Abstract
Background
Elderly patients have high ischemic and bleeding rates after acute coronary syndrome; however, the occurrence of these complications over time has never been studied. This study sought to characterize average daily ischemic rates (ADIRs) and average daily bleeding rates (ADBRs) over 1 year in patients aged >74 years with acute coronary syndrome undergoing percutaneous coronary intervention who were randomized in the Elderly ACS 2 trial, comparing low‐dose prasugrel (5 mg daily) with clopidogrel (75 mg daily).
Methods and Results
ADIRs and ADBRs were calculated as the total number of events, including recurrent events, divided by the number of patient‐days of follow‐up and assessed within different clinical phases: acute (0–3 days), subacute (4–30 days), and late (31–365 days). Generalized estimating equations were used to test the least squares mean differences for the pairwise comparisons of ADIRs and ADBRs and the pairwise comparison of clopidogrel versus prasugrel effects. Globally, ADIRs were 2.6 times (95% CI, 2.4–2.9) higher than ADBRs. ADIRs were significantly higher in the clopidogrel arm than in the low‐dose prasugrel arm in the subacute phase (P adj<0.001) without a difference in ADBRs (P adj=0.35). In the late phase, ADIRs remained significantly higher with clopidogrel (P adj<0.001), whereas ADBRs were significantly higher with low‐dose prasugrel (P adj<0.001).
Conclusions
Ischemic burden was greater than bleeding burden in all clinical phases of 1‐year follow‐up of elderly patients with acute coronary syndrome treated with percutaneous coronary intervention. Low‐dose prasugrel reduced ischemic events in the subacute and chronic phases compared with clopidogrel, whereas bleeding burden was lower with clopidogrel in the late phase.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01777503.
Keywords: acute coronary syndrome, antiplatelet drug, elderly
Subject Categories: Aging, Catheter-Based Coronary and Valvular Interventions, Pharmacology, Stent
Short abstract
See Editorial by Kinnaird
Clinical Perspective
What Is New?
The analysis of recurrent events in elderly patients (aged >74 years) undergoing percutaneous coronary intervention for acute coronary syndrome shows that ischemic events are 2 times higher than bleeding events in all time periods up to 1 year of follow‐up.
Prasugrel and clopidogrel have variable impact on the balance of ischemic versus bleeding events during the time course of follow‐up.
Low‐dose prasugrel reduced ischemic events in the subacute and chronic phases compared with clopidogrel, whereas bleeding burden was increased in the late phase.
What Are the Clinical Implications?
This analysis may help clinicians individualize antithrombotic treatment of elderly patients who are invasively managed after acute coronary syndrome according to their baseline thrombotic and bleeding risk and to their time period after acute coronary syndrome.
Introduction
Although elderly patients with acute coronary syndromes (ACS) represent at least a third of the overall ACS population1, 2 and are increasingly treated with percutaneous coronary intervention (PCI),3, 4, 5 few trials are devoted to this growing group of patients. In the recently published Elderly ACS 2 trial, which compared the effects of low‐dose prasugrel (5 mg daily) with standard dose clopidogrel (75 mg daily) for 12 months in patients aged >74 years who had an ACS and were undergoing PCI, no significant difference was found between the 2 pharmacological treatment modalities regarding the primary composite end point of ischemic and bleeding events. However, bleeding rates tended to be lower with clopidogrel, whereas the rates of stent thrombosis, cardiovascular death, myocardial infarction (MI), and stroke were numerically lower with the use of low‐dose prasugrel.6 Although it is known that elderly patients have increased rates of both ischemic and bleeding events compared with younger patients,7, 8 the occurrence of these complications over time has never been studied. If peaks of ischemic and bleeding events occur at different times, a more potent antiplatelet regimen may be considered when ischemic events prevail, whereas a less aggressive treatment could be proposed when bleeding complications are more frequent than ischemic complications.9, 10 In this analysis, we aimed to characterize the total temporally related burden of ischemic and bleeding events in patients randomized in the 2 arms of the Elderly ACS 2 trial.
Methods
Study Design and Objectives
The study design of the Elderly ACS 2 trial has been described previously.5, 11 The authors declare that all supporting data are available within the article (and the online supplementary files). Briefly, the study was a randomized, open‐label, blinded‐end‐point trial carried out at 32 centers in Italy. We enrolled patients aged >74 years with either ST‐segment–elevation MI or non–ST‐segment–elevation ACS undergoing PCI during the index admission. Patients with non–ST‐segment–elevation ACS were eligible if they had one of the following additional characteristics: elevated troponin levels, diabetes mellitus, prior MI, ≥1 new ischemic episode while on standard treatment during the index hospitalization, or stent thrombosis. We excluded patients with prior stroke, gastrointestinal or genitourinary bleeding within the previous 6 weeks, hemoglobin level <10 g/dL on admission unless this was considered to be secondary to renal dysfunction or known myelodysplasia, platelet count <90 000 cells/mL, secondary causes of ischemia, ongoing oral anticoagulant treatment or a spontaneous international normalized ratio >1.5 at the time of screening, concomitant severe obstructive lung disease, malignancy, or neurological deficit limiting follow‐up or adherence to the study protocol. Institutional review board approval was obtained according to the guidelines of the Journal of the American Heart Association. The study was approved by an institutional review committee, and patients gave informed consent; patients who were unable to give at least verbal informed consent to the study or were already under treatment with prasugrel or ticagrelor were excluded. Participants were randomly assigned to either clopidogrel (300‐ to 600‐mg loading dose [at investigator discretion] followed by 75 mg once daily) or prasugrel (60‐mg loading dose followed by 5 mg once daily) with a 1:1 allocation. Treatment assignment was stratified by center and type of ACS (ST‐segment–elevation versus non–ST‐segment–elevation). Follow‐up visits were to take place at 30 days, 6 months, and 12 months after randomization.
