicin vs. paromomycin alone; p = 0.94). The typical response of a treated lesion is shown in Fig 2.
Table 2. Efficacy outcomes.
| Outcome | Paromomycin-Gentamicin | Paromomycin Alone | p-value* |
|---|---|---|---|
| Patients with final clinical cure of index lesion (mITT): no./total (%) | 158/201 (78.6)*† | 154/198 (77.8)* | 0.841 |
| Patients with final clinical cure of all lesions (PP): no. /total (%) | 157/195 (80.5) | 154/192 (80.2) | 0.940 |
| Patients with final clinical cure of all lesions (mITT): no. /total (%) | 151/201 (75.1) | 151/198 (76.3) | 0.791 |
| Patients with final clinical cure of all lesions (PP): no. /total (%) | 150/195 (76.9) | 151/192 (78.6) | 0.684 |
| Patients with final clinical cure of index lesion (mITT) by species: no. /total (%) | |||
| L. panamensis | 123/159 (77.4) | 118/153 (77.1) | 0.961 |
| L. guyanensis | 34/42 (81.0) | 29/36 (80.6) | 0.965 |
| L. braziliensis | 2/3 (66.7) | 5/5 (100.0) | 0.168 |
| Patients with final clinical cure of index lesion (mITT) by patient age: no. /total (%) | |||
| Under 12 years | 48/61 (78.7) | 42/46 (91.3) | 0.077 |
| 12 to 17 years | 31/35 (88.6) | 32/42 (76.2) | 0.161 |
| Over 17 years | 79/105 (75.2) | 80/110 (72.7) | 0.675 |
| Reasons for early discontinuation | N = 201 | N = 199** | |
| Improperly consented minor | 0 (0.0) | 1 (0.5) | |
| Lost to follow-up | 1 (0.5) | 0 (0.0) | |
| Withdrawal of consent by subject | 3 (1.5) | 4 (2.0) | |
| Withdrawal of subject by investigator | 1 (0.5) | 0 (0.0) | |
| Continued presence of disease | 13 (6.5) | 20 (10.1) | |
| Worsening of disease | 4 (2.0) | 4 (2.0) | |
| Recurrence of disease | 12 (6.0) | 7 (3.5) | |
| Reasons for clinical failure (documented failure) | N = 43 | N = 44 | |
| No initial clinical cure by Day 63 | 10 (23.3) | 16 (36.4) | |
| No cure by Day 100 | 4 (9.3) | 7 (15.9) | |
| Disease recurred | 20 (46.5) | 12 (27.3) | |
| No clinical response at Day 49 | 1 (2.3) | ||
| Disease worsened at Day 35 | 1 (2.3) | ||
| Other reasons for clinical failure | |||
| Withdrawn–due to new multiple lesions | 3 (7.0) | ||
| Withdrawn—developed mucosal disease | 1 (2.3) | 3 (6.8) | |
| Withdrew consent | 3 (7.0) | 4 (9.1) | |
| Withdrawn by investigator | 1 (2.3) | ||
| Lost to follow-up | 1 (2.3) |
* Uncorrected chi-square test (2-sided).
** Improperly consented minor was not included in any analyses.
Fig 2. Photographs of lesion #1 in a subject treated with paromomycin over time (baseline, day 100 and day 168).
Of the 87 subjects in both groups who were final clinical failures of the index lesion, 71 (34 in the paromomycin-gentamicin group and 37 in the paromomycin alone group) had documented clinical failure (disease persistence, disease worsening, or disease relapse), and the other 16 were failures due to withdrawal of consent or lost to follow-up without meeting the protocol definition of failure to cure. The primary reasons for failure in both groups were lack of initial clinical response of disease by Day 100 (49 subjects) or recurrence of disease (32 subjects). There was no significant difference in the percentage of subjects with all lesions cured between groups in the mITT (75% vs. 76%; paromomycin-gentamicin vs. paromomycin alone; p = 0.79, a difference of 1.14% (95% CI -7.28 to 9.55) nor in the evaluable population (77% vs. 79%; paromomycin-gentamicin vs. paromomycin alone; p = 0.68, a difference of 1.72% (95% CI -6.56 to 10.00). Although the final clinical cure rate was the same between the two treatment groups, the median time to initial clinical cure of the index lesion was 36 days (95% CI 35 to 49) for paromomycin-gentamicin and 48 days (95% CI 36 to 49) for paromomycin alone. However, this difference was not significant (p = 0.22) (Table 2).
