Abstract
Background
The accumulation of myocardial triglycerides and remodeling of the left ventricle are common features in type 2 diabetes mellitus and represent potential risk factors for the development of diastolic and systolic dysfunction. A few studies have investigated the separate effects of diet and exercise training on cardiac function, but none have investigated myocardial changes in response to a combined diet and exercise intervention. This 12‐week randomized study assessed the effects of a Paleolithic diet, with and without additional supervised exercise training, on cardiac fat, structure, and function.
Methods and Results
Twenty‐two overweight and obese subjects with type 2 diabetes mellitus were randomized to either a Paleolithic diet and standard‐care exercise recommendations (PD) or to a Paleolithic diet plus supervised exercise training 3 hours per week (PD‐EX). This study includes secondary end points related to cardiac structure and function, ie, myocardial triglycerides levels, cardiac morphology, and strain were measured using cardiovascular magnetic resonance, including proton spectroscopy, at baseline and after 12 weeks. Both groups showed major favorable metabolic changes. The PD‐EX group showed significant decreases in myocardial triglycerides levels (−45%, P=0.038) and left ventricle mass to end‐diastolic volume ratio (−13%, P=0.008) while the left ventricle end‐diastolic volume and stroke volume increased significantly (+14%, P=0.004 and +17%, P=0.008, respectively). These variables were unchanged in the PD group.
Conclusions
Exercise training plus a Paleolithic diet reduced myocardial triglycerides levels and improved left ventricle remodeling in overweight/obese subjects with type 2 diabetes mellitus.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01513798.
Keywords: cardiovascular magnetic resonance imaging, diet, exercise, myocardial metabolism, type 2 diabetes mellitus
Subject Categories: Cardiomyopathy; Magnetic Resonance Imaging (MRI); Diabetes, Type 2; Diet and Nutrition; Exercise
Clinical Perspective
What Is New?
Exercise training in addition to a Paleolithic diet reduced myocardial triglyceride levels and improved left ventricle remodeling over a 12‐week period in overweight/obese subjects with type 2 diabetes mellitus.
Despite causing profound metabolic improvement, the Paleolithic diet alone had no beneficial effects on cardiac structure and function.
No associations were seen between reduction of myocardial triglyceride levels and changes in cardiac function, arguing against a simple relationship between these parameters.
What Are the Clinical Implications?
Exercise training may be an important component to achieve positive cardiometabolic effects in subjects with type 2 diabetes mellitus.
Long‐term follow‐up including detailed studies of cardiac metabolism with this type of intervention is of future interest.
Introduction
Individuals with type 2 diabetes mellitus have a 2‐fold higher risk of hospitalization because of heart failure than those without diabetes mellitus.1 The underlying cause remains a matter of debate, but 1 possible mechanism is that the development of structural and functional changes in the left ventricle (LV) leads to diastolic dysfunction, to alterations in strain patterns, and later to systolic dysfunction and clinical signs of heart failure.2, 3 These diabetes mellitus–associated changes, which are not accounted for by hypertension and coronary artery disease, are described as LV concentric remodeling, which is characterized by an increased LV mass/LV end‐diastolic volume ratio but a normal LV mass index. The accumulation of myocardial triglycerides (MTGs) has been suggested to be a possible driver of concentric remodeling in which an excess of intracellular lipids leads to hypertrophic signaling.4, 5 The use of proton magnetic resonance spectroscopy to noninvasively measure lipid content revealed an association between MTG levels and concentric remodeling in both animals and humans.6 In addition, recent data indicate that the MTG level predicts concentric LV remodeling in type 2 diabetes mellitus.7
A combination of diet and exercise is a cornerstone of treatment in type 2 diabetes mellitus,8, 9 as it improves glycemic control, increases insulin sensitivity, reduces fat mass (including visceral fat), and lowers serum triglycerides.10 Importantly, the cardiac effects of a combined diet and exercise intervention in this patient group are unknown. Weight reduction in obese subjects who have type 2 diabetes mellitus and who are following a very low‐calorie diet (450 kcal/d) decreases the MTG levels and improves diastolic LV function.11, 12 In contrast, an exercise intervention alone does not affect MTG levels13, 14 but may have beneficial effects on systolic LV function,13, 15 although some earlier data are not in accordance with this.14
We and others have shown that a Paleolithic‐type diet (PD) may have powerful metabolic effects in type 2 diabetes mellitus.10, 16 Notably, we found previously that there was a substantial reduction of ectopic fat accumulation in liver in subjects who were allowed ad libitum intake of this diet,17 which included high intake of vegetables, nuts, fruit, eggs, fish, and lean meat but excluded dairy products, grains, legumes, salt, and refined sugar. It is therefore of interest to investigate the myocardial effects of a PD alone or in combination with exercise in type 2 diabetes mellitus. Our study included 22 overweight individuals with type 2 diabetes mellitus. We hypothesized that a 12‐week PD intervention would decrease MTG accumulation and improve cardiac structure and function. We also hypothesized that additional supervised exercise training would further improve heart function.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
Overweight and obese individuals with type 2 diabetes mellitus consumed a PD for 12 weeks and in addition, the subjects were randomized to standard‐care exercise recommendations (PD group) or to receive supervised exercise training 3 hours per week (PD‐EX group). The main results of the intervention study have been previously published.10 The outcomes of this article are secondary end points: structural and functional cardiac measurements using cardiovascular magnetic resonance (CMR), including proton spectroscopy.