All definitions of the primary‐end‐point components have been published previously.5, 10 Because the study compared 2 antiplatelet agents with different P2Y12 receptor‐blocking potency, the primary end point of the study was a “net clinical benefit” composite of all‐cause death, MI, disabling stroke, and rehospitalization for cardiovascular causes or bleeding. All events were adjudicated by an independent event adjudication committee (including 3 expert cardiologists and 1 neurologist) blinded to study group assignment.
The objectives of the present study were as follows: (1) to determine the average daily ischemic rate (ADIR) and average daily bleeding rate (ADBR) during the first year following the index event and within different clinical phases (acute, subacute, late) and (2) to determine how the randomized antiplatelet treatments (clopidogrel 75 mg once daily versus prasugrel 5 mg once daily) affected ADIR and ADBR in the various clinical phases.
End‐Point and Time‐Interval Definitions
Ischemic events included in the ADIR were cardiovascular death, MI, and stroke. Events included in the ADBR were bleeding events defined according to Bleeding Academic Research Consortium (BARC) types 2, 3, and 5, excluding types 1 and 4 (related to coronary artery bypass grafting).12 The average daily rates (ADRs) for ischemic and bleeding events were categorized according to the time of their occurrence at the following time intervals: acute phase (0–3 days), subacute phase (4–30 days), and late phase (31–365 days).
Statistical Analysis
Ischemic and bleeding events were examined in the intention‐to‐treat Elderly ACS 2 population according to the approach described by Giustino et al.13 By including recurrent events, instead of just first event, this approach will increase power to capture subtle differences in outcome; furthermore, it allows comparison of absolute differences in outcome rates to show the degree of benefit or harm of a given randomized treatment. All ischemic or bleeding events per patient were considered in the analysis, as opposed to the conventional approach of time to first‐event survival. Ischemic events leading to a bleeding event (or vice versa) were considered as 2 separate, discrete events. Recurrent ischemic or bleeding events, respectively, were considered to be related and counted as 1 discrete event if occurring within 24 hours or if otherwise specified in medical records. The ADR was defined as the total number of events (numerator) divided by the patient‐days at risk (denominator). Patient‐days at risk were defined as the number of patients multiplied by how many days each patient was at risk in that given period, including partial intervals of patients who died or were lost to follow‐up. A generalized estimating equation was fitted for analysis and to generate the least squares mean differences among the acute, subacute, and late time periods, with the patient‐days at risk as a repeated measure and assuming a Poisson distribution; type of event (ischemic versus bleeding), randomized treatment and clinical phase (acute, subacute, and late) were also included in the model as covariates. This analysis compared the ADRs per patient and was used to estimate the least squares mean difference and 95% CI for the pairwise comparisons of ischemic versus bleeding events within each specific interval and pairwise comparisons of clopidogrel versus prasugrel ADIR and ADBR within each specific interval. Pairwise post hoc comparison P values were adjusted with the Tukey correction. All statistical tests were 2‐sided. A P value <0.05 was considered statistically significant for hypothesis testing. Data were analyzed in R environment 3.4.3 (R Foundation for Statistical Computing) using the “geepack” and “lsmeans” packages.
Results
Baseline clinical characteristics of patients enrolled in the Elderly ACS 2 study are already published and shown in Table S1. Among the 1443 ACS patients enrolled in the Elderly ACS 2 trial, 109 discrete ischemic events (cardiovascular death, MI, and stroke) and 49 BARC types 2,3, and 5 bleeding events occurred within the first year following PCI (Tables 1 and 2); the rate of recurrent bleeding was 8.1%, whereas the rate of recurrent ischemic events was 5.5%. The proportion of ischemic and bleeding events occurring in the acute, subacute, and late time intervals, as per randomized treatment, are shown in Table 3. Of note, 49 of 109 (44.9%) ischemic events, including 33 of 57 (58.9%) cardiovascular deaths, occurred within 30 days, compared with 17 of 49 (34.7%) bleeding events.
Table 1.
Events Included in the ADIR Numerator
| Clopidogrel 75 mg once daily | Prasugrel 5 mg once daily | Events in ADIR | |
|---|---|---|---|
| Cardiovascular deaths | 31 | 25 | 56 |
| Nonfatal re‐MI | 17 | 16 | 33 |
| Fatal re‐MI | 3 | 4 | 7 |
| Non fatal stroke | 13 | 7 | 20 |
| Fatal stroke | 1 | 0 | 1 |
| Total event in ADIR | 61 | 48 | 109 |
ADIR indicates average daily ischemic rate; MI, myocardial infarction.