There were no significant differences in the final clinical cure rate of the index lesion by age group, under 12 years; 12–17 years; and over 17 years (p = 0.92).
Of 399 subjects, 398 were typed using PCR/RFLP. Of those, a total of 312 (78%) of subjects were identified as infected with L. panamensis, 78 (20%) with L. guyanensis, and 8 (2%) with L. braziliensis. There was no significance difference in the final clinical cure rate between treatment groups for any of the species identified (Table 2).
Safety
All of the AEs were either mild (98.5%) or moderate (1.5%) in severity and none were severe or life-threatening. Adverse events that occurred with at least 5% incidence in any group are shown in Table 3. Application site reactions constituted the majority of AEs considered at least possibly related to investigational products. In order of frequency, these were application site dermatitis, pruritus, erythema, pain, and burning sensation.
Table 3. Adverse events occurring in greater than 5% of patients in any study group.
| Adverse Event Preferred Term | WR 279,396 (n = 201) |
Paromomycin (n = 198) |
|---|---|---|
| Contact Dermatitis | 90 (44.8) | 89 (44.9) |
| Nasopharyngitis | 90 (44.8) | 82 (41.4) |
| Pruritus | 54 (26.9) | 50 (25.3) |
| Headache | 28 (13.9) | 19 (9.6) |
| Lymphangitis | 23 (11.4) | 14 (7.1) |
| Skin erosion | 22 (10.9) | 11 (5.6) |
| Bacterial Superinfection | 19 (9.5) | 19 (9.6) |
| Application site injury | 19 (9.5) | 34 (17.2) |
| Application site dermatitis | 19 (9.5) | 13 (6.6) |
| Arthropod bite | 17 (8.5) | 14 (7.1) |
| Application site pain | 14 (7.0) | 16 (8.1) |
| Rhinitis | 12 (6.0) | 13 (6.6) |
| Application site pruritus | 11 (5.5) | 8 (4.0) |
| Folliculitis | 7 (3.5) | 13 (6.6) |
| Lymphadenopathy | 6 (3.0) | 10 (5.1) |
Eleven subjects (2.8%, 95% CI 1.1–4.4) developed nasal mucosal lesions that were positive for Leishmania by histopathology, culture, or PCR. Four of these cases were in the paromomycin-gentamicin group and 7 were in the paromomycin alone group. Mucosal lesions were mild in all cases, with erythema and superficial ulcerations in most of the patients. No septal perforation or nasal deformity was detected. In 9 of the 11 cases, subjects were treated with meglumine antimoniate and all nasal lesions resolved. The other two subjects were lost to follow up.
Discussion
Neither the primary nor secondary efficacy endpoints provided evidence for the superiority of paromomycin-gentamicin over paromomycin alone for the treatment of CL in Panama. Rather, the results of this study mirror the results of the Phase 3 study in Tunisia, which demonstrated virtually identical final clinical cure rates for both topical creams. Phase 3 clinical trial results against epidemiologically important Old World and New World species make it clear that the addition of gentamicin to paromomycin topical cream provides no additional clinical benefit [13].
Children had a favorable response to the paromomycin investigational products. The percentage of subjects under 12 years and 12 to 17 years of age who achieved final clinical cure rate of the index lesion was 84% and 82%, respectively. A high cure rate in children with a topical treatment is important because of poor adherence and higher rates of metabolic elimination with parenteral antimonials both of which contribute to lower cure rates [18].
Neither of the two topical creams was associated with any serious or severe systemic toxicity. Specifically, no aminoglycoside-related nephrotoxicity or ototoxicity was observed. Application site reactions associated with the topical therapy and contact dermatitis related to the tape dressing were common. However, no patient had to withdraw from the study because of these local adverse events. These findings were in agreement with those reported in other studies [13].
