Abstract
Background
Myocarditis is an important cause of acute and chronic heart failure. Men with myocarditis have worse recovery and an increased need for transplantation compared with women, but the reason for the sex difference remains unclear. Elevated sera soluble (s)ST2 predicts mortality from acute and chronic heart failure, but has not been studied in myocarditis patients.
Methods and Results
Adults with a diagnosis of clinically suspected myocarditis (n=303, 78% male) were identified according to the 2013 European Society of Cardiology position statement. Sera sST2 levels were examined by ELISA in humans and mice and correlated with heart function according to sex and age. Sera sST2 levels were higher in healthy men (P=8×10−6) and men with myocarditis (P=0.004) compared with women. sST2 levels were elevated in patients with myocarditis and New York Heart Association class III‐IV heart failure (P=0.002), predominantly in men (P=0.0003). Sera sST2 levels were associated with New York Heart Association class in men with myocarditis who were ≤50 years old (r=0.231, P=0.0006), but not in women (r=0.172, P=0.57). Sera sST2 levels were also significantly higher in male mice with myocarditis (P=0.005) where levels were associated with cardiac inflammation. Gonadectomy with hormone replacement showed that testosterone (P<0.001), but not estradiol (P=0.32), increased sera sST2 levels in male mice with myocarditis.
Conclusions
We show in a well‐characterized subset of heart failure patients with clinically suspected and biopsy‐confirmed myocarditis that elevated sera sST2 is associated with an increased risk of heart failure based on New York Heart Association class in men ≤50 years old.
Keywords: biomarkers, heart failure, myocarditis, sex differences, sST2
Subject Categories: Heart Failure, Inflammatory Heart Disease, Translational Studies, Biomarkers, Animal Models of Human Disease
Clinical Perspective
What Is New?
Men with myocarditis have worse recovery than women and we show that sera soluble ST2 is increased in men with myocarditis and this increase is associated with worse heart failure based on New York Heart Association class in men ≤50 years of age, but not in women.
What Are the Clinical Implications?
Sera soluble ST2 has been found to be clinically useful as a sera biomarker to predict heart failure, and our findings indicate that sera soluble ST2 levels could be added to current biomarkers used to diagnose patients with myocarditis but should be interpreted according to sex and age.
Introduction
In spite of advances in diagnosis and treatment, heart failure remains a growing medical problem associated with hospitalization and high mortality.1, 2 Myocarditis is an important cause of acute death and chronic heart failure with an estimated worldwide prevalence of 0.5% to 4.0%.3, 4 Biopsy‐proven myocarditis has been reported to occur in up to 16% of adult patients with unexplained nonischemic cardiomyopathy.5 Men have worse recovery from acute dilated cardiomyopathy (DCM) following suspected myocarditis with an increased need for transplantation compared with women,6 but the reason for the sex difference remains unknown. We have previously shown in an autoimmune model of coxsackievirus B3 (CVB3)‐induced myocarditis that male mice develop more severe inflammation compared with females and progress to DCM.7, 8
Historically, sera soluble interleukin‐1 receptor‐like 1 (IL1R1 also known as ST2) has not been considered to be a diagnostic marker for heart failure, yet elevated levels predict acute and chronic heart failure mortality regardless of cause.9 When combined with N‐terminal pro‐brain natriuretic peptide, sera sST2 levels provide additive value in predicting sudden death in patients with heart failure and left ventricular (LV) systolic dysfunction with a LV ejection fraction (LVEF) ≤45%.10 Recently, sST2 was found to independently predict all‐cause mortality and heart failure hospitalization in patients with chronic heart failure.11 In patients with New York Heart Association (NYHA) class III or IV heart failure, change in sera sST2 levels over time predicts subsequent mortality and the need for transplantation.12 Elevated sera sST2 values have also been significantly correlated with LVEF and NYHA class.12, 13 The ST2 gene encodes 4 isoforms of ST2 by alternative splicing: ST2L, a transmembrane receptor for interleukin (IL)‐33; sST2, a secreted soluble form of ST2; ST2V, a variant of ST2 found in the gut; and ST2LV, which is secreted by the eye, heart, lung, and liver.14, 15 sST2 is a cytokine induced by biomechanical strain in cardiac fibroblasts, cardiomyocytes, and vascular endothelial cells that binds IL‐33, thereby inhibiting its effect.9, 16
The IL‐33/ST2 signaling pathway is regulated by Toll‐like receptor 4 (TLR4), caspase‐1, and IL‐1β in CD11b+ immune cells.15, 17, 18 We previously showed that testosterone increases TLR4 and IL‐1β expression on CD11b+GR1+F4/80+ macrophages and CD11b+CD117+ mast cells in male BALB/c mice with myocarditis.7, 8, 19 We also demonstrated that men with myocarditis have higher CD11b expression in the heart compared with women.7, 8, 20 Although serum sST2 is known to predict progression to acute and chronic heart failure, there are currently no studies describing whether it has a role in predicting heart failure in patients with myocarditis.
Methods
Transparency and Openness
The authors will make the data and analytic methods used to conduct the research for clinical and animal studies available to any researcher for purposes of reproducing the results or replicating the procedure. However, the authors will not make the materials available to other researchers.
Patients With Myocarditis
Adult patients with myocarditis (n=303) were identified from existing registries/biobanks in the United States, Germany, Korea, and the Czech Republic. Patient demographics are described in Table. Criteria for inclusion in the study included a diagnosis of clinically suspected myocarditis according to the 2013 European Society of Cardiology position statement and heart failure symptoms of <6 months duration.21 Sera were collected at each site during the patient's first visit (baseline). Cardiac function was assessed for all subjects at each site using echocardiography and/or cardiac magnetic resonance imaging. Heart failure symptoms were categorized by NYHA classification. Sera samples were stored at −80°C locally and sST2 was analyzed by ELISA at each site. BNP/N‐terminal pro‐brain natriuretic peptide levels were not available for most (≈90%) patients in the study. Testosterone, 17β‐estradiol, and menopause status were not available for any of the patients. Sera from control subjects who did not have cardiovascular disease were analyzed for sST2 levels at the University of Oklahoma Health Sciences Center. Approval was obtained from the local Institutional Review Board (IRB) or ethics board at each institution for studies involving patients including: Johns Hopkins Bloomberg School of Public Health (IRB No: 00003212), Mayo Clinic (IRB No: 08‐002108, 12‐003533, 14‐008222, 17‐004148), University of Oklahoma Health Sciences Center (IRB No: 2712), St. Anne's University Hospital Brno (Reference No: 19V/2014), and Charite University of Medicine in Berlin (EA2/140/16). Patients from Marburg were included within Subproject 9 of the German Competence Network Heart Failure, which conformed to the principles outlined in the Declaration of Helsinki and was approved by the German Heart Failure Network and the local ethics committee. Informed and written consent was obtained from all patients.
Table 1.
Demographics of Patients With Myocarditis According to Sex (n=303, 78% Male)
| Total | Sexes Combined | Men (M) | Women (W) | P Value (M vs W) | |
|---|---|---|---|---|---|
| Age (y) | (n=303) Mean 40, Range 18–81 | 40±15 | 38±14 | 46±16 | 0.0003 |
| NYHA class | (n=268) | 2.3±0.8 | 2.2±0.8 | 2.4±0.7 | 0.16 |
| LVEF (%) | (n=280) | 48.4±20.3 | 48.6±20.2 | 47.8±20.9 | 0.79 |
| NYHA | Class I | Class II | Class III | Class IV |
|---|---|---|---|---|
| (n=268) | (n=42) 16% | (n=133) 49% | (n=77) 29% | (n=16) 6% |
| LVEF | LVEF >45% | LVEF ≤45% |
|---|---|---|
| (n=280) | (n=162) 58% | (n=118) 42% |
LVEF indicates left ventricular ejection fraction; M, men; NYHA, New York Heart Association; W, women.
Age 50 years was chosen for analyzing the effect of age in women because we did not have information on menopause status and this age is often used as a surrogate when menopause status is not known.22, 23, 24 We examined data according to age in men because we analyzed data in that manner for women. Additionally, age <50 years has been used in the literature to define young patients in the context of cardiovascular disease.25, 26
Mouse Model of Myocarditis
Male and female BALB/c (6–8 week old) mice were obtained from the Jackson Laboratory (Bar Harbor, ME), fed standard chow, and housed with corncob bedding in animal rooms where temperature was monitored. Mice were maintained under pathogen‐free conditions in the animal facility at the Johns Hopkins School of Medicine. Approval was obtained from the Animal Care and Use Committee of Johns Hopkins University and Mayo Clinic for all procedures. Mice were inoculated intraperitoneally with 103 plaque‐forming units of a heart‐passaged stock of CVB3 diluted in PBS or PBS alone (controls) on day 0 and myocarditis examined at day 10 postinfection.27 Knowledge of which mice were infected with CVB3 versus PBS controls was necessary so that samples could be handled safely by the researcher because CVB3 is a BSL2 pathogen. Individual experiments were conducted 2 to 4 times with 8 to 10 mice per group. Mice were randomly assigned to groups using simple randomization. Myocarditis was assessed histologically as the percentage of the heart section with inflammation compared with the overall size of the heart section. Analysis of samples was blinded to the researcher using a code. No deaths of mice occurred in any of the experiments reported in this article prior to harvest.
