Abstract
Background
The involvement of vaspin (visceral adipose tissue–derived serpin) in the development of atherosclerotic cardiovascular diseases has been documented. This study was designed to explore the prognostic value of serum vaspin in patients with acute myocardial infarction (AMI).
Methods and Results
We included 1036 AMI patients in a cohort study and determined the association between serum vaspin and major adverse cardiac events (MACE) using Cox regression analysis. The receiver operating characteristic curve indicated that serum vaspin could significantly differentiate patients with MACE, and the optimal cutoff value was 0.62 ng/mL. The Kaplan–Meier survival curve showed that patients with lower vaspin levels had higher incidence of MACE. Multivariate Cox regression analysis revealed that low vaspin was an independent predictor of MACE (hazard ratio: 0.74; 95% CI, 0.48–0.96; P=0.029), together with age; previous histories of AMI, heart failure, hypertension, and diabetes mellitus; Killip class; revascularization; CRP (C‐reactive protein); and NT‐proBNP (N‐terminal pro–B‐type natriuretic peptide). Integrated discrimination and net reclassification improvements for MACE were significantly improved by addition of vaspin to the model of traditional risk factors. Moreover, low vaspin was a valuable predictor of heart failure hospitalization (hazard ratio: 0.58; 95% CI, 0.37–0.89; P=0.005) and recurrent AMI (hazard ratio: 0.72; 95% CI, 0.53–0.95; P=0.036) after adjustment for conventional cardiovascular risk factors.
Conclusions
Our study suggests that serum vaspin is a significant prognostic marker of MACE in AMI patients.
Keywords: major adverse cardiac events, myocardial infarction, prognosis
Subject Categories: Cardiovascular Disease, Risk Factors
Clinical Perspective
What Is New?
Patients with acute myocardial infarction and low levels of vaspin (visceral adipose tissue‐derived serpin) have high incidence of major adverse cardiac events.
Multivariable regression analysis indicates that low vaspin is an independent predictor of major adverse cardiac events in acute myocardial infarction.
Low vaspin is also a valuable predictor of heart failure hospitalization and recurrent myocardial infarction.
What Are the Clinical Implications?
Serum vaspin is a significant prognostic marker of major adverse cardiac events in patients with acute myocardial infarction.
Serum vaspin can improve early risk stratification for patients with acute myocardial infarction.
Introduction
Vaspin (visceral adipose tissue–derived serpin), which belongs to the serine protease inhibitor family, has been newly identified to be an adipokine associated with obesity and its metabolic consequences. Vaspin may act as an insulin sensitizer with an anti‐inflammatory effect and function as a compensatory mechanism in response to impaired glucose metabolism or decreased insulin sensitivity.1, 2 Administration of vaspin to obese mice markedly improves insulin sensitivity and glucose tolerance.3 A previous study reported that vaspin‐deficient mice developed glucose intolerance related to endoplasmic reticulum stress, whereas vaspin‐transgenic mice were protected from diet‐induced obesity and glucose intolerance.4 In addition, a cross‐sectional study showed that elevated serum vaspin levels were correlated with obesity and impaired insulin sensitivity.5
In recent years, growing evidence has demonstrated that vaspin is actively involved in the development of atherosclerotic cardiovascular diseases. Esaki et al indicated that serum vaspin levels were positively related to carotid atherosclerosis, independent of insulin resistance in a general population.6 Kadoglou et al showed that serum vaspin levels were reduced in patients with coronary artery disease (CAD), and low vaspin seemed to be associated with disease severity.7 Moreover, a recent cohort study suggested that vaspin could be a prognostic biomarker in patients with acute myocardial infarction (AMI) and that patients with lower vaspin levels might have higher incidence of major adverse cardiac events (MACE)8; however, this is a single‐center study with a relatively small sample size. We conducted a large‐scale prospective cohort study to assess the prognostic value of serum vaspin in AMI patients.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
This study enrolled 1036 patients with ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation myocardial infarction admitted to the affiliated hospitals of Soochow University and Nanjing Medical University from May 2014 to December 2017. AMI was diagnosed based on chest pain symptoms, serial ECG changes, and increased cardiac troponin levels, according to the criteria of the American Heart Association.9 This study was conducted in agreement with the Declaration of Helsinki and was approved by the clinical institutional review board. Patients with end‐stage renal disease or known malignancy were excluded from this study. Demographic, clinical, and biochemical data were collected from the medical records. Written informed consent was obtained from each patient before enrollment.
