Abstract
Background
The 2013 American College of Cardiology/American Heart Association cholesterol guidelines recognize cardiovascular disease and diabetes mellitus but not chronic kidney disease (CKD) as high‐risk conditions warranting statin therapy. Statin use may be lower for adults with CKD compared with adults with conditions that have guideline indications for statin use.
Methods and Results
We analyzed data from the National Health and Nutrition Examination Surveys from 1999–2002 through 2011–2014 to determine trends in the percentage of US adults ≥20 years of age with and without CKD taking statins. CKD was defined by an estimated glomerular filtration rate <60 mL/min per 1.73m2 or albumin‐to‐creatinine ratio ≥30 mg/g. Statin use was identified through a medication inventory. Between 1999–2002 and 2011–2014, the percentage of adults taking statins increased from 17.6% to 35.7% among those with CKD and from 6.8% to 14.7% among those without CKD. After multivariable adjustment, adults with CKD were not more likely to be taking statins compared with those without CKD (prevalence ratio, 1.01; 95% CI] 0.96–1.08). Among adults without a history of cardiovascular disease, those with CKD but not diabetes mellitus were less likely to be taking statins compared with those with diabetes mellitus but not CKD (prevalence ratio, 0.54; 95% CI, 0.44–0.66). Among adults with a history of cardiovascular disease, there was no difference in statin use between those with CKD but not diabetes mellitus versus those with diabetes mellitus but not CKD (prevalence ratio, 0.95; 95% CI, 0.79–1.15).
Conclusions
CKD does not appear to be a major stimulus for statin use among US adults.
Keywords: chronic kidney disease, statin, trends
Subject Categories: Epidemiology
Clinical Perspective
What Is New?
Approximately two thirds (65%) of adults with chronic kidney disease (CKD) were recommended a statin based on the 2013 American College of Cardiology/American Heart Association guideline; however, only 36% of adults with CKD were taking a statin in 2011–2014.
The prevalence of statin use was lower among adults with CKD in the absence of another condition with high CVD risk (ie, diabetes mellitus) compared with adults with diabetes mellitus in the absence of CKD.
What Are the Clinical Implications?
These data demonstrate the underutilization of statins among patients with CKD and this suggests that its use may increase with its recognition as a risk‐enhancing factor in the 2018 American College of Cardiology/American Heart Association cholesterol management guideline.
Introduction
A number of studies over the past 15 years have demonstrated that adults with chronic kidney disease (CKD) have high cardiovascular disease (CVD) risk.1, 2, 3, 4 For example, in an analysis of 3 community‐based cohort studies, the incidence of coronary heart disease and stroke events was 24.5 and 13.4 per 1000 person‐years, respectively, among those with CKD and 8.4 and 4.8 per 1000 person‐years, respectively, among their counterparts without CKD.5 High low‐density lipoprotein‐cholesterol (LDL‐C) is a risk factor for CVD, both in the general population6 and among adults with CKD.7 Statins, which lower LDL‐C, are a key therapy for preventing CVD8, 9 and randomized controlled trials have demonstrated as much as a 25% risk reduction in atherosclerotic events and mortality with statin therapy in adults with CKD not requiring dialysis.10, 11, 12
Statin use has increased markedly among US adults between 1999–2000 and 2011–2012.13 Additionally, there has been increased awareness of the high CVD risk among adults with CKD.14 Studies using data collected in the early 2000s reported that adults with CKD were less likely to be taking a statin compared with their counterparts without CKD.15 In 2013, the American College of Cardiology/American Heart Association (ACC/AHA) updated the US guideline on the treatment of blood cholesterol and shifted to an atherosclerotic cardiovascular disease (ASCVD) risk‐based approach to guide treatment.9 While a history of CVD, diabetes mellitus, LDL‐C ≥190 mg/dL, and 10‐year predicted ASCVD risk ≥7.5% were indications for statin therapy, CKD was not included as a statin benefit group in this guideline. A population‐based study of middle‐aged and older adults recruited between 2003 and 2007 found that 92% of adults with CKD had an indication for a statin based on the 2013 ACC/AHA cholesterol guideline.15 However, only 50% of participants were taking a statin. The 2018 ACC/AHA cholesterol guideline defined CKD as a risk‐enhancing factor for the primary prevention of ASCVD and recommends statins for adults with non–dialysis‐dependent CKD who have LDL‐C ≥70 mg/dL and a 10‐year ASCVD risk ≥7.5%.16 It is unclear whether a difference in statin use still exists between adults with versus without CKD. Therefore, we examined trends in statin use from 1999–2002 through 2011–2014 among US adults with and without CKD. We additionally compared statin use among adults with CKD to those with diabetes mellitus, a specific indication for a statin based on the 2013 ACC/AHA cholesterol guideline. Finally, we examined trends in nonstatin lipid‐lowering therapy (NSLLT) use among adults with and without CKD.
Methods
The data and study materials are publicly available.17 The analytic methods have been made available within the article to other researchers for purposes of reproducing the results or replicating the procedure.
The National Health and Nutrition Examination Survey (NHANES) is conducted in 2‐year cycles by the National Center for Health Statistics of the Centers for Disease Control and Prevention.18 Each 2‐year cycle includes a sample of the non‐institutionalized civilian US population identified through a stratified, multistage probability sampling design. Two‐year cycles can be combined to produce more stable prevalence estimates. For the current analysis, we included adults who were ≥20 years of age (n=38 336) in the eight 2‐year cycles conducted from 1999–2000 through 2013–2014. The protocol for each NHANES cycle was approved by the National Center for Health Statistics of the Centers for Disease Control and Prevention Institutional Review Board. Written informed consent was obtained from each participant.
