Abstract
Background:
Inadequate response to infliximab [IFX] therapy in Crohn’s disease [CD] may necessitate dose intensification. We evaluated safety and efficacy of high-dose IFX [HD IFX] [greater than 10mg/kg every 8 weeks] in CD and characterized predictors of response to HD IFX intensification.
Methods:
Electronic medical records were queried for CD patients between 2010 and 2012 who received HD IFX and were reviewed for history, medications, laboratory data, efficacy, and safety.
Results:
In all, 86 patients received HD IFX for CD at doses between 10 and 22.5mg/kg every 4 to 7 weeks. In early HD IFX therapy [week 1–16], 25.8% and 59.1% experienced full and partial response, respectively. In later HD IFX therapy [week 38–100], 27.9% and 34.4% experienced full and partial response, respectively. Median serum IFX levels increased from 1.7 to 7.3µ/mL [p = 0.017], and median C-reactive protein [CRP] values decreased from 20.5 at baseline to 4.7mg/L after 16 weeks [p < 0.001]. Baseline CRP values were significantly elevated in the group that responded at 1–16 weeks compared with nonresponders [22.0 vs 3.5mg/L, p < 0.01]. HD IFX therapy was discontinued in 26% and 7.3% of patients for inadequate response and adverse events, respectively. Eleven cases of infection required hospitalization for a serious infection rate of 7.41 events per 100 patient-years.
Conclusions:
HD IFX therapy may benefit CD patients who have failed standard doses of IFX. HD IFX therapy may be associated with more serious adverse events compared with standard dosing. Baseline CRP value may predict clinical response to HD IFX.
Keywords: Crohn’s disease, infliximab, biologic therapy, anti-TNF, loss of response, dose intensification
1. Introduction
Infliximab [IFX] is a monoclonal antibody against tumor necrosis factor alpha [TNF] used to induce and maintain remission in Crohn’s disease [CD]. Failure to initially respond to IFX therapy, known as primary non-response, has been noted to occur in as many as 29–41% of patients with luminal CD1–2 and 31% of patients with fistulizing CD.3 The loss of a previously achieved clinical response, known as secondary non-response, occurs by week 54 in 61% of patients receiving IFX at 5mg/kg every 8 weeks and in 42% of patients receiving 10mg/kg every 8 weeks.1
One strategy for addressing primary and secondary non-response to IFX is to escalate therapy either by increasing the dosage administered or decreasing the interval between infusions. Studies examining dose escalation have demonstrated its efficacy in regaining clinical response in non-responders and it has been suggested that IFX intensification yields more quality-adjusted life-years compared with switching to adalimumab.4 Retrospective studies have shown dose escalation response rates of 66–96% among all patients who have lost response to doses of 5mg/kg every 8 weeks,5–7 and one study observed the response rate to IFX escalation to be 88% in patients without antibodies to infliximab [ATI] compared with 33% among those with ATIs.8 The sustained response rate to IFX intensification at 12 months is estimated to be 47%.7
Current literature examining IFX dose escalation in CD has largely focussed on dose doubling from the initial regimen of 5mg/kg every 8 weeks to 10mg/kg every 8 weeks or 5mg/kg every 4 weeks.5–7 However, a significant proportion of patients remain symptomatic despite dosing at these levels, and there are limited data on the safety and efficacy of IFX dosing above 10mg/kg every 8 weeks or equivalent in CD. Therefore, we evaluated the efficacy, safety, and predictors of response among a group of CD patients who received ‘high-dose IFX’ [HD IFX] therapy, which included IFX at doses equal to or greater than 10mg/kg every 7 weeks as maintenance therapy.
2. Methods
2.1 Study design and patients
We queried the electronic medical record [EPIC] to identify patients with a diagnosis of Crohn’s disease according to International Classification of Diseases [ICD-9] 555.x, who received IFX treatment between January 2010 and December 2012 at the outpatient infusion suite at the Faculty Practice Associates at Mount Sinai Medical Center, New York, NY. Patients whose primary gastroenterologists were outside the institution, who therefore had no clinical documentation in EPIC, were excluded. The remaining medical records were reviewed to stratify patients according to IFX dose. This study was approved by the Mount Sinai Institutional Review Board with a waiver of consent.
Standard dose infliximab [SD IFX] was defined as any regimen delivering less than or equal to 10mg/kg every 8 weeks, including 5mg/kg every 4 weeks. High-dose infliximab was defined as any regimen that delivered IFX at an average rate higher than 10mg/kg every 8 weeks, such as 10mg/kg every 4 to 7 weeks, or 15 to 22.5mg/kg every 4 to 8 weeks. The HD IFX start date was defined as the day of the first infusion of an HD IFX regimen.
Clinical evaluation included age at diagnosis and at HD IFX initiation, gender, disease location, phenotype, severity, and prior and concurrent medication use. Disease severity was assessed at HD IFX initiation, based on clinician description of symptoms, and subsequently categorized according to the American College of Gastroenterology guidelines as asymptomatic [remission], steroid dependent, mild-to-moderate, moderate-to-severe, or severe-to-fulminant.9 The decision to escalate to HD IFX therapy was made by the treating physician’s clinical judgment on whether the patient’s current therapy failed to provide adequate therapeutic response. An IFX hiatus was pre-defined as any period of 24 or more weeks without an IFX infusion after previously receiving IFX maintenance therapy, as a conservative estimate of IFX clearance. Based upon available data, clinical response was determined by the physician’s assessment of symptoms at early [1–16 weeks] and late [38–100 weeks] time periods from initiation as being either full, partial, or none, indicating complete, incomplete, or lack of resolution of symptoms, respectively. Treatment failure was defined as the discontinuation of HD IFX therapy due to inadequate clinical response or due to an adverse event.
Baseline serum IFX levels, titers of antibodies to infliximab [ATI], C-reactive protein [CRP] values, erythrocyte sedimentation rate [ESR], complete blood count, and metabolic panel were gathered from laboratory measurements immediately prior to the first HD IFX infusion. For all laboratory tests except serum IFX values, follow-up values were gathered from the first available measurement after 14 weeks of HD IFX therapy or, if unavailable, the most recent measurement up to 14 weeks. Follow-up values for serum IFX measurements were gathered from the first available measurement after starting HD IFX therapy, including those before 14 weeks. Serum IFX and ATI assays were performed by Prometheus Laboratories [San Diego, CA], and consisted of either a solid-phase enzyme-linked immunosorbent assay [ELISA] with lower limit of quantification of 1.4 µg/mL for IFX and 1.69 µg/mL for ATI, or by homogenous mobility shift assay [HMSA] with lower limit of quantification of 1.0 µg/mL for IFX and 0.036 µg/mL for ATI.10 A therapeutic serum IFX level was defined as greater than or equal to 3.0µ/mL.11
Adverse events including infectious, autoimmune, and neoplastic events, as well as instances of new-onset heart failure or demyelinating disease that occurred while a patient received HD IFX, were recorded. Infusion reactions were categorized by severity as outlined by Cheifetz et al.12
2.2 Statistical analysis
Patients were divided according to those with and without clinical responses at the early and late time periods for further analysis. Survival analysis was conducted using length of HD IFX treatment with treatment failure as an endpoint. Adverse event rates were calculated by dividing the number of events by the total amount of time that patients received HD IFX, for which outpatient follow-up data were available.
