Abstract
Introduction:
Childhood obesity remains high in prevalence. Sugar-sweetened beverages containing high fructose corn syrup (HFCS) are a common source of excess calories among children and adolescents. Fructose metabolism differs from glucose metabolism, which may also differ from fructose + glucose metabolism in HFCS consumption. The purpose of this study was to determine the acute metabolic effects of HFCS ingestion after soft drink consumption in adolescents who are lean, have overweight/obesity, or have type 2 diabetes (T2DM).
Methods:
Adolescents age 13–19 years were recruited into three groups: lean controls (n = 10), overweight/ obese without diabetes (n = 10), or uncomplicated T2DM on metformin monotherapy (n = 5). After an overnight fast, subjects drank 12 ounces of soda containing HFCS. Blood samples were collected at time zero and every 15 min for 120 min to be analyzed for fructose, glucose, and insulin levels.
Results:
Glucose and fructose concentrations rose quickly in the first 15 min. Fructose, which was very low at baseline, rose to 100–200 μM and remained higher than fasting concentrations even at 120 min in all groups. Glucose increased after soft drink consumption, with the highest concentrations among subjects with T2DM, but returned to baseline fasting levels at 120 min. Insulin levels increased 15 min after soft drink consumption and were the highest in the obese group. Lactate rose non-significantly in all subjects, with no differences between groups.
Conclusion:
Among adolescents who are lean, overweight/obese, or have T2DM, drinking an HFCS-containing soft drink exposes the liver to fructose. Glucose excursions in T2DM may be impacted by exaggerated glucose cycling, or fructose metabolism to glucose. The context of fructose consumption with or without other carbohydrates is an important consideration in studies of fructose metabolism.
Keywords: Obesity, Diabetes, High fructose corn syrup, Glucose
1. Introduction
High fructose corn syrup (HFCS) was introduced in the 1960s-70s as an added sweetener substituting for sucrose in the U.S [1,2]. This sweetener contains 42–55% fructose (with the remainder as glucose), and is commonly used in many food and beverage products in the U.S. today. Consumption of HFCS containing beverages (soda and sports drinks) contributes 34.4% of added sugars to the diet of the U.S. population ≥2 years old [3]. Epidemiology shows that the increased consumption of sugar-sweetened beverages has paralleled the rise in childhood obesity [4], and now 9.4% of 2–5 year olds, 17.4% of 6–11 year olds, and 20.6% of 12–19 year olds can be classified as obese [5], with an increasing rate of extreme obesity.
Many studies have been published on fructose metabolism in the liver. Fructose enters hepatocytes via insulin independent GLUT-2 and GLUT-5 transporters [1]. In the hepatocytes, fructose is phosphorylated to fructose-1-phosphate and further metabolized to produce dihydroxyacetone phosphate bypassing a key regulatory step in glycolysis (the conversion of glucose-6-phosphate to fructose 1,6-bisphosphate) [6]. For these reasons, ingestion of fructose provokes hormonal and metabolic responses that are different from those of glucose [7]. Fructose is known to cause an increased lactate production and glycogen synthesis within 2 h after ingestion [8,9]. Following the acute effects, there is an increase in triglyceride production and increase in uric acid concentration.
The acute effects of fructose ingestion have been previously studied among adults [2,10]. Fructose ingestion does not stimulate insulin release and most of the fructose consumed is metabolized resulting in minimal elevation of serum fructose levels. These effects have been observed in clinical studies of obese men and women consuming glucose or fructose-containing beverages with meals [7]. Additionally, the 24-h area under the curve for triglyceride is higher for individuals consuming fructose-containing beverages with meals than that for those consuming glucose containing beverages. In the standard western diet, fructose is commonly ingested together with glucose, and often in the form of high fructose-corn syrup (HFCS), and would therefore promote a different metabolic response than fructose or glucose alone.
While fructose ingestion does not stimulate insulin release, it has been implicated in the development of adverse metabolic consequences. Fructose has been shown in tracer studies to be converted to glucose and contribute to endogenous glucose output [11]. However, its metabolism in obese subjects with insulin resistance in comparison to those with type 2 diabetes (T2DM) with relative insulin insufficiency requires further study in the context of every day consumption as HFCS. Such information may help us better understand the consequences of dietary exposure to fructose-containing soft drinks in obesity and T2DM. The objective of this study was to determine the metabolic response of adolescents to HFCS-sweetened beverage consumption, and to compare the response among subjects who are lean, overweight/obese, and with T2DM over 2 h. We proposed that consumption from HFCS would lead to high fructose and glucose excursions T2DM in comparison to those with overweight/obesity or normal weight.