Data Collection
Data were collected through questionnaires and a physical examination. Questionnaires were used to obtain information on age, race, sex, education, income, smoking, history of CVD, and antihypertensive and glucose‐lowering medication use. During the physical examination, blood pressure, height, and weight were measured and blood and a random spot urine sample were collected. Body mass index was calculated using weight and height as measured during the study visit and categorized as <25, 25 to <30, and ≥30 kg/m2. Blood pressure was measured 3 times following a standardized protocol and averaged for all analyses. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or antihypertensive medication use. Glucose, glycated hemoglobin, creatinine, total and high‐density lipoprotein‐cholesterol (HDL‐C), and triglycerides were measured using blood collected during the study visit. LDL‐C was calculated using the Friedewald equation.19 Non–HDL‐C was calculated as total cholesterol minus HDL‐cholesterol.
Estimated glomerular filtration rate (eGFR) was calculated using serum creatinine and the Chronic Kidney Disease Epidemiology Collaboration equation.20 Reduced eGFR was defined by levels <60 mL/min per 1.73 m2.21 With the use of a random spot collection, urinary albumin and creatinine were measured and the albumin‐to‐creatinine ratio was calculated. Albuminuria was defined as an albumin‐to‐creatinine ratio ≥30 mg/g. CKD was defined as reduced eGFR or albuminuria. Stage 1 CKD was defined as eGFR ≥90 mL/min per 1.73 m2 and albumin‐to‐creatinine ratio ≥30 mg/g, stage 2 CKD as eGFR 60 to 89 mL/min per 1.73 m2 and albumin‐to‐creatinine ratio ≥30 mg/g, stage 3 CKD as eGFR 30 to 59 mL/min per 1.73 m2, and stage 4 to 5 CKD as eGFR <30 mL/min per 1.73 m2.22
Use of statins and NSLLT medications were determined through a pill bottle review that was conducted as part of the study examination. During the household interview, survey participants were asked to show all prescription medications taken in the past 30 days. Statins included atorvastatin, simvastatin, rosuvastatin, fluvastatin, pitavastatin, lovastatin, and cerivastatin. NSLLT included fibric acid derivatives, bile acid sequestrants, cholesterol absorption inhibitors, and niacin. Medication doses were not available.
CVD Risk Groups
There are 4 broad indications for statin therapy in the 2013 ACC/AHA guideline: history of CVD, diabetes mellitus, LDL‐C ≥190 mg/dL, and 10‐year predicted ASCVD risk ≥7.5% as defined by the Pooled Cohort risk equations.9 In the current study, history of CVD was defined as a self‐report of a prior diagnosis of myocardial infarction, coronary heart disease, or stroke. Diabetes mellitus was defined as a fasting plasma glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL, glycated hemoglobin level ≥6.5%, or self‐report of a history of diabetes mellitus with concurrent glucose‐lowering medication use. Adults were categorized into 5 hierarchical and mutually exclusive CVD risk groups defined by the first of the following conditions met: (1) history of CVD, (2) diabetes mellitus, (3) LDL‐C ≥190 mg/dL (non–HDL‐C ≥220 mg/dL for nonfasting adults), (4) 10‐year predicted ASCVD risk ≥7.5%, and (5) 10‐year predicted ASCVD risk <7.5%. A small number of NHANES participants had LDL‐C ≥190 mg/dL or non–HDL‐C ≥220 mg/dL in the absence of CVD or diabetes mellitus (n<50 in each calendar period). Therefore, results from this group are not presented.
Statistical Analysis
NHANES cycles were pooled into 4‐year calendar periods (1999–2002, 2003–2006, 2007–2010, 2011–2014) for all analyses. Characteristics of US adults and the proportion of US adults taking statins were calculated within each calendar period by CKD status, overall, and within CVD risk groups. Trends in the percentage of adults taking statins across calendar periods were calculated by modeling statin use with 4‐year calendar periods as a continuous independent variable. Differences in trends in the proportion of adults taking a statin between those with and without CKD were tested by including an interaction term between calendar period, modeled as a continuous variable, and CKD status. We also compared the percentage of adults taking a statin in 2011–2014 versus 1999–2002 using a chi‐squared test.
Using Poisson regression with progressive adjustment, we calculated prevalence ratios (PRs) and 95% CIs for statin use comparing US adults with versus without CKD during the overall study period (1999–2014). Model 1 was unadjusted. Model 2 included adjustment for age, race/ethnicity, sex, and calendar period. Model 3 included adjustment for all covariates simultaneously. The percentage of US adults in each calendar period and the PRs for taking statins in 1999–2014 were also calculated by CKD stage (no CKD, stage 1 or 2, stage 3, and stage 4 or 5) and for US adults with and without albuminuria.
To contrast statin use for adults with CKD versus their counterparts with a specific indication for treatment, we calculated the percentage of US adults with CKD in the absence of diabetes mellitus and with diabetes mellitus in the absence of CKD who were taking a statin within each calendar period. This analysis was conducted among those with and without a history of CVD, separately. Poisson regression was used to calculate PRs for statin use in 2011–2014 comparing US adults with CKD in the absence of diabetes mellitus versus diabetes mellitus in the absence of CKD with 3 levels of adjustment as described above.
Finally, we calculated the percentage of US adults with and without CKD adults who were taking NSLLT overall, NSLLT monotherapy (ie, NSLLT without a statin), and combination statin/NSLLT therapy, separately. Using Poisson regression with 3 levels of adjustment as described above, we calculated PRs for NSLLT use comparing US adults with versus without CKD during the overall study period (1999–2014). All statistical tests were 2 sided with a P<0.05 considered statistically significant. NHANES sampling weights were used in all calculations to obtain US nationally representative prevalence estimates, and the complex sampling design of NHANES was taken into account in all analyses. Stata 13.1 (StataCorp, College Station, TX) was used for all analyses.
