Abstract
Background
We evaluated the ability of various grading scales including platelet-albumin-bilirubin (PALBI) and albumin-bilirubin (ALBI) grades to predict overall survival (OS) according to treatment modality in patients with hepatocellular carcinoma (HCC).
Methods
The cohort of 6,669 patients with HCC was selected randomly from the 2008–2012 national cohort of the Korean Central Cancer Registry. The OS of 6,507 of these patients was evaluated using the Child-Turcotte-Pugh (CTP) class, Model for End-stage Liver Disease (MELD) score, and ALBI and PALBI grades.
Results
The patient’s mean age was 59.7 years. The most patients were hepatitis B virus (63.4%) and CTP class A (71.8%). The median OS durations of PALBI grade1 (38.4%), grade2 (33.2%), and grade3 (28.4%) patients were 81, 30, and 5 months, respectively (P<0.001). The PALBI grade had a larger area under the receiver operator characteristic curve (AUC) than did the CTP class, MELD score, and ALBI grade (overall AUC: 0.675 vs. 0.633, 0.645, and 0.642, respectively; P < 0.001). Moreover, the PALBI and ALBI grades enabled sub-classification of CTP A patients (P < 0.001). In a multivariate analysis, the PALBI and ALBI grades were significant risk factors for OS (P < 0.05). According to treatment modality, the PALBI grade was predictive of OS in patients receiving transarterial chemoembolization or supportive care. The ALBI grade was predictive of OS in patients undergoing surgical resection or radiofrequency ablation.
Conclusion
The PALBI and ALBI grades are more reliable for accessing liver function and predicting OS in patients with HCC. Moreover, according to treatment modality, appropriate use of the ALBI and PALBI grades will enable accurate determination of the prognosis of patients with HCC.
Introduction
Hepatocellular carcinoma (HCC) is a common type of cancer and a major cause of death worldwide [1]. Despite the development of new therapies, HCC remains difficult to treat because it typically occurs in advanced liver disease or cirrhosis [2]. Treatment decisions and prognosis prediction for patients with HCC are based on performance status, liver function, and tumor burden [3]. Thus, the evaluation of liver function is important in the management of HCC. The Child-Turcotte-Pugh (CTP) class and the Model for End-stage Liver Disease (MELD) score are used widely to assess liver function [4].
The CTP class was developed to predict mortality in patients undergoing surgery for portal hypertension especially variceal bleeding [5, 6], and is currently used to estimate the liver functional reserve and to predict overall survival (OS) in patients with HCC. The CTP class has several limitations: (1) it includes subjective factors, such as ascites and hepatic encephalopathy; (2) each variable is assigned the same weight; (3) some of the variables included, such as ascites and the albumin level, may be related; and (4) arbitrary cut-off levels result in the assignment of the same score to patients with different bilirubin levels [4, 7]. In addition, the inability to discriminate liver function and clinical outcomes among patients with HCC and well-preserved liver function is a major drawback of the CTP class system, as the majority of patients with HCC are of CTP class A [8, 9]. The MELD score is reliable for stratification of the risk of dropout in patients with HCC [10]. However, this score has limitations when applied to patients with less-severe HCC, and has been evaluated only with those awaiting liver transplantation (LT) with “exception” points [4, 9, 11]. Therefore, a new index of the liver functional reserve is needed.
The albumin-bilirubin (ALBI) grade and platelet-albumin-bilirubin (PALBI) grade were introduced to assess liver function in patients with HCC [9]. The ALBI grade is based on laboratory findings, together with the albumin and bilirubin levels, and may be reliable for the assessment of liver function in patients with HCC [12–14]. The PALBI grade, based on the ALBI grade, was developed to reflect the effect of portal hypertension. As a surrogate for portal hypertension, it includes consideration of the platelet count [15]. However, there was no study for evaluating the highest performance scoring system including PALBI and ALBI grades in each treatment modalities. Therefore, we investigated the prognostic performance of the ALBI grade, PALBI grade, CTP class, and MELD score in Korean patients with HCC according to treatment modality.
Materials and methods
The Institutional Review Board of the Catholic University of Korea approved this study (DC17RESI097) and waived the requirement for informed consent. This study was conducted in accordance with the Declaration of Helsinki.
Patients
The South Korean Ministry of Health and Welfare has maintained the Korean Central Cancer Registry (KCCR) since 1980. All new diagnosed cancer cases are registered in the database of KCCR. The Korean Liver Cancer Association (KLCA) has been randomly extracted and registered HCC cohort data from the database of KCCR. In this study, 6,669 patients who are registered in KLCA cohort with newly diagnosed HCC between 2008 and 2012 are enrolled. Of them, 162 patients were excluded for the following reasons: (1) age < 18 years (n = 1), (2) erroneous date of HCC diagnosis (n = 4), and (3) loss to follow-up due to treatment refusal (n = 157). Finally, 6,507 patients with HCC were included in this study (Fig 1). Survival data were obtained from the records of hospitals and/or the National Health Insurance Service of Korea through December 2016.
Fig 1. Flow chart of this study.
Clinical and laboratory data
HCC was diagnosed according to the Korean Liver Cancer Study Group (KLCSG) and National Cancer Center (NCC) of Korea guidelines as follows: (1) pathological diagnosis; (2) diagnosis by one or two imaging modalities with ≥1 cm liver nodules in high-risk patients, such as those with hepatitis B virus (HBV) or hepatitis C virus (HCV) infection or liver cirrhosis; or (3) diagnosis by two or more imaging modalities with <1 cm liver nodules and a steadily increasing serum alpha-fetoprotein (AFP) level in high-risk patients. Diagnosis by imaging modalities was based on the following hallmarks of HCC: hypervascularity in the arterial phase and washout in the portal or delayed phase of dynamic computed tomography, dynamic magnetic resonance imaging (MRI), or gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid–enhanced MRI [16].
The CTP class and MELD score were calculated at the time of diagnosis (Table 1) [17]. The MELD score was classified as grade 1 (<10), grade 2 (10–14), or grade 3 (>14) [18]. The ALBI grade was classified as 1 (≤−2.60), 2 (>−2.60 to ≤−1.39), or 3 (>−1.39) [9]. The PALBI grade was classified as 1 (≤−2.53) 2 (>−2.53 to ≤−2.09), or 3 (>−2.09) [15].
Table 1. Equation for calculating each grade including CTP score, MELD score, ALBI grade and PALBI grade.
| CTP score | Adding points of five categories below | ||
| CTP class | Class A, 5–6 points | Class B, 7–9 points | Class C, 10–15 points |
| 1 point | 2 points | 3 points | |
| Albumin (g/dL) | > 3.5 | 2.8–3.5 | < 2.8 |
| Bilirubin (mg/dL) | < 2 | 2–3 | >3 |
| INR | <1.7 | 1.7–2.3 | >2.3 |
| Ascites | None | Mild | Severe |
| Encephalopathy | None | Grade I or II | Grade III or IV |
| MELD score | 3.78 x loge serum bilirubin (mg/dL) + 11.20 x loge INR + 9.57 x loge serum creatinine (mg/dL) + 6.43 | ||
| MELD grade | Grade 1, <10 | Grade 2, 10–14 | Grade 3, >14 |
| ALBI score | (log10 bilirubin × 0.66) + (albumin × -0.085), where bilirubin is in μmol/L and albumin in g/L | ||
| ALBI grade | Grade 1, ≤-2.60 | Grade 2, >-2.60 to ≤-1.39 |
Grade 3, >-1.39 |
| PALBI score | 2.02 × log10 bilirubin − 0.37 × (log10 bilirubin)2 − 0.04 × albumin − 3.48 × log10 platelets + 1.01 × (log10 platelets) 2 | ||
| PALBI grade | Grade 1, ≤-2.53 | Grade 2, >-2.53 to ≤-2.09 |
Grade 3, >-2.09 |
CTP, Child-Turcotte-Pugh; INR, International normalized ratio; MELD, Model for end-stage liver disease; ALBI, albumin-bilirubin; PALBI, platelet-albumin-bilirubin
Tumor staging and treatment group
At the time of HCC diagnosis, tumors were staged using the Barcelona Clinic Liver Cancer (BCLC) and modified Union for Cancer Control staging systems [19, 20]. A multidisciplinary expert group in each hospital decided on the optimum initial treatment plan for each patient according to tumor staging following the BCLC and/or KLCSG-NCC guidelines. LT, surgical resection (SR), radiofrequency ablation (RFA), transarterial chemoembolization (TACE), sorafenib, and supportive-care treatments were administered by experts.
