Abstract
Background
The diagnosis of cardiac sarcoidosis (CS) is challenging because endomyocardial biopsy has only a 20% to 30% sensitivity rate for diagnosis and it presents with similar clinical features of idiopathic dilated cardiomyopathy (DCM). Lymphatic vessel proliferation in pulmonary sarcoidosis has been previously demonstrated. In this study, we compared endomyocardial biopsy samples obtained from patients with CS and DCM to determine whether lymph vessel counts using D2‐40 immunostaining can be utilized as a complementary tool to distinguish CS from DCM.
Methods and Results
Endomyocardial biopsy tissues were obtained from 62 patients with CS (30 patients with a diagnosis made histologically, 32 patients with a diagnosis made clinically), and hematoxylin/eosin, Masson trichrome, and D2‐40 immunostaining were performed. Their results were compared with those from 53 patients with DCM. The histological CS group showed significantly increased lymphatic vessels (12.0 [4.0–40.0] versus 2.6 [1.9–3.4], P<0.0001) and more severe mosaic fibrosis (P<0.0001) compared with the DCM group. The optimal threshold was 7.5 lymphatic vessels, and this resulted in a sensitivity of 0.67 and specificity of 0.96. The clinical CS group diagnosed according to Japanese Circulation Society 2016 criteria showed increased lymphatic vessels (4.0 [3.3–9.0] versus 2.6 [1.9–3.4], P<0.0001), more severe mosaic fibrosis (P<0.0001), more inflammatory cell infiltration (53% versus 0%, P<0.0001), and fatty infiltration within fibroblasts (50% versus 17%, P=0.0012) compared with the DCM group. The optimal threshold of lymphatic vessels was 3.5, which resulted in a sensitivity of 0.75 and specificity of 0.68.
Conclusions
Lymphatic vessel counts using D2‐40 immunostaining may help to distinguish clinical CS without granuloma from DCM.
Keywords: cardiac sarcoidosis, D2‐40 immunostaining, endomyocardial biopsy, lymphatic vessel
Subject Categories: Pathophysiology, Inflammatory Heart Disease, Diagnostic Testing
Clinical Perspective
What Is New?
Endomyocardial biopsy for cardiac sarcoidosis often shows false‐negative results because of sampling error.
Our study presented that endomyocardial biopsy specimens obtained from patients with suspected cardiac sarcoidosis revealed significant proliferation of lymphatic vessels and more severe mosaic pattern fibrosis compared with biopsy specimens from patients with dilated cardiomyopathy.
D2‐40 immunostaining for confirmation of lymphatic vessel proliferation is helpful for diagnosis of cardiac sarcoidosis without epithelioid granuloma in endomyocardial biopsies.
What Are the Clinical Implications?
Cardiovascular manifestations of cardiac sarcoidosis are often similar to those of dilated cardiomyopathy.
Our study showed that D2‐40 immunostaining added to routine hematoxylin/eosin staining may improve diagnostic accuracy of cardiac sarcoidosis in the differential diagnosis from dilated cardiomyopathy.
Our study showed the importance of endomyocardial biopsy for diagnosis of cardiac sarcoidosis.
Introduction
The recognition of cardiac sarcoidosis (CS) among patients with nonischemic cardiomyopathy or fatal arrhythmias is important, since the mainstay of treatment for patients with CS is steroids1 and immunosuppressive drugs,2, 3 which are not used in the treatment of nonischemic cardiomyopathy. Thus, the misdiagnosis of CS may result in further deterioration and poor outcome.1, 4 However, the diagnosis of CS is challenging because patients present with symptoms (ventricular arrhythmias and profound heart failure) that are similar to those in patients with idiopathic dilated cardiomyopathy (DCM). Furthermore, definitive histological diagnosis of CS by endomyocardial biopsy (EMB) is limited, as it has only a 20% to 30% sensitivity rate caused by sampling error.5 To overcome the disadvantages of EMB, several guidelines have suggested a clinical diagnosis of CS when EMB is not performed or shows nonspecific findings6, 7, 8 (Tables 1, 2, 3 through 4).
Table 1.
Highly Probable Laboratory Manifestations for Sarcoidosis
| 1 | Bilateral hilar lymphadenopathy |
| 2 | Elevated serum angiotensin‐converting enzyme or lysozyme |
| 3 | Elevated soluble interleukin 2 receptor |
| 4 | Significant positive uptake on gallium citrate scintigraphy or 18F‐fluorodeoxyglucose positron emission tomography imaging |
| 5 | Increased CD4/CD8 ratio >3.5 in bronchoalveolar lavage fluid |
Consider as highly probable laboratory manifestations if ≥2 of 5 criteria have been met.
Table 2.
Highly Probable Clinical Manifestations for Cardiac Sarcoidosis
| 1 | Major criteria |
| (a) | High‐degree atrioventricular block (include complete atrioventricular block) or lethal ventricular arrhythmia (persistent ventricular tachycardia, ventricular fibrillation) |
| (b) | Basal septum thinning or anatomical abnormalities of ventricular wall (ventricular aneurysm, focal thinning, focal thickening) |
| (c) | Left ventricular systolic dysfunction (ejection fraction <50%) or focal ventricular wall motion abnormalities |
| (d) | Significant positive uptake in the heart on gallium citrate scintigraphy or 18F‐fluorodeoxyglucose positron emission tomography imaging |
| (e) | Late gadolinium enhancement on cardiac magnetic resonance imaging |
| 2 | Minor criteria |
| (f) | Ventricular arrhythmia in ECG (nonsustained ventricular tachycardia, multifocal or frequent premature ventricular contraction), bundle branch block, axis deviation, abnormal Q wave |
| (g) | Focal defect on single‐photon emission computed tomography |
| (h) | Monocyte infiltration and moderate‐severe fibrosis on endomyocardial biopsy |
Consider as disease highly probable clinical manifestations if ≥2 of 5 major criteria (a–e) have been met. Consider as highly probable clinical manifestations if 1 of 5 major criteria (a–e) and 2 of 3 minor criteria (f–h) have been met.
Table 3.
Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis (JCS 2016)
| A | Diagnostic criteria for cardiac sarcoidosis |
| 1 | Histological diagnosis |
| Histological evidence of noncaseating epithelioid granuloma on endomyocardial biopsy or surgical resection of the heart | |
| 2 | Clinical diagnosis (negative granulomatous inflammation on endomyocardial biopsy) |
| a | (1) Histological evidence of noncaseating epithelioid granuloma in extracardiac organ and (2) meets criteria for highly probable clinical manifestations for cardiac sarcoidosis (Table 2) |
| b | (1) Clinical evidence of pulmonary sarcoidosis or ocular sarcoidosis and (2) meets criteria for highly probable laboratory manifestations for sarcoidosis (Table 1) and (3) meets criteria for highly probable clinical manifestations for cardiac sarcoidosis (Table 2) |
| B | Diagnostic criteria for limited cardiac sarcoidosis exclusion criteria |
| a | No evidence of sarcoidosis in extracardiac organs |
| b | No significant positive uptake on gallium citrate scintigraphy or 18F‐FDG PET in extracardiac organs |
| c | No sign of lymph duct tract on lung fields or bilateral hilar lymphadenopathy (short diameter >10 mm) |
| 1 | Histological diagnosis |
| (1) Meets exclusion criteria (a–c) and (2) histological evidence of noncaseating epithelioid granuloma on endomyocardial biopsy or surgical resection of the heart | |
| 2 | Clinical diagnosis |
| (1) Meets exclusion criteria (a–c) and (2) meets 4 of 5 criteria, including (d) on highly probable clinical manifestations for cardiac sarcoidosis (Table 2) | |
| Additional information | |
| (1) | Perform coronary angiography, coronary computed tomography, or cardiac magnetic resonance imaging to differentiate from ischemic heart disease |
| (2) | Cardiac manifestations of sarcoidosis occasionally appear a few years after extracardiac sarcoidosis has been diagnosed. Thus, a routine follow‐up with ECG and echocardiogram are recommended |
| (3) | Be aware of limited cardiac sarcoidosis |
| (4) | 18F‐FDG PET requires 12 h of fasting and dietary modification before the study in order to suppress myocardial physiological uptake. The methods of acquiring imaging follow the recommendation of the Japanese Society of Nuclear Cardiology |
| (5) | Cases of noncaseating epithelioid granuloma are not observed frequently on endomyocardial biopsy; thus, multiple samples should be taken |
| (6) | Histological diagnosis is made if there is evidence of noncaseating epithelioid granuloma by endomyocardial biopsy or surgical resection of the heart, and known cause of granuloma or focal granulomatous reaction has been excluded |
| (7) | Attention should be paid to the stipulation of the Japanese health insurance system. 18F‐FDG PET is approved only for the detection of inflammation sites in patients who have a diagnosis of cardiac sarcoidosis |
18F‐FDG PET indicates 18F‐fluorodeoxyglucose positron emission tomography imaging; JCS, Japanese Circulation Society.