Results
Population Characteristics
In each calendar period, the majority of US adults with CKD were older than 60 years of age compared with fewer than 25% of their counterparts without CKD (Table 1). Those with CKD were less likely to be male compared with their counterparts without CKD. Adults with CKD were more likely to have less than a high school education; have an annual household income <$20 000; be former smokers; and have a body mass index ≥30 kg/m2, hypertension, diabetes mellitus, and a history of CVD compared with those without CKD. Also, among those without a history of CVD, individuals with CKD were more likely to have a 10‐year ASCVD risk ≥7.5%. In 2011–2014, 65.3% of adults with CKD versus 27.4% of adults without CKD had an indication for statins based on the 2013 ACC/AHA cholesterol guideline.
Table 1.
Characteristics of US Adults Aged ≥20 Years With and Without Chronic Kidney Disease, NHANES 1999–2014
| CKD (n=7153) | No CKD (n=31 183) | |||||||
|---|---|---|---|---|---|---|---|---|
| 1999–2002 (n=1528) | 2003–2006 (n=1689) | 2007–2010 (n=2039) | 2011–2014 (n=1897) | 1999–2002 (n=7140) | 2003–2006 (n=7076) | 2007–2010 (n=8787) | 2011–2014 (n=8180) | |
| Age, y | ||||||||
| 20–39 | 16.9 | 14.4 | 14.6 | 15.9 | 43.7 | 42.7 | 41.3 | 40.3 |
| 40–59 | 30.3 | 28.0 | 27.1 | 29.0 | 38.9 | 40.7 | 40.6 | 39.1 |
| ≥60 | 52.8 | 57.6 | 58.3 | 55.1 | 17.4 | 16.6 | 18.2 | 20.6 |
| Race/ethnicity | ||||||||
| Non‐Hispanic White | 72.9 | 73.2 | 72.7 | 69.9 | 70.7 | 71.8 | 68.1 | 65.5 |
| Non‐Hispanic Black | 10.9 | 11.3 | 11.2 | 11.9 | 10.6 | 11.3 | 11.2 | 11.3 |
| Hispanic | 11.9 | 9.3 | 11.2 | 12.0 | 14.3 | 11.7 | 13.8 | 15.0 |
| Other | 4.3 | 6.2 | 4.9 | 6.2 | 4.4 | 5.2 | 6.9 | 8.2 |
| Male | 42.0 | 40.5 | 41.2 | 41.1 | 48.7 | 49.4 | 49.5 | 49.5 |
| Less than HS education | 31.5 | 25.1 | 25.3 | 21.3 | 20.1 | 16.5 | 18.6 | 14.7 |
| Income <$20 000 | 31.5 | 25.8 | 21.2 | 22.9 | 17.5 | 14.2 | 13.8 | 14.1 |
| Smoking | ||||||||
| Never | 48.7 | 50.0 | 52.5 | 49.9 | 51.0 | 50.4 | 54.4 | 57.7 |
| Former | 31.6 | 32.3 | 30.9 | 33.4 | 24.2 | 23.8 | 23.3 | 22.2 |
| Current | 19.7 | 17.7 | 16.6 | 16.7 | 24.9 | 25.8 | 22.3 | 20.1 |
| BMI, kg/m2 | ||||||||
| <25 | 31.2 | 27.6 | 28.4 | 25.7 | 35.7 | 34.1 | 32.0 | 30.9 |
| 25 to <30 | 31.3 | 33.6 | 30.0 | 30.8 | 35.4 | 33.7 | 34.6 | 34.1 |
| ≥30 | 37.5 | 38.8 | 41.6 | 43.4 | 28.9 | 32.2 | 33.3 | 35.1 |
| Hypertension | 58.1 | 61.5 | 61.6 | 59.9 | 23.7 | 24.8 | 25.1 | 26.1 |
| Non–HDL‐C, mg/dL | ||||||||
| <100 | 7.4 | 12.9 | 15.2 | 18.9 | 8.9 | 12.6 | 12.9 | 15.5 |
| 100–129 | 18.8 | 24.4 | 28.7 | 26.7 | 23.5 | 25.1 | 26.0 | 27.8 |
| 130–159 | 30.6 | 27.0 | 25.5 | 24.9 | 29.2 | 29.0 | 28.2 | 28.8 |
| 160–189 | 24.9 | 18.4 | 16.6 | 16.8 | 21.7 | 18.9 | 19.7 | 17.3 |
| 190–219 | 10.7 | 10.1 | 8.8 | 7.9 | 10.9 | 9.3 | 8.6 | 7.5 |
| ≥220 | 7.6 | 7.2 | 5.2 | 4.8 | 5.9 | 5.1 | 4.6 | 3.1 |
| LDL‐C, mg/dLa | ||||||||
| <70 | 5.8 | 11.5 | 12.9 | 15.2 | 3.9 | 7.6 | 6.4 | 7.8 |
| 70–99 | 19.9 | 28.9 | 28.5 | 30.4 | 22.3 | 26.0 | 26.3 | 27.4 |
| 100–129 | 35.6 | 28.7 | 32.3 | 27.8 | 34.1 | 34.0 | 34.9 | 34.0 |
| 130–159 | 24.9 | 20.0 | 16.0 | 17.6 | 25.4 | 21.4 | 21.6 | 21.5 |
| 160–189 | 10.8 | 6.9 | 7.8 | 6.4 | 10.8 | 8.0 | 7.9 | 7.0 |
| ≥190 | 3.0 | 4.0 | 2.4 | 2.6 | 3.5 | 3.0 | 2.9 | 2.4 |
| Diabetes mellitus | 24.0 | 25.4 | 28.6 | 27.8 | 5.7 | 6.5 | 7.8 | 8.2 |
| History of CVD | 17.6 | 21.2 | 18.3 | 19.1 | 4.8 | 4.8 | 4.8 | 4.4 |
| 10‐y predicted ASCVD risk ≥7.5%b | 55.7 | 55.4 | 56.9 | 52.3 | 20.2 | 19.1 | 19.4 | 21.2 |
| History of CVD, diabetes mellitus, LDL‐C ≥190 mg/dL or ASCVD risk ≥7.5%c | 67.0 | 67.9 | 67.3 | 65.3 | 26.1 | 25.4 | 26.1 | 27.4 |
All numbers presented are percentages, weighted to the US population according to NHANES analytic guidelines. All values in the table are percentages. ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; HS, high school; NHANES, National Health And Nutrition Examination Survey; LDL‐C, low‐density lipoprotein‐cholesterol; non–HDL‐C, non–high‐density lipoprotein‐cholesterol.