Statistics
The baseline characteristics of the patients are presented as means ± standard deviations or as counts with percentages, as appropriate [21]. The Kaplan–Meier survival method with the log-rank test was used to assess the CTP class, MELD grade, BCLC stage, ALBI grade, and PALBI grade. Areas under the receiver operating characteristic curve (AUCs) were calculated for the 1-, 3-, and 5-year mortality rates. Harrell’s c statistic was also calculated for each grade. The ability of the ALBI and PALBI grades to predict OS stratified by CTP class and treatment modality was evaluated using the Kaplan–Meier method. A multivariate Cox regression analysis was performed to identify risk factors for OS according to treatment modality. In this analysis, model 1 included the ALBI grade, but not the PALBI grade; model 2 included the PALBI grade, but not the ALBI grade; and model 3 included the ALBI and PALBI grades. All statistical analyses were performed by biostatistics team of Catholic university of Korea using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Baseline characteristics
The mean age of the patients was 59.7 ± 11.4 years (range, 52.0–68.0 years), and the majority (n = 5,144, 79.1%) was male. HBV (63.4%) was the most frequent etiology of HCC, followed by alcohol (22.7%) and HCV (12.5%). The majority (n = 4,669, 71.8%) of patients was of CTP class A, and the mean MELD score was 9.8 ± 4.0 (range, 7.0–11.0). The most frequent ALBI grade was 2 (49.3%) and the most frequent PALBI grade was 1 (38.4%), followed by 2 (33.2%).
Most (61.2%) patients had single tumors, and the mean tumor size was 4.8 ± 3.9 cm (range, 2.0–6.3 cm). The largest proportion (40.5%) of patients was of BCLC stage A, followed by stage C (33.2%). The most frequent treatment modality was TACE (45.8%), followed by SR (18.2%), supportive care (17.6%), and RFA (11.6%). The baseline characteristics of the patients are summarized in Table 2.
Table 2. Baseline characteristics of included patients.
| All patients (n = 6507) | |
|---|---|
| Age, years | 59.7±11.4 |
| gender (male/female) | 5144(79.1%)/1363 (20.9%) |
| Etiologies | |
| Hepatitis B, n (%) | 4036(63.4%) |
| Hepatitis C, n (%) | 778(12.5%) |
| alcohol, n (%) | 1479(22.7%) |
| non-B, C hepatitis, n (%) | 214(3.4%) |
| Diabetes mellitus, n (%) | 1530(23.7%) |
| Hypertension, n (%) | 2027(31.4%) |
| Laboratory values | |
| Alpha-fetoproteina, ng/mL, n (%) | 12411.7±97615.7 |
| ≤20 | 2542(41.5%) |
| 20–400 | 1819(29.7%) |
| >400 | 1760(28.8%) |
| PIVKA-IIb, mAU/mL | 3128.1±16406.5 |
| ≤40 | 1155 (33.6%) |
| >40 | 2282 (66.4%) |
| Albumin, g/dL | 3.7±0.7 |
| <2.8 | 586 (9.0%) |
| 2.8–3.5 | 1893 (29.1%) |
| >3.5 | 4028 (61.9%) |
| Total bilirubin, mg/dL | 1.7±3.0 |
| <2 | 5386 (82.8%) |
| ≥2-≤3 | 574 (8.8%) |
| >3 | 547 (8.4%) |
| Platelets, 1000/μL | 156.4±90.3 |
| Child-Turcotte-Pugh (CTP) Class, n (%) | |
| A | 4669 (71.8%) |
| B | 1522 (23.4%) |
| C | 316 (4.8%) |
| ALBI score | -2.3±0.7 |
| ALBI grade, n (%) | |
| grade 1 (≤-2.60) | 2575 (39.6%) |
| grade 2 (>-2.60 to -1.39) | 3211 (49.3%) |
| grade 3 (>-1.39) | 721 (11.1%) |
| PALBI score | -2.3±0.5 |
| PALBI grade | |
| grade 1 (≤-2.53) | 2499 (38.4%) |
| grade 2 (-2.53 to -2.09) | 2158 (33.2%) |
| grade 3 (>-2.09) | 1850 (28.4%) |
| MELD score | 9.8±4.0 |
| MELD grade, n (%) | |
| grade 1 (<10) | 4075 (62.6%) |
| grade 2 (10 to 14) | 1738 (26.7%) |
| grade 3 (>14) | 694 (10.7%) |
| Tumor number c, n (%) | |
| 1 | 3981 (61.2%) |
| 2 | 940 (14.4%) |
| ≥3 | 1585 (24.4%) |
| Maximal tumor diameterd, cm | 4.8±3.9 |
| ≤2 cm, n (%) | 1734 (29.2%) |
| 2–5 cm, n (%) | 2336 (39.3%) |
| >5 cm, n (%) | 1877 (31.5%) |
| Portal vein invasion, n (%) | 1511 (23.2%) |
| BCLC stages, n (%) | |
| 0 | 593 (9.1%) |
| A | 2635 (40.5%) |
| B | 718 (11.0%) |
| C | 2156 (33.2%) |
| D | 405 (6.2%) |
| TNM stages, n (%) | |
| I | 983 (15.1%) |
| I | 2425 (37.3%) |
| III | 1707 (26.2%) |
| IV-A | 760 (11.7%) |
| IV-B | 632 (9.7%) |
| Initial treatment modalities, n (%) | |
| Surgical resection | 1187 (18.2%) |
| Liver transplantation | 60 (0.9%) |
| Radiofrequency ablation (RFA) | 757 (11.6%) |
| Trans-arterial chemoembolization (TACE) | 2982 (45.8%) |
| Sorafenib | 212 (3.3%) |
| Radiation therapy | 82 (1.3%) |
| Supportive care | 1147 (17.6%) |
| Systemic chemotherapy | 80 (1.2%) |
ALBI, albumin-bilirubin; PALBI, platelet-albumin-bilirubin; MELD, model for end-stage liver disease; BCLC stage, Barcelona Clinic Liver Cancer stage; TNM stage, Tumor, Node, Metastasis
an = 386 missing data is not included to analysis;
bn = 3070 missing data is not included to analysis;
cn = 1 missing data is not included to analysis;
dn = 560 missing data is not included to analysis
OS and AUC according to liver function grade
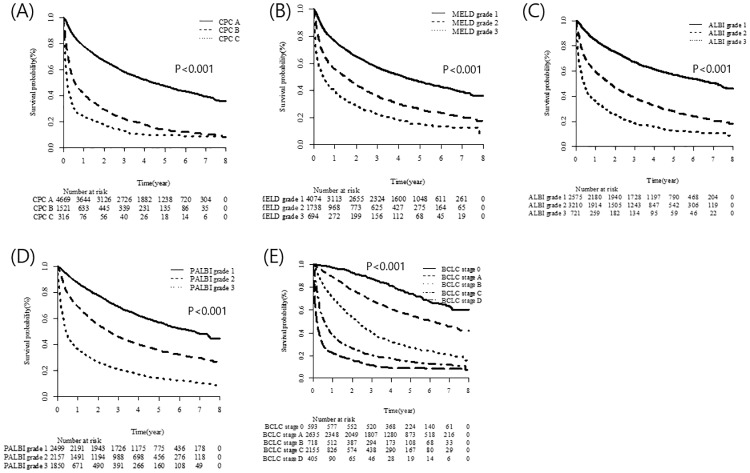
The median follow-up period was 32 months (range: 0–95 months) and the median OS of the entire cohort was 32 months (95% Confidence Interval (CI): 30–34 months). The 5-year survival rate of our nationwide cohort was 0.38 (95% CI: 0.36–0.39). OS was stratified according to the various liver function–grading systems (Fig 2). The CTP class, MELD score, ALBI grade, and PALBI grade were associated significantly with OS (P<0.001 for each grade, Fig 2A and 2D). The BCLC stage also enabled stratification of OS (P<0.001, Fig 2E).
Fig 2. OS stratified by liver function assessment grade and BCLC stage.
Harrell’s c statistic for OS was significantly higher for the PALBI grade than for the CTP class, MELD grade, and ALBI grade (0.675 vs. 0.633, 0.607, and 0.642, respectively; P<0.001). Moreover, the PALBI grade had the highest AUC values for the 1-, 3-, and 5-year mortality rates (0.750, 0.711, and 0.696, respectively; P<0.001; Table 3).
