Abstract
Background
It is believed that most sudden cardiac arrests (SCAs) in young people occur in previously healthy people with rare risk factors for sudden death. Few studies have investigated large populations with complete ascertainment. Our objective was to use multisource records to identify and classify all out‐of‐hospital cardiac arrests in the Greater Toronto Area (population 6.6 million) in people aged 2 to 45 years from 2009 to 2012.
Methods and Results
Expert reviewers employed a systematic process, with emergency medical services, in‐hospital and coroner records, to adjudicate the cause of death as SCA from cardiac or noncardiac causes. We report the adjudicated etiologies, circumstances, triggers, and characteristics of the SCA cohort. Of 2937 eligible out‐of‐hospital cardiac arrest cases, 608 (20.7%) SCAs had an adjudicated etiology of cardiac cause (120 survivors and 488 nonsurvivors). Two thirds of these SCA patients had a history of cardiovascular disease, and over 50% had been diagnosed with ≥1 cardiovascular disease risk factor. Moreover, 20.1% of SCAs were diagnosed with psychiatric disease and 30% had central nervous system drugs prescribed. Over 30% of SCA patients had central nervous system active drugs, including drugs of abuse detected postmortem, with opioids and ethanol being detected most frequently. Potentially heritable structural cardiac diseases accounted for only 6.9% of SCA events, with acquired cardiac diseases comprising the rest.
Conclusions
The underlying causes of SCA, in people aged 2 to 45 years, often occur in those with previously diagnosed cardiovascular diseases, and are associated with contributory factors including prescribed medications, recreational drugs, and a concomitant psychiatric history.
Keywords: cardiovascular disease risk factors, etiology, psychiatric comorbidity, sudden cardiac arrest, sudden cardiac death, triggers, young
Subject Categories: Sudden Cardiac Death, Etiology, Risk Factors, Mental Health, Epidemiology
Short abstract
See Editorial by Atkins
Clinical Perspective
What Is New?
This study adds to the sudden cardiac arrest literature by describing in detail the etiologies, circumstances, triggers, and characteristics of a large urban, population‐based sudden cardiac arrest cohort (both survivors and deceased) aged 2 to 45 years from 2009 to 2012.
The results highlight that the underlying causes of sudden cardiac arrest in younger people are complex, often occurring in people with previously diagnosed cardiac diseases, and are associated with contributory factors including prescribed medications, recreational drugs, and a concomitant psychiatric history.
What Are the Clinical Implications?
The high prevalence of cardiovascular disease risk factors among younger patients with sudden cardiac arrest highlights the importance of cardiac risk management aimed at those under age 45, in particular those aged 35 to 45.
Furthermore, the high rates of psychiatric disease and prescribed central nervous system active drugs warrant caution with respect to the use of psychotropic therapies in patients diagnosed with mental illness and concomitant cardiac disease.
Introduction
Sudden cardiac arrest (SCA) in younger people, although rare, is often a highly publicized event, particularly among athletes.1 These occurrences have a devastating impact on both the family and community, due to the perception that these individuals are “too young to die suddenly.”2 Because of various methodological differences between studies, reported incidence rates for sudden cardiac death (SCD) in younger people, which refers solely to nonsurvivors of SCA, can vary up to 6‐fold, emphasizing the lack of clarity with respect to the true scope of this phenomenon.3, 4, 5
In younger people, the cardiac causes of SCA are diverse and assumed to be most often caused by underlying potentially heritable cardiac diseases or “primary arrhythmia syndromes” with no identified risk factors.6 In almost all studies of SCA in younger people, cardiomyopathies, “primary electrical disease,” and premature coronary disease are the most frequently identified cardiac etiologies.7, 8, 9 Yet the exact distribution of these etiologies differs substantially between studies.10, 11 Moreover, it is generally assumed that these individuals have few comorbidities and that the cardiac disorder is the only relevant condition leading to the SCA event. This may not accurately reflect the spectrum of etiologies of SCA in this younger population. A more detailed examination of causes, circumstances, and potential triggers that contribute to the presentation of SCA in the younger population may address this confusion and help guide future studies in the prediction and prevention of SCA.
Accurate assessment of these aspects requires a comprehensive evaluation of all SCAs within a geographic area. To date only 2 other studies have systematically tracked and described all young people with SCAs within defined geographic areas using multisource records.12, 13 Both Meyer13 and Jayaraman12 used similar adjudication processes to classify and evaluate cohorts of young people (age <35) with SCAs from large US cities (populations ≈1–1.2 million), reporting on the incidence, etiologies, circumstances, and outcomes. Our study adds to these findings by comprehensively evaluating all out‐of‐hospital cardiac arrest (OHCA) events that had a 911 response in a larger urban area within the Greater Toronto Area of Ontario, Canada (population 6.6 million).
A population‐based database interface of all OHCAs within the Greater Toronto Area (Rescu Epistry) has been successfully linked to detailed coroner and in‐hospital records.14 Previously, we found that the majority of OHCAs labeled as “presumed cardiac” in younger people were not primarily cardiac but occurred because of drug overdoses, acute noncardiac illnesses, or terminal diseases.14 The current study expands the cohort of interest to include all cases of OHCA classified as “presumed cardiac” and, in addition, all motor vehicle accidents and drownings with documented cardiac arrest to align with prior literature.15 Our objective was to systematically examine each of these SCA cases and describe the etiologies, circumstances, triggers, and characteristics in this expanded cohort.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
The methods used for this study have been described previously in detail.14 In brief, this was an observational cohort study using data collected with a waiver of consent from the Rescu Epistry interface; Rescu Epistry is composed of data points from the Resuscitation Outcomes Consortium Epistry cardiac arrest database and the Strategies for Post Arrest Resuscitation Care database; the methodologies of which are described elsewhere.16, 17 The study was approved by the St. Michael's Hospital Research Ethics Board (REB #10‐105) and data were collected under a waiver of consent.
Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to Katherine Allan at St. Michael's Hospital. Contact information is listed under address for correspondence.
Study Setting and Population
Rescu Epistry is a single web‐based data management interface that links electronic data from Emergency Medical Services (EMS) and Fire Services; device data from monitors, defibrillators, and laptops; and clinical data from hospitals. The in‐hospital data are entered into Rescu Epistry manually by trained data guardians after chart review with several built‐in automated features to minimize errors. It captures all patients with OHCAs or trauma for which there was a 911 response occurring in the City of Toronto and adjacent regions (Halton, Peel, Simcoe, Muskoka, York, and Durham) serving a population of 6.6 million people. This group of end users is collectively referred to as Toronto Regional RescuNET.
From the Rescu Epistry interface we identified OHCAs and traumas in people of all ages from January 1, 2009, to December 31, 2012. From these, we identified OHCAs in people aged 2 to 45 years that were designated by trained data abstractors as presumed cardiac cause (ie, assumed to be attributable to an underlying cardiac disease) per the standardized Utstein criteria.18 We also identified OHCAs in people aged 2 to 45 years that were designated as obvious, noncardiac causes, including those categorized as motor vehicle collisions or drownings, both with documented cardiac arrest, which could have been potential SCAs.
In our database search strategy, we included all motor vehicle collisions with documented cardiac arrest in either the driver or passenger. All other cases attributed to trauma including burns were excluded.
The lower age cutoff was chosen to exclude sudden infant death syndrome cases, as these are considered to be a different entity from SCA.19 Age 45 was chosen as the upper age cutoff to maximize capture rates for heritable cardiac syndromes and prevent overlap with coronary artery disease.1
Data Collection
We used the following sources of information to adjudicate the cause of death and to collect past medical history, circumstances, and triggers on patients with SCA: (1) ambulance call and fire reports; (2) in‐hospital data; (3) medical certificates of death; (4) coroner investigative statements; (5) police reports; and (6) autopsies, toxicology, and molecular autopsy reports.
The coroner investigative statement contains the deceased's personal information, medical history obtained from medical records, and a narrative summary including the circumstances, manner of death, and medical cause of death with contributory factors.