Among participants without CKD (n=14 660) and with CKD (n=3231) who have measured LDL‐C.
Among those without a history of cardiovascular disease.
Among the overall population.
Statin Use Among Those With and Without CKD
Between 1999–2002 and 2011–2014, the percentage of US adults ≥20 years taking statins increased from 17.6% to 35.7% for those with CKD and from 6.8% to 14.7% for those without CKD. Among adults with and without CKD, statin use increased in each CVD risk group except for those who had CKD and a 10‐year predicted ASCVD risk <7.5% (Figure 1 and Table S1). In the overall population and within CVD risk groups, trends in the percentage of US adults taking statins between 1999–2002 and 2011–2014 were not statistically significantly different for those with and without CKD (P=0.17). After multivariable adjustment, CKD was not associated with statin use (PR 1.01; 95% CI 0.96; 1.08; Table 2). The percentage of US adults taking statins was higher with progressively more severe CKD and for those with versus without albuminuria (Table S2). Statin use increased from 1999–2002 to 2011–2014 within each CKD stage and for participants with and without albuminuria. After multivariable adjustment, individuals with stage 3 CKD compared with no CKD were more likely to be taking a statin (Table S3). Having albuminuria was not associated with statin use after multivariable adjustment (Table S4).
Figure 1.
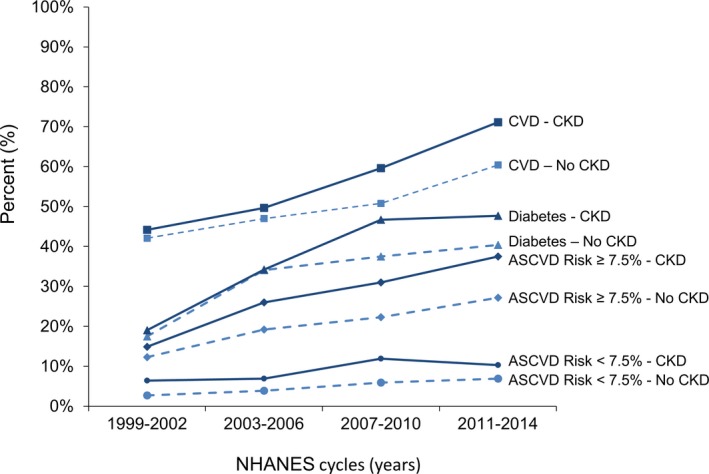
Percentage of statin use among US adults ≥20 years old in CVD risk groups, NHANES 1999–2014. ASCVD indicates atherosclerotic cardiovascular disease; CKD, chronic kidney disease; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey.
Table 2.
Prevalence Ratios and 95% CIs Associated With Statin Use Among US Adults ≥20 Years Old, NHANES 1999–2014
| Characteristics | Prevalence Ratio (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| CKD | 2.61 (2.46–2.77) | 1.38 (1.30–1.47) | 1.01 (0.96–1.08) |
| Calendar year | |||
| 1999–2002 | 1 (ref) | 1 (ref) | 1 (ref) |
| 2003–2006 | 1.45 (1.26–1.68) | 1.41 (1.26–1.58) | 1.36 (1.21–1.52) |
| 2007–2010 | 1.83 (1.61–2.07) | 1.73 (1.57–1.91) | 1.64 (1.49–1.81) |
| 2011–2014 | 2.17 (1.91–2.48) | 1.98 (1.78–2.21) | 1.88 (1.68–2.11) |
| Age, y | |||
| 20 to 39 | 1 (ref) | 1 (ref) | 1 (ref) |
| 40 to 59 | 12.00 (9.66–14.91) | 11.72 (9.42–14.58) | 8.12 (6.52–10.11) |
| ≥60 | 33.21 (26.77–41.19) | 32.16 (25.86–40.00) | 14.17 (11.27–17.83) |
| Race/ethnicity | |||
| Non‐Hispanic White | 1 (ref) | 1 (ref) | 1 (ref) |
| Non‐Hispanic Black | 0.69 (0.63–0.76) | 0.89 (0.82–0.97) | 0.75 (0.70–0.82) |
| Hispanic | 0.43 (0.38–0.48) | 0.70 (0.63–0.77) | 0.69 (0.62–0.75) |
| Other | 0.73 (0.63–0.85) | 0.98 (0.86–1.11) | 0.92 (0.81–1.05) |
| Male | 1.17 (1.10–1.25) | 1.27 (1.19–1.34) | 1.13 (1.06–1.21) |
| Less than HS education | 1.10 (1.01–1.20) | 1.00 (0.92–1.07) | 0.94 (0.88–1.00) |
| Income <$20 000 | 1.07 (0.97–1.17) | 0.96 (0.89–1.04) | 0.91 (0.84–0.97) |
| Smoking | |||
| Never | 1 (ref) | 1 (ref) | 1 (ref) |
| Former | 1.82 (1.70–1.95) | 1.17 (1.10–1.24) | 1.10 (1.03–1.17) |
| Current | 0.75 (0.67–0.85) | 0.92 (0.83–1.02) | 0.96 (0.87–1.06) |
| BMI, kg/m2 | |||
| <25 | 1 (ref) | 1 (ref) | 1 (ref) |
| 25 to <30 | 1.85 (1.68–2.03) | 1.48 (1.36–1.60) | 1.33 (1.22–1.44) |
| ≥30 | 2.17 (1.97–2.39) | 1.82 (1.67–1.99) | 1.30 (1.19–1.42) |
| Hypertension | 4.73 (4.38–5.12) | 2.18 (2.00–2.38) | 1.86 (1.71–2.02) |
| Diabetes mellitus | 3.93 (3.68–4.20) | 2.28 (2.14–2.44) | 1.80 (1.67–1.93) |
| History of CVD | 4.77 (4.50–5.07) | 2.24 (2.10–2.38) | 1.90 (1.77–2.04) |
Model 1 is unadjusted. Model 2 includes age, race, sex, and calendar period, plus individual covariates listed in the table one at a time. Model 3 includes all covariates listed in table. BMI indicates body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; HS, high school; NHANES, National Health and Nutrition Examination Survey.
