Abstract
Background
Cardiac biomarkers and left ventricular hypertrophy (LVH) are related to the risk of stroke and death in patients with atrial fibrillation. We investigated the interrelationship between LVH and cardiac biomarkers and their independent associations with outcomes.
Methods and Results
Plasma samples were obtained at baseline in 5275 patients with atrial fibrillation in the RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial. NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide), cardiac troponin I and T, and growth differentiation factor‐15 were determined using high‐sensitivity (hs) assays. LVH was defined by ECG. Cox models were adjusted for baseline characteristics, LVH, and biomarkers. LVH was present in 1257 patients. During a median follow‐up of 2.0 years, 165 patients developed a stroke and 370 died. LVH was significantly (P<0.0001) associated with higher levels of all biomarkers in linear regression analyses adjusting for baseline characteristics. Geometric mean ratios (95% CIs) were as follows: NT‐proBNP, 1.32 (1.25–1.38); hs cardiac troponin I, 1.67 (1.57–1.78); hs troponin T, 1.38 (1.32–1.44); and growth differentiation factor‐15, 1.09 (1.05–1.12). For stroke, the hazard ratios (95% CIs) per 50% increase were as follows: NT‐proBNP, 1.09 (1.00–1.19); hs cardiac troponin I, 1.09 (1.03–1.15); hs troponin T, 1.14 (1.06–1.24); and growth differentiation factor‐15, 1.22 (1.08–1.38) (all P<0.05). For death, hazard ratios (95% CIs) were as follows: NT‐proBNP, 1.24 (1.17–1.31); hs cardiac troponin I, 1.13 (1.10–1.17); hs troponin T, 1.28 (1.23–1.34); and growth differentiation factor‐15, 1.31 (1.22–1.42) (all P<0.0001). LVH was not significantly associated with stroke or death after adjustment for biomarkers.
Conclusions
Cardiac biomarkers are significantly associated with LVH. The prognostic value of biomarkers for stroke and death is not affected by LVH. The prognostic information of LVH is attenuated in the presence of cardiac biomarkers.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00262600.
Keywords: atrial fibrillation, biomarker, left ventricular hypertrophy, risk prediction
Subject Categories: Atrial Fibrillation, Biomarkers, Hypertrophy, Ischemic Stroke, Mortality/Survival
Clinical Perspective
What Is New?
The prognostic value of cardiovascular biomarkers (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide], growth differentiation factor‐15, and troponin) for stroke, death, and major bleeding is not affected by left ventricular hypertrophy in patients with atrial fibrillation.
What Are the Clinical Implications?
Cardiac biomarkers are able to further identify patients with lower or higher risk both in the presence and in the absence of left ventricular hypertrophy by ECG.
Introduction
Atrial fibrillation (AF), the most common sustained arrhythmia, substantially increases the risk of stroke and mortality.1, 2 Left ventricular hypertrophy (LVH) is a recognized risk factor for cardiovascular disease and mortality.3 Recently, LVH diagnosed by ECG was also confirmed to be a predictor in patients with AF for risk of stroke and death.4 Similarly, cardiac biomarkers are well‐known predictors of cardiovascular risk and their independent association with stroke and mortality outcomes in AF has consistently been demonstrated.5, 6, 7, 8, 9 Several factors influence the concentrations of cardiac biomarkers, including the presence of LVH.10, 11, 12, 13 To date, there are limited data on the association of cardiac biomarkers with LVH in patients with AF, neither on the combination of these 2 indicators, to further increase the understanding of and improve the risk stratification in AF.
In the present RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy)14 trial, we investigated the relationship between cardiovascular biomarkers (NT‐proBNP [N‐terminal pro‐B‐type natriuretic peptide], high‐sensitivity cardiac troponin I [hs‐cTnI] and T [hs‐cTnT], and growth differentiation factor‐15 [GDF‐15]) and LVH diagnosed by ECG and their individual independent associations with outcomes in 5275 anticoagulated patients with AF using baseline plasma samples.
Methods
The data and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population and Trial Design
The study organization, trial design, patient characteristics, and outcomes of the RE‐LY trial have been published previously.14, 15 Patients were recruited from 967 centers in 44 countries between November 2005 and December 2007. A total of 18 113 patients with AF, with at least 1 additional risk factor for stroke, were randomized in a 1:1:1 manner to receive either fixed doses of dabigatran (110 or 150 mg twice daily) in a blinded manner or adjusted‐dose warfarin (target international normalized ratio, 2.0–3.0) in an unblinded manner for a median of 2 years.15 All patients were centrally randomized through an interactive voice response system located at the Coordinating Centre at Population Health Research Institute (Hamilton, Ontario, Canada). Estimated glomerular filtration rate <30 mL/min, according to Cockcroft‐Gault, was an exclusion criterion. The primary efficacy outcome was fatal and nonfatal stroke (ischemic, hemorrhagic, or unspecified) or systemic embolism. The main safety outcome was major bleeding, defined as (1) a reduction in hemoglobin level of at least 20 g/L, (2) transfusion of at least 2 units of blood, or (3) symptomatic bleeding in a critical area or organ. Approval by the appropriate ethics committees was obtained at all sites. All patients provided written informed consent.
Of the 18 113 patients included in the RE‐LY trial, LVH data by ECG were available in 10 372. Of these, 5275 patients also had baseline measurements available for at least 1 of the cardiovascular biomarkers (NT‐proBNP, hs‐cTnI, hs‐cTnT, and GDF‐15). The data set for this study was accordingly 5275 patients. A flowchart is presented in Figure S1.
