Abstract
Findings
Here, we demonstrate that OP2113 (5-(4-Methoxyphenyl)-3H-1,2-dithiole-3-thione, CAS 532-11-6), synthesized and used as a drug since 1696, does not act as an unspecific antioxidant molecule (i.e., as a radical scavenger) but unexpectedly decreases mitochondrial reactive oxygen species (ROS/H2O2) production by acting as a specific inhibitor of ROS production at the IQ site of complex I of the mitochondrial respiratory chain. Studies performed on isolated rat heart mitochondria also showed that OP2113 does not affect oxidative phosphorylation driven by complex I or complex II substrates. We assessed the effect of OP2113 on an infarct model of ex vivo rat heart in which mitochondrial ROS production is highly involved and showed that OP2113 protects heart tissue as well as the recovery of heart contractile activity.
Conclusion / Significance
This work represents the first demonstration of a drug authorized for use in humans that can prevent mitochondria from producing ROS/H2O2. OP2113 therefore appears to be a member of the new class of mitochondrial ROS blockers (S1QELs) and could protect mitochondrial function in numerous diseases in which ROS-induced mitochondrial dysfunction occurs. These applications include but are not limited to aging, Parkinson’s and Alzheimer's diseases, cardiac atrial fibrillation, and ischemia-reperfusion injury.
Introduction
The free radical theory of aging suggests that free radical-induced damage to cellular structures is a crucial event in aging [1]; however, clinical trials on antioxidant supplementation in various populations have not successfully demonstrated an anti-aging effect [2]. Current explanations include the lack of selectivity of available antioxidants for the various sources of oxygen radicals and the poor distribution of antioxidants to mitochondria, which are now believed to be both the primary sources of reactive oxygen species (ROS) and primary targets of ROS-induced damage [3]. Indeed, mitochondrial dysfunction that occurs due to accumulation of oxidative damage [4] is implicated in the pathogenesis of virtually all human age-related diseases [5, 6], including cardiovascular and neurodegenerative diseases, cancer, and diabetes [7–12], as well as ischemia-reperfusion injury [13].
Given the key role of age-dependent mitochondrial deterioration in aging [4], there is currently a great interest in approaches to protect mitochondria from ROS-mediated damage. Mitochondria are not only a major source of ROS but also particularly susceptible to oxidative damage. Consequently, mitochondria accumulate oxidative damage with age that contribute to mitochondrial dysfunction [4]. Cells and even organelles possess several protection pathways against this ROS-mediated damage given that local protection is fundamental to circumvent the high reactivity of ROS. Therefore, mitochondria appear as the main victims of their own ROS production, and evidence suggests that the best mitochondrial protection will be obtained from inside mitochondria. This conclusion has driven several potential therapeutic strategies to improve mitochondrial function in aging and pathologies. Antioxidants designed for accumulation by mitochondria in vivo have been developed [2, 14] and are currently being thoroughly tested for mitochondrial protection [15–17]. Given that functional mitochondria are characterized by a very high proton gradient, mainly represented by a negative-internal membrane potential gradient [18], lipophilic cationic compounds accumulate inside the mitochondrial matrix as they may cross the lipid bilayer barrier given the electrical gradient. Therefore, mitochondria-targeted antioxidants are essentially cationic lipophilic drugs combined with a quinone moiety with radical scavenging properties. The growing interest in ROS production associated with diseases has elicited numerous clinical trials that have also demonstrated that uncontained ROS reduction in cells is deleterious, and it appears that an adequate balance of ROS production is necessary for correct cell function [2]. As a consequence, there is also a growing interest in the selective inhibition of ROS production of mitochondrial origin that would not affect cellular signalization involving either mitochondrial [19] or cytosolic ROS production [20, 21]. Conditions of high ROS production in mitochondria are now better characterized [7, 22–24], and it appears that ROS may be produced at multiple sites of the respiratory chain in mitochondria. Maximal ROS production occurs under conditions of high reduction of electron transporters, chiefly quinones, and high membrane potential values. Paradoxically, these conditions are satisfied when mitochondrial oxidative phosphorylation is low (low cellular ATP turnover) [25, 26] or under low oxygen conditions (hypoxia, inhibition of terminal oxidase) [13].
The molecule OP2113 (Anetholtrithion, or 5-(4-methoxyphenyl)dithiole-3-thione—CAS number 532-11-6) has been marketed in many countries and used in human therapy in certain countries including France, Germany, and China for its choleretic and sialogogic properties. Anetholtrithion also exhibits chemoprotective effects against cancer and various kinds of toxicity caused by some drugs and xenobiotics [27]. These chemoprotective effects appear to be mainly due to its antioxidant properties [28–30]. The most typical indications for which anetholtrithion is currently used include increasing salivary secretion in patients experiencing dry mouth. It is also indicated as an adjunctive therapy for cholecystitis, gallstone, indigestion, and acute/chronic hepatitis (see DrugBank database [31]). Anetholtrithion and derivatives have also been tested for their properties as H2S donors and therapeutic effects [27, 32–34].
However, until now, no precise mechanism of action has been described for this molecule. Considering the high lipophilicity of OP2113, which represents a promising characteristic for mitochondrial targeting, we investigated the effect of OP2113 on mitochondrial ROS/H2O2 production. Here we show that OP2113 decreases ROS/H2O2 production by isolated rat heart mitochondria. Interestingly, it does not act as an unspecific antioxidant molecule (i.e. as a radical scavenger), but as a direct specific inhibitor of ROS production at site IQ of complex I of the mitochondrial respiratory chain, without impairing electron transfer.
Results
OP2113 does not inhibit mitochondrial oxidative phosphorylation
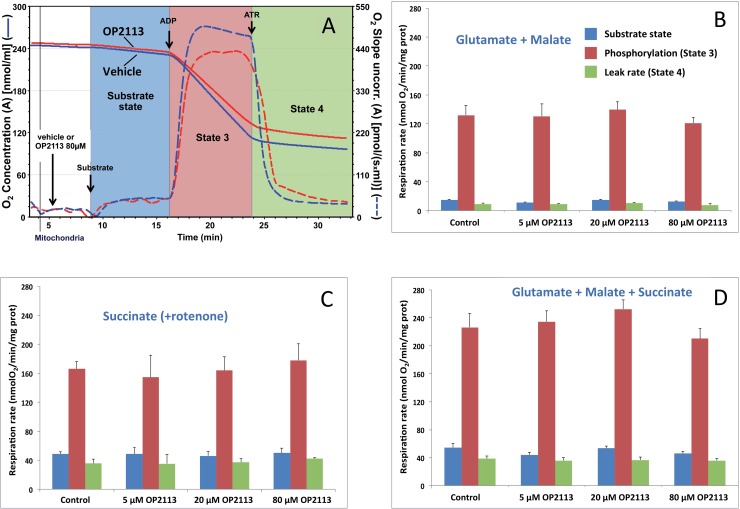
Using the classical oxygraph method, we first verified directly that the OP2113 compound did not affect oxidative phosphorylation or mitochondrial integrity using mitochondria isolated from rat heart. The substrate combination that fed electrons to the entire respiratory chain (see legend to Fig 1) was chosen given that it most closely resembles in vivo conditions where metabolism and Krebs cycle are active inside mitochondria and both NADH (complex I) and succinate (complex II) are oxidized by the respiratory chain. No statistically significant differences were observed regarding the presence of OP2113 for the large range of concentrations tested here (Fig 1), demonstrating that OP2113 has no effect on mitochondrial oxidative phosphorylation, respiratory chain activity, ATP synthesis, or mitochondrial inner membrane integrity (leak rate, in green) under these conditions. Overall, these results confirm the absence of any harmful effect of OP2113 on mitochondrial energetics under these conditions.
Fig 1. The lack of an effect of OP2113 on mitochondrial oxidative phosphorylation.