Gonadectomy and Ovariectomy
Male or female BALB/c mice were either bilaterally gonadectomized (Gdx), ovariectomized (Ovx), or received a sham operation (Sham) under a ketamine (50 mg/kg) (Westward, Eatontown, NJ)/xylazine (5 mg/kg) (Bayer Health Care, Shawnee Mission, KS) anesthesia and either testosterone (Te) (testosterone C‐III; Sigma, St. Louis, MO), estradiol (E2) (β‐estradiol, Sigma, St. Louis, MO) or control capsules (empty) implanted subcutaneously, as previously described.8, 28 The analgesic meloxicam (4 mg/kg) (ZooPharm, Laramie, WY) was administered subcutaneously 72 hours before surgery and every 72 hours postoperation, as needed. Sera levels of testosterone were 12 to 15 ng/mL per 5‐mm capsule. The estradiol pellet maintained physiological circulating levels of estrogen at concentrations of 100 to 200 mL per 5‐mm capsule (data not shown). Successful operations were assessed using serum testosterone concentrations by ELISA or uterine horn weights.29 Mice were allowed 2 weeks to recover from the operation before myocarditis was induced. Mice received CVB3 on day 0 and hearts and sera were obtained at day 10 postinfection during acute myocarditis.
Mouse Model of Cardiac Function
Cardiac function was assessed in mice using a pressure‐volume catheter (1.2F Scisense Inc, London, Ontario) placed in the left ventricle via the apex in open‐chest mice anesthetized with 3% isoflurane, as previously described.30, 31
Histology
Mouse hearts were cut longitudinally, fixed in 10% phosphate‐buffered formalin, and embedded in paraffin for histological analysis. Five‐micron‐thick sections were stained with hematoxylin and eosin to detect myocardial inflammation. Myocarditis was assessed as the percentage of the heart section with inflammation compared with the overall size of the heart section using an eyepiece grid at low power (×25), as described previously.8, 31
ELISAs
The US Food and Drug Administration–approved Critical Diagnostics human sST2 ELISA kit was not available when the study began, so all clinical sites used the human R&D Systems sST2 ELISA kit (Minneapolis, MN) and the same dilution series for consistency. For a subset of myocarditis samples (n=21) the human Critical Diagnostics Presage sST2 kit (San Diego, CA) was compared with the R&D Systems kit. Samples were run comparing the 2 kits on the same day from the same dilution series. Mouse hearts were homogenized at 10% w/v in 2% minimal essential medium for use in sST2 or testosterone ELISAs (R&D Systems, Minneapolis, MN), as previously described.8, 31
IL‐1β Treatment
Male BALB/c mice received 0.1 mL PBS or recombinant mouse rIL‐1β/IL‐1F2 (R&D Systems, Cat#401‐ML, 7.8 ng/0.1 mL diluted in PBS) intraperitoneally every other day from day 1 to 9 postinfection following infection with CVB3 on day 0. Sera were collected on day 10 postinfection for analysis of sST2 levels.
Statistical Analysis
Clinical data are expressed as jitter scatter plots, so that each patient's sST2 value can be visualized, and bar graphs depict mean±SEM. Clinical data were adjusted for multiple sites using linear regression analysis. Two‐group analysis of normally distributed data were performed using Student t test. The Mann–Whitney rank test was used to evaluate nonparametric data comparing 2 groups. Multiple comparisons were analyzed by 1‐ or 2‐way ANOVA. Post hoc pairwise comparisons were calculated using Sidak's or Tukey's multiple comparisons test. Associations were analyzed using Pearson's correlation and adjusted for multiple sites using linear regression analysis for clinical data. A value of P<0.05 was considered significant. Power calculations for sex differences in sST2 levels in patients with myocarditis were limited by the number of retrospective samples that were available in biorepositories. Power calculations for sex differences in sST2 levels in mice with myocarditis are not provided because no studies of sST2 levels in mouse models of myocarditis exist to base a power calculation on.
Results
Sera sST2 Is Elevated in Healthy Men and Men With Myocarditis Compared With Women
Patient demographics are described in Table. We found that sera sST2 levels were significantly increased in healthy men compared with healthy women (data were obtained from 1 site) (7.9±0.9 versus 12.8±0.9, P=7.6×10−6) (Figure 1A). Among patients with clinically suspected myocarditis and symptom duration <6 months, men had significantly higher sera levels of sST2 than women (n=303, 14.1±1.5 versus 20.2±1.0, P=0.004) (Figure 1B). In a subset of patients with biopsy‐confirmed myocarditis and symptom duration <6 months, sera sST2 levels were significantly higher in men than women (n=265, 13.3±1.7 versus 19.6±1.0, P=0.006) (Figure 2). There was no significant difference in sST2 levels when clinically suspected myocarditis (Figure 1B) was compared with biopsy‐confirmed myocarditis (Figure 2) in women (P=0.17) or men (P=0.12).
Figure 1.
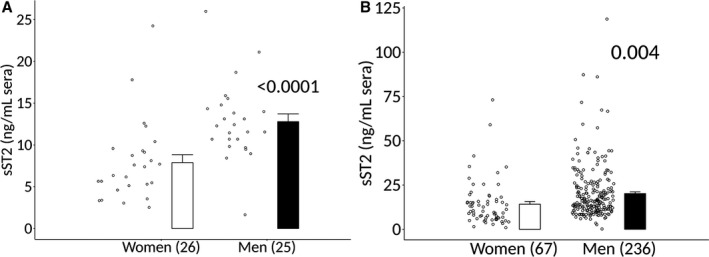
Sera sST2 elevated in healthy men and men with clinically suspected myocarditis compared with women. Soluble ST2 (sST2) in sera of (A) healthy men and women (n) with no known cardiovascular diseases (1 site). Data are shown as jitter scatter plots and mean±SEM and P values were calculated using a 2‐way Mann–Whitney rank test. B, Men and women with clinically suspected myocarditis with symptom duration <6 mo (multiple sites) were determined by ELISA. Data are shown as jitter scatter plots, and mean±SEM and P values were calculated after adjusting for multiple sites using linear regression analysis.
Figure 2.
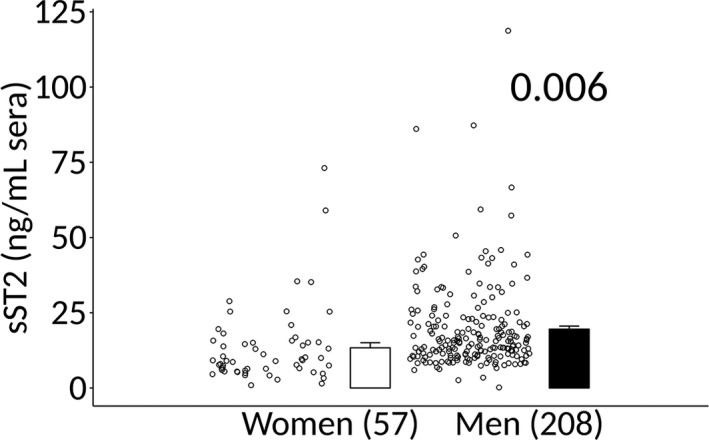
Sera sST2 elevated in men with biopsy‐confirmed myocarditis. Soluble ST2 (sST2) in sera of men and women with biopsy‐confirmed myocarditis were determined by ELISA. Data are shown as jitter scatter plots, and mean±SEM and P values were calculated after adjusting for multiple sites using linear regression analysis.
Analyses of sST2 were performed with R&D Systems kits because the US Food and Drug Administration–approved Critical Diagnostic kit was not available when the study was initiated. For a subset of samples obtained from 1 site we examined sST2 levels using both kits. We found a high correlation between sera sST2 levels when the values were compared between the kits (n=21, r=0.7422, P=0.0001) (Figure 3A). The average sST2 value for the R&D Systems kit was 19.45 ng/mL while the Critical Diagnostics kit was 48.39 ng/mL for the same samples (Figure 3B). This is a factor of 2.488. Thus, based on this factor, women with clinically suspected myocarditis in our study (Figure 1B) are estimated to have a value around 35.2 ng/mL and men a value around 50.1 ng/mL if the Critical Diagnostics kit had been used.
Figure 3.
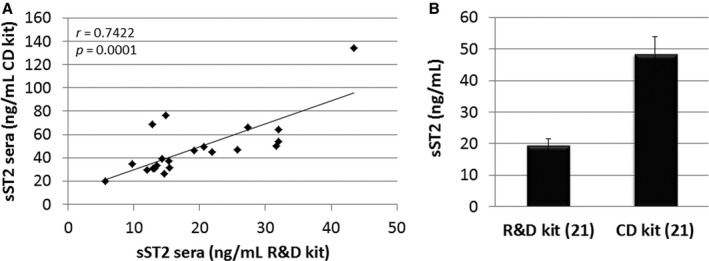
Comparison of R&D Systems and Critical Diagnostics sST2 ELISA kits. A, Sera samples from a subset of patients with myocarditis from 1 site were analyzed for soluble ST2 (sST2) using the human R&D Systems (R&D) kit vs the Critical Diagnostics (CD) ELISA kit. Associations were calculated using 2‐tailed Pearson's correlation. B, Average values obtained for the R&D vs the CD kit for the same 21 (n) samples. Data are shown as mean±SEM.
Sera sST2 Is Elevated in Men With Myocarditis ≤50 Years Old
In this study, the male‐to‐female ratio was 3.5:1 among patients with myocarditis. Men comprised 236 of the 303 patients in the study (78%) (Table), and 193 (64%) of the men were ≤50 years old (Figure 4). In contrast, only 67 (22%) of the 303 patients were women and of these 8 (12%) were ≤50 years old (Figure 4). In patients who were ≤50 years old, sera sST2 levels were significantly higher in men compared with women (12.2±2.1 versus 20.0±1.0, P=0.004) (Figure 5). In contrast, there was no statistically significant difference in sST2 levels between men and women >50 years old (16.4±2.2 versus 20.7±2.2, P=0.21) (Figure 5).
Figure 4.