ECG Procedure and LVH Definition
The ECG procedure has been described in detail previously.4 Briefly, a 25‐mm/s 12‐lead ECG obtained at study entry was examined by an expert reader (G.M.), blinded to the patients’ features and randomized treatment. Electrographic LVH was defined according to the Cornell voltage criteria (R wave in aVL+S wave in V3 >2.0 mV in women or >2.4 mV in men) or by presence of strain pattern (ST‐segment depression of at least 0.5 mm and inverted T wave in at least 1 of the leads in I, II, aVL, or V4 to V6). LVH diagnosis by electrocardiography was thus a binary variable (yes/no). This definition of electrographic LVH is considered simple and applicable in large populations and has been validated in large studies.4, 16, 17 In a large validation study in hypertensive patients, this definition yielded 34% sensitivity and 91% specificity with echocardiographic LVH as reference, performed better than traditional ECG criteria of LVH, and identified subjects at higher risk of cardiovascular outcomes.17
Laboratory Methods
Venous blood was drawn at randomization, before initiation of study treatment, using a 21/22‐gauge needle into vacutainer tubes containing EDTA. The blood was centrifuged within 30 minutes at 2000g for 10 minutes. The tubes were thereafter immediately frozen at −20°C or colder. Aliquots were stored at −70°C to allow for central batch analysis.
Plasma concentration of GDF‐15 was determined by Elecsys GDF‐15 precommercial assay kit P03 with the same standardization as the recently introduced routine reagent (Roche Diagnostics).9, 18 cTnI concentrations were measured on Architect i1000SR (Abbott Diagnostics) using hs assays. cTnT and NT‐proBNP were analyzed with high‐sensitivity assays on Cobas Analytics e601 and c501 Immunoanalyzer (Roche Diagnostics). These biochemical analyses were performed centrally at Uppsala Clinical Research Center laboratory (Uppsala, Sweden), according to the instructions of the manufacturer. Details about the characteristics of these assays have been reported previously.19, 20
Statistical Analyses
The numbers of patients with available GDF‐15, NT‐proBNP, hs‐cTnI, and hs‐cTnT were 4850, 5239, 4948, and 4892, respectively. There were relatively few missing data (<1%) on other covariates, and a complete case analysis was therefore implemented. In a univariate analysis comparing baseline characteristics of patients between categories of LVH, continuous variables were reported with median and first and third quartiles and compared by Wilcoxon rank sum test. Categorical variables were reported as number and column percentage and compared by χ2 tests.
Continuous biomarker levels were log transformed and used as outcome in linear regression models, including LVH category (no/yes), age, sex, body mass index, current smoking, heart failure, hypertension, prior myocardial infarction, diabetes mellitus, systolic blood pressure, permanent AF, creatinine clearance, digoxin use, and angiotensin‐converting enzyme inhibitors/angiotensin II receptor blocker as explanatory variables, on the basis of clinical importance. The results are presented as model adjusted geometric mean ratios of biomarker levels between LVH categories, with nominal CIs and P values. Observed marginal distributions were used in the model adjustment.
The impact of biomarkers at baseline on the association between LVH and outcomes was analyzed by adding biomarkers (continuous, log‐transformed values) to Cox regression models, including LVH category, randomized treatment, CHA2DS2‐VASc score, permanent AF at entry, smoking, digoxin use, and creatinine clearance. Biomarkers were added individually to the model, as well as simultaneously in prespecified combinations. The interactions between LVH category and biomarkers were analyzed by Cox regression models, including LVH category, biomarker, and interaction between LVH category and biomarker. The biomarkers were included as continuous, log transformed, and fitted using restricted cubic splines with 4 knots, located at the 5th, 35th, 65th, and 95th percentiles. Plots of estimated probability of event at 1 year against biomarker values for each LVH category were constructed. The P values for the tests of interactions are reported.
The impact of LVH on the association between biomarkers and outcomes was analyzed by adding LVH to Cox regression models, including 1 biomarker (continuous, log transformed) and randomized treatment, CHA2DS2‐VASc score, permanent AF at entry, smoking, digoxin use, and creatinine clearance.
All statistical tests were 2 tailed and performed at the 0.05 significance level. Because the analyses were exploratory, no adjustments for multiple comparisons were made. The proportional hazards assumption was evaluated by plotting Schoenfeld residuals against rank time and fitting a smooth curve. All statistical analyses were performed using SAS software, version 9.4 (SAS Institute Inc, Cary, NC).
Results
Baseline Data and Clinical Characteristics According to Presence of LVH
The median age was 72.0 years, and 3431 patients (65%) were men. LVH was present in 1257 patients (23.8%). Baseline characteristics and comorbidities according to presence of LVH are shown in Table 1.
Table 1.