Panel A: This diagram illustrates a classical respiration assay and presents O2 concentration (solid lines) and O2 consumption slope (dotted lines) of rat heart mitochondria after a short incubation in the presence of the vehicle (blue lines) or 80 μM OP2113 (red lines). Respiratory substrates (glutamate + malate in this assay) were added, triggering the onset of oxygen consumption (substrate state, blue background) and then phosphorylation was promoted by the addition of 1 mM ADP in order to obtain the maximal oxidative phosphorylation rate (State 3, red background) [4, 35]. Finally, atractyloside (ATR), which inhibits the ADP/ATP translocator, was added to yield the mitochondrial leak rate (green background) under non phosphorylating conditions (State 4). Panels B to D: The three energetic states were studied in the presence of increasing concentrations of OP2113 (5, 20, and 80 μM), and with mitochondria oxidizing different respiratory substrate combinations: glutamate + malate (B), which feeds electrons to complex I; succinate in the presence of rotenone (C), supplying electrons to complex II; and glutamate + malate + succinate (D) feeding electrons to both complexes I & II. Results are based on 3 independent experiments. No significant differences in mitochondrial respiration rates were noted after the addition of OP2113.
Since it was not possible to measure more precisely the effect of OP2113 on the specific activity of complex I due to spectrophotometric interferences, we have been advised to assay by polarography the effect of OP2113 on rotenone-sensitive NADH oxidase activity by broken (frozen-thawed) mitochondria (S1 File), assimilated to complex I [36]. Interestingly, this activity turned out to be almost 30 times higher than the activity of oxidative phosphorylation driven by complex I substrates presented in Fig 1 (respectively 3500 versus 130 nmol O2/min/mg prot). Due to this huge NADH oxidase activity as compared to complex I oxidative phosphorylation, we had to use a much lower protein content in the assay, while keeping the same drug to protein ratio. We could detect an inhibition (about 8%) of NADH oxidase activity on broken mitochondria starting at the equivalent of 20 μM (200 nmol OP2113 / mg of mitochondrial protein) and increasing to 45% for 80 μM (800 nmol OP2113 / mg of mitochondrial protein). However, due to the difference in activity, even at the higher OP2113 concentration the activity of rotenone-sensitive NADH oxidase is still 15 times higher than complex I driven oxidative phosphorylation rate. These results explain the total absence of effect of OP2113, even at very high concentration (80 μM), on complex I-driven oxidative phosphorylation by intact heart mitochondria.
OP2113 specifically inhibits mitochondrial superoxide/H2O2 production
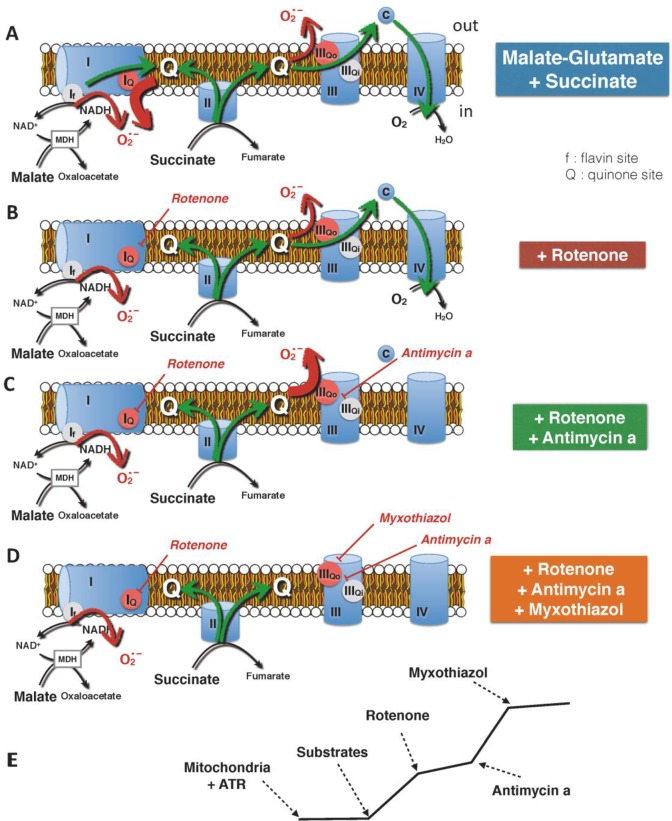
We further tested the effects of OP2113 on ROS production by mitochondria under various conditions. As previously stated, mitochondrial ROS production is highly dependent on mitochondrial bioenergetic state, and maximal production occurs under conditions of high reduction of electron transporters and high membrane potential. These conditions are fulfilled in the presence of ATR (inhibition of ATP/ADP translocator; green bars, Fig 1B to 1D). Under these conditions ROS are produced at different sites of the respiratory chain [7, 24]. The main sites of production are located at complex I and III, where large changes in potential energy of electrons occur [5, 22, 25]. These complexes also allow proton pumping [18]. Based on the work of M.D Brand and colleagues [21, 24, 25], we designed a series of inhibitor titrations to decipher OP2113 action on ROS production by the mitochondrial respiratory chain under conditions of maximal ROS production. The schematic representation of these experiments and of their rationale is presented in Fig 2.
Fig 2. Main sites of oxygen radical production by isolated mitochondria.
This scheme gives background information regarding the potential sites for ROS production, using different mitochondrial-targeted drugs in a protocol specifically designed for a study of the effect of a compound on mitochondrial ROS/H2O2 production. When mitochondria are energized by a combination of complex I (malate-glutamate) and complex II (succinate) substrates and in the absence of specific inhibitors of the complexes, ROS production is considered as mainly derived from reverse electron transport (RET) at site IQ (A). Of note, ROS produced by complex I, either at site IQ (quinone site) or at site If (flavin site), are delivered to the inner- (matrix-) side of the inner mitochondrial membrane. In the presence of rotenone, a specific inhibitor of complex I which blocks RET, ROS production is thought to occur predominantly at site IIIQO, possibly with residual production at site If (B) [24]. If complex III is inhibited as it is the case in the presence of antimycin a, the reduced to oxidized quinone ratio increases due to complex II activity and triggers an increase in ROS production, essentially at site IIIQO (C). Finally, myxothiazol (inhibitor of complex III site IIIQO) is supposed to block complex III ROS production, and the remaining production is usually ascribed to the flavin site of complex I for which there is no known inhibitor (D)[25]. However, due to matrix antioxidant machinery, the possibility that some ROS/H2O2 produced in the matrix may escape to the measurement has been suggested from experiments carried out with submitochondrial particles [37]. A typical recording of ROS production kinetics by mitochondria during the designed inhibitor sequence is presented in E.
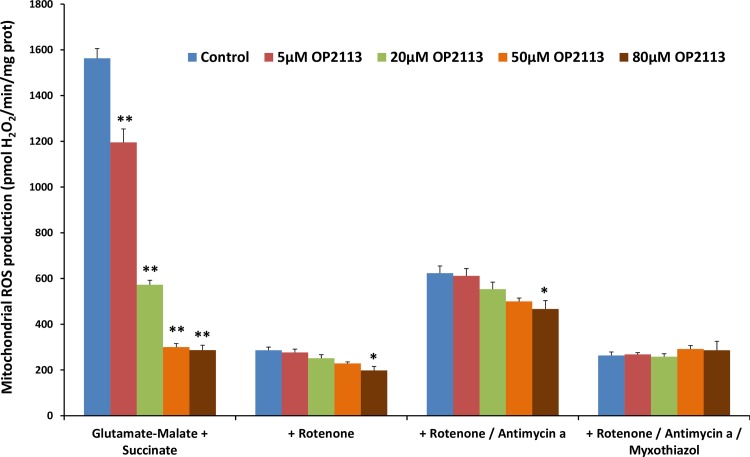
Fig 3 presents the effect of increasing OP2113 concentrations (from 5 to 80 μM) on ROS/H2O2 production measured under the different conditions depicted in Fig 2. Results chiefly show that once rotenone has been added to the assay, ROS/H2O2 production becomes insensitive to OP2113 even at high concentrations, irrespective of the site involved. Thus, the only condition under which OP2113 is active in our assay is the condition where ROS are produced at the level of complex I (site IQ sensitive to rotenone). These results demonstrate that OP2113 significantly decreases ROS/H2O2 production (by approximately 80%) under conditions where complex I is the main producer (before addition of rotenone), while no significant effect was observed under all other conditions (after addition of rotenone).