The significance of mucosal lesions detected in eleven subjects is unclear, but it warrants evaluation of the nasal mucosa in all patients with New World CL caused by the subgenus Viannia, in order to consider whether systemic therapy is appropriate. To date there is no conclusive evidence that systemic treatment of CL prevents the development of mucosal leishmaniasis [19,20].
This trial demonstrates that topical therapy with a paramomycin-based cream offers a potential alternative to the current standard of care for the treatment of CL in Panama. A topical therapy offers possible advantages over systemic treatments, such as pentavalent antimonials, and might be an alternative to treat CL in children or in settings where parenteral therapy is not feasible. Topical treatment could also be studied in future trials as part of combination treatment with oral or parenteral agents. A topical treatment for uncomplicated CL is also of great interest in the United States, where current treatment options all have suboptimal risk/benefit profiles for this disease.
Supporting information
(DOC)
(PDF)
(DOCX)
(PDF)
Acknowledgments
We acknowledge the Regional Directorates of Health of Coclé, and Bocas del Toro; Juan Mendez and Sheila Peel at the Leishmaniasis Diagnostic Laboratory (LDL), Walter Reed Army Institute of Research (WRAIR), Silver Spring, Maryland, US for performance of MLEE, study team members at Gorgas, Penonomé and Changuinola: Jessica Rodriguez, Vanessa Almengor, Betsy Ortiz, José Antonio Suárez, Robert Samuels, Elvia Valdés, Zeuz Capitán-Barrios, Fenny Abrego, Ismael Arroyo, José Batista, José Calzada, Azael Saldaña, Kadir González, Aracelys Miranda, Vanessa Pineda, Gerald Darling, Betsy Tunón, Xochitl Rugama, Luis Lezcano, Jorge Tuñón, Maurice Coronado, Laurencio Aguilar and Franz Barnes; Dianne Urittia and Kerime Cose for clinical monitoring; and Senior Leaders of USAMMDA and USAMRMC for their support. To the National Research System (SNI-SENACYT-PANAMA) for supporting Dr. JM Pascale. Above all, we acknowledge the participation of the volunteers and parents who allowed their children to participate in the study.
Data Availability
All relevant data are within the manuscript and its Supporting Information files. In addition, there is a Clinical Trial number where further information can be obtained.
Funding Statement
This study was funded by the U.S. Army Medical Materiel Development Activity (USAMMDA), U.S. Army Medical Research and Materiel Command. USAMMDA also provided the investigational product for the study. The investigators have adhered to the policies for protection of human subjects as prescribed in Army Regulation 70-25. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J et al. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS One. 2012;7(5):e35671 10.1371/journal.pone.0035671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hepburn NC. Cutaneous leishmaniasis: an overview. J Postgrad Med. 2003; 49(1):50–54. [DOI] [PubMed] [Google Scholar]
- 3.Gontijo B, de Carvalho Mde L. American cutaneous leishmaniasis (Article in Portuguese). Rev Soc Bras Med Trop. 2003; 36(1):71–80. [DOI] [PubMed] [Google Scholar]
- 4.Control of the leishmaniasis: WHO technical report series no. 949. Geneva: World Health Organization, 2010. [Google Scholar]
- 5.Chaves LF, Calzada JE, Valderrama A, Saldana A. Cutaneous leishmaniasis and sand fly fluctuations are associated with el Niño in Panamá. PLoS Negl Trop Dis. 2014. October 2;8(10):e3210 10.1371/journal.pntd.0003210 eCollection 2014 Oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miranda A, Carrasco R, Paz H, Pascale J, Samudio F, Saldana A, et al. Molecular epidemiology of American tegumentary leishmaniasis in Panama. Am J Trop Med Hyg. 2009; 81(4):565–571. 10.4269/ajtmh.2009.08-0265 [DOI] [PubMed] [Google Scholar]