Measurement of Serum Vaspin
Fasting blood samples were collected from AMI patients on admission. Serum was obtained by centrifugation at 1000 g for 10 minutes and then stored at −80°C. Serum levels of vaspin were determined using a commercial ELISA kit (ALPCO Diagnostics) according to the manufacturer's instructions.
End Points
The primary composite end point was MACE, including cardiovascular death, recurrent AMI, or hospitalization for heart failure (HF). Recurrent AMI was diagnosed according to the criteria as described earlier.9 HF hospitalization was defined as a hospital readmission for which HF was the main cause, requiring treatment with intravenous diuretics, inotropes, or vasodilators. The secondary end points were the individual components of the primary end point. End points were obtained by reviewing the hospital database and by contacting patients or their families.
Statistical Analysis
Categorical variables were expressed as percentages and compared with the χ2 test. Continuous variables were presented as median and quartile ranges, and comparisons were analyzed by the Mann–Whitney U test. The normality of distribution was evaluated by the Kolmogorov–Smirnov test. A receiver operating characteristic curve was used to determine the optimal cutoff value of vaspin to predict MACE. Cox regression analyses were performed to determine the association between baseline variables and MACE. The following variables were entered into the Cox proportional hazards models: age, sex, body mass index; previous histories of AMI, HF, hypertension, diabetes mellitus, hyperlipidemia, and smoking; AMI type; Killip class; revascularization; CRP (C‐reactive protein); troponin T; NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide); and vaspin. Troponin T, NT‐proBNP, CRP, and vaspin levels were normalized by log10 transformation. Kaplan–Meier analysis was undertaken to estimate survival rates in the groups stratified by serum vaspin levels. Integrated discrimination improvement and net reclassification improvement were calculated to assess the incremental value of vaspin in the prognosis of AMI. P<0.05 was considered statistically significant.
Results
Patient Characteristics
The ROC curve analysis was conducted to evaluate the prognostic value of vaspin, and the results are shown in Figure 1. Serum levels of vaspin could significantly differentiate patients with MACE (area under the curve: 0.785; P<0.001) and the optimal cutoff value for predicting MACE was 0.62 ng/mL.
Figure 1.
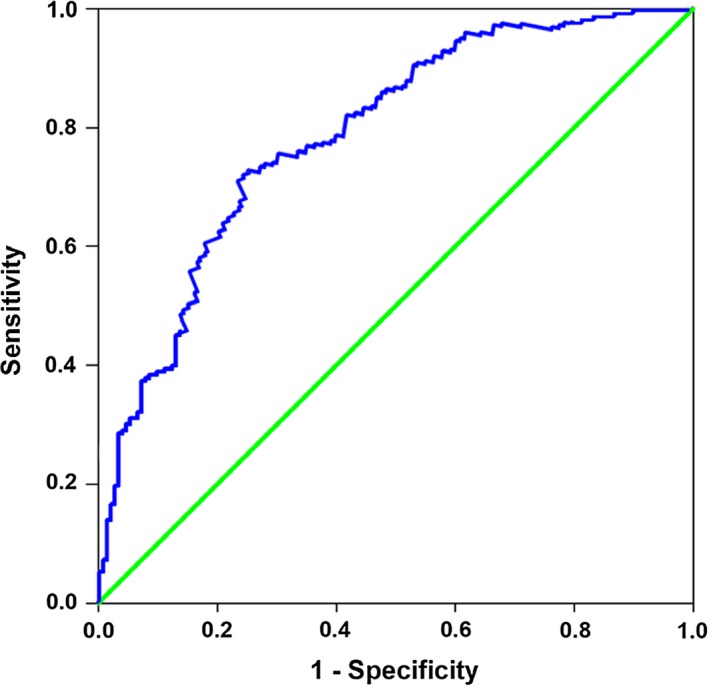
Receiver operating characteristic curve of serum vaspin for predicting major adverse cardiac events in acute myocardial infarction patients.
AMI patients were divided into 2 groups according to the cutoff value of vaspin level. The baseline characteristics of patients are shown in Table 1. Patients with higher vaspin levels were more likely to be female, obese, and diabetic. In addition, vaspin seemed to be inversely associated with CRP.