Demographics and Clinical Characteristics According to LVH Category
| Baseline Data | No LVH (N=4018) | LVH (N=1257) | P Valuea |
|---|---|---|---|
| Age, median (quartile 1–quartile 3), y | 72.0 (67.0–77.0) | 72.0 (66.0–78.0) | 0.74 |
| Age ≥75 y, n (%) | 1531 (38.1) | 503 (40.0) | 0.22 |
| Male sex, n (%) | 2683 (66.8) | 748 (59.5) | <0.0001 |
| Current smoker, n (%) | 303 (7.5) | 107 (8.5) | 0.26 |
| Weight, median (quartile 1–quartile 3), kg | 82.0 (71.0–95.0) | 78.0 (67.0–90.0) | <0.0001 |
| Body mass index, median (quartile 1–quartile 3), kg/m2 | 28.3 (25.3–32.0) | 27.6 (24.8–31.0) | <0.0001 |
| Systolic blood pressure, median (quartile 1–quartile 3), mm Hg | 130.0 (120.0–140.0) | 132.0 (120.0–145.0) | <0.0001 |
| Diastolic blood pressure, median (quartile 1–quartile 3), mm Hg | 80.0 (70.0–85.0) | 80.0 (70.0–86.0) | 0.75 |
| Heart rate, median (quartile 1–quartile 3), bpm | 76.0 (68.0–86.0) | 76.0 (66.0–87.0) | 0.94 |
| Type of atrial fibrillation, n (%) | |||
| Paroxysmal | 632 (15.7) | 154 (12.3) | 0.005 |
| Persistent | 1285 (32.0) | 397 (31.6) | |
| Permanent | 2099 (52.3) | 706 (56.2) | |
| Heart failure, n (%) | 1348 (33.5) | 651 (51.8) | <0.0001 |
| Diabetes mellitus, n (%) | 837 (20.8) | 329 (26.2) | <0.0001 |
| Coronary artery disease, n (%) | 896 (22.3) | 319 (25.4) | 0.0237 |
| Hypertension, n (%) | 3105 (77.3) | 1010 (80.4) | 0.0217 |
| Vascular disease, n (%)b | 669 (16.7) | 266 (21.2) | 0.0003 |
| History of stroke/SEE/TIA, n (%) | 872 (21.7) | 279 (22.2) | 0.71 |
| VKA use class at study entry, n (%) | |||
| Naive | 1869 (46.5) | 625 (49.7) | 0.0469 |
| Statin at baseline, n (%) | 1649 (41.0) | 474 (37.7) | 0.0355 |
| ARB and/or ACEi at baseline, n (%) | 2598 (64.7) | 938 (74.6) | <0.0001 |
| β Blocker at baseline, n (%) | 2558 (63.7) | 809 (64.4) | 0.65 |
| Digoxin at baseline, n (%) | 1209 (30.1) | 638 (50.8) | <0.0001 |
| CrCL at baseline, median (quartile 1–quartile 3), mL/min | 70.4 (55.6–88.6) | 64.3 (50.0–81.1) | <0.0001 |
| CrCL class at baseline, n (%) | |||
| <50 mL/min | 639 (16.1) | 312 (25.1) | <0.0001 |
| 50–<80 mL/min | 1929 (48.5) | 601 (48.3) | |
| ≥80 mL/min | 1412 (35.5) | 332 (26.7) | |
| Left ventricular ejection fraction, n (%) | |||
| ≤40% | 343 (8.5) | 232 (18.5) | NAc |
| >40% | 1493 (37.2) | 424 (33.7) | |
| Unknown | 2182 (54.3) | 501 (47.8) | |
| CHA2DS2VASc score, median (quartile 1–quartile 3) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | <0.0001 |
| CHA2DS2VASc score category, n (%) | |||
| ≤2 | 1051 (25.3) | 239 (19.0) | <0.0001 |
| >2 | 3003 (74.7) | 1018 (81.0) | |
| NT‐proBNP | |||
| Median (quartile 1–quartile 3), ng/L | 931 (575–1453) | 1354 (775–2277) | <0.0001 |
| n | 1250 | 3989 | |
| Troponin I | |||
| Median (quartile 1–quartile 3), ng/L | 6.0 (4.0–10.0) | 12.0 (6.6–21.0) | <0.0001 |
| n | 1191 | 3793 | |
| Troponin T | |||
| Median (quartile 1–quartile 3), ng/L | 11.2 (7.4–17.2) | 17.1 (10.8–27.1) | <0.0001 |
| n | 1167 | 3725 | |
| GDF‐15 | |||
| Median (quartile 1–quartile 3), ng/L | 1472 (1103–2090) | 1785 (1271–2601) | <0.0001 |
| n | 1159 | 3691 | |
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; bpm, beats per minute; CrCL, creatinine clearance; GDF‐15, growth differentiation factor‐15; LVH, left ventricular hypertrophy; NA, not applicable; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; SEE, systemic embolism; TIA, transient ischemic attack; VKA, vitamin K antagonist.
The P value is for the comparison between groups and is based on the χ2 test for categorical variables and the Kruskal‐Wallis test for continuous variables.
Vascular disease: peripheral artery disease or prior myocardial infarction.
Not calculated because of a large proportion with unknown values.
All the cardiovascular biomarkers and several clinical characteristics were significantly associated with presence of LVH. Patients with AF and LVH more often had heart failure, poor renal function, diabetes mellitus, hypertension, permanent AF, and vascular disease. They had also more commonly been prescribed digoxin, angiotensin‐converting enzyme inhibitors, and angiotensin II receptor blocker treatment, but less commonly statins. A slightly larger proportion of patients with both AF and LVH were naïve to oral anticoagulation therapy. In linear regression analyses adjusting for baseline characteristics, LVH was significantly (P<0.0001) associated with higher levels of all analyzed biomarkers (Table 2).
Table 2.
Linear Regression Analysis of Biomarker Level According to LVH Category
| Biomarker | Without LVH | With LVH | Ratio | P Value |
|---|---|---|---|---|
| NT‐proBNP, ng/L | 924 (903–946) | 1220 (1169–1273) | 1.32 (1.26–1.39) | <0.0001 |
| Troponin I, ng/L | 7.1 (6.9–7.3) | 11.8 (11.2–12.5) | 1.67 (1.57–1.78) | <0.0001 |
| Troponin T, ng/L | 11.6 (11.4–11.9) | 16.2 (15.6–16.9) | 1.40 (1.34–1.46) | <0.0001 |
| GDF‐15, ng/L | 1589 (1567–1612) | 1735 (1690–1780) | 1.09 (1.06–1.12) | <0.0001 |
Data are given as geometric mean (95% CI). Multiple linear regression analyses with log‐transformed continuous biomarker levels as outcome in models including LVH category (no/yes), age, sex, body mass index, current smoking, heart failure, hypertension, prior myocardial infarction, diabetes mellitus, systolic blood pressure, permanent atrial fibrillation, creatinine clearance, digoxin use, and angiotensin‐converting enzyme inhibitor/angiotensin II receptor blocker as explanatory variables. GDF‐15 indicates growth differentiation factor‐15; LVH, left ventricular hypertrophy; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
The baseline characteristics of the present cohort with available data on both biomarkers and LVH were similar to the larger cohort with available LVH data (Table S1).