- 7.Reithinger R, Dujardin J, Louzir H, Pirmez C, Alexander B, Brooker S, et al. Cutaneous leishmaniasis. Lancet Infect Dis. 2007; 7(9):581–596. 10.1016/S1473-3099(07)70209-8 [DOI] [PubMed] [Google Scholar]
- 8.Guía para el abordaje integral de la leishmaniasis en Panamá, 2015. Ministry of Health of Panama (MINSA), Social Security Hospital (CSS) and Panamerican Health Organization (PAHO). [Google Scholar]
- 9.Soto J, Toledo J, Vega J, Berman J. Short Report: Efficacy of Pentavalent Antimony for Treatment or Colombian Cutaneous Leishmaniasis. Am J Trop Med Hyg. 2005; 72(4):421–422. [PubMed] [Google Scholar]
- 10.Soto J, Soto P. Current situation and future of antileishmanial therapy in Colombia. Biomédica 2006; 26(Supl.1):194–206. Review. Spanish. PMID: 15827279 [PubMed] [Google Scholar]
- 11.Gasser R Jr, Magill A, Oster C, Franke E, Grögl M, Berman J. Pancreatitis induced by pentavalent antimonial agents during treatment of leishmaniasis. Clin Infect Dis. 1994;18(1):83–90. [DOI] [PubMed] [Google Scholar]
- 12.Palacios R, Osorio L, Grajalew L, Ochoa M. Treatment failure in children in a randomized clinical trial with 10 and 20 days of meglumine antimonate for cutaneous leishmaniasis due to Leishmania viannia species. Am J Trop Med Hyg. 2001; 64 (3–4):187–193. . [DOI] [PubMed] [Google Scholar]
- 13.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, et al. Topical paromomycin with or without gentamicin for cutaneous leishmaniasis. N Engl J Med. 2013;369:524–532. 10.1056/NEJMoa1202657 . [DOI] [PubMed] [Google Scholar]
- 14.Grogl M, Schuster BG, Ellis WY, Berman J. Successful topical treatment of murine cutaneous leishmaniasis with a combination of paromomycin (Aminosidine) and gentamicin. J Parasitol. 1999; 85(2):354–359. . [PubMed] [Google Scholar]
- 15.Sosa N, Capitán Z, Nieto J, Nieto M, Calzada J, Paz H, et al. Randomized, Double-blinded, Phase 2 Trial of WR 279,396 (Paromomycin and Gentamicin) for Cutaneous Leishmaniasis in Panama. Am J Trop Med Hyg. 2013; 89(3):557–563. 10.4269/ajtmh.12-0736 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montalvo AM, Fraga J, Maes I, Dujardin J, Van der Auwera G, et al. Three new sensitive and specific heat-shock protein 70 PCRs for global Leishmania species identification. Eur J Clin Microbiol Infect Dis. 2012;31:1453–1461. 10.1007/s10096-011-1463-z . [DOI] [PubMed] [Google Scholar]
- 17.Thomaz-Soccol V, Velez ID, Pratlong F, Agudelos S, Lanotte G, Rioux J, et al. Enzymatic polymorphism and phylogenetic relationships in Leishmania Ross, 1903 (Sarcomastigophora: Kinetoplastida): a case study in Colombia. Syst Parasitol. 2000;46(1):59–68. . [DOI] [PubMed] [Google Scholar]
- 18.Cruz A, Rainey P, Herwaldt B, Stagni G, Palacios R, Trujillo R, et al. Pharmacokinetics of antimony in children treated for leishmaniasis with meglumine antimoniate. J Infect Dis. 2007;195(4):602–608. 10.1086/510860 . [DOI] [PubMed] [Google Scholar]
- 19.Machado-Coelho G, Caiaffa W, Genaro O, Magalhães P, Mayrink W. Risk factors for mucosal manifestation of American cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 2005; 99(1):55–61. 10.1016/j.trstmh.2003.08.001 . [DOI] [PubMed] [Google Scholar]
- 20.Blum J, Lockwood D, Visser L, Harms G, Bailey M, Caumes E, et al. Local or systemic treatment for New World cutaneous leismaniasis? Re-evaluating the evidence for the risk of mucosal leishmanisis. Int Health. 2012; 4(3):153–163 10.1016/j.inhe.2012.06.004 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(PDF)
(DOCX)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files. In addition, there is a Clinical Trial number where further information can be obtained.