Abstract
Sex-biased infections are a recurrent observation in vertebrates. In many species, males are more parasitized than females. Two potentially complementary mechanisms are often suggested to explain this pattern: sexual differences in susceptibility mainly caused by the effect of sex hormones on immunity and differential exposure to parasites. Exposure is mostly a consequence of host behavioural traits, but vector-borne parasitic infections involve another degree of complexity due to the active role of vectors in transmission. Blood-sucking insects may make choices based on cues produced by hosts. Regarding malaria, several studies highlighted a male-biased infection by Plasmodium sp in great tits (Parus major). We hypothesize that the mosquito vector, Culex pipiens, might at least partially cause this bias by being more attracted to male birds. Intrinsic variation associated to bird sex would explain a preference of mosquitoes for males. To test this hypothesis, we provide uninfected mosquitoes with a choice between uninfected male and female nestlings. Mosquito choice is assessed by sex typing of the ingested blood. We did not observe any preference for a given sex. This result does not support our prediction of a preference of mosquitoes for male great tits during the nestling period. In conclusion, mosquitoes do not seem to have an intrinsic preference for male nestlings. However, sexually divergent traits (e.g. behaviour, odour, metabolic rate) present in adults may play a role in the attraction of mosquitoes and should be investigated.
Introduction
Prevalence and intensity of parasitism in vertebrates, is often higher in males than females ([1–6] but see [7]). Sex differences in susceptibility, development and exposure (reviewed in [8]) can explain sex-biased infection. Firstly, male-biased parasitism may be due to how parasites perform in each sex. Sex hormones have different influences on the immune system. Both androgens and oestrogens suppress cell-mediated immunity, but oestrogens can stimulate humoral immunity [9]. In particular, some studies on birds show evidence for the immunosuppressive role of testosterone ([10–12] but see [13, 14]). From this perspective, we might expect parasites to perform better within a male host.
Secondly, sex differences in behaviours linked to reproduction [15, 16], foraging [17] or social status [18, 19] may also induce a sex-biased exposition to parasite. For example, a reduction of grooming activities at the expense of time spent for territorial defences during the breeding season may imply that males harbour higher ectoparasite load than females as showed in impalas infected by ticks [20]. In contrast, females might be more exposed than males if they aggregated in nursery colonies, as shown for example in different bat species [7].
In vector-borne parasite diseases, vectors add another level of complexity. For instance, blood-feeding invertebrates such as mosquitoes actively seek for a blood meal and may differentially encounter male or female vertebrate hosts. In that case, sex-biased parasitism will result from specific host/vector interaction [21, 22]. In addition, mosquitoes mostly use olfaction during host-seeking and volatile organic compounds (VOC) and CO2 are crucial cues for them [23–25]. Because males are often bigger than females in both mammals and birds, they produce more CO2 [26–28] and may therefore be more easily detected by vectors. In addition, males’ odours are known to differ from females’ [29–32]. Both factors may result into a higher attractiveness of males to mosquitoes. Wild caught mosquitoes were found to feed more on male birds (64.0%) than females (36.0%, of 308 samples), consistently across mosquito species [33].
Other factors may influence mosquitoes’ host-choice, as for example relative humidity, parasitic infection, defensive behaviours or body heat (reviewed in [34]). In particular, mosquitoes are attracted to heat [35–38] or heated baits [39, 40]. However, this cue seems to act on mosquito behaviour at close proximity to the host mainly [41]. Former studies on different bird species found that females have a slightly higher temperature than males in most cases [42, 43], but it is not known whether it can influence mosquito choices.
Plasmodium is a mosquito-borne haemosporidian parasite that infects many different vertebrate host species. A male biased infection was observed in many host / parasite pairs [16, 44–48]. Differential exposure to mosquito bites between sexes may thus explain the observed sex-biased infection.
In this study, we investigated the role of Culex pipiens, the natural mosquito vector of Plasmodium in great tits in sex-biased malaria infection. Indeed, male-biased infection in great tits was observed in several studies [16, 49, 50]. A study of 13 years on two natural populations of P. major also found a sex effect on Plasmodium infection prevalence, with males being more infected than females (S1 Table). In the present study, we tested whether juvenile male birds were more attractive to mosquitoes by placing 18 pairs composed of a male and a female chick coming from a same nest in a 1-meter-long box together with 25 mosquitoes for one hour and by identifying the sex of the host of each mosquito by PCR.
Materials and methods
Ethical statement
This experiment was approved by the Ethical Committee of the Vaud Canton veterinary authorities, licence number 1730.4. Birds were caught and ringed under licence with the permit number F044-0799 of the Swiss Federal Office for the Environment.