Table 1.
Baseline Characteristics of Patients With AMI
| All Patients (n=1036) | Vaspin <0.62 ng/mL (n=495) | Vaspin ≥0.62 ng/mL (n=541) | P Value | |
|---|---|---|---|---|
| Age, y | 67 (59–78) | 68 (61–79) | 66 (58–76) | NS |
| Male (%) | 729 (70.4) | 402 (81.2) | 327 (60.4) | <0.001 |
| BMI (kg/m2) | 27 (24–29) | 25 (23–27) | 29 (26–31) | <0.001 |
| Previous history | ||||
| AMI (%) | 98 (9.5) | 63 (12.7) | 35 (6.5) | <0.001 |
| HF (%) | 63 (6.1) | 34 (6.9) | 29 (5.4) | NS |
| Hypertension (%) | 614 (59.3) | 278 (56.2) | 336 (62.1) | NS |
| Diabetes mellitus (%) | 261 (25.2) | 89 (18.0) | 172 (31.8) | <0.001 |
| Hyperlipidemia (%) | 435 (42.0) | 190 (38.4) | 245 (45.3) | NS |
| Smoking (%) | 523 (50.5) | 237 (47.9) | 286 (52.9) | NS |
| STEMI (%) | 654 (63.1) | 301 (60.8) | 353 (65.2) | NS |
| Killip class >1 (%) | 406 (39.2) | 185 (37.4) | 221 (40.9) | NS |
| Revascularization (%) | 812 (78.4) | 398 (80.4) | 414 (76.5) | NS |
| Medical treatment | ||||
| Aspirin (%) | 935 (90.3) | 450 (90.9) | 485 (89.6) | NS |
| Statin (%) | 959 (92.6) | 462 (93.3) | 497 (91.9) | NS |
| β‐Blocker (%) | 736 (71.0) | 345 (69.7) | 391 (72.3) | NS |
| ACEI/ARB (%) | 812 (78.4) | 379 (76.6) | 433 (80.0) | NS |
| Metformin (%) | 187 (18.1) | 98 (19.8) | 89 (16.5) | NS |
| CRP (mg/L) | 24 (15–32) | 43 (31–56) | 15 (8–23) | <0.001 |
| Troponin T (ng/mL) | 652 (283–1365) | 594 (218–1132) | 708 (356–1520) | NS |
| NT‐proBNP (pg/mL) | 765 (320–1879) | 810 (392–2045) | 725 (268–1653) | NS |
Values are median (interquartile range) or n (%). ACEI indicates angiotensin‐converting enzyme inhibitor; AMI, acute myocardial infarction; ARB, angiotensin receptor blocker; BMI, body mass index; CRP, C‐reactive protein; HF, heart failure; NS, not significant; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; STEMI, ST‐segment–elevation myocardial infarction.
A total of 654 patients with ST‐segment–elevation myocardial infarction and 382 patients with non–ST‐segment–elevation myocardial infarction were enrolled in this study. The median length of follow‐up was 574 days. During the follow‐up period, 86 patients died from cardiovascular causes, 145 were readmitted with HF, and 59 had recurrent AMI.
Kaplan–Meier Analysis
Kaplan–Meier analysis was performed to compare the survival rates of patients with high and low vaspin levels. Our results indicated that low vaspin was a valuable predictor of MACE in patients with AMI (Figure 2). Patients with vaspin levels <0.62 ng/mL had significantly higher incidence of MACE compared with those with vaspin levels >0.62 ng/mL (log‐rank test, P<0.001).
Figure 2.