Prognostic Value of LVH and Cardiac Biomarker Levels
During a median follow‐up of 2.0 years, 165 patients developed a stroke, 370 died, and 258 had a major bleeding event. For stroke, the hazard ratios (95% CIs) per 50% increase after adjustment for clinical risk factors and LVH were as follows: NT‐proBNP, 1.09 (1.00–1.19); hs‐cTnI, 1.09 (1.03–1.15); hs‐cTnT, 1.14 (1.06–1.24); and GDF‐15, 1.22 (1.08–1.38) (all P<0.05) (Figure 1). For death, the hazard ratios (95% CIs) per 50% increase were as follows: NT‐proBNP, 1.24 (1.17–1.31); hs‐cTnI, 1.13 (1.10–1.17); hs‐cTnT, 1.28 (1.23–1.34); and GDF‐15, 1.31 (1.22–1.42) (all P<0.0001) (Figure 1). Elevated biomarker levels were consistently associated with poorer prognosis in patients with AF, regardless of LVH (Figure 2). Results were similar for the association between biomarker levels and major bleeding (Figure 1).
Figure 1.
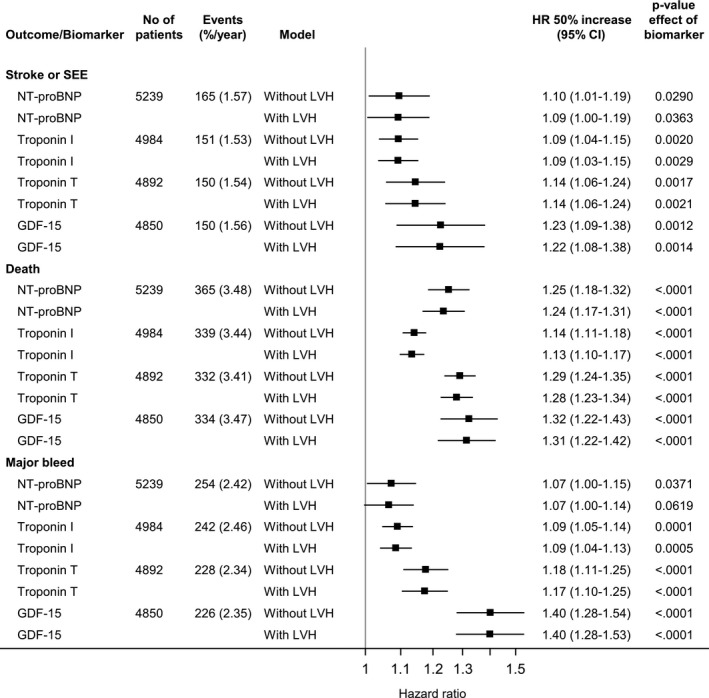
Impact of left ventricular hypertrophy (LVH) on the association between baseline biomarkers and stroke or systemic embolism (SEE), all‐cause mortality, and major bleeding outcomes. GDF‐15 indicates growth differentiation factor‐15; HR, hazard ratio; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
Figure 2.
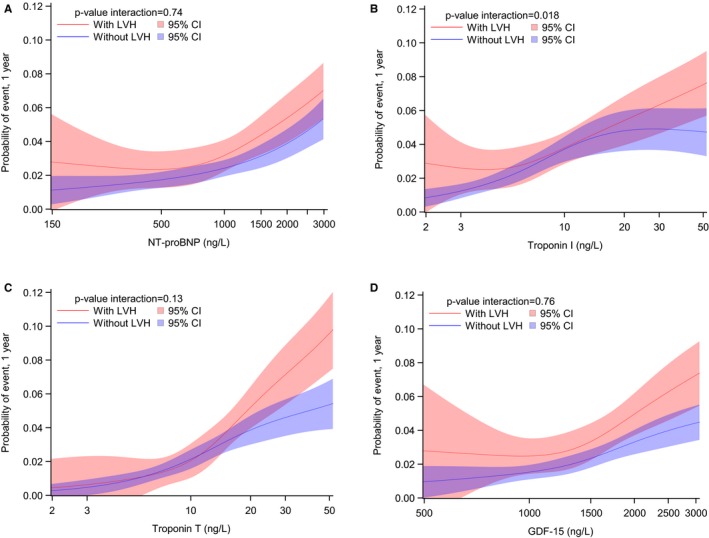
One‐year risk for all‐cause mortality by left ventricular hypertrophy (LVH) category according to levels of NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide; A), troponin I (B), troponin T (C), and growth differentiation factor‐15 (GDF‐15; D). The biomarkers were included as continuous, log transformed, and fitted using restricted cubic splines with 4 knots, located at the 5th, 35th, 65th, and 95th percentiles.
The influence of baseline biomarker levels on the association between LVH and outcomes was analyzed by adding biomarkers (continuous, log transformed) to Cox regression models adjusted for several baseline characteristics (Table 3).
Table 3.