Experimental system
Great tit collection and rearing
A total of 38 Great tits (P. major) were used during this study. Hatching date of new-born nestlings was determined by monitoring nest boxes in three different study sites: Dorigny (46°31’26”N; 6°34’48”E; alt. 409 m), Monod (46°34’27”N; 6°23’32”E; alt. 686 m) and La Praz (46°40’12”N; 6°25’06”E, 1032m) from March to June. Three to five days after hatching (day 0), the nests were microwaved to eliminate all ectoparasites. Nestlings aged between six and nine days were blood sampled to determine their sex and infection status and ringed with an individual metallic ring. Five to 10μL of blood were sampled from the medial metatarsal vein and stored in PBS at 4°C until DNA extraction and molecular sexing (see Molecular analyses). At day 14, nestlings were weighted (electronic scales ± 0.1 g), tarsus and wing length were measured using an electronic metal calliper (± 0.01mm).
Mosquito rearing
We used a Culex pipiens lab colony, issued from egg rafts sampled in September 2016 in the Dorigny forest. Mosquitoes were reared in an insectary at 25°C ± 1°C, 70 ± 5% RH and with 12L:12D photoperiod. On the hatching day, larvae were seeded into plastic trays containing 500 mL of mineral water (Fonte Tavina Naturale, Italia) at a constant density (130 ± 5 individuals per tray). Larvae were fed ad libitum every two days until pupation with a mixture composed by TetraMin Junior fish pellets, Schweizer Classic rabbit pellets and JBL Novo Malawi fish flakes (1:1:1 ratio). Tray water was changed every 3 days. On pupation day, plastic trays provided with 10% sugar solution were placed in emergence cages. Experimentally naïve female were deprived of sugar solution 20 h prior to the experiment, in order to maximize the biting rate. Water was provided from 20 h to 6 h before the experiment to prevent dehydration.
Experimental procedure
Host choice behaviour experiments were performed by simultaneously presenting an average of 25 (range: 20–30) female mosquitoes to a 14 days old male and female great tit. To reduce individual and family effects all couples of nestlings were formed by a male and a female coming from the same nest and were of similar weight. We chose to perform this experiment with 14-day-old nestlings (as in [51]) because their tarsus length have reached their final size at this age. If they are disturbed when they are older (more than 16 days old), this may provoke early fledging. We also estimated that at this age, they had not fully acquired anti-ectoparasite behaviours, so we would not need to consider this parameter. Moreover, when 14 days old, haemosporidian parasites were never detected in their blood. Because infection status may influence mosquito biting choice [52, 53], performing the experiment at this stage allows avoiding this confounding factor.
Birds’ cloacal body temperature were taken 30 minutes before the assay then each bird was placed in the cup at one of the extremities of a rectangular, transparent Plexiglas box (100cm x 20cm x 20cm, 0.6 cm thickness, see S1 Fig). An ambient air flow was created by an axial flow propeller pump, and ambient air was pumped inside the box from the two sides. Incoming airflow (20 ± 1 cm/s) was controlled on both sides using a hot-wire anemometer. Mosquitoes were released in the middle of the box in a section closed by two mosquito-net screens and were allowed to settle for 5min before the start of the experiment. Then the screens were removed and the mosquitoes were given the opportunity to select and bite one of the birds. The experiments lasted for one hour and fed mosquitoes were then collected and stored at -80°C. After each trial, all the material was cleaned using 96% ethanol and then air dried.
Molecular analyses
For the mosquito choice experiments, DNA was extracted from the blood meal of mosquitoes using a Qiagen BioSprint 96 workstation following the tissue protocol for extraction (Qiagen, Hilden, Germany). A PCR was used to assess sex of great tit nestlings at the beginning of the study and to assess the host upon which mosquitoes fed during the choice experiment. We used three primers targeting CHD1, a gene located on birds’ sex chromosome that contains an intron with a constant size difference between W and Z chromosomes (2987 F, 3007 F, 3112 R, [54]). Sex was determined by examination of the agarose gel (2%) after electrophoresis, where migrated samples of female DNA produced two bands, while males only one [54]. A nested PCR [55] followed by an electrophoresis on agarose gel (2%) was performed to detect haemosporidian (Plasmodium, Haemoproteus and Leucocytozoon) infection status in birds, in order to ensure that we were testing only uninfected nestlings.
Statistical analyses
All analyses were performed in R 3.3.2. The effect of sex on bird attractiveness was tested using a repeated G-test of goodness of fit [53, 56]. The observed preference for male great tits (proportion of mosquitoes fed on the male relative to the total number of blood-fed mosquitoes) was tested against the predicted no-choice value of p = 0.5. We also performed the repeated G-test of goodness of fit on subsets of the data, separating the trials according to the site of origin of the birds, as well as on the total data, pooling the trials per site, in order to check whether the proportion of mosquitoes biting the male bird would differ according to the origin of the pair of birds. To assess whether other variables had an effect on bird attractiveness, the proportion of mosquitoes fed on male birds was analysed using Generalized Linear Mixed Models (GLMM) with a binomial error distribution [57]. Models were fitted by specifying the differences in body mass and temperature as fixed effects. The date of the experiment and the origin (site) were included as random effects. Model selection was performed by stepwise elimination of variables that were not significant (p > 0.05) by Likelihood Ratio Tests (LRT), using a χ2 test to estimate the significance (“lme4” package, [57]). In previous studies with Cx pipiens, the percentage of mosquitoes fed with a mix of blood (mosquitoes fed on more than one bird) reached up to 10% of fed mosquitoes in trials lasting 12 hours [58] and 3–6.2% in trials lasted two hours [53, 56]. In our experiment mixed blood meal would overlap in the agarose gel and appear as a female blood meal. Therefore, although our trials lasting only 1 hour, we ran a conservative parallel analysis on data where 10% of mosquitoes fed on females were excluded, to simulate the exclusion of potential mixed blood meals.