Subjects and Randomization
The dietary and exercise interventions were described in detail previously.10 Briefly, we recruited patients with type 2 diabetes mellitus by advertising in local newspapers and by posting flyers at Umeå University Hospital. The main inclusion criteria were age 30 to 70 years, body mass index between 25 and 40 kg/m2, type 2 diabetes mellitus <10 years, hemoglobin A1c 6.5% to 10.8%, and the use of lifestyle modification and/or metformin. Only postmenopausal women were included, a group associated with metabolic disturbances and increased risk of cardiovascular complications. The exclusion criteria were smoking, use of β‐blockers, blood pressure >160/100, known cardiovascular disease, macroalbuminuria, weight loss (>5% weight loss in the past 6 months), severe illness, and higher level of exercise training (resistance training once every other week, moderate endurance training 5 times a week or more).
A total of 261 individuals were interested in participating; of these 32 (12.3%) participants fulfilled the inclusion criteria and were randomized into the 2 groups (Figure 1).10 The participants were assigned to the PD and PD‐EX groups using biased coin minimization with an allocation ratio of 1:1. A subpopulation of 22 participants underwent complete CMR analysis, and these 22 comprised the final study population. The staff who performed the examinations and dietary counseling was blinded to the allocation. The statistician who randomized the participants and the research assistant who informed the participants of their randomization outcomes were not involved in later data collection or data analyses. All participants provided written informed consent. The study protocol fulfilled the tenets of the Helsinki declaration and was approved by the Regional Ethical Review Board, Umeå, Sweden.
Figure 1.
CONSORT flow diagram. CMR indicates cardiovascular magnetic resonance; CONSORT, Consolidated Standards of Reporting Trials; PD, Paleolithic diet.
Dietary Intervention
The PD was based on vegetables, fruits, berries, nuts, seafood, eggs, fish, and lean meat. Dairy products, cereals, legumes, refined fats, added sugar, and salt were excluded. Energy intake was ad libitum. Each group participated in 5 group sessions led by a trained dietitian, and the group members could contact the dietitian between meetings. Dietary intake was assessed at baseline and at 12 weeks using self‐reported weighed food records.10
Exercise Training Intervention
Before randomization, both intervention groups were advised to perform at least 30 minutes of moderate exercise daily as recommended by the current guidelines for patients with type 2 diabetes mellitus. The PD‐EX group additionally participated in a program that combined aerobic and resistance training in 1‐hour sessions 3 times a week at the Sports Medicine Unit at Umeå University. The training protocol had a progressive design in accord with the guidelines of the American College of Sports Medicine.18 The exercise sessions were performed on weekdays, with at least 1 day of rest between sessions. The first session every week consisted of moderate‐intensity aerobic exercise (≈70% of maximum heart rate) for 20 to 30 minutes on a cross‐trainer. The second session of the week comprised 10 repetitions of 10‐ to 60‐s high‐intensity intermittent training (with 50–90‐s of active recovery in between each repetitions) on a cycle‐ergometer; these were performed at 100% to 110% of the initial VO2max workload. The third session of the week consisted of 6 moderate‐intensity 5‐minute intervals between 45% and 60% of maximal workload on a cycle‐ergometer. All aerobic training sessions were combined with 30 to 40 minutes of resistance training that included 10 to 15 repetitions in 2 to 4 sets. These exercises engaged multiple muscle groups in both the upper and lower body, including leg curls, leg extensions, leg presses, hip raises, flat and incline bench presses, seated rows, dumbbell rows, lat pull‐downs, back extensions, shoulder raises, burpees, step‐ups, wall ball shots, and sit‐ups. Once participants could manage all repetitions, the workload was increased for the following session.
Anthropometry and Cardiopulmonary Exercise Testing
The participants were examined at baseline and after 12 weeks of the intervention at the Clinical Research Center at Umeå University Hospital, Umeå, Sweden. Height was measured using a calibrated height‐measuring gauge. Weight was measured on a calibrated electronic digital scale (Tanita BWB‐800 MA, Umedico AB, Rosersberg, Sweden) with the participants wearing light clothing. Waist circumference was assessed between the lowest rib and iliac crest during gentle exhalation. Systolic and diastolic blood pressure were measured twice in the right arm with the subject in a sitting position at 2‐minute intervals and once after 5 minutes of rest using an automated blood pressure meter (Boso Medicus, Bosch, Germany). Maximal oxygen uptake (VO2 max) and maximal workload were measured during a cardiopulmonary exercise test using a Jaeger Oxycon Pro system (Erich Jaeger GmbH, Hoechberg, Germany) and an 894E Monark cycle ergometer (Monark, Varberg, Sweden).
Blood Samples
Fasting venous blood samples were analyzed to measure the serum triglycerides, serum insulin, and hemoglobin A1c at the Department of Clinical Chemistry, Umeå University Hospital. Fasting glucose was analyzed in a capillary sample (HemoCue 201 RT; Radiometer Medical Aps, Brønshøj, Denmark). Aliquots of plasma were immediately stored at −80°C. Nonesterified fatty acids were analyzed with NEFA‐HR2 (WAKO Chemicals, Neuss, Germany). Insulin sensitivity was calculated according to the homeostatic model assessment of insulin resistance as (fasting glucose×fasting insulin)/22.5.