Table 4.
Comparison of Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis
| Guidelines for Diagnosis and Treatment of Cardiac Sarcoidosis (JCS 2016) and Their Comparisons With Other Criteria (JMHW/JSSOG 2015 and WASOG 2014) | JCS 2016 | JMHW/JSSOG 2015 | WASOG 2014 | |
|---|---|---|---|---|
| A | Diagnostic criteria for cardiac sarcoidosis | |||
| 1 | Histological diagnosis | |||
| Histological evidence of noncaseating epithelioid granuloma on endomyocardial biopsy or surgical resection of the heart | ○ | ○ | ○ | |
| 2 | Clinical diagnosis (negative granulomatous inflammation on endomyocardial biopsy) | |||
| a | (1) Histological evidence of noncaseating epithelioid granuloma in extracardiac organ and (2) meets criteria for highly probable clinical manifestations for cardiac sarcoidosis (Table 2) | ○ | ○ | ○ |
| b | (1) Clinical evidence of pulmonary sarcoidosis or ocular sarcoidosis and (2) meets criteria for highly probable laboratory manifestations for sarcoidosis (Table 1) and (3) meets criteria for highly probable clinical manifestations for cardiac sarcoidosis (Table 2) | ○ | ○ | × |
| B | Diagnostic criteria for limited cardiac sarcoidosis exclusion criteria | |||
| a | No evidence of sarcoidosis in extracardiac organs | |||
| b | No significant positive uptake on gallium citrate scintigraphy or 18F‐FDG PET in extracardiac organs | |||
| c | No sign of lymph duct tract on lung fields or bilateral hilar lymphadenopathy (short diameter >10 mm) | |||
| 1 | Histological diagnosis | |||
| (1) Meets exclusion criteria (a–c) and (2) histological evidence of noncaseating epithelioid granuloma on endomyocardial biopsy or surgical resection of the heart | ○ | ○ | ○ | |
| 2 | Clinical diagnosis | |||
| (1) Meets exclusion criteria (a–c) and (2) meets 4 of 5 criteria, including (d) on highly probable clinical manifestations for cardiac sarcoidosis (Table 2) | ○ | × | × | |
| Additional information | ||||
| (1) | Perform coronary angiography, coronary computed tomography, or cardiac magnetic resonance imaging to differentiate from ischemic heart disease | |||
| (2) | Cardiac manifestations of sarcoidosis occasionally appear a few years after extracardiac sarcoidosis has been diagnosed. Thus, a routine follow‐up with ECG and echocardiogram are recommended | |||
| (3) | Be aware of limited cardiac sarcoidosis | |||
| (4) | 18F‐FDG PET requires 12 h of fasting and dietary modification before the study in order to suppress myocardial physiological uptake. The methods of acquiring imaging follow the recommendation of the Japanese Society of Nuclear Cardiology | |||
| (5) | Cases of noncaseating epithelioid granuloma are not observed frequently on endomyocardial biopsy; thus, multiple samples should be taken | |||
| (6) | Histological diagnosis is made if there is evidence of noncaseating epithelioid granuloma by endomyocardial biopsy or surgical resection of the heart, and known cause of granuloma or focal granulomatous reaction has been excluded | |||
| (7) | Attention should be paid to the stipulation of the Japanese health insurance system. 18F‐FDG PET is approved only for the detection of inflammation sites in patients who have a diagnosis of cardiac sarcoidosis | |||
A‐2‐a: included in the Japanese Ministry of Health and Welfare (JMHW)/Japan Society of Sarcoidosis and Other Granuloma Disorders (JSSOG) 2015 and World Association of Sarcoidosis and Other Granuloma Diseases (WASOG) 2014 diagnosis. A‐2‐b: included in the JMHW/JSSOG 2015 diagnosis, but not included in the WASOG 2014 diagnosis. B‐1: included in the JMHW/JSSOG 2015 and WASOG 2014 diagnosis. B‐2: not included in the JMHW/JSSOG 2015 and WASOG 2014 diagnosis. 18F‐FDG PET indicates 18F‐fluorodeoxyglucose positron emission tomography imaging; JCS, Japanese Circulation Society.
Pathological evidence of suspected granuloma, including monocyte infiltrates and moderate to severe fibrosis without giant cells, is one of the minor diagnostic criteria for CS (Table 2). We thought that additional diagnostic evidence might be obtained when a suspected granuloma could not be visualized with standard hematoxylin and eosin staining. Kambouchner et al9 described the relationship between granuloma and the lymphatic network in lung biopsies of patients with pulmonary sarcoidosis. D2‐40 is a commercially available monoclonal antibody directed against human podoplanin, a transmembrane mucoprotein expressed in lymphatic endothelial cells. Unlike the vascular markers CD31 and CD34, which cannot discriminate between blood vessel and lymphatic endothelium, the immunoreactivity of D2‐40 is restricted to lymphatic endothelium.10 D2‐40 immunoexpression has also been described in a variety of lymphovascular neoplasms including lymphangioma, Kaposi sarcoma, and hemangioendothelioma, as well as nonvascular neoplasms such as epithelioid mesothelioma, seminoma, and hemangioblastoma.10, 11 To our knowledge, there are no previous studies that have demonstrated the histopathological structure of lymphatic vessels in formalin‐fixed, paraffin‐embedded myocardial tissues. We hypothesized that the lymphatic system, which may have a unique presentation in granulomatous tissue, may serve as a surrogate marker for granulomatous tissue.
We utilized D2‐40 immunostaining and compared histopathology of biopsy samples obtained from patients with CS and DCM to evaluate whether lymphatic vessel counts can be utilized as a complementary tool to distinguish between CS and DCM.
Diagnostic Criteria
Recently, several guidelines have been revised for the diagnosis of CS, as new technologies have been developed. A sarcoidosis organ assessment instrument was developed by the steering committee of A Case Control Etiologic Study of Sarcoidosis published in 1999.12 It was revised by the World Association of Sarcoidosis and Other Granuloma Diseases in 2014 (WASOG 2014),6 which was expanded by the Heart Rhythm Society in 2014 (HRS 2014).13 Separately, the guideline from the Japanese Ministry of Health and Welfare (JMHW) was originally published in 1993,7 revised in 20068, 14 and a latest guideline was published in collaboration with the Japan Society of Sarcoidosis and Other Granuloma Disorders (JSSOG) in 2015 (JMHW/JSSOG 2015) (http://jssog.com/www/top/shindan/shindan2-1new.html). The Japanese Circulation Society (JCS) expanded the JMHW/JSSOG 2015 guideline to include criteria for the diagnosis of limited CS in 2016 (JCS 2016) (Table 3) (URL www.j-circ.or.jp/guideline/pdf/JCS2016_terasaki_h.pdf).
