Abstract
Glioblastomas (GBMs) are the most aggressive primary brain tumors, with an average survival of less than 15 months. Therefore, there is a critical need to develop novel therapeutic strategies for GBM. This study aimed to assess the prognostic value of miR-4516 and investigate its oncogenic functions and the underlying cellular and molecular mechanisms in GBM. To determine the correlation between miR-4516 expression and overall survival of patients with GBM, total RNAs were isolated from 268 FFPE tumor samples, miR expression was assayed (simultaneously) using the nCounter human miRNA v3a assay followed by univariable and multivariable survival analyses. Further, in vitro and in vivo studies were conducted to define the role of miR-4516 in GBM tumorigenesis and the underlying molecular mechanisms. Upon multivariable analysis, miR-4516 was correlated with poor prognosis in GBM patients (HR=1.49, 95%CI: 1.12–1.99, p=0.01). Interestingly, the significance of miR-4516 was retained including MGMT methylation status. Overexpression of miR-4516 significantly enhanced cell proliferation and invasion of GBM cells both in vitro and in vivo. While conducting downstream targeting studies, we found that the tumor-promoting function of miR-4516, in part, was mediated by direct targeting of PTPN14 (protein tyrosine phosphatase, non-receptor type 14) which, in turn, regulated the Hippo pathway in GBM. Taken together, our data suggest that miR-4516 represents an independent negative prognostic factor in GBM patients and acts as a novel oncogene in GBM, which regulates the PTPN14/Hippo pathway. Thus, this newly identified miR-4516 may serve as a new potential therapeutic target for GBM treatment.
Keywords: miR-4516, prognosis, PTPN14, Hippo pathway, GBM
INTRODUCTION
Glioblastoma (GBM), is the most common and the most aggressive of all adult primary tumors of the human brain with an average patient survival time of less than 15 months despite standard treatment with maximum safe resection followed by radio-chemotherapy (1). Recently, the World Health Organization has reclassified GBM, based on molecular features, into two broad subgroups: GBM with wild-type isocitrate dehydrogenase (IDH)1/2 and GBM with mutant IDH1/2. However, less than 10% of primary GBMs have IDH1/2 mutations (2). Significant prognostic biomarkers providing relative high-risk assessments for early clinical treatment selection and potential targets for novel therapies have remained to be identified and characterized in GBM (3, 4). To meet this critical need, we undertook high-throughput molecular profiling approaches to identify micro-RNAs (miRs) that correlate with clinical outcomes of patients with GBM harboring the wild-type IDH, who had significantly longer survival times compared with those harboring mutant IDH (2). Further, we employed molecular genetics approaches, in vitro and in vivo, to define the potential roles of candidate biomarker miRs that contribute to GBM pathogenesis and treatment response.
miRNAs are small, endogenous non-coding RNAs that act as negative regulators of mRNA translation at the post-transcriptional level by annealing with 3’-untranslated regions (UTRs) (5). To date, miRNAs have been reported as an important player in regulating tumor cellular functions such as cell motility, apoptosis, proliferation, and stress response (6, 7). Moreover, current literature indicates that miRNAs could be useful in the diagnosis and prognosis of human cancers (8, 9). Although the mechanisms of tumorigenesis are still being elucidated, miRNAs are emerging as novel promising, prognostic biomarkers in GBM. A few miRNAs including miR-326, miR-218, miR-377, and miR-423–5p have been validated as having functional significance in GBM biology (10–13). However, little is known about the novel role of miR-4516 in the development of cancer including GBM. This is the first study, to our knowledge, reporting an involvement of miR-4516 in GBM, one of the most lethal human malignancies, which remains relatively understudied. In the present study, we aimed to investigate the expression and function of miR-4516 in GBM and its potential targets. For the first time, we showed that miR-4516 is a novel prognostic biomarker of GBM patients and that protein-tyrosine phosphatase non-receptor type 14 (PTPN14) is a direct functional target of miR-4516. PTPN14, also called Pez, PTP36, PTPD2 plays a role in the regulation of cell-cell adhesion, growth, and cell motility (14, 15). PTPN14 acts as a negative regulator of the oncogenic activity of YAP, a downstream signal transducer of the Hippo pathway, and functions as a tumor suppressor in various cancer types (16, 17). Thus, the current study provides insights into the molecular mechanisms underlying miR-4516-mediated tumorigenesis in GBM.