Cardiovascular Magnetic Resonance
Cardiac structure and function was assessed with a 1.5‐T ACS NT MRI scanner (Philips, Best, The Netherlands). The subjects lay in a supine position, head first in the bore of the magnet. A balanced steady‐state free precession sequence was used with ECG‐gating and breath holding to measure cardiac function and morphology. The heart was imaged in short‐axis orientation from the apex to the base with slices. LV end‐systolic volume, end‐diastolic volume, and mass were quantified by manually drawing endocardial and epicardial contours (including papillary musculature as part of the LV mass) in end‐diastole and end‐systole in short‐axis orientation from the apex to the base. Dedicated software (Segment)19 was used to assess the LV end‐diastolic volume, end‐systolic volume, stroke volume, cardiac output, ejection fraction, and LV mass. One operator analyzed all CMR scans and was blinded to treatment assignment and sequence. Intraobserver variability was assessed using the Bland‐Altman method.
Myocardial strain was calculated using 4‐chamber and 2‐chamber long‐axis views of the ventricle. The LV was segmented according to the standard 17‐segment model. The apical cap (segment 17) was not considered in the analysis. Retrospective image analyses were conducted using 2‐dimensional CPA CMR Feature tracking software (TomTec Imaging Systems, Munich, Germany). This tool comprises a software‐based feature‐tracking algorithm that has been validated previously in experimental and clinical studies.20, 21, 22 Feature tracking was used to measure the radial and longitudinal strain along user‐defined endocardial and epicardial borders throughout the cardiac cycle on standard CMR balanced steady‐state free precession sequences. In our study, the strain values were measured by a blinded observer and were calculated as follows. The peak segmental values of the longitudinal and transverse strains of the LV derived from the 4‐chamber LAX view were measured, and values were obtained for the average longitudinal strain, average radial strain, global longitudinal strain, and global radial strain of the LV. In addition, we measured the peak segmental values of the longitudinal and transverse (radial) strains of the LV as derived from the 2‐chamber view.
Spectroscopy
High‐resolution cardiac images aligned to 3 orthogonal planes of the heart were acquired in order to locate the interventricular septum. Images at end‐systole were used to position a spectroscopic sample volume of 10×20×30 mm3 within the ventricular septum. During spectroscopic data acquisition, ECG triggering at end‐systole (with the delay from the R‐wave calculated using cine images) and respiratory triggering at end‐exhalation (using a navigator sequence) were performed simultaneously using the physiological gating capabilities of the scanner's spectroscopy package. CMR spectra were recorded using the PRESS sequence (point‐resolved spectroscopy) with a repetition time that was typically set to 6 heart beats, giving a repetition time of at least 4 s and an echo time of 28.7 ms, which was the shortest achievable echo time. Sixty‐four signal averages were acquired from the spectroscopic volume over 1024 data points covering a 1000‐Hz spectral width. Two spectra were acquired from each volume, 1 with and 1 without water suppression.
The spectra were analyzed using LCModel software version 6.3–1J (Stephen Provencher, Oakville, Ontario, Canada) using the built‐in model for lipid spectra and using an analysis window of 3.6 to −1.0 ppm. Signal intensities were extrapolated to zero echo time using the assumption that the T2 relaxation time of water is 40 ms and the T2 relaxation time for triglycerides is 78 ms at 1.5 T, while the signal was acquired at 28.7 ms echo time. The results are reported as the ratios of the metabolite resonance areas to the unsuppressed water resonance. The metabolite resonance that was used encompassed the sum of the lipid resonances at 1.6, 1.3, and 0.9 ppm. This corresponds well to the “lipid signal” reported by other groups that used simple integration of the lipid signals from MR spectra rather than the more sophisticated metabolite models built into LCModel.23
Statistical Analysis
Statistical analyses were performed using SPSS Statistics 23 (IBM Corp, Armonk, NY). The analyses reflect a per‐protocol approach. The null hypothesis was rejected for 2‐sided P<0.05. Because several variables had skewed distribution, all data were reported as medians with interquartile ranges. The nonparametric Wilcoxon signed‐rank test was used to compare changes within the groups over time. The Mann–Whitney U test was used to compare changes between the intervention groups. In pairwise comparisons, each test was evaluated separately for missing values. For bivariate correlation analyses, we used a natural logarithm to achieve a normal distribution of the data.