In the current study, we selected patients with clinical CS who met 1 of the criteria from the 3 recently published guidelines that included JCS 2016, JMHW/JSSOG 2015, and WASOG 2014 (Tables 1, 2, 3 through 4).
Methods
The data that support the findings of this study are available to other researchers on request.
Patient Selection
A total of 114 patients with suspected CS underwent EMB at our institute between 1990 and 2017. All patients underwent coronary angiography before EMB to exclude coronary artery disease. CS was diagnosed in 68 patients, and 46 patients who did not meet current clinical diagnostic criteria for CS (JCS 2016, JMHW/JSSOG 2015, or WASOG 2014) were excluded. Among the 68 patients with CS, histological CS was diagnosed in 32 cases based on evidence of granuloma in the EMB from the right ventricular septum. CS was clinically diagnosed in the other 36 cases by JCS 2016 criteria. Of those with clinical CS, 15 patients met CS 2016, JMHW/JSSOG 2015, and WASOG 2014 criteria. Finally, the biopsy samples of 62 patients underwent D2‐40 immunostaining for evaluation of lymphatic vessels, and 6 patients with CS were excluded because of lack of tissue samples for additional D2‐40 immunostaining (Figure 1).
Figure 1.
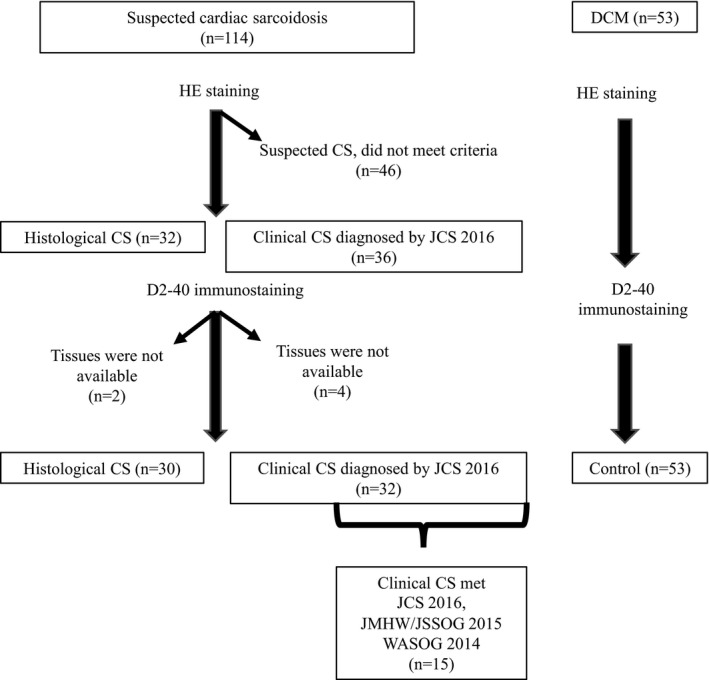
Patient selection. CS indicates cardiac sarcoidosis; DCM, dilated cardiomyopathy; HE, hematoxylin‐eosin; JCS, Japanese Circulation Society; JMHW, Japanese Ministry of Health and Welfare; JSSOG, Japan Society of Sarcoidosis and Other Granuloma Disorders; WASOG, World Association of Sarcoidosis and Other Granuloma Diseases.
The control group consisted of 53 randomly selected patients with DCM who underwent EMB and had D2‐40 immunostaining between 2002 and 2017. DCM, characterized by ventricular enlargement and systolic dysfunction with exclusion of secondary causes, was defined according to the criteria from the American Heart Association15 and European Society of Cardiology.16 The cases were adjusted by age to match study groups.
The following clinical data were collected at the time of diagnosis: age, sex, history of permanent pacemaker implantation, implantable cardioverter‐defibrillator implantation, steroid treatment, ECG parameters, echocardiographic findings, chest x‐ray or computed tomography scan of the chest, gallium scintigraphy, thallium‐201 or technetium‐99m‐sesta methoxyisobutyl isonitrile single‐photon emission computed tomography, 18F‐fluorodeoxyglucose positron emission tomography, and serology.
The study conformed to the principles outlined in the Declaration of Helsinki. The study protocol (M25‐47‐2) was approved by our institutional review committee of the National Cerebral and Cardiovascular Center. Informed consent was waived because of the retrospective design; however, detailed information of the study was posted at the official institutional website to allow study participants to opt out of enrollment.
Light Microscopy and Immunohistochemistry
All myocardial tissue obtained by EMB were fixed in formalin and embedded in paraffin. Thin sections were stained with hematoxylin and eosin. All samples were analyzed by 2 trained pathologists without any knowledge of the patients’ backgrounds. Thin sections were observed under ×100 original magnification on light microscopy, which yield 1 mm2 field in the entire view, and tissue characteristics including granulomatous changes, inflammatory cell infiltration, the degree of fibrosis, and fatty tissue infiltration were examined. Inflammatory cells were considered present if more than 5 cells/mm2 were seen. The degree of fibrosis was graded as mild, moderate, or severe. Fatty infiltration was categorized by its location either within fibroblasts or between myocytes and/or isolated connective tissue that are considered anatomical areas of normal deposition. Additional subserial sections were created for immunostaining with D2‐40 antibody. The number of D2‐40–positive lymphatic vessels was counted under ×100 original magnification in the densest field (count per mm2). The sample from a single biopsy usually fit into the entire view.
Statistical Analyses
Continuous data are expressed as median (interquartile range). Two groups were compared using a Wilcoxon rank sum test for continuous variables. Categorical variables were reported as frequencies along with percentages and compared between groups using the Fisher exact test. Differences were considered significant if the P value was <0.05. Statistical analyses were performed using JMP (SAS Institute). Receiver operating characteristic (ROC) curve analysis was used to determine optimal cutoff values of lymphatic vessel counts to discriminate CS from DM. The area under each curve was determined, and the sensitivity and specificity with their 95% CIs were calculated for the optimal cutoff value. The R language (URL https://www.R-project.org/) and the “pROC” package were used for all statistical analyses.17
Results
Patient Characteristics
The patient characteristics of the 4 groups, which include histological CS, clinical CS by JCS 2016 criteria, clinical CS by 3 criteria (JCS 2016, JMHW/JSSOG 2015, and WASOG 2014), and DCM as a control group, are shown in Table 5. Each CS group was compared with the DCM group. Patients with histological CS were more likely to have the following characteristics: atrioventricular block (P=0.08), complete right bundle branch block (P=0.02), pacemaker implantation (P=0.046), bilateral hilar lymphadenopathy (P<0.0001) on chest x‐ray or computed tomography scan, focal wall motion abnormalities on echocardiography (P=0.005), positive uptake on gallium citrate scintigraphy (P<0.0001), focal defect on thallium‐201 or technetium‐99m‐sesta methoxyisobutyl isonitrole single‐photon emission computed tomography (P=0.02), and higher serum angiotensin‐converting enzyme levels (P=0.01). There were no significant differences in age, sex, or left ventricular ejection fraction.
Table 5.