Continuous variables were compared using a two-tailed Student’s t-test. Logarithmic transformation was utilized as appropriate. Categorical variables were compared using Fisher’s exact test or the chi-square test, as appropriate. Kaplan–Meier analysis with log-rank test was used to compare survival among groups with treatment failure as the endpoint. Statistical analysis was completed using Medcalc software [Mariakerke, Belgium]. A two-tailed p-value of < 0.05 was considered significant.
3. Results
3.1 Patients
After the screening and exclusion criteria, a total of 318 patients receiving IFX for CD were identified [Figure 1]. Of these, 86 [27%] received HD IFX. Baseline characteristics are shown in Table 1. The median age at diagnosis was 16 years and the median age at initiation of HD IFX was 28.6 years. There was a slight predominance of females at 52.3% in this population. The majority of patients had both small and large bowel disease, a history of penetrating or penetrating with fibrostenotic symptoms, and many had at least one prior hospital admission for CD. All but one patient exhibited either moderate-to-severe or steroid-dependent disease severity. Of patients with available data, receiving thiopurines, 19 of 25 patients on 6-mercaptopurine were dosed at > 1.0mg/kg and 4 of 4 patients on azathioprine were dosed at > 2.5mg/kg at the time of HD IFX initiation. Among all the patients, 49% had one or more previous exposures to IFX with at least a 24-week hiatus, whereas the remaining 51% had been receiving their first SD IFX induction or maintenance regimen continuously when the decision was made to escalate to HD IFX therapy.
Figure 1.
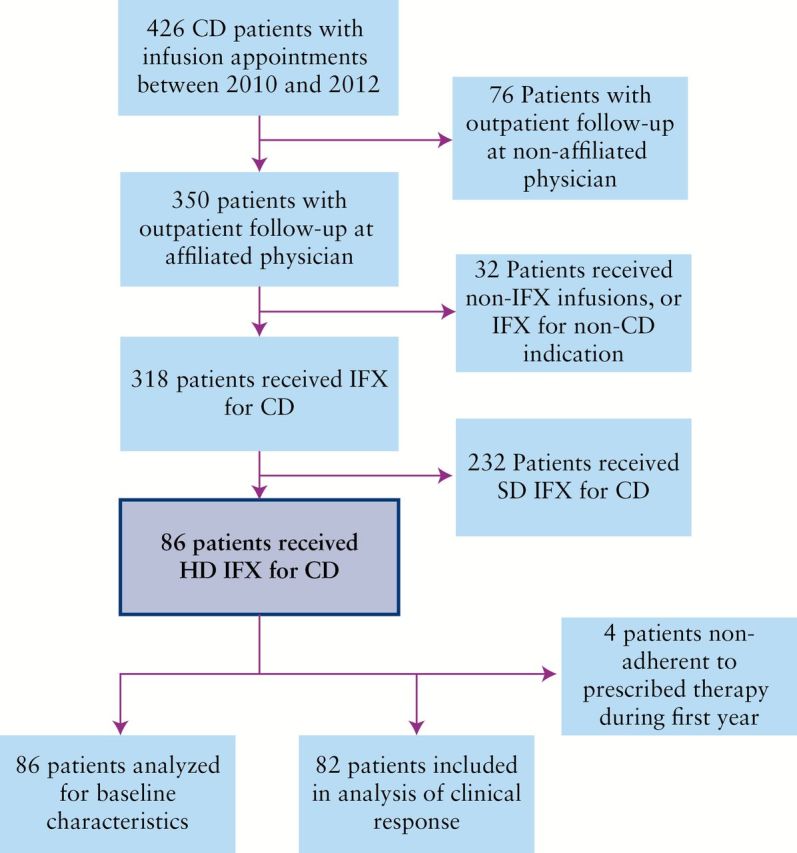
Patient selection process.
Table 1.
Demographics and disease characteristics of HD IFX patients.
| n = 86 | n | % | n | % | |
|---|---|---|---|---|---|
| Age at diagnosis, [median years ±SD] | 16 | ±10.8 kg/m2 | Medical hospitalizations | ||
| Age at HD IFX start, [median years ±SD] | 28.6 | ±12.0 kg/m2 | No prior hospitalizations | 46 | 53.5% |
| BMI, [median kg/m2 ±SD] | 20.39 | ±5.22 kg/m2 | 1 prior hospitalization | 16 | 18.6% |
| Female | 45 | 52.3% | 2 prior hospitalizations | 13 | 15.1% |
| 3+ prior hospitalizations | 11 | 12.8% | |||
| Smokinga | Surgical hospitalizations | ||||
| Never | 57 | 66.3% | No prior hospitalizations | 47 | 54.7% |
| Former | 12 | 14.0% | 1 prior hospitalization | 17 | 19.8% |
| Current | 3 | 3.5% | 2 prior hospitalizations | 6 | 7.0% |
| Locationa | 3+ prior hospitalizations | 16 | 18.6% | ||
| Small bowel only | 13 | 15.1% | Prior medication usage | ||
| Large bowel only | 21 | 24.4% | Any immunomodulator | 74 | 86.1% |
| Small and large bowel | 51 | 59.3% | 6MP or azathioprine | 70 | 81.4% |
| Any upper involvement | 11 | 12.8% | Methotrexate | 21 | 24.4% |
| Any perianal involvement | 41 | 47.7% | Other immunomodulatore | 2 | 2.3% |
| Phenotype | |||||
| Inflammatory | 33 | 38.4% | Any non-IFX biologic agent | 25 | 29.1% |
| Penetrating | 36 | 41.9% | Adalimumab | 19 | 22.1% |
| Fibrostenotic | 6 | 7.0% | Certolizumab pegol | 8 | 9.3% |
| Penetrating, fibrostenotic | 11 | 12.8% | Other non-IFX biologic agentf | 4 | 4.7% |
| Disease severitya | |||||
| Asymptomatic | 1 | 1.2% | Prior IFX treatment with Hiatus > 24 weeks |
42 | 48.8% |
| Steroid-dependent | 4 | 4.7% | Concomitant medication usage | ||
| Mild-moderateb | 0 | 0.0% | 6MP or azathrioprine | 38 | 44.2% |
| Moderate-severec | 77 | 89.5% | Methotrexate | 4 | 4.7% |
| Severe-fulminantd | 0 | 0.0% | Other immunomodulatord | 0 | 0% |
HD IFX, high-dose infliximab; SD, standard deviation; 6MP, 6-mercaptopurine.