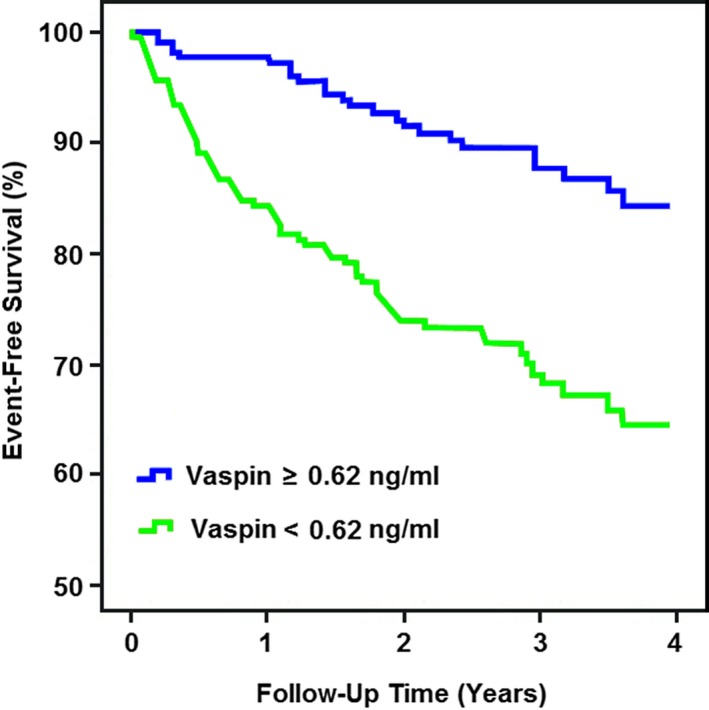
Kaplan–Meier survival analysis. The event‐free survival for major adverse cardiac events in patients with acute myocardial infarction, stratified according to the cutoff value of serum vaspin.
Primary End Point
Cox regression analysis indicated that age; previous histories of AMI, HF, hypertension, diabetes mellitus, and hyperlipidemia; Killip class; revascularization; CRP; NT‐proBNP; and vaspin were univariate predictors of the primary end point of MACE (Table 2). In addition, multivariate Cox regression analysis revealed that low vaspin remained an independent predictor of MACE (hazard ratio: 0.74; 95% CI, 0.48–0.96; P=0.029), together with age; previous histories of AMI, HF, hypertension, and diabetes mellitus; Killip class; revascularization; CRP; and NT‐proBNP (Table 2). The integrated discrimination and net reclassification improvements for MACE were significantly improved by the addition of vaspin to the model of conventional risk factors (integrated discrimination improvement: 0.072; 95% CI, 0.045–0.126; net reclassification improvement: 0.098; 95% CI, 0.053–0.164).
Table 2.
Cox Regression Analysis for MACE in AMI Patients
| Univariable Analysis | P Value | Multivariable Analysis | P Value | |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Age | 1.15 (1.08–1.24) | 0.015 | 1.10 (1.05–1.16) | 0.021 |
| Male | 0.84 (0.53–1.40) | NS | 1.02 (0.81–1.68) | NS |
| BMI | 1.21 (0.82–1.75) | NS | 1.13 (0.74–1.59) | NS |
| Previous history | ||||
| AMI | 3.16 (2.41–4.38) | <0.001 | 1.82 (1.43–2.75) | <0.001 |
| HF | 2.78 (2.26–3.90) | <0.001 | 1.69 (1.27–2.56) | <0.001 |
| Hypertension | 1.67 (1.29–2.45) | <0.001 | 1.35 (1.08–1.97) | 0.032 |
| Diabetes mellitus | 1.59 (1.18–2.64) | 0.007 | 1.46 (1.12–2.10) | 0.013 |
| Hyperlipidemia | 1.31 (1.04–1.83) | 0.042 | 1.19 (0.86–1.71) | NS |
| Smoking | 1.03 (0.62–1.79) | NS | 0.87 (0.53–1.62) | NS |
| STEMI | 0.87 (0.50–1.65) | NS | 1.14 (0.89–1.76) | NS |
| Killip class >1 | 2.40 (2.08–3.52) | <0.001 | 1.53 (1.20–2.27) | <0.001 |
| Revascularization | 0.45 (0.21–0.76) | <0.001 | 0.68 (0.42–0.91) | <0.001 |
| Log CRP* | 1.56 (1.25–2.18) | <0.001 | 1.30 (1.06–1.85) | 0.038 |
| Log troponin T* | 1.23 (0.80–2.07) | NS | 1.06 (0.73–1.69) | NS |
| Log NT‐proBNP* | 2.57 (1.83–4.12) | <0.001 | 1.75 (1.31–2.64) | <0.001 |
| Log vaspin* | 0.60 (0.34–0.89) | <0.001 | 0.74 (0.48–0.96) | 0.029 |
AMI indicates acute myocardial infarction; BMI, body mass index; CRP, C‐reactive protein; HF, heart failure; HR, hazard ratio; MACE, major adverse cardiac events; NS, not significant; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; STEMI, ST‐segment–elevation myocardial infarction.