Baseline Characteristics of Study Population
| Histological CS | Clinical CS Diagnosed by JCS 2016 Criteria | Clinical CS Diagnosed by JCS 2016, JMHW/JSSOG 2015, WASOG 2014 Criteria | DCM | Histological CS vs DCM (P Value) | Clinical CS JCS 2016 vs DCM (P Value) | Clinical CS JCS 2016, JMHW/JSSOG 2015, WASOG 2014 vs DCM (P Value) | |
|---|---|---|---|---|---|---|---|
| n=30 | n=32 | n=15 | n=53 | ||||
| Age, median (IR), y | 57 (47–68) | 59 (51–66) | 57 (45–65) | 53 (50–56) | 0.29 | 0.07 | 0.71 |
| Women, No. (%) | 17 (57) | 24 (75) | 11 (73) | 19 (36) | 0.06 | 0.0005 | 0.0098 |
| Steroid treatment, No. (%) | 20 (67) | 25 (78) | 12 (80) | 0 | <0.0001 | <0.0001 | <0.0001 |
| Cardiac device, No. (%) | |||||||
| ICD (lethal ventricular arrhythmia) | 4 (13) | 9 (28) | 3 (20) | 5 (9) | 0.71 | 0.035 | 0.36 |
| PPM (high‐degree atrioventricular block) | 10 (33) | 10 (31) | 5 (33) | 7 (13) | 0.046 | 0.04 | 0.1 |
| Chest x‐ray or CT scan, No. (%) | |||||||
| BHL | 11 (37) | 14 (44) | 7 (47) | 0 | <0.0001 | <0.0001 | <0.0001 |
| Echocardiography | |||||||
| Basal septum thinning, No. (%) | 6 (21) | 12 (38) | 4 (27) | 6 (11) | 0.25 | 0.004 | 0.2 |
| Asymmetrical wall thickness (/IVST‐PWT/≥3 mm), No. (%) | 7 (24) | 14 (44) | 5 (33) | 7 (13) | 0.2 | 0.0016 | 0.1 |
| Anatomical abnormalities of ventricular wall (focal thinning, focal thickening, ventricular aneurysm), No. (%) | 3 (10) | 5 (16) | 4 (27) | 1 (2) | 0.12 | 0.026 | 0.07 |
| LVEF, median (IR), % | 26 (22–37) | 40 (32–48) | 46 (35–55) | 27 (25–30) | 0.3 | <0.0001 | <0.0001 |
| Focal ventricular wall motion abnormalities, asynergy, No. (%) | 18 (62) | 23 (72) | 9 (60) | 16 (30) | 0.005 | 0.0002 | 0.03 |
| Moderate‐severe MR (≥2/4), No. (%) | 16 (55) | 20 (63) | 7 (47) | 24 (45) | 0.39 | 0.12 | 0.9 |
| ECG, No. (%) | |||||||
| Atrioventricular block (1–3°) | 12 (41) | 20 (63) | 10 (67) | 8 (15) | 0.008 | <0.0001 | <0.0001 |
| Complete left bundle branch block | 4 (14) | 0 | 0 | 8 (15) | 1 | 0.02 | 0.18 |
| Complete right bundle branch block | 8 (28) | 12 (38) | 4 (27) | 4 (8) | 0.02 | 0.001 | 0.06 |
| Nonsustained ventricular tachycardia, multifocal or frequent premature ventricular contraction | 11 (37) | 17 (53) | 10 (67) | 16 (30) | 0.54 | 0.035 | 0.01 |
| Left axial deviation | 7 (24) | 7 (22) | 3 (20) | 9 (17) | 0.43 | 0.57 | 0.79 |
| ST changes | 13 (45) | 17 (53) | 11 (73) | 27 (51) | 0.59 | 0.85 | 0.12 |
| RI, positive/No. (%) | |||||||
| Positive uptake on gallium citrate scintigraphy | 16/28 (57) | 18/30 (60) | 7/15 (47) | 1/23 (4) | <0.0001 | <0.0001 | 0.003 |
| Positive uptake on 18F‐FDG PET | 17/19 (89) | 23/25 (92) | 8/10 (80) | 7/10 (70) | 0.18 | 0.09 | 0.6 |
| Focal defect on SPECT (201Tl or 99mTc‐MIBI) | 17/18 (94) | 20/21 (95) | 9/10 (90) | 19/29 (66) | 0.02 | 0.01 | 0.14 |
| Serologies, median (IR) | |||||||
| ACE (normal range: 7.7–29.4 IU/L) | 18.0 (10.3–25.1) | 12.7 (5.0–18.0) | 14.6 (10.6–36.4) | 11 (9–14) | 0.01 | 0.28 | 0.061 |
| Lysozyme (normal range: 4.2–11.5 μg/mL) | 9.7 (6.9–14.7) | 8.3 (5.7–12.6) | 8.5 (10.6–36.4) | 8 (4–13) | 0.29 | 0.67 | 0.46 |
Continuous variables are presented as median (interquartile range [IR]). 18F‐FDG PET indicates 18F‐fluorodeoxyglucose positron emission tomography imaging; 99mTc‐MIBI, technetium‐99m‐sesta methoxyisobutyl isonitrile; 201Tl, thallium‐201; ACE, angiotensin‐converting enzyme; BHL, bihilar lymphadenopathy; CS, cardiac sarcoidosis; DCM, idiopathic dilated cardiomyopathy; ICD, implantable cardioverter‐defibrillator; IVST, interventricular septum thickness; JCS, Japanese Circulation Society; JMHW, Japanese Ministry of Health and Welfare; JSSOG, Japan Society of Sarcoidosis and Other Granuloma Disorders; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; PPM, permanent pacemaker; PWT, posterior wall thickness; RI, radio isotope; SPECT, single‐photon emission computed tomography; WASOG, World Association of Sarcoidosis and Other Granuloma Diseases.
Patients with clinical CS diagnosed by JCS 2016 criteria were more likely to have the following characteristics: female sex (P=0.0005), atrioventricular block (P<0.0001), complete right bundle branch block (P=0.001), complete left bundle branch block (P=0.02), pacemaker implantation (P=0.04), nonsustained ventricular tachycardia or frequent premature ventricular contractions (P=0.035), implantable cardioverter‐defibrillator implantation (P=0.035), bilateral hilar lymphadenopathy (P<0.0001) on chest x‐ray or computed tomography scan, basal septal thinning (P=0.004), asymmetrical wall thickness (P=0.0016), focal wall motion abnormalities on echocardiography (P=0.005), positive uptake on gallium citrate scintigraphy (P<0.0001), and focal defect on thallium‐201 or technetium‐99m‐sesta methoxyisobutyl isonitrole single‐photon emission computed tomography (P=0.01). Left ventricular ejection fraction was higher in this group than in the DCM control group (40% [32–48%] versus 27% [25–30%], P<0.0001).
Patients with clinical CS who met JCS 2016, JMHW/JSSOG 2015, and WASOG 2014 criteria were more likely to have the following characteristics: female sex (P=0.0098), atrioventricular block (P<0.0001), nonsustained ventricular tachycardia or frequent premature ventricular contractions (P=0.01), bilateral hilar lymphadenopathy (P<0.0001) on chest x‐ray or computed tomography scan, focal wall motion abnormalities on echocardiography (P=0.03), and positive uptake on gallium citrate scintigraphy (P=0.03). Left ventricular ejection fraction was higher in this group than in the DCM control group (46% [35–55%] versus 27% [25–30%], P<0.0001). There were no statistical differences in clinical characteristics between patients with clinical CS diagnosed by JCS 2016 criteria and patients with clinical CS who met JCS 2016, JMHW/JSSOG 2015, and WASOG 2014 criteria.
Characteristics of Endomyocardial Biopsy
Representative pathological specimens are shown in Figure 2, and the characteristics of EMB are summarized in Table 6.
Figure 2.