aItems for which not all patients’ data were available.
bMild-moderate: ambulatory, tolerates oral alimentation, without manifestations of dehydration, toxicity, abdominal tenderness, painful mass, obstruction, or >10% weight loss.
cModerate-severe: failed to respond to treatment for mild-moderate disease, with fevers, significant weight loss, abdominal pain or tenderness, intermittent nausea or vomiting, or significant anemia.
dSevere-fulminant: persisting symptoms despite the introduction of steroids as outpatients or with high fever, persistent vomiting, evidence of intestinal obstruction, rebound tenderness, cachexia, or evidence of an abscess.
eOther immunomodulators included cyclosporine, tacrolimus.
fOther non-IFX biologics included abatacept, natalizumab, vedolizumab.
3.2 High-dose IFX therapy
At the time of escalation to an HD IFX regimen, 13 patients [15%] were receiving one of the first three induction doses to an SD IFX regimen; 57 patients [66%] were receiving SD IFX maintenance treatment; 6 patients [7%] were receiving non-IFX biologic therapy [adalimumab, certolizumab pegol, or clinical trial]; and 7 [8%] patients were receiving non-biologic treatment [steroids or immunomodulators only]. The context of high-dose escalation was unknown in 3 patients. All patients receiving non-IFX therapy at the time of HD IFX escalation had received SD IFX in the past before switching to the non-IFX therapy. All patients started on HD IFX were intended to continue with HD IFX maintenance therapy.
The initial and maximum dosage regimens of HD IFX prescribed are outlined in Table 2. Of the 86 patients, 57 [66%] were maintained on the initial HD IFX regimen throughout the time of data collection; 22 patients [25%] underwent one subsequent escalation, 14 patients [16%] underwent two subsequent escalations, and 1 patient [1%] underwent three subsequent escalations. The highest prescribed dose of IFX was 22.5mg/kg every 4 weeks. The median time from the first HD IFX escalation to subsequent escalations was 33.5 weeks. A total of 42 patients [48.9%] received combination immunomodulator therapy such as 6-mercaptopurine, azathioprine, or methotrexate at the initiation of HD IFX therapy. Seven patients [8.1%] were receiving systemic corticosteroids at the time of HD IFX escalation. Four patients were excluded from the analysis of clinical response due to non-adherence to their prescribed HD IFX regimens within the first year of being prescribed treatment.
Table 2.
Initial and maximum HD IFX regimens prescribed.
| n = 86 | Interval | Initial delivery prescribed | Maximum delivery prescribed | ||
|---|---|---|---|---|---|
| Dose | n | % | n | % | |
| 10mg/kg | 7 weeks | 8 | 9.3% | 6 | 7.0% |
| 6 weeks | 31 | 36.1% | 21 | 24.4% | |
| 5 weeks | 2 | 2.3% | 2 | 2.3% | |
| 4 weeks | 33 | 38.4% | 29 | 33.7% | |
| 15mg/kg | 8 weeks | 7 | 8.1% | 4 | 4.7% |
| 7 weeks | 1 | 1.2% | 1 | 1.2% | |
| 6 weeks | 1 | 1.2% | 2 | 2.3% | |
| 4 weeks | 2 | 2.3% | 6 | 7.0% | |
| 20mg/kg | 8 weeks | 0 | 0.0% | 1 | 1.2% |
| 6 weeks | 1 | 1.2% | 1 | 1.2% | |
| 4 weeks | 0 | 0.0% | 12 | 14.0% | |
| 22.5mg/kg | 4 weeks | 0 | 0.0% | 1 | 1.2% |
HD IFX, high dose infliximab
3.2 Clinical response and duration of treatment
At the early time period [1–16 weeks from initiation], a total of 66 patients had available data to assess clinical response; 17 patients [25.8%] responded fully, 39 patients [59.1%] responded partially, and 10 patients [15.2%] had no response to HD IFX treatment. At the late time period [38–100 weeks] a total of 61 patients had available data, where 17 patients [27.9%] responded fully, 21 patients [34.4%] responded partially, and 23 patients [37.7%] either had no response or had discontinued treatment. All but 1 of the 7 patients initially receiving systemic corticosteroids were successfully tapered from steroids while receiving HD IFX therapy after a median of 13.2 weeks, and remained steroid-free for the duration of follow-up.
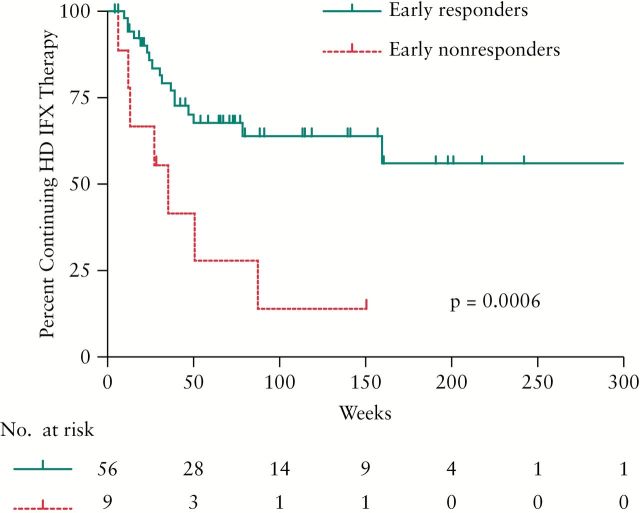
At the time of data collection, 37 of 82 patients [45.1%] were still receiving HD IFX therapy for a median of 113.4 weeks; 27 patients [32.9%] discontinued HD IFX due to treatment failure after a median of 31.3 weeks; and of these, 21 [25.6%] were due to inadequate response and 6 [7.3%] were due to adverse events or infusion reactions. HD IFX was discontinued in three patients with undetectable IFX levels and positive ATI, and in one patient who remained symptomatic despite adequate serum IFX levels. The time until treatment failure among patients with and without early clinical response is shown in Figure 2. Patients who captured response during the early time period had a statistically longer duration of treatment compared with those who had no response [p = 0.0006]. Six patients [7.3%] were successfully de-escalated to SD IFX therapy, and 12 patients [14.6%] discontinued treatment due to other factors [e.g. switching providers, loss to follow-up, insurance].
Figure 2.
Time to treatment failure. Kaplan–Meier survival analysis showing time to treatment failure due to inadequate response or adverse events among early clinical responders vs non-responders.