Results
None of the tested nestlings were infected with haemosporidian parasite. Eighteen trials were performed and the blood meal origin of 387 mosquitoes was identified. The mean (± SE) number of mosquitoes that fed per trial was 21.5 ± 1.68 (81% ± 0.04 SE, range: 35%-100%). The outcome of each trial is reported in (S2 Table).
A mean of 47.9% ± 0.03 SE of fed mosquitoes took blood from male nestlings (range: 31.8%-69%). Nestling sex did not affect the mosquito feeding preference, as the replicated G-test of goodness of fit did not show statistically significant departure from the proportion in the absence of choice (total-G = 27.64, 18df, p = 0.068; pooled-G = 1.14, 1df, p = 0.286, heterogeneity-G = 26.50, 17df, p = 0.066). Similar results were obtained in statistical analyses with data where 10% of mosquitoes fed on females were excluded (total-G = 25.116, 18df, p = 0.092; pooled-G = 0.001, 1df, p = 0.975, heterogeneity-G = 25.115, 17df, p = 0.0922). When separating the data according to the site of origin of the birds, there was also no significant departure from the no-choice proportion for any of the site, and there was no significant difference in the proportion of mosquitoes biting the male bird when comparing the sites of origin of the birds (S3 Table).
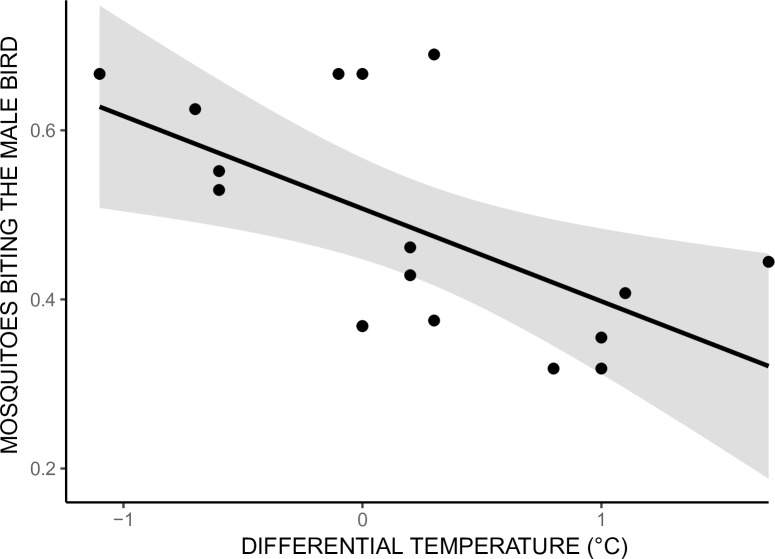
The difference in bird temperature significantly influenced mosquitoes’ choice (χ2 = 6.969, p = 0.008). Individuals with lower temperature were preferentially chosen independently of their sex (Fig 1). There was no effect of body weight (χ2 = 1.166, p = 0.280) on mosquitoes’ choice. Results were similar when 10% of mosquitoes fed on females were excluded to correct for potential bias due to mixed blood meals (body temperature: χ2 = 7.864, p = 0.005, body weight χ2 = 1.110, p = 0.292).
Fig 1. Proportion of mosquitoes biting the male in function of the difference in temperature.
Each dot represents a trial in which a pair composed two nestlings (brother and sister) was exposed to mosquito bites. The x-axis represents the male nestling’s temperature minus the female nestling’s temperature. The y-axis represents the proportion of mosquitoes that have bitten the male bird.
Discussion
In this study, we tested whether mosquitoes bite preferentially juvenile male great tits. We found no preference for one of the sexes in great tit nestlings. These results suggest that the sex-biased infection observed in natural great tits populations [S1 Table] was not caused by an intrinsic preference of mosquitoes for juvenile male birds.
There is evidence that vertebrate hosts vary in their attractiveness to mosquitoes [59] and that host-vector contact is far from random [60]. Indeed, the composition of the host’s odour profile and its CO2 emission intensity may influence mosquitoes’ choice for specific host profile [61, 62]. Traits such as odour and metabolic rates are predicted to differ more between sexes in sexually mature birds than in nestlings. In zebra finches, no difference in metabolic rates between male and female nestlings was reported [63], but adult females had a higher metabolic rate than males [64, 65]. A difference was also observed in adult great tits [28]. Nevertheless, in this species the adult males have higher metabolic rate than females [28]. Regarding odour, nestlings of both sexes live together in a closed environment and therefore their odours probably mix. In addition, the uropygial gland, involved in volatile organic compounds emission [31, 66, 67], is not yet fully functional in nestlings [68, 69]. Conversely, in adult birds, several studies reported a difference in odour profile between sexes [31, 70–73]. The absence of a sex-based preference in mosquitoes in our experiment might therefore be due to the use of nestlings which are not sexually mature and probably do not differ strongly in their odour profile. Using adult great tits to test the effect of Plasmodium infection on attraction of Cx pipiens, [52] found a marginally higher attractiveness of females.