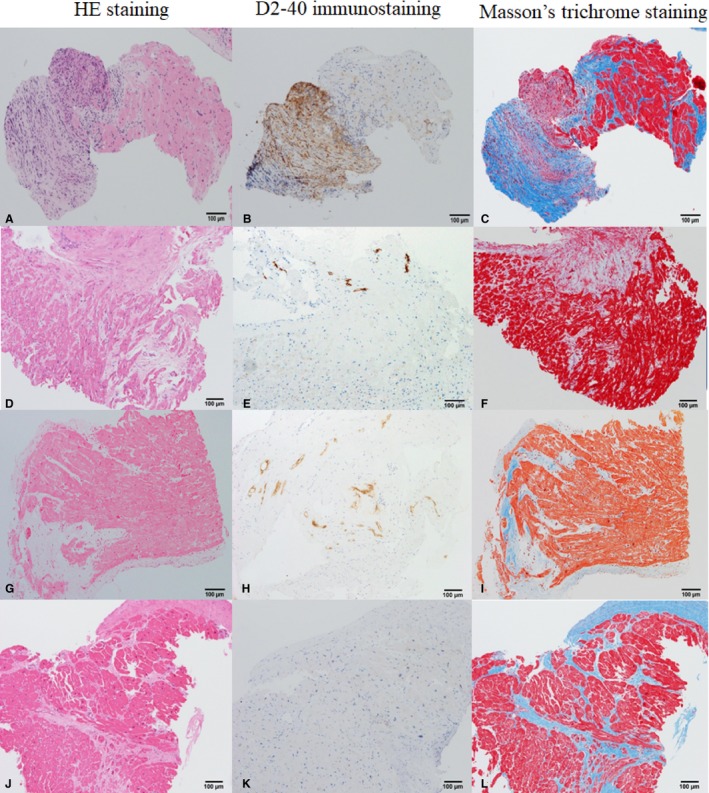
Representative cases of histological cardiac sarcoidosis (A through C), clinical cardiac sarcoidosis (CS; D through I), and idiopathic dilated cardiomyopathy (DCM) (J through L). A, D, G, and J, Hematoxylin‐eosin (HE) staining ×100 original magnification. B, E, H, and K, D2‐40 immunostaining ×100 original magnification. C, F, I, and L, Masson trichrome staining ×100 original magnification. In histological CS, routine HE staining (A) shows noncaseous granuloma consisting of giant cells, epithelioid cells, and fibroblasts. With D2‐40 immunostaining (B), numerous numbers of small lymphatic capillaries were elucidated within granuloma. Endomyocardial granuloma with fibrosis is shown by Masson trichrome staining (C). In clinical CS, no granuloma is seen in standard HE staining (D and G). There is an increased number of lymphatic vessels within connective tissues of fibrosis area (E and H). Fibrosis tends to be mosaic pattern with relatively preserved myocardium (F and I). In DCM, there are extensive myocardial damages seen in HE staining (J). No lymph duct or few sporadic lymph ducts are observed (K). Replacement fibrosis is seen in a diffuse or focal pattern of myocardium that is extensively damaged (L).
Table 6.
Characteristics of Endomyocardial Biopsy in the Study Population
| Histological CS | Clinical CS Diagnosed by JCS 2016 Criteria | Clinical CS Diagnosed by JCS 2016, JMHW/JSSOG 2015, WASOG 2014 Criteria | DCM | Histological CS vs DCM (P Value) | Clinical CS JCS 2016 vs DCM (P Value) | Clinical CS JCS 2016, JMHW/JSSOG 2015, WASOG 2014 vs DCM (P Value) | |
|---|---|---|---|---|---|---|---|
| n=30 | n=32 | n=15 | n=53 | ||||
| Endomyocardial biopsy | |||||||
| Positive D2‐40 immunostaining, No. (%) | 27 (90) | 30 (94) | 13 (87) | 39 (74) | 0.075 | 0.021 | 0.29 |
| Lymphatic vessel count per mm2, median (IR) | 12.0 (4.0–40.0) | 4.0 (3.3–9.0) | 4.0 (4.0–7.0) | 2.6 (1.9–3.4) | <0.0001 | <0.0001 | 0.0015 |
| Granuloma with giant cell, No. (%) | 30 (100) | 0 | 0 | 0 | <0.0001 | n/a | n/a |
| Inflammatory cell infiltration, No. (%) | 30 (100) | 17 (53) | 8 (53) | 0 | <0.0001 | <0.0001 | <0.0001 |
| Mild (focal or diffuse) fibrosis, No. (%) | 2 (7) | 13 (41) | 4 (27) | 31 (58) | <0.0001 | 0.11 | 0.04 |
| Moderate mosaic fibrosis, No. (%) | 4 (13) | 6 (19) | 6 (40) | 13 (25) | 0.26 | 0.54 | 0.33 |
| Severe mosaic fibrosis, No. (%) | 21 (70) | 12 (38) | 4 (27) | 2 (4) | <0.0001 | <0.0001 | 0.02 |
| Fatty tissue located within fibroblasts, No. (%) | 5 (17) | 16 (50) | 7 (47) | 9 (17) | 1 | 0.0012 | 0.02 |
| Fatty tissue located in myocyte or isolated connective tissue, No. (%) | 5 (17) | 3 (9) | 1 (7) | 12 (23) | 0.58 | 0.15 | 0.16 |
Continuous variables are presented as median (interquartile range [IR]). CS indicates cardiac sarcoidosis; DCM, idiopathic dilated cardiomyopathy; JCS, Japanese Circulation Society; JMHW, Japanese Ministry of Health and Welfare; JSSOG, Japan Society of Sarcoidosis and Other Granuloma Disorders; WASOG, World Association of Sarcoidosis and Other Granuloma Diseases.
In the histological CS group, D2‐40 immunostaining was positive in 90% of patients and there were no significant differences compared with the DCM group (90% versus 74%). However, the number of lymphatic vessels was significantly increased (12.0 [4.0–40.0] versus 2.6 [1.9–3.4], P<0.0001) and showed more severe mosaic fibrosis (P<0.0001).
In the group with clinical CS diagnosed by JCS 2016 criteria, D2‐40 immunostaining was positive in 94% of patients and there was a significant difference compared with the DCM group (94% versus 74%, P=0.021). The number of lymphatic vessels was also increased (6.2 [4.6–7.7] versus 2.6 [1.9–3.4], P<0.0001). Furthermore, there was more severe mosaic fibrosis (P<0.0001), more inflammatory cell infiltration (53% versus 0%, P<0.0001), and fatty infiltration within fibroblasts (50% versus 17%, P=0.0012).
In the group with clinical CS who met 3 criteria (JCS 2016, JMHW/JSSOG 2015, and WASOG 2014), D2‐40 immunostaining was positive in 87% of patients and there were no significant differences compared with the DCM group (87% versus 74%). The number of lymphatic vessels was increased (4.0 [4.0–7.0] versus 2.6 [1.9–3.4], P=0.0015), there was more severe mosaic fibrosis (P<0.02), more inflammatory cell infiltration (53% versus. 0%, P<0.0001), and greater fatty infiltration within fibroblasts (47% versus 17%, P=0.02). There were no significant differences between the group with clinical CS diagnosed by JCS 2016 criteria and the group with clinical CS diagnosed by 3 criteria (JCS 2016, JMHW/JSSOG 2015, and WASOG 2014).
Diagnostic Accuracy of Lymphatic Vessel Counts to Discriminate Between CS and DCM
Figure 3 shows ROC curves of lymphatic vessel counts to discriminate between patients with CS and those with DCM. From the box plots, there were higher lymphatic vessel counts in patients with histological and clinical CS than in those with DCM (Figure 3A, 3C, and 3E). The ROC curve generated to discriminate between histological CS and DCM had an area under the curve of 0.831 (95% CI, 0.725–0.938) (Figure 3B). The optimal threshold was 7.5 lymphatic vessels, and this resulted in a sensitivity of 0.67 and specificity of 0.96 (Table 7). The ROC curve generated to discriminate between clinical CS diagnosed by JCS 2016 criteria and DCM had an area under the curve of 0.765 (95% CI, 0.661–0.869) (Figure 3D). The optimal threshold was 3.5 lymphatic vessels, and this resulted in a sensitivity of 0.75 and specificity of 0.68 (Figure 3E). The ROC curve generated to discriminate between clinical CS diagnosed by JCS 2016, JMHW/JSSOG 2015, and WASOG 2014 and DCM had an area under the curve of 0.745 (95% CI, 0.593–0.897). The optimal threshold was 3.5 lymphatic vessels, and this resulted in a sensitivity of 0.80 and specificity of 0.68 (Figure 3F).