Table 2.
Events Included in ADBR Numerator
| Clopidogrel 75 mg once daily | Prasugrel 5 mg once daily | Events in ADBR | |
|---|---|---|---|
| All bleeding events | 21 | 29 | 50 |
| CABG related* | 1 | 0 | 1 |
| Fatal | 0 | 1 | 0 |
| Recurrent events† | 3 | 0 | 0 |
| Patients with both bleeding and ischemic events | 0 | 4 | 0 |
| Bleeding preceding unrelated ischemic event | 0 | 3 | 0 |
| Ischemic event preceding unrelated bleeding event | 0 | 1 | 0‡ |
| Total bleeding events in ADBR | 20 | 29 | 49‡ |
ADBR indicates average daily bleeding rate; CABG, coronary artery bypass grafting.
Excluded by ADBR.
Considered as 2 unrelated discrete events.
Table 3.
Discrete Events Within Each Clinical Phase Divided by Randomization Treatment
| Clopidogrel 75 mg once daily | Prasugrel 5 mg once daily | |
|---|---|---|
| Events, n | Events, n | |
| Acute phase (days 0–3) | ||
| Ischemic events | 9 | 8 |
| Cardiovascular death | 4 | 5 |
| MI | 2 | 2 |
| Stroke | 3 | 1 |
| Bleeding events | 3 | 4 |
| Subacute phase (days 4–30) | ||
| Ischemic events | 22 | 10 |
| Cardiovascular death | 16 | 8 |
| MI | 3 | 1 |
| Stroke | 3 | 1 |
| Bleeding events | 6 | 4 |
| Late phase (days 31–365) | ||
| Ischemic events | 29 | 30 |
| Cardiovascular death | 11 | 12 |
| MI | 12 | 13 |
| Stroke | 6 | 5 |
| Bleeding events | 11 | 21 |
MI indicates myocardial infarction.
Average Daily Ischemic and Bleeding Rates Within 1 Year
Both ADIR and ADBR peaked in the first day after PCI (0.97 and 0.47 events per 100 patients‐days at risk, respectively) and exponentially decreased thereafter. ADIRs and ADBRs occurring in each clinical phase are shown in Figure 1 for comparison; globally, ADIRs were, on average, 2.6 times (95% CI, 2.4–2.9) higher than ADBR and were significantly higher in each clinical phase. A complete set of pairwise comparisons is shown in Table S2.
Figure 1.
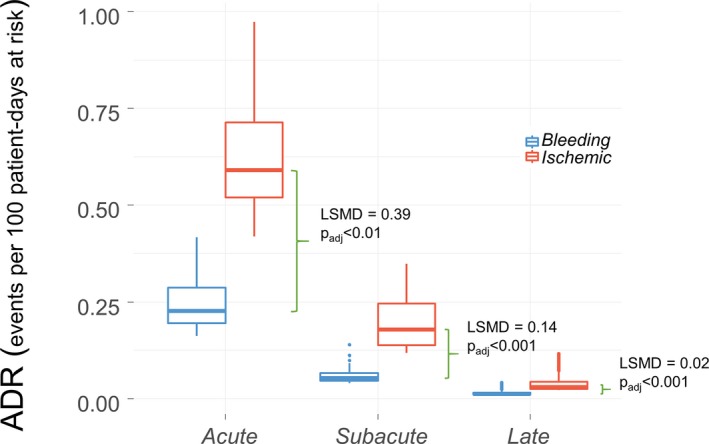
Bleeding vs ischemic average event rate in the Elderly 2 ACS study patients. The 2 box plots show the distribution of average daily rate (ADR) of ischemic (red) and bleeding (blue) events within each clinical phase. The ADR was defined as the total number of events (numerator) divided by the patient‐days at risk (denominator). Patient‐days at risk were defined as number of patients multiplied by how many days each patient was at risk in that given period including partial intervals of patients who died or were lost to follow‐up. Green brackets show the least squares mean difference (LSMD), that is, the absolute difference of ADR of ischemic events vs ADR of bleeding events in the time course of 1‐year follow‐up. Positive LSMD indicates higher ADR for ischemic events than ADR for bleeding.
Average Daily Ischemic, Bleeding, and Net Clinical Benefit Rates According to the Randomized Arms
Differences in ADR between patients receiving clopidogrel versus prasugrel after PCI are shown in Figures 2 and 3 and in Table 4. The ADIRs were higher in the clopidogrel arm, with a maximum absolute rate difference occurring in the subacute phase, whereas ADBR, almost overlapping between the study arms until the 30th day, became progressively higher in patients receiving prasugrel. In the acute phase, we found no difference in ADIR and ADBR between the study arms, whereas in the subacute phase, patients receiving clopidogrel had a significantly higher absolute difference of ADIR (least squares mean difference: 0.088; 95% CI, 0.069–0.106; P adj<0.001), and a nonsignificantly lower ADBR (least squares mean difference: 0.014; 95% CI, 0.021–0.007; P adj=0.35) than patients receiving low‐dose prasugrel.