Results
Patient Characteristics and Cardiometabolic Risk Markers
The full results from the intervention study regarding compliance with the diet and exercise program, energy balance, glucose metabolism, cardiovascular fitness, and adipokine levels were published previously (Table 1).10 In this substudy group, the PD group had significantly higher diastolic blood pressure (P=0.03), lower levels of nonesterified fatty acids (P=0.02), and lower fasting glucose levels (P=0.003) compared with the PD‐EX group at baseline. During the intervention, there were significant decreases in both groups in terms of weight (PD −9% and PD‐EX −8%), body mass index (PD −9% and PD‐EX −8%), waist circumference (PD −9% and PD‐EX −7%), systolic blood pressure (PD −10% and PD‐EX −6%), diastolic blood pressure (PD −14% and PD‐EX −9%), fasting triglycerides (PD −56% and PD‐EX −41%), fasting glucose (PD −17% and PD‐EX −26%), hemoglobin A1c (PD −22% and PD‐EX −24%), fasting insulin (PD −38% and PD‐EX −39%), and homeostatic model assessment of insulin resistance (PD −46% and PD‐EX −51%). The maximal oxygen uptake increased significantly in both groups (PD +19% and PD‐EX +19%), although the increase in the PD‐EX group was more pronounced. Mean resting heart rate decreased and the maximum workload increased significantly in the PD‐EX group (−12% and +16%, respectively), while no changes were seen for these measures in the PD group.
Table 1.
Anthropometric and Metabolic Measurements in Participants During an Intervention With a Paleolithic Diet or a Paleolithic Diet With Additional Supervised Exercise Training
| PD (n=11) | PD‐EX (n=11) | Group Change Difference (P Value) | |||
|---|---|---|---|---|---|
| Baseline | 12 Wks | Baseline | 12 Wks | ||
| Age, y | 59 (52–64) | 61 (58–66) | |||
| Sex, male/female | 7/4 | 7/4 | |||
| Weight, kg | 95 (82–102) | 85 (78–97)† | 97 (84–116) | 90 (76–112)† | 0.533 |
| BMI, kg/m2 | 31 (30–31) | 29 (27–30)† | 31 (29–37) | 29 (27–34)† | 0.533 |
| Waist circumference, cm | 112 (104–119) | 103 (98–109)† | 107 (104–116) | 99 (94–116)† | 0.189 |
| Systolic BP, mm Hg | 135 (127–158) | 128 (112–128)* | 130 (119–138) | 119 (112–132)* | 0.189 |
| Diastolic BP, mm Hg | 87 (84–95) | 77 (72–81)† | 81 (74–91)‡ | 72 (68–81)† | 0.305 |
| Heart rate, beats/min | 71 (65–80) | 72 (61–81) | 71 (66–81) | 63 (55–75)* | 0.056 |
| VO2max, mL/min per kg | 22 (21–25) | 24 (23–30)†, § | 22 (20–25) | 27 (25–30)† | 0.005 |
| VO2max total, mL/min | 2100 (1900–2600) | 2100 (1900–2500)§ | 2200 (1700–2800) | 2600 (1800–2900)† | 0.048 |
| Maximum workload, Watts | 180 (140–200) | 180 (170–210)§ | 190 (130–240) | 220 (150–260)† | 0.002 |
| Fasting triglycerides, mmol/L | 2.4 (1.2–3.3) | 1.2 (0.7–1.9)†, § | 1.7 (1.0–2.5) | 1.1 (0.9–1.2)† | 0.503 |
| Fasting nonesterified fatty acids, mmol/L | 0.65 (0.54–0.77) | 0.76 (0.49–0.81)∥ | 0.85 (0.66–0.96)‡ | 0.77 (0.63–0.91) | 0.063 |
| Fasting glucose (HemoCue), mmol/L | 7.8 (7.0–8.2) | 6.1 (5.5–6.9)*, ∥ | 10.0 (8.3–12.3)‡ | 7.2 (6.4–7.9)† | 0.149 |
| HbA1C, mmol/mol | 52 (47–56) | 42 (41–43)†, § | 57 (51–71) | 43 (40–45)* | 0.562 |
| Fasting P‐insulin, mIU/L | 23 (15–28) | 12 (9–17)*, § | 17 (11–23) | 10.0 (8.7–15.0)† | 0.648 |
| HOMA‐IR | 7.0 (5.4–8.6) | 3.2 (2.1–5.5)*, ∥ | 8.1 (6.0–10.2) | 3.2 (2.7–4.5)† | 0.621 |
Data are reported as medians (interquartile ranges). BMI indicates body mass index; BP, blood pressure; HbA1c, hemoglobin A1c; HOMA‐IR, homeostatic model assessment of insulin resistance; VO2max, maximal oxygen uptake. The nonparametric Wilcoxon signed‐rank test was used to compare values within groups.
* P<0.05, † P<0.01. The Mann–Whitney U test was used to compare changes between the 2 groups.
The baseline value differs significantly between groups (P<0.05).
One missing value in statistical analyses.
Two missing values in statistical analyses.
Structural/Functional Cardiac Changes and MTGs
The LV mass, LV end‐systolic volume, ejection fraction, endocardial longitudinal strain, and epicardial longitudinal strain did not change in either group during the intervention (Table 2 and Figure 2). Cardiac output decreased significantly in the PD group (−14%) but was unchanged in the PD‐EX group. In the PD‐EX group, the stroke volume and LV end‐diastolic volume increased significantly (+17% and +14%, respectively), and there was a nonsignificant increase in the global radial strain (+13%, P=0.05). The ratio of the LV mass to the end‐diastolic volume and the MTGs were both significantly reduced in the PD‐EX group (−13% and −45%) but were unchanged in the PD group. Changes over time in stroke volume, LV end‐diastolic volume, and endocardial global longitudinal strain differed significantly between the groups (P=0.023, 0.039 and 0.039, respectively), with a relative increase seen in the PD‐EX group.