Autopsies are conducted by a forensic pathologist and follow a standardized protocol in which organs are examined both macroscopically and microscopically. When the death is suspected to be attributable to an underlying cardiac cause, the heart is sent to a specialized cardiovascular pathologist for further examination.
Standard protocols for toxicology testing were followed, including a general drug screen for ethanol and drugs of abuse and a comprehensive drug screen for 300 prescription and over‐the‐counter drugs. Testing is performed sequentially, such that if a drug is detected at a level considered to be either toxicologically significant or fatal, further testing is not performed. Toxicology was performed only as part of the autopsy, and we did not have toxicology results for survivors or those who died in‐hospital without autopsy. Cases with toxicologic findings were reviewed by a toxicologist, coroner, and cardiac pathologist. Any SCAs for which the etiology was considered to be drug overdose on the basis of the drug concentrations and the clinical and autopsy findings were excluded from the study. In patients categorized as having SCA, none of the detected drug levels, including the few SCAs with “toxic” levels of drugs were deemed by toxicologists and cardiac pathologists to be solely causative of the SCA event.
Toxicology reports classify “toxic” drug levels on the basis of ranges reported in the literature; however, the actual effect on an individual is highly dependent on several factors including prior tolerance, type of drugs, dosage, and the like, all of which are taken into account by toxicologists and coroners when assigning cause of death. In such cases, the drug may have been considered contributory but not causative.
Review and Adjudication Process
Initially, all deceased people with OHCAs were matched to a coroner investigative statement and autopsy/toxicology report where available; all resuscitated people with OHCAs were matched to their in‐hospital reports, where available. If there was a clear medical cause of death ascribed by the coroner or if there was a cause of death that was obviously noncardiac (ie, electrocution), the adjudication process was considered complete. OHCAs that were attributed to drug overdose; suicide; homicide; drowning; motor vehicle collisions; blunt, penetrating, or burn injury trauma; cancer; complex chronic care; acute noncardiac illnesses; and vascular noncardiac causes were excluded (Figure 1). OHCAs that remained unmatched across source documents or that had unclear etiology (either cardiac or noncardiac) were also excluded.
Figure 1.
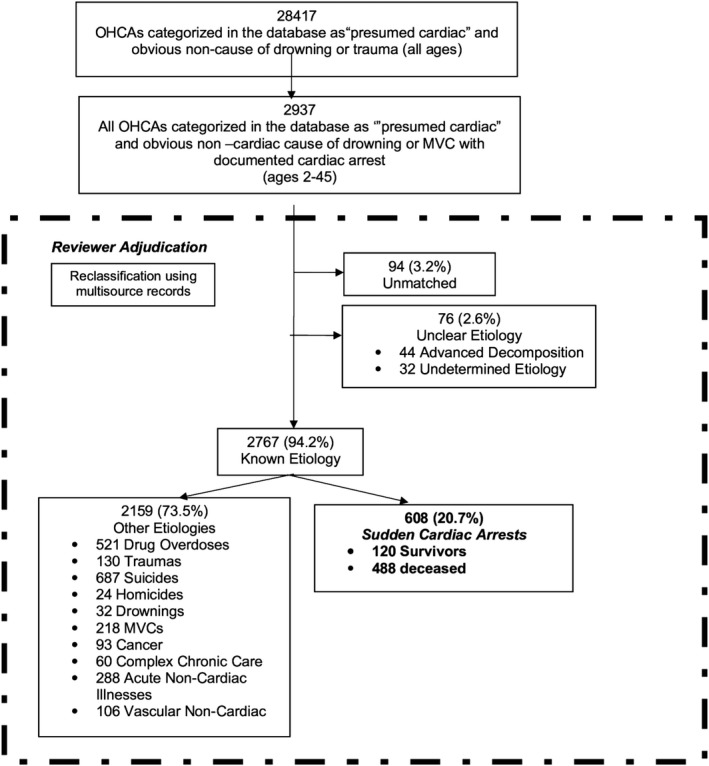
Review and classification of all sudden cardiac arrests from 2009 to 2012. Unmatched were cases that had no corresponding emergency medical services, in‐hospital record, or death certificate. Unclear etiologies included cases with advanced decomposition and cases with limited clinical information regarding diagnosis or contributing noncardiac factors. MVC indicates motor vehicle collision.
In OHCAs for which the cause of death was unclear; or OHCAs with contributing factors, such as positive toxicology, and OHCAs with only EMS and in‐hospital data (eg, survivors and people who died in‐hospital without autopsy), a joint deliberation process ensued between the primary author and 2 additional experts (P.D., A.P.), using all available sources of information to assign cause of death, cardiac or noncardiac. OHCAs with contributing toxicology results were further reviewed with a coroner, cardiac pathologist, and toxicologist. For all cases, a decision was reached by consensus. The macroscopic and microscopic criteria used for different cardiac and noncardiac etiologies have been published previously.14
Key Definitions
Presumed cardiac: As per the standardized Utstein criteria for reporting cardiac arrest data,18 an OHCA etiology is classified as “presumed cardiac” when “there is evidence of a primary cardiac etiology or when an obvious non‐cardiac cause for the OHCA has been ruled out. Obvious non‐cardiac causes include: drowning, electrocution, trauma, anaphylaxis, drug overdose, other medical cause or asphyxia.” Paramedics and trained data abstractors who assign this definition can also list contributing causes for an arrest, which are thought to contribute to the event but are not the direct cause (eg, patient was a known drug user and suspect drug poisoning but no direct evidence currently available). At the time of etiology assignment, paramedics have access to EMS data, and trained data abstractors have access to EMS and in‐hospital data. They do not have access to autopsy or toxicology findings.
Sudden cardiac arrest was defined as an abrupt collapse with documented loss of vital signs and attended by EMS in response to a 911 call. The etiology was considered to be cardiac only after the exclusion of SCAs attributable to vascular noncardiac disease, acute noncardiac illnesses, drug overdose, metabolic causes, or terminal disease. Cases could be witnessed or unwitnessed, with no time limit restrictions from the onset of the event to when the individual was found. SCA included both patients who did not survive (previously defined as SCD) and those successfully resuscitated.
Sudden unexplained deaths (SUDs) were those unexplained by preexisting disease after clinical investigation or without identifiable anatomic or toxicologic cause on autopsy.
Physical activity level at the time of the event was defined as any form of physical activity that the individual was performing immediately before or within 1 hour of experiencing SCA. An estimated metabolic equivalent score was assigned to each type of physical activity based on the criteria described by Ainsworth et al.20 Physical activity was classified into 4 groups: Rest (metabolic equivalent, 0.9–1.3), light activity (metabolic equivalent, 1.4–5.9), moderate activity (metabolic equivalent score ≥6), or unknown.
Cardiac disease was based on documented evidence of a previous diagnosis of a cardiac disease or having been prescribed a cardiac medication, after chart review of multisource records including EMS/fire reports, in‐hospital data, and autopsy reports when available.
Cardiovascular disease risk factors included diabetes mellitus, hyperlipidemia, hypertension (HTN), smoking, and obesity. Diabetes mellitus and hyperlipidemia were defined as such after observing a documented chart history or if the individual was prescribed a medication to treat those conditions. HTN and smoking were defined by a chart history, and obesity was defined as a body mass index ≥30 kg/m2.
Psychiatric disease: A patient with psychiatric disease was defined as such if (1) there was a documented previous diagnosis of psychiatric disease or (2) there was documented evidence that the patient was prescribed an antidepressant or antipsychotic medication. These details were obtained after chart review of multisource records, as described above.
Statistical Analysis
Descriptive statistics were used to assess the distribution of all variables. Continuous variables were summarized as means and standard deviations or medians and interquartiles, while categorical variables were summarized as counts and percentages. Cases were compared on the basis of 3 age groups: 2 to 24, 25 to 34, and 35 to 45, which is reflective of the age cutoffs used in similar studies.7, 8 Selected categorized data were compared using the Pearson χ2 or Fisher's exact test. When continuous data satisfied criteria for normality, we compared groups using the Student t test; when they did not satisfy these criteria, we used the Mann–Whitney test. All calculations and data analyses were performed with SPSS software (IBM Corp., IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY).