Fig 3. Effect of OP2113 on ROS/H2O2 production by isolated rat heart mitochondria.
The rate of ROS/H2O2 production by isolated rat heart mitochondria respiring on glutamate + malate + succinate was measured under the different conditions described in Fig 2 in the presence of increasing concentrations of OP2113 (5 to 80 μM). In the absence of specific inhibitors of the complexes (see Fig 2A), ROS/H2O2 production is maximal and is mainly derived from reverse electron transport at site IQ (see comments in Fig 2). Following blockade of reverse electron transport by addition of rotenone (1.5 μM) (Fig 2B), ROS/H2O2 production is reduced and occurs essentially at site IIIQO. The subsequent addition of Antimycin a (2 μM) (Fig 2C), which blocks the transfer of electrons to oxygen, increases this ROS/H2O2 production. Finally, myxothiazol (0.2 μM) blocks ROS/H2O2 production at site IIIQO (Fig 2D). Data are based on 4 independent experiments, each performed in duplicate. *P < 0.05, **P < 0.005 versus control group.
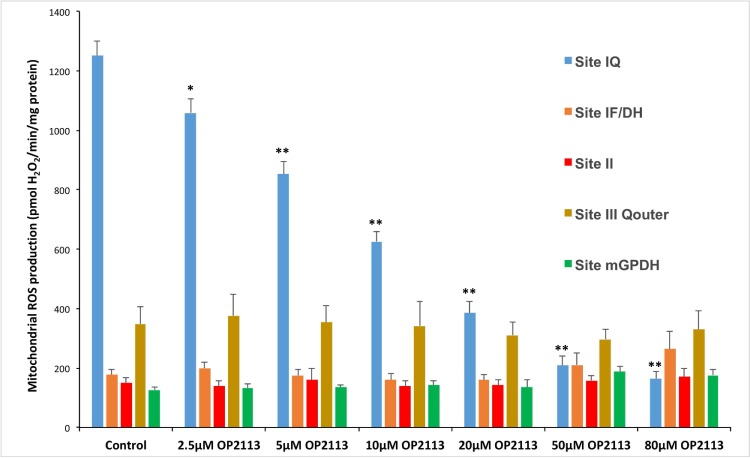
Given that OP2113 surprisingly did not inhibit all mitochondrial ROS/H2O2 production, further investigations were conducted to obtain better insight into the action of the compound on the different mitochondrial sites based on the excellent pioneering work of MD Brand's group [21, 22, 24, 26, 38]. These experiments confirmed that OP2113 affects complex I ROS production, without any measurable effect on the other main sites of ROS/H2O2 production tested (Fig 4). These results allowed the calculation of a half maximal effective concentration (EC50) of 10.2 ± 0.9 μM for OP2113 on the inhibition of ROS/H2O2 production by complex I in isolated rat heart mitochondria under our experimental conditions.
Fig 4. Specific effects of OP2113 on ROS/H2O2 production at different sites in isolated rat heart mitochondria.
The rates of ROS/H2O2 production were measured under conditions specifically designed by MD brand's group for the identification of the different mitochondrial ROS production sites [21, 22, 24, 38](see Methods section for details). The effects of increasing concentrations of OP2113 (from 2.5 to 80 μM) were tested for each condition of ROS/H2O2 production. Data are based on 3 to 5 independent experiments, each performed in duplicate or triplicate. *P < 0.01, **P < 0.005 versus control group.
The specific effect of OP2113 on a unique site of mitochondrial ROS/H2O2 production is not only surprising but raises interesting questions about the mechanism of action of OP2113 on mitochondria. These results exclude the hypothesis that the therapeutic effects of OP2113 arise from a mere radical scavenger property as previously reported [28–30]. Indeed, we show in this study that OP2113 does not trap all the ROS produced by mitochondrial respiratory chain, independently of their site of production.
Although the mechanism of action requires further investigations, evidence is presented here that OP2113 directly interferes with mitochondrial complex I ROS production and selectively inhibits superoxide production from the ubiquinone-binding site of complex I (site IQ) with no effect on superoxide production from other sites or on oxidative phosphorylation processes. As can be seen in Fig 4, the half maximal effective concentration of OP2113 inhibiting mitochondrial Complex I driven ROS production is equal to 10 μM. This concentration corresponds to 100 nmol /mg protein, an OP2113 quantity that is affecting neither the rotenone-sensitive NADH oxidase activity (see above) nor the oxidative phosphorylation in our experimental conditions (see Fig 1).
Another compound also inhibiting mitochondrial complex I ROS production, N-cyclohexyl-4-(4-nitrophenoxy) benzenesulfonamide, has very recently been described by Brand's group [7] along with other molecules [38, 39]. Similarly with OP2113, these chemicals (S1QELs) do not modify the activity of complex I-driven oxidative phosphorylation [38, 39].
The specificity of OP2113 for mitochondrial ROS production by complex I was further tested in vitro on the ROS/H2O2 production by NAD(P)H oxidase (S2 File). We did not observe any inhibition of resorufin production under these conditions, indicating that OP2113 does not interfere either with the ROS/H2O2 measurement system (amplex red) or by direct interaction with H2O2. These results also suggest that OP2113 may not inhibit ROS/H2O2 production by cytosolic NAD(P)H oxidases, which are likely the major non-mitochondrial ROS/H2O2 producers in the cells. These results are in striking contrast with previous assertions on the putative effect of OP2113 as a radical scavenger [28–30].
OP2113 protects heart form ischemia-reperfusion injury (infarct model)
The role of mitochondrial ROS production in ischemia reperfusion injury is now heavily documented and complex I appears to play a central role, during both ischemia and reperfusion [40]. Recent works [13] have for instance demonstrated that the mechanism by which extensive ROS generation occurs at reperfusion involves reverse electron transport at mitochondrial complex I [38] is due to succinate accumulation during ischemia [13, 41], although we did question this mechanism in the context of ischemic preconditioning [42, 43]. It appears also that mitochondrial respiratory chain—and specifically complex I—damages occur during ischemia [40, 44], and that these damages were paralleled by further ROS production and infarct development. Indeed, previous works have shown that a ROS production sensitive to complex I inhibitors occurs during the ischemic phase that may be involved in the mechanisms of heart pharmacological protection [45–48]. Interestingly, ROS production sensitive to complex I inhibitors has been shown to be involved in the damages to complex I occurring during ischemia [44] which increase complex I capacity of ROS production [49] at reperfusion. Reversible ischemia-induced conformational change of complex I to a deactive form has also been shown [50].
Considering the crucial role of ROS from complex I in the complex mechanisms of heart ischemia-reperfusion and the effect of OP2113 on complex I driven ROS production, we appraised that protection of the infarcted heart from ischemia-reperfusion damage may represent a demonstrative experiment to test the effect of OP2113 on mitochondria in living tissues.
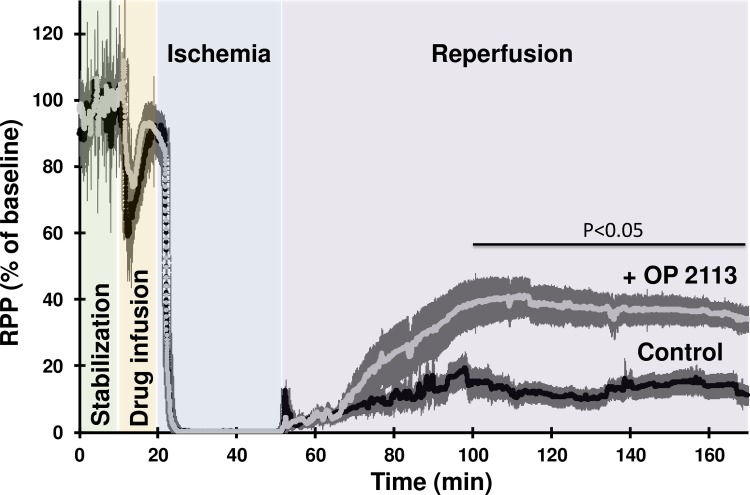
This hypothesis was assessed by investigating the cardioprotective effects of OP2113 pre-treatment of Langendorff-perfused rat hearts submitted to an ischemia-reperfusion protocol (see S3 File). Cardioprotection was assessed from the recovery of contractile performance during reperfusion (Fig 5) and quantification of infarct size by triphenyltetrazolium chloride (TTC) staining at the end of the reperfusion period (Fig 6). Fig 5 shows the time course of the rate-pressure product (RPP calculated as the product of left ventricular developed pressure by heart rate). After the stabilization period, the presence of ethanol in the vehicle during the infusion period caused a similar transitory decrease of the RPP in the two groups studied (Fig 5, orange background). Contraction was stopped during the 30 min ischemia and restart at reperfusion. Fig 5 shows that pre-treatment with OP2113 significantly improves the recovery of RPP at the end of the reperfusion period (34% vs 11% of baseline value in OP2113 and control conditions, respectively, p°<°0.05).