Table 2.
Selected Cardiovascular Magnetic Resonance Measurements in Participants During an Intervention With a Paleolithic Diet or a Paleolithic Diet With Additional Supervised Exercise Training
| PD (n=11) | PD‐EX (n=11) | Group Change Difference (P Value) | |||
|---|---|---|---|---|---|
| Baseline | 12 Wks | Baseline | 12 Wks | ||
| LV mass, g | 142 (124–179) | 140 (124–171) | 157 (108–179) | 152 (107–177) | 0.511 |
| LV end‐systolic volume, mL | 27 (24–32) | 26 (24–34) | 34 (24–36) | 30 (24–40) | 0.743 |
| Ejection fraction, % | 74 (71–79) | 75 (72–78) | 73 (72–77) | 76 (73–83) | 0.264 |
| Cardiac output, L/min | 6.5 (5.8–7.3) | 6.0 (5.1–6.8)* | 7.3 (5.8–8.8) | 6.8 (5.7–8.5) | 0.023 |
| Epicardial global longitudinal strain, % | −17 (−19 to −16) | −17 (−17 to −15) | −18 (−19 to −17) | −19 (−20 to −18) | 0.189 |
Data are reported as medians (interquartile ranges). LV indicates left ventricle. The nonparametric Wilcoxon signed‐rank test was used to compare values within groups.
*P<0.05. The Mann–Whitney U test was used to compare changes between the 2 groups.
Figure 2.
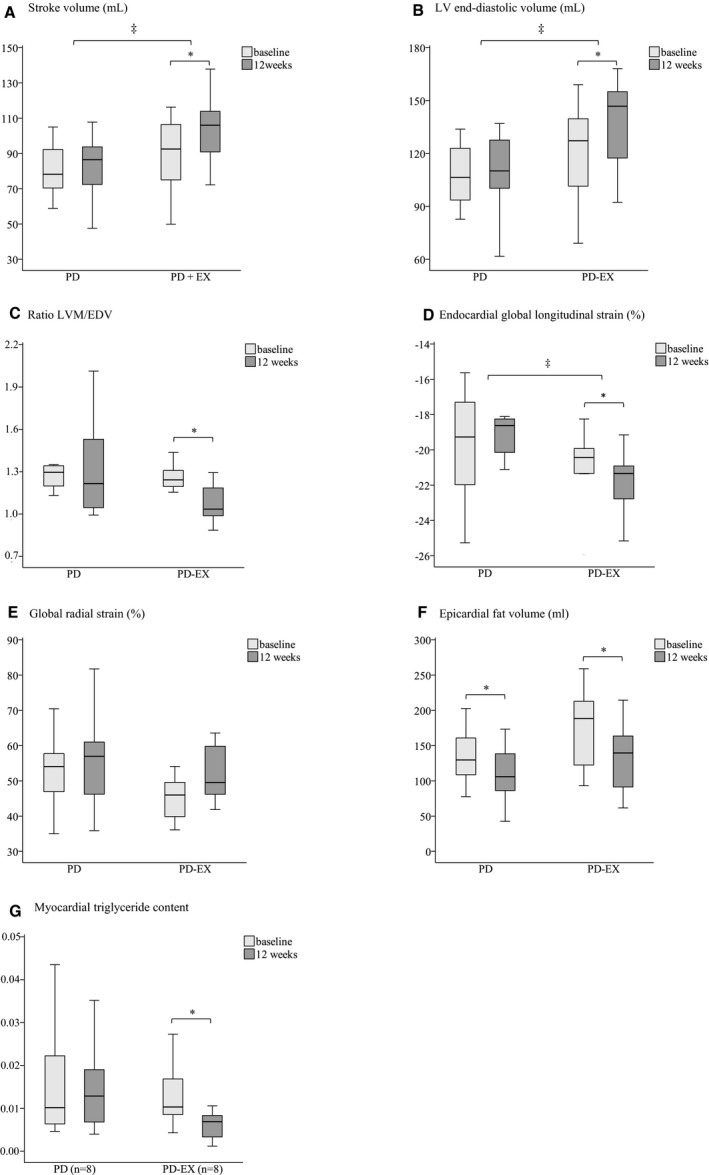
Selected cardiovascular magnetic resonance and spectroscopy findings in participants during an intervention with a Paleolithic diet (PD) or a Paleolithic diet with additional supervised exercise training (PD‐EX) (A through G). The boxes represent medians and interquartile ranges, and whiskers represent the range of values, not including the outliers. LVM indicates left ventricle mass; EDV, end‐diastolic volume. The nonparametric Wilcoxon signed‐rank test was used to compare values within the groups. *P<0.05. The Mann–Whitney U test was used to compare changes between the 2 groups. ‡Significant differences in the changes between the groups (P<0.05).
Bivariate correlation analyses at baseline showed associations between MTG levels and LVM (P=0.004, r=0.679) and between MTG levels and cardiac output (P=0.009, r=0.631). However, no associations were found between changes in the MTG levels and changes in other functional or structural cardiac parameters over time (data not shown). There were associations between changes in the maximal oxygen uptake (VO2max total) and changes in end‐diastolic volume, stroke volume, and cardiac output (P=0.041, r=0.449; P=0.03, r=0.474; and P=0.01, r=−0.538, respectively). We did not find any correlations between fasting glucose levels and functional/structural cardiac parameters at baseline or between changes in these parameters over time (data not shown). Furthermore, no associations were found between weight loss and functional cardiac changes.