Association Between LVH and Outcomes According to Adjustment for Biomarkers
| Outcome | Biomarker Added in Model | Events, %/Year | Adjusted Cox Model | ||
|---|---|---|---|---|---|
| Without LVH (N=3616) | With LVH (N=1136) | HR (95% CI) | P Valuea | ||
| Stroke or systemic embolism | LVH without biomarkers in model | 105 (1.46) | 43 (1.94) | 1.14 (0.79–1.64) | 0.49 |
| Troponin I | 1.02 (0.70–1.50) | 0.91 | |||
| Troponin T | 1.03 (0.71–1.49) | 0.89 | |||
| NT‐proBNP | 1.06 (0.73–1.54) | 0.75 | |||
| GDF‐15 | 1.10 (0.77–1.59) | 0.60 | |||
| Troponin T+NT‐proBNP | 0.99 (0.68–1.44) | 0.95 | |||
| Troponin T+NT‐proBNP+GDF‐15 | 1.00 (0.69–1.46) | 0.99 | |||
| All‐cause mortality | LVH without biomarkers in model | 204 (2.83) | 119 (5.38) | 1.60 (1.27–2.02) | 0.0001 |
| Troponin I | 1.33 (1.04–1.70) | 0.0249 | |||
| Troponin T | 1.28 (1.01–1.63) | 0.0408 | |||
| NT‐proBNP | 1.35 (1.07–1.72) | 0.0143 | |||
| GDF‐15 | 1.53 (1.21–1.93) | 0.0005 | |||
| Troponin T+NT‐proBNP | 1.17 (0.92–1.49) | 0.21 | |||
| Troponin T+NT‐proBNP+GDF‐15 | 1.19 (0.93–1.51) | 0.16 | |||
| Major bleed | LVH without biomarkers in model | 155 (2.15) | 67 (3.03) | 1.27 (0.95–1.70) | 0.12 |
| Troponin I | 1.20 (0.88–1.63) | 0.25 | |||
| Troponin T | 1.12 (0.83–1.51) | 0.47 | |||
| NT‐proBNP | 1.24 (0.92–1.68) | 0.16 | |||
| GDF‐15 | 1.22 (0.91–1.64) | 0.19 | |||
| Troponin T+NT‐proBNP | 1.12 (0.83–1.51) | 0.47 | |||
| Troponin T+NT‐proBNP+GDF‐15 | 1.18 (0.87–1.59) | 0.30 | |||
Biomarkers included as continuous, log‐transformed, variables. Patients with no missing data for clinical risk factors, troponin T, GDF‐15, and NT‐proBNP were included in the analysis. GDF‐15 indicates growth differentiation factor‐15; HR, hazard ratio; LVH, left ventricular hypertrophy; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide.
P value for effect of LVH.
LVH did not remain significantly associated with stroke or death after adjustment for biomarker levels. Similarly, there was no improvement in C‐indexes for stroke/systemic embolism, all‐cause mortality, or major bleeding events by adding LVH data to risk models containing the cardiovascular biomarkers (Table S2). The biomarkers, however, provided significant improvements when added on top of models containing LVH data.
Discussion
The present study provides new insight into the association between the cardiovascular biomarkers NT‐proBNP, troponin, and GDF‐15 and LVH in patients with AF. There was an independent association between LVH and increased levels of each biomarker in multiple linear regression analyses, in particular for the cardiac biomarkers (NT‐proBNP and troponin). Overall, the biomarkers remained independently associated with cardiovascular outcomes after adjustment for information on LVH status. On the other hand, LVH did not remain an independent predictor for stroke or mortality after adjustment for the cardiac biomarkers NT‐proBNP and troponin. Accordingly, in patients with AF, cardiac biomarkers were able to further identify patients with lower or higher risk both in the presence and in the absence of LVH by ECG.
LVH is a strong marker for adverse outcomes in several populations ranging from the general population to those with cardiovascular diseases,3, 17, 21, 22 but is not included among the traditional risk factors for stroke or death in AF.1, 2 LVH according to either ECG or echocardiography was recently shown to be a marker of increased risk for adverse outcomes also in patients with AF.4, 23 Similarly, the cardiovascular biomarkers NT‐proBNP,5, 8, 24, 25, 26 troponin,6, 7, 11, 27, 28 and GDF‐159, 29, 30, 31 are powerful risk markers for cardiovascular outcomes in both the general population and in patients with cardiovascular diseases, including AF.32 In the present study, we confirmed the significant independent associations between the levels of these cardiovascular biomarkers and adverse outcomes in patients with AF, even after adjustment for LVH by ECG. Biomarkers thus remained significant predictors for cardiovascular outcomes both in the presence as well as in the absence of LVH. In contrast, the association between LVH and outcomes did not remain after adjustment for biomarker levels. Previous studies on the prognostic impact of these biomarkers in other cohorts have accounted for the presence of LVH to a varying degree. This issue was recently investigated comprehensively in a general population in the DHS (Dallas Heart Study) cohort, which confirmed the prognostic value of the cardiac biomarkers.13 In the present study, we extended these observations and showed a prognostic value of the levels of the cardiovascular biomarkers beyond information on LVH also in patients with AF.
Causes of LVH mainly include hemodynamic states with increased afterload, such as hypertension and aortic valvular disease, besides specific genetic myocardial diseases, such as hypertrophic cardiomyopathies.33, 34 In addition, the levels of troponin, natriuretic peptides, and GDF‐15 have been shown to increase with progression of LVH.11, 12, 13, 35 LVH and elevated levels of cardiac biomarkers thus seem to have several causes in common. On the other hand, biomarker levels not only reflect structural abnormalities in the heart, but are also related to cardiac and vascular function.11, 12, 36 Besides the positive interaction between these variables, the reasons that cardiovascular biomarkers are stronger risk markers than LVH may be because of the fact that they also indicate underlying potentially clinically silent cardiovascular disease processes or dysfunctions that are also related to cardiovascular outcomes.7, 8, 9, 37 For example, the levels of cardiac biomarkers seem to signal adverse remodeling in patients with LVH, indicating a transition from structural LVH to clinical heart failure.13 This transition was recently termed “malignant LVH” and confers a state of even poorer prognosis.38 There are thus several reasons that cardiovascular biomarkers provide additional dimensions of information on risk of cardiovascular events that also seem to include the signal provided by LVH in patients with AF. This notion is also supported in the current findings, which show no added prognostic value of LVH by ECG in predictive models already containing the plasma biomarkers.