Table 3. AUC value and Harrell’s C-statics for comparing each grades.
| Harrell’s C-statistic (95% CI) | 1-year mortality | 3-year mortality | 5-year mortality | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AUC (95% CI) | p valuea | p valueb | AUC (95% CI) | p valuea | p valueb | AUC (95% CI) | p valuea | p valueb | ||
| All patients | ||||||||||
| MELD grade | 0.607 (0.599–0.615) | 0.645 (0.632–0.658) | reference | <0.001 | 0.625 (0.613–0.636) | reference | <0.001 | 0.618 (0.604–0.632) | reference | <0.001 |
| CTP class | 0.633 (0.625–0.640) | 0.685 (0.673–0.697) | <0.001 | <0.001 | 0.656 (0.646–0.666) | <.0001 | <0.001 | 0.647 (0.636–0.658) | <.0001 | <0.001 |
| ALBI grade | 0.642 (0.634–0.649) | 0.688 (0.676–0.700) | <0.001 | <0.001 | 0.676 (0.664–0.688) | <.0001 | <0.001 | 0.669 (0.654–0.684) | <.0001 | <0.001 |
| PALBI grade | 0.675 (0.667–0.682) | 0.750 (0.738–0.762) | <0.001 | reference | 0.711 (0.699–0.723) | <.0001 | reference | 0.696 (0.682–0.711) | <.0001 | reference |
AUC, The area under the receiver operating characteristics curve; MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh; ALBI, albumin-bilirubin; PALBI, platelet-albumin-bilirubin
aP value in the table denotes for comparison between MELD with other scores;
bP value in the table denotes for comparison between PALBI with other scores
Stratification of CTP class according to ALBI grade and PALBI grade
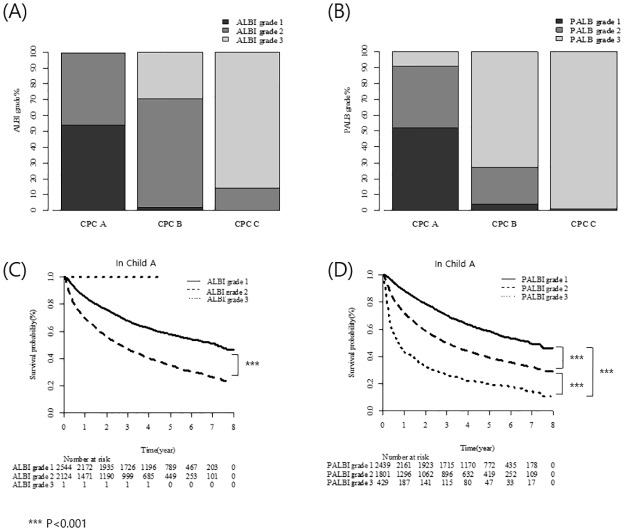
The CTP class was stratified according to the ALBI and PALBI grades. Of the CTP A patients (n = 4,669), 54.5% (n = 2,544), 45.5% (n = 2,124), and 0.02% (n = 1) were of ALBI grades 1, 2, and 3, respectively. Of the CTP B patients (n = 1,522), 31 (2.0%), 1,042 (68.5%), and 449 (29.5%) were of ALBI grades 1, 2, and 3, respectively. Of the CTP C patients, 14.2% (n = 45) and 85.8% (n = 271) were of ALBI grades 2 and 3, respectively. No patient was of ALBI grade 1 (Fig 3A).
Fig 3. CTP class stratified by ALBI and PALBI grades.
Of the CTP A patients (n = 4,669), 52.2% (n = 2,439), 38.6% (n = 1,801), and 9.2% (n = 429) were of PALBI grades 1, 2, and 3, respectively. Of the CTP B patients (n = 1,522), 23.3% (n = 60), 23.3% (n = 354), and 72.8% (n = 1,108) were of PALBI grades 1, 2, and 3, respectively. Of the CTP C patients (n = 316), 0% (n = 0), 1% (n = 3), and 99.1% (n = 313) were of PALBI grades 1, 2, and 3, respectively (Fig 3B).
The ALBI and PALBI grades enabled prediction of OS in patients with CTP class A HCC (Fig 3C and 3D). OS was significantly longer for ALBI grade 1 than for ALBI grade 2 patients (median, 86 vs. 31.5 months, respectively; P < 0.001). PALBI grade 1 patients had the longest OS, followed by those of grades 2 and 3 (median, 83 vs. 35 and 8 months, respectively; P < 0.001 between each grade). However, the ALBI and PALBI grades were not predictive of OS for CTP B and C patients, with the exception of grade 2 vs. 3 in CTP B patients (P < 0.001).
Ability of ALBI and PALBI grades for OS according to different etiologies and level of tumor marker
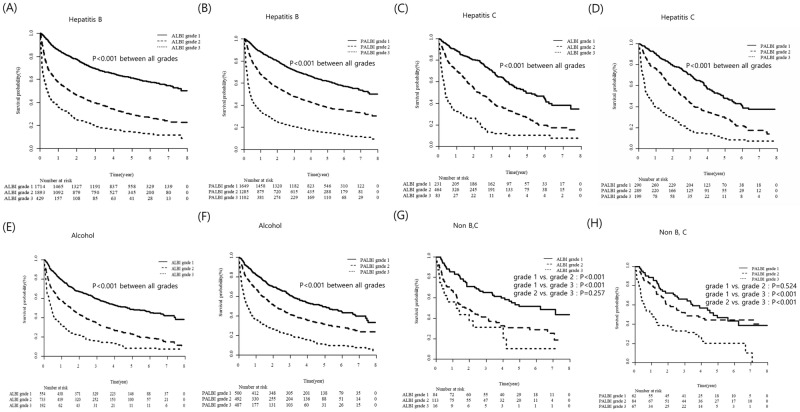
The ability of ALBI and PALBI grade in predicting OS was evaluated according to etiologies (Fig 4). In hepatitis B patients, which is the major etiology of this cohort, both ALBI and PALBI grades were significant in predicting OS by grades (P < 0.001 for each grade; Fig 4A and 4B). Both ALBI and PALBI grades are also significantly differentiated OS by grades in patients with hepatitis C (P <0.001 for each grade; Fig 4C and 4D) and alcohol (P < 0.001 for each grade; Fig 4E and 4F). In Non-B, C patients, ALBI and PALBI grade showed good predictive ability for OS (P <0.001), with the exception of ALBI grade 2 vs. grade 3 (P = 0.257) and PALBI grade 1 vs. grade 2 (P = 0.524; Fig 4G and 4H).
Fig 4. Utility of ALBI and PALBI grades according to different etiologies.
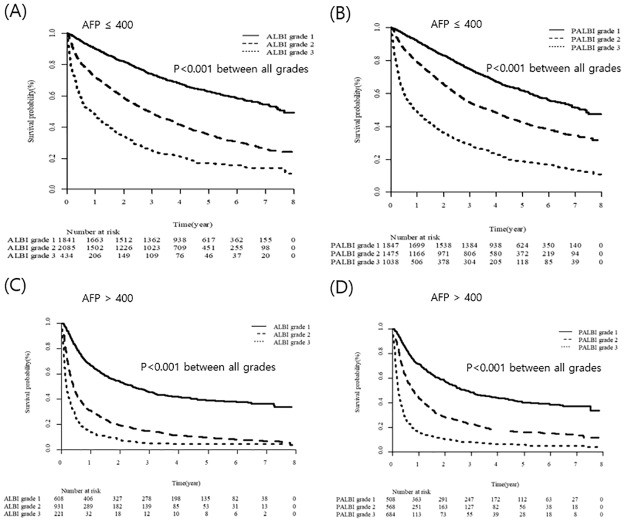
We also investigated the predictive value of ALBI and PALBI grades for OS according to the level of AFP (Fig 5). The cut off level of AFP (400 ng/mL) was classified into low AFP (AFP ≤ 400 ng/mL) and high AFP group (AFP > 400 ng/mL) [22, 23]. In low AFP group, both ALBI and PALBI grade could stratified OS by their grades (P < 0.001 for each grade; Fig 5A and 5B). Moreover, in high AFP group, OS was significantly differentiated by both ALBI and PALBI grades (P < 0.001 for each grade; Fig 5C and 5D).
Fig 5. Utility of ALBI and PALBI grades according to tumor marker.
Predictive power of ALBI and PALBI grades according to treatment modality
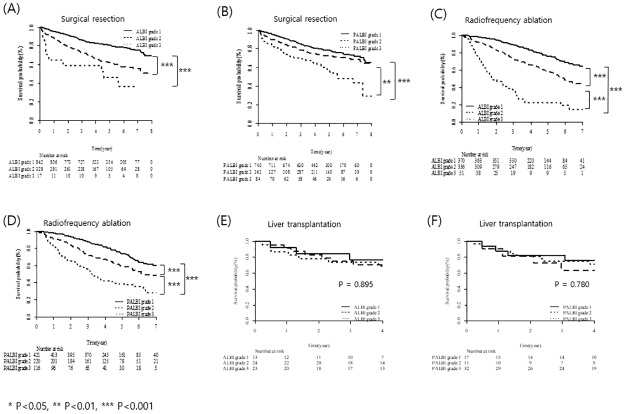
The predictive power of the ALBI and PALBI grades for OS was assessed according to curative treatment modality (SR, RFA, and LT; Fig 6). In patients undergoing SR, the ALBI grade was significantly predictive of OS, with the exception of grade 2 vs. 3 (grade 1 vs. 2 and 3, P < 0.001; grade 2 vs. 3, P = 0.230; Fig 6A). The PALBI grade also significantly differentiated OS by grades, with the exception of grade 1 vs. 2 (grade 1 vs. 2, P = 0.107; grade 2 vs. 3, P < 0.05; grade 3 vs. 1, P < 0.001; Fig 6B). The ALBI and PALBI grades showed good predictive performance for OS in patients receiving RFA (P < 0.001 for each grade; Fig 6C and 6D). However, in patients receiving LT, the ALBI and PALBI grades were not predictive of OS (ALBI grade, P = 0.895; PALBI grade, P = 0.780; Fig 6E and 6F).