2. Materials and methods
2.1. Subject recruitment and study visit
The study was approved by the Human Subjects Committee at the Los Angeles Biomedical Research Institute at Harbor-UCLA (LABioMed), in accordance with the Declaration of Helsinki. Three groups of subjects, age 13–19 years, were recruited from the Pediatric clinics at Harbor-UCLA Medical Center. The parent or legal guardian of the subjects provided written informed consent, and the subjects provided assent (age 13–17 years) or informed consent (age 18–19 years). 10 lean controls, 10 overweight/obese subjects without diabetes, and 5 subjects with T2DM treated with metformin completed the study. Overweight was defined as having a BMI ≥85% for age and gender, and obesity was defined as having a BMI≥95% for age and gender. Subjects with T2DM were diagnosed previously by the subject’s personal physician based on the American Diabetes Association criteria (HbA1c ≥ 6.5%, fasting glucose ≥126 mg/dL, or glucose ≥200 mg/dL drawn randomly or in response to a 2 h oral glucose tolerance test) [12]. For inclusion in this study, their HbA1c was within target (≤7.5%). Subjects were excluded in the T2DM group if medications included insulin, or if they had complications of diabetes such as microalbuminuria or retinopathy. Prior to the study visit, the subjects were instructed to maintain their usual dietary intake. After an overnight fast, subjects arrived at the Clinical and Translational Research Center (CTRC) at LABioMed. A 24-h food recall was collected from each subject. Study subject dietary intake was estimated by 24 h recall for the time period before the overnight fast. The quantification of dietary intake was generated by The Nutrition Data System for Research Software based on the University of Minnesota Nutrition Coordinating Center Food and Nutrient Database. A physical exam was performed, including anthropometric measurements for height, weight, and waist circumference. Percent body fat was determined by bioimpedance.
2.2. HFCS ingestion and evaluation of response
Fasting blood samples were drawn for a basic chemistry panel, lipid profile, hemoglobin A1c, C peptide, insulin, leptin, lactate, fructose, and glucose concentrations. The subjects were instructed to drink a 12 ounce can of soda containing 40 g of HFCS. Blood samples were collected every 15 min over 2 h after ingestion to determine concentrations of insulin, leptin, lactate, fructose, and glucose.
2.3. Biochemical analysis
The plasma concentrations of insulin were measured by a two-site fluoroimmunometric assay (AutoDELFIA Insulin), and leptin was measured by radioimmunoassay (Millipore). The HOMA-IR was calculated from the insulin and glucose values [13].
The plasma concentrations of fructose and glucose were measured using a previously reported isotope dilution gas chromatography/mass spectrometry (GC/MS) method [6]. Into each 200 μL serum sample, equal volumes of [1,2–13C2]-D-glucose (1 mg/mL) and [1,2,3–13C3]-Dfructose (10 μg/mL) were added as internal standards, prior to protein precipitation. The supernatant, which contains glucose and fructose, was transferred into a glass tube and air dried at room temperature. Conversion of glucose and fructose into their respective methoxyamineperacetate derivatives was carried out prior to the GC/MS analysis.
The lactate concentration was determined using the method of Jeong and colleagues [14]. Uniformly labeled 13C-lactate was added to 50 μl of plasma, and lactate was extracted from plasma with ethyl acetate. After drying, the residue was treated with bis-trimethylsilyl trifluoroacetamide and trimethylchlorosilane (99:1, v/v) (Sulpelco). GC/MS analyses were performed using a ZB5 capillary column (Phenomenex), and chemical ionization with methane as the carrier gas. The clusters around m/z 219 was monitored for lactate isotopomer calculation. Lactate concentration was calculated from the ratio of unlabeled and labeled lactate.
2.4. Statistical analysis
SYSTAT version 13 (Systat Software, San Jose, CA) was used for statistical analysis. Subject characteristics including the body measurements, nutrition intake, baseline laboratory values, and metabolite concentrations were compared among the three groups by ANOVA, followed by Tukey’s test for pairwise comparisons. Area under the curve for measures repeated over time was also compared among groups by ANOVA. Age and sex were examined as covariates, but these factors did not significantly affect the comparisons made in this study, and therefore unadjusted data is presented. Repeated measures ANOVA was performed to compare the multiple time points to baseline for fructose, glucose, and insulin concentrations. Kruskal-Wallis one-way analysis of variance on ranks was used if the normality or equal variance tests failed.
3. Results
3.1. Subject characteristics
A total of 25 subjects enrolled in this study. Subject characteristics, body measurements, and previous 24-h macronutrient intake are shown in Table 1. The average age for the subjects was 16 years old. Consistent with the ethnic distribution of patients served at the hospital affiliated with the study site, the majority of subjects in this study were of Hispanic descent. There was no difference in height among the three groups. Weight, BMI, waist circumference, and percent body fat measured by bioimpedance were greater in the obese subjects and nonsignificantly in the T2DM subjects compared to the controls. From the 24-h dietary recall, the estimated nutritional intake among the three groups was similar. While the sucrose/equivalent intake (including HFCS) were not significantly different among groups, the intake appeared less in the T2DM group than in the lean and overweight groups.
Table 1.
Subject characteristics.