Results
From the Rescu Epistry interface we identified 28 417 OHCAs and traumas in people of all ages from January 1, 2009, to December 31, 2012. Of these, 2937 of 28 417 (10.3%) were between the ages of 2 and 45 (Figure 1). From the 2937 OHCAs, 1892 of 2937 (64.4%) were designated by trained data guardians as “presumed cardiac cause” as per the standardized Utstein criteria.18 There were 1045 of 2937 (35.6%) designated as obvious noncardiac OHCAs, and of these, 250 of 1045 (23.9%) were categorized as motor vehicle collisions or drowning etiologies that could have been potential SCAs. This combined cohort of all OHCAs (n=2937) classified as motor vehicle collisions, drownings, or presumed cardiac formed the source data set for this study.
Review and Adjudication Process
EMS/fire reports were available in 2937 of 2937 (100%) OHCAs. In the deceased individuals with OHCAs (n=2763), coroner investigative statements were available in 2403 of 2763 (87.0%), and autopsy reports were available in 2231 of 2763 (80.7%). Of the OHCAs transported to a hospital (n=956), in‐hospital reports were available in 100%.
A clear cause of death, cardiac or noncardiac, was confirmed by the primary author in 2255 of 2937 (76.7%) OHCAs using EMS/fire reports; in‐hospital medical records; coroner investigative statements; and autopsy, toxicology, and police reports. The other 682 of 2937 (23.2%) OHCAs were adjudicated jointly by the primary author and 2 cardiologists using the same information sources as outlined above. Of the 682 adjudicated cases, 79 of 682 (11.6%) cases were jointly reviewed with a coroner, cardiac pathologist, and toxicologist. After this review process, 130 of 2937 (4.4%) OHCAs were attributed to blunt, penetrating, or burn injury traumas; 687 of 2937 (23.4%) as suicide; 218 of 2937 (7.4%) as motor vehicle collisions; 24 of 2937 (0.82%) as homicide; 521 of 2937 (17.7%) as drug overdose; 32 of 2937 (1.1%) as drownings; 288 of 2937 (9.8%) as acute noncardiac illness (eg, terminal illness); and 106 of 2937 (3.6%) as vascular noncardiac causes. There were 94 of 2937 (3.2%) OHCAs unmatched to discharge summaries/death certificates and 76 of 2937 (2.6%) cases with unclear etiologies (either cardiac or noncardiac). Of the unclear etiology cases, 44 (57.9%) had advanced decomposition, 10 (13.2%) were survivors with sparse clinical information, 9 (11.8%) died in‐hospital without autopsy or coroner investigation, 6 (7.9%) were coroner cases without autopsy and spare clinical information, 5 (6.6%) had significant contributory toxicology, and 2 (2.6%) had mixed cardiac and noncardiac findings.
This left a total of 608 of 2937 (20.7%) adjudicated OHCAs of confirmed cardiac cause comprising the SCA cohort of interest. In the SCA cohort, 120 of 608 (19.7%) survived and 488 of 608 (80.3%) died. Within the deceased cohort, 448 of 488 (91.8%) were coroner cases and 399 of 488 (81.8%) had autopsy‐explained cardiac etiology; the other 18.2% (89 of 488) did not have autopsy reports and were adjudicated as cardiac etiology using EMS reports, including initial rhythm, discharge summaries, and all other available information.
Patient Characteristics
Three quarters of the SCA cohort (N=608) were male, with an average age of 35.4±8.9 (Table 1). Past medical history and prescribed medications were available in 582 of 608 (95.7%) and 504 of 608 (82.9%), respectively, of all SCAs. Prior to their SCA event, 395 of 582 (67.9%) of the entire cohort had a history of diagnosed cardiac disease or were prescribed a cardiac medication, and 320 of 582 (55%) were diagnosed with ≥1 cardiovascular disease risk factors. Just over 20% (117 of 582) were diagnosed with psychiatric disease. At the time of their SCA event, over 60% (61.7%; 311 of 504) had been prescribed ≥1 cardiac or central nervous system active medication, including cardiac (65.3%; 329 of 504), diabetic (10.3%; 52 of 504) and psychiatric (19.0%; 96 of 504).
Table 1.
Past Medical History of Sudden Cardiac Arrests
| Variablea | Age, y 2–24 (n=82) | Age, y 25–34 (n=131) | Age, y 35–45 (n=395) | P Value |
|---|---|---|---|---|
| Age±SD | 17.6±5.7 | 30.1±3.0 | 40.8±2.8 | NA |
| Male sex | 53 (64.6) | 100 (76.3) | 311 (78.7) | 0.02 |
| Hypertension | 2 (2.5) | 15 (12.2) | 123 (32.4) | <0.0001 |
| Lipid disorder | 0 | 5 (4.1) | 51 (13.4) | <0.0001 |
| Diabetes mellitus | 3 (3.8) | 10 (8.1) | 65 (17.1) | 0.001 |
| Obesity | 4 (5.1) | 31 (25.2) | 71 (18.7) | 0.001 |
| Smoking | 5 (6.3) | 22 (17.9) | 119 (31.3) | <0.0001 |
| ≥1 Cardiovascular disease risk factorsb | 10 (12.7) | 61 (49.6) | 249 (65.5) | <0.0001 |
| ≥2 Cardiovascular disease risk factorsb | 3 (3.8) | 16 (13.0) | 114 (30.0) | <0.0001 |
| History of cardiovascular diseasec | 28 (35.4) | 83 (67.5) | 284 (74.7) | <0.0001 |
| Prior myocardial infarction | 0 | 6 (4.9) | 38 (10.0) | 0.004 |
| Arrhythmia | 5 (6.3) | 10 (8.1) | 26 (6.9) | NS |
| ICD/pacemaker | 2 (2.5) | 6 (4.9) | 7 (1.8) | NS |
| Seizure disorder | 9 (11.4) | 8 (6.5) | 18 (4.7) | NS |
| Mood disorderd | 1 (1.3) | 20 (16.3) | 60 (15.8) | 0.02 |
| Psychosis | 1 (1.3) | 10 (8.1) | 19 (5.0) | NS |
| Other psychiatric historye | 3 (3.8) | 6 (4.9) | 21 (5.5) | NS |
| Any psychiatric historyf | 6 (7.6) | 32 (26.0) | 79 (20.8) | 0.005 |
| Illicit drug use | 11 (13.9) | 25 (20.3) | 62 (16.3) | NS |
| Alcohol abuse | 1 (1.3) | 15 (12.2) | 57 (15.0) | 0.004 |
ICD indicates implantable cardioverter defibrillator; NA, not applicable; NS, nonsignificant; SD, standard deviation.
Data were missing in 4.3% of cases.
Includes 1 of hypertension, diabetes mellitus, lipids, obesity, or smoking.
Defined as any previously diagnosed cardiac disease or prescribed cardiac medication.
Includes 1 of depression, anxiety, bipolar, schizoaffective disorder, or manic depressive.
Includes 1 of obsessive‐compulsive disorder, borderline personality disorder, or suicide attempt.
Includes any diagnosed psychiatric disease or prescribed antidepressant/antipsychotic.
Increasing age (age >25) was associated with higher proportions of cardiovascular disease risk factors than younger (age <25), with the exception of obesity (5.1% ages 2–24 versus 25.2% ages 25–34 versus 18.7% ages 35–45). Proportions of psychiatric disease were similar between the older age groups (7.6% ages 2–24 versus 26.0% ages 25–34 versus 20.8% ages 35–45). Sex‐based comparisons can be seen in Table 2.
Table 2.