Statin Use Among Those With CKD and Diabetes Mellitus
Among adults without a history of CVD, statin use increased between 1999–2002 and 2011–2014 from 10.0% to 21.0% for those with CKD in the absence of diabetes mellitus and from 17.5% to 40.4% among their counterparts with diabetes mellitus in the absence of CKD (Figure 2 and Table S5). The proportion of US adults with a history of CVD taking a statin increased from 40.9% to 67.2% between 1999–2002 and 2011–2014 for those with CKD in the absence of diabetes mellitus and from 55.3% to 76.1% among their counterparts with diabetes mellitus in the absence of CKD. Among US adults without CVD, those with CKD in the absence of diabetes mellitus were less likely to be taking a statin compared with their counterparts with diabetes mellitus in the absence of CKD (multivariable‐adjusted PR 0.54; 95% CI, 0.44–0.66; Table 3). Among US adults with a history of CVD, the PR for taking statins was 0.95 (95% CI, 0.79–1.15) comparing those with CKD in the absence of diabetes mellitus versus their counterparts with diabetes mellitus in the absence of CKD.
Figure 2.

Percentage of statin use among US adults ≥20 years old with CKD and no diabetes vs diabetes and no CKD, by history of CVD, NHANES 1999–2014. CKD indicates chronic kidney disease; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey.
Table 3.
Prevalence Ratios and 95% CIs Associated With Statin Use Among US Adults ≥20 Years Old With CKD in the Absence of Diabetes Mellitus, by History of CVD, NHANES 2011–2014
| Prevalence Ratio (95% CI) | |||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| No history of CVD | |||
| Diabetes mellitus and no CKD | 1 (ref) | 1 (ref) | 1 (ref) |
| CKD and no diabetes mellitus | 0.52 (0.44–0.62) | 0.52 (0.42–0.64) | 0.54 (0.44–0.66) |
| History of CVD | |||
| Diabetes mellitus and no CKD | 1 (ref) | 1 (ref) | 1 (ref) |
| CKD and no diabetes mellitus | 0.88 (0.74–1.06) | 0.91 (0.76–1.08) | 0.95 (0.79–1.15) |
Model 1 is unadjusted. Model 2 includes age, race, sex, and calendar period. Model 3 includes Model 2 covariates plus education, income, smoking, body mass index, hypertension, diabetes mellitus, and history of cardiovascular disease. CKD, chronic kidney disease; CVD, cardiovascular disease; NHANES, National Health and Nutrition Examination Survey.
NSLLT Use
Between 1999–2002 and 2011–2014, the percentage of US adults taking NSLLT increased from 2.6% to 7.1% among those with CKD and from 1.1% to 2.1% among those without CKD (Table S6). In unadjusted analyses, US adults with CKD were 3.26 times (95% CI, 2.77–3.83) more likely than their counterparts without CKD to be taking NSLLT (Table S7). This association was attenuated but remained statistically significant after multivariable adjustment (PR, 1.34; 95% CI, 1.12–1.59). Statin monotherapy and combination statin/NSLLT therapy increased, while there was no evidence of a change in the proportion taking NSLLT monotherapy among participants both with and without CKD (Table S8).
Discussion
There are several key findings from the current study. Between 1999–2002 and 2011–2014 the percentage of US adults with and without CKD taking statins increased. There was no difference in the percentage of US adults with versus without CKD taking statins after multivariable adjustment. Although over 65% of adults with CKD had an indication for a statin based on the 2013 ACC/AHA cholesterol guideline in 2011–2014, only 35.7% were taking a statin. Among US adults without a history of CVD, those with CKD in the absence of diabetes mellitus were less likely to be taking a statin relative to those with diabetes mellitus in the absence of CKD. Finally, NSLLT use was more common among adults with versus without CKD.