Fig 6. Utility of the ALBI and PALBI grades according to curative treatment modality.
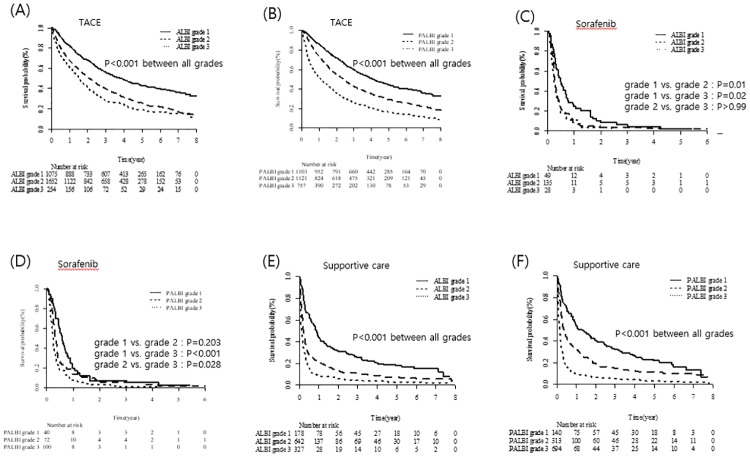
The predictive power of the ALBI and PALBI grades for OS was next assessed according to palliative treatment modality (TACE, sorafenib, and supportive care). In patients receiving TACE, the ALBI and PALBI grades were significantly predictive of OS (P < 0.001 for each grade; Fig 7A and 7B). In patients on sorafenib, the ALBI grade was significantly predictive of OS, with the exception of grade 2 vs. 3 (grade 1 vs. 2, P = 0.01; grade 1 vs. 3, P = 0.02; grade 2 vs. 3, P > 0.99; Fig 7C). OS differed significantly between PALBI grades 1 and 2 vs. 3 (P < 0.001 and 0.028, respectively). However, OS did not differ between PALBI grades 1 and 2 in the sorafenib group (P = 0.203; Fig 7D). In patients on supportive care, the ALBI and PALBI grades were significantly predictive of OS (P < 0.001 for each grade; Fig 7E and 7F).
Fig 7. Utility of the ALBI and PALBI grades according to palliative treatment modality.
Analysis of OS according to treatment modality with BCLC stage
The results of univariate and multivariate Cox regression analyses for OS are shown in Table 4. Age, male sex, Non-B&C, maximum tumor diameter, AFP level, and CTP class were independent risk factors for OS. The ALBI and PALBI grades were associated significantly with OS in the multivariate analysis.
Table 4. Cox regression analysis including ALBI and PALBI grade on overall survival.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p value | Hazard ratio (95% CI) | p value | |
| Age, years | 1.01 (1.01–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| Gender (Female vs. male) | 0.84 (0.77–0.90) | <0.001 | 0.83 (0.76–0.91) | <0.001 |
| CTP Class | ||||
| Class B vs. Class A | 2.91 (2.72–3.12) | <0.001 | 1.91(1.72–2.13) | <0.001 |
| Class C vs. Class A | 4.66 (4.12–5.27) | <0.001 | 3.27 (2.66–4.01) | <0.001 |
| Etiology | ||||
| Hepatitis B | 0.81 (0.76–0.87) | <0.001 | 1.04 (0.79–1.36) | 0.802 |
| Hepatitis C | 1.11 (1.01–1.22) | 0.024 | 1.15 (0.89–1.49) | 0.277 |
| Non-B, C | 0.94 (0.79–1.11) | 0.464 | 0.71 (0.51–0.99) | 0.043 |
| Alcohol | 1.27 (1.18–1.36) | <0.001 | 1.16 (0.88–1.54) | 0.297 |
| Maximal tumor diameter | ||||
| 2–5 cm vs. ≤2 cm | 1.43 (1.30–1.56) | <0.001 | 1.41 (1.28–1.56) | <0.001 |
| >5 cm vs. ≤2 cm | 4.16 (3.81–4.55) | <0.001 | 3.72 (3.36–4.12) | <0.001 |
| Alpha-fetoprotein, ng/mL | ||||
| > 400 vs. ≤ 400 | 2.43 (2.27–2.59) | <0.001 | 1.74 (1.61–1.89) | <0.001 |
| ALBI grade | ||||
| grade 2 vs. grade 1 | 2.39 (2.23–2.57) | <0.001 | 1.70 (1.53–1.89) | <0.001 |
| grade 3 vs. grade 1 | 4.43 (4.02–4.89) | <0.001 | 1.56 (1.30–1.86) | <0.001 |
| PALBI grade | ||||
| grade 2 vs. grade 1 | 1.89(1.75–2.05) | <0.001 | 1.13 (1.02–1.26) | 0.023 |
| grade 3 vs. grade 1 | 4.31(3.98–4.65) | <0.001 | 1.57 (1.37–1.80) | <0.001 |
CTP, Child-Turcotte-Pugh; ALBI, albumin-bilirubin; PALBI, platelet-albumin-bilirubin
The patients were evaluated according to initial treatment modality and BCLC stage. First, predictive factors for OS in patients of BLCC stage 0 or A undergoing curative treatment were evaluated (Table 5). Among the 591 patients receiving RFA, 390 survived and 201 died. In the multivariate analysis, the ALBI grade was an independent predictive factor for OS in model 1 (grade 2 vs. 1, P = 0.009; grade 3 vs. 1, P = 0.002). However, the PALBI grade was not a significant factor in patients receiving RFA. In model 3, the ALBI grade was an independent predictive factor for OS (grade 2 vs. 1, P = 0.038; grade 3 vs. 1, P = 0.001). Among the 855 patients undergoing SR, 672 survived and 183 died. The ALBI grade was a significant factor for OS in the multivariate analysis (model 1, grade 2 vs. 1, P < 0.001). However, only seven ALBI grade 3 patients underwent SR, and there was no significance between ALBI grades 3 and 1 (model 1; P = 0.705). The PALBI grade was not a significant risk factor in model 2. In model 3, the ALBI and PALBI grades had significance between grade 2 vs. 1 (P < 0.001 and 0.015, respectively).
Table 5. Multivariate cox regression analysis on survival according to curative treatment modalities with BCLC stage.
| Initial treatment modality | Number of patients | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||
| Alive | Dead | crude HR (95% CI) |
p value | adjust HR (95% CI) |
p value | adjust HR (95% CI) |
p value | adjust HR (95% CI) |
p value | |
| RFA patients & BCLC 0,A (n = 591,alive = 390,death = 201) | ||||||||||
| Age, years (mean age) | 58.7±.9 | 62.8±10.9 | 1.04(1.02–1.05) | <0.001 | 1.04(1.02–1.05) | <0.001 | 1.03(1.01–1.05) | 0.001 | 1.03(1.02–1.05) | <0.001 |
| Gender (Female vs. male) | 119/307 | 59/166 | 0.93(0.69–1.26) | 0.651 | 0.78(0.55–1.10) | 0.152 | 0.83(0.59–1.16) | 0.273 | 0.80(0.57–1.13) | 0.208 |
| CTP Class (Class B vs. Class A) | 33/393 | 59/166 | 3.26(2.42–4.39) | <0.001 | 2.28(1.53–3.42) | <0.001 | 3.52(2.23–5.57) | <0.001 | 3.11(1.93–5.00) | <0.001 |
| Etiology | ||||||||||