Figure 3.
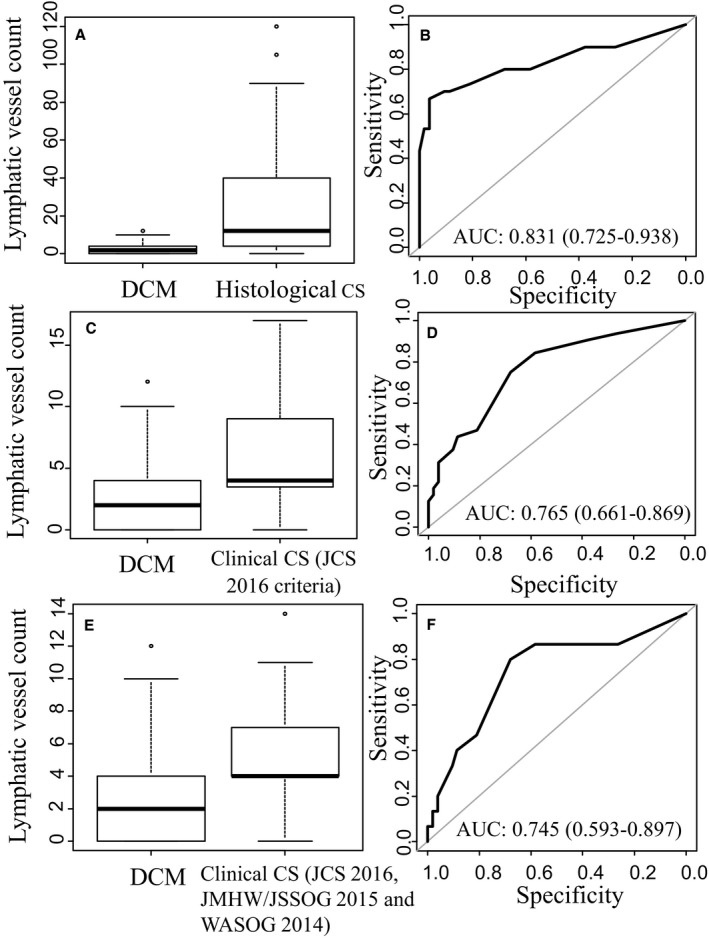
Distribution of lymph duct count (A, C, and E) and the area under the receiver operating characteristic (AUC) curve (B, D, and F). CS indicates cardiac sarcoidosis; DCM, idiopathic dilated cardiomyopathy; JCS, Japanese Circulation Society; JMHW, Japanese Ministry of Health and Welfare; JSSOG, Japan Society of Sarcoidosis and Other Granuloma Disorders; WASOG, World Association of Sarcoidosis and Other Granuloma Diseases.
Table 7.
Thresholds for the Sensitivity and Specificity of Lymphatic Vessels (With 95% CI)
| Thresholds of Lymphatic Vessels | Sensitivity | Specificity | |
|---|---|---|---|
| Histological CS | 7.5 | 0.67 (0.50–0.83) | 0.96 (0.9–1.00) |
| Clinical CS diagnosed by JCS 2016 criteria | 3.5 | 0.75 (0.59–0.91) | 0.68 (0.54–0.81) |
| Clinical CS diagnosed by JCS 2016, JMHW/JSSOG 2015 and WASOG 2014 criteria | 3.5 | 0.80 (0.60–1.00) | 0.68 (0.55–0.79) |
CS indicates cardiac sarcoidosis; JCS, Japanese Circulation Society; JMHW, Japanese Ministry of Health and Welfare; JSSOG, Japan Society of Sarcoidosis and Other Granuloma Disorders; WASOG, World Association of Sarcoidosis and Other Granuloma Diseases.
Discussion
Histological Cardiac Sarcoidosis
Our study demonstrated that lymphatic vessels were present in EMB specimens from patients with both CS and DCM. This is most likely based on the fact that lymphatic vessels are normally present in the heart. The normal anatomy and structure of the lymphatic system in the heart has been reviewed.18, 19 Lymphatics of the heart consist of terminal capillaries of various diameters; capillary plexuses that continuously drain the subendocardial, myocardial, and subepicardial layers; and draining (collecting) vessels that lead the lymph out of the heart. Lymphatic capillaries are located uniformly in the myocardial, subepicardial, and subendocardial layers forming a continuous plexus. Lymphatic capillaries and drainage vessels often form networks and meshes that are irregular in contour and can be found within interfascicular connective tissue located between the lining endothelium and Purkinje fibers.20
Although lymphatic vessels are considered normal structures of the heart, our study demonstrated that the number of lymphatic vessels was significantly increased in granulomatous tissue from patients with CS compared with patients with DCM. Since these vessels were smaller in diameter than typical lymphatic vessels, they are presumably lymphatic capillaries. The role of increased lymphatic vessels is uncertain. In the normal heart, the lymphatic vessel network regulates many physiological processes important for heart function, such as fluid balance, transport of extravasated protein, and trafficking of immune cells.21, 22 Previous studies have shown that lymphangiogenesis may take place under certain pathological situations, including inflammation (inflammation‐associated lymphangiogenesis) and tissue repair (repair‐associated lymphangiogenesis).23, 24 Upon initiation of inflammation, the lymphatic system is activated, and both the extranodal lymphatic vessels in peripheral tissues and the intranodal lymphatic vessels display exuberant growth and vigorously expand.25 Large quantities of immune cells are transported through blood vessels and gather in the inflamed peripheral tissue. Lymphatic vessels locally promote the recruitment of macrophages and dendric cells that have gathered in the peripheral tissue. Antigen‐bearing dendric cells and macrophages enter the lymphatic vessels in peripheral tissue through discontinuous endothelial junctions or preformed portals and continue to travel to regional lymph nodes.25, 26 Repair‐associated lymphangiogensis, on the other hand, is observed after myocardial infarction. Ishikawa et al24 demonstrated that newly formed lymphatics first appeared in the early stage of granulation in lesions with coagulation necrosis and increased in the late stage of granulation. The newly formed lymphatics remained up to the phase of scar formation.
Sarcoidosis is a noncaseating granulomatous disease most likely attributable to an autoimmune disorder, and it causes inflammation and tissue damage in multiple organs.27 It is thought to be caused by either extrinsic antigen‐driven immune activation or intrinsic, genetically determined overactivation of inflammatory pathways. Recently, a critical role of dendric cells was suggested regardless of the initiating factors.28 Although the pathogenesis of sarcoidosis remains unclear, it is conceivable that the demand for lymphatic drainage increases during granuloma formation. Increased lymphatic capillaries suggest that lymphogenesis might have occurred during granuloma formation. To our knowledge, this is the first study to describe the structure of the lymphatic system in granulomatous tissue seen in EMB. The increased number of lymph ducts may serve as a surrogate marker for the appropriate diagnosis of CS.
Clinical Cardiac Sarcoidosis
Currently, the histological diagnosis of CS depends on typical features of granuloma including giant cells, clusters of epithelioid cells, and fibrosis. In clinical CS, there is no typical granulomatous tissue in the EMB; thus, the diagnosis is based on other clinical futures. In this study, we found that there was an increased number of lymphatic vessels within connective tissues along with patchy fibrosis in EMB specimens from patients with CS.