In the general US population, statin use has increased over the past 2 decades.13 Before 2013, statin use was recommended based on cholesterol levels in US guidelines.23 The National Kidney Foundation's Kidney Disease Outcomes Quality Initiative clinical practice guidelines recommend using statins or combination statin/NSLLT therapy to reduce the risk of major atherosclerotic events in patients with CKD.24 The National Lipid Association and the American Association of Clinical Endocrinologists/American College of Endocrinology recommendations for dyslipidemia management also considered adults with mild and moderate CKD as a high‐risk group for atherosclerotic events, and recommend statins to lower the risk of CVD.25, 26 The 2013 Kidney Disease Improving Global Outcomes clinical practice guideline recommended statin use for adults ≥50 years of age with non–dialysis‐dependent CKD or with a kidney transplant and for adults <50 years of age with high CVD risk.27 However, adults with CKD are often diagnosed and managed in a primary care setting and a low proportion receives secondary care from a nephrologist,28 who may be more likely to utilize Kidney Disease Improving Global Outcomes guidelines.29 Many adults with CKD have an increased risk for CVD5 and lipid management is likely to occur in a primary care setting. Differences in guideline recommendations and statin underutilization in adults with CKD may be attributable to the high‐risk nature of CKD being underrecognized and perceived lack of benefit from statins in adults with CKD. There are also limited data from large randomized controlled trials on statin side effects in CKD populations. The Study of Heart and Renal Protection randomized trial found no evidence that statin use is associated with adverse effects in adults with CKD.10, 30 Similarly, results from the 4‐D trial and Assessment of Lescol in Renal Transplantation trial reported a low incidence of statin‐related side effects in adults with CKD, and these rates were similar for adults with and without CKD.31, 32
In 2013, the ACC/AHA updated recommendations for the management of blood cholesterol and adopted an approach that uses ASCVD risk in conjunction with LDL‐C to guide statin treatment.9 The National Kidney Foundation's task force on CVD concluded in 1998 that the incidence of ASCVD was higher in adults with CKD compared with the general population and that CKD should be a risk equivalent for coronary heart disease.33 However, the 2013 ACC/AHA cholesterol guideline writing committee did not include CKD as a condition warranting treatment with a statin.9 In the current analysis of nationally representative data, almost two thirds of US adults with CKD had an indication for taking a statin but only 35.7% were taking one. More recently, the 2018 ACC/AHA cholesterol treatment guidelines considered CKD as a risk‐enhancing factor for ASCVD. While it is unknown what impact the 2018 ACC/AHA guideline will have on the proportion of US adults with CKD taking statins, results from the current study highlight the underutilization of statins among adults with CKD.
Statins are effective in preventing cardiovascular events and delaying mortality in adults with mild and moderate CKD. In a meta‐analysis of 26 randomized controlled trials and quasi‐randomized controlled trials that included 25 017 adults with stage 3 or 4 CKD, statins decreased the risk of all‐cause mortality, cardiovascular death, and nonfatal cardiovascular events.11 Studies of observational data collected in the early 2000s have shown that despite an increased CVD risk, many patients with CKD do not receive a statin. An analysis of 1428 adults with stage 1 to 4 CKD in NHANES 2001–2010 showed that while 28.4% had a history of CVD, only 50.7% with CVD and CKD and 30.4% overall were taking a statin.34 While the current results demonstrate that statin use has increased over time, by 2011–2014 still only 35.7% of US adults with CKD were taking a statin. Although US adults with CKD were more likely than those without CKD to be taking an NSLLT, the absolute percentage of those with CKD taking NSLLT was low.
Including CKD as an indication for statins may result in treatment uptake in this high‐risk population. In the current study, we compared adults with CKD who lack a specific indication for a statin to adults with diabetes mellitus who have a specific indication for treatment. There was no difference in the proportion of US adults with CKD in the absence of diabetes mellitus versus with diabetes mellitus in the absence of CKD taking statins in the presence of CVD, which is an indication for statin therapy. However, among those without a history of CVD, US adults with CKD in the absence diabetes mellitus were less likely to be taking a statin when compared with those with diabetes mellitus in the absence of CKD. The lower rate of statin use in those with CKD versus diabetes mellitus may represent a missed treatment opportunity that could potentially be eliminated as the 2018 ACC/AHA cholesterol management includes CKD as a risk‐enhancing factor for statin treatment.16
There are several strengths to the current analysis. NHANES provides nationally representative estimates for noninstitutionalized US adults. Data were collected using standardized questionnaires, and procedures used were consistent over the 16‐year study period. We acknowledge some limitations to the current analysis. Enrollment in NHANES was restricted to non‐institutionalized US adults and did not include nursing home residents. NHANES participants are not followed, and we are unable to identify those who initiated a statin following their study visit. NHANES data on statin use are currently available only through 2014, only 1 year following the publication of the 2013 ACC/AHA cholesterol guideline. As there is often a delay in implementation of treatment guidelines, these data may not reflect the full impact of the 2013 ACC/AHA cholesterol guideline on the prevalence of statin use. Between 2009 and 2015, the proportion of patients in a large, integrated health system remained stable for those with ASCVD, high LDL‐C, and diabetes mellitus but increased substantially for those with 10‐year ASCVD risk ≥7.5%; there was no information on contemporary statin use in populations with CKD.35 In older cohorts of CKD populations, however, statins are consistently underused.15, 36, 37
In conclusion, statin use increased between 1999–2002 and 2011–2014 among US adults with and without CKD. Despite over 65% of adults with CKD being recommended a statin based on the 2013 ACC/AHA guideline, only 35.7% of adults with CKD were taking a statin in 2011–2014. Statin use was lower among adults with CKD in the absence of diabetes mellitus compared with those with diabetes mellitus in the absence of CKD. This difference highlights the importance of recognizing the high CVD risk in patients with CKD and potential benefits of increased statin utilization. Implementation of the 2018 ACC/AHA cholesterol guideline, which considers CKD a risk‐enhancing factor, may increase the appropriate use of statins in this high‐risk population.