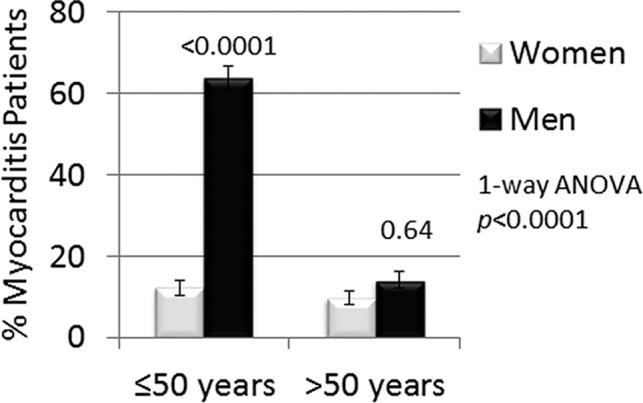
Percentage of men and women by age in the study. Percent of women and men with clinically suspected myocarditis that were ≤50 or >50 years of age. Data are shown as mean±SEM (n) and P values calculated using 1‐way ANOVA with Sidak's multiple comparisons test.
Figure 5.
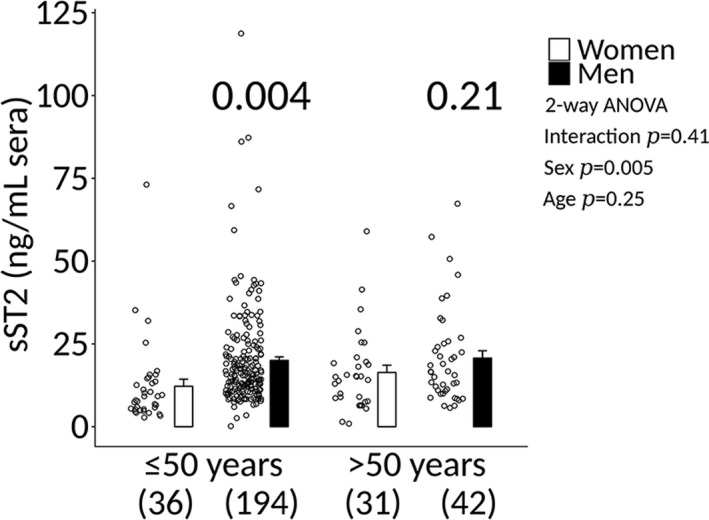
sST2 elevated in men ≤50 years old with clinically suspected myocarditis. Sera soluble ST2 (sST2) levels in clinically suspected patients with myocarditis according to sex and age. Data are shown as jitter scatter plots, and mean±SEM (n) and P values were calculated after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between sex and age (P=0.41), sex and sST2 (P=0.005), and age and sST2 (P=0.25).
Sera sST2 Is Not Significantly Altered in Myocarditis Patients With LVEF ≤45%
We found that sST2 levels in patients with clinically suspected myocarditis were not significantly associated with LVEF ≤45% when men and women were combined (17.8±1.1 versus 20.3±1.4, P=0.15) (Figure 6A). sST2 levels were also not higher in men with clinically suspected myocarditis with a LVEF ≤45% compared with women (16.3±2.4 versus 21.6±1.7, P=0.14) (Figure 6B). sST2 levels were higher in men with clinically suspected myocarditis with a LVEF >45% compared with women (13.0±2.3 versus 18.9±1.2, P=0.03) (Figure 6B), similar to men without heart disease (Figure 1A). sST2 levels also did not vary significantly according to LVEF ≤45% in patients with biopsy‐confirmed myocarditis (Figure 7). Additionally, sST2 levels did not differ significantly by LVEF ≤45% when data were analyzed according to sex and age (Figure 8).
Figure 6.
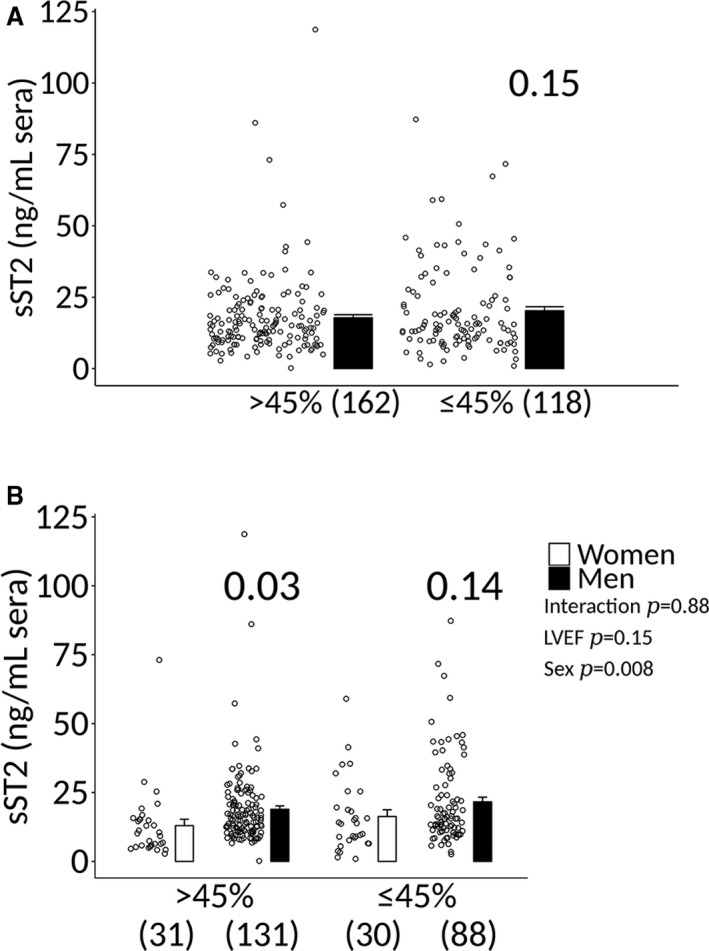
Sera sST2 levels in men and women with clinically suspected myocarditis based on LVEF. A, Soluble ST2 (sST2) levels in patients with clinically suspected myocarditis (men and women combined) based on a left ventricular ejection fraction (LVEF) > or ≤45%. Data are shown as jitter scatter plots, and mean±SEM and P value calculated after adjusting for multiple sites using linear regression analysis. B, Sera sST2 levels in patients with myocarditis according to sex and LVEF. Data are shown as jitter scatter plots mean±SEM (n), and P values were calculated after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between LVEF and sex (P=0.88), LVEF and sST2 (P=0.15), and sex and sST2 (P=0.008).
Figure 7.
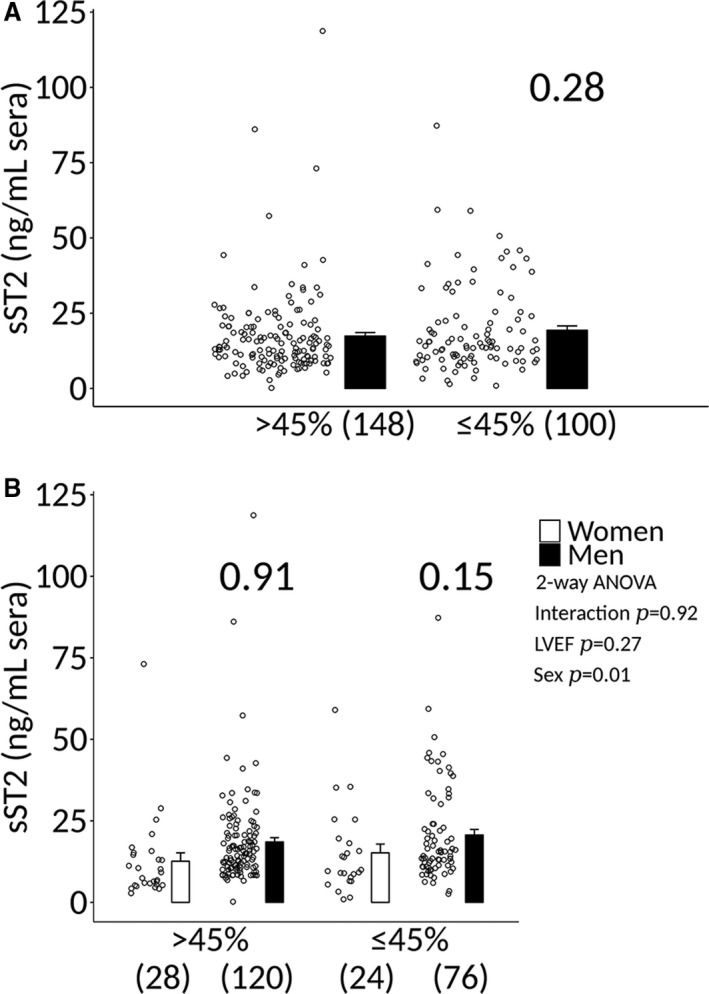
Sera sST2 levels in men and women with biopsy‐proven myocarditis based on LVEF. A, Soluble ST2 (sST2) levels in patients with biopsy‐proven myocarditis (men and women combined) based on a left ventricular ejection fraction (LVEF) > or ≤45%. Data are shown as jitter scatter plots, and mean±SEM and P value calculated after adjusting for multiple sites using linear regression analysis. B, Sera sST2 levels in patients with myocarditis according to sex and LVEF. Data are shown as jitter scatter plots and mean±SEM (n) and P values were calculated after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between LVEF and sex (P=0.92), LVEF and sST2 (P=0.27), and sex and sST2 (P=0.01).
Figure 8.