In the clinical setting, ECG recording is fundamental in the management of patients with AF to obtain information on heart rhythm and heart rate.1, 2 It therefore provides easy accessible screening of other possible abnormalities, such as LVH, and may aid in the decision about rate or rhythm control strategies and/or the selection of antiarrhythmic drugs.1, 2, 39 For risk stratification, electrographic LVH data may still provide practical prognostic information for cardiovascular events in AF. However, if plasma biomarker measurements are available, they can improve risk prediction in patients with AF both in the presence or absence of LVH.4, 40, 41 Measurement of the cardiac biomarkers NT‐proBNP and troponin would thus probably be useful as part of the routine evaluation of a patient with AF.
Limitations
The present study was a post hoc analysis based on a subgroup of participants in the RE‐LY trial with ECGs suitable for LVH analysis and biomarker measurements at baseline available. Generalizability of our results may thus be limited. Although baseline characteristics in patients with available biomarker measurements were almost identical to the total cohort with available LVH data, the annual event rate was somewhat lower in those with available biomarker measurements. This may have influenced the results on the association of LVH with outcomes in presence of biomarkers.4 LVH was assessed by ECG and not magnetic resonance imaging or echocardiography. ECG assessment of LVH traditionally indicates the presence or absence of LVH, not the actual severity. However, a validated definition for electrographic LVH was applied in the analysis, which also encompassed ECG strain patterns in addition to traditional voltage criteria, thereby providing additional prognostic information because strain pattern is associated with higher left ventricular mass indexes.21 Moreover, ECG screening is often used in large‐scale screening programs for practical reasons.
Conclusions
Levels of cardiac biomarkers are significantly associated with LVH. The prognostic value of biomarkers on stroke, death, and major bleeding is not affected by LVH. The association of LVH with outcomes is attenuated in the presence of cardiac biomarkers.
Sources of Funding
The RE‐LY (Randomized Evaluation of Long‐Term Anticoagulation Therapy) trial was funded by Boehringer Ingelheim Pharmaceuticals.
Disclosures
Dr Hijazi reports receiving lecture fees from Boehringer Ingelheim, Bristol‐Myers Squibb (BMS), and Pfizer; consulting fees from Merck Sharp & Dohme and BMS/Pfizer; and institutional research grants from Boehringer Ingelheim, BMS, and Pfizer. Dr Verdecchia reports research grants, consulting fees, and lecture fees from Boehringer Ingelheim. Dr Oldgren reports consulting and lecture fees from Boehringer‐Ingelheim, Bayer, BMS, and Pfizer. Ms Andersson reports an institutional research grant from BMS/Pfizer. Dr Di Pasquale reports research grants, consulting fees, and lecture fees from Boehringer Ingelheim. Dr Connolly reports consulting and research grants from Boehringer Ingelheim. Dr Ezekowitz is a consultant for and/or has received consulting/honoraria fees from Boehringer Ingelheim, Pfizer, Sanofi, BMS, Portola, Bayer, Daiichi‐Sankyo, Medtronic, Aegerion, Merck, Johnson & Johnson, Gilead, Janssen Scientific Affairs, Pozen Inc, Amgen, Coherex, and Armetheon. Dr Yusuf reports research grants from Boehringer Ingelheim. Dr Wallentin reports institutional research grants, consultancy and lecture fees, and travel support from AstraZeneca, BMS, Pfizer, Boehringer Ingelheim, and GlaxoSmithKline; honoraria from GlaxoSmithKline; institutional research grant from Merck & Co and Roche; consultancy fees from Abbot; and holds 2 patents involving growth differentiation factor‐15. The remaining authors have no disclosures to report.
Supporting information
Figure S1. Flowchart.
Table S1. Demographics and Clinical Characteristics for the Present Subgroup With Biomarkers and LVH‐Data Available in Comparison to the Cohort With LVH‐Data Available
Table S2. Discriminative Ability of Models With and Without Biomarkers and/or Left Ventricular Hypertrophy
(J Am Heart Assoc. 2019;8:e010107 DOI: 10.1161/JAHA.118.010107.)
References
- 1. Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010;31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC Jr, Cigarroa JE, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322:1561–1566. [DOI] [PubMed] [Google Scholar]
- 4. Verdecchia P, Reboldi G, Di Pasquale G, Mazzotta G, Ambrosio G, Yang S, Pogue J, Wallentin L, Ezekowitz MD, Connolly SJ, Yusuf S; Re‐Ly Study Investigators . Prognostic usefulness of left ventricular hypertrophy by electrocardiography in patients with atrial fibrillation (from the Randomized Evaluation of Long‐Term Anticoagulant Therapy Study). Am J Cardiol. 2014;113:669–675. [DOI] [PubMed] [Google Scholar]
- 5. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Ezekowitz MD, Hohnloser SH, Reilly PA, Vinereanu D, Siegbahn A, Yusuf S, Wallentin L. Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a Randomized Evaluation of Long‐term Anticoagulation Therapy (RE‐LY) substudy. Circulation. 2012;125:1605–1616. [DOI] [PubMed] [Google Scholar]
- 6. Hijazi Z, Siegbahn A, Andersson U, Granger CB, Alexander JH, Atar D, Gersh BJ, Mohan P, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L; Aristotle Investigators . High‐sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;129:625–634. [DOI] [PubMed] [Google Scholar]