A recent study showed that house sparrows with lower metabolic rate suffered more mosquito bites than individuals with higher metabolic rate [58]. This result is surprising because a higher metabolic rate is expected to be associated with higher CO2 emission and therefore should induce a higher attractiveness [58, 74]. However, a higher metabolic rate might also result from a high activity level [58, 75]. At the time of biting, mosquitoes tend to avoid individuals that are more active [58, 76]. Indeed, the defensive behaviour displayed by birds may reduce the ability of mosquitoes to take a blood meal. Interestingly we also observed that birds with a lower body temperature were preferentially chosen regardless of their sex. Although mosquitoes are attracted to heat when host seeking [35, 36, 38], to our knowledge, there was previously no evidence that mosquitoes choose the warmer or colder host, when they have the choice. The negative association between mosquito feeding preference and bird body temperature could be explained by the fact that mosquitoes avoid active individuals [58, 76], which may have a higher body temperature due to their activity [77, 78].
Our study leaves the question of why a male-biased infection exists in P. major unanswered. Several life-history traits of birds could be involved. Firstly, male great tits may be more infected due to a higher exposure to mosquitoes. For instance, during incubation and brooding period, female great tits sleep in the nest, built in cavities that may offer better protection from mosquitoes [79]. Thus, males should be unavoidably more exposed to mosquitoes, which reach a peak of abundance during late spring and early summer [80]. Secondly, susceptibility to Plasmodium infection may differ between the two sexes. Males may be more susceptible to parasite infection than females because sex hormones may reduce male’s immunocompetence [5, 81, 82], but also because sex hormones affect disease resistance genes and behaviours [82, 83]. Finally, mosquitoes may prefer males only when being vectors of avian malaria parasite. Some parasites induce remarkable and complex behavioural modifications in their hosts [84]. Lefèvre & Thomas (2008) [85] suggested that parasites may manipulate several phenotypic traits of their vectors to attain a higher transmission probability. Amongst many altered traits, such as biting rate [86], parasites may influence how infected vectors select their vertebrate hosts [53, 87]. According to the ‘‘qualitative manipulation” hypothesis [88], which states that in infected vectors host choice should match the preference of the parasite, it is suggested that the manipulation could occur “at the inter- and/or intra- specific level”, with hosts suitable for the parasite being preferentially chosen [85]. Assuming that males have a weaker immune system than females, parasites should benefit when transmitted to males. Thus, a manipulation of Cx pipiens by Plasmodium spp. may have evolved and be partly responsible for the sex difference in infection found for P. major. The most efficient way to test this prediction would be to perform choice experiments with infected vectors.
Conclusion
We found no sex-biased biting preference of mosquitoes in great tit nestlings. We hypothesize that this is due to the nestlings, as being sexually immature, having undifferentiated body odours. Thus, our results are not in line with the hypothesis that the male-biased Plasmodium infection observed in P. major is caused by an intrinsic preference of Cx pipiens mosquitoes for male great tits at this stage of development. A more complex interaction between host, vector and parasite might be a more plausible explanation for the difference in infection found in nature. In particular, we suggest the development of a study that tests adult host preference of uninfected but also infected mosquitoes.
Supporting information
The schema represents the setup in which the host-choice trials were performed.
(TIF)
Results of likelihood ratio test performed on generalized linear mixed model fitted using Plasmodium infection status as a response variable (binomial error distribution; infected: 1, uninfected:0), sex, age, scaled mass index (MI) and site as explanatory variables and year of sampling as fixed factor. The dataset is the one presented in [51].
(XLSX)
Each line of the table corresponds to a trial in which a male and a female nestlings coming from the same nest were exposed to mosquito bites during 1 hour.
(XLSX)
Dorigny, Monod and La Praz are the three sites.