Discussion
The main finding of our study was that 12 weeks of a PD plus exercise training intervention improved several indices of LV function with a concomitant decrease in MTG levels in overweight and obese subjects with type 2 diabetes mellitus. To our knowledge, this is the first study to examine the detailed cardiac effects of a combined diet and training intervention in type 2 diabetes mellitus.
Previous investigations of the cardiac effects of lifestyle interventions in this patient group have focused mainly on dietary interventions. The PD alone had a powerful impact on several important metabolic and cardiovascular risk markers in our study, but there were no specific beneficial effects on cardiac structure and function. Two previous studies found reduced MTG levels and improved diastolic function following a 16‐week very low‐calorie diet.11, 12 This discrepancy could be explained by the fact that more extreme metabolic manipulation (ie, 450 kcal/d) is needed to decrease MTG levels. Another possible explanation is that the macronutrient composition may have influenced the MTG levels. However, a study of overweight/obese women reported that moderate dietary weight loss after reducing carbohydrate or fat intake also reduced cardiac MTGs, which seems to contradict these theories.24 Notably, the PD emphasizes increased intake of mono‐ and polyunsaturated fatty acids, and this may have important metabolic effects.25, 26
The additive effect of exercise training on MTG levels and heart function was a novel finding in our study. The lower level of MTGs in the PD‐EX group could theoretically improve LV hemodynamics. However, we did not find any association between reduced MTG levels and changes in cardiac function, arguing against a simple relationship between triglyceride content and cardiac function. This is line with the study by Jonker et al, who found that the improvement in diastolic function remained after 14 months of follow‐up despite a return of MTGs to baseline levels.11 Notably, physical activity energy expenditure (kcal/d and kcal/kg per day) measured from a combined accelerometer and heart monitor did not show any significant differences between groups at baseline or at the 12‐week follow‐up as reported in a previous publication.10
The lack of associations between glucose levels and structural or functional cardiac parameters partly contrasts with the results of an earlier study in which fasting blood glucose was identified as the most important predictor of impairment of cardiac function in type 2 diabetes mellitus.27 This may be related mainly to altered myocardial elastic properties (fibrosis) or to prolonged ventricular relaxation.27 However, the referenced study showed associations between glucose levels and diastolic measures (E/A and diastolic filling rate), parameters not measured in our study. The change over time in endocardial global longitudinal strain differed between the PD and the PD‐EX groups, suggesting improved subendocardial function in the PD‐EX group.27, 28 More detailed studies on the effect of the PD‐EX intervention on cardiac metabolism, including possible effects on glucose and fatty acid utilization, are therefore of major interest.29 This includes studies on the potential reversibility of myocardial resistance to insulin‐mediated glucose uptake, which may augment fatty acid metabolism.30 In addition, the lower heart rate, which might be because of altered autonomic balance, together with the reduction in blood pressure and decreased blood volume (accompanying weight loss), might have improved LV performance.
The use of CMR was a major strength of our study, since this is the criterion standard for obtaining reproducible, observer‐independent measures of LV volume and mass31 and cardiac strain for detecting subclinical LV dysfunction, which has important prognostic value.32 The majority of earlier studies that evaluated LV function and dimensions in subjects with diabetes mellitus used echocardiography, which has several limitations, particularly in obese populations. A limitation of our study was that coronary angiography was not performed. Therefore, we cannot exclude the possibility that subclinical coronary artery disease affected myocardial function in the study subjects. Furthermore, differences between groups at baseline in some parameters could have affected the statistical analyses. Hypertension is a common comorbidity in type 2 diabetes mellitus, which complicates the assessment of interventions on cardiac function, but hopefully this affected the 2 groups to a similar extent. The population size was relatively small, and we did not include a control group. Furthermore, the optimal time point studying cardiac effects of different interventions is unknown. We chose 12 weeks based on previous literature. A long‐term follow‐up of the effects of the PD plus exercise training on MTG levels and cardiac remodeling using this protocol would be of great interest.
In conclusion, exercise training plus a PD was associated with beneficial changes in cardiovascular structure and function in overweight/obese patients with type 2 diabetes mellitus. The long‐term effects of this intervention and the prognostic value of our findings need to be addressed in larger prospective studies.
Sources of Funding
This work was supported by grants from the Swedish Heart and Lung Foundation (20120450); King Gustav V and Queen Victoria's Foundation; The Swedish Diabetes Research Foundation (2014‐096); the County Council of Västerbotten (VLL‐460481); and Umeå University, Sweden.
Disclosures
None.
Acknowledgments
We thank research nurses Inger Arnesjö, Liv‐Helene Bergman, Katarina Iselid, Camilla Ring, and Lena Uddståhl for their expert technical assistance and Caroline Mellberg for help with planning the studies. We thank Marie Eriksson at the Department of Statistics, Umeå University, Sweden, for performing the randomization and Magnus Hedström at the Heart Center, Umeå University Hospital, Sweden for planning the cardiopulmonary exercise test and interpreting the results.