RESULTS
miR-4516 expression is negatively correlated with GBM patient overall survival (OS) and overexpressed in GBM cell lines.
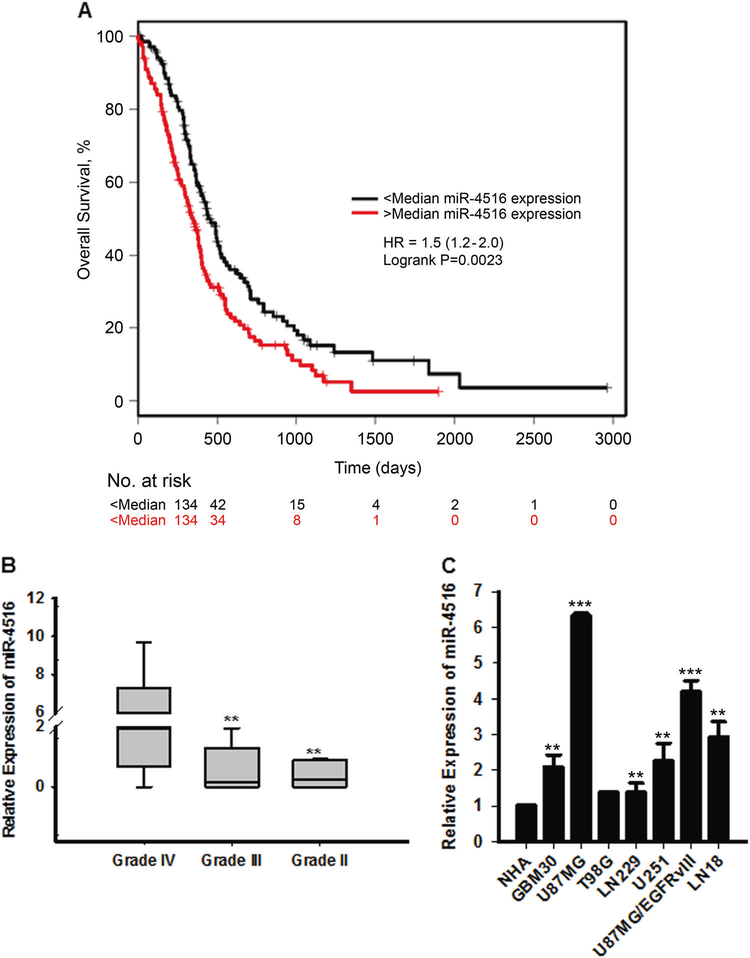
To investigate the correlation between miRNA expression and GBM patient clinical outcome, we first profiled miRNA expression in GBM tumor samples using Nanostring v3 miRNA assay. miR-4516 was one of the top miRNAs correlated with OS (FDR p<0.05). Upon UVA (HR=1.29, 95%CI: 1.12–1.48, p<0.001, Table 1), miR-4516 was significantly correlated with worse OS. Figure 1A shows the median dichotomized miR association with survival. Multivariable analyses (n=185 patients with non-missing data available across all covariables) showed that miR-4516 was associated with poor prognosis which was independent of clinical variables (HR=1.49, 95%CI: 1.12–1.99, p=0.01, Table 1). Importantly, a multi-marker multivariable analysis was performed with MGMT methylation status and miR-4516 retained its statistical significance (Supplementary Table 2). To determine if miR-4516 expression was associated with glioma grade, we evaluated expression of miR-4516 in glioma tumor samples by Taqman microRNA assay-based qRT-PCR. By categorizing all glioma RNA samples into grade II, grade III, and grade IV (8 glioma tissues each group) using the traditional WHO classification, we found that miR-4516 level was significantly upregulated in grade IV compared with grade II and III (Figure 1B). The endogenous expression of miR-4516 was further assessed by qRT-PCR in normal human astrocytes (NHAs) and 7 GBM cell lines (GBM30, U87MG, T98G, LN229, U251, U87MGvIII, and LN18). The results show that all GBM cell lines expressed varying levels of miR-4516, which were significantly higher than miR-4516 level in NHAs (Figure 1C). These results suggest that miR-4516 is significantly upregulated in different GBM cell lines and correlates with poor prognosis in GBM.