Fig 5. OP2113 improves the recovery of the contractile performance of Langendorff-perfused rat heart during the reperfusion phase following ischemia.
This figure shows the time course of the rate-pressure product of two groups of isolated langendorff-perfused rat hearts (n = 6 in each group) during a protocol of ischemia-reperfusion. Rate Pressure Product (RPP), the product of the left ventricular developed pressure (mmHg/beat) by heart rate (beat/min) is used as an index of contractile performance and is expressed as % of baseline value measured at the end of the stabilization period (control 28932±2467 mmHg/min, OP2113 31653±4611 mmHg/min). Each heart was allowed to stabilize during 10 min (green background) before perfusion of vehicle (control group, black trace) or 10 μM OP2113 (+ OP2113 group, grey trace) during 10 more minutes (orange background). Hearts were then submitted to 30 min zero-flow ischemia (blue background) before 120 min of reperfusion in the absence of vehicle or OP2113 (purple background). For more details on the perfusion protocol see also the Methods section and S3 File. Data are expressed as the mean ± SEM for 6 independent experiments. The thickness of the line represents the error bars.
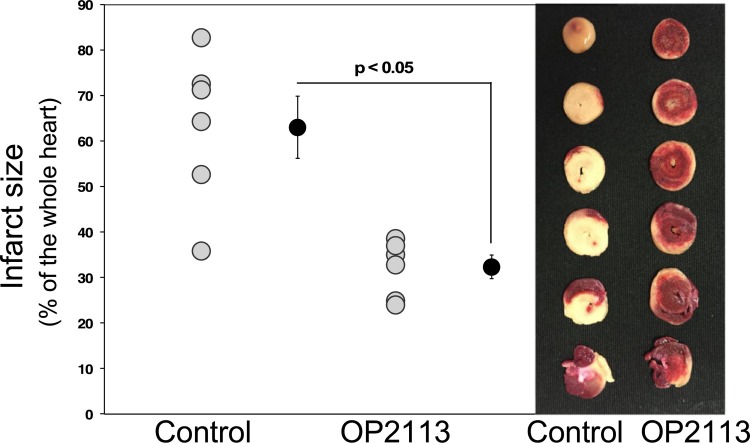
Fig 6. Effect of OP2113 on infarct size.
Hearts were pre-treated (OP2113) or not (Control) with OP2113 before 30 min of ischemia followed by 120 min of reperfusion (see Fig 5). Left panel, grey dots represent individual experiments and black dots mean ± SEM (see Methods, n = 6 independent experiments for each condition). Right panel, typical pictures obtained in the control and OP2113 groups following TTC staining. The tissues appearing in red are living tissues stained with TTC, whereas damaged tissue appears white.
The infarct size was analyzed by TTC staining and quantified at the end of the reperfusion period for a series of 6 experiments per condition (see typical photographs of heart slices in Fig 6 right panel). Results clearly demonstrate that OP2113 significantly decreased infarct size at the end of reperfusion by approximately 50% (Fig 6, left panel). This observation fits well with the better recovery of heart contractile performance of hearts treated with OP2113 as compared to controls (see Fig 5).
Discussion
When tested on isolated mitochondria from rat heart, OP2113 effectively decreases mitochondrial ROS/H2O2 production (in isolated mitochondria, H2O2 is produced from the reduction of superoxide anion by mitochondrial superoxide dismutase). Moreover, the results presented here clearly demonstrate that OP2113 presents a very strong selectivity towards the formation of ROS by site IQ in complex I (Fig 1), which demonstrates that OP2113 does not simply interact with superoxide radicals but specifically prevents their formation by complex I. In that respect, OP2113 therefore appears as a member of the brand new class of oxidative stress protectants. Whereas antioxidants generally do not interfere directly with electron transport and scavenge ROS and/or H2O2 downstream from production and therefore can never fully suppress the effect of ROS [7], OP2113 may act differently by preventing ROS formation and thus being more active to protect mitochondria from their own ROS. Data presented here further demonstrate that OP2113 is a specific inhibitor of ROS formation at site IQ of complex I of the mitochondrial respiratory chain. Further experiments are however required to ascertain whether OP2113 has no effect on other mitochondrial ROS production sites, but this does not preclude the above conclusions. Incidentally, the observed inhibition of the rotenone-sensitive NADH oxidase activity in broken mitochondria may reflect a different process, since it occurs at higher concentrations than ROS inhibition and without any effect on oxidative phosphorylation. Nonetheless, at this stage we cannot rule out that in our ex vivo experiments the cardioprotective effect brought by OP2113 could be secondary to complex I inhibition during the ischemic phase, a cardioprotective strategy as shown by Lesnefsky and collaborators [44, 45, 51].
We also present evidence that OP2113 may interact only with mitochondria without affecting ROS formation in the cytosol and therefore would not affect intracellular signaling. Selective modulators of superoxide production from site IQ would offer unique opportunities to probe the putative role of mitochondrial ROS production in normal and pathological processes [7] and during a life span [2]. OP2113’s specificity towards mitochondrial ROS production ("bad" ROS) would preserve cytosolic ROS signaling ("good" ROS) and therefore appears as a very promising property that may circumvent the bias of the use of non-specific antioxidants in clinical trials.
Since OP2113 does not present a permanent positive charge, it may not accumulate in mitochondria due to the inner mitochondrial membrane potential difference. However, to our knowledge, all previous chemical and pharmacological data [31] confirm the high lipophilicity of the molecule and its large distribution in tissues. The observed cardioprotective effect of OP2113 against damages induced by ischemia-reperfusion strongly suggest that, at least when added before reperfusion, the drug reaches mitochondrial membranes.
As discussed previously, the mechanisms leading to ischemia-reperfusion injury are highly complex and ROS, especially from complex I, are involved during both ischemia and reperfusion [40]. At this stage, we cannot effectively conclude if OP2113 acts by protecting complex I from his own ROS during the ischemic period [44–49] or by inhibiting reverse electron transfer and consequent ROS production at the onset of reperfusion [13, 38, 41]. Furthermore, as underlined in the introduction, besides these new specific properties, we must also consider the role of OP2113 as a potential H2S donor [27, 32–34] in the isolated heart ischemia-reperfusion experiments. Indeed H2S is now considered as a signaling molecule with potential therapeutic applications [52–54], including ischemia-reperfusion and cardiac pathologies [55]. H2S at low concentration may be oxidized by mammalian mitochondria [56] and protect mitochondria during the ischemic phase [40] and reperfusion [54, 57]. These mechanisms may also be involved in heart protection by OP2113 during ischemia-reperfusion (Fig 6).