(XLSX)
Acknowledgments
We thank Jérôme Wassef for his important contribution to the fieldwork as well as Jason Buser, Jérémy Gremion, Laélia Maumary, Jézaëlle Rufener and Tom Kay for their invaluable assistance in hand-raising the birds. We also thank Franck Chalard for building and improving the experimental setup, Aline Revel for technical support, and Tania Jenkins for her advices. Finally, we thank the anonymous reviewers whose comments remarkably improved this manuscript.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This project was funded by the Swiss National Science Foundation [grants 31003A-159600 and 31003A-179378] awarded to P.C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Poulin R. Sexual inequalities in helminth infections: A Cost of Being a Male? Am Nat. 1996;147:287–95. [Google Scholar]
- 2.Klein SL. Hormonal and immunological mechanisms mediating sex differences in parasite infection. Parasite Immunol. 2004;26:247–64. 10.1111/j.0141-9838.2004.00710.x [DOI] [PubMed] [Google Scholar]
- 3.Vardo AM, Wargo AR, Schall JJ. PCR detection of lizard malaria parasites: prevalence of Plasmodium infections with low-level parasitemia differs by site and season. J Parasitol. 2005;91:1509–11. 10.1645/GE-589R.1 [DOI] [PubMed] [Google Scholar]
- 4.Schmid-Hempel P. Evolutionary parasitology: the integrated study of infections, immunology, ecology, and genetics. OUP Oxford; 2011. [Google Scholar]
- 5.Nunn CL, Lindenfors P, Pursall ER, Rolff J, Nunn CL, Lindenfors P, et al. On sexual dimorphism in immune function. Philos Trans R Soc B Biol Sci. 2009;364:61–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16:626 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 7.Christe P, Glaizot O, Evanno G, Bruyndonckx N, Devevey G, Yannic G, et al. Host sex and ectoparasites choice: preference for, and higher survival on female hosts. J Anim Ecol. 2007;76:703–10. 10.1111/j.1365-2656.2007.01255.x [DOI] [PubMed] [Google Scholar]
- 8.Duneau D, Ebert D. Host sexual dimorphism and parasite adaptation. PLOS biology. 2012;10(2):e1001271 10.1371/journal.pbio.1001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grossman CJ. Interactions between the gonadal steroids and the immune system. Science. 1985;227:257–61. [DOI] [PubMed] [Google Scholar]
- 10.Duffy DL, Bentley GE, Drazen DL, Ball GF. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behav Ecol. 2000;11:654–62. [Google Scholar]
- 11.Buchanan KL, Evans MR, Goldsmith AR. Testosterone, dominance signalling and immunosuppression in the house sparrow, Passer domesticus. Behav Ecol Sociobiol. 2003;55:50–9. [Google Scholar]
- 12.Owen-Ashley NT, Hasselquist D, Wingfield JC. Androgens and the immunocompetence handicap hypothesis: unraveling direct and indirect pathways of immunosuppression in song sparrows. Am Nat. 2004;164:490–.50.5 10.1086/423714 [DOI] [PubMed] [Google Scholar]
- 13.Hasselquist D, Marsh JA, Sherman PW, Wingfield JC. Is avian humoral immunocompetence suppressed by testosterone? Behav Ecol Sociobiol. 1999;45:167–75. [Google Scholar]
- 14.Greenman CG, Martin LB, Hau M. Reproductive state, but not testosterone, reduces immune function in male house sparrows (Passer domesticus). Physiol Biochem Zool PBZ. 2005;78:60–8. 10.1086/425194 [DOI] [PubMed] [Google Scholar]
- 15.Tinsley RC. The effects of host sex on transmission success. Parasitol Today. 1989;5:190–5. [DOI] [PubMed] [Google Scholar]
- 16.Richner H, Christe P, Oppliger A. Paternal investment affects prevalence of malaria. Proc Natl Acad Sci. 1995;92:1192–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drobney RD, Train CT, Fredrickson LH. Dynamics of the platyhelminth fauna of wood ducks in relation to food habits and reproductive state. J Parasitol. 1983;69:375–80. [PubMed] [Google Scholar]
- 18.Habig B, Doellman MM, Woods K, Olansen J, Archie EA. Social status and parasitism in male and female vertebrates: a meta-analysis. Sci Rep. 2018;8:3629 10.1038/s41598-018-21994-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habig B, Archie EA. Social status, immune response and parasitism in males: a meta-analysis. Philos Trans R Soc Lond B Biol Sci. 2015;370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mooring MS, Hart BL. Differential grooming rate and tick load of territorial male and female impala, Aepyceros melampus. Behav Ecol. 1995;6:94–101. [Google Scholar]
- 21.Sol D, Jovani R, Torres J. Geographical variation in blood parasites in feral pigeons: the role of vectors. Ecography. 2000;23:307–14. [Google Scholar]
- 22.Martinez-Abrain, Esparza B, Oro D. Lack of blood parasites in bird species: does absence of blood parasite vectors explain it all? Ardeola. 2004;51:225–32. [Google Scholar]