| Characteristic | Lean Control | Overweight/Obese | T2DM |
|---|---|---|---|
| Number of Subjects (N) | 10 | 10 | 5 |
| F,M (N) | 6,4 | 5,5 | 1,4 |
| Age (yrs) | 16.8 ± 1.2 | 15.5 ± 1.3 | 16.8 ± 1.4 |
| Race/Ethnicity | 4 Hispanic, 5 Asian, 1 Indian | 7 Hispanic, 2 African American, 1 Pacific Islander | 4 Hispanic 1 African American |
| Height (cm) | 165.68 ± 4.79 | 166.5 ± 3.46 | 169.6 ± 6.2 |
| Weight (kg) | 57.5 ± 4.12 | 96.1 ± 10.99* | 85 ± 14.5* |
| BMI (kg/m2) | 20.9 ± 1.07 | 34.6 ± 3.58* | 30 ± 7.49* |
| Percent Body Fat (%) | 25.56 ± 3.91 | 38.82 ± 5.16* | 31.8 ± 10.4 |
| Waist circumference (cm) | 71.52 ± 4.36 | 103.45 ± 7.82* | 97.81 ± 16.93* |
| Energy intake (kcal/day) | 1971 ± 612 | 1907 ± 514 | 1552 ± 673 |
| Total carbohydrates (g)/day | 249 ± 64 | 230 ± 97 | 179 ± 131 |
| Starch (g)/day | 94 ± 27 | 105 ± 43 | 75 ± 38 |
| Glucose (g)/day | 28 ± 21 | 24 ± 19 | 24 ± 46 |
| Fructose (g)/day | 28 ± 24 | 23 ± 26 | 28 ± 57 |
| Sucrose/equivalent# (g)/day | 44 ± 24 | 46 ± 41 | 20 ± 18 |
| Other sugars@ (g)/day | 20 ± 24 | 12 ± 10 | 17 ± 17 |
| Total fat (g)/day | 75 ± 31 | 76 ± 25 | 69 ± 45 |
| Total protein (g)/day | 84 ± 35 | 79 ± 25 | 60 ± 18 |
Data are expressed as the mean ± standard deviation (SD) of the mean.
p < 0.05 vs. controls.
sucrose or high fructose corn syrup (HFCS).
includes lactose,galactose and maltose.
Baseline laboratory values are presented in Table 2. There were no differences among the groups for total cholesterol, LDL, triglycerides, HbA1c, or HOMA-IR. HDL concentrations were greater in the control group versus the T2DM and overweight/obese subjects. C-peptide concentrations were greater in the overweight/obese group compared to the controls.
Table 2.
Baseline fasting biochemical measures.
| Lean Control (n = 10) | Overweight/Obese (n = 10) | T2DM (n = 5) | |
|---|---|---|---|
| Glucose (mmol/L) | 4.57 ± 0.24 | 4.57 ± 0.23 | 5.26 ± 0.89 |
| C peptide (nmol/L) | 0.20 ± 0.07 | 0.47 ± 0.15* | 0.33 ± 0.14 |
| HbA1c (%) | 5.3 ± 0.2 | 5.6 ± 0.1 | 6.1 ± 0.6 |
| Fructose (μmol/L) | 14.18 ± 3.7 | 17.56 ± 4.77 | 13.99 ± 6.08 |
| HOMA-IR | 2.00 ± 0.69 | 4.37 ± 1.61 | 2.78 ± 2.6 |
| Total Cholesterol (mmol/L) | 3.85 ± 0.44 | 3.93 ± 0.57 | 3.85 ± 0.78 |
| LDL (mmol/L) | 2.28 ± 0.31 | 2.53 ± 0.36 | 2.48 ± 0.70 |
| HDL (mmol/L) | 1.19 ± 0.16 | 0.88 ± 0.13* | 0.72 ± 0.18* |
| TG (mmol/L) | 0.82 ± 0.19 | 1.16 ± 0.44 | 1.43 ± 0.81 |
Data are presented as the mean ± standard deviation (SD) of the mean.
p < 0.05 vs. controls.
3.2. Fructose
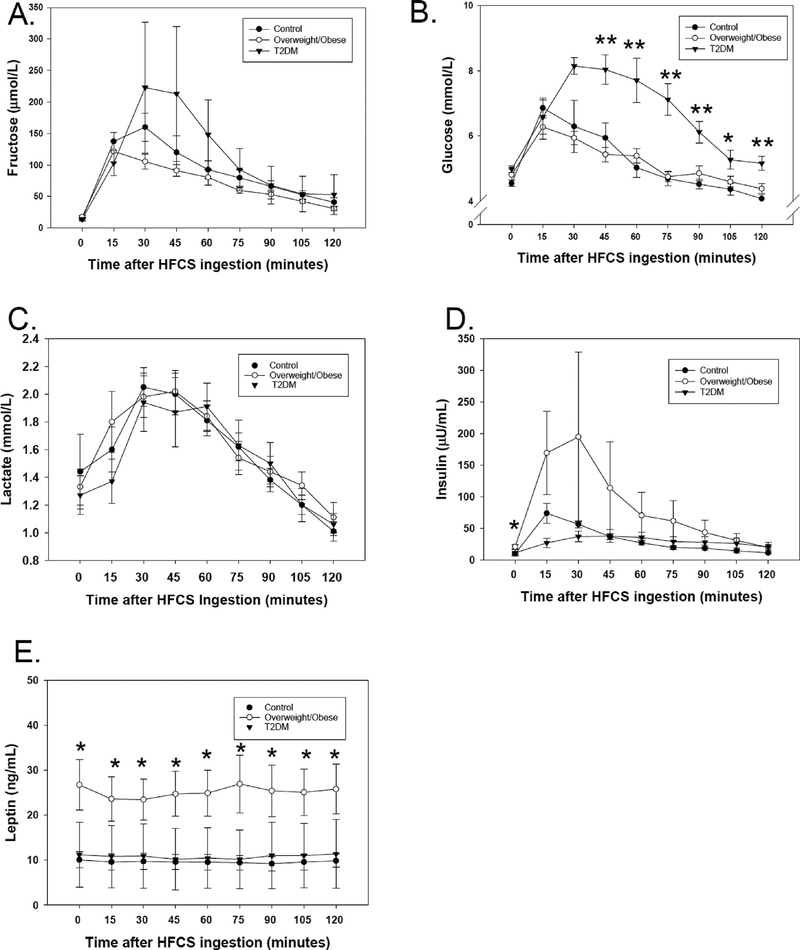
The baseline fasting concentrations of fructose were measured in low concentrations in the three groups of subjects. After consuming the soft drink, fructose levels rose quickly in the first 15 min (Fig. 1A). Within the control and overweight/obese groups, fructose concentrations achieved mean peaks in the 100–200 μmol/L range and remained significantly higher than baseline at 120 min. Among T2DM subjects, the mean concentration of fructose was as high as 222 μmol/L, but the mean concentrations at any time point were not significantly different compared to baseline. There were no statistically significant differences in fructose concentrations among groups at any time point, although one T2DM subject appeared to achieve much higher concentrations than the other subjects. Similarly, there were no significant differences in the areas under the curve (AUC) calculated for fructose in the three groups of subjects (11031 ± 2986 μmol/L-min for controls, 8657 ± 2159 μmol/L-min for obese, and 14009 ± 13079 μmol/L-min among T2DM), p = 0.28.