Figure 2.
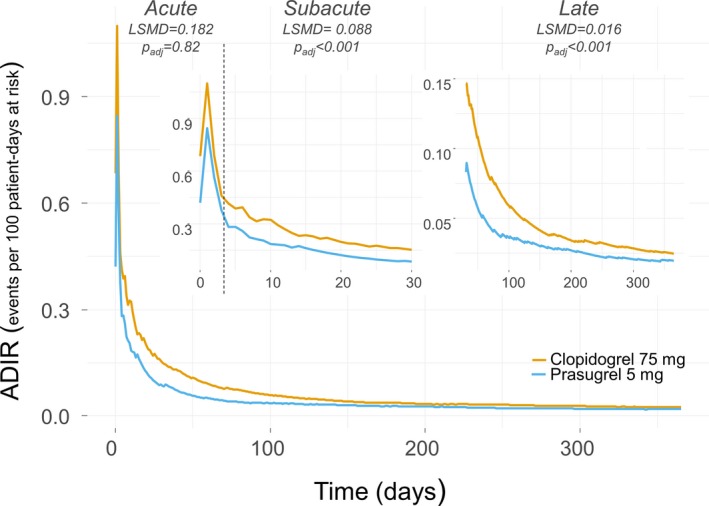
Average daily ischemic rates (ADIRs) for prasugrel 5 mg (light blue line) vs clopidogrel 75 mg (orange line) daily. The lines plot the ADIR as a continuous variable in the time course of follow‐up. The main panel shows the overall follow‐up time, the upper small panels show (with ad hoc magnification) the first 30 d (upper left) including the acute and the subacute phases, which are separated by a dotted vertical line, and the 31 to 365 d of follow‐up (upper right). The least squares mean difference (LSMD) indicates, within each clinical phase, the absolute difference of ADIRs between study arms; for example, positive LSMD indicates more events occurring in patients receiving clopidogrel 75 than in patients receiving prasugrel 5 mg. The higher the LSMD, the greater the magnitude of effect.
Figure 3.
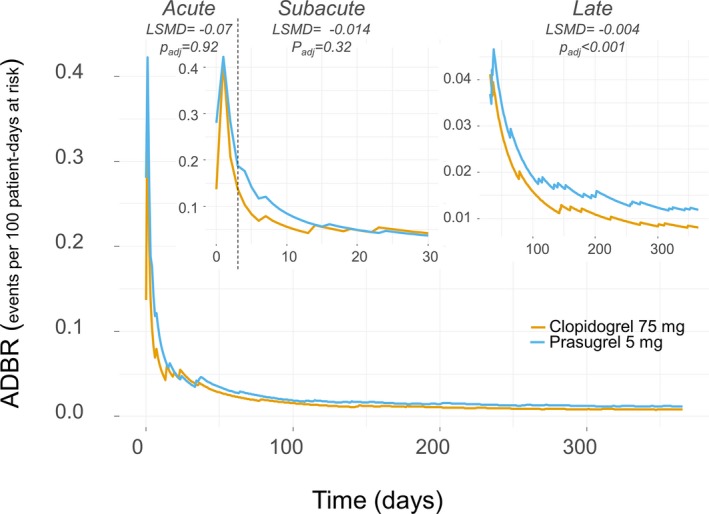
Average daily bleeding rate (ADBR) for prasugrel 5 mg (light blue line) vs clopidogrel 75 mg (orange line) daily. The lines plot the ADBR as a continuous variable in the time course of follow‐up. The main panel shows the overall follow‐up time; the upper small panels show (with ad hoc magnification) the first 30 d (upper left) including the acute and the subacute phases, which are separated by a dotted vertical line, and the 31 to 365 d of follow‐up (upper right). The least squares mean difference (LSMD) indicates, within each clinical phase, the absolute difference of ADBR between study arms; for example, negative LSMD indicates more events in patients receiving prasugrel 5 mg compared with patients receiving clopidogrel 75 mg.
Table 4.
LSMD of ADIR and ADBR Between Patients Randomized to Receive Clopidogrel 75 mg vs Prasugrel 5 mg Once Daily for 12 Months
| Absolute LSMD (Clopidogrel–Prasugrel) | Lower 95% CI | Upper 95% CI | Adjusted P Value | |
|---|---|---|---|---|
| ADIR | ||||
| Acute | 0.182 | 0.034 | 0.329 | 0.8215 |
| Subacute | 0.088 | 0.069 | 0.106 | <0.0001 |
| Late | 0.016 | 0.014 | 0.017 | <0.0001 |
| ADBR | ||||
| Acute | −0.070 | −0.140 | 0.000 | 0.9195 |
| Subacute | −0.014 | −0.021 | −0.007 | 0.3524 |
| Late | −0.004 | −0.005 | −0.004 | <0.0001 |
Positive values indicate higher ADRs for clopidogrel 75 mg daily compared with prasugrel 5 mg daily; negative values indicate higher ADRs for prasugrel compared with clopidogrel; the higher the LSMD in absolute value, the greater the magnitude of effect. ADBR indicates average daily bleeding rate; ADIR, average daily ischemic rate; ADR, average daily rate; LSMD, least squares mean difference.