3.3 Laboratory markers of response
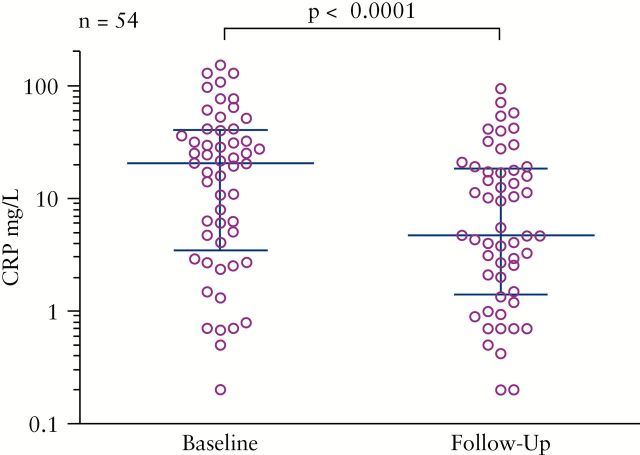
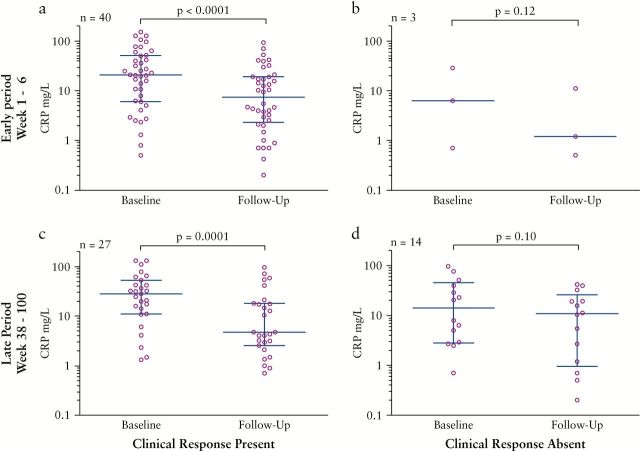
CRP values were available for 54 patients; baseline values were drawn immediately before the initiation of HD IFX therapy [week 0] and follow-up CRP values were drawn at a median of 16.4 weeks [range week 4–50]. Among all HD IFX patients with available laboratory data, CRP values dropped from a median of 20.5mg/L to 4.7mg/L [p < 0.001; Figure 3]. A significant decrease in CRP was observed in patients who responded at both early [22.9 to 7.45mg/L, p <0.0001, Figure 4a] and late [27.5 to 4.7mg/L, p = 0.0001, Figure 4c] time periods, whereas there was no significant decrease among those who did not respond during these periods [6.3 to 1.2mg/L, p = 0.12, Figure 4b, and 14.2 to 10.8mg/L, p = 0.10, Figure 4d, respectively].
Figure 3.
CRP response. Serum CRP measurements drawn at baseline and follow-up [median week 16.4, range 4–50] for all patients with available data. Errors bars indicate median and interquartile range.
Figure 4.
CRP by clinical response. Baseline and follow-up CRP measurements among patients with [a]or without [b] clinical response at the early period of 1–16 weeks, and with [c] or without [d] clinical response at the late period of 38–100 weeks. Error bars indicate median and interquartile range.
Serum albumin and platelet levels also demonstrated improvement to treatment with HD IFX. Among 51 patients with available albumin measurements, median levels increased from a baseline of 3.60g/dL to 3.90g/dL [p < 0.001] at a median of 16.1 weeks [range week 4–28]. Platelet levels among 49 patients with available data decreased from a median baseline of 333 to 305 thousand cells/µL [p = 0.004] at a median of 16.4 weeks [range week 4–38]. ESRs among 45 patients with available data showed no significant difference between baseline and measurement at week 18 [31.5 to 28.0mm/h, p = 0.26, range week 4–28].
3.4 Therapeutic drug monitoring
Of the 86 HD IFX patients, 25 patients had serum IFX troughs drawn immediately before starting HD IFX therapy: 24 by ELISA and 1 by HMSA. Of these, 16 [64%] patients had subtherapeutic serum IFX levels less than 3.0 µg/mL, and 5 [36%] patients had therapeutic IFX levels with a median of 8.0 µg/mL [range 3.2–16.4 µg/mL]. Among patients with available data, there were no statistically significant differences in rates of early clinical response [n = 18] or CRP improvement [n = 17] among those with subtherapeutic vs therapeutic serum IFX levels drawn immediately prior to HD IFX initiation.
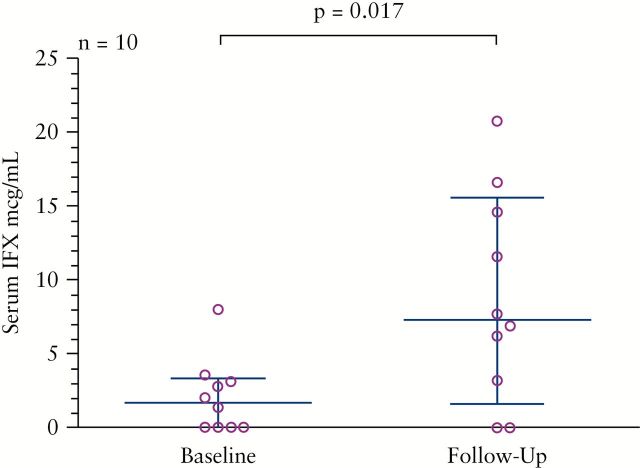
A total of 10 patients had serum IFX trough levels drawn both before and after the initiation of HD IFX therapy at a median of 20.7 weeks [range 4–78 weeks]. After the initiation of HD IFX therapy, the median serum IFX trough level significantly increased from 1.7 to 7.3 µg/mL [p = 0.017], Figure 5. Among patients with available data, there were no statistically significant differences in the rates of early clinical response [n = 9], or CRP improvement [n = 8] among those demonstrating an increase in serum IFX levels after initiating HD IFX therapy vs those who did not demonstrate an increase.
Figure 5.
Serum IFX response. Serum IFX measurements drawn at baseline and follow-up [median week 20.7, range 4–78] for patients with available data. Error bars indicate median and interquartile range.
In all, 31 [35.6%] of the 86 HD IFX patients had titers of antibodies to infliximab [ATI] drawn either before [n = 8] or after [n = 23] the initiation of HD IFX therapy; 23 by ELISA assay and 8 by HMSA. ATI measurement was indeterminate in 15 patients who underwent ELISA testing, due to the presence of serum IFX. ATIs were detected in 9 [29%] of patients. Among 13 patients with available clinical and ATI data, there was no difference in the rate of early clinical response [88.9% vs 100%, p = 1.0] among those with [n = 9] and without [n = 4] detectable ATIs. Among 12 patients with available CRP and ATI data, those without detectable ATIs had a significant decrease in CRP from a median of 31.3 to 8.65mg/dL [p = 0.03, n = 6], whereas those with detectable ATIs did not demonstrate a significant decrease in CRP [14.05 to 8.35mg/dL, p = 0.90, n = 6].