- 7. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Alexander JH, Atar D, Gersh BJ, Hanna M, Harjola VP, Horowitz JD, Husted S, Hylek EM, Lopes RD, McMurray JJ, Granger CB; Aristotle Investigators . High‐sensitivity troponin T and risk stratification in patients with atrial fibrillation during treatment with apixaban or warfarin. J Am Coll Cardiol. 2014;63:52–61. [DOI] [PubMed] [Google Scholar]
- 8. Hijazi Z, Wallentin L, Siegbahn A, Andersson U, Christersson C, Ezekowitz J, Gersh BJ, Hanna M, Hohnloser S, Horowitz J, Huber K, Hylek EM, Lopes RD, McMurray JJ, Granger CB. N‐terminal pro‐B‐type natriuretic peptide for risk assessment in patients with atrial fibrillation: insights from the ARISTOTLE Trial (Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation). J Am Coll Cardiol. 2013;61:2274–2284. [DOI] [PubMed] [Google Scholar]
- 9. Wallentin L, Hijazi Z, Andersson U, Alexander JH, De Caterina R, Hanna M, Horowitz JD, Hylek EM, Lopes RD, Asberg S, Granger CB, Siegbahn A; Aristotle Investigators . Growth differentiation factor 15, a marker of oxidative stress and inflammation, for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and Other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;130:1847–1858. [DOI] [PubMed] [Google Scholar]
- 10. deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lind L, Wallentin L, Kempf T, Tapken H, Quint A, Lindahl B, Olofsson S, Venge P, Larsson A, Hulthe J, Elmgren A, Wollert KC. Growth‐differentiation factor‐15 is an independent marker of cardiovascular dysfunction and disease in the elderly: results from the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) Study. Eur Heart J. 2009;30:2346–2353. [DOI] [PubMed] [Google Scholar]
- 13. Neeland IJ, Drazner MH, Berry JD, Ayers CR, deFilippi C, Seliger SL, Nambi V, McGuire DK, Omland T, de Lemos JA. Biomarkers of chronic cardiac injury and hemodynamic stress identify a malignant phenotype of left ventricular hypertrophy in the general population. J Am Coll Cardiol. 2013;61:187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L; Re‐Ly Steering Committee and Investigators . Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 15. Ezekowitz MD, Connolly S, Parekh A, Reilly PA, Varrone J, Wang S, Oldgren J, Themeles E, Wallentin L, Yusuf S. Rationale and design of RE‐LY: randomized evaluation of long‐term anticoagulant therapy, warfarin, compared with dabigatran. Am Heart J. 2009;157:805–810, 810.e1‐2. [DOI] [PubMed] [Google Scholar]
- 16. Verdecchia P, Sleight P, Mancia G, Fagard R, Trimarco B, Schmieder RE, Kim JH, Jennings G, Jansky P, Chen JH, Liu L, Gao P, Probstfield J, Teo K, Yusuf S; Ontarget Transcend Investigators . Effects of telmisartan, ramipril, and their combination on left ventricular hypertrophy in individuals at high vascular risk in the Ongoing Telmisartan Alone and in Combination With Ramipril Global End Point Trial and the Telmisartan Randomized Assessment Study in ACE Intolerant Subjects With Cardiovascular Disease. Circulation. 2009;120:1380–1389. [DOI] [PubMed] [Google Scholar]
- 17. Verdecchia P, Angeli F, Reboldi G, Carluccio E, Benemio G, Gattobigio R, Borgioni C, Bentivoglio M, Porcellati C, Ambrosio G. Improved cardiovascular risk stratification by a simple ECG index in hypertension. Am J Hypertens. 2003;16:646–652. [DOI] [PubMed] [Google Scholar]
- 18. Kempf T, Horn‐Wichmann R, Brabant G, Peter T, Allhoff T, Klein G, Drexler H, Johnston N, Wallentin L, Wollert KC. Circulating concentrations of growth‐differentiation factor 15 in apparently healthy elderly individuals and patients with chronic heart failure as assessed by a new immunoradiometric sandwich assay. Clin Chem. 2007;53:284–291. [DOI] [PubMed] [Google Scholar]
- 19. Hijazi Z, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Yusuf S, Siegbahn A, Wallentin L. Growth‐differentiation factor 15 and risk of major bleeding in atrial fibrillation: insights from the Randomized Evaluation of Long‐Term Anticoagulation Therapy (RE‐LY) trial. Am Heart J. 2017;190:94–103. [DOI] [PubMed] [Google Scholar]
- 20. Hijazi Z, Siegbahn A, Andersson U, Lindahl B, Granger CB, Alexander JH, Atar D, Gersh BJ, Hanna M, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L. Comparison of cardiac troponins I and T measured with high‐sensitivity methods for evaluation of prognosis in atrial fibrillation: an ARISTOTLE substudy. Clin Chem. 2015;61:368–378. [DOI] [PubMed] [Google Scholar]
- 21. Shah AS, Chin CW, Vassiliou V, Cowell SJ, Doris M, Kwok TC, Semple S, Zamvar V, White AC, McKillop G, Boon NA, Prasad SK, Mills NL, Newby DE, Dweck MR. Left ventricular hypertrophy with strain and aortic stenosis. Circulation. 2014;130:1607–1616. [DOI] [PubMed] [Google Scholar]
- 22. Westerhout CM, Lauer MS, James S, Fu Y, Wallentin L, Armstrong PW; Gusto IV ACS Investigators . Electrocardiographic left ventricular hypertrophy in GUSTO IV ACS: an important risk marker of mortality in women. Eur Heart J. 2007;28:2064–2069. [DOI] [PubMed] [Google Scholar]
- 23. Apostolakis S, Sullivan RM, Olshansky B, Lip GY. Left ventricular geometry and outcomes in patients with atrial fibrillation: the AFFIRM Trial. Int J Cardiol. 2014;170:303–308. [DOI] [PubMed] [Google Scholar]