*LSMD and SE are calculated by fitting a generalized estimated equation model, with patients as repeated measure and assuming Poisson distribution. The Tukey honest significant difference method was used to adjust P values for post hoc multiple comparisons.
In the late phase, the magnitude of absolute differences between the 2 treatments was smaller than in the subacute phase. Although ADBRs were significantly higher with low‐dose prasugrel than with clopidogrel, ADIRs remained significantly higher with clopidogrel than with low‐dose prasugrel (Table 4).
Discussion
The relevant data of this analysis are as follows: First, ischemic events were more than twice as frequent as bleeding events during the 12‐month follow‐up period, with this difference being more pronounced in the acute and subacute phases and decreasing exponentially thereafter. Second, daily ischemic and bleeding burdens peaked in the first 3 days after the ACS, remained high in the first month, and then gradually and steadily decreased throughout the follow‐up period. However, about a half of the ischemic events occurred within the first 30 days (with a peak of 61% for cardiovascular death), whereas only a third of the bleeding complications occurred during the first month, and two‐thirds occurred thereafter. Third, except for the first 3 days of treatment, ischemic events were significantly lower in patients assigned low‐dose prasugrel than in those assigned clopidogrel throughout the 1‐year follow‐up period, whereas bleeding events were significantly higher with prasugrel starting from the first month of treatment to the end of follow‐up.
In this analysis, we used the methodology proposed by Giustino et al13 to calculate ADRs of ischemic and bleeding burdens. The advantage of this analysis is that it considers all ischemic and bleeding events that occurred throughout the follow‐up period rather than censoring patients after occurrence of the first event, as in conventional time‐to‐event analyses (Kaplan–Meier methods and Cox proportional hazards models).14, 15 The evaluation of multiple events over time allows full appreciation of the overall disease burden and the effects of concomitant treatments.14 Our findings differ from those presented in the analysis of patients undergoing primary PCI in the HORIZONS‐AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial.16 That study showed that bleeding risk exceeded ischemic risk in the first 3 days after the acute event, whereas ischemic events outnumbered bleeding events from the first month to 1 year. The lower bleeding burden observed in our patients in the early phase could be ascribed to the lower use of glycoprotein IIb/IIIa inhibitors (globally 17% in the Elderly ACS 2 trial), whereas their use was part of the randomized protocol in the HORIZONS‐AMI trial.16 Moreover, radial access was used in three‐quarters of the patients enrolled in the Elderly ACS 2 trial compared with the prevailing femoral access, linked to a greater number of bleeding events, used in the HORIZONS‐AMI population. A low bleeding rate was also observed in the recent SENIOR trial,17 in which the radial approach was used in 80% of cases. Finally, elderly patients may have different time distribution of bleeding events compared with younger ACS patients.
Effects of Prasugrel and Clopidogrel on Ischemic and Bleeding Events
In the Elderly ACS 2 randomized trial, we found no difference in the composite primary end point (the aggregate of all‐cause death, MI, disabling stroke, and rehospitalization for cardiovascular causes or bleeding within 1 year) between patients assigned to low‐dose prasugrel and those assigned to clopidogrel. In the present analysis of the temporal‐related burden of adverse events, we found that treatment with low‐dose prasugrel significantly reduced the ischemic burden (including all cardiovascular deaths, MIs, and ischemic strokes) from the fourth day to the end of follow‐up compared with treatment with clopidogrel. In the original analysis, low‐dose prasugrel reduced a composite ischemic end point (cardiovascular death, MI, and stroke) by 21% compared with clopidogrel, a difference that did not reach statistical significance.18 The discrepancy observed between the original analysis and this one is due to the fact that ADR computation of ischemic burden considers all ischemic complications rather than the first occurring event. Moreover, the time‐related analysis may permit identification of periods of follow‐up in which one treatment could be found to be superior to another. ADBR was found to be higher with low‐dose prasugrel than with clopidogrel from the 31st day to the end of follow‐up, whereas during the same period, ischemic events were lower with low‐dose prasugrel than with clopidogrel. On the basis of these data, the clinician could personalize dual‐antiplatelet therapy after the first month, switching to a less potent antiplatelet regimen in patients with high bleeding risk.19, 20