3.5 Predictors of clinical response
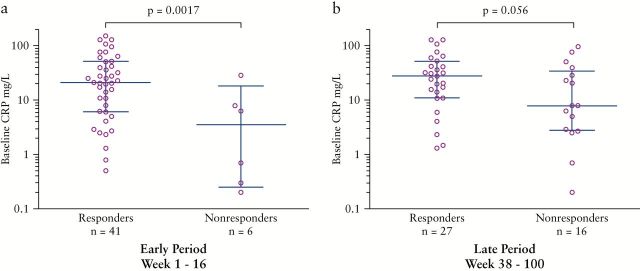
Continuous variables were analyzed for correlation to clinical response. Among patients with available data for baseline CRP measurements before the first HD IFX dose and clinical response at the early time period [n = 47], the median baseline CRP of patients who responded was significantly elevated compared with those who did not respond at this time [median 22.0 vs 3.5mg/L, p = 0.0017, Figure 6a]. Baseline CRP values of those who responded at the late time period also trended toward being elevated [n = 43, median 27.5 vs 7.9mg/L, p = 0.056, Figure 6b]. Body mass index [BMI] at HD IFX initiation was significantly lower among responders compared with non-responders at the early time period [median 19.5 vs 24.7kg/m2, p = 0.046, n = 58], though was not significantly different between responders and non-responders at the late time period [median 21.4 vs 20.7kg/m2, p = 0.32, n = 53]. Age at diagnosis, disease duration, baseline serum IFX level, serum albumin, platelet, and ESR measurements were examined but not found to correlate with clinical response.
Figure 6.
Baseline CRP by clinical response. Baseline CRP measurements sorted by the presence or absence of clinical response at [a] and b] the early time period [1–16 weeks, and [c] and [d] at the late time period [38–100 weeks,rspectively. Error bars indicate median and interquartile range.
Univariate analysis of categorical variables including gender, disease location and phenotype, smoking status, prior medication history, prior IFX hiatus of greater than 24 weeks, context of HD IFX escalation, concurrent immunomodulator therapy, or presence of ATIs, did not reveal any significant differences between those patients who responded clinically and those who did not.
3.6 Safety and adverse events
Medical records were examined for safety data in 86 HD IFX patients throughout periods of compliance with therapy. A total of 148.5 patient-years were examined with a median of 64.9 [range 3.7–577] weeks of safety data per patient. Table 3 describes the adverse events experienced by patients while receiving HD IFX therapy. A total of 14 serious adverse events [requiring hospital admission or surgery] occurred in 9 patients. One death occurred in a 27-year-old female due to hemoperitoneum from necrotizing vasculitis. One month previously she underwent drainage and received antibiotics for a peritracheal abscess growing oxacillin-sensitive Staphylococcus aureus. Blood and wound cultures at the time of death were negative. She had been receiving IFX at 15mg/kg every 4 weeks and her last infusion was 59 days before she died. Another patient who received IFX at 10mg/kg every 4 weeks in combination with 6-mercaptopurine developed coccidioidomycosis after traveling to Arizona and was successfully treated with oral fluconazole. One other patient who received IFX at 10mg/kg every 4 weeks in combination with azathioprine and corticosteroids developed disseminated histoplasmosis after travelling to Bangladesh. He was admitted for intravenous antifungal therapy and successfully treated; however, IFX therapy was discontinued. Vulvar squamous cell carcinoma of the skin developed in one patient with longstanding vulvar hidradenitis suppurativa, who had received IFX at 15mg/kg every 4 weeks in combination with tacrolimus for 178 weeks. Two other patients were diagnosed with melanoma of the skin in situ; one had received IFX 15mg/kg every 6 weeks for 17 months, and the other had received 20mg/kg every 4 weeks for 10 months in combination with 6-mercaptopurine. Both patients underwent successful surgical resection.
Table 3.
Adverse events for HD IFX patients.
| 148.5 pt-yrs | Events, n [per 100 pt-yrs] | Events occurring on combination therapya, n | Events requiring admission or surgery, n [per 100 pt-yrs] | Events leading to discontinuation of HD IFX, n [per 100 pt-yrs] | ||||
|---|---|---|---|---|---|---|---|---|
| Infection | 59 | [38.7] | 24 | 40.7% | 11 | [7.41] | 1 | [0.67] |
| Respiratory infection | 21 | [14.1] | 11 | 2 | 0 | |||
| Skin infection | 10 | [6.73] | 2 | 1 | 0 | |||
| Herpes zoster | 6 | [4.04] | 2 | 0 | 0 | |||
| Non-perianal abscess | 5 | [3.37] | 1 | 2 | 0 | |||
| Local fungal infection | 4 | [2.63] | 2 | 0 | 0 | |||
| Catheter/surgical material infection | 3 | [2.02] | 1 | 2 | 0 | |||
| Urinary / gynecological infection | 3 | [2.02] | 1 | 0 | 0 | |||
| Systemic fungal infection | 2 | [1.37] | 2 | 1 | 1 | |||
| Bacterial diarrheab | 2 | [1.37] | 1 | 1 | 0 | |||
| Perianal abscess | 2 | [1.37] | 0 | 2 | 0 | |||
| Coxsackie virus infection | 1 | [0.67] | 1 | 0 | 0 | |||
| Mycobacterial infection | 0 | [0.00] | 0 | 0 | 0 | |||
| Autoimmune | 8 | [5.39] | 2 | 25% | 0 | [0.00] | 1 | [0.67] |
| Inflammatory skin disorder | 4 | [2.63] | 1 | 0 | 1 | |||
| Drug-induced lupus-like reaction | 3 | [2.02] | 1 | 0 | 0 | |||
| Asymptomatic +dsDNA titer | 1 | [0.67] | 0 | 0 | 0 | |||
| Neoplasia | 3 | [2.02] | 2 | 66.7% | 3 | [2.02] | 0 | [0.00] |
| Squamous cell carcinoma of skin | 1 | [0.67] | 1 | 1 | 0 | |||
| Melanoma in situ | 2 | [1.37] | 1 | 2 | 0 | |||
HD IFX, high dose infliximab; pt-yrs, patient-years.
aCombination therapy included 6-mercaptourine, azathioprine, methotrexate, or tacrolimus.
bOne patient was stool culture positive for salmonella; one was positive for Clostridium difficile.