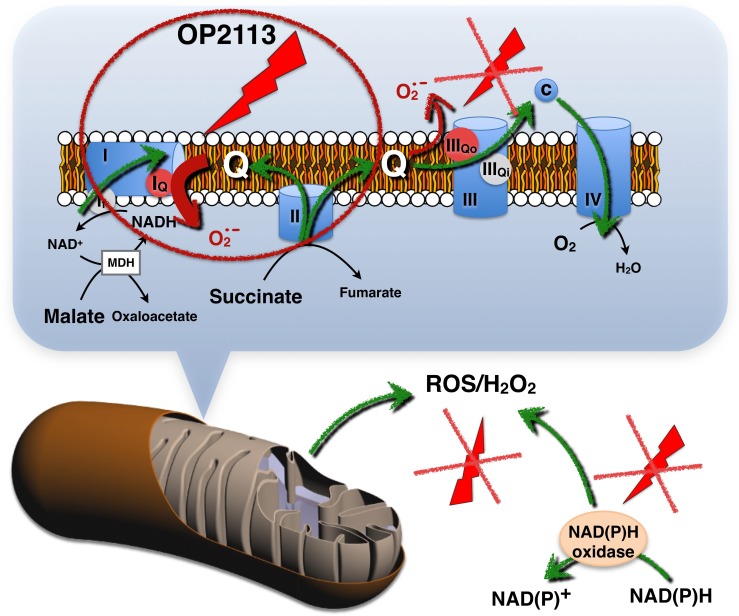
In summary, OP2113 acts upstream from ROS production, therefore insuring increased protection compared with standard antioxidants. OP2113 acts specifically on mitochondrial ROS production to ensure mitochondrial protection, and this action is crucial for numerous pathologies, especially cardiac diseases. OP2113 does not seem to interfere with cell signaling ("good" ROS) (Fig 7). OP2113 acts specifically on site IQ in complex I, which is the main mitochondrial site and may be implicated in important diseases, including Parkinson's and cardiac arrhythmias.
Fig 7. Scheme presenting the specific effects of OP2113 on ROS production by complex I.
To conclude, OP2113’s properties may represent a break-through in the search for specific modulators of ROS/H2O2 production in cells. This is a timely and important discovery given that OP2113 has a great advantage over newly discovered molecules as it is currently authorized for use in humans [31] and may therefore be rapidly included in clinical trials [58–60]. OP2113 may represent the first medicinal member of a new class of "protectants" that specifically prevent ROS production inside mitochondria and may therefore be used for mitochondrial protection during various oxidative stresses, therefore preventing diseases with minimal effects on crucial cellular ROS signaling.
Methods
Animal procedures and ethics statement
All experiments described adhered to the guidelines in the National and European Research Council Guide for the care and use of laboratory animals. P. Diolez has a valid license to conduct experiments on animals from the Service Vétérinaire de la Santé et de la Protection Animale of the Ministère de l’agriculture et de la Forêt, France (03/17/1999, license number 3308010). All procedures conformed to the UK Animals (Scientific Procedures) Act 1986 and the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23. revised 1996).
Materials
All the chemicals were reagent grade and purchased from Sigma Chemical (St. Louis, MO), except for sucrose and NADH oxidase (Merck, Darmstadt, Germany). OP2113 [anetholtrithion, 5-(4-Methoxyphenyl)-3H-1,2-dithiole-3-thione, CAS 532-11-6] was a gift from the private company OP2 drugs (Pessac, France). A 15 mM stock solution of OP2113 was prepared in DMSO and stored away from light at 0°C for only few days. For heart perfusion a daily prepared solution was used.
Isolation of mitochondria
Male Wistar rats (250–325 g; obtained from Janvier Labs, Le Genest-Saint-Isle, France) were anesthetized using 3% isoflurane, heparinized and euthanized by a lethal intra-peritoneal injection of pentobarbital (130 mg/kg), and the heart was quickly removed and washed in cold isolation medium containing 100 mM sucrose, 180 mM KCl, 50 mM Tris, 5 mM MgCl2, 10 mM EDTA, and 0.1% (w/v) defatted BSA (pH 7.2).
Isolation of heart mitochondria was performed in a cold chamber. Before homogenization, hearts (approximately 1.5 g) were minced with scissors and treated for 5 min in 5 ml of the same medium supplemented with protease (2 mg of bacterial proteinase type XXIV per ml of isolation buffer) with stirring. The tissue suspension was poured into a 50-ml glass Potter homogenizer, diluted with 20 ml of isolation buffer, and then homogenized for 3 min using a motorized Teflon pestle. The homogenate was filtered through bolting cloth (Sefar Nitex) to remove debris and centrifuged at 8,000 g for 10 min. The resulting pellet was rinsed with 5 ml of isolation buffer, resuspended in 25 ml of the same buffer, and then subjected to low speed centrifugation (400 g) for 8 min. The resulting supernatant was centrifuged twice at 7,000 g for 15 min to yield a washed mitochondrial pellet that was gently resuspended in 150 μl of isolation buffer. Protein concentration was determined by the Bradford method (Sigma, kit # B6916) using BSA as a standard. Mitochondria were kept on ice at a final concentration of 40–50 mg/ml for less than 5 hours.
Mitochondrial respiration
Oxygen consumption rates of heart mitochondria (0.1 mg/ml) incubated in the absence or presence of OP2113 at increasing doses (from 0 to 80 μM final concentration) were recorded polarographically under constant stirring at 25°C using a high resolution oximeter (Oxygraph-2K, Oroboros Instruments, Austria). The respiration medium consisted of 140 mM sucrose, 100 mM KCl, 1 mM EGTA, 20 mM MgCl2, 10 mM KH2PO4, and 1 g/L (w/v) BSA essentially fatty acid free (pH 7.2). Oxidative phosphorylation has been carried out using various substrate combinations: Glutamate (5 mM)/malate (2.5 mM) as complex I substrates, succinate (5 mM in the presence of 1.5 μM rotenone) as complex II substrates, and the combination Glutamate + Malate + Succinate.
Mitochondrial ROS/H2O2 production
Rates of ROS/H2O2 production from heart mitochondria were assessed through the oxidation of the colorless, non-fluorescent indicator Amplex Red in the presence of exogenous horseradish peroxidase (HRP, EC 1.11.1.7, Sigma). H2O2 reacts with Amplex Red in a 1:1 stoichiometry, yielding the fluorescent compound resorufin (excitation: 560 nm; emission: 585 nm), which is stable once formed. Fluorescence was measured continuously with a spectrofluorometer equipped with temperature control and stirring (SAFAS Xenius, Monaco).
Isolated mitochondria (0.1 mg/ml) were incubated in the same experimental buffer as previously described supplemented with 15 μM Amplex Red and 10 μg/ml HRP. Glutamate (5 mM)/malate (2.5 mM) together with succinate (5 mM) were used as complex I and complex II substrates, respectively. Experiments were conducted under non-phosphorylating conditions in the presence of 15 μM atractyloside (inhibitor of adenine nucleotide translocator), i.e., under conditions where the mitochondrial membrane potential is maximal. Afterwards, rotenone (1.5 μM), antimycin A (2 μM), and myxothiazol (0.2 μM) were sequentially added to inhibit the redox centers within the electron transfer chain (see Fig 2), namely sites IQ, IF (with rotenone), IIIQi (with antimycin A) and IIIQO (with myxothiazol). The assay was finally calibrated with known amounts of H2O2 (steps of 300 nM) in the presence of all relevant compounds, including OP2113. The control test of the absence of effect of OP2113 on the amplex red assay itself and NAD(P)H oxidase ROS/H2O2 production was performed in the absence of cardiac mitochondria and the presence of NAD(P)H oxidase (EC 1.6.3.3, 1 mU/ml) and NADH (150 μM) solutions.
The measurement of the rates of ROS/H2O2 production from major separate mitochondrial sites was performed as described by MD Brand's group [21, 24]. Sites of mitochondrial superoxide/H2O2 production were targeted individually using distinct combinations of mitochondrial substrates and inhibitors [21, 24] designed to generate maximal rates of ROS/H2O2 production predominantly from a single site within the respiratory chain. The sites of production targeted and the solutions used to drive H2O2 production were as follows: site IQ with 5 mM succinate; site IF/DH with 5 mM glutamate + 2.5 mM malate and 4 μM rotenone; site IIIQo with 5 mM succinate, 4 μM rotenone and 2.5 μM antimycin A; site IIF with 15 μM palmitoylcarnitine, 2.5 μM antimycin A and 2 μM myxothiazol; and mitochondrial glycerol-3-phosphate dehydrogenase (mGPDH) with 25 mM glycerolphosphate, 4 μM rotenone, 2.5 μM antimycin A, 1 mM malonate and 2 μM myxothiazol.
The final effect of the OP2113 compound was scaled to positive controls included in each assay. In addition, 4 μM CCCP, 20 mM aspartate, 2 μM myxothiazol, and 10 mM malonate were used as positive controls for site IQ, IF, IIIQo and IIF, respectively [21, 24]. No positive control is available in the case of mGPDH.