Past Medical History of Sudden Cardiac Arrests by Sex
| Variable, N (%)a | Males (n=464) | Females (n=144) | P Value |
|---|---|---|---|
| Age±SD | 35.6±8.5 | 34.4±9.9 | NS |
| Hypertension | 96 (21.6) | 44 (32.1) | 0.01 |
| Lipid disorder | 50 (11.2) | 6 (4.4) | 0.02 |
| Diabetes mellitus | 53 (11.9) | 25 (18.2) | 0.06 |
| Obesity | 76 (17.1) | 30 (21.9) | NS |
| Smoking | 114 (25.6) | 32 (23.4) | NS |
| ≥1 Cardiovascular disease risk factorsb | 242 (54.4) | 78 (56.9) | NS |
| ≥2 Cardiovascular disease risk factorsb | 94 (21.1) | 39 (28.5) | 0.07 |
| History of cardiovascular diseasec | 303 (68.1) | 92 (67.2) | NS |
| Prior myocardial infarction | 34 (7.6) | 10 (7.3) | NS |
| Arrhythmia | 33 (7.4) | 8 (5.9) | NS |
| ICD/pacemaker | 10 (2.2) | 5 (3.6) | NS |
| Seizure disorder | 31 (7.0) | 4 (2.9) | NS |
| Mood disorderd | 51 (11.5) | 30 (21.9) | 0.002 |
| Psychosis | 24 (5.4) | 6 (4.4) | NS |
| Other psychiatric historye | 20 (4.5) | 10 (7.3) | NS |
| Illicit drug use | 80 (18) | 18 (13.1) | NS |
| Any psychiatric historyf | 80 (18.0) | 37 (27.0) | 0.02 |
| Alcohol abuse | 65 (14.6) | 8 (5.8) | 0.007 |
ICD indicates implantable cardioverter defibrillator; NS, nonsignificant; SD, standard deviation.
Data were missing in 4.3% of cases.
Includes 1 of hypertension, diabetes mellitus, lipids, obesity, or smoking.
Defined as any previously diagnosed cardiac disease or prescribed cardiac medication.
Includes 1 of depression, anxiety, bipolar, schizoaffective disorder, or manic depressive.
Includes 1 of obsessive‐compulsive disorder, borderline personality disorder, or suicide attempt.
Includes any diagnosed psychiatric disease or prescribed antidepressant/antipsychotic.
Detailed Causes of SCA Events
In the entire SCA cohort, (243 of 608) (40%) of cases were attributed to coronary heart disease (CHD), 174 of 608 (28.6%) attributed to structural diseases of the myocardium, 98 of 608 (16.1%) attributed to SUD, and 15 of 608 (2.5%) attributed to other cardiac causes (anomalous coronary arteries, congenital heart disease, and tamponade). In 78 of 608 (12.8%) of cases, the exact subtype of cardiac disease (either structural or arrhythmic but not CHD) remained unspecified after adjudication. Of the 78 unspecified cases, 31 (39.7%) were coroner cases without autopsy and sparse clinical information, 32 (41.0%) were survivors with sparse clinical information, 2 (2.6%) had competing cardiac etiologies, and 13 (16.7) had competing noncardiac etiologies including SUD in epilepsy (n=2) and central nervous system active drugs (n=11).
The most common etiologies among patients with SCA aged <35 and ≥35 years, respectively, were CHD (16.4% versus 52.7%; P<0.0001) and structural diseases of the myocardium (32.9% versus 26.3%; P=0.09), followed by SUDs (31.5% versus 7.8%; P<0.0001) (Figure 2). In 14.1% of patients aged <35 years and 12.2% of patients aged ≥35 years, the exact subtype of cardiac disease (either structural or arrhythmic but not CHD) remained unspecified.
Figure 2.
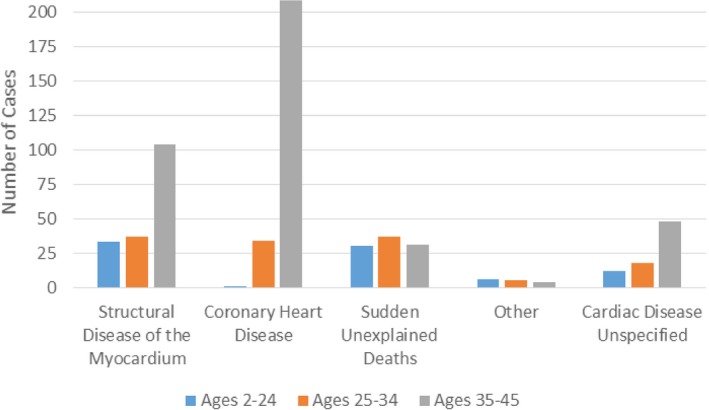
Age distribution of cardiac etiologies in sudden cardiac arrests. Other includes congenital heart disease, anomalous coronary arteries, and tamponade. Cardiac disease unspecified includes cases in whom the etiology was considered to be cardiac, either structural or arrhythmic, but not coronary heart disease.
In the group aged <25 years, the most frequent causes of death were SUDs (36.6%) and structural diseases of the myocardium (40.2%) (Figure 2).
Males had a higher proportion of CHD in comparison to females (43.5% versus 28.5%; P=0.001), while proportions of structural diseases of the myocardium were higher in females versus males (26.7% males versus 34.7% females; P=0.06). Females had significantly higher proportions of SUDs compared with males (21.5% versus 14.4%; P=0.04) (Table 3).
Table 3.
Sudden Cardiac Arrest Etiologies by Sex
| Etiology Type, N (%) | Males (n=464) | Females (n=144) | P Value |
|---|---|---|---|
| Structural diseases of the myocardium | 124 (26.7) | 50 (34.7) | 0.06 |
| Coronary heart disease | 202 (43.5) | 41 (28.5) | 0.001 |
| Sudden unexplained deaths | 67 (14.4) | 31 (21.5) | 0.04 |
| Othera | 12 (2.6) | 3 (2.1) | NS |
| Cardiac disease unspecifiedb | 59 (12.7) | 19 (13.2) | NS |
NS indicates nonsignificant.
Other includes congenital heart disease, anomalous coronary arteries, and tamponade.
Cardiac disease unspecified includes patients in whom the etiology was considered to be cardiac, either structural or arrhythmic, but not coronary heart disease.
The distribution of specific structural diseases of the myocardium for all SCAs stratified by age can be seen in Figure 3. Younger age was associated with higher prevalence of arrhythmogenic right ventricular cardiomyopathy (ARVC; 7.3% ages 2–24 versus 1.5% ages 25–34 versus 1.3% ages 35–45) and HCM (6.1% ages 2–24 versus 0% ages 25–34 versus 0.8% ages 35–45). Sex‐based comparisons can be found in Table 4.
Figure 3.
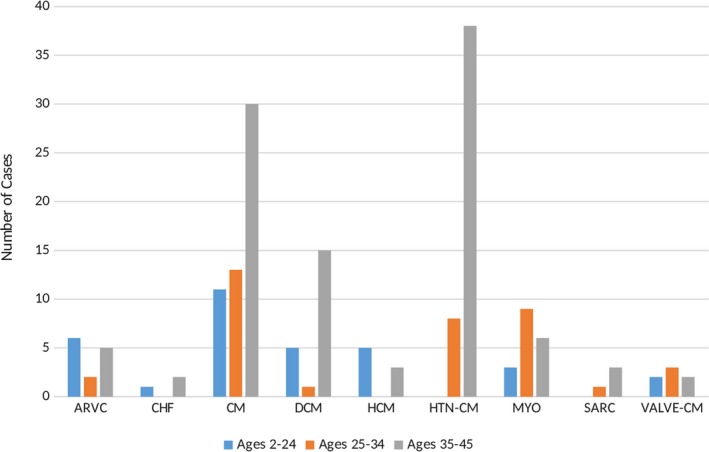
Age distribution of detailed structural causes of sudden cardiac arrests. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; CHF, congestive heart disease; CM, cardiomyopathy; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; HTN CM, hypertensive cardiomyopathy; MYO, myocarditis; SARC, sarcoidosis; VALVE‐CM, valvular cardiomyopathy.