Fig. 1.
Fructose, glucose, lactate, insulin, and leptin concentrations at baseline, and at multiple time points over 120 min after high fructose corn syrup (HFCS) soda ingestion. Subjects who are lean controls are represented by filled circles, subjects with overweight/obesity are represented by open circles, and subjects with T2DM are represented by filled inverted triangles. The mean and standard error of the mean at each time point are shown. *p < 0.05, **p < 0.01 in comparison of the three groups by ANOVA. A. Fructose concentrations (μmol/L) rose after HFCS consumption and remained higher than baseline at every time point but did not significantly differ among groups. B. Glucose (mmol/L) was significantly higher among T2DM subjects after HFCS ingestion from 45 to 120 min. C. Lactate (mmol/L) rose non-significantly after HFCS consumption but did not differ among the three groups. D. Insulin (1μIU/mL = 7.175 pmol/L) was significantly higher in overweight/obese subjects at baseline. E. Leptin (ng/mL) did not change after HFCS consumption, but was significantly higher in the overweight/obese group across all time points.
3.3. Lactate
As fructose enters the glycolytic pathway, this results in production of the intermediate dihydroxyacetone phosphate (DHAP), which is metabolized via formation of methylgloxal, to form lactate. Consequently, fructose ingestion leads to a rise in lactate level [2]. The baseline lactate concentrations were: 1.4 ± 0.9 mmol/L (control), 1.3 ± 0.4 mmol/L (overweight/obese), and 1.3 ± 0.3 mmol/L (T2DM). The highest mean values were observed at 45 min (control 2.0 ± 0.5 mmol/L, overweight/obese 2.0 ± 0.5 mmol/L and T2DM 1.9 ± 0.6 mg/dL groups). Non-significant increases in lactate were observed after HFCS ingestion (Fig. 1C). When the three groups were compared, there were no significant differences in lactate concentrations at any time point. The non-significant increases observed in lactate concentration after HFCS ingestion indicate that a relatively small amount of the fructose is turned over into lactate.
3.4. Glucose
After ingestion of glucose, changes in plasma glucose concentration reflect the glucose ingested, insulin secretion, and insulin sensitivity. Initial glucose values were 4.5 ± 0.3 mmol/L (80 mg/dL) and 4.8 ± 0.6 mmol/L in the lean and obese subjects, respectively, compared to 5.0 ± 0.2 mmol/L (90 mg/dL) in subjects with T2DM (Fig. 1B). In all subjects, the glucose concentration rose quickly in the first 15 min. In the control and overweight/obese subjects, glucose concentrations peaked at 15–30 min to 6.8 ± 1.0 and 6.5 ± 1.2 mmol/L respectively, then decreased rapidly, returning to values similar to baseline by 60 min. In the subjects with T2DM, glucose concentrations peaked at 8.1 ± 0.6 mmol/L at 30 min and remained significantly higher longer until 105 min. At all time points, the mean glucose concentrations were significantly different among groups. Mean values from the T2DM group were higher than both the control and overweight/obese groups at 45 min (p = 0.001), at 60 min (p = 0.0002), at 75 min (p = 5 × 10−6), at 90 min (p = 6.0 × 10−4), and at 120 min (p = 0.00081). At 105 min there was a significant difference in glucose among groups (p = 0.025), with glucose concentrations in the T2DM group significantly higher than controls (p = 0.020), but non-significantly higher than the overweight/obese group (p = 0.09). The glucose AUC for the T2DM group (860 ± 96 mmol/ L × min) was significantly higher than the AUC in both the control (661 ± 69 mmol/L × min, p < 0.00007), and the overweight/obese groups (634 ± 49 mmol/L × min, p = 0.00002).
3.5. Insulin
At baseline, the obese group demonstrated the highest insulin concentrations (p < 0.05). Insulin concentrations increased 15 min after soft drink consumption (Fig. 1D). The concentration of insulin increased the highest in the obese group of subjects, and at 15 min the insulin level was higher in the overweight/obese group compared to the T2DM group. Insulin secretion was the lowest in the T2DM group and the acute rise in insulin concentration was blunted. The insulin AUC appeared to be the highest among the overweight/obese subjects (8450 ± 11,005 μU/mL × min), although this was not statistically significantly different from the other groups (3859 ± 1282 μU/ mL × min in the control group, 3548 ± 2165 μU/mL × min in the T2DM group).