There were 59 infectious events experienced by 31 patients on HD IFX therapy. Respiratory infections were the most common [21 events] consisting mainly of nonspecific viral syndromes [8 events] and sinus infections [5 events]. However, two patients required hospitalization for pneumonia; and 24 infectious events [40.7%] occurred on combination therapy with an immunomodulator. No mycobacterial infections, demyelinating events, or instances of new-onset heart failure were documented.
The incidence rates of serious infections are stratified by average weekly IFX dose in Table 4. Five serious infections occurred among five patients receiving 10mg/kg every 4 weeks, and five serious infections occurred among two patients receiving 20mg/kg every 4 weeks. One of the patients receiving 20mg/kg every 4 weeks for fistulizing Crohn’s disease also had axillary and perianal hidradenitis suppurativa, had declined recommended surgical therapy [diverting ostomy with wide excision] and was frequently noncompliant with prescribed medical therapy. During an 18-month period of compliance with HD IFX she experienced four serious infections: two perianal abscesses requiring drainage, one central line infection, and one pneumonia.
Table 4.
Serious infections grouped by average weekly dose of IFX.
| Average IFX mg/kg/week [range] | Doses included | Pt-years follow-up | Serious infections | Serious infection rate per 100 pt-years [95% CI] |
|---|---|---|---|---|
| < 2.5mg/kg/week [1.43–2.14] |
10q7, 10q6, 15q8, 15q7, 10q5 | 53.55 | 1 | 1.87 [0.05 – 10.41] |
| 2.5mg/kg/week [2.5] |
10q4, 15q6, 20q8 | 58.63 | 5 | 8.53 [2.77 – 19.9] |
| > 2.5mg/kg/week [3.33–5.63] |
15q4, 20q6, 20q4, 22.5q4 | 36.33 | 5a | 13.76b [4.47 – 32.12] |
| Total | 148.50 | 11 | 7.41c [3.69 – 13.25] |
CI, confidence interval.
aA single patient accounted for four events. See the text for details.
bThe serious infection rate for this group with the above-mentioned patient excluded becomes 2.87 [0.07–15.97] events per 100 patient-years [95% CI].
cThe serious infection rate for all patients with the above-mentioned patient excluded becomes 4.76 [3.73–13.38] events per 100 patient-years [95% CI].
Acute and delayed infusion reactions are shown in Table 5. Ten patients [11.6%] experienced acute infusion reactions, nine of whom subsequently tolerated infusions after premedication with acetaminophen, steroids, and/or antihistamines. One patient receiving IFX at 15mg/kg every 6 weeks discontinued treatment due to chest tightness and dyspnea with infusions which did not improve with premedications; this patient was subsequently switched to adalimumab. Five patients [5.8%] experienced delayed infusion reactions, and premedications for subsequent infusions successfully prevented symptoms in four of them. One patient receiving IFX at 10mg/kg every 7 weeks after infusions experienced headaches that were not controlled with premedications but resolved after a decrease in dosage to 5mg/kg every 7 weeks.
Table 5.
Infusion reactions during HD IFX therapy.
| n = 86 | Patients affected | Tolerated with premedications, n | Discontinued HD IFX, n | |
|---|---|---|---|---|
| n | % | |||
| Acute infusion reactions | 10 | 11.6% | 9 | 1 |
| Milda | 2 | 3.5% | 2 | 0 |
| Moderateb | 8 | 9.3% | 7 | 1 |
| Severec | 0 | 0.0% | 0 | 0 |
| Delayed infusion reactions | 5 | 5.8% | 4 | 1 |
HD IFX, high dose infliximab.
aMild: hyperemia, palpitations, diaphoresis, headache, dizziness, nausea.
bModerate: mild hypo/hypertension, chest discomfort, shortness of breath, elevated temperature, palpitations, urticaria.
cSevere: significant hypo/hypertension, elevated temperature with rigors, significant shortness of breath, stridor.
4. Discussion
This study aims to characterize the use of HD IFX therapy for CD patients in whom standard dose therapy [up to 10mg/kg every 8 weeks] is not adequate to control symptoms. The current literature has not explored the characteristics of patients selected to undergo high-dose infliximab escalation, or how they respond to and tolerate high-dose treatment. The present study suggests that a certain population of patients who have failed to respond, or lost response, to IFX even after standard dose escalation may benefit from further IFX dose intensification to the doses described here. The rates of clinical response we observed after escalating to an HD IFX regimen [84.9% and 62.3% at the early and late time periods, respectively] closely parallel the response rates of 66–96% seen in other studies examining standard escalation from 5mg/kg every 8 weeks to 5mg/kg every 4 weeks or to 10mg/kg every 8 weeks.5–7 Clinical response was accompanied by a drop in inflammatory markers and was associated with a longer duration of HD IFX treatment. Furthermore, the durations of HD IFX therapy received by patients suggest that it is tolerated and represents a feasible treatment option for select patients in whom other medical options have been exhausted.
Whereas it is not fully understood why primary and secondary non-response occur in CD, investigators have proposed that free IFX concentrations may become inadequate due to altered clearance of the drug from multiple causes including the presence of antibodies to infliximab [ATIs], and that some patients’ disease may be mediated through non-TNF pathways.13 Detectable serum trough IFX levels have been associated with improved clinical, laboratory, and endoscopic responses in a number of observational and prospective studies.1,2,14–16 The measurement of serum IFX levels and ADA titers has been proposed to guide the decision to escalate IFX dosing or switch to another agent in the setting of non-response,11 and the clinical significance of therapeutic drug monitoring to guide IFX dosing is under investigation.17
In this study, we were unable to determine the ratio of primary to secondary non-responders in the HD IFX cohort due to the mixed population of both IFX-naïve and non-naïve patients and the availability of clinical data. In addition, because the clinical management of these patients was prior to the publication of the SONIC trial, only 49% of patients were receiving combination therapy before starting HD IFX. However, the incidence of primary or secondary non-response to standard IFX dosing can be approximated by examining the context in which each patient was escalated to HD IFX. Patients who were escalated to HD IFX during the first three induction doses of an SD IFX regimen likely exhibited primary non-response, whereas those who were escalated to HD IFX after receiving maintenance therapy with SD IFX likely exhibited secondary non-response. We found no statistical difference in clinical response rates between those who were escalated to HD IFX during the induction period vs during maintenance.
As this retrospective study was aimed at investigating the safety and efficacy of HD IFX, we recognize its limitation of few patients undergoing serum IFX and ATI measurements, since this was not considered standard practice at the time these data were collected. However, our results suggest that HD IFX escalation can significantly increase serum IFX levels, allowing for more patients to fall within the therapeutic range. Although we did not observe a statistical correlation between increased serum IFX levels and clinical or CRP response, this is likely due to the low sample size. Additionally, patients with detectable ATIs were seen to have less improvement to CRP than those without ATIs. These findings support the idea that HD IFX may have a role within the paradigm of dose escalation based on serum IFX and ATI measurements, and may be necessary to overcome drug clearance or counteract inflammatory burden in the appropriate pharmacokinetic milieu [subtherapeutic serum IFX and absent ATIs] when SD IFX is inadequate to achieve successful results.