In the TRITON–TIMI 38 (Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel—Thrombolysis in Myocardial Infarction 38) trial, comparing the 10‐mg maintenance dose of prasugrel versus clopidogrel in ACS patients,21 bleeding risk increased with age, particularly in patients aged ≥75 years, in whom fatal bleeding was significantly higher with prasugrel 10 mg. 22 In our study, fatal bleeding was an exceptional occurrence, with only 1 case observed among those randomized to low‐dose prasugrel. In the United States, use of prasugrel 10 mg in patients aged ≥75 years is generally not recommended except in high‐risk situations (patients with diabetes mellitus or history of MI). In Europe, this dosage is also generally not recommended in elderly patients23 but may be considered after careful individual benefit–risk evaluation. In such cases, a reduced 5‐mg maintenance dose should be prescribed, although it had never been tested in a randomized clinical trial of ACS patients treated invasively.24 Data from the TRILOGY‐ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) platelet function substudy of patients with non‐ST‐segment–elevation ACS treated conservatively showed that prasugrel 5 mg induced a significantly higher level of platelet inhibition compared with clopidogrel; however, this favorable pharmacodynamic profile did not result in clinical superiority of prasugrel versus clopidogrel in that clinical setting.25
Study Limitations
In the present study we assessed ischemic events by using a composite end point (including cardiovascular death, MI, and stroke) that was not the primary net‐benefit end point of the original analysis, which also included revascularizations for cardiovascular causes or bleeding. However, the composite of cardiovascular death, MI, and stroke was the primary efficacy end point in most trials comparing the effects on outcome of different antiplatelet agents.19, 26 The results of our analysis should be applied with caution and only to an elderly population with a globally low risk of bleeding events, such as that enrolled in the Elderly ACS 2 trial.6 The Elderly ACS 2 trial was stopped prematurely after an interim analysis showed a lower‐than‐expected rate of the primary outcome and a virtually null between‐group difference in the primary outcome. Nevertheless, we found that bleeding rates were significantly higher with low‐dose prasugrel than with clopidogrel after the first month of treatment. It is therefore possible that in patients with a high bleeding risk, as expressed by high values of the PRECISE‐DAPT27 or PARIS20, 28 scores, a de‐escalation strategy—starting with low‐dose prasugrel in the first month to counteract the early high ischemic risk, followed by a switch to clopidogrel thereafter—could result in better 1‐year clinical outcomes than using a regimen of low‐dose prasugrel for 12 months. Conversely, if the ischemic risk prevails over the bleeding risk, low‐dose prasugrel could be continued after the first month of treatment.
In conclusion, this analysis shows that the overall ischemic burden is greater than the bleeding burden in elderly patients with ACS treated with PCI, particularly in the first month after the acute event. Low‐dose prasugrel reduces the ischemic events in the subacute and late phases compared with clopidogrel, whereas the bleeding burden is lower using clopidogrel in the late treatment phase. The analysis of temporal‐related ischemic and bleeding events and thoughtful assessment of the ischemic and bleeding risk may permit tailoring the most appropriate treatment strategy in the individual patient with ACS.
Disclosures
Dr Savonitto reports research grants through his Institution from Novartis, Eli Lilly and Daiichi Sankyo; personal fees from AstraZeneca, Bayer, Bristol‐Myers Squibb, and Pfizer, outside the submitted work. Dr Piatti reports personal fee from Volcano Europe. Dr Grosseto reports personal fees from Pfizer, Sanofi and Boehringer‐Ingelheim, outside the submitted work. Dr L. De Luca reports personal fees from Amgen, Aspen, AstraZeneca, Bayer, Boehringer‐Ingelheim, Chiesi, Eli Lilly, Daiichi Sankyo, Pharmevo, Menarini and The Medicines Company, outside the submitted work. Professor De Servi reports personal fees from AstraZeneca, Bristol‐Myers Squibb, Eli Lilly, Daichi Sankyo, MSD, Pfizer. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Clinical Characteristics
Table S2. Pairwise Comparisons
(J Am Heart Assoc. 2019;8:e010956 DOI: 10.1161/JAHA.118.010956.)
References