(J Am Heart Assoc. 2019;8:e010634 DOI: 10.1161/JAHA.118.010634.)
References
- 1. Rawshani A, Rawshani A, Franzen S, Eliasson B, Svensson AM, Miftaraj M, McGuire DK, Sattar N, Rosengren A, Gudbjornsdottir S. Mortality and cardiovascular disease in type 1 and type 2 diabetes. N Engl J Med. 2017;376:1407–1418. [DOI] [PubMed] [Google Scholar]
- 2. Gjesdal O, Bluemke DA, Lima JA. Cardiac remodeling at the population level—risk factors, screening, and outcomes. Nat Rev Cardiol. 2011;8:673–685. [DOI] [PubMed] [Google Scholar]
- 3. Korosoglou G, Humpert PM, Ahrens J, Oikonomou D, Osman NF, Gitsioudis G, Buss SJ, Steen H, Schnackenburg B, Bierhaus A, Nawroth PP, Katus HA. Left ventricular diastolic function in type 2 diabetes mellitus is associated with myocardial triglyceride content but not with impaired myocardial perfusion reserve. J Magn Reson Imaging. 2012;35:804–811. [DOI] [PubMed] [Google Scholar]
- 4. Glenn DJ, Cardema MC, Ni W, Zhang Y, Yeghiazarians Y, Grapov D, Fiehn O, Gardner DG. Cardiac steatosis potentiates angiotensin II effects in the heart. Am J Physiol Heart Circ Physiol. 2015;308:H339–H350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Glenn DJ, Wang F, Nishimoto M, Cruz MC, Uchida Y, Holleran WM, Zhang Y, Yeghiazarians Y, Gardner DG. A murine model of isolated cardiac steatosis leads to cardiomyopathy. Hypertension. 2011;57:216–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Szczepaniak LS, Dobbins RL, Metzger GJ, Sartoni‐D'Ambrosia G, Arbique D, Vongpatanasin W, Unger R, Victor RG. Myocardial triglycerides and systolic function in humans: in vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn Reson Med. 2003;49:417–423. [DOI] [PubMed] [Google Scholar]
- 7. Levelt E, Pavlides M, Banerjee R, Mahmod M, Kelly C, Sellwood J, Ariga R, Thomas S, Francis J, Rodgers C, Clarke W, Sabharwal N, Antoniades C, Schneider J, Robson M, Clarke K, Karamitsos T, Rider O, Neubauer S. Ectopic and visceral fat deposition in lean and obese patients with type 2 diabetes. J Am Coll Cardiol. 2016;68:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thomas DE, Elliott EJ, Naughton GA. Exercise for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2006;3:CD002968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Joslin EP, Lombard HL, Burrows RE, Manning MD. Diabetes and cancer. N Engl J Med. 1959;260:486–488. [DOI] [PubMed] [Google Scholar]
- 10. Otten J, Stomby A, Waling M, Isaksson A, Tellstrom A, Lundin‐Olsson L, Brage S, Ryberg M, Svensson M, Olsson T. Benefits of a paleolithic diet with and without supervised exercise on fat mass, insulin sensitivity, and glycemic control: a randomized controlled trial in individuals with type 2 diabetes. Diabetes Metab Res Rev. 2017;33:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jonker JT, Snel M, Hammer S, Jazet IM, van der Meer RW, Pijl H, Meinders AE, de Roos A, Smit JW, Romijn JA, Lamb HJ. Sustained cardiac remodeling after a short‐term very low calorie diet in type 2 diabetes mellitus patients. Int J Cardiovasc Imaging. 2014;30:121–127. [DOI] [PubMed] [Google Scholar]
- 12. Hammer S, Snel M, Lamb HJ, Jazet IM, van der Meer RW, Pijl H, Meinders EA, Romijn JA, de Roos A, Smit JW. Prolonged caloric restriction in obese patients with type 2 diabetes mellitus decreases myocardial triglyceride content and improves myocardial function. J Am Coll Cardiol. 2008;52:1006–1012. [DOI] [PubMed] [Google Scholar]
- 13. Schrauwen‐Hinderling VB, Meex RC, Hesselink MK, van de Weijer T, Leiner T, Schar M, Lamb HJ, Wildberger JE, Glatz JF, Schrauwen P, Kooi ME. Cardiac lipid content is unresponsive to a physical activity training intervention in type 2 diabetic patients, despite improved ejection fraction. Cardiovasc Diabetol. 2011;10:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jonker JT, de Mol P, de Vries ST, Widya RL, Hammer S, van Schinkel LD, van der Meer RW, Gans RO, Webb AG, Kan HE, de Koning EJ, Bilo HJ, Lamb HJ. Exercise and type 2 diabetes mellitus: changes in tissue‐specific fat distribution and cardiac function. Radiology. 2013;269:434–442. [DOI] [PubMed] [Google Scholar]