Results were analyzed using one-way analysis of variance, followed by Bonferroni’s test to check for significant differences, using Statview Software. Significance was accepted at P < 0.05.
Heart perfusion
Male Wistar rats (250–300 g) were anesthetized by 3% isoflurane, heparinized and euthanized by a lethal IP injection of pentobarbital (130 mg/kg). Hearts (~0.95 g fresh weight) were rapidly harvested and placed into ice-cold Krebs-Henseleit buffer containing (in mmol/L): NaCl 118, NaHCO3 25, KCl 4.8, KH2PO4 1.2, MgSO4 1.2, glucose 11 and CaCl2 1.8. The solution was gassed with 95% O2/5% CO2 at 37°C (pH 7.4). Langendorff heart perfusions were performed as described previously [61], and isometric contractile performance (rate-pressure product (RPP)) was assessed from continuous monitoring of the left ventricular developed pressure (LVDP) via a balloon placed in the left ventricle and connected to a pressure transducer (RPP (mmHg/min) = LVDP (mmHg/beat) x heart rate (beat/min)) [61]. Hearts were perfused in a constant flow mode (12 ml/min) during 10 min for stabilization followed by 10 min treatment with the vehicle (Control; final concentrations: 0.83% ethanol + 0.07% Dimethyl-Sulfoxide) or 10 μM OP2113 solution in the same vehicle. Global normothermic ischemia was induced by halting perfusion flow for 30 min while immersing the heart in perfusion buffer thermostabilized at 37°C. Then, hearts were reperfused for 2 hours.
Assessment of infarct size
At the end of the 2-hour reperfusion period, hearts were stained with triphenyltetrazolium chloride (TTC). Hearts were stained by perfusion for 7 min at 12 ml/min with a 1% (w / v) TTC solution. Hearts were then detached from the cannula and incubated for 4 min at 37°C before being sliced perpendicularly to the longitudinal axis into 6 slices. The slices were then treated in 4% (w/v) formalin solution overnight at 4°C and weighed before both sides of each slice were photographed. The surface of the necrotic and at risk areas of each side were determined for each photograph by planimetry (AlphaEase v5.5). Infarct size was expressed as the percentage of the total cross-sectional area of the heart given that the total heart was subjected to ischemia under our conditions. Data in supporting information S3 File.
Statistical analysis
Data from 6 independent heart perfusions are expressed as the means ± SEM. As the number in each group was less than 20, the distribution was considered non-normal. Consequently, a non-parametric Mann-Whitney test (SPSS statistics 17.0) was performed for comparisons between the control and OP2113 groups. The results were considered statistically significant if the p-value was less than 0.05.
Supporting information
After freeze-thaw treatment, rat heart mitochondria were used to assess mitochondrial rotenone-sensitive NADH oxidase activity by polarography as described in the supplementary Materials and Methods. Panel A: Typical polarographic trace showing the rotenone-sensitive NADH oxidase activity and the effect of the addition of increasing quantity of OP2113 from 50 to 800 nmol / mg mitochondrial protein on oxygen consumption. Panel B: Bar graph representing the mean oxygen consumption expressed in nmol O2 / min / mg mitochondrial protein. Rotenone addition completely stop oxygen consumption suggesting that the activity is mainly supported by the mitochondrial complex I. Data are presented as means ± SD. 4 independent mitochondrial preparation were used for the assay and for each mitochondrial batch the assay was realized in quadruplicate. High quantity of OP2113 inhibit partly the mitochondrial rotenone-sensitive NADH oxidase activity.
(ZIP)
The rates of H2O2 production were measured in the presence of NAD(P)H oxidase (1 mU/ml) and NADH (150 μM), and in the absence of heart mitochondria. Data are based on 3 independent experiments, each performed in duplicate. No significant effect of OP2113 on this experimental H2O2 production was noted.
(ZIP)
Supporting data contain supplementary informations concerning the experiments on isolated rat heart ischemia and reperfusion. Raw data presents contractile activity (RPP), whole heart oxygen consumption (MVO2) during the pre-schemic and post-ischemic (reperfusion) phases for all the experiments, as well as all data used for the determination of infarct size. Separate files describe the results of all the statistical analyses presented in Figs 5 and 6. Finally, supplementary figures present pre-ischemic RPP and MVO2 and reperfusion phases (MVO2 and RPP to MVO2 ratio), as well as a graphic description of the protocols used in the study.
(ZIP)
Acknowledgments
The authors thank Pippa McKelvie-Sebileau of the LIRYC Institute for editorial assistance, Emma Abell for technical assistance and Y. Chatenet for 3D mitochondria drawing (Striking image).
Abbreviations
- ATR
atractyloside
- BSA
bovine serum albumin
- CCCP
Carbonylcyanure m-chlorophénylhydrazone
- DMSO
dimethyl sulfoxide
- HRP
Horseradish peroxidase
- LVDP
Left ventricular developed pressure
- mGPDH
mitochondrial glycerol-3-P dehydrogenase
- RET
reverse electron transfer
- ROS
reactive oxygen species
- RPP
rate-pressure product
- S1QELs
Suppressors of complex 1 site Q electron leak
- TTC
Triphenyl tetrazolium chloride
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Centre National de la Recherche Scientifique (P.D. salary), the French Government as part of the ‘investments for the future’ program managed by the National Research Agency (ANR), Grant reference ANR-10-IAHU-04, and the grant MITOCARD ANR-17-CE11-0041 (P.D.). The authors thanks the company OP2drugs (Pessac, France) for providing the anethol trithione (OP2113) compound. The funding sources had no role in the study design or the analysis and interpretation of data or in the decision to submit the article for publication.