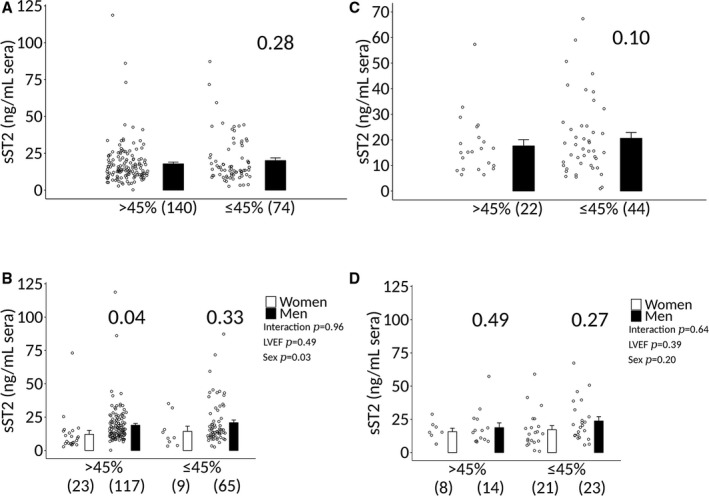
Analysis of sST2 levels according to LVEF, sex, and age in clinically suspected myocarditis. Soluble ST2 (sST2) levels in patients with clinically suspected myocarditis (n) that are (A and B) ≤50 vs (C and D) >50 y old. A and C, Analysis of sST2 levels according to left ventricle ejection fraction (LVEF) > or ≤45% when data from men and women were combined. Data are shown as jitter scatter plots and mean±SEM (n) and P value calculated after adjusting for multiple sites using linear regression analysis. B and D, Analysis of sST2 levels according to sex and LVEF. Data are shown as jitter scatter plots, and mean±SEM and P values calculated after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between (B) ≤50: LVEF and sex (P=0.96), LVEF and sST2 (P=0.49), and sex and sST2 (P=0.03) and (D) >50: LVEF and sex (P=0.64), LVEF and sST2 (P=0.39), and sex and sST2 (P=0.20).
Sera sST2 Is Significantly Higher in Men With Myocarditis and Correlates With NYHA Class III–IV Heart Failure
Sera sST2 levels were significantly higher in patients with clinically suspected myocarditis with NYHA class III–IV heart failure compared with NYHA class I–II (17.3±0.9 versus 21.8±1.9, P=0.005) (Figure 9A), and in men with NYHA class III–IV heart failure (15.2±2.7 versus 24.3±2.4, P=0.04) compared with women (Figure 9B). Sera sST2 levels were also significantly higher in patients with biopsy‐proven myocarditis with NYHA class III–IV heart failure compared with NYHA class I–II (17.3±0.9 versus 21.8±1.9, P=0.002) (Figure 10A). The increase in sST2 in men compared with women with NYHA class III–IV heart failure was also observed in patients with biopsy‐proven myocarditis (13.9±1.0 versus 24.4±2.5, P=0.03) (Figure 10B). sST2 levels correlated to NYHA class in men and women with clinically suspected or biopsy‐proven myocarditis (P<0.0001) (Figures 9C and 10C); however, the associations were not significant in women (P=0.10 and 0.08, respectively) (Figures 9D and 10D) but significant in men (P<0.0003 and <0.0001, respectively) (Figures 9E and 10E).
Figure 9.
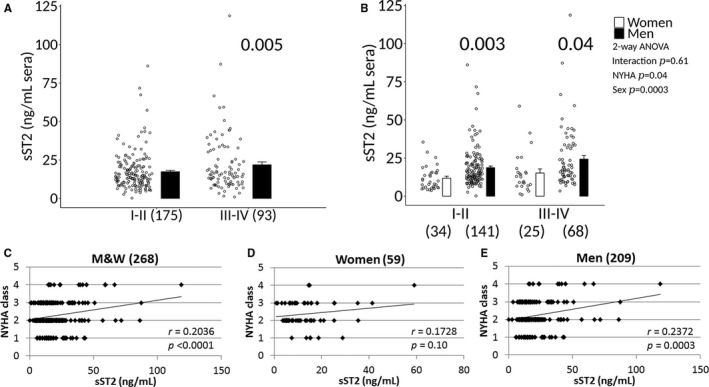
Sera sST2 associated with NYHA class III‐IV heart failure in patients with clinically suspected myocarditis. A, Soluble ST2 (sST2) levels in men and women (M&W) combined with clinically suspected myocarditis based on New York Heart Association (NYHA) class. Data are shown as jitter scatter plots, and mean±SEM (n) and P value calculated after adjusting for multiple sites using linear regression analysis. B, Sera sST2 levels based on NYHA class according to sex. Data are shown as jitter scatter plots and mean±SEM and P values determined after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between NYHA and sex (P=0.61), NYHA and sST2 (P=0.04) and sex and sST2 (P=0.0003). Comparisons between NYHA class I‐IV in (C) M&W combined, (D) women, or (E) men. C through E, P values calculated using 2‐tailed Pearson correlation and adjusted for multiple sites using linear regression analysis.
Figure 10.
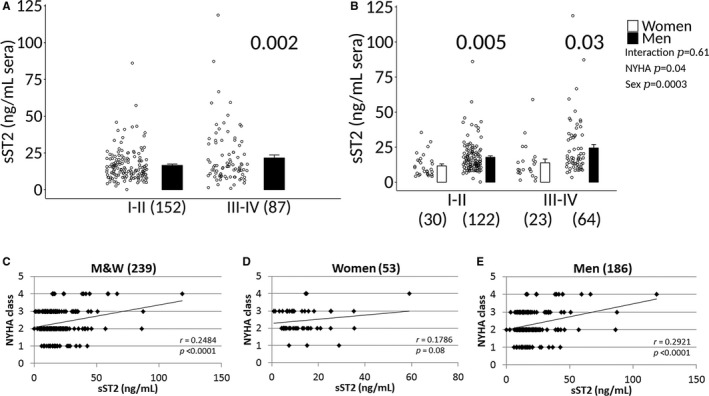
Sera sST2 associated with NYHA class III‐IV heart failure in men with biopsy‐confirmed myocarditis. A, Soluble ST2 (sST2) levels in men and women (M&W) combined with biopsy‐confirmed myocarditis based on New York Heart Association (NYHA) class. Data are shown as jitter scatter plots, and mean±SEM (n) and P value determined after adjusting for multiple sites using linear regression analysis. B, Sera sST2 levels based on NYHA class according to sex. Data are shown as jitter scatter plots, and mean±SEM and P values calculated after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between NYHA and sex (P=0.61), NYHA and sST2 (P=0.04), and sex and sST2 (P=0.0003). Comparisons between NYHA class I‐IV in (C) M&W combined, (D) women, or (E) men. C through E, P values were calculated using 2‐tailed Pearson correlation and adjusted for multiple sites using linear regression analysis.
Sera sST2 Correlates With NYHA Class III–IV Heart Failure in Men ≤50 Years Old With Myocarditis
Sera sST2 levels were significantly higher in myocarditis patients with NYHA class III–IV heart failure who were ≤50 years old (16.9±1.0 versus 23.3±2.5, P=0.002), but not in patients older than age 50 years old (19.0±2.4 versus 18.7±2.5, P=0.69) (Figure 11A and 11C). sST2 levels correlated to NYHA class in myocarditis patients who were ≤50 years old (P=0.0006) (Figure 12A). However, there was no significant association in women ≤50 years (P=0.57) (Figure 12B). In contrast, there was a significant association between sST2 levels and NYHA class in men who were ≤50 years of age (P=0.0004) (Figure 12C). sST2 levels were not significantly associated with NYHA class in men or women with myocarditis who were older than age 50 years (Figure 12D through 12F).
Figure 11.
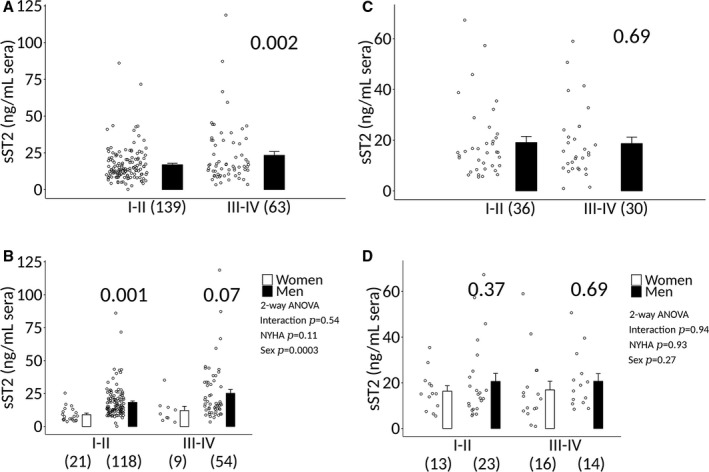
Sera sST2 according to NYHA class, sex, and age in patients with clinically suspected myocarditis. Soluble ST2 (sST2) levels in patients with clinically suspected myocarditis (n) that are (A and B) ≤50 y old vs (C and D) >50 y old. A and C, Analysis of sST2 levels according to New York Heart Association (NYHA) class when data from men and women are combined. Data are shown as jitter scatter plots and mean±SEM (n) and P value determined after adjusting for multiple sites using linear regression analysis. B and D, Analysis of sST2 levels according to sex, age, and NYHA class. Data are shown as jitter scatter plots and mean±SEM and P values calculated after adjusting for multiple sites using linear regression analysis. Two‐way ANOVA P values indicate interaction between (B) ≤50: NYHA and sex (P=0.54), NYHA and sST2 (P=0.11), and sex and sST2 (P=0.0003) and (D) >50: NYHA and sex (P=0.94), NYHA and sST2 (P=0.93), and sex and sST2 (P=0.27).
Figure 12.
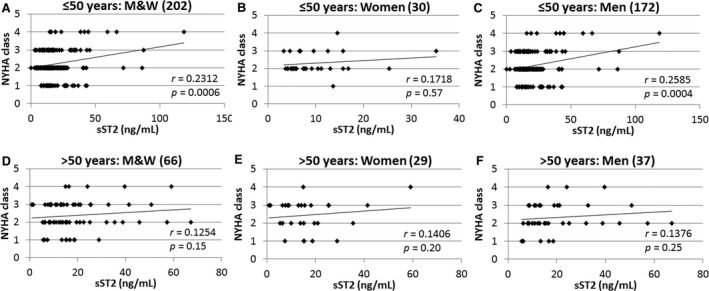
Sera sST2 correlates with heart failure based on NYHA class in men ≤50 y old with myocarditis. Correlations between New York Heart Association (NYHA) class and soluble ST2 (sST2) levels in patients with clinically suspected myocarditis (n) (A through C) ≤50 or (D through F) >50 y old were examined in men and women (M&W) combined (A and D), women (B and E), or men (C and F). P values determined using 2‐tailed Pearson correlation and adjusted for multiple sites using linear regression analysis.