Table 1.
Univariable and Multivariable analyses of clinic-pathologic parameters of overall survival in patient with GBM
| UVA | MVA | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | Categorical | 1.13 | 0.85–1.50 | 0.39 | 1.23 | 0.92–1.64 | 0.16 |
| Age | Continuous | 1.03 | 1.02–1.04 | <0.001 | 1.00 | 0.99–1.02 | 0.23 |
| KPS-70 | < 70 vs 70 and above | 0.55 | 0.40–0.76 | <0.001 | 0.91 | 0.64–1.29 | 0.60 |
| Treatment | None vs TMZ or IR alone | 0.12 | 0.07–0.20 | <0.001 | 0.12 | 0.070–0.21 | <0.001 |
| Treatment | None vs TMZ+IR (Stupp protocol) | 0.03 | 0.02–0.05 | <0.001 | 0.04 | 0.02–0.06 | <0.001 |
| miR-4516 | Continuous | 1.29 | 1.12–1.48 | <0.001 | 1.49 | 1.12–1.99 | 0.01 |
Figure 1. Expression of miR-4516 in glioma patient samples and cell lines.
(A) Correlation between miR-4516 expression and OS by Kaplan-Meier analysis of GBM patients with high (n=134) and low (n=134) expression of miR-4516. (B) qRT-PCR analysis of miR-4516 expression in grade II (n=8), grade III (n=8), and grade IV (n=8) glioma patient samples by qRT-PCR. Normalized by miR-361–5p. Alterations in miR-4516 expression are shown as box plot. **P<0.01. (C) Expression of miR-4516 in 7 glioblastoma cell lines (GBM30, U87MG, T98G, LN229, U251, U87MGvIII, and LN18) and NHA. Normalized by miR-361–5p. n=3, **P<0.01, ***P<0.001.
miR-4516 promotes proliferation and invasion of GBM cells
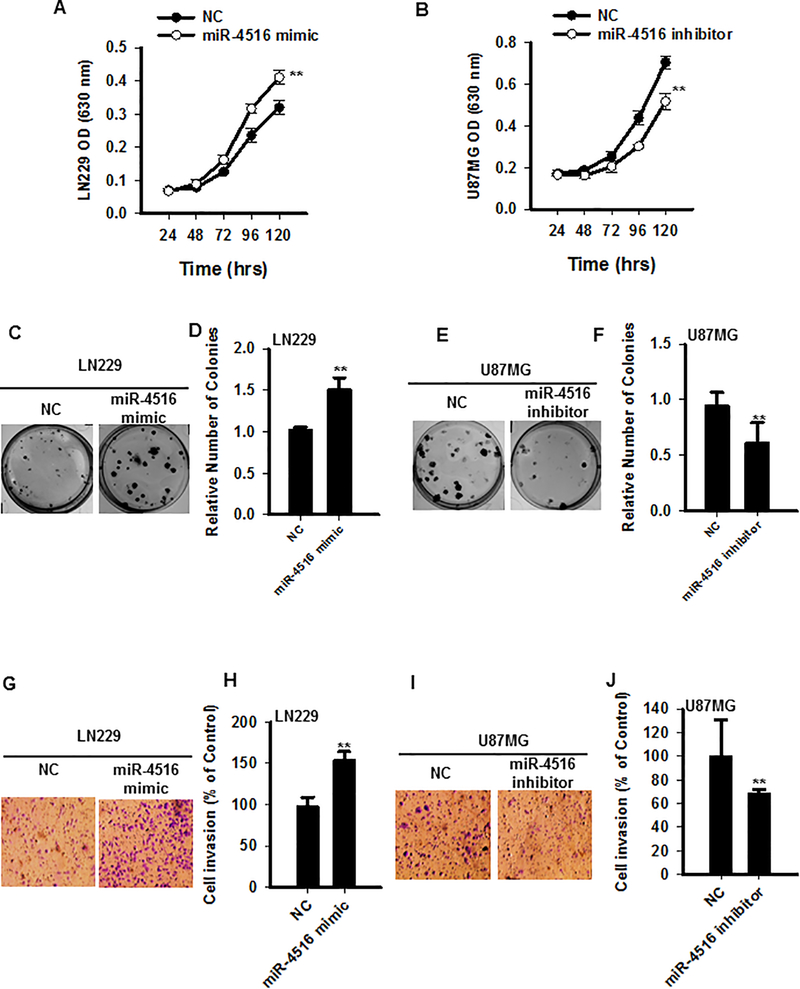
Given the fact that high expression of miR-4516 in GBM patients is associated with worse overall survival, we hypothesized that miR-4516 might play a role in the progression of GBM. To investigate the function of miR-4516 in GBM cells, LN229 cells with low endogenous expression of miR-4516 compared with other GBM cells (Figure 1C) were transfected with miRNA negative control (NC) or miR-4516 mimic, and overexpression of miR-4516 was confirmed by qRT-PCR (Figure S1A). In parallel, U87MG and LN18 cells that expressed relatively high levels of endogenous miR-4516 