References
- 1.Harman D. The free radical theory of aging: effect of age on serum copper levels. Journal of gerontology. 1965. April;20:151–3. . [DOI] [PubMed] [Google Scholar]
- 2.Dai DF, Chiao YA, Marcinek DJ, Szeto HH, Rabinovitch PS. Mitochondrial oxidative stress in aging and healthspan. Longevity & healthspan. 2014;3:6 10.1186/2046-2395-3-6 . Pubmed Central PMCID: 4013820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harman D. The biologic clock: the mitochondria? Journal of the American Geriatrics Society. 1972. April;20(4):145–7. . [DOI] [PubMed] [Google Scholar]
- 4.Gouspillou G, Bourdel-Marchasson I, Rouland R, Calmettes G, Biran M, Deschodt-Arsac V, et al. Mitochondrial energetics is impaired in vivo in aged skeletal muscle. Aging cell. 2014. February;13(1):39–48. 10.1111/acel.12147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005. February 25;120(4):483–95. 10.1016/j.cell.2005.02.001 . [DOI] [PubMed] [Google Scholar]
- 6.Murphy MP. How mitochondria produce reactive oxygen species. The Biochemical journal. 2009. January 1;417(1):1–13. 10.1042/BJ20081386 . Pubmed Central PMCID: 2605959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Orr AL, Ashok D, Sarantos MR, Shi T, Hughes RE, Brand MD. Inhibitors of ROS production by the ubiquinone-binding site of mitochondrial complex I identified by chemical screening. Free radical biology & medicine. 2013. December;65:1047–59. 10.1016/j.freeradbiomed.2013.08.170 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000. November 9;408(6809):239–47. 10.1038/35041687 . [DOI] [PubMed] [Google Scholar]
- 9.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006. October 19;443(7113):787–95. 10.1038/nature05292 . [DOI] [PubMed] [Google Scholar]
- 10.Muller FL, Lustgarten MS, Jang Y, Richardson A, Van Remmen H. Trends in oxidative aging theories. Free radical biology & medicine. 2007. August 15;43(4):477–503. 10.1016/j.freeradbiomed.2007.03.034 . [DOI] [PubMed] [Google Scholar]
- 11.Nunnari J, Suomalainen A. Mitochondria: in sickness and in health. Cell. 2012. March 16;148(6):1145–59. 10.1016/j.cell.2012.02.035 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006. December 14;444(7121):875–80. 10.1038/nature05487 . [DOI] [PubMed] [Google Scholar]
- 13.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL, et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014. November 20;515(7527):431–5. 10.1038/nature13909 . Pubmed Central PMCID: 4255242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyssokikh MY, Antonenko YN, Lyamzaev KG, Rokitskaya TI, Skulachev VP. Methodology for use of mitochondria-targeted cations in the field of oxidative stress-related research. Methods in molecular biology. 2015;1265:149–59. 10.1007/978-1-4939-2288-8_12 . [DOI] [PubMed] [Google Scholar]
- 15.Smith RA, Murphy MP. Mitochondria-targeted antioxidants as therapies. Discovery medicine. 2011. February;11(57):106–14. . [PubMed] [Google Scholar]
- 16.Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox biology. 2013;1(1):86–93. 10.1016/j.redox.2012.11.009 . Pubmed Central PMCID: 3647698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber J, Fong S, Chen CB, Yoong S, Pastorin G, Schaffer S, et al. Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnology advances. 2013. Sep-Oct;31(5):563–92. 10.1016/j.biotechadv.2012.09.005 . [DOI] [PubMed] [Google Scholar]
- 18.Nicholls DG, Ferguson S. Bioenergetics 4. Amsterdam: Elsevier; 2013. [Google Scholar]
- 19.Mailloux RJ. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox biology. 2015;4:381–98. 10.1016/j.redox.2015.02.001 . Pubmed Central PMCID: PMC4348434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Finkel T. Signal transduction by reactive oxygen species. The Journal of cell biology. 2011. July 11;194(1):7–15. 10.1083/jcb.201102095 . Pubmed Central PMCID: 3135394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinlan CL, Treberg JR, Perevoshchikova IV, Orr AL, Brand MD. Native rates of superoxide production from multiple sites in isolated mitochondria measured using endogenous reporters. Free radical biology & medicine. 2012. November 1;53(9):1807–17. 10.1016/j.freeradbiomed.2012.08.015 . Pubmed Central PMCID: 3472107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand MD. The sites and topology of mitochondrial superoxide production. Experimental gerontology. 2010. August;45(7–8):466–72. 10.1016/j.exger.2010.01.003 . Pubmed Central PMCID: 2879443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brand MD. The role of mitochondria in longevity and healthspan. Longevity & healthspan. 2014;3:7 10.1186/2046-2395-3-7 . Pubmed Central PMCID: 4030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Orr AL, Brand MD. Sites of reactive oxygen species generation by mitochondria oxidizing different substrates. Redox biology. 2013;1:304–12. 10.1016/j.redox.2013.04.005 . Pubmed Central PMCID: PMC3757699. Epub 2013/09/12. Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goncalves RL, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, Brand MD. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. The Journal of biological chemistry. 2014. November 11;290(1):209–27. 10.1074/jbc.M114.619072 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong HS, Dighe PA, Mezera V, Monternier PA, Brand MD. Production of superoxide and hydrogen peroxide from specific mitochondrial sites under different bioenergetic conditions. The Journal of biological chemistry. 2017. August 24;292(41):16804–9. 10.1074/jbc.R117.789271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dulac M, Sassi A, Nagarathinan C, Christen MO, Dansette PM, Mansuy D, et al. Metabolism of Anethole Dithiolethione by Rat and Human Liver Microsomes: Formation of Various Products Deriving from Its O-Demethylation and S-Oxidation. Involvement of Cytochromes P450 and Flavin Monooxygenases in These Pathways. Drug Metab Dispos. 2018. October;46(10):1390–5. 10.1124/dmd.118.082545 . [DOI] [PubMed] [Google Scholar]
- 28.Pouzaud F, Christen MO, Warnet JM, Rat P. [Anethole dithiolethione: an antioxidant agent against tenotoxicity induced by fluoroquinolones]. Pathol Biol (Paris). 2004. July;52(6):308–13. 10.1016/j.patbio.2003.11.001 . L'anethole dithiolethione: un agent cytoprotecteur contre la tenotoxicite induite par les fluoroquinolones. [DOI] [PubMed] [Google Scholar]
- 29.Christen MO. Anethole dithiolethione: biochemical considerations. Methods Enzymol. 1995;252:316–23. . [DOI] [PubMed] [Google Scholar]
- 30.Ben-Mahdi MH, Gozin A, Driss F, Andrieu V, Christen MO, Pasquier C. Anethole dithiolethione regulates oxidant-induced tyrosine kinase activation in endothelial cells. Antioxid Redox Signal. 2000. Winter;2(4):789–99. 10.1089/ars.2000.2.4-789 . [DOI] [PubMed] [Google Scholar]
- 31.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 2018. January 4;46(D1):D1074–D82. 10.1093/nar/gkx1037 . Pubmed Central PMCID: PMC5753335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen P, Luo Y, Hai L, Qian S, Wu Y. Design, synthesis, and pharmacological evaluation of the aqueous prodrugs of desmethyl anethole trithione with hepatoprotective activity. Eur J Med Chem. 2010. July;45(7):3005–10. 10.1016/j.ejmech.2010.03.029 . [DOI] [PubMed] [Google Scholar]
- 33.Kashfi K, Olson KR. Biology and therapeutic potential of hydrogen sulfide and hydrogen sulfide-releasing chimeras. Biochem Pharmacol. 2013. March 1;85(5):689–703. 10.1016/j.bcp.2012.10.019 . Pubmed Central PMCID: PMC3566320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sparatore A, Santus G, Giustarini D, Rossi R, Del Soldato P. Therapeutic potential of new hydrogen sulfide-releasing hybrids. Expert Rev Clin Pharmacol. 2011. January;4(1):109–21. 10.1586/ecp.10.122 . [DOI] [PubMed] [Google Scholar]
- 35.Gouspillou G, Rouland R, Calmettes G, Deschodt-Arsac V, Franconi JM, Bourdel-Marchasson I, et al. Accurate determination of the oxidative phosphorylation affinity for ADP in isolated mitochondria. PLoS One. 2011;6(6):e20709 10.1371/journal.pone.0020709 . Pubmed Central PMCID: PMC3111431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoppel CL, Kerr DS, Dahms B, Roessmann U. Deficiency of the reduced nicotinamide adenine dinucleotide dehydrogenase component of complex I of mitochondrial electron transport. Fatal infantile lactic acidosis and hypermetabolism with skeletal-cardiac myopathy and encephalopathy. J Clin Invest. 1987. July;80(1):71–7. 10.1172/JCI113066 . Pubmed Central PMCID: PMC442203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ. Production of reactive oxygen species by mitochondria: central role of complex III. The Journal of biological chemistry. 2003. September 19;278(38):36027–31. 10.1074/jbc.M304854200 . [DOI] [PubMed] [Google Scholar]