3.6. Leptin
Leptin concentrations did not change from baseline values after HFCS ingestion (Fig. 1E). However, at all time points, the leptin values were significantly higher in the overweight/obese group than in the control and T2DM groups (control 9.60 ± 1.60 ng/mL, T2DM 10.77 ± 4.52 ng/mL, overweight/obese 25.18 ± 5.15 ng/mL, p < 0.05). Leptin values did not change over time in any group. The AUC was significantly higher in the overweight/obese group (3005 ± 2013 mg/mL × min) compared to lean controls (1147 ± 625 mg/mL × min), p = 0.027, but not T2DM(1285 ± 1882 mg/mL × min) (p = 0.98) due to the high variation in T2DM.
4. Discussion
This study examined the metabolic effects of consuming HFCS in adolescents with overweight/obesity, T2DM, or lean body composition. In analysis of our data, the unique considerations included an adolescent study cohort, using HFCS as the ingested source of fructose rather than fructose alone, and observing responses of lean, overweight/obese, and T2DM subjects. There were 3 main observations in our study: 1) plasma fructose concentrations rise and remain higher than baseline 120 min after HFCS ingestion, 2) glucose rises in all subjects despite a lower glucose load than that of a traditional OGTT, and 3) insulin rises in response to glucose after ingestion of HFCS.
Results from this study reveal that consuming a 12 oz. beverage containing about 40 g of HFCS causes fructose concentrations to rise quickly after consumption. In all three groups studied, mean plasma fructose concentrations remained higher than fasting values 2 h after consuming a beverage containing HFCS (though non-significantly in T2DM). The range of plasma fructose concentrations at baseline have been previously published [6]. Our baseline and peak values of fructose after ingestion of HFCS were comparable to those of McDonald et al. [2], who showed baseline values ranging from 15 μmol/L to 35 μmol/L with a peak value of 195 ± 20 μmol/L, in adults ingesting 75 gm/ 100 kg body weight of sucrose. While we did not demonstrate statistically significant differences between subjects with and without T2DM, one of the subjects with T2DM reached much higher peak fructose concentrations than the lean control or overweight/obese subjects. Previous published data suggests that individuals with T2DM exhibit higher plasma fructose concentrations than individuals without diabetes [15]. The higher concentration of fructose achieved in response to HFCS ingestion in our T2DM subject may not be fully explained by the ingested fructose since all subjects consumed the same quantity of HFCS. Therefore, one possibility is that an elevation of fructose concentrations in T2DM may be related to less effective disposal of fructose in the liver. Adults with T2DM have been shown to oxidize fructose less efficiently than lean individuals [16]. While overall carbohydrate oxidation has been shown to increase when glucose is administered with a fructose source (sucrose) [17], it is less clear how this process may be affected by T2DM. We speculate one possibility is that the higher rise in fructose in T2DM may be due to impaired clearance by lower oxidation.
Recent evidence indicates that the small intestine may play an important role in fructose metabolism. In a series of mouse studies, Jang et al. [18] used stable isotopes to demonstrate that the small intestine metabolized most free fructose that was ingested together with glucose. The authors concluded that fructose that reaches the liver is the portion that exceeds the small intestinal clearance capacity, thereby impacting liver metabolism and the microbiome. While these animal studies did not address the setting of T2DM, we speculate that if the human small intestine might play a similar role, then higher serum fructose concentrations may result from differences in the small intestinal ability to metabolize fructose in order to protect the liver from its exposure.
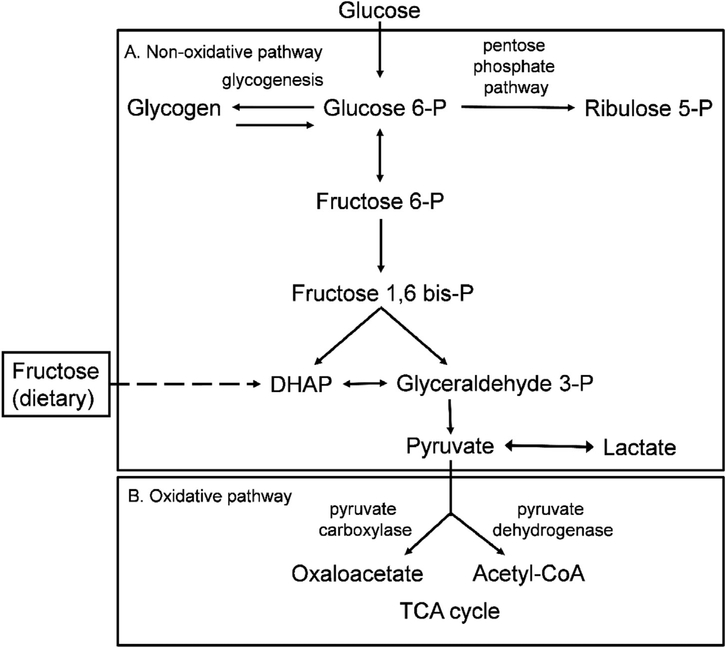
Pathways of fructose metabolism in the liver intersect with those of glycolysis and the pentose phosphate pathway (PPP) (Fig. 2). Major metabolic sinks for fructose that is absorbed through the small intestine to reach the liver may involve lactate production contributing to gluconeogenesis, glycogen synthesis, or pentose synthesis. Fructose may be converted to lactate, and the presence of fructose with glucose has been shown to increase lactate levels during exercise more than glucose alone [19]. However, in light of the baseline similar lactate levels seen across groups in our study and the non-significant rise after soft-drink consumption, we do not have evidence of impact on this pathway by HFCS ingestion. Metabolism of fructose through the PPP in T2DM has not been fully investigated. However, it is known that PPP is an important pathway in the development of hyperglycemia (as in thiamine responsive megaloblastic anemia syndrome [20]). Therefore, an alternate consideration is that the higher concentrations of fructose achieved by one of the T2DM subjects may be due to impaired clearance possibly at the level of the PPP.