A previous study of D2‐40‐immunolabelled sections in lung biopsies from patients with pulmonary sarcoidosis performed 3‐dimensional reconstruction and showed a woven‐like arrangement around a sarcoid granuloma that consisted of small clusters of closely packed epithelioid and giant cells surrounded by a connective tissue sheath traversed by a lymphatic network.9 In patients with clinical CS in the present study, the biopsy was probably not taken from a core of the granuloma; thus, granulomatous tissue could not be identified. However, increased lymphatic vessel counts suggest that surrounding tissues of traditional granuloma with giant cells may be captured by the biopsy. Moreover, with hematoxylin and eosin and Masson trichrome staining, fibrosis was found to occur in a mosaic pattern with relatively preserved myocardium, as opposed to the diffuse or focal pattern of fibrosis between extensively damaged myocardial cells seen in DCM. This finding may help to distinguish granulomatous tissue from simple fibrosis, even if the specimen does not show the typical features of granuloma. Since biopsy specimens from patients with DCM may show inflammatory cells in up to 50% of cases,29, 30 the presence of inflammatory cells is not enough to distinguish between CS and DCM. Some cases that were initially thought to be inflammatory DCM pathologically were diagnosed as clinical CS by the clinical criteria used in our study. The addition of D2‐40 immunostaining to standard hematoxylin and eosin staining may allow the appropriate diagnosis of CS.
In addition to fibrosis and lymphatic vessels, fatty tissues were also increased in clinical CS, typically appearing within loose connective tissue. There was no fatty tissue in densely populated granulomatous tissue or the diffuse fibrosis seen in DCM. Fatty infiltration is often seen in arrhythmogenic right ventricular cardiomyopathy.31 Interestingly, arrhythmogenic right ventricular cardiomyopathy and CS have similar clinical features, such as sudden cardiac death, ventricular tachycardia, right ventricular structural abnormalities, and similar cardiac magnetic resonance findings.32 However, it is not clear whether fatty infiltration is an end product of postinflammation or a primary deposition that causes electrical disturbances. Further study is warranted to elucidate the role of fatty tissue infiltration in the myocardium.
Limitations
Although EMB is the gold standard for the diagnosis CS, this method has several technical limitations: (1) the disease progresses in a patchy manner with preservation of normal myocardium; (2) the location of the biopsy is limited to the subendocardial layer of the right ventricular septum; (3) granulomas are composed of clusters of epithelioid and giant cells surrounded by a rim of lymphocytes and fibroblasts, and a missing core of granuloma leads to a nonspecific diagnosis; and (4) granuloma tends to convert into “nonspecific” fibrotic tissue as the disease progresses.33 Therefore, the negative biopsy results may not reflect overall cardiac conditions, although positive biopsy results have been linked to poor prognosis.5 Other new diagnostic modalities such as cardiac magnetic resonance imaging or 18F‐fluorodeoxyglucose positron emission tomography may help to evaluate overall cardiac conditions.
In the current study, 40% (46 of 114) of cases with suspected CS were excluded, as they did not meet the diagnostic criteria for CS. Usually, these cases are categorized as DCM by exclusion criteria; however, to clearly discriminate CS from DCM, we excluded cases that were nondiagnostic but had clinical features of CS. Even with that effort, the possibility of existing CS in the DCM group cannot be excluded. In fact, we experienced a case that was initially thought to be DCM based only on fibrotic changes in the EMB; however, it was diagnosed as histological CS with an additional deeper cut that showed granuloma with giant cells.
Importantly, we used patients with DCM as a control group, and there was no comparison between patients with CS and patients with ischemic cardiomyopathy, other types of cardiomyopathies, other inflammatory cardiac diseases, or normal hearts. Therefore, the specificity of D2‐40 staining for CS remains uncertain. A previous autopsy study in patients with acute myocardial infarction and/or old myocardial infarction demonstrated abundant D2‐40–positive lymphatics in the interstitium of the subepicardium and subendocardium within normal cardiac tissue.24 Lymphatic capillaries were sporadically scattered among cardiomyocytes, and relatively abundant lymphatics were observed in the interstitium around arteries and veins running in the myocardium.24 Dashkevich et al34 obtained biopsies from donor hearts and transplant recipients and described the microvascular structure of lymphatics and blood vessels in DCM, ischemic cardiomyopathy, and normal myocardium. In DCM, there was a higher density of lymphatic vessel endothelial hyaluronan receptor 1–positive lymphatics than in normal myocardium, whereas there was a difference in D2‐40–positive lymphatics. Lymphatic vessel endothelial hyaluronan receptor 1 is one of the markers of lymphatic endothelial cells and a homolog of hyaluronan receptor CD44, a type I integral membrane glycoprotein. It is also expressed in the liver and the spleen sinusoid endothelium and activated macrophages.35 In the study by Dashkevich et al,34 results from biopsies from patients with ischemic cardiomyopathy showed a higher density of D2‐40–positive lymphatics and a lower density of vascular endothelial growth factor receptor 2–positive capillaries compared with normal myocardium. To our knowledge, there is no study that has compared CS with ischemic cardiomyopathy, thus further studies are warranted.
Last, because of technical limitation of EMB, serpiginous lymphatic vessels may have been counted more than once if they passed repeatedly through the cross‐section of myocardium.
Conclusions
Although EMB has limited sensitivity and is invasive, it is the only method that provides a definitive diagnosis of CS. It potentially contains a tremendous amount of pathophysiological information. With D2‐40 immunostaining, EMB becomes more valuable, especially for specimens that do not allow a definitive diagnosis. Lymphatic vessel counts by D2‐40 immunostaining may help to distinguish CS from DCM, especially in cases of clinical CS without histological granuloma, although the specificity of D2‐40 staining for CS remains uncertain.
Sources of Funding
This study was supported by Sciences Research Grant for Cardiovascular Diseases (20C‐5) from the Japanese Ministry of Health, Labor and Welfare, and Intramural Research Fund (24‐4‐2) for Cardiovascular Diseases of National Cerebral and Cardiovascular Center, Japan.
Disclosures
None.
Acknowledgments
We thank Mayumi Oka for her skillful technical assistance throughout the study.
(J Am Heart Assoc. 2019;8:e010967 DOI: 10.1161/JAHA.118.010967)
This study was presented in part at the Cardiac Biopsy Conference, November 1–2, 2013, in Tokyo, Japan.