| Hepatitis B | 283 | 107 | 0.49(0.38–0.64) | <0.001 | 0.74(0.25–2.16) | 0.576 | 0.75(0.26–2.19) | 0.602 | 0.79(0.27–2.30) | 0.658 |
| Hepatitis C | 63 | 54 | 1.63(1.19–2.22) | 0.002 | 1.22(0.43–3.51) | 0.709 | 1.23(0.43–3.51) | 0.694 | 1.26(0.44–3.60) | 0.666 |
| NBNC | 16 | 4 | 0.47(0.18–1.28) | 0.140 | 0.32(0.07–1.49) | 0.147 | 0.30(0.07–1.40) | 0.126 | 0.31(0.07–1.44) | 0.136 |
| Alcohol | 68 | 58 | 1.82(1.35–2.445) | <0.001 | 1.16(0.37–3.58) | 0.800 | 1.20(0.39–3.70) | 0.749 | 0.35(0.04–2.98) | 0.339 |
| Maximal tumor diameter | ||||||||||
| 2–5 cm vs. ≤2 cm | 105/319 | 82/141 | 1.48(1.13–1.95) | 0.005 | 1.41(1.05–1.89) | 0.022 | 1.45(1.09–1.95) | 0.012 | 1.40(1.05–1.87) | 0.024 |
| >5 cm vs. ≤2 cm | 2/319 | 2/141 | 1.74(0.43–7.04) | 0.436 | 1.53(0.21–11.29) | 0.676 | 1.22(0.17–8.96) | 0.843 | 1.35(0.18–9.90) | 0.771 |
| Alpha-fetoprotein, ng/mL | ||||||||||
| > 400 vs. ≤ 400 | 25/273 | 15/192 | 1.31 (0.77–2.22) | 0.315 | 1.93(1.10–3.38) | 0.021 | 1.79(1.03–3.13) | 0.04 | 1.95(1.11–3.40) | 0.019 |
| ALBI grade | ||||||||||
| grade 2 vs. grade 1 | 169/251 | 127/81 | 1.95(1.48–2.58) | <0.001 | 1.55(1.12–2.14) | 0.009 | 1.55(1.02–2.35) | 0.038 | ||
| grade 3 vs. grade 1 | 6/251 | 17/81 | 5.10(3.02–8.60) | <0.001 | 3.05(1.53–6.08) | 0.002 | 4.27(1.89–9.66) | <0.001 | ||
| PALBI grade | ||||||||||
| grade 2 vs. grade 1 | 116/277 | 80/105 | 1.56(1.17–2.09) | 0.003 | 1.37(0.99–1.90) | 0.059 | 1.05(0.69–1.59) | 0.815 | ||
| grade 3 vs. grade 1 | 33/277 | 40/105 | 2.41(1.67–3.47) | <0.001 | 0.93(0.52–1.67) | 0.806 | 0.51(0.25–1.05) | 0.068 | ||
| Surgical resection & BCLC 0,A (n = 855,alive = 672,death = 183) | ||||||||||
| Age, years | 56.1±10.3 | 58.2±11.2 | 1.02(1.01–1.04) | 0.004 | 1.01(1.00–1.03) | 0.099 | 1.02(1.00–1.03) | 0.058 | 1.01(1.00–1.03) | 0.088 |
| Gender (Female vs. male) | 139/572 | 37/159 | 0.97(0.68–1.38) | 0.845 | 0.85(0.58–1.26) | 0.427 | 0.85(0.58–1.26) | 0.427 | 0.79(0.53–1.17) | 0.242 |
| CTP Class‡ (Class B vs. Class A) | 14/697 | 14/182 | 3.03(1.76–5.21) | <0.001 | 1.92(0.91–4.04) | 0.086 | 2.21(1.02–4.79) | 0.044 | 1.84(0.82–4.11) | 0.137 |
| Etiology | ||||||||||
| Hepatitis B | 526 | 130 | 0.78(0.57–1.05) | 0.102 | 1.00(0.67–1.35) | 0.781 | 1.01(0.71–1.44) | 0.943 | 0.95(0.67–1.36) | 0.789 |
| Hepatitis C | 38 | 25 | 2.19(1.44–3.34) | <0.001 | 1.65(0.56–4.87) | 0.361 | 2.02(0.70–5.85) | 0.196 | 1.40(0.45–4.31) | 0.559 |
| NBNC | 17 | 2 | 0.41(0.10–1.64) | 0.207 | 0.32(0.06–1.80) | 0.194 | 0.30(0.05–1.70) | 0.176 | 0.29(0.05–1.71) | 0.170 |
| Alcohol | 132 | 39 | 1.10(0.77–1.56) | 0.602 | 0.95(0.29–3.06) | 0.926 | 0.95(0.30–3.04) | 0.927 | 0.83(0.25–2.81) | 0.765 |
| Maximal tumor diameter | ||||||||||
| 2–5 cm vs. ≤2 cm | 415/185 | 100/35 | 1.23(0.84–1.81) | 0.292 | 1.23(0.82–1.83) | 0.294 | 1.25(0.83–1.87) | 0.281 | 1.24(0.83–1.86) | 0.294 |
| >5 cm vs. ≤2 cm | 110/185 | 59/35 | 2.39(1.57–3.63) | <0.001 | 2.53(1.60–3.99) | <0.001 | 2.48(1.57–3.92) | 0.000 | 2.40(1.52–3.77) | <0.001 |
| Alpha-fetoprotein, ng/mL | ||||||||||
| > 400 vs. ≤ 400 | 122/552 | 39/148 | 1.19(0.83–1.70) | 0.350 | 1.18(0.80–1.75) | 0.400 | 1.13(0.76–1.67) | 0.555 | 1.19(0.80–1.76) | 0.390 |
| ALBI grade | ||||||||||
| grade 2 vs. grade 1 | 155/553 | 84/108 | 2.44(1.83–3.24) | <0.001 | 2.16(1.58–2.95) | <0.001 | 2.76(1.89–4.04) | <0.001 | ||
| grade 3 vs. grade 1 | 3/553 | 4/108 | 4.20(1.55–11.40) | 0.005 | 1.37(0.27–6.86) | 0.705 | 1.64(0.32–8.52) | 0.555 | ||
| PALBI grade | ||||||||||
| grade 2 vs. grade 1 | 201/487 | 61/114 | 1.22(0.89–1.67) | 0.209 | 1.04(0.75–1.45) | 0.807 | 0.62(0.42–0.91) | 0.015 | ||
| grade 3 vs. grade 1 | 23/487 | 21/114 | 2.91(1.83–4.64) | <0.001 | 1.58(0.84–2.97) | 0.160 | 0.83(0.43–1.62) | 0.584 | ||
RFA, radiofrequency ablation; BCLC, Barcelona clinic liver cancer stage; CTP, Child-Turcotte-Pugh; ALBI, albumin-bilirubin; PALBI, platelet-albumin-bilirubin
The factors predictive of OS according to palliative treatment modality are listed in Table 6. Among the 1,715 patients of BCLC stage 0 to B receiving TACE, 684 survived and 1,030 died. The PALBI grade was a significant risk factor for OS (model 2: grade 2 vs. 1, P < 0.001; grade 3 vs. 1, P < 0.001). In model 3, PALBI grade 3 vs. grade 1 had significant difference (P < 0.001), but there was no significant difference between PALBI grades 2 and 1 (P = 0.053). Only ALBI grade 2 vs. 1 was a risk factor for OS (model 1, P < 0.001; model 3, P < 0.001). Of the patients of BCLC stage C on sorafenib (n = 111), 4 survived and 107 died. The ALBI and PALBI grades were not predictive of OS in patients on sorafenib. Among the patients on supportive care (n = 1,147; 81 survived, 1,066 died), the ALBI and PALBI grades were effective factor for survival in models 1 and 2, respectively. According to model 3, the predictive power of the PALBI grade for OS was superior to that of the ALBI grade (PALBI grade 2 vs. 1, P = 0.002; grade 3 vs. 1, P < 0.001).
Table 6. Multivariate cox regression analysis on survival according to palliative treatment modalities with BCLC stage.