- 23.Suh E, Bohbot J, Zwiebel LJ. Peripheral olfactory signaling in insects. Curr Opin Insect Sci. 2014;6:86–92. 10.1016/j.cois.2014.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Breugel F, Riffell J, Fairhall A, Dickinson MH. Mosquitoes use vision to associate odor plumes with thermal targets. Curr Biol CB. 2015;25:2123–9. 10.1016/j.cub.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Busula AO, Takken W, de Boer JG, Mukabana WR, Verhulst NO. Variation in host preferences of malaria mosquitoes is mediated by skin bacterial volatiles. Med Vet Entomol. 2017;31(3):320–6. 10.1111/mve.12242 [DOI] [PubMed] [Google Scholar]
- 26.Nagy KA. Field metabolic rate and food requirement scaling in mammals and birds. Ecol Monogr. 1987;57:112–28. [Google Scholar]
- 27.Buchholz AC, Rafii M, Pencharz PB. Is resting metabolic rate different between men and women? Br J Nutr. 2001;86:641–6. [DOI] [PubMed] [Google Scholar]
- 28.Mathot KJ, Abbey-Lee RN, Kempenaers B, Dingemanse NJ. Do great tits (Parus major) suppress basal metabolic rate in response to increased perceived predation danger? A field experiment. Physiol Behav. 2.1.6;164:400–6. 10.1016/j.physbeh.2016.06.029 [DOI] [PubMed] [Google Scholar]
- 29.Penn DJ, Oberzaucher E, Grammer K, Fischer G, Soini HA, Wiesler D, et al. Individual and gender fingerprints in human body odour. J R Soc Interface. 2007;4:331–40. 10.1098/rsif.2006.0182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.MacDonald EA, Fernandez-duque E, Evans S, Hagey LR. Sex, age, and family differences in the chemical composition of owl monkey (Aotus nancymaae) subcaudal scent secretions. Am J Primatol. 2008;70:12–8. 10.1002/ajp.20450 [DOI] [PubMed] [Google Scholar]
- 31.Whittaker DJ, Soini HA, Atwell JW, Hollars C, Novotny MV, Ketterson ED. Songbird chemosignals: volatile compounds in preen gland secretions vary among individuals, sexes, and populations. Behav Ecol. 2.1.0;21:608–14. 10.1093/beheco/arq033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whittaker DJ, Gerlach NM, Soini HA, Novotny MV, Ketterson ED. Bird odour predicts reproductive success. Anim Behav. 2013;86:697–703. [Google Scholar]
- 33.Burkett-Cadena ND, Bingham AM, Unnasch TR. Sex-biased avian host use by arbovirus vectors. R Soc Open Sci. 2014;1:140262 10.1098/rsos.140262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–53. 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- 35.Peterson DG, Brown AW. Studies of the responses of the female Aedes mosquito. Part III. The response of Aedes aegypti (L.) to a warm body and its radiation. Bull Entomol Res. 1951;42(3):535–41. [Google Scholar]
- 36.Eiras AE, Jepson PC. Responses of female Aedes aegypti (Diptera: Culicidae) to host odours and convection currents using an olfactometer bioassay. Bull Entomol Res. 1994;84:207–11 [Google Scholar]
- 37.Spitzen J, Spoor CW, Grieco F, ter Braak C, Beeuwkes J, van Brugge SP, et al. A 3D analysis of flight behavior of Anopheles gambiae sensu stricto malaria mosquitoes in response to human odor and heat. PloS one. 2013;8(5):e62995 10.1371/journal.pone.0062995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zermoglio PF, Robuchon E, Leonardi MS, Chandre F, Lazzari CR. What does heat tell a mosquito? Characterization of the orientation behaviour of Aedes aegypti towards heat sources. J Insect Physiol. 2017;100:9–14. 10.1016/j.jinsphys.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 39.Healy TP, Copland MJ, Cork A, Przyborowska A, Halket JM. Landing responses of Anopheles gambiae elicited by oxocarboxylic acids. Med Vet Entomol. 2002;16(2):126–32. [DOI] [PubMed] [Google Scholar]
- 40.Olanga EA, Okal MN, Mbadi PA, Kokwaro ED, Mukabana WR. Attraction of Anopheles gambiae to odour baits augmented with heat and moisture. Malaria Journal. 2010;9(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Breugel F, Riffell J, Fairhall A, Dickinson MH. Mosquitoes use vision to associate odor plumes with thermal targets. Current Biology. 2015;25(16):2123–9. 10.1016/j.cub.2015.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simpson S, Galbraith JJ. An investigation into the diurnal variation of the body temperature of nocturnal and other birds, and a few mammals. J Physiol. 1905;33(3):225–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gilbreath JC, Ko RC. Sex differential for body temperature in Japanese quail. Poultry science. 1970;49(1):34–6. 10.3382/ps.0490034 [DOI] [PubMed] [Google Scholar]
- 44.Schall JJ, Pearson AR, Perkins SL. Prevalence of malaria parasites (Plasmodium floridense and Plasmodium azurophilum) infecting a Puerto Rican lizard (Anolis gundlachi): a nine-year study. J Parasitol. 2000;86:511–5. 10.1645/0022-3395(2000)086[0511:POMPPF]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- 45.Lachish S, Knowles SCL, Alves R, Wood MJ, Sheldon BC. Infection dynamics of endemic malaria in a wild bird population: parasite species-dependent drivers of spatial and temporal variation in transmission rates. J Anim Ecol. 2011;80:1207–16. 10.1111/j.1365-2656.2011.01893.x [DOI] [PubMed] [Google Scholar]