Fig. 2.
Non-oxidative and oxidative pathways of carbohydrate metabolism. A. The non-oxidative pathway of glycolysis. Glucose is converted to glucose-6-phosphate (glucose-6-P) which may be used as a substrate in the pentose phosphate pathway for pyrimidine synthesis, or continue down glycolysis to be converted to pyruvate. Lactate may be formed from pyruvate under anaerobic conditions. Dietary fructose is converted to fructose-1-phosphate, which then enters glycolysis through production of dihydroxyacetone phosphate (DHAP). B. Pyruvate enters the oxidative steps of the tricarboxylic acid cycle (TCA) to produce acetyl CoA via pyruvate dehydrogenase or oxaloacetate through pyruvate carboxylase.
Our study subjects all demonstrated a rise in blood glucose after HFCS ingestion, with the T2DM subjects demonstrating the greatest rise. While this is not an unexpected finding, consideration of the origin of the blood glucose in the context of HFCS ingestion is of interest. In general, a rise in blood glucose may be attributed to increased glucose production or decreased glucose disposal. Previous studies of acute ingestion of fructose in normal adult male volunteers have shown that ingestion of fructose alone causes a very minor elevation of glucose with no elevation of insulin [2,21]. However, when fructose was administered together with glucose (e.g. sucrose), the rise in glucose was demonstrated to be higher than expected [2,22] (since fructose makes up only half the sucrose molecule), and insulin levels were less than or equal to that observed after glucose ingestion alone [2]. While fructose ingestion has not been shown to cause a rise in total endogenous glucose output in lean, obese, or T2DM individuals, the glucose includes a contribution from fructose-to-glucose conversion [16]. The recent tracer-based mouse studies by Jang et al., also suggest that the small intestine may have a role in metabolizing fructose to glucose before its appearance in the portal vein [18]. Therefore, we speculate that conversion of fructose to glucose might be a contributing factor to higher glucose in T2DM, which should be explored in future investigations in the context of both small intestinal function and liver.
Glucose disposal also depends on glucose cycling, which is the hepatic uptake, partial metabolism, and recycling of glucose. Tracer studies using simultaneous infusion of 2-H3-glucose and 6-H3-glucose [23,24] have measured the difference between glucose uptake and glucose release, showing that glucose cycling is significantly increased in T2DM subjects accounting for 30–50% of the glucose production rate, and could account for the blood glucose elevation in T2DM. Since fructose promotes hepatic glucose output and glucose cycling [25], the glucose excursion seen in our study after consuming a HFCS containing soft drink might be partially attributable to increased glucose cycling. Specifically, in T2DM, glucose cycling may be exacerbated by the decrease in insulin release.
HFCS soft drink consumption resulted in increased insulin production in our lean and obese subjects, but the insulin response was blunted in T2DM. Fructose alone does not produce an insulin response [26]. However, glucose normally stimulates insulin secretion in a biphasic pattern with an initial spike followed by a sustained plateau. The initial phase is from preformed insulin in response to glucose in the enterohepatic circulation, and the second phase is from newly synthesized insulin [27]. The insulin excursions observed in our subjects within 30 min after soft drink ingestion in our study likely represent the first phase of insulin secretion, in response to the glucose component of HFCS and any glucose produced from metabolism of fructose. The notable elevation in obese subjects that persists is consistent with the greater responsiveness in their islet cells in comparison to the lean subjects, resulting in high second phase levels reflecting the insulin resistant state, and the ability to compensate in order to control their blood glucose. The blunted initial pattern observed among the T2DM subjects reflects impaired first phase insulin release, consistent with decreased glucose uptake and the resulting elevated blood glucose curve.
Fructose interactions with other nutrient components is complex depending on the dietary source and proportion of fructose as shown by our example of ingestion of HFCS. One study in adults with T2DM showed that 7.5 g of fructose added to an oral glucose tolerance test led to a decrease in insulin and glucose production (lower glycemic response) compared to results of the test without fructose [28]. Teft KL et al. showed in men and women with obesity, that fructose consumption in meals instead of glucose resulted in a lower post-prandial rise in blood glucose and insulin levels than when glucose was consumed. On the other hand, the rise in lactate was higher with fructose than with glucose in the diet [7]. Stanhope et al. reported similar results in normal lean subjects [29]. These studies suggest that the effects of fructose on metabolism may be dependent on the amount consumed and whether it is mixed with glucose. A small amount (e.g. 10 g/meal) in addition to glucose may result in a lower glycemic response, whereas a larger amount (e.g. 60 g) may have potentially adverse consequences on metabolism such as increase in triglyceride concentrations [30]. Le et al. compared the ingestion of free fructose in HFCS to the ingestion of bound fructose in sucrose, and found that the plasma fructose concentration was reflective of its higher proportion in HFCS [22]. However, the glucose concentration after HFCS appeared higher than expected based on the proportion.