Increased sST2 Levels in Male Mice With Myocarditis Correlate With Myocardial Inflammation and Poor Heart Function
Male BALB/c mice with CVB3‐induced myocarditis had significantly increased sera sST2 levels compared with females (n=10/group, P=0.005) (Figure 13A). PBS‐inoculated male and female control mice did not develop myocarditis and had undetectable serum levels of sST2 (data not shown). Serum sST2 levels correlated with cardiac inflammation in mice with CVB3‐induced myocarditis (r=0.3259, P=0.04) (Figure 14). Serum sST2 levels were also associated with multiple metrics that reflect poor heart function or hypertrophy in male BALB/c mice (n=18–27): LVEF, R 2=0.1865, P=0.07; fractional shortening, R 2=0.1864, P=0.07; left LV mass, R 2=0.272, P=0.03; LV end systolic dimension, R 2=0.2461, P=0.04; LV end diastolic dimension, R 2=0.1956, P=0.07; and interventricular septal dimension, R 2=0.3371, P=0.002 (Figure 13B).
Figure 13.
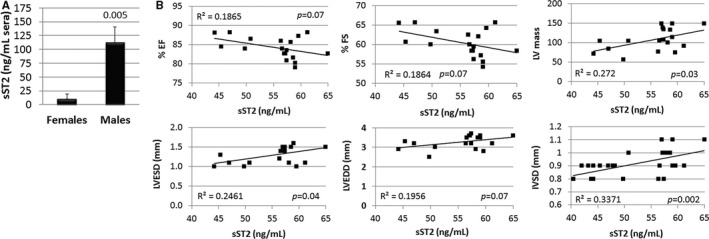
Sera sST2 increased in male mice with myocarditis and correlates with worse heart function and size. A, Soluble ST2 (sST2) levels in the sera of male and female mice with myocarditis determined by ELISA (n=10/group). Data are shown as mean±SEM (n) and P value determined using 2‐way Student t test. B, Correlation between sST2 levels and cardiac functional parameters in male mice with myocarditis (n=18–27). Experiments were repeated 2 to 4 times. P values calculated using a 2‐tailed Pearson correlation. EF indicates ejection fraction; FS, fractional shortening; IVSD, interventricular septal dimension; LV, left ventricular; LVEDD, LV end diastolic dimension; LVESD, LV end systolic dimension.
Figure 14.
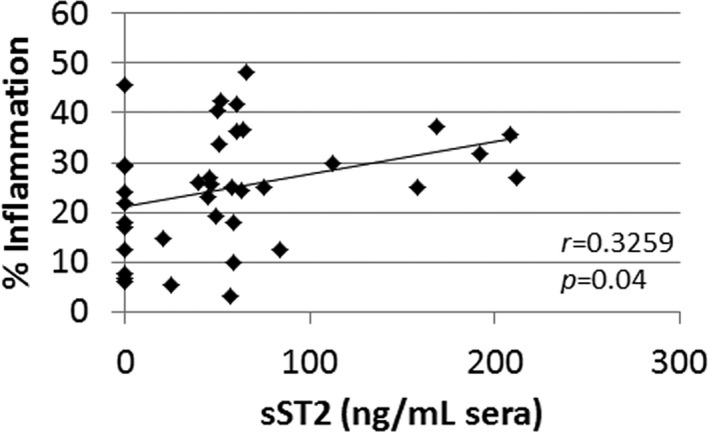
Sera sST2 levels correlate with myocardial inflammation in male BALB/c mice with CVB3 myocarditis. P value determined using 2‐tailed Pearson's correlation (n=38). CVB3 indicates coxsackievirus B3; sST2, soluble ST2.
Testosterone, But Not Estradiol, Increased Serum sST2 Levels in Mice With Myocarditis
To determine whether testosterone could be responsible for the increased serum sST2 levels found in men and male mice with myocarditis, we compared gonadectomized (Gdx‐Con) to sham operated (Sham‐Con) or gonadectomized male BALB/c mice that received testosterone supplementation (Gdx‐Te). Gonadectomy (Gdx‐Con) resulted in significantly reduced myocardial inflammation compared with sham‐operated males (Sham‐Con) (P=0.02) (Figure 15A and 15B). Myocarditis was restored with testosterone supplementation (Gdx‐Te versus Gdx‐Con) (P=0.03) (Figure 15A and 15B). Sera testosterone levels were within the normal range for sham‐operated mice (Sham‐Con, ≈10 ng/mL) and were significantly reduced by gonadectomy (Gdx‐Con) (P<0.0001) (Figure 15C). Testosterone replacement of gonadectomized mice restored sera testosterone levels to sham‐operated control levels (Gdx‐Te versus Gdx‐Con, P=0.0002) (Figure 15C). Gonadectomy (Gdx‐Con) significantly reduced sera sST2 levels compared with sham‐operated mice (Sham‐Con) (P=0.02) (Figure 15D). Testosterone replacement in gonadectomized mice significantly increased sST2 levels compared with gonadectomized mice (Gdx‐Te versus Gdx‐Con, P<0.0001) (Figure 15D). In contrast, testosterone supplementation did not increase sST2 levels in the heart of male mice with myocarditis that had undergone gonadectomy (Gdx‐Te versus Gdx‐Con, P=0.87) (Figure 16).
Figure 15.
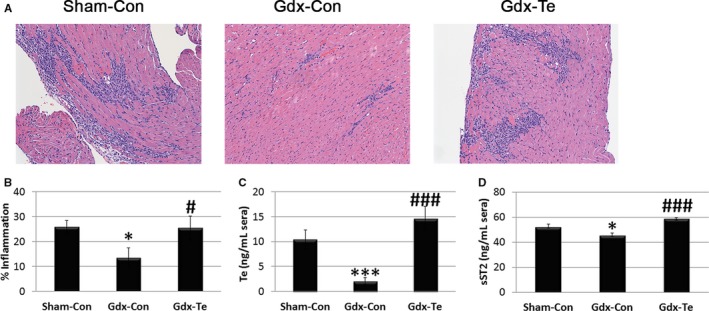
Testosterone increases cardiac inflammation and sera sST2 levels in male mice with myocarditis. A, Representative histology of myocarditis at day 10 postinfection (magnification ×64). B, Myocarditis assessed as the % inflammation in histology sections. Sera (C) testosterone (Te) and (D) soluble ST2 (sST2) levels were assessed using ELISA. B through D, Data show the mean±SEM and are representative of 3 separate experiments (n=9/group), *compares gonadectomy (Gdx‐Con) with Sham‐Con and #compares Gdx‐Te with Gdx‐Con; * and #, P<0.05; *** and ###, P<0.001. P values determined using 1‐way ANOVA with Tukey's multiple comparisons test. ANOVA (B) myocarditis P=0.02, (C) testosterone P<0.0001, (D) sST2 P<0.0001.
Figure 16.
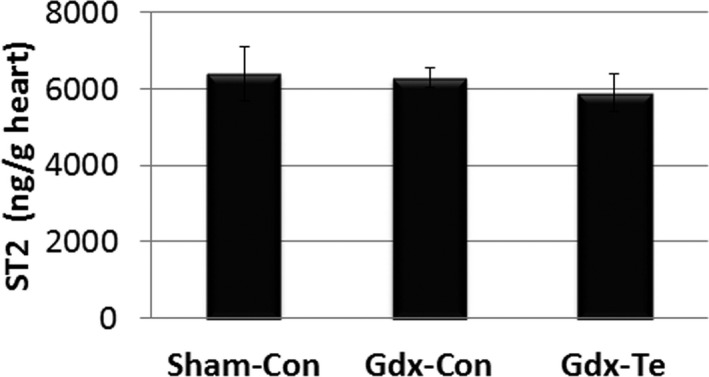
Testosterone does not significantly alter soluble ST2 (sST2) levels in the heart of male mice with myocarditis. Male BALB/c mice received a sham operation and a control capsule without Te (Sham‐Con), or a gonadectomy (Gdx) and a control capsule (Gdx‐Con), or a Gdx and a Te replacement capsule (Gdx‐Te). Data show mean±SEM and are representative of 3 separate experiments (n=9/group). P values determined using 1‐way ANOVA with Tukey's multiple comparisons test (overall P=0.49, individual comparisons not significant).
To determine whether estrogen alters sera sST2 levels during myocarditis, we performed ovariectomy on female mice with or without estradiol replacement. Ovariectomy significantly increased myocardial inflammation compared with sham‐operated females (Ovx+Con versus Sham+Con, P=0.001) (Figure 17A and 17C). Estradiol supplementation reduced myocardial inflammation back to sham‐control levels (Ovx+E2 versus Ovx+Con, P=0.03) (ANOVA P=0.002) (Figure 17A and 17C). Evidence that the ovariectomy procedure was successful was demonstrated by a significant reduction in uterine horn weight in ovariectomized mice (Ovx+Con versus Sham+Con, P=0.0002). Uterine horn weight was restored with estradiol supplementation (Ovx+E2 versus Ovx+Con, P=4×10−7) (ANOVA P=0.007) (Figure 17A and 17D). Ovariectomy had no significant effect on sera sST2 levels compared with sham‐operated mice (Ovx+Con versus Sham+Con, P=0.99) or with estradiol supplementation (Ovx+E2 versus Ovx+Con, P=0.34) (ANOVA P=0.32) (Figure 17E).
Figure 17.