- 46.Bernin H, Lotter H. Sex bias in the outcome of human tropical infectious diseases: influence of steroid hormones. J Infect Dis. 2014;209:S107–13. 10.1093/infdis/jit610 [DOI] [PubMed] [Google Scholar]
- 47.Calero-Riestra M, García JT. Sex-dependent differences in avian malaria prevalence and consequences of infections on nestling growth and adult condition in the Tawny pipit, Anthus campestris. Malar J.2016;15:178 10.1186/s12936-016-1220-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonneaud C, Sepil I, Wilfert L, Calsbeek R. Plasmodium infections in natural populations of Anolis sagrei reflect tolerance rather than susceptibility. Integr Comp Biol. 2017;57:352–61. 10.1093/icb/icx044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christe P, Glaizot O, Strepparava N, Devevey G, Fumagalli L. Twofold cost of reproduction: an increase in parental effort leads to higher malarial parasitaemia and to a decrease in resistance to oxidative stress. Proc R Soc B. 2012;279:1142–9. 10.1098/rspb.2011.1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jenkins T, Delhaye J, Christe P. Testing local adaptation in a natural great tit-malaria system: an experimental approach. PLOS ONE. 2015;10:e0141391 10.1371/journal.pone.0141391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pigeault R, Cozzarolo CS, Choquet R, Strehler M, Jenkins T, Delhaye J, et al. Haemosporidian infection and co-infection affect host survival and reproduction in wild populations of great tits. Int J Parasitol. 2018;48(14):1079–87. 10.1016/j.ijpara.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 52.Lalubin F, Bize P, van Rooyen J, Christe P, Glaizot O. Potential evidence of parasite avoidance in an avian malarial vector. Animal Behav. 2012;84(3):539–45. [Google Scholar]
- 53.Cornet S, Nicot A, Rivero A, Gandon S. Both infected and uninfected mosquitoes are attracted toward malaria infected birds. Malar J. 2013;12:179 10.1186/1475-2875-12-179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fridolfsson A-K, Ellegren H. A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol. 1999;30:116–21. [Google Scholar]
- 55.Hellgren O, Waldenström J, Bensch S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J Parasitol. 2004;90:797–802. 10.1645/GE-184R1 [DOI] [PubMed] [Google Scholar]
- 56.Cornet S, Nicot A, Rivero A, Gandon S. Malaria infection increases bird attractiveness to uninfected mosquitoes. Ecol Lett. 2013;16:323–9. 10.1111/ele.12041 [DOI] [PubMed] [Google Scholar]
- 57.Bates D, Mächler M, Bolker B, Walker S. Fitting Linear Mixed-Effects Models Using lme4. J Stat Softw. 2015;067. [Google Scholar]
- 58.Yan J, Broggi J, Martínez-de la Puente J, Gutiérrez-López R, Gangoso L, Soriguer R, et al. Does bird metabolic rate influence mosquito feeding preference? Parasit Vectors. 2018;11:110 10.1186/s13071-018-2708-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiu YT, Smallegange RC, Van Loon JJA, Ter Braak CJF, Takken W. Interindividual variation in the attractiveness of human odours to the malaria mosquito Anopheles gambiae s. s. Med Vet Entomol. 2006;20:2.80–7. [DOI] [PubMed] [Google Scholar]
- 60.Kelly DW. Why are some people bitten more than others? Trends Parasitol. 2001;17:578–81. [DOI] [PubMed] [Google Scholar]
- 61.Mukabana WR, Takken W, Coe R, Knols BGJ. Host-specific cues cause differential attractiveness of Kenyan men to the African malaria vector Anopheles gambiae. Malar J. 2002;1:17 10.1186/1475-2875-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.de Boer JG, Robinson A, Powers SJ, Burgers SLGE, Caulfield JC, Birkett MA, et al. Odours of Plasmodium falciparum-infected participants influence mosquito-host interactions. Sci Rep. 2017;7:9283 10.1038/s41598-017-08978-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tobler M, Nilsson J-Å, Nilsson JF. Costly steroids: egg testosterone modulates nestling metabolic rate in the zebra finch. Biol Lett. 2007;3:408–10. 10.1098/rsbl.2007.0127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Moe B, Rønning B, Verhulst S, Bech C. Metabolic ageing in individual zebra finches. Biol Lett. 2009;5:86–9. 10.1098/rsbl.2008.0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rønning B, Moe B, Berntsen HH, Noreen E, Bech C. Is the rate of metabolic ageing and survival determined by basal metabolic rate in the zebra finch? PLOS ONE. 2014;9:e108675 10.1371/journal.pone.0108675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Burger BV, Reiter B, Borzyk O, Plessis MA du. Avian exocrine secretions. I. Chemical characterization of the volatile fraction of the uropygial secretion of the green woodhoopoe, Phoeniculus purpureus. J.Chem Ecol. 2004;30:1603–11. [DOI] [PubMed] [Google Scholar]