There are limited data correlating the incidence rate of adverse events with IFX dosage or serum IFX levels. An early study examining IFX for fistulizing CD suggested an increased rate of adverse events in patients receiving scheduled treatment with 10mg/kg vs 5mg/kg [84% and 65%, respectively].18 Data from the ACCENT I trial did not suggest an increased rate of serious infections or serious events leading to discontinuation of therapy among those receiving scheduled infusions of 10mg/kg vs 5mg/kg.1 Similarly, the authors of the TREAT registry note that there was no significant difference in serious infections between those receiving 10mg/kg vs 5mg/kg.19
In the present study, HD IFX therapy demonstrated a high rate of adverse events compared with historical data on SD IFX therapy, although with similar rates of discontinuation due to complications from therapy. Of patients who attempted HD IFX, 7% discontinued therapy due to adverse events, which is similar to the rate of discontinuation due to reasonably related adverse events seen among SD IFX users in ACCENT I [6–8%].1 Although we found the rate of serious infections requiring hospitalization in patients receiving HD IFX to be increased compared with that seen among SD IFX patients in the TREAT registry,19 this is likely confounded by the higher prevalence of more severe disease in this HD IFX cohort, which has been identified as an independent predictor of serious infections. Our sample size limits us from drawing conclusions about rare events such as malignancy.
When stratified by average weekly dose, our data suggest a positive correlation between IFX delivery and serious infections. Those receiving HD IFX at the lower end [less than 2.5mg/kg/week] exhibit a rate of only 1.87 serious infections per 100 patient-years, similar to the rate of 2.01 serious infections per 100 patient-years seen in TREAT. Doses at these levels have also been shown to have similar serious infection rates compared with lower IFX doses and placebo in the ATTRACT trial in patients with rheumatoid arthritis.20 The high rate of serious infections seen at the upper end of HD IFX dosages [greater than 2.5mg/kg/week] suggests that higher IFX delivery is likely associated with more serious infections. Furthermore the fact that these events were experienced by a relatively few number of patients, with one patient accounting for 80% of serious infections in this dosage group, outlines the fact that a high degree of vigilance is necessary to identify those patients most at risk for adverse outcomes and to make appropriate accommodations when selecting a therapy. The high overall burden of complications in this population necessitates caution when prescribing IFX escalation. Furthermore, additional discretion should be exercised in geographic areas with high rates of endemic mycobacterial or fungal infections.
A challenge remains to identify the patients for whom HD IFX represents the most effective and safe therapeutic option compared with other available treatments. Overall, our cohort of HD IFX patients is characterized by a high burden of disease with many patients exhibiting extensive anatomical involvement, a high prevalence of penetrating symptoms, high rates of hospitalization for CD, and a history of repeated treatment failure with other therapies. None of these factors were predictive of response to HD IFX therapy. The finding that a 24-week hiatus from IFX therapy was not associated with altered clinical response rates to HD IFX escalation suggests that HD IFX therapy may be a viable salvage treatment for patients who have previously discontinued IFX in favor of other agents, but have failed to achieve adequate control with these agents. A recent study similarly reported that re-initiation of SD IFX therapy after discontinuation due to loss of response or sustained remission is efficacious and safe.21 The finding that a lower BMI was found among patients who responded clinically than those who did not respond is in accordance with current theories about the pharmacokinetics of serum IFX clearance.11
Inflammatory burden as approximated by CRP measurements drawn before the initiation of HD IFX therapy correlated with clinical response, with a higher baseline CRP value associated with a higher likelihood of benefitting from escalation to HD IFX therapy, reflecting similar findings as a 2011 retrospective study correlating elevated baseline CRP with increased likelihood of response to IFX.22 Although a recent study found that higher pre-IFX CRP values were associated with a decreased likelihood of response to standard IFX dosing regimens,23 it remains possible that patients with significant inflammatory burdens may only achieve symptom control with sufficiently high delivery of IFX that is not provided by standard dosing. IFX’s intravenous administration makes it more practical to deliver these high doses than anti-TNF agents delivered subcutaneously.
The decision to intensify a poorly controlled CD patient’s IFX regimen or switch to another agent should be based on a combination of clinical and laboratory parameters, and through monitoring of serum IFX and ATIs.11,24 Similar algorithms have been proposed for adalimumab dosing as well.25 Although the present study was limited by its retrospective nature, relatively small sample size, and limited data for IFX pharmacokinetics, it provides preliminary evidence to suggest that HD IFX may have a role in the treatment of CD. This role probably lies in patients already dose-escalated to 10mg/kg every 8 weeks, who continue to exhibit a high CRP burden with low serum IFX levels and undetectable ATI. Given the rate of serious adverse events in this current study, HD IFX should be considered and reserved for patients with high inflammatory burden and no other therapeutic alternative. Furthermore, the significant financial costs associated with administering high doses of infliximab should be considered when deciding upon treatment. Further prospective studies and validation in large populations will help determine the optimal role of HD IFX therapy in the treatment of CD.
Funding
This study was funded in part by the Crohn’s & Colitis Foundation of America [CCFA], Student Research Fellowship Award [#3830]. The CCFA had no role in the study design, data collection, interpretation, writing of the manuscript, or decision to submit the manuscript for publication.
Conflict of Interest
JC has served as speaker, consultant, and advisory board member for Abbvie, Bristol Meyers Squibb, Ferring, Genentech, Giuliani SPA, Given Imaging, Merck, Millenium Pharmeceuticals, Pfizer, Prometheus Laboratories, Sanofi, Schering Plough, Takeda, Teva Pharmeceuticals, and UCB Pharma. BS has served as a consultant and an advisory board member for AbbVie, Amgen, AstraZeneca, Avaxia Biologics, Bristol-Myers Squibb, Forest Research Institute, Janssen Biotech, Luitpold Pharmaceuticals, MedImmune, Millennium Pharmaceuticals, Pfizer, Prometheus Laboratories, Puretech Ventures LLC, Salix, Shire, Takeda, Topivert Pharma, and Vedanta Biosciences, has received research funding from AbbVie, AstraZeneca, Amgen, MedImmune, Janssen Biotech, Pfizer, Millennium Pharmaceuticals, Takeda and Osiris, and has been a speaker for Creative Educational Concepts, Focus Medical Communications, Curtio CME, and IMEDEX. Dr. BS owns stocks and shares in Avaxia Biologics. LM has served as consultant for Centocor [now Janssen Biotech], Abbott, UCB, and Medorex. SA has received research funding from Prometheus Laboratories.