Table 4.
Types of Structural Diseases of the Myocardium by Sex
| Type of Structural HD, N (%) | Males (n=124) | Females (n=50) | P Value |
|---|---|---|---|
| Arrhythmogenic right ventricular cardiomyopathy | 10 (8.1) | 3 (6.0) | NS |
| Congestive HD | 1 (0.8) | 2 (4.0) | NS |
| Cardiomyopathy nonspecifica | 40 (32.3) | 14 (28.0) | NS |
| Dilated cardiomyopathy | 15 (12.1) | 6 (12.0) | NS |
| Hypertrophic cardiomyopathy | 6 (4.8) | 2 (4.0) | NS |
| Hypertensive cardiomyopathy | 31 (25.0) | 15 (30.0) | NS |
| Myocarditis | 13 (10.5) | 5 (10.0) | NS |
| Sarcoidosis | 3 (2.4) | 1 (2.0) | NS |
| Valvular cardiomyopathy | 5 (4.0) | 2 (4.0) | NS |
HD indicates heart disease; NS, nonsignificant.
Includes fibrotic cardiomyopathies, endocarditis, and cardiomyopathies attributable to Duchenne muscular dystrophy.
There were 140 SCAs (23.0%) attributable to potentially heritable cardiac diseases: 13 (2.1%) with ARVC, 21 (3.5%) with dilated cardiomyopathy, 8 (1.3%) with hypertrophic cardiomyopathy (HCM) and 98 (16.1%) with SUD. In contrast, acquired cardiac diseases such as CHD, sarcoidosis, myocarditis, congestive heart failure and cardiomyopathies due to HTN or valvular disease, constituted 52.8% (321 of 608) of the total SCA population.
Toxicology Results
Toxicology testing was performed in almost 60% (233 of 399) of autopsied SCAs, and 31.1% (124 of 399) had ≥1 drugs detected at any level (Table 5). The most frequently detected central nervous system active drugs were ethanol (n=50), opioids (n=26), selective serotonin reuptake inhibitors (n=18) and cocaine/benzyolecgonine (n=16), benzodiazapines (n=16), and dopamine antagonists (n=13), with most drugs detected at or below therapeutic levels (Table 6).
Table 5.
Toxicology of Autopsied SCAs
| Variable | Autopsied SCAs (n=399) |
|---|---|
| Toxicology results | N (%) |
| Positivea | 124 (31.1) |
| Negativeb | 109 (27.3) |
| Not performed | 166 (41.6) |
| Positive toxicology in autopsied cases | N=124 |
| Nontoxic levelc | 82 (66.1) |
| Significant leveld | 26 (21.0) |
| Toxic levele | 16 (12.9) |
SCAs indicates sudden cardiac arrests.
Positive toxicology screen means ≥1 drugs were detected.
Negative toxicology screen means no drugs were detected.
Indicates a drug detected at a low level such as therapeutic (according to toxicology drug standards) and would be considered noncontributory to cause of death.
Indicates a drug detected at a significant level (according to toxicology drug standards) and would be considered to have contributed to the cause of death.
Indicates a drug detected at a toxic level (according to toxicology drug standards) and would be considered to have contributed to the cause of death.
Table 6.
CNS Active Medications in Tox‐Positive SCAs (n=124)
| Type of Medication or Drug | Detected, N (%) | Therapeutic Levels, N (%) | Significant Levels, N (%) | Toxic Levels, N (%) |
|---|---|---|---|---|
| Illicit drugs | ||||
| Cocaine/benzyolecgonine (n=16) | 5 (31.3) | 1 (6.3) | 3 (18.7) | 7 (43.7) |
| Opioids (n=26) | 2 (7.7) | 10 (38.5) | 4 (15.3) | 10 (38.5) |
| Ethanol (n=50) | 3 (6.0) | 31 (62.0) | 13 (26.0) | 3 (6.0) |
| Psychotropic medications | ||||
| Selective serotonin reuptake inhibitors (n=18) | 10 (55.5) | 6 (33.3) | 1 (5.6) | 1 (5.6) |
| Serotonin and norepinephrine reuptake inhibitors (n=2) | 2 (100) | 0 (0) | 0 (0) | 0 (0) |
| Tricyclic antidepressants (n=8) | 0 (0) | 7 (87.5) | 0 (0) | 1 (12.5) |
| Benzodiazapines (n=16) | 11 (68.7) | 4 (25.0) | 1 (6.3) | 0 (0) |
| Dopamine antagonists (n=13) | 5 (38.5) | 6 (46.1) | 2 (15.4) | 0 (0) |
| Anticholinergics (n=6) | 1 (16.7) | 5 (83.3) | 0 (0) | 0 (0) |
| Other (n=8)a | 2 (25.0) | 3 (37.5) | 3 (37.5) | 0 (0) |
CNS indicates central nervous system; SCAs, sudden cardiac arrests.
Consists of domperidone, anticonvulsants, nonbenzodiazepine hypnotics, amphetamines such as dextroamphetamine, cyclobenzaprine, amantadine, and pregabalin.
In the SUD group (n=98), (31 of 98) (31.6%) had a positive toxicology, with alcohol and opioids (n=12) detected most frequently, followed by antidepressants and antipsychotics (n=11) and over‐the‐counter prescription drugs (n=8).
Despite some SCAs having toxic levels of drugs detected postmortem, the toxicologists and cardiac pathologists agreed that the cause of death in these individuals was primarily attributable to a cardiac etiology, and the significant or toxic drug levels were considered contributory but not causative.
Circumstances and Triggers
Most SCA events occurred at home and during rest (Table 7). Patients aged <35 with SCA of cardiac cause more often had their SCA event occur during or after exercise (31.5% versus 23.7%; P=0.04). Sex comparisons can be seen in Table 8.
Table 7.
Circumstances and Activity Levels at the Time of Sudden Cardiac Arrest
| Variable, N (%) | Age, y 2–24 (n=82) | Age, y 25–34 (n=131) | Age, y 35–45 (n=395) | P Value |
|---|---|---|---|---|
| Environment | ||||
| Private residence | 51 (62.2) | 97 (74.0) | 289 (73.2) | NS |
| Public location | 31 (37.8) | 34 (25.9) | 106 (26.8) | NS |
| Public nonrecreationala | 25 (30.5) | 25 (19.1) | 89 (22.5) | NS |
| Public recreationalb | 6 (7.3) | 9 (6.9) | 17 (4.3) | NS |
| Circumstancesc | ||||
| In bed | 22 (26.8) | 36 (27.5) | 111 (28.2) | NS |
| Rest | 49 (59.8) | 96 (73.3) | 300 (75.9) | 0.08 |
| During exercise | 25 (30.5) | 25 (19.1) | 56 (14.2) | 0.002 |
| 1–60 min after exercise | 8 (9.8) | 9 (6.9) | 37 (9.4) | NS |
| Exercise level (n=160) | ||||
| Light | 10 (30.3) | 13 (38.2) | 55 (59.1) | 0.007 |
| Vigorous | 23 (69.7) | 21 (61.8) | 38 (40.9) | 0.007 |
NS indicates nonsignificant.
Defined as 1 of office, roadway, restaurant, mall, etc.
Defined as a recreational facility such as a gymnasium, athletic center, pool, park, or community center.
Data were unknown in 0.3% of cases.
Table 8.
Sex‐Based Comparisons of Circumstances and Activity Level
| Variable | Males (n=464) | Females (n=144) | P Value |
|---|---|---|---|
| N (%) | N (%) | ||
| Environment | |||
| Private residence | 330 (71.1) | 107 (74.3) | NS |
| Public location | 134 (28.9) | 37 (25.7) | NS |
| Public nonrecreationala | 105 (22.6) | 34 (23.6) | NS |
| Public recreationalb | 29 (6.3) | 3 (2.1) | NS |
| Circumstancesc | |||
| Rest | 325 (69.8) | 121 (84.0) | 0.001 |
| In bed | 124 (26.8) | 45 (31.3) | NS |
| During exercise | 90 (19.4) | 16 (11.1) | 0.02 |
| 1–60 min after exercise | 47 (10.1) | 7 (4.9) | 0.05 |
| Exercise level (n=160) | |||
| Light | 64 (46.7) | 14 (60.9) | NS |
| Vigorous | 73 (53.3) | 9 (39.1) | NS |
NS indicates nonsignificant.