- 67.Campagna S, Mardon J, Celerier A, Bonadonna F. Potential semiochemical molecules from birds: a practical and comprehensive compilation of the last 20 years studies. Chem Senses. 2012;37:3–25. 10.1093/chemse/bjr067 [DOI] [PubMed] [Google Scholar]
- 68.Kolattukudy PE, Sawaya WN. Age dependent structural changes in the diol esters of uropygial glands of chicken. Lipids. 1974;9:290–2. [DOI] [PubMed] [Google Scholar]
- 69.Shanbhag BA, Sharp PJ. Immunocytochemical localization of androgen receptor in the comb, uropygial gland, testis, and epididymis in the domestic chicken. Gen Comp Endocrinol. 1996;101:76–82. 10.1006/gcen.1996.0009 [DOI] [PubMed] [Google Scholar]
- 70.Zhang J-X, Wei W, Zhang J-H, Yang W-H. Uropygial gland-secreted alkanols contribute to olfactory sex signals in budgerigars. Chem Senses. 2010;35:375–82. 10.1093/chemse/bjq025 [DOI] [PubMed] [Google Scholar]
- 71.Whittaker DJ, Soini HA, Gerlach NM, Posto AL, Novotny MV, Ketterson ED. Role of testosterone in stimulating seasonal changes in a potential avian chemosignal. J Chem Ecol. 2011;37:1349–57. 10.1007/s10886-011-0050-1 [DOI] [PubMed] [Google Scholar]
- 72.Amo L, Avilés JM, Parejo D, Peña A, Rodríguez J, Tomás G. Sex recognition by odour and variation in the uropygial gland secretion in starlings. J Anim Ecol. 2012;81:605–13. 10.1111/j.1365-2656.2011.01940.x [DOI] [PubMed] [Google Scholar]
- 73.Caro SP, Balthazart J, Bonadonna F. The perfume of reproduction in birds: Chemosignaling in avian social life. Horm Behav. 2015;68:25–42. 10.1016/j.yhbeh.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol. 2013;58:433–53. 10.1146/annurev-ento-120811-153618 [DOI] [PubMed] [Google Scholar]
- 75.Careau V, Thomas D, Humphries MM, Réale D. Energy metabolism and animal personality. Oikos. 2008;117:641–53. [Google Scholar]
- 76.Day JF, Edman JD. Mosquito engorgement on normally defensive hosts depends on host activity patterns. J Med Entomol. 1984;21:732–40. [DOI] [PubMed] [Google Scholar]
- 77.Prinzinger R, Preßmar A, Schleucher E. Body temperature in birds. Comp Biochem Physiol A Physiol. 1991;99:499–506. [Google Scholar]
- 78.Carere C, van Oers K. Shy and bold great tits (Parus major): body temperature and breath rate in response to handling stress. Physiol Behav. 2004;82:905–12. 10.1016/j.physbeh.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 79.Hinde RA. The behaviour of the great tit (Parus major) and some other related species. Behav Suppl. 1952;III–201. [Google Scholar]
- 80.Lalubin F, Delédevant A, Glaizot O, Christe P. Temporal changes in mosquito abundance (Culex pipiens), avian malaria prevalence and lineage composition. Parasit Vectors. 2013;6:307 10.1186/1756-3305-6-307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. Am Nat. 1992;139:603–22. [Google Scholar]
- 82.Roved J, Westerdahl H, Hasselquist D. Sex differences in immune responses: hormonal effects, antagonistic selection, and evolutionary consequences. Horm Behav. 2017;88:95–105. 10.1016/j.yhbeh.2016.11.017 [DOI] [PubMed] [Google Scholar]
- 83.Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–38. [DOI] [PubMed] [Google Scholar]
- 84.Poulin R. Chapter 5—Parasite manipulation of host behavior: an update and frequently asked questions. In: Brockmann HJ, Roper TJ, Naguib M, Wynne-Edwards KE, Mitani JC, Simmons LW (Eds). Academic Press; 2010; p. 151–86. [Google Scholar]
- 85.Lefèvre T, Thomas F. Behind the scene, something else is pulling the strings: emphasizing parasitic manipulation in vector-borne diseases. Infect Genet Evol. 2008;8:504–19. 10.1016/j.meegid.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 86.Koella JC, Sørensen FL, Anderson RA. The malaria parasite, Plasmodium falciparum, increases the frequency of multiple feeding of its mosquito vector, Anopheles gambiae. Proc R Soc B Biol Sci. 1998;265:.76.3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gandon S. Evolution and manipulation of vector host choice. Am Nat. 2018;192:23–34. 10.1086/697575 [DOI] [PubMed] [Google Scholar]
- 88.Lefèvre T, Koella JC, Renaud F, Hurd H, Biron DG, Thomas F. New prospects for research on manipulation of insect vectors by pathogens. PLOS Pathog. 2006;2:e72 10.1371/journal.ppat.0020072 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The schema represents the setup in which the host-choice trials were performed.
(TIF)
Results of likelihood ratio test performed on generalized linear mixed model fitted using Plasmodium infection status as a response variable (binomial error distribution; infected: 1, uninfected:0), sex, age, scaled mass index (MI) and site as explanatory variables and year of sampling as fixed factor. The dataset is the one presented in [51].
(XLSX)
Each line of the table corresponds to a trial in which a male and a female nestlings coming from the same nest were exposed to mosquito bites during 1 hour.
(XLSX)
Dorigny, Monod and La Praz are the three sites.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.