- 1. De Luca L, Marini M, Gonzini L, Boccanelli A, Casella G, Chiarella F, De Servi S, Di Chiara A, Di Pasquale G, Olivari Z, Caretta G, Lenatti L, Gulizia MM, Savonitto S. Contemporary trends and age‐specific sex differences in management and outcome for patients with ST‐segment elevation myocardial infarction. J Am Heart Assoc. 2016;5:e004202 DOI: 10.1161/JAHA.116.004202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Puymirat E, Aissaoui N, Cayla G, Lafont A, Riant E, Mennuni M, Saint‐Jean O, Blanchard D, Jourdain P, Elbaz M, Henry P, Bataille V, Drouet E, Mulak G, Schiele F, Ferrières J, Simon T, Danchin N; FAST‐MI Investigators . Changes in one‐year mortality in elderly patients admitted with acute myocardial infarction in relation with early management. Am J Med. 2017;130:555–563. [DOI] [PubMed] [Google Scholar]
- 3. De Luca L, Leonardi S, Cavallini C, Lucci D, Musumeci G, Caporale R, Abrignani MG, Lupi A, Rakar S, Gulizia MM, Bovenzi FM, De Servi S; EYESHOT Investigators . Contemporary antithrombotic strategies in patients with acute coronary syndrome admitted to cardiac care units in Italy: the EYESHOT Study. Eur Heart J Acute Cardiovasc Care. 2015;4:441–452. [DOI] [PubMed] [Google Scholar]
- 4. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, Gjertsen E, Dahl‐Hofseth O, Ranhoff AH, Gullestad L, Bendz B; After Eighty Study Investigators . Invasive versus conservative strategy in patients aged 80 years or older with non‐ST‐elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open‐label randomised controlled trial. Lancet. 2016;387:1057–1065. [DOI] [PubMed] [Google Scholar]
- 5. Savonitto S, Cavallini C, Petronio AS, Murena E, Antonicelli R, Sacco A, Steffenino G, Bonechi F, Mossuti E, Manari A, Tolaro S, Toso A, Daniotti A, Piscione F, Morici N, Cesana BM, Jori MC, De Servi S; Italian Elderly ACS Trial Investigators . Early aggressive versus initially conservative treatment in elderly patients with non‐ST‐segment elevation acute coronary syndrome: a randomized controlled trial. JACC Cardiovasc Interv. 2012;5:906–916. [DOI] [PubMed] [Google Scholar]
- 6. Savonitto S, Ferri LA, Piatti L, Grosseto D, Piovaccari G, Morici N, Bossi I, Sganzerla P, Tortorella G, Cacucci M, Ferrario M, Murena E, Sibilio G, Tondi S, Toso A, Bongioanni S, Ravera A, Corrada E, Mariani M, Di Ascenzo L, Petronio AS, Cavallini C, Vitrella G, Rogacka R, Antonicelli R, Cesana BM, De Luca L, Ottani F, De Luca G, Piscione F, Moffa N, De Servi S; on behalf of the Elderly ACS 2 Investigators . A comparison of reduced‐dose prasugrel and standard‐dose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization. Circulation. 2018;137:2435–2445. [DOI] [PubMed] [Google Scholar]
- 7. Levine GN, Bates ER. It is time to end the dualistic short versus long duration of dual antiplatelet therapy debates. Circulation. 2017;135:2451–2453. [DOI] [PubMed] [Google Scholar]
- 8. De Rosa R, Palmerini T, De Servi S, Belmonte M, Crimi G, Cornara S, Calabrò P, Cattaneo M, Maffeo D, Toso A, Bartorelli A, Palmieri C, De Carlo M, Capodanno D, Genereux P, Angiolillo D, Piscione F, Galasso G. High on‐treatment platelet reactivity and outcome in elderly with non ST‐segment elevation acute coronary syndrome—insight from the GEPRESS study. Int J Cardiol. 2018;259:20–25. [DOI] [PubMed] [Google Scholar]
- 9. Sibbing D, Aradi D, Jacobshagen C, Gross L, Trenk D, Geisler T, Orban M, Hadamitzky M, Merkely B, Kiss RG, Komócsi A, Dézsi CA, Holdt L, Felix SB, Parma R, Klopotowski M, Schwinger RHG, Rieber J, Huber K, Neumann FJ, Koltowski L, Mehilli J, Huczek Z, Massberg S; TROPICAL‐ACS Investigators . Guided de‐escalation of antiplatelet treatment in patients with acute coronary syndrome undergoing percutaneous coronary intervention (TROPICAL‐ACS): a randomised, open‐label, multicentre trial. Lancet. 2017;390:1747–1757. [DOI] [PubMed] [Google Scholar]
- 10. Franchi F, Rollini F, Rivas Rios J, Rivas A, Agarwal M, Kureti M, Nagaraju D, Wali M, Shaikh Z, Briceno M, Nawaz A, Moon JY, Been L, Suryadevara S, Soffer D, Zenni MM, Bass TA, Angiolillo DJ. Pharmacodynamic effects of switching from ticagrelor to clopidogrel in patients with coronary artery disease: results of the SWAP (Switching Antiplatelet Therapy)‐4 study. Circulation. 2018;137:2450–2462. [DOI] [PubMed] [Google Scholar]
- 11. Ferri LA, Morici N, Grosseto D, Tortorella G, Bossi I, Sganzerla P, Cacucci M, Sibilio G, Tondi S, Toso A, Ferrario M, Gandolfo N, Ravera A, Mariani M, Corrada E, Di Ascenzo L, Petronio AS, Cavallini C, Moffa N, De Servi S, Savonitto S. A comparison of reduced‐dose prasugrel and standarddose clopidogrel in elderly patients with acute coronary syndromes undergoing early percutaneous revascularization: design and rationale of the randomized Elderly‐ACS 2 study. Am Heart J. 2016;181:101–106. [DOI] [PubMed] [Google Scholar]
- 12. Cutlip DE, Windecker S, Mehran R, Boam A, Cohen DJ, van Es GA, Steg PG, Morel MA, Mauri L, Vranckx P, McFadden E, Lansky A, Hamon M, Krucoff MW, Serruys PW; Academic Research Consortium . Clinical end points in coronary stent trials: a case for standardized definitions. Circulation. 2007;115:2344–2351. [DOI] [PubMed] [Google Scholar]