CRP, troponin T, NT‐proBNP, and vaspin were normalized by log10 transformation.
Secondary End Points
A Cox proportional hazards model was constructed for the secondary end points of cardiovascular death, HF hospitalization, and recurrent AMI. Our results showed that low vaspin was a significant predictor of HF hospitalization (hazard ratio: 0.58; 95% CI, 0.37–0.89; P=0.005) and recurrent AMI (hazard ratio: 0.72; 95% CI, 0.53–0.95; P=0.036) following adjustment for age; sex; body mass index; previous histories of AMI, HF, hypertension, diabetes mellitus, hyperlipidemia, and smoking; AMI type; Killip class; revascularization; CRP; troponin T; and NT‐proBNP (Table 3). However, low vaspin was not found to be a predictor of cardiovascular death.
Table 3.
Cox Regression Analysis for Cardiovascular Death, HF Hospitalization, and Recurrent AMI
| Cardiovascular Death | P Value | HF Hospitalization | P Value | Recurrent AMI | P Value | |
|---|---|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||||
| Age | 1.16 (1.10–1.25) | 0.008 | 1.07 (1.03–1.12) | 0.043 | 1.04 (0.95–1.16) | NS |
| Male | 1.23 (0.94–1.81) | NS | 0.92 (0.68–1.40) | NS | 1.09 (0.76–1.67) | NS |
| BMI | 1.10 (0.72–1.65) | NS | 0.86 (0.59–1.31) | NS | 1.27 (0.84–1.78) | NS |
| Previous history | ||||||
| AMI | 1.68 (1.29–2.54) | <0.001 | 2.15 (1.60–3.27) | <0.001 | 1.36 (0.81–2.39) | NS |
| HF | 1.75 (1.32–2.70) | <0.001 | 2.39 (1.83–3.56) | <0.001 | 1.21 (0.72–2.13) | NS |
| Hypertension | 1.57 (1.20–2.38) | 0.004 | 1.30 (0.92–1.87) | NS | 1.12 (0.78–1.94) | NS |
| Diabetes mellitus | 1.32 (1.05–1.84) | 0.032 | 1.17 (0.85–1.69) | NS | 1.60 (1.25–2.38) | <0.001 |
| Hyperlipidemia | 1.08 (0.67–1.73) | NS | 0.95 (0.58–1.42) | NS | 1.29 (0.93–2.06) | NS |
| Smoking | 0.84 (0.51–1.62) | NS | 1.02 (0.61–1.74) | NS | 0.76 (0.45–1.32) | NS |
| STEMI | 0.89 (0.60–1.37) | NS | 1.24 (0.88–1.79) | NS | 1.05 (0.70–1.57) | NS |
| Killip class >1 | 1.46 (1.18–2.03) | 0.016 | 2.08 (1.65–3.16) | <0.001 | 1.13 (0.64–1.98) | NS |
| Revascularization | 0.41 (0.26–0.87) | <0.001 | 0.53 (0.30–0.91) | 0.012 | 0.98 (0.61–1.59) | NS |
| Log CRP* | 1.25 (0.82–1.76) | NS | 1.09 (0.73–1.84) | NS | 1.50 (1.23–2.15) | 0.010 |
| Log troponin T* | 1.12 (0.79–1.95) | NS | 1.20 (0.82–2.05) | NS | 0.81 (0.49–1.54) | NS |
| Log NT‐proBNP* | 1.69 (1.34–2.48) | <0.001 | 2.14 (1.63–2.86) | <0.001 | 1.28 (0.76–2.10) | NS |
| Log vaspin* | 0.90 (0.56–1.63) | NS | 0.58 (0.37–0.89) | 0.005 | 0.72 (0.53–0.95) | 0.036 |
AMI indicates acute myocardial infarction; BMI, body mass index; CRP, C‐reactive protein; HF, heart failure; HR, hazard ratio; NS, not significant; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; STEMI, ST‐segment–elevation myocardial infarction.
CRP, troponin T, NT‐proBNP, and vaspin were normalized by log10 transformation.