Sources of Funding
The current study was funded by an industry/academic collaboration between Amgen Inc, University of Alabama at Birmingham, and the Icahn School of Medicine at Mount Sinai.
Disclosures
Dr Rosenson receives research support from Akcea, Amgen, AstraZeneca Medicines Company, and Sanofi. He has participated in advisory boards for Akcea and Amgen. He consults for C5 and CVS Caremark. He receives honoraria from Akcea, Kowa, and Pfizer, and royalties from UpToDate. Dr Bittner receives research grants to her institution from Amgen, AstraZeneca, DalCor, Sanofi‐Regeneron, Bayer Healthcare, and Esperion. She has participated in advisory boards for Sanofi. Dr Safford receives research support from Amgen. She has participated in advisory boards for Amgen. Drs Coll, Mues, and Monda are employees and stockholders of Amgen. Dr Muntner receives grant support from Amgen. The remaining authors have no disclosures to report.
Supporting information
Table S1. Percentage of US Adults ≥20 Years of Age Taking a Statin by CVD Risk Groups, NHANES 1999–2014
Table S2. Percentage of US Adults ≥20 Years of Age Taking a Statin by CKD Stage and Albuminuria Status, NHANES 1999–2014
Table S3. Prevalence Ratios and 95% CIs Associated With Statin Use Among US Adults ≥20 Years Old, NHANES 1999–2014
Table S4. Prevalence Ratios and 95% CIs Associated With Statin Use Among US Adults ≥20 Years Old, NHANES 1999–2014
Table S5. Percentage of US Adults Aged ≥20 Years With Chronic Kidney Disease in the Absence of Diabetes Mellitus and Diabetes Mellitus in the Absence of Chronic Kidney Disease Taking Statins, by CVD Status, NHANES 1999–2014
Table S6. Percentage of US Adults Aged ≥20 Years Taking Nonstatin Lipid‐Lowering Therapy by CKD Status, NHANES 1999–2014
Table S7. Prevalence Ratios and 95% CIs Associated With NSLLT Use Among US Adults ≥20 Years Old, NHANES 1999–2014
Table S8. Percentage of US Adults ≥20 Years of Age Taking and Not Taking Lipid‐Lowering Therapy by CVD Risk Groups, NHANES 1999–2014
(J Am Heart Assoc. 2019;8:e010640 DOI: 10.1161/JAHA.118.010640)
References
- 1. Segura J, Campo C, Gil P, Roldan C, Vigil L, Rodicio JL, Ruilope LM. Development of chronic kidney disease and cardiovascular prognosis in essential hypertensive patients. J Am Soc Nephrol. 2004;15:1616–1622. [DOI] [PubMed] [Google Scholar]
- 2. Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. [DOI] [PubMed] [Google Scholar]
- 3. Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium . Associations of kidney disease measures with mortality and end‐stage renal disease in individuals with and without diabetes: a meta‐analysis. Lancet. 2012;380:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matsushita K, Coresh J, Sang Y, Chalmers J, Fox C, Guallar E, Jafar T, Jassal SK, Landman GW, Muntner P, Roderick P, Sairenchi T, Schottker B, Shankar A, Shlipak M, Tonelli M, Townend J, van Zuilen A, Yamagishi K, Yamashita K, Gansevoort R, Sarnak M, Warnock DG, Woodward M, Arnlov J; CKD Prognosis Consortium . Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta‐analysis of individual participant data. Lancet Diabetes Endocrinol. 2015;3:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bansal N, Katz R, Robinson‐Cohen C, Odden MC, Dalrymple L, Shlipak MG, Sarnak MJ, Siscovick DS, Zelnick L, Psaty BM, Kestenbaum B, Correa A, Afkarian M, Young B, de Boer IH. Absolute rates of heart failure, coronary heart disease, and stroke in chronic kidney disease: an analysis of 3 community‐based cohort studies. JAMA Cardiol. 2017;2:314–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 7. Manttari M, Tiula E, Alikoski T, Manninen V. Effects of hypertension and dyslipidemia on the decline in renal function. Hypertension. 1995;26:670–675. [DOI] [PubMed] [Google Scholar]
- 8. Cholesterol Treatment Trialists Collaboration , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 10. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt‐Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellstrom B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Gronhagen‐Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; Sharp Investigators . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Navaneethan SD, Pansini F, Perkovic V, Manno C, Pellegrini F, Johnson DW, Craig JC, Strippoli GF. HMG CoA reductase inhibitors (statins) for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009:CD007784. [DOI] [PubMed] [Google Scholar]
- 12. Palmer SC, Craig JC, Navaneethan SD, Tonelli M, Pellegrini F, Strippoli GF. Benefits and harms of statin therapy for persons with chronic kidney disease: a systematic review and meta‐analysis. Ann Intern Med. 2012;157:263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA. 2015;314:1818–1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chronic Kidney Disease Prognosis Consortium , Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all‐cause and cardiovascular mortality in general population cohorts: a collaborative meta‐analysis. Lancet. 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Colantonio LD, Baber U, Banach M, Tanner RM, Warnock DG, Gutierrez OM, Safford MM, Wanner C, Howard G, Muntner P. Contrasting cholesterol management guidelines for adults with CKD. J Am Soc Nephrol. 2015;26:1173–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd‐Jones D, Lopez‐Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018. Available at: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000624. Accessed January 9, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Centers for Disease Control and Prevention. National Health and Nutrition Examination Survey . Continuous NHANES Data, Questionnaires, and Related Documentation. Available at: https://wwwn.cdc.gov/nchs/nhanes/Default.aspx. Accessed June 30, 2017.