- 13. Giustino G, Mehran R, Dangas GD, Kirtane AJ, Redfors B, Généreux P, Brener SJ, Prats J, Pocock SJ, Deliargyris EN, Stone GW. Characterization of the average daily ischemic and bleeding risk after primary PCI for STEMI. J Am Coll Cardiol. 2017;70:1846–1857. [DOI] [PubMed] [Google Scholar]
- 14. Pocock SJ, Stone GW. The primary outcome fails—what next? N Engl J Med. 2016;375:861–870. [DOI] [PubMed] [Google Scholar]
- 15. Ferreira‐González I, Alonso‐Coello P, Solà I, Pacheco‐Huergo V, Domingo‐Salvany A, Alonso J, Montori V, Permanyer‐Miralda G. Composite endpoints in clinical trials. Rev Esp Cardiol. 2008;61:283–290. [PubMed] [Google Scholar]
- 16. Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R; HORIZONS‐AMI Trial Investigators . Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–2230. [DOI] [PubMed] [Google Scholar]
- 17. Varenne O, Cook S, Sideris G, Kedev S, Cuisset T, Carrié D, Hovasse T, Garot P, El Mahmoud R, Spaulding C, Helft G, Diaz Fernandez JF, Brugaletta S, Pinar‐Bermudez E, Mauri Ferre J, Commeau P, Teiger E, Bogaerts K, Sabate M, Morice MC, Sinnaeve PR; SENIOR Investigators . Drug‐eluting stents in elderly patients with coronary artery disease (SENIOR): a randomised single‐blind trial. Lancet. 2018;391:41–50. [DOI] [PubMed] [Google Scholar]
- 18. Bangalore S. Prasugrel in the elderly. Circulation. 2018;137:2446–2449. [DOI] [PubMed] [Google Scholar]
- 19. Deharo P, Quilici J, Camoin‐Jau L, Johnson TW, Bassez C, Bonnet G, Fernandez M, Ibrahim M, Suchon P, Verdier V, Fourcade L, Morange PE, Bonnet JL, Alessi MC, Cuisset T. Benefit of switching dual antiplatelet therapy after acute coronary syndrome according to on‐treatment platelet reactivity: the TOPIC‐VASP pre‐specified analysis of the TOPIC randomized study. JACC Cardiovasc Interv. 2017;10:2560–2570. [DOI] [PubMed] [Google Scholar]
- 20. Capodanno D, Greco A. Risk stratification for bleeding in the elderly wth acute coronary syndrome: not so simple. Thromb Haemost. 2018;118:949–952. [DOI] [PubMed] [Google Scholar]
- 21. Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM; TRITON‐TIMI 38 Investigators . Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. [DOI] [PubMed] [Google Scholar]
- 22. Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, Dalby AJ, Montalescot G, Braunwald E. Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel–Thrombolysis in Myocardial Infarction 38 (TRITON‐TIMI 38). Circulation. 2011;123:2681–2689. [DOI] [PubMed] [Google Scholar]
- 23. De Servi S, Goedicke J, Schirmer A, Widimsky P. Clinical outcomes for prasugrel versus clopidogrel in patients with unstable angina or non‐ST‐elevation myocardial infarction: an analysis from the TRITON‐TIMI 38 trial. Eur Heart J Acute Cardiovasc Care. 2014;3:363–372. [DOI] [PubMed] [Google Scholar]
- 24. Roe MT, Armstrong PW, Fox KA, White HD, Prabhakaran D, Goodman SG, Cornel JH, Bhatt DL, Clemmensen P, Martinez F, Ardissino D, Nicolau JC, Boden WE, Gurbel PA, Ruzyllo W, Dalby AJ, McGuire DK, Leiva‐Pons JL, Parkhomenko A, Gottlieb S, Topacio GO, Hamm C, Pavlides G, Goudev AR, Oto A, Tseng CD, Merkely B, Gasparovic V, Corbalan R, Cinteză M, McLendon RC, Winters KJ, Brown EB, Lokhnygina Y, Aylward PE, Huber K, Hochman JS, Ohman EM; TRILOGY ACS Investigators . Prasugrel versus clopidogrel for acute coronary syndromes without revascularization. N Engl J Med. 2012;367:1297–1309. [DOI] [PubMed] [Google Scholar]
- 25. Gurbel PA, Erlinge D, Ohman EM, Neely B, Neely M, Goodman SG, Huber K, Chan MY, Cornel JH, Brown E, Zhou C, Jakubowski JA, White HD, Fox KA, Prabhakaran D, Armstrong PW, Tantry US, Roe MT; TRILOGY ACS Platelet Function Substudy Investigators . Platelet function during extended prasugrel and clopidogrel therapy for patients with ACS treated without revascularization: the TRILOGY ACS platelet function substudy. JAMA. 2012;308:1785–1794. [DOI] [PubMed] [Google Scholar]
- 26. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Freij A, Thorsén M; PLATO Investigators . Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 27. Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, Steg PG, Zanchin T, Palmerini T, Wallentin L, Bhatt DL, Stone GW, Windecker S, Steyerberg EW, Valgimigli M; PRECISE‐DAPT Study Investigators . Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE‐DAPT) score: a pooled analysis of individual‐patient datasets from clinical trials. Lancet. 2017;389:1025–1034. [DOI] [PubMed] [Google Scholar]
- 28. Baber U, Mehran R, Giustino G, Cohen DJ, Henry TD, Sartori S, Ariti C, Litherland C, Dangas G, Gibson CM, Krucoff MW, Moliterno DJ, Kirtane AJ, Stone GW, Colombo A, Chieffo A, Kini AS, Witzenbichler B, Weisz G, Steg PG, Pocock S. Coronary thrombosis and major bleeding after PCI with drug‐eluting stents: risk scores from PARIS. J Am Coll Cardiol. 2016;67:2224–2234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Clinical Characteristics
Table S2. Pairwise Comparisons