- 24. James SK, Lindahl B, Siegbahn A, Stridsberg M, Venge P, Armstrong P, Barnathan ES, Califf R, Topol EJ, Simoons ML, Wallentin L. N‐terminal pro‐brain natriuretic peptide and other risk markers for the separate prediction of mortality and subsequent myocardial infarction in patients with unstable coronary artery disease: a Global Utilization of Strategies To Open occluded arteries (GUSTO)‐IV substudy. Circulation. 2003;108:275–281. [DOI] [PubMed] [Google Scholar]
- 25. Tsutamoto T, Wada A, Maeda K, Hisanaga T, Maeda Y, Fukai D, Ohnishi M, Sugimoto Y, Kinoshita M. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation. 1997;96:509–516. [DOI] [PubMed] [Google Scholar]
- 26. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 27. Latini R, Masson S, Anand IS, Missov E, Carlson M, Vago T, Angelici L, Barlera S, Parrinello G, Maggioni AP, Tognoni G, Cohn JN. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation. 2007;116:1242–1249. [DOI] [PubMed] [Google Scholar]
- 28. Omland T, de Lemos JA, Sabatine MS, Christophi CA, Rice MM, Jablonski KA, Tjora S, Domanski MJ, Gersh BJ, Rouleau JL, Pfeffer MA, Braunwald E. A sensitive cardiac troponin T assay in stable coronary artery disease. N Engl J Med. 2009;361:2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anand IS, Kempf T, Rector TS, Tapken H, Allhoff T, Jantzen F, Kuskowski M, Cohn JN, Drexler H, Wollert KC. Serial measurement of growth‐differentiation factor‐15 in heart failure: relation to disease severity and prognosis in the Valsartan Heart Failure Trial. Circulation. 2010;122:1387–1395. [DOI] [PubMed] [Google Scholar]
- 30. Daniels LB, Clopton P, Laughlin GA, Maisel AS, Barrett‐Connor E. Growth‐differentiation factor‐15 is a robust, independent predictor of 11‐year mortality risk in community‐dwelling older adults: the Rancho Bernardo Study. Circulation. 2011;123:2101–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, Ponikowski P, Filippatos GS, Rozentryt P, Drexler H, Anker SD, Wollert KC. Prognostic utility of growth differentiation factor‐15 in patients with chronic heart failure. J Am Coll Cardiol. 2007;50:1054–1060. [DOI] [PubMed] [Google Scholar]
- 32. Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem. 2017;63:152–164. [DOI] [PubMed] [Google Scholar]
- 33. Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, Fauad‐Tarazi F, Horan MJ, Marcus M, Massie B. The heart in hypertension. N Engl J Med. 1992;327:998–1008. [DOI] [PubMed] [Google Scholar]
- 34. Authors/Task Force Members , Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 35. Chin CW, Shah AS, McAllister DA, Joanna Cowell S, Alam S, Langrish JP, Strachan FE, Hunter AL, Maria Choy A, Lang CC, Walker S, Boon NA, Newby DE, Mills NL, Dweck MR. High‐sensitivity troponin I concentrations are a marker of an advanced hypertrophic response and adverse outcomes in patients with aortic stenosis. Eur Heart J. 2014;35:2312–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Redfield MM, Rodeheffer RJ, Jacobsen SJ, Mahoney DW, Bailey KR, Burnett JC Jr. Plasma brain natriuretic peptide to detect preclinical ventricular systolic or diastolic dysfunction: a community‐based study. Circulation. 2004;109:3176–3181. [DOI] [PubMed] [Google Scholar]
- 37. Nadir MA, Rekhraj S, Wei L, Lim TK, Davidson J, MacDonald TM, Lang CC, Dow E, Struthers AD. Improving the primary prevention of cardiovascular events by using biomarkers to identify individuals with silent heart disease. J Am Coll Cardiol. 2012;60:960–968. [DOI] [PubMed] [Google Scholar]
- 38. Peters MN, Seliger SL, Christenson RH, Hong‐Zohlman SN, Daniels LB, Lima JAC, de Lemos JA, Neeland IJ, deFilippi CR. “Malignant” left ventricular hypertrophy identifies subjects at high risk for progression to asymptomatic left ventricular dysfunction, heart failure, and death: MESA (Multi‐Ethnic Study of Atherosclerosis). J Am Heart Assoc. 2018;7:e006619 DOI: 10.1161/JAHA.117.006619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Badheka AO, Shah N, Grover PM, Patel NJ, Chothani A, Mehta K, Singh V, Deshmukh A, Savani GT, Rathod A, Panaich SS, Patel N, Arora S, Bhalara V, Coffey JO, Mitrani RD, Halperin JL, Viles‐Gonzalez JF. Outcomes in atrial fibrillation patients with and without left ventricular hypertrophy when treated with a lenient rate‐control or rhythm‐control strategy. Am J Cardiol. 2014;113:1159–1165. [DOI] [PubMed] [Google Scholar]
- 40. Hijazi Z, Lindback J, Alexander JH, Hanna M, Held C, Hylek EM, Lopes RD, Oldgren J, Siegbahn A, Stewart RA, White HD, Granger CB, Wallentin L; Aristotle and Stability Investigators . The ABC (age, biomarkers, clinical history) stroke risk score: a biomarker‐based risk score for predicting stroke in atrial fibrillation. Eur Heart J. 2016;37:1582–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hijazi Z, Oldgren J, Lindback J, Alexander JH, Connolly SJ, Eikelboom JW, Ezekowitz MD, Held C, Hylek EM, Lopes RD, Siegbahn A, Yusuf S, Granger CB, Wallentin L; Aristotle, Re‐Ly Investigators . The novel biomarker‐based ABC (age, biomarkers, clinical history)‐bleeding risk score for patients with atrial fibrillation: a derivation and validation study. Lancet. 2016;387:2302–2311. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flowchart.
Table S1. Demographics and Clinical Characteristics for the Present Subgroup With Biomarkers and LVH‐Data Available in Comparison to the Cohort With LVH‐Data Available
Table S2. Discriminative Ability of Models With and Without Biomarkers and/or Left Ventricular Hypertrophy