- 18. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1. 1994:1–407. [PubMed] [Google Scholar]
- 19. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low‐density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD‐EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Levin A, Kellum JA. Definition and classification of kidney diseases. Am J Kidney Dis. 2013;61:686–688. [DOI] [PubMed] [Google Scholar]
- 22. Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int. 2011;80:17–28. [DOI] [PubMed] [Google Scholar]
- 23. National Cholesterol Education Program Expert Panel on Detection Evaluation and Treatment of High Blood Cholesterol in Adults . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 24. National Kidney Foundation . KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. [DOI] [PubMed] [Google Scholar]
- 25. Jacobson TA, Ito MK, Maki KC, Orringer CE, Bays HE, Jones PH, McKenney JM, Grundy SM, Gill EA, Wild RA, Wilson DP, Brown WV. National lipid association recommendations for patient‐centered management of dyslipidemia: part 1—full report. J Clin Lipidol. 2015;9:129–169. [DOI] [PubMed] [Google Scholar]
- 26. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah‐Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1–87. [DOI] [PubMed] [Google Scholar]
- 27. Kidney Disease: Improving Global Outcomes Lipid Work Group . KDIGO clinical practice guideline for lipid management in chronic kidney disease. Kidney Int Suppl. 2013;3:259–305. [Google Scholar]
- 28. Agrawal V, Jaar BG, Frisby XY, Chen SC, Qiu Y, Li S, Whaley‐Connell AT, McCullough PA, Bomback AS; KEEP Investigators . Access to health care among adults evaluated for CKD: findings from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis. 2012;59:S5–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Budzisz E, Nowicki M. Opinions of nephrologists on the efficacy and tolerance of statins in hemodialysis patients. Ren Fail. 2017;39:277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Haynes R, Lewis D, Emberson J, Reith C, Agodoa L, Cass A, Craig JC, de Zeeuw D, Feldt‐Rasmussen B, Fellstrom B, Levin A, Wheeler DC, Walker R, Herrington WG, Baigent C, Landray MJ; Sharp Collaborative Group . Effects of lowering LDL cholesterol on progression of kidney disease. J Am Soc Nephrol. 2014;25:1825–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 32. Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Gronhagen‐Riska C, Neumayer HH, Maes B, Ambuhl P, Hartmann A, Staffler B, Jardine AG; Assessment of Lescol in Renal Transplantation Study Investigators . Long‐term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005;5:2929–2936. [DOI] [PubMed] [Google Scholar]
- 33. Levey AS, Beto JA, Coronado BE, Eknoyan G, Foley RN, Kasiske BL, Klag MJ, Mailloux LU, Manske CL, Meyer KB, Parfrey PS, Pfeffer MA, Wenger NK, Wilson PW, Wright JT Jr. Controlling the epidemic of cardiovascular disease in chronic renal disease: what do we know? What do we need to learn? Where do we go from here? National Kidney Foundation Task Force on Cardiovascular Disease. Am J Kidney Dis. 1998;32:853–906. [DOI] [PubMed] [Google Scholar]
- 34. Kuznik A, Mardekian J, Tarasenko L. Evaluation of cardiovascular disease burden and therapeutic goal attainment in US adults with chronic kidney disease: an analysis of National Health and Nutritional Examination Survey data, 2001–2010. BMC Nephrol. 2013;14:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harrison TN, Scott RD, Cheetham TC, Chang SC, Hsu JY, Wei R, Ling Grant DS, Boklage SH, Romo‐LeTourneau V, Reynolds K. Trends in statin use 2009–2015 in a large integrated health system: pre‐ and post‐2013 ACC/AHA guideline on treatment of blood cholesterol. Cardiovasc Drugs Ther. 2018;32:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tonelli M, Bohm C, Pandeya S, Gill J, Levin A, Kiberd BA. Cardiac risk factors and the use of cardioprotective medications in patients with chronic renal insufficiency. Am J Kidney Dis. 2001;37:484–489. [PubMed] [Google Scholar]
- 37. Chow FY, Polkinghorne KR, Chadban SJ, Atkins RC, Kerr PG. Cardiovascular risk in dialysis patients: a comparison of risk factors and cardioprotective therapy between 1996 and 2001. Nephrology. 2003;8:177–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Percentage of US Adults ≥20 Years of Age Taking a Statin by CVD Risk Groups, NHANES 1999–2014
Table S2. Percentage of US Adults ≥20 Years of Age Taking a Statin by CKD Stage and Albuminuria Status, NHANES 1999–2014
Table S3. Prevalence Ratios and 95% CIs Associated With Statin Use Among US Adults ≥20 Years Old, NHANES 1999–2014
Table S4. Prevalence Ratios and 95% CIs Associated With Statin Use Among US Adults ≥20 Years Old, NHANES 1999–2014
Table S5. Percentage of US Adults Aged ≥20 Years With Chronic Kidney Disease in the Absence of Diabetes Mellitus and Diabetes Mellitus in the Absence of Chronic Kidney Disease Taking Statins, by CVD Status, NHANES 1999–2014
Table S6. Percentage of US Adults Aged ≥20 Years Taking Nonstatin Lipid‐Lowering Therapy by CKD Status, NHANES 1999–2014
Table S7. Prevalence Ratios and 95% CIs Associated With NSLLT Use Among US Adults ≥20 Years Old, NHANES 1999–2014
Table S8. Percentage of US Adults ≥20 Years of Age Taking and Not Taking Lipid‐Lowering Therapy by CVD Risk Groups, NHANES 1999–2014