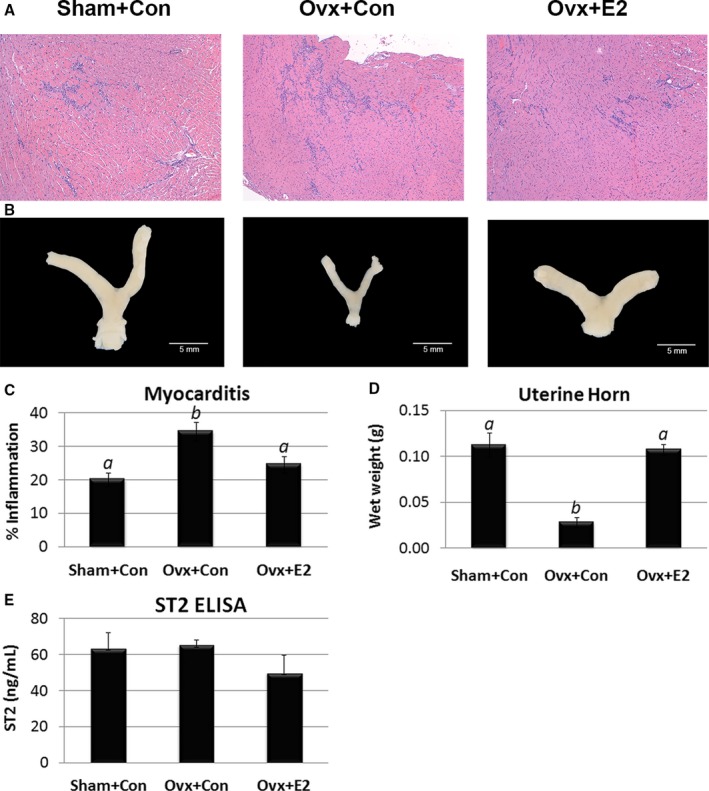
Estradiol decreases myocarditis but has no significant effect on sera soluble ST2 (sST2) levels in female mice with myocarditis. Female BALB/c mice received a sham operation and a control capsule without estradiol (E2) (Sham+Con), or an ovariectomy (Ovx) and a control capsule (Ovx+Con), or Ovx and E2 replacement (Ovx+E2). A, Representative histology of myocarditis at day 10 postinfection (magnification ×64). B, Representative photographs of uterine horns. C, Myocarditis assessed as the % inflammation in histology sections. D, Uterine horn weights. E, Sera sST2 levels were assessed using ELISA. C through E, Data show mean±SEM and are representative of 3 separate experiments (n=8–9/group) a is significantly different from b with P<0.05. P values determined using 1‐way ANOVA with Tukey's multiple comparisons test. ANOVA (C) myocarditis P=0.002, (D) uterine horn weight P<0.0001, (E) sST2 heart P=0.32, individual comparisons not significant.
IL‐1β Administration Increased and Correlated With Serum sST2 Levels in Male Mice During Myocarditis
sST2 is induced by biomechanical strain in cardiac fibroblasts, cardiomyocytes, and vascular endothelial cells,9 and its expression is increased in cardiac myocytes by IL‐1β because of the ST2 promotor being responsive to IL‐1β.16 We found here that recombinant IL‐1β administration in male mice significantly increased circulating levels of sST2 during myocarditis (P<0.001) (Figure 18). We hypothesized that cardiac inflammation that releases IL‐1β may lead to elevated sera sST2 levels.8 We found that sera sST2 correlated with cardiac IL‐1β levels in BALB/c mice during myocarditis at day 10 postinfection (r=0.4421, P=0.03) (Figure 19); however, this does not distinguish whether IL‐1β originates from cardiac tissue or immune cells.
Figure 18.
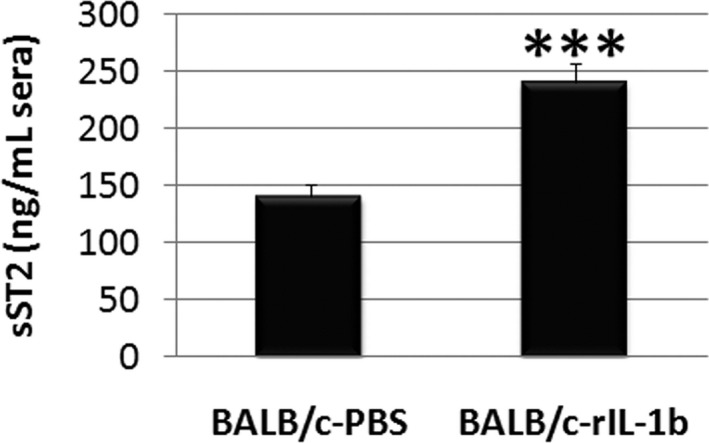
IL‐1β administration increases sera soluble ST2 (sST2) levels during myocarditis in BALB/c mice. Data show mean±SEM of n=10/group, ***P<0.001. P value determined using a 2‐way Student t test.
Figure 19.
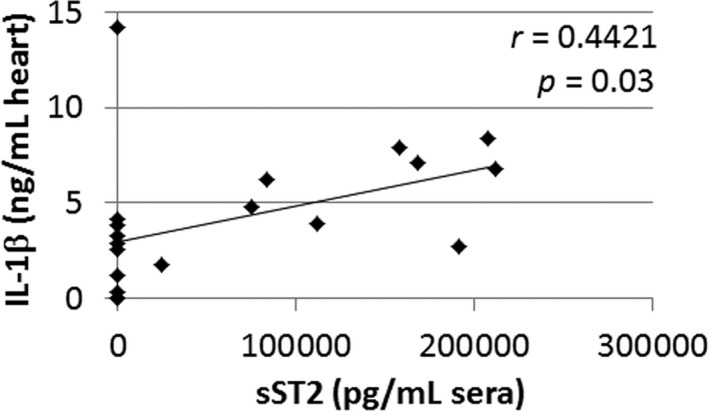
Cardiac IL‐1β levels correlate with sera soluble ST2 (sST2) levels during myocarditis in BALB/c mice. P value calculated using a 2‐tailed Pearson's correlation (n=20).
Discussion
Sera sST2 has emerged as a new biomarker that may be used to improve management of heart failure patients beyond the diagnostic value of natriuretic peptides.9, 32 In particular, sera sST2 has been found to be a powerful predictor of mortality.10, 12, 33, 34 Historically sST2 has not been considered a diagnostic marker for heart failure, even though its levels were significantly higher in heart failure patients compared with controls, because it is not specific to heart failure but also rises with infection and in autoimmune diseases.9, 34 In this study we found that sera sST2 levels were higher in men and women with clinically suspected myocarditis and biopsy‐confirmed myocarditis compared with healthy individuals who did not have cardiovascular disease. Similar to the results of other heart failure studies,13 the current study revealed that elevated sST2 levels were associated with worse heart failure symptoms in patients with myocarditis based on NYHA class, but uniquely, the association occurred in men but not women. In contrast to NYHA class, LVEF ≤45% has been found to only weakly correlate with elevated sST2,13 a finding similarly observed in this study. The findings of this study suggest that myocarditis should be added to the growing list of cardiovascular diseases where circulating sST2 levels may provide clinically relevant information about the risk of death from heart failure.11
The US Food and Drug Administration–approved Critical Diagnostics sST2 ELISA kit provides diagnostic information on the risk of death from heart failure for circulating sST2 levels >35 ng/mL, but does not describe whether sex differences in sera sST2 exist for various forms of heart failure.35 The Critical Diagnostics kit was not available when the study began, so the R&D Systems kit was used at all sites for consistency. Comparison of the R&D Systems and Critical Diagnostics kits found a 2.488‐fold difference between the kits. Thus, women with myocarditis in this study had an estimated sST2 value of 35.2 ng/mL, which is a borderline risk for death, while men with myocarditis had an estimated sST2 value of 51.53 ng/mL, indicating an increased risk of death based on extrapolation of our values to estimated Critical Diagnostics kit values. Further investigation as to whether sex differences exist in circulating sST2 levels for other cardiovascular diseases is warranted.
Two studies have reported that sST2 levels are increased in men compared with women with heart failure.36, 37 Myocarditis has been reported to occur most often in young men.38, 39 We are the first to report that elevated sST2 levels in patients with myocarditis correlate with symptoms of heart failure based on NYHA class, but only in men ≤50 years old. Dieplinger et al reported that sST2 was not associated with circulating sex hormones in men or women with heart failure.40 In contrast, we found that testosterone significantly increased sera sST2 levels in male mice with myocarditis (Figure 15), but estradiol did not significantly alter sST2 levels in females with myocarditis (Figure 17). These findings suggest that increased sST2 in men could be because of, at least in part, testosterone.
In a well‐characterized, community‐based cohort of subjects without cardiovascular disease from the Framingham Heart Study (n=1136), sera sST2 levels were found to be significantly elevated in healthy men compared with healthy women.41 In another study of 528 healthy men and women, sST2 was found to be significantly elevated in men compared with women.40 The current study also found that sera sST2 levels were significantly elevated in healthy men compared with healthy women without cardiovascular disease.