Defined as 1 of office, roadway, school, mall, etc.
Defined as a recreational facility such as a gym, athletic center, park, or community center.
Data were unknown in 0.3% of cases.
Discussion
This study is one of a few to describe in detail the distribution of adjudicated underlying cardiac causes, circumstances, triggers, and characteristics of a large, population‐based cohort of younger patients with SCA, where all EMS‐attended cardiac arrests in a large urban area were evaluated. The main finding is that most SCA events were not completely “unexpected” in that they occurred in people who were previously diagnosed with conditions known to be associated with sudden death, such as cardiac and psychiatric disease. In contrast to other studies, potentially heritable structural cardiac diseases such as HCM, ARVC, and dilated cardiomyopathy constituted <10% of the combined total cohort, while rates of SCAs attributable to SUDs were comparable to other recent studies in this area.7, 9, 12
Our data suggest that, in younger people, SCA events do not mainly occur in “healthy individuals,” as has been reported by other studies in this area.4, 9, 21 We observed that 67.9% of SCA events in people aged <45 years occurred in patients with a past cardiac medical history and in 20.1% of those with a past psychiatric history. Comparisons with other SCD studies in younger people are difficult, as few report this level of detail.4, 9, 21 Two large population‐based SCD autopsy studies from Denmark reported that up to 24% of people aged <35 years with SCD had a reported previous medical history,4 while in a larger, older cohort (age <50 years), 58.2% had a past medical history.9 The overall rate of HTN (24%) among our SCA cohort was comparable to that reported for the general Canadian population22; however, compared with age‐matched cohorts, the rates of HTN and smoking were substantially higher among older (age >35 years) SCA cases (HTN: 32.4% ours versus 10.3% reported) and smoking (31.3% ours versus 18.9% reported).23, 24 Moreover, the rate of diabetes mellitus (13.4%) in our study was twice as high as what has been reported in the general Canadian population.25 Similar findings of higher rates of diabetes mellitus, HTN, and obesity among patients with SCA aged 5 to 34 years were observed by the Oregon SUDS (Sudden Unexpected Death Study) group, with 58% of patients having ≥1 cardiovascular disease risk factors, suggesting that these risk factors may play a larger role in SCA among younger adults than was previously thought.12
Our study is the only North American study to report on the prevalence of a psychiatric history in younger patients with SCA within a population‐based cohort.26, 27 A Danish study reported that 20% of young people (age <50 years) with SCDs and 14% of sudden noncardiac deaths had a past medical history of psychiatric disease.26 We observed that a history of depression and psychosis were more frequent than expected among SCAs compared with the general Canadian population.28 In 2012, the Canadian Community Health Survey on Mental Health reported that 5.4% of the Canadian population aged >15 years reported symptoms that met the criteria for a mood disorder in the past year, including 4.7% for major depression.28 In our study, the rate of mood disorders was 13.9% overall and was significantly higher among females compared with males. The reported rate of psychosis is also significantly higher than what has been reported in the general Canadian population (≈1%).29 Depression and other major psychiatric disorders such as schizophrenia have been independently linked to increased rates of SCD.30, 31 Risgaard et al reported nationwide incidence rates of SCD among younger individuals with and without prior psychiatric disease and found that the SCD incidence rate in psychiatric patients was almost 4 times that of individuals without psychiatric disease.27
Moreover, in our study, one third of SCA patients were prescribed ≥1 psychotropic medications (defined here as antidepressants, antipsychotics, opioids, benzodiazapines, and non‐benzodiazapine hypnotics), which could have contributed to their event. In addition, the main central nervous system active drugs detected at autopsy in our cohort following toxicology testing were drugs of abuse, including opioids, ethanol, and cocaine, while the 2 main psychotropic medications detected were antipsychotics and antidepressants. The potential proarrhythmic properties of psychotropic drugs such as antipsychotics32 or opioids33 could have induced a fatal arrhythmia in these patients through prolongation of the QT interval. A study by the Oregon SUDS group observed that among SCA cases in people aged ≥18 years from the general population, antipsychotic drugs were significant and independent determinants of pulseless electrical activity.34
It is widely believed that potentially heritable structural cardiac diseases such as HCM, ARVC, and dilated cardiomyopathy are the most frequent causes of SCD among those aged <35 years,11 while in adults aged >35 years, CHD accounts for up to 70% to 75% of all SCD events.35 In our study, we observed similar rates of CHD and SUDs compared with other North American studies among younger people,7, 12, 13 including a recent study from the Oregon SUDS group.12 They observed that SUDs (31%), CHD (22%), and HCM (14%) were the most frequent SCA etiologies in those aged 5 to 34 years. However, our rates of potentially inheritable cardiomyopathies such as HCM, ARVC, and dilated cardiomyopathy (42 of 608; 6.9% combined) were substantially lower.
The most likely explanations for these discrepancies are the differing case ascertainment strategies and patient inclusion criteria used between studies, as was highlighted in a recent meta‐analysis on HCM as a cause of SCD among younger people.36 In contrast to Jayaraman et al, who used traditional, time‐based SCD definitions to identify their cases, we used the underlying etiology with no time‐based restrictions to classify SCAs, thus increasing the overall denominator. Furthermore, although there are recommended guidelines for performing autopsies, few jurisdictions have adopted these standardized approaches.36 Within our catchment area, forensic autopsies are performed using a standardized protocol, and any SCD cases with a diagnosis of a heritable cardiac disease, including HCM, are reviewed by a specialized cardiac pathologist. Finally, although autopsies are recommended for all SCD cases,37 autopsy rates among most SCD studies range between 12% and 75%.9, 38 A strength of our study is that our autopsy rates are among some of the highest reported in the published literature, following the POST SCD (Postmortem Systematic Investigation of Sudden Cardiac Death) group in San Francisco.39
The proportion of SCD individuals diagnosed on autopsy with an “acquired” heart disease such as hypertensive cardiomyopathy (HTN‐CM) in our cohort, is higher than what has been reported elsewhere.4, 9, 40 A Finnish study observed a rate of HTN‐CM at 15.5%, while recent studies in Denmark reported HTN‐CM rates of 5.7% in individuals aged 1 to 35 years and 7.7% in those aged 1 to 49 years.4, 9, 40 A diagnosis of HTN‐CM is based on both macroscopic and microscopic findings in the heart in the absence of any other disease (eg, coronary artery disease or valvular disease), and a history of HTN or evidence of renovascular hypertensive changes at autopsy.9, 41 Alternatively, as this study involves a population‐based sample that is derived from a large and ethnically diverse population in Ontario, population diversity could account for the observed differences in etiologies.42 Interestingly, in individuals aged 2 to 34 years, CHD accounted for 16.4% of all SCA events and over half of SCA events in the 35 to 45 age group. The high prevalence of CHD and HTN‐CM among young patients with SCA highlights the importance of cardiovascular disease risk management in much younger patients.
Limitations
In our jurisdiction, toxicology is performed in 2 situations: (1) when cause of death is not evident from macroscopic or microscopic investigation and/or (2) when there is an indication that drugs may have contributed to the cause of death. If there is a clear cause of death, toxicology testing is usually not performed unless drugs are considered to be contributory. Only 60% of all autopsied cases received toxicology testing. We were not able to report the toxicology levels of the rest of the autopsied cases and the nonautopsied cases, including survivors. Thus, we may have overestimated the number of patients with SCA with contributory levels of medications on board, particularly in survivors.
Ascertainment of past medical history and prescribed medications was not possible for 4.3% and 17.1% of our population, respectively; therefore, there is a potential for bias.