Discussion
Over the past decade, significant progress has been made to identify various biomarkers that can facilitate risk stratification for AMI patients. Vaspin, a newly discovered adipokine, has been found to be involved in the development of metabolic syndrome and atherosclerosis.10 In the present study, we included 1036 AMI patients in a prospective cohort study and determined the relationship between serum vaspin and cardiovascular outcomes. Our findings demonstrate that vaspin might be a valuable biomarker of MACE and could be used to improve risk stratification for AMI patients.
In this study, our results showed that high vaspin levels were associated with obesity and diabetes mellitus. Multivariate Cox regression analysis suggested that low vaspin was a significant predictor of MACE, together with age; previous histories of AMI, HF, hypertension, and diabetes mellitus; Killip class; revascularization; CRP; and NT‐proBNP. In addition, low vaspin was also an independent predictor of HF hospitalization and recurrent AMI following adjustment for conventional risk factors. Furthermore, addition of vaspin to the traditional model resulted in better integrated discrimination and net reclassification improvements for MACE prediction. Our findings were consistent with another prospective study with a small sample size that suggested vaspin might be a prognostic biomarker in patients with AMI.8 A recent study by Su et al showed that low vaspin was independently associated with an increased risk for MACE in a cohort of patients with CAD of differing severity.11
The mechanisms by which vaspin is involved in the progression and prognosis of CAD have not been fully clarified. In this study, low vaspin was found to be associated with increased inflammation in AMI patients. A previous study indicated that vaspin could exert anti‐inflammatory action in vascular smooth muscle cells and prevent TNF‐α (tumor necrosis factor α)–induced ICAM1 (intercellular adhesion molecule 1) expression by inhibiting reactive oxygen species–dependent NF‐κB (nuclear factor κB) and PRKCQ (protein kinase C θ) activation.12 Recently, accumulating evidence has suggested that inflammation plays an important role in the pathogenesis of CAD.13, 14 Moreover, ventricular remodeling is a critical mechanism in the development of HF.15 Inflammation has been reported to be involved in the pathogenesis of cardiac remodeling after AMI.16 Therefore, vaspin might attenuate myocardial remodeling and improve cardiovascular prognosis in AMI patients partly through anti‐inflammatory effects.
In recent years, several experimental studies have been performed to investigate the effects of vaspin on vascular function, which may partially explain the beneficial roles of vaspin in CAD. Nakatsuka et al showed that vaspin served as a ligand for the GRP78 (78‐kDa glucose‐regulated protein)/voltage‐dependent anion channel complex in endothelial cells and could promote proliferation, suppress apoptosis, and attenuate vascular injury in diabetes mellitus.17 Moreover, Jung et al indicated that vaspin increased nitric oxide bioavailability in vascular endothelial cells by reducing asymmetric dimethylarginine, which might provide a novel molecular mechanism of antiatherogenic actions of vaspin.18
This study has some limitations. First, this prospective study was conducted in a Chinese population, so the results should be extrapolated cautiously to other populations. Second, we did not perform serial measurements of serum vaspin after the occurrence of AMI. Third, we did not combine vaspin with other biomarkers to predict the prognosis of AMI.
In conclusion, our study suggests that serum vaspin is an independent prognostic marker of MACE in AMI patients. Further studies with longer follow‐up are needed to investigate the predictive value of vaspin, to determine a cutoff value, and to clarify its potential mechanisms.
Sources of Funding
This study was financially supported by the National Natural Science Foundation of China (81770370) and Scientific Research Program for Young Talents of China National Nuclear Corporation (51001).
Disclosures
None.
(J Am Heart Assoc. 2019;8:e010934 DOI: 10.1161/JAHA.118.010934.)