References
- 1. Nagai T, Nagano N, Sugano Y, Asaumi Y, Aiba T, Kanzaki H, Kusano K, Noguchi T, Yasuda S, Ogawa H, Anzai T. Effect of corticosteroid therapy on long‐term clinical outcome and left ventricular function in patients with cardiac sarcoidosis. Circ J. 2015;79:1593–1600. [DOI] [PubMed] [Google Scholar]
- 2. Uthman I, Touma Z, Khoury M. Cardiac sarcoidosis responding to monotherapy with infliximab. Clin Rheumatol. 2007;26:2001–2003. [DOI] [PubMed] [Google Scholar]
- 3. Barnabe C, McMeekin J, Howarth A, Martin L. Successful treatment of cardiac sarcoidosis with infliximab. J Rheumatol. 2008;35:1686–1687. [PubMed] [Google Scholar]
- 4. Padala SK, Peaslee S, Sidhu MS, Steckman DA, Judson MA. Impact of early initiation of corticosteroid therapy on cardiac function and rhythm in patients with cardiac sarcoidosis. Int J Cardiol. 2017;227:565–570. [DOI] [PubMed] [Google Scholar]
- 5. Ardehali H, Howard DL, Hariri A, Qasim A, Hare JM, Baughman KL, Kasper EK. A positive endomyocardial biopsy result for sarcoid is associated with poor prognosis in patients with initially unexplained cardiomyopathy. Am Heart J. 2005;150:459–463. [DOI] [PubMed] [Google Scholar]
- 6. Judson MA, Costabel U, Drent M, Wells A, Maier L, Koth L, Shigemitsu H, Culver DA, Gelfand J, Valeyre D, Sweiss N, Crouser E, Morgenthau AS, Lower EE, Azuma A, Ishihara M, Morimoto S, Tetsuo Yamaguchi T, Shijubo N, Grutters JC, Rosenbach M, Li HP, Rottoli P, Inoue Y, Prasse A, Baughman RP; Organ Assessment Instrument Investigators TW . The WASOG Sarcoidosis Organ Assessment Instrument: an update of a previous clinical tool. Sarcoidosis Vasc Diffuse Lung Dis. 2014;31:19–27. [PubMed] [Google Scholar]
- 7. Hulten E, Aslam S, Osborne M, Abbasi S, Bittencourt MS, Blankstein R. Cardiac sarcoidosis‐state of the art review. Cardiac sarcoidosis‐state of the art review. Cardiovasc Diagn Ther. 2016;6:50–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Diagnostic standard and guidelines for sarcoidosis. Jpn J Sarcoidosis Granulomatous Disord. 2007;27:89–102. [Google Scholar]
- 9. Kambouchner M, Pirici D, Uhl JF, Mogoanta L, Valeyre D, Bernaudin JF. Lymphatic and blood microvasculature organisation in pulmonary sarcoid granulomas. Eur Respir J. 2011;37:835–840. [DOI] [PubMed] [Google Scholar]
- 10. Kalof AN, Cooper K. D2‐40 immunohistochemistry–so far! Adv Anat Pathol. 2009;16:62–64. [DOI] [PubMed] [Google Scholar]
- 11. Ordóñez NG. D2‐40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol. 2005;36:372–380. [DOI] [PubMed] [Google Scholar]
- 12. Judson MA, Baughman RP, Teirstein AS, Terrin ML, Yeager H Jr. Defining organ involvement in sarcoidosis: the ACCESS proposed instrument. ACCESS Research Group. A Case Control Etiologic Study of Sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. 1999;16:75–86. [PubMed] [Google Scholar]
- 13. Birnie DH, Sauer WH, Bogun F, Cooper JM, Culver DA, Duvernoy CS, Judson MA, Kron J, Mehta D, Cosedis Nielsen J, Patel AR, Ohe T, Raatikainen P, Soejima K. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart Rhythm. 2014;11:1305–1323. [DOI] [PubMed] [Google Scholar]
- 14. Soejima K, Yada H. The work‐up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. J Cardiovasc Electrophysiol. 2009;20:578–583. [DOI] [PubMed] [Google Scholar]
- 15. Maron BJ, Towbin JA, Thiene G, Antzelevitch C, Corrado D, Arnett D, Moss AJ, Seidman CE, Young JB; American Heart Association; Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; Council on Epidemiology and Prevention . Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation. 2006;113:1807–1816. [DOI] [PubMed] [Google Scholar]
- 16. Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, Dubourg O, Kühl U, Maisch B, McKenna WJ, Monserrat L, Pankuweit S, Rapezzi C, Seferovic P, Tavazzi L, Keren A. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. [DOI] [PubMed] [Google Scholar]
- 17. Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, Müller M. pROC: an open‐source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ratajska A, Gula G, Flaht‐Zabost A, Czarnowska E, Ciszek B, Jankowska‐Steifer E, Niderla‐Bielinska J, Radomska‐Lesniewska D. Comparative and developmental anatomy of cardiac lymphatics. ScientificWorldJournal. 2014;2014:183170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Loukas M, Abel N, Tubbs RS, Grabska J, Birungi J, Anderson RH. The cardiac lymphatic system. Clin Anat. 2011;24:684–691. [DOI] [PubMed] [Google Scholar]
- 20. Lupinski RW. Aortic fat pad and atrial fibrillation: cardiac lymphatics revisited. ANZ J Surg. 2009;79:70–74. [DOI] [PubMed] [Google Scholar]
- 21. Vuorio T, Tirronen A, Ylä‐Herttuala S. Cardiac lymphatics—a new avenue for therapeutics? Trends Endocrinol Metab. 2017;28:285–296. [DOI] [PubMed] [Google Scholar]
- 22. Randolph GJ, Angeli V, Swartz MA. Dendritic‐cell trafficking to lymph nodes through lymphatic vessels. Nat Rev Immunol. 2005;5:617–628. [DOI] [PubMed] [Google Scholar]
- 23. Paupert J, Sounni NE, Noël A. Lymphangiogenesis in post‐natal tissue remodeling: lymphatic endothelial cell connection with its environment. Mol Aspects Med. 2011;32:146–158. [DOI] [PubMed] [Google Scholar]
- 24. Ishikawa Y, Akishima‐Fukasawa Y, Ito K, Akasaka Y, Tanaka M, Shimokawa R, Kimura‐Matsumoto M, Morita H, Sato S, Kamata I, Ishii T. Lymphangiogenesis in myocardial remodelling after infarction. Histopathology. 2007;51:345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H, Kataru RP, Koh GY. Regulation and implications of inflammatory lymphangiogenesis. Trends Immunol. 2012;33:350–356. [DOI] [PubMed] [Google Scholar]
- 26. Baluk P, Fuxe J, Hashizume H, Romano T, Lashnits E, Butz S, Vestweber D, Corada M, Molendini C, Dejana E, McDonald DM. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349–2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller‐Quernheim J. Sarcoidosis. Lancet. 2014;383:1155–1167. [DOI] [PubMed] [Google Scholar]
- 28. Zaba LC, Smith GP, Sanchez M, Prystowsky SD. Dendritic cells in the pathogenesis of sarcoidosis. Am J Respir Cell Mol Biol. 2010;42:32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kühl U, Noutsias M, Seeberg B, Schultheiss HP. Immunohistological evidence for a chronic intramyocardial inflammatory process in dilated cardiomyopathy. Heart. 1996;75:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Trachtenberg BH, Hare JM. Inflammatory cardiomyopathic syndromes. Circ Res. 2017;121:803–818. [DOI] [PubMed] [Google Scholar]
- 31. Burke AP, Farb A, Tashko G, Virmani R. Arrhythmogenic right ventricular cardiomyopathy and fatty replacement of the right ventricular myocardium: are they different diseases? Circulation. 1998;97:1571–1580. [DOI] [PubMed] [Google Scholar]
- 32. Murphy DT, Shine SC, Cradock A, Galvin JM, Keelan ET, Murray JG. Cardiac MRI in arrhythmogenic right ventricular cardiomyopathy. AJR Am J Roentgenol. 2010;194:W299–W306. [DOI] [PubMed] [Google Scholar]
- 33. Ohira H, Tsujino I, Ishimaru S, Oyama N, Takei T, Tsukamoto E, Miura M, Sakaue S, Tamaki N, Nishimura M. Myocardial imaging with 18F‐fluoro‐2‐deoxyglucose positron emission tomography and magnetic resonance imaging in sarcoidosis. Eur J Nucl Med Mol Imaging. 2008;35:933–941. [DOI] [PubMed] [Google Scholar]
- 34. Dashkevich A, Bloch W, Antonyan A, Goebel H, Fries JU, Schlensak C, Beyersdorf F, Geissler HJ. Immunohistochemical study of remodeling of myocardial lymphatic and blood microvascular structures in terminal heart failure: differences between ischemic and dilated cardiomyopathy. Lymphology. 2010;43:110–117. [PubMed] [Google Scholar]
- 35. Mouta Carreira C, Nasser SM, di Tomaso E, Padera TP, Boucher Y, Tomarev SI, Jain RK. LYVE‐1 is not restricted to the lymph vessels: expression in normal liver blood sinusoids and down‐regulation in human liver cancer and cirrhosis. Cancer Res. 2001;61:8079–8084. [PubMed] [Google Scholar]