Despite a comprehensive review of each case, there are inherent limitations in current autopsy protocols and coroner diagnoses that can complicate the determination of a specific disorder as the cause for the SCD event. However, a lack of autopsy information was counterbalanced by the high availability of coroner investigative statements, from which we were able to extract important information about the cause and circumstances of death.
Conclusions
Our results highlight that the underlying causes of SCA in younger people are complex, often occurring in people with previously diagnosed cardiovascular diseases and cardiovascular disease risk factors, and are associated with contributory factors including prescribed medications, recreational drugs, and a concomitant psychiatric history.
Appendix
Rescu Investigators
Barto Nascimiento, Damon Scales, Dennis Ko, Jamie Hutchison, Katie Dainty, Laurie Morrison, Paul Dorian, Richard Swartz, Richard Verbeek, Sandro Rizoli, Sheldon Cheskes, Steven Brooks, Steve Lin, and Tim Chan.
Sources of Funding
Dr Allan was funded by a Canadian Institutes of Health Research Doctoral Award Frederick Banting and Charles Best Canada Graduate Scholarship. The ROC (Resuscitation Outcomes Consortium) Registry study is supported by a cooperative agreement (5U01 HL077863) with the National Heart, Lung, and Blood Institute in partnership with the National Institute of Neurological Disorders and Stroke, Canadian Institutes of Health Research–Institute of Circulatory and Respiratory Health, Defense Research and Development Canada, Heart and Stroke Foundation of Canada, and American Heart Association. The Rescu Epistry is funded by a center grant from the Laerdal Foundation, and knowledge translation collaborative grants and operating grants from Canadian Institutes of Health Research and the Heart and Stroke Foundation of Canada.
Disclosures
Dr Tu was supported by a Tier 1 Canada Research Chair in Health Services Research and an Eaton Scholar award. Dr Morrison is supported by an endowed chair; The Robert and Dorothy Pitt Chair in Acute Care and Emergency Medicine Research. Dr Dorian is a Network Investigator of the Cardiac Arrhythmia Network of Canada (CANet) as part of the Networks of Centres of Excellence (NCE) and is supported by a CANet Program grant. Dr Allan is a member of CANet HQP Association for Trainees (CHAT) and receives salary support from a CANet Program grant. The remaining authors have no disclosures to report.
Acknowledgments
The authors thank the Rescu Epistry investigators and all emergency medical service operators, providers, and medical directors as well as the in‐hospital staff in the SPARC network hospitals working together in the front line of emergency patient care for their continued commitment and contributions to high‐quality care and primary data collection in resuscitation research at Rescu, Li Ka Shing Knowledge Institute, St Michael's Hospital, Toronto Ontario, Canada. The authors wish to dedicate this manuscript to the memory of Dr Jack Tu who died before this was published.
(J Am Heart Assoc. 2019;8:e010330 DOI: 10.1161/JAHA.118.010330.)
Contributor Information
Katherine S. Allan, Email: allank@smh.ca.
the Rescu Investigators:
Barto Nascimiento, Damon Scales, Dennis Ko, Jamie Hutchison, Katie Dainty, Richard Swartz, Richard Verbeek, Sandro Rizoli, Sheldon Cheskes, Steven Brooks, Steve Lin, and Tim Chan
References
- 1. Ackerman M, Atkins DL, Triedman JK. Sudden cardiac death in the young. Circulation. 2016;133:1006–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poirier P, Sharma S, Pipe A. The Atlantic Rift: guidelines for athletic screening‐where should Canada stand? Can J Cardiol. 2016;32:400–406. [DOI] [PubMed] [Google Scholar]
- 3. Eckart RE, Shry EA, Burke AP, McNear JA, Appel DA, Castillo‐Rojas LM, Avedissian L, Pearse LA, Potter RN, Tremaine L, Gentlesk PJ, Huffer L, Reich SS, Stevenson WG; Department of Defense Cardiovascular Death Registry Group . Sudden death in young adults: an autopsy‐based series of a population undergoing active surveillance. J Am Coll Cardiol. 2011;58:1254–1261. [DOI] [PubMed] [Google Scholar]
- 4. Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunso S, Tfelt‐Hansen J. Nationwide study of sudden cardiac death in persons aged 1–35 years. Eur Heart J. 2011;32:983–990. [DOI] [PubMed] [Google Scholar]
- 5. Wisten A, Forsberg H, Krantz P, Messner T. Sudden cardiac death in 15–35‐year olds in Sweden during 1992–99. J Intern Med. 2002;252:529–536. [DOI] [PubMed] [Google Scholar]
- 6. Semsarian C, Maron BJ. Sudden cardiac death in the young. Med J Aust. 2002;176:148–149. [DOI] [PubMed] [Google Scholar]
- 7. Bagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam L, Davis AM, Thompson T, Connell V, Wallace J, Naylor C, Crawford J, Love DR, Hallam L, White J, Lawrence C, Lynch M, Morgan N, James P, du Sart D, Puranik R, Langlois N, Vohra J, Winship I, Atherton J, McGaughran J, Skinner JR, Semsarian C. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. [DOI] [PubMed] [Google Scholar]
- 8. Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M, Mellor G, Merghani A, Malhotra A, Behr E, Sharma S, Sheppard MN. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol. 2016;67:2108–2115. [DOI] [PubMed] [Google Scholar]
- 9. Risgaard B, Winkel BG, Jabbari R, Behr ER, Ingemann‐Hansen O, Thomsen JL, Ottesen GL, Gislason GH, Bundgaard H, Haunso S, Holst AG, Tfelt‐Hansen J. Burden of sudden cardiac death in persons aged 1 to 49 years: nationwide study in Denmark. Circ Arrhythm Electrophysiol. 2014;7:205–211. [DOI] [PubMed] [Google Scholar]
- 10. Fishbein MC. Cardiac disease and risk of sudden death in the young: the burden of the phenomenon. Cardiovasc Pathol. 2010;19:326–328. [DOI] [PubMed] [Google Scholar]
- 11. Kaltman JR, Thompson PD, Lantos J, Berul CI, Botkin J, Cohen JT, Cook NR, Corrado D, Drezner J, Frick KD, Goldman S, Hlatky M, Kannankeril PJ, Leslie L, Priori S, Saul JP, Shapiro‐Mendoza CK, Siscovick D, Vetter VL, Boineau R, Burns KM, Friedman RA. Screening for sudden cardiac death in the young: report from a National Heart, Lung, and Blood Institute Working Group. Circulation. 2011;123:1911–1918. [DOI] [PubMed] [Google Scholar]
- 12. Jayaraman R, Reinier K, Nair S, Aro AL, Uy‐Evanado A, Rusinaru C, Stecker EC, Gunson K, Jui J, Chugh SS. Risk factors of sudden cardiac death in the young: a multiple‐year community‐wide assessment. Circulation. 2018;137:1561–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Meyer L, Stubbs B, Fahrenbruch C, Maeda C, Harmon K, Eisenberg M, Drezner J. Incidence, causes, and survival trends from cardiovascular‐related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30‐year review. Circulation. 2012;126:1363–1372. [DOI] [PubMed] [Google Scholar]
- 14. Allan KS, Morrison LJ, Pinter A, Tu JV, Dorian P. “Presumed cardiac” arrest in children and young adults: a misnomer? Resuscitation. 2017;117:73–79. [DOI] [PubMed] [Google Scholar]
- 15. Pilmer CM, Porter B, Kirsh JA, Hicks AL, Gledhill N, Jamnik V, Faught BE, Hildebrandt D, McCartney N, Gow RM, Goodman J, Krahn AD. Scope and nature of sudden cardiac death before age 40 in Ontario: a report from the cardiac death advisory committee of the office of the chief coroner. Heart Rhythm. 2013;10:517–523. [DOI] [PubMed] [Google Scholar]