- 15. Cassidy S, Thoma C, Hallsworth K, Parikh J, Hollingsworth KG, Taylor R, Jakovljevic DG, Trenell MI. High intensity intermittent exercise improves cardiac structure and function and reduces liver fat in patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2016;59:56–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jonsson T, Granfeldt Y, Ahren B, Branell UC, Palsson G, Hansson A, Soderstrom M, Lindeberg S. Beneficial effects of a paleolithic diet on cardiovascular risk factors in type 2 diabetes: a randomized cross‐over pilot study. Cardiovasc Diabetol. 2009;8:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Otten J, Mellberg C, Ryberg M, Sandberg S, Kullberg J, Lindahl B, Larsson C, Hauksson J, Olsson T. Strong and persistent effect on liver fat with a paleolithic diet during a two‐year intervention. Int J Obes (Lond). 2016;40:747–753. [DOI] [PubMed] [Google Scholar]
- 18. Garber CE, Blissmer B, Deschenes MR, Franklin BA, Lamonte MJ, Lee IM, Nieman DC, Swain DP; American College of Sports M . American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334–1359. [DOI] [PubMed] [Google Scholar]
- 19. Soneson H, Ubachs JF, Ugander M, Arheden H, Heiberg E. An improved method for automatic segmentation of the left ventricle in myocardial perfusion SPECT. J Nucl Med. 2009;50:205–213. [DOI] [PubMed] [Google Scholar]
- 20. Buss SJ, Breuninger K, Lehrke S, Voss A, Galuschky C, Lossnitzer D, Andre F, Ehlermann P, Franke J, Taeger T, Frankenstein L, Steen H, Meder B, Giannitsis E, Katus HA, Korosoglou G. Assessment of myocardial deformation with cardiac magnetic resonance strain imaging improves risk stratification in patients with dilated cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2015;16:307–315. [DOI] [PubMed] [Google Scholar]
- 21. Hor KN, Gottliebson WM, Carson C, Wash E, Cnota J, Fleck R, Wansapura J, Klimeczek P, Al‐Khalidi HR, Chung ES, Benson DW, Mazur W. Comparison of magnetic resonance feature tracking for strain calculation with harmonic phase imaging analysis. JACC Cardiovasc Imaging. 2010;3:144–151. [DOI] [PubMed] [Google Scholar]
- 22. Pirat B, Khoury DS, Hartley CJ, Tiller L, Rao L, Schulz DG, Nagueh SF, Zoghbi WA. A novel feature‐tracking echocardiographic method for the quantitation of regional myocardial function: validation in an animal model of ischemia‐reperfusion. J Am Coll Cardiol. 2008;51:651–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001;14:260–264. [DOI] [PubMed] [Google Scholar]
- 24. Utz W, Engeli S, Haufe S, Kast P, Bohnke J, Haas V, Hermsdorf M, Wiesner S, Pofahl M, Traber J, Luft FC, Boschmann M, Jordan J, Schulz‐Menger J. Moderate dietary weight loss reduces myocardial steatosis in obese and overweight women. Int J Cardiol. 2013;167:905–909. [DOI] [PubMed] [Google Scholar]
- 25. Ryberg M, Sandberg S, Mellberg C, Stegle O, Lindahl B, Larsson C, Hauksson J, Olsson T. A palaeolithic‐type diet causes strong tissue‐specific effects on ectopic fat deposition in obese postmenopausal women. J Intern Med. 2013;274:67–76. [DOI] [PubMed] [Google Scholar]
- 26. Estruch R, Ros E, Salas‐Salvado J, Covas MI, Corella D, Aros F, Gomez‐Gracia E, Ruiz‐Gutierrez V, Fiol M, Lapetra J, Lamuela‐Raventos RM, Serra‐Majem L, Pinto X, Basora J, Munoz MA, Sorli JV, Martinez JA, Martinez‐Gonzalez MA; Investigators PS . Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–1290. [DOI] [PubMed] [Google Scholar]
- 27. Cassidy S, Hallsworth K, Thoma C, MacGowan GA, Hollingsworth KG, Day CP, Taylor R, Jakovljevic DG, Trenell MI. Cardiac structure and function are altered in type 2 diabetes and non‐alcoholic fatty liver disease and associate with glycemic control. Cardiovasc Diabetol. 2015;14:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fonseca CG, Dissanayake AM, Doughty RN, Whalley GA, Gamble GD, Cowan BR, Occleshaw CJ, Young AA. Three‐dimensional assessment of left ventricular systolic strain in patients with type 2 diabetes mellitus, diastolic dysfunction, and normal ejection fraction. Am J Cardiol. 2004;94:1391–1395. [DOI] [PubMed] [Google Scholar]
- 29. Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. [DOI] [PubMed] [Google Scholar]
- 30. Rijzewijk LJ, van der Meer RW, Lamb HJ, de Jong HW, Lubberink M, Romijn JA, Bax JJ, de Roos A, Twisk JW, Heine RJ, Lammertsma AA, Smit JW, Diamant M. Altered myocardial substrate metabolism and decreased diastolic function in nonischemic human diabetic cardiomyopathy: studies with cardiac positron emission tomography and magnetic resonance imaging. J Am Coll Cardiol. 2009;54:1524–1532. [DOI] [PubMed] [Google Scholar]
- 31. Keenan NG, Pennell DJ. CMR of ventricular function. Echocardiography. 2007;24:185–193. [DOI] [PubMed] [Google Scholar]
- 32. Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351–369; quiz 453‐355. [DOI] [PubMed] [Google Scholar]