| Initial treatment modality | Number of patients | Univariate analysis | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | ||||||||
| Alive | Dead | crude HR (95% CI) |
p value | adjust HR (95% CI) |
p value | adjust HR (95% CI) |
p value | adjust HR (95% CI) |
p value | |
| TACE patients& BCLC 0,A,B (n = 1715,alive = 684,death = 1030) | ||||||||||
| Age, years | 59.7±9.6 | 62.8±10.8 | 1.02(1.02–1.03) | <0.001 | 1.02(1.01–1.03) | <0.001 | 1.02(1.02–1.03) | <0.001 | 1.02(1.02–1.03) | <0.001 |
| Gender (Female vs. male) | 178/572 | 262/898 | 0.94(0.82–1.08) | 0.385 | 0.85(0.73–0.99) | 0.034 | 0.89(0.76–1.03) | 0.118 | 0.86(0.74–1.00) | 0.043 |
| CTP Class (Class B vs. Class A) | 78/672 | 288/872 | 1.87(1.64–2.14) | <0.001 | 1.82(1.53–2.17) | <0.001 | 1.43(1.17–1.75) | 0.001 | 1.43(1.17–1.75) | 0.001 |
| Etiology | ||||||||||
| Hepatitis B | 504 | 622 | 0.70(0.62–0.79) | <0.001 | 0.85 (0.54–1.33) | 0.480 | 0.81 (0.51–1.27) | 0.355 | 0.84 (0.53–1.33) | 0.457 |
| Hepatitis C | 87 | 196 | 1.24 (1.07–1.45) | 0.006 | 0.94 (0.60–1.46) | 0.772 | 0.90 (0.57–1.42) | 0.655 | 0.91 (0.58–1.44) | 0.696 |
| NBNC | 24 | 35 | 0.98 (0.70–1.37) | 0.913 | 0.66 (0.38–1.15) | 0.144 | 0.57 (0.32–1.01) | 0.054 | 0.60 (0.34–1.05) | 0.074 |
| Alcohol | 135 | 299 | 1.37 (1.20–1.57) | <0.001 | 0.98 (0.62–1.57) | 0.943 | 0.90 (0.56–1.45) | 0.663 | 0.95 (0.59–1.52) | 0.824 |
| Maximal tumor diameter | ||||||||||
| 2–5 cm vs. ≤2 cm | 329/343 | 522/331 | 1.45(1.27–1.67) | <0.001 | 1.37(1.18–1.58) | <0.001 | 1.36(1.17–1.57) | <0.001 | 1.38(1.19–1.60) | <0.001 |
| >5 cm vs. ≤2 cm | 77/343 | 270/331 | 2.67(2.28–3.14) | <0.001 | 2.65(2.21–3.17) | <0.001 | 2.53(2.12–3.03) | <0.001 | 2.64(2.20–3.16) | <0.001 |
| Alpha-fetoprotein, ng/mL | ||||||||||
| > 400 vs. ≤ 400 | 88/611 | 227/867 | 1.61 (1.38–1.87) | <0.001 | 1.45 (1.23–1.70) | <0.001 | 1.40 (1.20–1.65) | <0.001 | 1.41 (1.20–1.66) | <0.001 |
| ALBI grade | ||||||||||
| grade 2 vs. grade 1 | 332/395 | 690/385 | 1.62(1.43–1.83) | <0.001 | 1.55(1.34–1.79) | <0.001 | 1.37(1.15–1.62) | <0.001 | ||
| grade 3 vs. grade 1 | 23/395 | 85/385 | 2.26(1.79–2.86) | <0.001 | 1.39(1.02–1.90) | 0.036 | 1.00(0.72–1.40) | 0.992 | ||
| PALBI grade | ||||||||||
| grade 2 vs. grade 1 | 250/428 | 469/424 | 1.51(1.32–1.72) | <0.001 | 1.38(1.20–1.60) | <0.001 | 1.18(1.00–1.39) | 0.053 | ||
| grade 3 vs. grade 1 | 72/428 | 267/424 | 2.32(1.99–2.70) | <0.001 | 2.09(1.67–2.62) | <0.001 | 1.86(1.46–2.38) | <0.001 | ||
| sorafenib & BCLC C (n = 111, alive = 4, death = 107) | ||||||||||
| Age, years | 62.0±7.4 | 55.5±11.5 | 0.99(0.98–1.00) | 0.086 | 0.99(0.97–1.01) | 0.402 | 0.99(0.97–1.02) | 0.436 | 0.99(0.96–1.01) | 0.288 |
| Gender (Female vs. male) | 0/4 | 23/150 | 1.15(0.74–1.79) | 0.524 | 1.45(0.73–2.88) | 0.296 | 1.47(0.73–2.95) | 0.285 | 1.49(0.74–3.03) | 0.266 |
| CTP Class (Class B vs. Class A) | 0/4 | 75/98 | 1.91(1.41–2.60) | <0.001 | 2.32(1.44–3.73) | 0.001 | 1.95(1.20–3.19) | 0.007 | 2.04(1.24–3.37) | 0.005 |
| Etiology | ||||||||||
| Hepatitis B | 3 | 123 | 1.28(0.91–1.80) | 0.157 | 2.17 (0.71–6.62) | 0.175 | 2.09 (0.72–6.10) | 0.178 | 2.12 (0.70–6.40) | 0.185 |
| Hepatitis C | 0 | 18 | 0.83 (0.51–1.36) | 0.455 | 1.67 (0.57–4.90) | 0.348 | 1.62 (0.59–4.46) | 0.349 | 1.63 (0.57–4.69) | 0.362 |
| NBNC | 0 | 8 | 0.89 (0.43–1.80) | 0.736 | 2.42 (0.51–11.4) | 0.265 | 2.36 (0.52–10.66) | 0.263 | 2.57 (0.54–12.18) | 0.236 |
| Alcohol | 1 | 27 | 0.85 (0.57–1.29) | 0.45 | 1.27 (0.38–4.31) | 0.699 | 1.03 (0.32–3.32) | 0.961 | 1.07 (0.32–3.57) | 0.919 |
| Maximal tumor diameter | ||||||||||
| 2–5 cm vs. ≤2 cm | 0/1 | 15/3 | 4.10(1.16–14.40) | 0.028 | 3.59(0.94–13.7) | 0.062 | 3.27(0.82–13.02) | 0.093 | 3.16(0.80–12.51) | 0.102 |
| >5 cm vs. ≤2 cm | 1/3 | 95/3 | 2.67(0.84–8.49) | 0.097 | 2.44(0.70–8.46) | 0.160 | 2.04(0.58–7.22) | 0.267 | 1.99(0.56–7.06) | 0.290 |
| Alpha-fetoprotein, ng/mL | ||||||||||
| > 400 vs. ≤ 40 | 3/1 | 104/63 | 1.54 (1.05–2.25) | 0.029 | 1.63 (1.08–2.46) | 0.020 | 1.68 (1.12–2.54) | 0.013 | 1.68 (1.11–2.53) | 0.014 |
| ALBI grade | ||||||||||
| grade 2 vs. grade 1 | 3/1 | 119/40 | 1.58(1.10–2.27) | 0.013 | 1.38(0.77–2.49) | 0.280 | 1.20(0.63–2.30) | 0.579 | ||
| grade 3 vs. grade 1 | 0/1 | 14/40 | 2.55(1.37–4.73) | 0.003 | 1.25(0.50–3.17) | 0.632 | 0.81(0.30–2.20) | 0.683 | ||
| PALBI grade | ||||||||||
| grade 2 vs. grade 1 | 2/1 | 60/36 | 1.44(0.95–2.18) | 0.088 | 1.10(0.59–2.07) | 0.764 | 0.99(0.50–1.97) | 0.986 | ||
| grade 3 vs. grade 1 | 1/1 | 77/36 | 2.35(1.57–3.52) | <0.001 | 1.95(1.05–3.64) | 0.036 | 1.89(0.96–3.72) | 0.064 | ||
| supportive patients (n = 1147, alive = 81, death = 1066) | ||||||||||
| Age, years | 59.3±11.8 | 62.9±12.6 | 1.00(0.99–1.00) | 0.200 | 1.01(1.00–1.01) | 0.051 | 1.01(1.00–1.01) | 0.043 | 1.01(1.00–1.01) | 0.041 |
| Gender (Female vs. male) | 19/62 | 206/860 | 0.91(0.78–1.06) | 0.232 | 0.97(0.81–1.17) | 0.752 | 0.97(0.81–1.17) | 0.748 | 0.96(0.80–1.16) | 0.670 |
| CTP Class | ||||||||||
| Class B vs. Class A | 20/61 | 474/400 | 2.03(1.77–2.32) | <0.001 | 1.74(1.43–2.11) | <0.001 | 1.61(1.32–1.95) | <0.001 | 1.53(1.24–1.89) | <0.001 |
| Class C vs. Class A | 0/61 | 192/400 | 3.41(2.85–4.07) | <0.001 | 3.09(2.30–4.14) | <0.001 | 3.03(2.36–3.94) | <0.001 | 2.68(1.98–3.63) | <0.001 |
| Etiology | ||||||||||
| Hepatitis B | 39 | 572 | 1.22(1.08–1.38) | 0.002 | 0.95 (0.61–1.46) | 0.798 | 0.96 (0.63–1.48) | 0.861 | 0.95 (0.62–1.47) | 0.824 |
| Hepatitis C | 3 | 131 | 0.93 (0.77–1.12) | 0.427 | 0.94 (0.63–1.42) | 0.773 | 0.96 (0.64–1.45) | 0.838 | 0.96 (0.64–1.45) | 0.842 |
| NBNC | 1 | 24 | 0.91 (0.60–1.36) | 0.629 | 0.95 (0.50–1.80) | 0.880 | 0.91 (0.48–1.71) | 0.767 | 0.90 (0.48–1.70) | 0.754 |
| Alcohol | 35 | 340 | 0.86 (0.75–0.97) | 0.018 | 0.96 (0.61–1.51) | 0.867 | 0.96 (0.61–1.52) | 0.871 | 0.96 (0.61–1.51) | 0.850 |
| Maximal tumor diameter | ||||||||||
| 2–5 cm vs. ≤2 cm | 32/30 | 217/93 | 1.47(1.16–1.88) | 0.002 | 1.51(1.16–1.96) | 0.002 | 1.44(1.11–1.87) | 0.007 | 1.46(1.12–1.90) | 0.005 |
| >5 cm vs. ≤2 cm | 16/30 | 516/93 | 2.87(2.29–3.59) | <0.001 | 3.23(2.51–4.15) | <0.001 | 2.96(2.31–3.79) | <0.001 | 3.08(2.39–3.96) | <0.001 |
| Alpha-fetoprotein, ng/mL | ||||||||||
| > 400 vs. ≤ 400 | 14/57 | 463/522 | 2.13(1.82–2.49) | <0.001 | 1.50 (1.28–1.75) | <0.001 | 1.49 (1.28–1.74) | <0.001 | 1.50 (1.28–1.75) | <0.001 |
| ALBI grade | ||||||||||
| grade 2 vs. grade 1 | 43/30 | 599/258 | 1.83(1.53–2.19) | <0.001 | 1.43(1.14–1.79) | 0.002 | 1.00(0.76–1.32) | 0.985 | ||
| grade 3 vs. grade 1 | 8/30 | 463/258 | 2.89(2.38–3.53) | <0.001 | 1.88(1.38–2.56) | <0.001 | 1.21(0.84–1.73) | 0.308 | ||
| PALBI grade | ||||||||||
| grade 2 vs. grade 1 | 34/28 | 279/112 | 1.58(1.27–1.97) | <0.001 | 1.60(1.24–2.07) | 0.000 | 1.60(1.20–2.15) | 0.002 | ||
| grade 3 vs. grade 1 | 19/28 | 675/112 | 3.18(2.59–3.89) | <0.001 | 2.22(1.69–2.91) | <0.001 | 2.16(1.55–2.99) | <0.001 | ||
TACE, transarterial chemoembolization; BCLC, Barcelona clinic liver cancer stage; CTP, Child-Turcotte-Pugh; ALBI, albumin-bilirubin; PALBI, platelet-albumin-bilirubin
Discussion
In this analysis of nationally representative data, we evaluated the ability of various scoring system focused on the ALBI and PALBI grades to predict the OS of patients with HCC. The ALBI and PALBI grades had higher AUC values than did the CTP class and MELD score. The ALBI and PALBI grades also showed good predictive performance for OS in patients with HCC. Moreover, each grade has different strength according to treatment modalities. Therefore, the ALBI and PALBI grades may be used to assess liver function and predict the survival of patients with HCC according to treatment modalities.
