OBJECTIVE:
To examine the diagnostic performance of proposed criteria for the research reporting of amniotic fluid embolism (AFE).1
STUDY DESIGN:
The Amniotic Fluid Embolism Registry is an international database established at Baylor College of Medicine in partnership with the Amniotic Fluid Embolism Foundation and the Perinatal Research Branch of the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health. Charts submitted to the registry between August 1, 2013, and June 31, 2017, underwent 2 separate analyses. First, a group of maternal-fetal medicine specialists with expertise in critical care (S.L.C., G.A.D., M.A.B.) conducted a detailed analysis of medical records to categorize these women into 3 groups, based on their consensus diagnosis: (1) AFE; (2) a distinct diagnosis other than AFE; and (3) indeterminate/uncertain diagnosis. Next, each chart was independently analyzed by a physician with no obstetric training to determine whether the medical record did or did not contain each of the recently published criteria for the diagnosis of AFE1 (Table). Agreement between expert record analysis and the presence of these strict, objective criteria was determined.
TABLE.
Uniform diagnostic criteria for research reporting of amniotic fluid embolism1
| 1. Sudden onset of cardiorespiratory arrest, or both hypotension (systolic blood pressure <90 mm Hg) and respiratory compromise (dyspnea, cyanosis, or peripheral capillary oxygen saturation [Sp02] <90%). |
| 2. Documentation of overt disseminated intravascular coagulation (DIC) following appearance of these initial signs or symptoms, using scoring system of Scientific and Standardization Committee on DIC of the International Society on Thrombosis and Haemostasis (ISTH), modified for pregnancy. Coagulopathy must be detected prior to loss of sufficient blood to itself account for dilutional or shock-related consumptive coagulopathy. |
| 3. Clinical onset during labor or within 30 minutes of delivery of placenta. |
| 4. No fever (≥ 38.0°C) during labor. |
RESULTS:
A total of 115 charts were reviewed. Expert review resulted in a diagnosis of AFE in 68 cases (59%), a clear alternative diagnosis in 26 cases (23%), and an indeterminate diagnosis in 21 cases (18%) (Figure).When analyzed according to the presence or absence of the proposed objective criteria, 54 cases were identified as AFE. In each of these cases, expert review had also made the diagnosis of AFE. The 14 cases (21%) identified by the expert panel as AFE that did not meet the objective criteria are believed to represent atypical presentations of this condition. The diagnostic performance of this criteria set was as follows: sensitivity, 79.4% (67.9–88.3); specificity, 100% (94.1–100.0); positive predictive value, 100%; negative predictive value, 81.3% (73.7–87.4).
Figure.
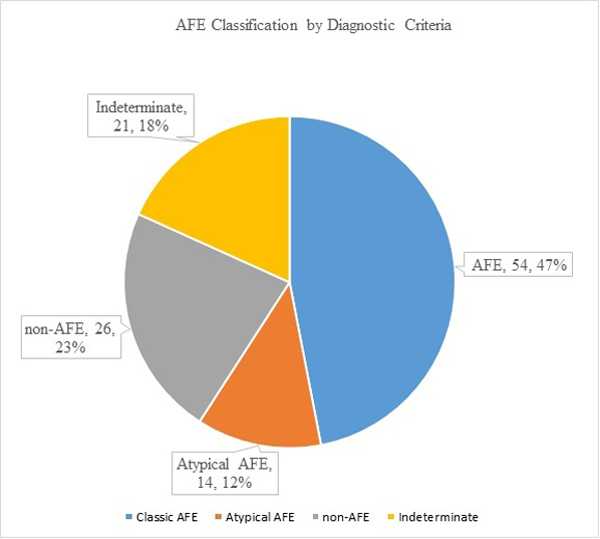
Amniotic fluid embolism classification by diagnostic criteria Stafford. Research reporting of amniotic fluid embolism. Am J Obstet Gynecol 2019.
CONCLUSION:
The guidelines for research reporting of AFE were developed by a multidisciplinary group in an effort to establish objective criteria that, if present, are not consistent with any known medical condition other than AFE.1 The authors of these guidelines acknowledged that although the use of these criteria may provide a clean data set for research analysis, some atypical cases would be excluded. Our review supports this assumption. Each case meeting the objective criteria for AFE diagnosis was independently determined by the expert panel to have AFE. However, as anticipated, these criteria appear to exclude 21% of women with less typical presentations.1–3 In this analysis, we used consensus expert review as the standard against which the proposed objective criteria were measured. Although we recognize potential problems with the use of such review as a standard, such opinions have, to date, been the only available diagnostic standard, and we believe that these particular reviewers are as qualified as any to render such opinions.4,5 In light of these data, we encourage the adoption of these objective criteria as the standard for research reporting pending future identification of additional valid, objective criteria that would allow inclusion of less typical cases, and further enhance our understanding of the spectrum of this condition.1
Acknowledgments
FUNDING: This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U.S. Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
DISCLOSURE: The authors report no conflicts of interest.
Contributor Information
Irene A. Stafford, Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine, 6651 Main Street, Suite 1096, Baylor College of Medicine, Houston, TX, 77030, Irene.Stafford@bcm.edu.
Amirhossein Moaddab, Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine, Baylor College of Medicine, Houston, TX.
Gary A. Dildy, Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine, Baylor College of Medicine, Houston, TX.
Miranda Klassen, Amniotic Fluid Embolism Foundation, Vista, CA.
Michael A. Belfort, Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine, Baylor College of Medicine, Houston, TX.
Roberto Romero, Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Bethesda, MD and Detroit, MI; Department of Obstetrics and Gynecology University of Michigan Ann Arbor, MI; Department of Epidemiology and Biostatistics Michigan State University East Lansing, MI; Center for Molecular Medicine and Genetics Wayne State University Detroit, MI.
Steven L. Clark, Department of Obstetrics and Gynecology, Division of Maternal-Fetal Medicine, Baylor College of Medicine, Houston, TX.
REFERENCES
- 1.Clark SL, Romero R, Dildy GA, et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol 2016; 215:408–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol 1995; 172:1158–1167. [DOI] [PubMed] [Google Scholar]
- 3.Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol 2015; 4:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacheco LD, Saade G, Hankins GD, Clark SL. Amniotic fluid embolism: diagnosis and management. Society for Maternal-Fetal Medicine (SMFM). Am J Obstet Gynecol 2016; 215:B16–24. [DOI] [PubMed] [Google Scholar]
- 5.Clark SL. Amniotic fluid embolism—expert review. Obstet Gynecol 2014; 123:337–348. [DOI] [PubMed] [Google Scholar]