The main limitation of this study is the smaller number of subjects in the T2DM group relative to the control and overweight/obese groups. The small sample size limits the ability to detect differences between the groups for many of the outcome measures. The T2DM group was more challenging to recruit since many of the subjects screened required treatment with agents in addition to metformin, and we aimed to minimize confounding from variation in treatment regimens by including subjects on metformin monotherapy. Metformin reduces gluconeogenesis and thus may affect the study results in the T2DM subjects [31]. However, metformin may not completely reduce it to control levels, and therefore, subjects on metformin still exhibit a T2DM metabolic phenotype [32]. The present study focused on “reallife” HFCS consumption only, and we did not test the responses to fructose-only or glucose-only beverages for direct comparison. Although all our subjects consumed the same brand of HFCS containing beverage, it is possible that the HFCS content in the beverage may differ from what the label showed [33].
5. Conclusions
Consumption of an HFCS-containing soft drink resulted in hepatic exposure to fructose, with higher-than-baseline concentrations up to 120 min after ingestion. We propose that HFCS may contribute to elevations in plasma glucose concentrations through promotion of glucose cycling, which may be excessive in T2DM, thus exacerbating hyperglycemia. Moreover, glucose concentrations may be affected by small intestinal carbohydrate absorption and fructose metabolism to glucose. Future investigations should include the role of the small intestine and the liver, and their impact on the metabolism of fructose in the presence or absence of glucose.
Supplementary Material
HIGHLIGHTS.
After high fructose corn syrup (HFCS) ingestion by adolescents, plasma fructose rises and peaks at 15–30 min.
At 120 min after HFCS ingestion, plasma fructose levels remain higher than baseline.
Insulin rises in response to the glucose component of HFCS.
Acknowledgements
The research described was supported by NIH/National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881 through the UCLA partner institute, the Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center. We appreciate laboratory assistance from Shu Lim, of the Biomedical Mass Spectrometry Facility at LABioMed, and biostatistical advice from Peter Christenson, PhD.
Abbreviations:
- HFCS
high fructose corn syrup
- T2DM
type 2 diabetes
- BMI
body mass idex
- HbA1c
hemoglobin A1c
- GC/MS
gas chromatography/mass spectrometry
- ANOVA
analysis of variance
- LDL
low density lipoprotein
- HDL
high density lipoprotein
- HOMA-IR
homeostasis model assessment as an index of insulin resistance
- DHAP
dihydroxyacetone phosphate
- PPP
pentose phosphate pathway
Footnotes
Conflicts of interest statement
The authors have no conflicts of interests to disclose.
Appendix A. Supplementary data
Supplementary data related to this article can be found at https://doi.org/10.1016/j.jnim.2018.08.004.
References
- [1].Bray GA, Nielsen SJ, Popkin BM, Consumption of high-fructose corn syrup in beverages may play a role in the epidemic of obesity, Am. J. Clin. Nutr 79 (4) (2004) 537–543. [DOI] [PubMed] [Google Scholar]
- [2].Macdonald I, Keyser A, Pacy D, Some effects, in man, of varying the load of glucose, sucrose, fructose, or sorbitol on various metabolites in blood, Am. J. Clin. Nutr 31 (8) (1978) 1305–1311. [DOI] [PubMed] [Google Scholar]
- [3].Drewnowski A, Rehm CD, Consumption of added sugars among Us children and adults by food purchase location and food source, Am. J. Clin. Nutr 100 (3) (2014) 901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu FB, Malik VS, Sugar-sweetened beverages and risk of obesity and type 2 diabetes: epidemiologic evidence, Physiol. Behav 100 (1) (2010) 47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. , Trends in obesity prevalence among children and adolescents in the United States, 19881994 through 2013–2014, JAMA 315 (21) (2016) 2292–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wahjudi PN, Patterson ME, Lim S, Yee JK, Mao CS, Lee WN, Measurement of glucose and fructose in clinical samples using gas chromatography/mass spectrometry, Clin. Biochem 43 (1–2) (2010) 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Teff KL, Grudziak J, Townsend RR, Dunn TN, Grant RW, Adams SH, et al. , Endocrine and metabolic effects of consuming fructose- and glucose-sweetened beverages with meals in obese men and women: influence of insulin resistance on plasma triglyceride responses, J. Clin. Endocrinol. Metab 94 (5) (2009) 1562–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Katz J, McGarry JD, The glucose paradox. Is glucose a substrate for liver metabolism? J. Clin. Invest 74 (6) (1984) 1901–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Marin S, Lee WN, Bassilian S, Lim S, Boros LG, Centelles JJ, et al. , Dynamic profiling of the glucose metabolic network in fasted rat hepatocytes using [1,2–13c2]Glucose, Biochem. J 381 (Pt 1) (2004) 287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Theytaz F, de Giorgi S, Hodson L, Stefanoni N, Rey V, Schneiter P, et al. , Metabolic fate of fructose ingested with and without glucose in a mixed meal, Nutrients 6 (7) (2014) 2632–2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Sun SZ, Empie MW, Fructose metabolism in humans - what isotopic tracer studies tell Us, Nutr. Metab. (Lond.) 9 (1) (2012) 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Classification and diagnosis of diabetes, Diabetes Care 38 (Supplement 1) (2015) S8–S16. [DOI] [PubMed] [Google Scholar]