Our analysis showed that the PALBI and ALBI grades enable prediction of OS. In patients with HCC of CTP class A, the PALBI and ALBI grades enabled discrimination of OS during the 5-year study period. The majority (71.8%) of patients with HCC were of CTP class A. Patients with CTP class A HCC can have various clinical courses, including no chronic liver disease, chronic inflammation only, and well-compensated cirrhosis [24, 25]. Although the CTP class has been used to estimate the liver functional reserve and predict OS in patients with HCC, the subclassification of same CTP class A enables prediction of OS in patients with HCC with well-preserved liver function. Thus, the PALBI and ALBI grades allow assessment of the liver functional reserve.
In the view of etiologies and tumor marker, both ALBI and PALBI grades could predict and stratify OS across all different etiologies and the level of tumor marker. However, because non-B,C patients are only small number in our study with possible heterogeneous cause of hepatitis, it may affect the negative result in the stratification of OS between ALBI grade 2 vs.3 and PALBI grade 1 vs. 2. In multivariate analysis, the ALBI and PALBI grade are independently predictive factor for OS regardless of etiologies and AFP level. Therefore, the PALBI and ALBI grades could be applied to predict OS irrespective of etiology and tumor marker level.
We also evaluated the ability of the ALBI and PALBI grades to predict OS according to BCLC stage and treatment modality. First, we investigated both grading systems according to curative treatment modalities. ALBI grades 1 and 2, but not the PALBI grade, were predictive of OS of patients with HCC undergoing SR. The lack of a difference between ALBI grades 2 and 3 may be due to the small number of ALBI grade 3 patients undergoing SR (n = 7). The OS of patients undergoing RFA differed significantly among ALBI grades 1 to 3, but not according to the PALBI grade. These results are in agreement with previous reports that the ALBI grade is predictive of survival after liver resection and RFA [26, 27]. However, the ALBI grade showed greater predictive power than the PALBI grade in this study. Patients undergoing curative treatments typically have good liver function and relatively high platelet counts [28, 29], which may explain our finding of greater predictive power in patients with HCC receiving curative treatments. In contrast, neither the ALBI grade nor the PALBI grade was predictive of OS in patients with HCC receiving LT. The ALBI and PALBI grades predict the risk of mortality related to underlying liver dysfunction. OS prediction using these grades in patients receiving LT may be difficult because LT may not only treat HCC, but also manage underlying liver dysfunction, and because many other factors affect survival during the perioperative period [30].
Second, we evaluated the predictive power for OS of the ALBI and PALBI grades according to palliative treatment modality. In patients with HCC receiving TACE, PALBI grades 1 to 3, and ALBI grades 1 to 2, were significantly predictive of OS. Similarly, the ALBI and PALBI grades are reportedly predictive of survival in patients with HCC undergoing TACE [27, 31]. In our study, ALBI grades 2 and 3 were not significantly predictive of OS. Some ALBI grade 3 patients treated with TACE had severe portal hypertension, resulting in reduced platelet counts, which cannot be distinguished using the ALBI grade. This limitation may have contributed to the negative results for ALBI grades 2 and 3. In patients with HCC of BCLC stage C on sorafenib, PALBI grades 1 and 3, but not the ALBI grades, were significantly predictive of OS. In model 3, both ALBI and PALBI grades are not significant factor of OS. The high risk of mortality of patients of these patients with sorafenib may be related to tumor progression, rather than underlying liver dysfunction. The OS of patients receiving supportive care differed significantly according to the ALBI grade and to PALBI grades 1 to 3. The PALBI grade had greater predictive power than the ALBI grade. Therefore, the evaluation of underlying liver function, including a surrogate for portal hypertension, is important, particularly in patients on supportive treatment. Therefore, the PALBI grade may enable more refined discrimination among patients with HCC receiving palliative treatments; this issue warrants further investigation.
This study has several strengths and limitations. First, we used Korean HCC registry data for a 5-year period. We evaluated a nationally representative cohort of patients with HCC, increasing the accuracy of the results. Second, our results highlight the importance of identifying candidates using more suitable grade system according to BCLC staging and treatment. Although other authors have demonstrated the utility of the ALBI and PALBI grades, these grades have not been studied to identify candidates for whom particular treatment modalities are suitable. Our results suggest that the ALBI grade is more useful than the PALBI grade in patients with HCC undergoing curative treatment, and vice versa for those receiving palliative treatment. Third, we analyzed data of treatment modality with BCLC stage. The treatment decision-making process differed among the participating centers, likely reflecting clinical practice, in which various factors are considered before making decision. To reduce bias, we defined treatment modality with BCLC stage. Forth, unfortunately, there was no data about antiviral therapy in HCC patients with HBV and HCV in our cohort. However, as Korean medical insurance covered HCC patients for antiviral treatment, HCC patients with HBV received and maintained antiviral treatment according to HCC guideline. In HCV treatment, Direct Acting Antivirals (DAA) therapy was not available in Korea during the study period. So, HCC patients with HCV did not receive DAA.
In conclusion, the ALBI and PALBI grades showed good performance for the assessment of liver function in patients with HCC. The ALBI grade showed greater predictive power for OS than did the PALBI grade in patients with HCC receiving curative treatment, and the opposite was true for those receiving palliative treatment. Therefore, appropriate use of the ALBI and PALBI grades will enable more-accurate prediction of the prognosis of patients with HCC.
Abbreviations
- AFP
alpha-fetoprotein
- ALBI
albumin-bilirubin
- AUC
area under the receiver operator characteristic curve
- BCLC
Barcelona Clinic Liver Cancer
- CTP
Child-Turcotte-Pugh
- DAA
Direct Acting Antivirals
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- KCCR
Korean Central Cancer Registry
- KLCA
The Korean Liver Cancer Association
- LT
liver transplantation
- MELD
Model for End-stage Liver Disease
- MRI
magnetic resonance imaging
- OS
overall survival
- PALBI
Platelet-albumin-bilirubin
- RFA
radiofrequency ablation
- SR
surgical resection
- TACE
transarterial chemoembolization
Data Availability
The data of this study was from the Korean Central Cancer Registry (KCCR) and it is ethical restriction data on sharing. Moreover, because of institution policy, data cannot be shared. Data are available after approval from the Ethics Committee of the Institutional Review Board (contact via email; ctcirb@cmcdj.or.kr) for researchers who meet the criteria for access to data.