- 38.Brand MD, Goncalves RL, Orr AL, Vargas L, Gerencser AA, Borch Jensen M, et al. Suppressors of superoxide-H2O2 production at site IQ of mitochondrial complex I protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 2016. October 11;24(4):582–92. 10.1016/j.cmet.2016.08.012 . Pubmed Central PMCID: PMC5061631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brand MD. Mitochondrial generation of superoxide and hydrogen peroxide as the source of mitochondrial redox signaling. Free radical biology & medicine. 2016. November;100:14–31. 10.1016/j.freeradbiomed.2016.04.001 . [DOI] [PubMed] [Google Scholar]
- 40.Lesnefsky EJ, Chen Q, Tandler B, Hoppel CL. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu Rev Pharmacol Toxicol. 2017. January 6;57:535–65. 10.1146/annurev-pharmtox-010715-103335 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy MP. Understanding and preventing mitochondrial oxidative damage. Biochem Soc Trans. 2016. October 15;44(5):1219–26. 10.1042/BST20160108 . Pubmed Central PMCID: PMC5095902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Andrienko T, Pasdois P, Rossbach A, Halestrap AP. Real-Time Fluorescence Measurements of ROS and [Ca2+] in Ischemic / Reperfused Rat Hearts: Detectable Increases Occur only after Mitochondrial Pore Opening and Are Attenuated by Ischemic Preconditioning. PLoS One. 2016;11(12):e0167300 10.1371/journal.pone.0167300 . Pubmed Central PMCID: PMC5131916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Andrienko TN, Pasdois P, Pereira GC, Ovens MJ, Halestrap AP. The role of succinate and ROS in reperfusion injury—A critical appraisal. J Mol Cell Cardiol. 2017. September;110:1–14. 10.1016/j.yjmcc.2017.06.016 . Pubmed Central PMCID: PMC5678286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen Q, Camara AK, Stowe DF, Hoppel CL, Lesnefsky EJ. Modulation of electron transport protects cardiac mitochondria and decreases myocardial injury during ischemia and reperfusion. Am J Physiol Cell Physiol. 2007. January;292(1):C137–47. 10.1152/ajpcell.00270.2006 . [DOI] [PubMed] [Google Scholar]
- 45.Aldakkak M, Stowe DF, Chen Q, Lesnefsky EJ, Camara AK. Inhibited mitochondrial respiration by amobarbital during cardiac ischaemia improves redox state and reduces matrix Ca2+ overload and ROS release. Cardiovasc Res. 2008. January 15;77(2):406–15. 10.1016/j.cardiores.2007.08.008 . [DOI] [PubMed] [Google Scholar]
- 46.Kevin LG, Camara AK, Riess ML, Novalija E, Stowe DF. Ischemic preconditioning alters real-time measure of O2 radicals in intact hearts with ischemia and reperfusion. Am J Physiol Heart Circ Physiol. 2003. February;284(2):H566–74. 10.1152/ajpheart.00711.2002 . [DOI] [PubMed] [Google Scholar]
- 47.Riess ML, Camara AK, Kevin LG, An J, Stowe DF. Reduced reactive O2 species formation and preserved mitochondrial NADH and [Ca2+] levels during short-term 17 degrees C ischemia in intact hearts. Cardiovasc Res. 2004. February 15;61(3):580–90. 10.1016/j.cardiores.2003.09.016 . [DOI] [PubMed] [Google Scholar]
- 48.Vanden Hoek TL, Becker LB, Shao Z, Li C, Schumacker PT. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. The Journal of biological chemistry. 1998. July 17;273(29):18092–8. . [DOI] [PubMed] [Google Scholar]
- 49.Chen Q, Moghaddas S, Hoppel CL, Lesnefsky EJ. Ischemic defects in the electron transport chain increase the production of reactive oxygen species from isolated rat heart mitochondria. Am J Physiol Cell Physiol. 2008. February;294(2):C460–6. 10.1152/ajpcell.00211.2007 . [DOI] [PubMed] [Google Scholar]
- 50.Galkin A, Abramov AY, Frakich N, Duchen MR, Moncada S. Lack of oxygen deactivates mitochondrial complex I: implications for ischemic injury? The Journal of biological chemistry. 2009. December 25;284(52):36055–61. 10.1074/jbc.M109.054346 . Pubmed Central PMCID: PMC2794721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mohsin AA, Chen Q, Quan N, Rousselle T, Maceyka MW, Samidurai A, et al. Mitochondrial Complex I Inhibition by Metformin Limits Reperfusion Injury. J Pharmacol Exp Ther. 2019. March 7 10.1124/jpet.118.254300 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wallace JL, Wang R. Hydrogen sulfide-based therapeutics: exploiting a unique but ubiquitous gasotransmitter. Nat Rev Drug Discov. 2015. May;14(5):329–45. 10.1038/nrd4433 . [DOI] [PubMed] [Google Scholar]
- 53.Wu D, Hu Q, Zhu Y. Therapeutic application of hydrogen sulfide donors: the potential and challenges. Front Med. 2016. March;10(1):18–27. 10.1007/s11684-015-0427-6 . Epub 2015/11/26. eng. [DOI] [PubMed] [Google Scholar]
- 54.Dyson A, Dal-Pizzol F, Sabbatini G, Lach AB, Galfo F, Dos Santos Cardoso J, et al. Ammonium tetrathiomolybdate following ischemia/reperfusion injury: Chemistry, pharmacology, and impact of a new class of sulfide donor in preclinical injury models. PLoS Med. 2017. July;14(7):e1002310 10.1371/journal.pmed.1002310 . Pubmed Central PMCID: PMC5497958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang L, Wang Y, Li Y, Li L, Xu S, Feng X, et al. Hydrogen Sulfide (H2S)-Releasing Compounds: Therapeutic Potential in Cardiovascular Diseases. Front Pharmacol. 2018;9:1066 10.3389/fphar.2018.01066 . Pubmed Central PMCID: PMC6160695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abou-Hamdan A, Guedouari-Bounihi H, Lenoir V, Andriamihaja M, Blachier F, Bouillaud F. Oxidation of H2S in mammalian cells and mitochondria. Methods Enzymol. 2015;554:201–28. 10.1016/bs.mie.2014.11.042 . [DOI] [PubMed] [Google Scholar]
- 57.Nandi S, Ravindran S, Kurian GA. Role of endogenous hydrogen sulfide in cardiac mitochondrial preservation during ischemia reperfusion injury. Biomed Pharmacother. 2018. January;97:271–9. 10.1016/j.biopha.2017.10.118 . [DOI] [PubMed] [Google Scholar]
- 58.Hamada T, Nakane T, Kimura T, Arisawa K, Yoneda K, Yamamoto T, et al. Treatment of xerostomia with the bile secretion-stimulating drug anethole trithione: a clinical trial. Am J Med Sci. 1999. September;318(3):146–51. . [DOI] [PubMed] [Google Scholar]
- 59.Lam S, MacAulay C, Le Riche JC, Dyachkova Y, Coldman A, Guillaud M, et al. A randomized phase IIb trial of anethole dithiolethione in smokers with bronchial dysplasia. J Natl Cancer Inst. 2002. July 3;94(13):1001–9. . [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Liu ZG, Peng J, Lin H, Zhong JX, Hu JY. [The clinical therapic efficiency of anethol trithione on dry eye]. Zhonghua Yan Ke Za Zhi. 2009. June;45(6):492–7. . [PubMed] [Google Scholar]
- 61.Garlid KD, Puddu PE, Pasdois P, Costa AD, Beauvoit B, Criniti A, et al. Inhibition of cardiac contractility by 5-hydroxydecanoate and tetraphenylphosphonium ion: a possible role of mitoKATP in response to inotropic stress. Am J Physiol Heart Circ Physiol. 2006. July;291(1):H152–60. 10.1152/ajpheart.01233.2005 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
After freeze-thaw treatment, rat heart mitochondria were used to assess mitochondrial rotenone-sensitive NADH oxidase activity by polarography as described in the supplementary Materials and Methods. Panel A: Typical polarographic trace showing the rotenone-sensitive NADH oxidase activity and the effect of the addition of increasing quantity of OP2113 from 50 to 800 nmol / mg mitochondrial protein on oxygen consumption. Panel B: Bar graph representing the mean oxygen consumption expressed in nmol O2 / min / mg mitochondrial protein. Rotenone addition completely stop oxygen consumption suggesting that the activity is mainly supported by the mitochondrial complex I. Data are presented as means ± SD. 4 independent mitochondrial preparation were used for the assay and for each mitochondrial batch the assay was realized in quadruplicate. High quantity of OP2113 inhibit partly the mitochondrial rotenone-sensitive NADH oxidase activity.
(ZIP)
The rates of H2O2 production were measured in the presence of NAD(P)H oxidase (1 mU/ml) and NADH (150 μM), and in the absence of heart mitochondria. Data are based on 3 independent experiments, each performed in duplicate. No significant effect of OP2113 on this experimental H2O2 production was noted.
(ZIP)
Supporting data contain supplementary informations concerning the experiments on isolated rat heart ischemia and reperfusion. Raw data presents contractile activity (RPP), whole heart oxygen consumption (MVO2) during the pre-schemic and post-ischemic (reperfusion) phases for all the experiments, as well as all data used for the determination of infarct size. Separate files describe the results of all the statistical analyses presented in Figs 5 and 6. Finally, supplementary figures present pre-ischemic RPP and MVO2 and reperfusion phases (MVO2 and RPP to MVO2 ratio), as well as a graphic description of the protocols used in the study.
(ZIP)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.