- [13].Salgado AL, Carvalho L, Oliveira AC, Santos VN, Vieira JG, Parise ER, Insulin resistance index (homa-Ir) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals, Arq. Gastroenterol 47 (2) (2010) 165–169. [DOI] [PubMed] [Google Scholar]
- [14].Jeoung NH, Rahimi Y, Wu P, Lee WN, Harris RA, Fasting induces ketoacidosis and hypothermia in pdhk2/pdhk4-double-knockout mice, Biochem. J 443 (3) (2012) 829–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kawasaki T, Akanuma H, Yamanouchi T, Increased fructose concentrations in blood and urine in patients with diabetes, Diabetes Care 25 (2) (2002) 353–357. [DOI] [PubMed] [Google Scholar]
- [16].Paquot N, Schneiter P, Jequier E, Gaillard R, Lefebvre PJ, Scheen A, et al. , Effects of ingested fructose and infused glucagon on endogenous glucose production in obese niddm patients, obese non-diabetic subjects, and healthy subjects, Diabetologia 39 (5) (1996) 580–586. [DOI] [PubMed] [Google Scholar]
- [17].Jentjens RL, Shaw C, Birtles T, Waring RH, Harding LK, Jeukendrup AE, Oxidation of combined ingestion of glucose and sucrose during exercise, Metabolism 54 (5) (2005) 610–618. [DOI] [PubMed] [Google Scholar]
- [18].Jang C, Hui S, Lu W, Cowan AJ, Morscher RJ, Lee G, et al. , The small intestine converts dietary fructose into glucose and organic acids, Cell Metabol. 27 (2) (2018) e353351–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lecoultre V, Benoit R, Carrel G, Schutz Y, Millet GP, Tappy L, et al. , Fructose and glucose Co-ingestion during prolonged exercise increases lactate and glucose fluxes and oxidation compared with an equimolar intake of glucose, Am. J. Clin. Nutr 92 (5) (2010) 1071–1079. [DOI] [PubMed] [Google Scholar]
- [20].Pacal L, Kuricova K, Kankova K, Evidence for altered thiamine metabolism in diabetes: is there a potential to oppose gluco- and lipotoxicity by rational supplementation? World J. Diabetes 5 (3) (2014) 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Jameel F, Phang M, Wood LG, Garg ML, Acute effects of feeding fructose, glucose and sucrose on blood lipid levels and systemic inflammation, Lipids Health Dis. 13 (2014) 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Le MT, Frye RF, Rivard CJ, Cheng J, McFann KK, Segal MS, et al. , Effects of highfructose corn syrup and sucrose on the pharmacokinetics of fructose and acute metabolic and hemodynamic responses in healthy subjects, Metabolism 61 (5) (2012) 641–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Efendic S, Wajngot A, Vranic M, Increased activity of the glucose cycle in the liver: early characteristic of type 2 diabetes, Proc. Natl. Acad. Sci. U. S. A 82 (9) (1985) 2965–2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Efendic S, Karlander S, Vranic M, Mild type ii diabetes markedly increases glucose cycling in the postabsorptive state and during glucose infusion irrespective of obesity, J. Clin. Invest 81 (6) (1988) 1953–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Dirlewanger M, Schneiter P, Jequier E, Tappy L, Effects of fructose on hepatic glucose metabolism in humans, Am. J. Physiol. Endocrinol. Metab 279 (4) (2000) E907–E911. [DOI] [PubMed] [Google Scholar]
- [26].Curry DL, Effects of mannose and fructose on the synthesis and secretion of insulin, Pancreas 4 (1) (1989) 2–9. [DOI] [PubMed] [Google Scholar]
- [27].Henquin JC, Ishiyama N, Nenquin M, Ravier MA, Jonas JC, Signals and pools Underlying biphasic insulin secretion, Diabetes 51 (Suppl 1) (2002) S60–S67. [DOI] [PubMed] [Google Scholar]
- [28].Moore MC, Davis SN, Mann SL, Cherrington AD, Acute fructose administration improves oral glucose tolerance in adults with type 2 diabetes, Diabetes Care 24 (11) (2001) 1882–1887. [DOI] [PubMed] [Google Scholar]
- [29].Stanhope KL, Havel PJ, Endocrine and metabolic effects of consuming beverages sweetened with fructose, glucose, sucrose, or high-fructose corn syrup, Am. J. Clin. Nutr 88 (6) (2008) 1733S–1737S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cozma AI, Sievenpiper JL, de Souza RJ, Chiavaroli L, Ha V, Wang DD, et al. , Effect of fructose on glycemic control in diabetes, A systematic review and meta-analysis of controlled feeding trials 35 (7) (2012) 1611–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Stumvoll M, Nurjhan N, Perriello G, Dailey G, Gerich JE, Metabolic effects of metformin in non-insulin-dependent diabetes mellitus, N. Engl. J. Med 333 (9) (1995) 550–554. [DOI] [PubMed] [Google Scholar]
- [32].Hundal RS, Krssak M, Dufour S, Laurent D, Lebon V, Chandramouli V, et al. , Mechanism by which metformin reduces glucose production in type 2 diabetes, Diabetes 49 (12) (2000) 2063–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Walker RW, Dumke KA, Goran MI, Fructose content in popular beverages made with and without high-fructose corn syrup, Nutrition 30 (7–8) (2014) 928–935. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.