Funding Statement
This work is supported by 2017R1C1B5017511 of national research foundation of Korea. The statistical consultation was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C1062). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International journal of cancer. 2015;136(5):E359–86. Epub 2014/09/16. 10.1002/ijc.29210 . [DOI] [PubMed] [Google Scholar]
- 2.Kanda T, Ogasawara S, Chiba T, Haga Y, Omata M, Yokosuka O. Current management of patients with hepatocellular carcinoma. World journal of hepatology. 2015;7(15):1913–20. Epub 2015/08/06. 10.4254/wjh.v7.i15.1913 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology (Baltimore, Md). 2018;67(1):358–80. Epub 2017/01/29. 10.1002/hep.29086 . [DOI] [PubMed] [Google Scholar]
- 4.Durand F, Valla D. Assessment of the prognosis of cirrhosis: Child-Pugh versus MELD. Journal of hepatology. 2005;42 Suppl(1):S100–7. Epub 2005/03/22. 10.1016/j.jhep.2004.11.015 . [DOI] [PubMed] [Google Scholar]
- 5.Child CG, Turcotte JG. Surgery and portal hypertension. Major problems in clinical surgery. 1964;1:1–85. Epub 1964/01/01. . [PubMed] [Google Scholar]
- 6.Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. The British journal of surgery. 1973;60(8):646–9. Epub 1973/08/01. . [DOI] [PubMed] [Google Scholar]
- 7.Durand F, Valla D. Assessment of prognosis of cirrhosis. Seminars in liver disease. 2008;28(1):110–22. Epub 2008/02/23. 10.1055/s-2008-1040325 . [DOI] [PubMed] [Google Scholar]
- 8.Lee YH, Hsu CY, Chu CW, Liu PH, Hsia CY, Huang YH, et al. A new Child-Turcotte-Pugh class 0 for patients with hepatocellular carcinoma: determinants, prognostic impact and ability to improve the current staging systems. PloS one. 2014;9(6):e99115 Epub 2014/06/07. 10.1371/journal.pone.0099115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson PJ, Berhane S, Kagebayashi C, Satomura S, Teng M, Reeves HL, et al. Assessment of liver function in patients with hepatocellular carcinoma: a new evidence-based approach-the ALBI grade. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2015;33(6):550–8. Epub 2014/12/17. 10.1200/jco.2014.57.9151 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrini GP, Pinelli D, Marini E, Corno V, Guizzetti M, Zambelli M, et al. Value of HCC-MELD Score in Patients With Hepatocellular Carcinoma Undergoing Liver Transplantation. Progress in transplantation (Aliso Viejo, Calif). 2018;28(1):63–9. Epub 2017/12/19. 10.1177/1526924817746686 . [DOI] [PubMed] [Google Scholar]
- 11.Rude MK, Crippin JS. Liver transplantation for hepatocellular carcinoma. Current gastroenterology reports. 2015;17(3):11 Epub 2015/03/06. 10.1007/s11894-015-0435-3 . [DOI] [PubMed] [Google Scholar]
- 12.Chan AWH, Leung HHW, Chong CCN, Chan SL. Validating the ALBI grade: Its current and future use in HCC prognostication. Journal of hepatology. 2017;66(3):661–3. Epub 2016/11/29. 10.1016/j.jhep.2016.10.037 . [DOI] [PubMed] [Google Scholar]
- 13.Pinato DJ, Sharma R, Allara E, Yen C, Arizumi T, Kubota K, et al. The ALBI grade provides objective hepatic reserve estimation across each BCLC stage of hepatocellular carcinoma. Journal of hepatology. 2017;66(2):338–46. Epub 2016/09/30. 10.1016/j.jhep.2016.09.008 . [DOI] [PubMed] [Google Scholar]
- 14.Hiraoka A, Kumada T, Michitaka K, Toyoda H, Tada T, Ueki H, et al. Usefulness of albumin-bilirubin grade for evaluation of prognosis of 2584 Japanese patients with hepatocellular carcinoma. Journal of gastroenterology and hepatology. 2016;31(5):1031–6. Epub 2015/12/10. 10.1111/jgh.13250 . [DOI] [PubMed] [Google Scholar]
- 15.Roayaie S, Jibara G, Berhane S, Tabrizian P, Park J-w, Yang J, et al. palbi-an Objective Score Based on Platelets, Albumin & Bilirubin Stratifies Hcc Patients Undergoing Resection & Ablation Better than Child’s Classification: 851. Hepatology (Baltimore, Md). 2015;62:631A–2A. [Google Scholar]
- 16.2014 KLCSG-NCC Korea Practice Guideline for the Management of Hepatocellular Carcinoma. Gut and liver. 2015;9(3):267–317. Epub 2015/04/29. 10.5009/gnl14460 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, et al. A model to predict survival in patients with end-stage liver disease. Hepatology (Baltimore, Md). 2001;33(2):464–70. Epub 2001/02/15. 10.1053/jhep.2001.22172 . [DOI] [PubMed] [Google Scholar]
- 18.Huo TI, Huang YH, Lin HC, Wu JC, Chiang JH, Lee PC, et al. Proposal of a modified Cancer of the Liver Italian Program staging system based on the model for end-stage liver disease for patients with hepatocellular carcinoma undergoing loco-regional therapy. The American journal of gastroenterology. 2006;101(5):975–82. Epub 2006/04/01. 10.1111/j.1572-0241.2006.00462.x . [DOI] [PubMed] [Google Scholar]
- 19.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet (London, England). 2012;379(9822):1245–55. Epub 2012/02/23. 10.1016/s0140-6736(11)61347-0 . [DOI] [PubMed] [Google Scholar]
- 20.Ueno S, Tanabe G, Nuruki K, Hamanoue M, Komorizono Y, Oketani M, et al. Prognostic performance of the new classification of primary liver cancer of Japan (4th edition) for patients with hepatocellular carcinoma: a validation analysis. Hepatology research: the official journal of the Japan Society of Hepatology. 2002;24(4):395–403. Epub 2002/12/14. . [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z. Univariate description and bivariate statistical inference: the first step delving into data. Annals of translational medicine. 2016;4(5):91 Epub 2016/04/06. 10.21037/atm.2016.02.11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu CY, Liu PH, Lee YH, Hsia CY, Huang YH, Lin HC, et al. Using serum alpha-fetoprotein for prognostic prediction in patients with hepatocellular carcinoma: what is the most optimal cutoff? PloS one. 2015;10(3):e0118825 Epub 2015/03/05. 10.1371/journal.pone.0118825 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye JZ, Chen JZ, Li ZH, Bai T, Chen J, Zhu SL, et al. Efficacy of postoperative adjuvant transcatheter arterial chemoembolization in hepatocellular carcinoma patients with microvascular invasion. World journal of gastroenterology. 2017;23(41):7415–24. Epub 2017/11/21. 10.3748/wjg.v23.i41.7415 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva MF, Sapisochin G, Strasser SI, Hewa-Geeganage S, Chen J, Wigg AJ, et al. Liver resection and transplantation offer similar 5-year survival for Child-Pugh-Turcotte A HCC-patients with a single nodule up to 5 cm: a multicenter, exploratory analysis. European journal of surgical oncology: the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2013;39(4):386–95. Epub 2013/02/05. 10.1016/j.ejso.2012.12.011 . [DOI] [PubMed] [Google Scholar]
- 25.Chen X, Zhai J, Cai X, Zhang Y, Wei L, Shi L, et al. Severity of portal hypertension and prediction of postoperative liver failure after liver resection in patients with Child-Pugh grade A cirrhosis. The British journal of surgery. 2012;99(12):1701–10. Epub 2012/11/08. 10.1002/bjs.8951 . [DOI] [PubMed] [Google Scholar]
- 26.Wang YY, Zhong JH, Su ZY, Huang JF, Lu SD, Xiang BD, et al. Albumin-bilirubin versus Child-Pugh score as a predictor of outcome after liver resection for hepatocellular carcinoma. The British journal of surgery. 2016;103(6):725–34. Epub 2016/03/24. 10.1002/bjs.10095 . [DOI] [PubMed] [Google Scholar]
- 27.Liu PH, Hsu CY, Hsia CY, Lee YH, Chiou YY, Huang YH, et al. ALBI and PALBI grade predict survival for HCC across treatment modalities and BCLC stages in the MELD Era. Journal of gastroenterology and hepatology. 2017;32(4):879–86. Epub 2016/10/04. 10.1111/jgh.13608 . [DOI] [PubMed] [Google Scholar]
- 28.Karasu Z, Tekin F, Ersoz G, Gunsar F, Batur Y, Ilter T, et al. Liver fibrosis is associated with decreased peripheral platelet count in patients with chronic hepatitis B and C. Digestive diseases and sciences. 2007;52(6):1535–9. Epub 2007/04/28. 10.1007/s10620-006-9144-y . [DOI] [PubMed] [Google Scholar]
- 29.Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. Journal of gastroenterology. 2011;46(11):1300–6. Epub 2011/07/14. 10.1007/s00535-011-0436-4 . [DOI] [PubMed] [Google Scholar]
- 30.Lucey MR, Terrault N, Ojo L, Hay JE, Neuberger J, Blumberg E, et al. Long-term management of the successful adult liver transplant: 2012 practice guideline by the American Association for the Study of Liver Diseases and the American Society of Transplantation. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2013;19(1):3–26. Epub 2013/01/03. 10.1002/lt.23566 . [DOI] [PubMed] [Google Scholar]
- 31.Hansmann J, Evers MJ, Bui JT, Lokken RP, Lipnik AJ, Gaba RC, et al. Albumin-Bilirubin and Platelet-Albumin-Bilirubin Grades Accurately Predict Overall Survival in High-Risk Patients Undergoing Conventional Transarterial Chemoembolization for Hepatocellular Carcinoma. Journal of vascular and interventional radiology: JVIR. 2017;28(9):1224–31.e2. Epub 2017/07/10. 10.1016/j.jvir.2017.05.020 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data of this study was from the Korean Central Cancer Registry (KCCR) and it is ethical restriction data on sharing. Moreover, because of institution policy, data cannot be shared. Data are available after approval from the Ethics Committee of the Institutional Review Board (contact via email; ctcirb@cmcdj.or.kr) for researchers who meet the criteria for access to data.