The sex differences in sera sST2 levels observed in this study may also be explained by the central role cardiac inflammation plays in the pathogenesis of acute and chronic heart failure in myocarditis and inflammatory DCM.7, 8, 20, 42, 43 Activation of the ST2 receptor by IL‐33 on innate immune cells such as macrophages and mast cells has been shown to be able to drive T helper (Th)1, Th2, and Th17‐type immune responses.15, 17, 18 Th1‐ and Th17‐associated cytokines increase acute inflammation while Th2‐ and Th17‐associated cytokines promote remodeling and fibrosis, all of which is known to influence the severity and progression of myocarditis/acute DCM in patients and mouse models.20, 31, 42, 44 IL‐33 signaling via the ST2 receptor increases inflammation in a number of animal models of auto/inflammatory diseases such as rheumatoid arthritis, inflammatory bowel disease, and asthma.15, 17, 18 We previously showed that treatment of male mice with recombinant IL‐33 during initiation of myocarditis significantly increased myocardial and pericardial inflammation, resulting in eosinophilic myocarditis.31 IL‐33 has been found to increase CD11b expression on immune cells in both humans and mice.15, 45, 46 We previously reported that the primary effect of testosterone in male mice with myocarditis was elevation of CD11b+TLR4+IL‐1β+ immune cells.7, 8, 20 Importantly, CD11b expression is also significantly elevated in biopsies from men compared with women with myocarditis.20 In animal models, sST2 has been found to be released predominantly from CD11b+ and CD4+ immune cells,15 which are the primary cardiac immune cells observed during acute myocarditis in mice and humans.7, 8, 20 IL‐33/ST2 signaling has been found to upregulate not only CD11b but also TLR4, caspase‐1, and IL‐1β in a number of human and animal studies of infection and autoimmune disease.15, 17, 18 Previously we reported that CD11b, TLR4, caspase‐1, and IL‐1β are increased by testosterone in male mice with myocarditis.7, 8, 20, 42
TLR4 mRNA expression has been found to be higher in patients with myocarditis than controls and to correlate with viral RNA levels in the heart.47 Satoh et al showed that myocarditis patients with active viral replication had higher levels of TLR4, which were associated with lower systolic function.47 We have previously shown that TLR4 and its downstream cytokines IL‐1β and IL‐18 are elevated in the heart of male but not female mice with CVB3 myocarditis.48 IL‐1β and IL‐18 have been shown to play a role in clinical and mouse models of myocarditis by inducing myocyte hypertrophy, contractile dysfunction, myocyte apoptosis, and by contributing to extracellular matrix remodeling, a step critical in the progression from myocarditis to DCM and chronic heart failure.49
Previous studies have shown that circulating IL‐1β levels correlate with NYHA classification and predict mortality in heart failure patients.50 A long‐term study of patients with myocarditis revealed that inflammation defined by immunoperoxidase‐based criteria on endomyocardial biopsies was the best predictor of death or heart transplantation following acute myocarditis.51 Bartunek et al found that ST2 production did not correlate with wall stress but rather that endothelial cells and/or inflammation contributed to the release of sST2.52 These authors further showed that IL‐1β induced sST2 secretion from human venous and arterial endothelial cells and suggested that the distinct ability of sST2 to predict heart failure aside from BNP, which is released by stress, indicates that inflammation may contribute to elevated sST2 levels in heart failure patients.52 Similarly, we found that sera sST2 levels correlated with cardiac inflammation in mice with myocarditis (Figure 14). Additionally, cardiac IL‐1β levels correlated with sera sST2 levels (Figure 19), and recombinant IL‐1β administration during the initiation of myocarditis increased circulating sST2 levels in male mice (Figure 18), suggesting that IL‐1β+ cardiac inflammation may contribute to elevated sera sST2 levels in males. This theory will need to be confirmed in future animal experiments and human studies.
Limitations of this study include a small sample size, particularly for women with myocarditis. The number of patients >50 years old is also relatively small. Menopause status was not obtained. Future studies should include higher numbers of subjects >50 years of age in order to determine whether sST2 is increased in older myocarditis patients, particularly postmenopausal women. Age 50 years was used as a cut‐off to assess the effect of aging on both men and women, but it remains unclear whether this is the best age cut‐off to use to assess the effects of aging in patients with myocarditis. Another limitation of the study is that the various sites had different study populations, which likely increased the variability in sST2 levels. However, we found that men with myocarditis had significantly higher sST2 levels than women at each study site (data not shown), and linear regression analysis revealed that sex differences dominated over site‐specific differences in sST2 variability. Examining sST2 at different sites provides information on the variability of sST2 as a biomarker of heart failure from patients with myocarditis in different regions of the world. Another potential limitation is that only baseline levels of sST2 were analyzed in this study. Baseline sST2 levels are able to predict outcome in acute and chronic heart failure patients, but serial measurements have been shown to be of even greater value.53, 54 Serial measures of sST2 are better at predicting long‐term prognosis than natriuretic peptides because sST2 has been found to significantly decrease from admission to discharge.9 Future studies should examine whether serial measurements of sST2 improve prediction of sudden death and/or the need for transplantation in patients with myocarditis with a LVEF <45%, similar to other forms of heart failure, and whether prediction differs by sex.
In conclusion, in this study of well‐characterized patients with myocarditis, we show that sST2 levels were elevated in men with myocarditis compared with women and that sST2 correlated with NYHA class in men with myocarditis, but only in men ≤50 years old. These findings highlight the importance of analyzing inflammatory biomarkers like sST2 according to sex and age. Biomarkers that predict increased risk of heart failure in women with myocarditis are needed.
Sources of Funding
This study was supported by grants from the National Institutes of Health (NIH) (R01 HL087033, R01 HL111938, R21 ES024414) and the American Heart Association (12GRNT12050000, 16GRNT30950007) to Fairweather; NIH HL129474 to Coronado; T32 ES007141 to Coronado, Bruno, and Abston; R01 HL56267 and R01 HL135165 to Cunningham; Mayo Clinic and Samsung Medical Center Collaborative Research Grant to Cooper, Blauwet, and Jeon; German Competence Network Heart Failure, TP9, FKZ 01GI0205 to Pankuweit; and CETOCOEN PLUS (CZ.02.1.01/0.0/0.0/15_003/0000469) to Bienertová‐Vašků.
Disclosures
None.
(J Am Heart Assoc. 2019;8:e008968 DOI: 10.1161/JAHA.118.008968.)
References
- 1. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Braunwald E. The war against heart failure: the Lancet lecture. Lancet. 2015;385:812–824. [DOI] [PubMed] [Google Scholar]
- 3. Cooper LT Jr. Myocarditis. N Engl J Med. 2009;360:1526–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cooper LT Jr, Keren A, Sliwa K, Matsumori A, Mensah GA. The global burden of myocarditis: part 1: a systematic literature review for the Global Burden of Diseases, Injuries, and Risk Factors 2010 study. Glob Heart. 2014;9:121–129. [DOI] [PubMed] [Google Scholar]
- 5. Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270–283. [DOI] [PubMed] [Google Scholar]
- 6. McNamara DM, Starling RC, Cooper LT, Boehmer JP, Mather PJ, Janosko KM, Gorcsan J III, Kip KE, Dec GW; IMAC Investigators . Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)‐2 study. J Am Coll Cardiol. 2011;58:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Frisancho‐Kiss S, Davis SE, Nyland JF, Frisancho JA, Cihakova D, Barrett MA, Rose NR, Fairweather D. Cutting edge: cross‐regulation by TLR4 and T cell Ig mucin‐3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178:6710–6714. [DOI] [PubMed] [Google Scholar]
- 8. Coronado MJ, Brandt JE, Kim E, Bucek A, Bedja D, Abston ED, Shin J, Gabrielson KL, Mitzner W, Fairweather D. Testosterone and interleukin‐1beta increase cardiac remodeling during coxsackievirus B3 myocarditis via serpin A 3n. Am J Physiol Heart Circ Physiol. 2012;302:H1726–H1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Villacorta H, Maisel AS. Soluble ST2 testing: a promising biomarker in the management of heart failure. Arq Bras Cardiol. 2016;106:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pascual‐Figal DA, Ordonez‐Llanos J, Tornel PL, Vazquez R, Puig T, Valdes M, Cinca J, de Luna AB, Bayes‐Genis A; MUSIC Investigators . Soluble ST2 for predicting sudden cardiac death in patients with chronic heart failure and left ventricular systolic dysfunction. J Am Coll Cardiol. 2009;54:2174–2179. [DOI] [PubMed] [Google Scholar]
- 11. Emdin M, Aimo A, Vergaro G, Bayes‐Genis A, Lupon J, Latini R, Meessen J, Anand IS, Cohn JN, Gravning J, Gullestad L, Broch K, Ueland T, Nymo SH, Brunner‐La Rocca HP, de Boer RA, Gaggin HK, Ripoli A, Passino C, Januzzi JL Jr. sST2 predicts outcome in chronic heart failure beyond NT‐proBNP and high‐sensitivity troponin T. J Am Coll Cardiol. 2018;72:2309–2320. [DOI] [PubMed] [Google Scholar]
- 12. Weinberg EO, Shimpo M, Hurwitz S, Tominaga S, Rouleau JL, Lee RT. Identification of serum soluble ST2 receptor as a novel heart failure biomarker. Circulation. 2003;107:721–726. [DOI] [PubMed] [Google Scholar]
- 13. Rehman SU, Mueller T, Januzzi JL Jr. Characteristics of the novel interleukin family biomarker ST2 in patients with acute heart failure. J Am Coll Cardiol. 2008;52:1458–1465. [DOI] [PubMed] [Google Scholar]