References
- 1. Blüher M. Vaspin in obesity and diabetes: pathophysiological and clinical significance. Endocrine. 2012;41:176–182. [DOI] [PubMed] [Google Scholar]
- 2. Wada J. Vaspin: a novel serpin with insulin‐sensitizing effects. Expert Opin Investig Drugs. 2008;17:327–333. [DOI] [PubMed] [Google Scholar]
- 3. Hida K, Wada J, Eguchi J, Zhang H, Baba M, Seida A, Hashimoto I, Okada T, Yasuhara A, Nakatsuka A, Shikata K, Hourai S, Futami J, Watanabe E, Matsuki Y, Hiramatsu R, Akagi S, Makino H, Kanwar YS. Visceral adipose tissue‐derived serine protease inhibitor: a unique insulin‐sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA. 2005;102:10610–10615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nakatsuka A, Wada J, Iseda I, Teshigawara S, Higashio K, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Hida K, Eguchi J, Horiguchi CS, Ogawa D, Matsuki Y, Hiramatsu R, Yagita H, Kakuta S, Iwakura Y, Makino H. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell‐surface GRP78/MTJ‐1 complex. Diabetes. 2012;61:2823–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Youn BS, Klöting N, Kratzsch J, Lee N, Park JW, Song ES, Ruschke K, Oberbach A, Fasshauer M, Stumvoll M, Blüher M. Serum vaspin concentrations in human obesity and type 2 diabetes. Diabetes. 2008;57:372–377. [DOI] [PubMed] [Google Scholar]
- 6. Esaki E, Adachi H, Hirai Y, Yamagishi S, Kakuma T, Enomoto M, Fukami A, Kumagai E, Ohbu K, Obuchi A, Yoshimura A, Nakamura S, Nohara Y, Fujiyama T, Fukumoto Y, Imaizumi T. Serum vaspin levels are positively associated with carotid atherosclerosis in a general population. Atherosclerosis. 2014;233:248–252. [DOI] [PubMed] [Google Scholar]
- 7. Kadoglou NP, Gkontopoulos A, Kapelouzou A, Fotiadis G, Theofilogiannakos EK, Kottas G, Lampropoulos S. Serum levels of vaspin and visfatin in patients with coronary artery disease‐Kozani study. Clin Chim Acta. 2011;412:48–52. [DOI] [PubMed] [Google Scholar]
- 8. Zhang B, Peng W, Wang K, Li H, Xu Y. Vaspin as a prognostic marker in patients with acute myocardial infarction. Heart Lung Circ. 2016;25:257–264. [DOI] [PubMed] [Google Scholar]
- 9. Thygesen K, Alpert JS, White HD. Joint ESC/ACCF/AHA/WHF task force for the redefinition of myocardial infarction. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 10. Dimova R, Tankova T. The role of vaspin in the development of metabolic and glucose tolerance disorders and atherosclerosis. Biomed Res Int. 2015;2015:823481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Su Y, Ming Q, Li W, Peng W, Li H, Xu Y. Serum vaspin: a reliable independent predictor for major adverse cardiac events. Int J Clin Exp Med. 2016;9:18133–18141. [Google Scholar]
- 12. Phalitakul S, Okada M, Hara Y, Yamawaki H. Vaspin prevents TNF‐α‐induced intracellular adhesion molecule‐1 via inhibiting reactive oxygen species‐dependent NF‐κB and PKCθ activation in cultured rat vascular smooth muscle cells. Pharmacol Res. 2011;64:493–500. [DOI] [PubMed] [Google Scholar]
- 13. Christodoulidis G, Vittorio TJ, Fudim M, Lerakis S, Kosmas CE. Inflammation in coronary artery disease. Cardiol Rev. 2014;22:279–288. [DOI] [PubMed] [Google Scholar]
- 14. Krintus M, Kozinski M, Kubica J, Sypniewska G. Critical appraisal of inflammatory markers in cardiovascular risk stratification. Crit Rev Clin Lab Sci. 2014;51:263–279. [DOI] [PubMed] [Google Scholar]
- 15. Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. [DOI] [PubMed] [Google Scholar]
- 16. Frangogiannis NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. 2014;11:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nakatsuka A, Wada J, Iseda I, Teshigawara S, Higashio K, Murakami K, Kanzaki M, Inoue K, Terami T, Katayama A, Hida K, Eguchi J, Ogawa D, Matsuki Y, Hiramatsu R, Yagita H, Kakuta S, Iwakura Y, Makino H. Visceral adipose tissue‐derived serine proteinase inhibitor inhibits apoptosis of endothelial cells as a ligand for the cell‐surface GRP78/voltage‐dependent anion channel complex. Circ Res. 2013;112:771–780. [DOI] [PubMed] [Google Scholar]
- 18. Jung CH, Lee WJ, Hwang JY, Lee MJ, Seol SM, Kim YM, Lee YL, Kim HS, Kim MS, Park JY. Vaspin increases nitric oxide bioavailability through the reduction of asymmetric dimethylarginine in vascular endothelial cells. PLoS One. 2012;7:e52346. [DOI] [PMC free article] [PubMed] [Google Scholar]