- 16. Lin S, Morrison LJ, Brooks SC. Development of a data dictionary for the Strategies for Post Arrest Resuscitation Care (SPARC) network for post cardiac arrest research. Resuscitation. 2011;82:419–422. [DOI] [PubMed] [Google Scholar]
- 17. Morrison LJ, Nichol G, Rea TD, Christenson J, Callaway CW, Stephens S, Pirrallo RG, Atkins DL, Davis DP, Idris AH, Newgard C. Rationale, development and implementation of the Resuscitation Outcomes Consortium Epistry‐Cardiac Arrest. Resuscitation. 2008;78:161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Perkins GD, Jacobs IG, Nadkarni VM, Berg RA, Bhanji F, Biarent D, Bossaert LL, Brett SJ, Chamberlain D, de Caen AR, Deakin CD, Finn JC, Grasner JT, Hazinski MF, Iwami T, Koster RW, Lim SH, Huei‐Ming MM, McNally BF, Morley PT, Morrison LJ, Monsieurs KG, Montgomery W, Nichol G, Okada K, Eng Hock OM, Travers AH, Nolan JP. Cardiac arrest and cardiopulmonary resuscitation outcome reports: update of the Utstein Resuscitation Registry Templates for Out‐of‐Hospital Cardiac Arrest: a statement for healthcare professionals from a task force of the International Liaison Committee on Resuscitation (American Heart Association, European Resuscitation Council, Australian and New Zealand Council on Resuscitation, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Southern Africa, Resuscitation Council of Asia); and the American Heart Association Emergency Cardiovascular Care Committee and the Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Circulation. 2015;132:1286–1300. [DOI] [PubMed] [Google Scholar]
- 19. Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, Corey T, Cutz E, Hanzlick R, Keens TG, Mitchell EA. Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics. 2004;114:234–238. [DOI] [PubMed] [Google Scholar]
- 20. Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, O'Brien WL, Bassett DR Jr, Schmitz KH, Emplaincourt PO, Jacobs DR Jr, Leon AS. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:S498–S504. [DOI] [PubMed] [Google Scholar]
- 21. Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN, Pearse LA, Virmani R. Sudden death in young adults: a 25‐year review of autopsies in military recruits. Ann Intern Med. 2004;141:829–834. [DOI] [PubMed] [Google Scholar]
- 22. Padwal RS, Bienek A, McAlister FA, Campbell NR. Epidemiology of hypertension in Canada: an update. Can J Cardiol. 2016;32:687–694. [DOI] [PubMed] [Google Scholar]
- 23. Table 13‐10‐0096‐10 smokers, by age group. Statistics Canada. Ottawa: The Government of Canada; Last Updated: 4‐12‐2018. Available at: https://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/health73b-eng.htm. Accessed November 22, 2017. [Google Scholar]
- 24. Table: 13‐10‐0096‐09 high blood pressure, by age group. Statistics Canada. Ottawa: The Government of Canada; Last Updated 14‐12‐2018. Available at: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1310009609. Accessed June 19, 2018. [Google Scholar]
- 25. Greiver M, Williamson T, Barber D, Birtwhistle R, Aliarzadeh B, Khan S, Morkem R, Halas G, Harris S, Katz A. Prevalence and epidemiology of diabetes in Canadian primary care practices: a report from the Canadian Primary Care Sentinel Surveillance Network. Can J Diabetes. 2014;38:179–185. [DOI] [PubMed] [Google Scholar]
- 26. Risgaard B, Lynge TH, Wissenberg M, Jabbari R, Glinge C, Gislason GH, Haunso S, Winkel BG, Tfelt‐Hansen J. Risk factors and causes of sudden noncardiac death: a nationwide cohort study in Denmark. Heart Rhythm. 2015;12:968–974. [DOI] [PubMed] [Google Scholar]
- 27. Risgaard B, Waagstein K, Winkel BG, Jabbari R, Lynge TH, Glinge C, Albert C, Correll CU, Haunso S, Fink‐Jensen A, Tfelt‐Hansen J. Sudden cardiac death in young adults with previous hospital‐based psychiatric inpatient and outpatient treatment: a nationwide cohort study from Denmark. J Clin Psychiatry. 2015;76:e1122–e1129. [DOI] [PubMed] [Google Scholar]
- 28. What is depression? Public Health Agency of Canada. Ottawa: The Government of Canada; Last updated: 30‐12‐2016. Available at: https://www.canada.ca/en/public-health/services/chronic-diseases/mental-illness/what-depression.html. Accessed October 19, 2017. [Google Scholar]
- 29. Hafner H, an der Heiden W. Epidemiology of schizophrenia. Can J Psychiatry. 1997;42:139–151. [DOI] [PubMed] [Google Scholar]
- 30. Bushe CJ, Taylor M, Haukka J. Mortality in schizophrenia: a measurable clinical endpoint. J Psychopharmacol. 2010;24:17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Empana JP, Jouven X, Lemaitre RN, Sotoodehnia N, Rea T, Raghunathan TE, Simon G, Siscovick DS. Clinical depression and risk of out‐of‐hospital cardiac arrest. Arch Intern Med. 2006;166:195–200. [DOI] [PubMed] [Google Scholar]
- 32. Ray WA, Chung CP, Murray KT, Hall K, Stein CM. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sticherling C, Schaer BA, Ammann P, Maeder M, Osswald S. Methadone‐induced torsade de pointes tachycardias. Swiss Med Wkly. 2005;135:282–285. [DOI] [PubMed] [Google Scholar]
- 34. Teodorescu C, Reinier K, Uy‐Evanado A, Chugh H, Gunson K, Jui J, Chugh SS. Antipsychotic drugs are associated with pulseless electrical activity: the Oregon Sudden Unexpected Death Study. Heart Rhythm. 2013;10:526–530. [DOI] [PubMed] [Google Scholar]
- 35. Myerburg RJ, Junttila MJ. Sudden cardiac death caused by coronary heart disease. Circulation. 2012;125:1043–1052. [DOI] [PubMed] [Google Scholar]
- 36. Ullal AJ, Abdelfattah RS, Ashley EA, Froelicher VF. Hypertrophic cardiomyopathy as a cause of sudden cardiac death in the young: a meta‐analysis. Am J Med. 2016;129:486–496. [DOI] [PubMed] [Google Scholar]
- 37. Al‐Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Hlatky MA, Granger CB, Hammill SC, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm. 2018;15:e253–e274. [DOI] [PubMed] [Google Scholar]
- 38. Chugh SS, Jui J, Gunson K, Stecker EC, John BT, Thompson B, Ilias N, Vickers C, Dogra V, Daya M, Kron J, Zheng ZJ, Mensah G, McAnulty J. Current burden of sudden cardiac death: multiple source surveillance versus retrospective death certificate‐based review in a large U.S. community. J Am Coll Cardiol. 2004;44:1268–1275. [DOI] [PubMed] [Google Scholar]
- 39. Tseng ZH, Olgin JE, Vittinghoff E, Ursell PC, Kim AS, Sporer K, Yeh C, Colburn B, Clark NM, Khan R, Hart AP, Moffatt E. Prospective countywide surveillance and autopsy characterization of sudden cardiac death: POST SCD Study. Circulation. 2018;137:2689–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hookana E, Junttila MJ, Puurunen VP, Tikkanen JT, Kaikkonen KS, Kortelainen ML, Myerburg RJ, Huikuri HV. Causes of nonischemic sudden cardiac death in the current era. Heart Rhythm. 2011;8:1570–1575. [DOI] [PubMed] [Google Scholar]
- 41. Burke A, Tavora F. Practical Cardiovascular Pathology: An Atlas. Philadelphis: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 42. Tu JV, Chu A, Rezai MR, Guo H, Maclagan LC, Austin PC, Booth GL, Manuel DG, Chiu M, Ko DT, Lee DS, Shah BR, Donovan LR, Sohail QZ, Alter DA. The incidence of major cardiovascular events in immigrants to Ontario, Canada: the CANHEART Immigrant Study. Circulation. 2015;132:1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]