Supplementary Material
Acknowledgments
The authors thank the clinical staff of the Therapeutic Infusion Center at Mount Sinai Medical Center: Monica Reiter-Wong, Aurora Barriga, Cynthia Medina, Damaris Mero, Rachel Mallore, Stanley Vano, Maria Rodriguez, Kate Leto, and Nicole Thomas. We dedicate this work to our colleague and friend, Lloyd Mayer, MD.
SH provided contributions in study planning, data collection, analysis and interpretation, and manuscript drafting. BC, JC, and BS provided contributions in data analysis and interpretation, and manuscript review. LM [deceased] provided contributions in study concept and planning, and data analysis and interpretation. SA provided contributions in study concept and planning, data analysis and interpretation, and manuscript review. All authors have read and approved the final draft of the manuscript submitted.
References
- 1. Hanauer SB, Feagan BG, Lichtenstein GR, et al. Maintenance infliximab for Crohn’s disease: the ACCENT I randomised trial. Lancet 2002; 359:1541–9. [DOI] [PubMed] [Google Scholar]
- 2. Targan SR, Hanauer SB, van Deventer SJ, et al. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor alpha for Crohn’s disease. Crohn’s Disease cA2 Study Group. N Engl J Med 1997; 337:1029–35. [DOI] [PubMed] [Google Scholar]
- 3. Sands BE, Anderson FH, Bernstein CN, et al. Infliximab maintenance therapy for fistulizing Crohn’s disease. N Engl J Med 2004; 350:876–85. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan GG, Hur C, Korzenik J, Sands BE. Infliximab dose escalation vs. initiation of adalimumab for loss of response in Crohn’s disease: a cost-effectiveness analysis. Aliment Pharmacol Ther 2007; 26:1509–20. [DOI] [PubMed] [Google Scholar]
- 5. Regueiro M, Siemanowski B, Kip KE, Plevy S. Infliximab dose intensification in Crohn’s disease. Inflamm Bowel Dis 2007; 13:1093–9. [DOI] [PubMed] [Google Scholar]
- 6. Chaparro M, Panes J, Garcia V, et al. Long-term durability of infliximab treatment in Crohn’s disease and efficacy of dose ‘escalation’ in patients losing response. J Clin Gastroenterol 2011; 45:113–8. [DOI] [PubMed] [Google Scholar]
- 7. Katz L, Gisbert JP, Manoogian B, et al. Doubling the infliximab dose versus halving the infusion intervals in Crohn’s disease patients with loss of response. Inflamm Bowel Dis 2012; 18:2026–33. [DOI] [PubMed] [Google Scholar]
- 8. Afif W, Loftus EV, Jr, Faubion WA, et al. Clinical utility of measuring infliximab and human anti-chimeric antibody concentrations in patients with inflammatory bowel disease. Am J Gastroenterol 2010; 105:1133–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanauer SB, Sandborn W. Practice Parameters Committee of the American College of Gastroenterology. Management of Crohn’s disease in adults. Am J Gastroenterol 2001; 96:635–43. [DOI] [PubMed] [Google Scholar]
- 10. Wang SL, Ohrmund L, Hauenstein S, et al. Development and validation of a homogenous mobility shift assay for the measurement of infliximab and antibodies-to-infliximab levels in patient serum. J Immunol Methods 2012;382:177–88. [DOI] [PubMed] [Google Scholar]
- 11. Ordas I, Feagan BG, Sandborn WJ. Therapeutic drug monitoring of tumor necrosis factor antagonists in inflammatory bowel disease. Clin Gastroenterol Hepatol 2012; 10:1079–87. [DOI] [PubMed] [Google Scholar]
- 12. Cheifetz A, Smedley M, Martin S, et al. The incidence and management of infusion reactions to infliximab: a large center experience. Am J Gastroenterol 2003; 98:1315–24. [DOI] [PubMed] [Google Scholar]
- 13. Danese S, Fiorino G, Reinisch W. Review article: Causative factors and the clinical management of patients with Crohn’s disease who lose response to anti-TNF-alpha therapy. Aliment Pharmacol Ther 2011; 34:1–10. [DOI] [PubMed] [Google Scholar]
- 14. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362:1383–95. [DOI] [PubMed] [Google Scholar]
- 15. Maser EA, Villela R, Silverberg MS, Greenberg GR. Association of trough serum infliximab to clinical outcome after scheduled maintenance treatment for Crohn’s disease. Clin Gastroenterol Hepatol 2006; 4:1248–54. [DOI] [PubMed] [Google Scholar]
- 16. Imaeda H, Bamba S, Takahashi K, et al. Relationship between serum infliximab trough levels and endoscopic activities in patients with Crohn’s disease under scheduled maintenance treatment. J Gastroenterol 2014; 49:674–82. [DOI] [PubMed] [Google Scholar]
- 17. Casteele NV, Gils A, Ballet V, et al. Randomised controlled trial of drug level versus clinically based dosing of infliximab maintenance therapy in IBD: Final results of the TAXIT study. United European Gastroenterology Journal 2013;1S:A1 [Google Scholar]
- 18. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn’s disease. N Engl J Med 1999; 340:1398–405. [DOI] [PubMed] [Google Scholar]
- 19. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infection and mortality in patients with Crohn’s disease: more than 5 years of follow-up in the TREAT registry. Am J Gastroenterol 2012; 107:1409–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet 1999; 354:1932–9. [DOI] [PubMed] [Google Scholar]
- 21. Baert F, Drobne D, Gils A, et al. Early trough levels and antibodies to infliximab predict safety and success of reinitiation of infliximab therapy. Clin Gastroenterol Hepatol 2014; 12:1474–81 [DOI] [PubMed] [Google Scholar]
- 22. Jurgens M, Mahachie John JM, Cleynen I, et al. Levels of C-reactive protein are associated with response to infliximab therapy in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2011; 9:421–7. [DOI] [PubMed] [Google Scholar]
- 23. Magro F, Rodrigues-Pinto E, Santos-Antunes J, et al. High C-reactive protein in Crohn’s disease patients predicts nonresponse to infliximab treatment. J Crohns Colitis 2014; 8:129–36. [DOI] [PubMed] [Google Scholar]
- 24. Bendtzen K. Anti-TNF-alpha biotherapies: perspectives for evidence-based personalized medicine. Immunotherapy 2012; 4:1167–79. [DOI] [PubMed] [Google Scholar]
- 25. Roblin X, Rinaudo M, Del Tedesco E, et al. Development of an algorithm incorporating pharmacokinetics of adalimumab in inflammatory bowel diseases. Am J Gastreoenterol 2014; 109: 1250–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.