- 14. Pascual‐Figal DA, Januzzi JL. The biology of ST2: the international ST2 consensus panel. Am J Cardiol. 2015;115:3B–7B. [DOI] [PubMed] [Google Scholar]
- 15. Griesenauer B, Paczesny S. The ST2/IL‐33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT. Expression and regulation of ST2, an interleukin‐1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002;106:2961–2966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lu J, Kang J, Zhang C, Zhang X. The role of IL‐33/ST2L signals in the immune cells. Immunol Lett. 2015;164:11–17. [DOI] [PubMed] [Google Scholar]
- 18. Rostan O, Arshad MI, Piquet‐Pellorce C, Robert‐Gangneux F, Gangneux JP, Samson M. Crucial and diverse role of the interleukin‐33/ST2 axis in infectious diseases. Infect Immun. 2015;83:1738–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frisancho‐Kiss S, Coronado MJ, Frisancho JA, Lau VM, Rose NR, Klein SL, Fairweather D. Gonadectomy of male BALB/c mice increases Tim‐3(+) alternatively activated M2 macrophages, Tim‐3(+) T cells, Th2 cells and Treg in the heart during acute coxsackievirus‐induced myocarditis. Brain Behav Immun. 2009;23:649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fairweather D, Coronado MJ, Garton AE, Dziedzic JL, Bucek A, Cooper LT Jr, Brandt JE, Alikhan FS, Wang H, Endres CJ, Choi J, Pomper MG, Guilarte TR. Sex differences in translocator protein 18 kDa (TSPO) in the heart: implications for imaging myocardial inflammation. J Cardiovasc Transl Res. 2014;7:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno‐Blanes J, Felix SB, Fu M, Helio T, Heymans S, Jahns R, Klingel K, Linhart A, Maisch B, McKenna W, Mogensen J, Pinto YM, Ristic A, Schultheiss HP, Seggewiss H, Tavazzi L, Thiene G, Yilmaz A, Charron P, Elliott PM; European Society of Cardiology Working Group on Myocardial and Pericardial Diseases . Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648, 2648a‐2648d. [DOI] [PubMed] [Google Scholar]
- 22. Jacobsen BK, Heuch I, Kvale G. Age at natural menopause and all‐cause mortality: a 37‐year follow‐up of 19,731 Norwegian women. Am J Epidemiol. 2003;157:923–929. [DOI] [PubMed] [Google Scholar]
- 23. Fairweather D. Autoimmune skin diseases: role of sex hormones, vitamin D and menopause In: Farage MA, Miller KW, Fugate‐Woods N, Maibach HI, eds. Skin, Mucosa and Menopause: Management of Clinical Issues. Heidelberg: Springer; 2015:359–381. [Google Scholar]
- 24. Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch‐Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol. 1998;51:1271–1276. [DOI] [PubMed] [Google Scholar]
- 25. Regitz‐Zagrosek V, Becher E, Mahmoodzadeh S, Schubert C. Sex steroid hormones In: Bader M, ed. Cardiovascular Hormone Systems. Weinheim: Wiley‐Blackwell; 2008:39–64. [Google Scholar]
- 26. Wu C, Singh A, Collins B, Fatima A, Qamar A, Gupta A, Hainer J, Klein J, Jarolim P, Di Carli M, Nasir K, Bhatt DL, Blankstein R. Causes of troponin elevation and associated mortality in young patients. Am J Med. 2018;131:284–292. e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers JM, Fairweather D, Huber SA, Cunningham MW. Autoimmune myocarditis, valvulitis, and cardiomyopathy. Curr Protoc Immunol. 2013;Chapter 15:Unit 15 14 11‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Robinson DP, Hall OJ, Nilles TL, Bream JH, Klein SL. 17beta‐estradiol protects females against influenza by recruiting neutrophils and increasing virus‐specific CD8 T cell responses in the lungs. J Virol. 2014;88:4711–4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen‐induced proliferation of uterine epithelial cells is independent of estrogen receptor alpha binding to classical estrogen response elements. J Biol Chem. 2006;281:26683–26692. [DOI] [PubMed] [Google Scholar]
- 30. Georgakopoulos D, Kass DA. Protocols for hemodynamic assessment of transgenic mice in vivo. Methods Mol Biol. 2003;219:233–243. [DOI] [PubMed] [Google Scholar]
- 31. Abston ED, Barin JG, Cihakova D, Bucek A, Coronado MJ, Brandt JE, Bedja D, Kim JB, Georgakopoulos D, Gabrielson KL, Mitzner W, Fairweather D. IL‐33 independently induces eosinophilic pericarditis and cardiac dilation: ST2 improves cardiac function. Circ Heart Fail. 2012;5:366–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Boer RA, Daniels LB, Maisel AS, Januzzi JL Jr. State of the art: newer biomarkers in heart failure. Eur J Heart Fail. 2015;17:559–569. [DOI] [PubMed] [Google Scholar]
- 33. Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT. Serum levels of the interleukin‐1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–2190. [DOI] [PubMed] [Google Scholar]
- 34. Januzzi JL Jr, Peacock WF, Maisel AS, Chae CU, Jesse RL, Baggish AL, O'Donoghue M, Sakhuja R, Chen AA, van Kimmenade RR, Lewandrowski KB, Lloyd‐Jones DM, Wu AH. Measurement of the interleukin family member ST2 in patients with acute dyspnea: results from the PRIDE (Pro‐Brain Natriuretic Peptide Investigation of Dyspnea in the Emergency Department) study. J Am Coll Cardiol. 2007;50:607–613. [DOI] [PubMed] [Google Scholar]
- 35. Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Houston‐Miller N, Fleg JL, Schulman KA, Pina IL; HF‐ACTION Trial Investigators . Heart failure and a controlled trial investigating outcomes of exercise training (HF‐ACTION): design and rationale. Am Heart J. 2007;153:201–211. [DOI] [PubMed] [Google Scholar]
- 36. Ky B, French B, McCloskey K, Rame JE, McIntosh E, Shahi P, Dries DL, Tang WH, Wu AH, Fang JC, Boxer R, Sweitzer NK, Levy WC, Goldberg LR, Jessup M, Cappola TP. High‐sensitivity ST2 for prediction of adverse outcomes in chronic heart failure. Circ Heart Fail. 2011;4:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Daniels LB, Maisel AS. Cardiovascular biomarkers and sex: the case for women. Nat Rev Cardiol. 2015;12:588–596. [DOI] [PubMed] [Google Scholar]
- 38. Kyto V, Sipila J, Rautava P. The effects of gender and age on occurrence of clinically suspected myocarditis in adulthood. Heart. 2013;99:1681–1684. [DOI] [PubMed] [Google Scholar]
- 39. Bohm P, Scharhag J, Meyer T. Data from a nationwide registry on sports‐related sudden cardiac deaths in Germany. Eur J Prev Cardiol. 2016;23:649–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dieplinger B, Egger M, Poelz W, Gabriel C, Haltmayer M, Mueller T. Soluble ST2 is not independently associated with androgen and estrogen status in healthy males and females. Clin Chem Lab Med. 2011;49:1515–1518. [DOI] [PubMed] [Google Scholar]
- 41. Coglianese EE, Larson MG, Vasan RS, Ho JE, Ghorbani A, McCabe EL, Cheng S, Fradley MG, Kretschman D, Gao W, O'Connor G, Wang TJ, Januzzi JL. Distribution and clinical correlates of the interleukin receptor family member soluble ST2 in the Framingham Heart Study. Clin Chem. 2012;58:1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fairweather D, Cooper LT Jr, Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr Probl Cardiol. 2013;38:7–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krejci J, Mlejnek D, Sochorova D, Nemec P. Inflammatory cardiomyopathy: a current view on the pathophysiology, diagnosis, and treatment. Biomed Res Int. 2016;2016:4087632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Myers JM, Cooper LT, Kem DC, Stavrakis S, Kosanke SD, Shevach EM, Fairweather D, Stoner JA, Cox CJ, Cunningham MW. Cardiac myosin‐Th17 responses promote heart failure in human myocarditis. JCI Insight. 2016;1:e85851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Suzukawa M, Koketsu R, Iikura M, Nakae S, Matsumoto K, Nagase H, Saito H, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. Interleukin‐33 enhances adhesion, CD11b expression and survival in human eosinophils. Lab Invest. 2008;88:1245–1253. [DOI] [PubMed] [Google Scholar]
- 46. Jovanovic IP, Pejnovic NN, Radosavljevic GD, Pantic JM, Milovanovic MZ, Arsenijevic NN, Lukic ML. Interleukin‐33/ST2 axis promotes breast cancer growth and metastases by facilitating intratumoral accumulation of immunosuppressive and innate lymphoid cells. Int J Cancer. 2014;134:1669–1682. [DOI] [PubMed] [Google Scholar]
- 47. Satoh M, Nakamura M, Akatsu T, Iwasaka J, Shimoda Y, Segawa I, Hiramori K. Expression of Toll‐like receptor 4 is associated with enteroviral replication in human myocarditis. Clin Sci (Lond). 2003;104:577–584. [DOI] [PubMed] [Google Scholar]
- 48. Fairweather D, Yusung S, Frisancho S, Barrett M, Gatewood S, Steele R, Rose NR. IL‐12 receptor beta 1 and Toll‐like receptor 4 increase IL‐1 beta‐ and IL‐18‐associated myocarditis and coxsackievirus replication. J Immunol. 2003;170:4731–4737. [DOI] [PubMed] [Google Scholar]
- 49. Cain BS, Meldrum DR, Dinarello CA, Meng X, Joo KS, Banerjee A, Harken AH. Tumor necrosis factor‐alpha and interleukin‐1beta synergistically depress human myocardial function. Crit Care Med. 1999;27:1309–1318. [DOI] [PubMed] [Google Scholar]
- 50. Vasan RS, Sullivan LM, Roubenoff R, Dinarello CA, Harris T, Benjamin EJ, Sawyer DB, Levy D, Wilson PW, D'Agostino RB; Framingham Heart S . Inflammatory markers and risk of heart failure in elderly subjects without prior myocardial infarction: the Framingham Heart Study. Circulation. 2003;107:1486–1491. [DOI] [PubMed] [Google Scholar]
- 51. Kindermann I, Kindermann M, Kandolf R, Klingel K, Bultmann B, Muller T, Lindinger A, Bohm M. Predictors of outcome in patients with suspected myocarditis. Circulation. 2008;118:639–648. [DOI] [PubMed] [Google Scholar]
- 52. Bartunek J, Delrue L, Van Durme F, Muller O, Casselman F, De Wiest B, Croes R, Verstreken S, Goethals M, de Raedt H, Sarma J, Joseph L, Vanderheyden M, Weinberg EO. Nonmyocardial production of ST2 protein in human hypertrophy and failure is related to diastolic load. J Am Coll Cardiol. 2008;52:2166–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Boisot S, Beede J, Isakson S, Chiu A, Clopton P, Januzzi J, Maisel AS, Fitzgerald RL. Serial sampling of ST2 predicts 90‐day mortality following destabilized heart failure. J Card Fail. 2008;14:732–738. [DOI] [PubMed] [Google Scholar]
- 54. Maisel AS, Richards AM, Pascual‐Figal D, Mueller C. Serial ST2 testing in hospitalized patients with acute heart failure. Am J Cardiol. 2015;115:32B–37B. [DOI] [PubMed] [Google Scholar]