were transfected with NC or miR-4516 inhibitor. Cell proliferation assays showed that overexpression of miR-4516 significantly promoted proliferation and that inhibition of miR-4516 suppressed proliferation of GBM cells (Figures 2A–B). Colony formation assays demonstrated that GBM cells which overexpressed miR-4516 produced significantly more colonies than control (NC) cells (Figures 2C–D). Reversely, there were significantly fewer colonies in miR-4516 inhibited cells than in NC cells (Figures 2E–F). Subsequently, we determined the role of miR-4516 in invasion using a Transwell Matrigel assay, which demonstrated that overexpression of miR-4516 enhanced invasion of GBM cells (Figures 2G–H), whereas inhibition of miR-4516 reduced the invasion (Figures 2I–J). Similar results were observed in LN18 cells (Figures S1B–D). A recent study shows that miR-4516 inhibition in human keratinocytes partially inhibits PUVA-induced apoptosis, suggesting that miR-4516 is involved in PUVA-induced apoptosis in these cells (18). However, in GBM cells, we found that inhibition of miR-4516 by anti-miR-4516 inhibitor produced a marginal increase in apoptosis compared with the control (Figures S2A–C) Taken together, these in vitro data suggest that miR-4516 promotes both proliferation and invasion of GBM cells thereby functioning as an onco-micro-RNA.
Figure 2. miR-4516 promotes proliferation and invasion of GBM cells.
LN229 cells were transiently transfected with miRNA negative control (NC) or miR-4516 mimics, respectively. U87MG cells were transiently transfected with NC or miR-4516 inhibitor. Cell proliferation was measured at the different time points. n=5, **P<0.01, compared with controls (A-B). Colony formation assay was performed after NC, miR-4516 mimics, or miR-4516 inhibitor transfection (C-F). n=3, **P<0.01. The transwell invasion assay was conducted and numbers of invaded cells were quantified (G-J). n=3, **P<0.01.
PTPN14 is a putative direct target of miR-4516
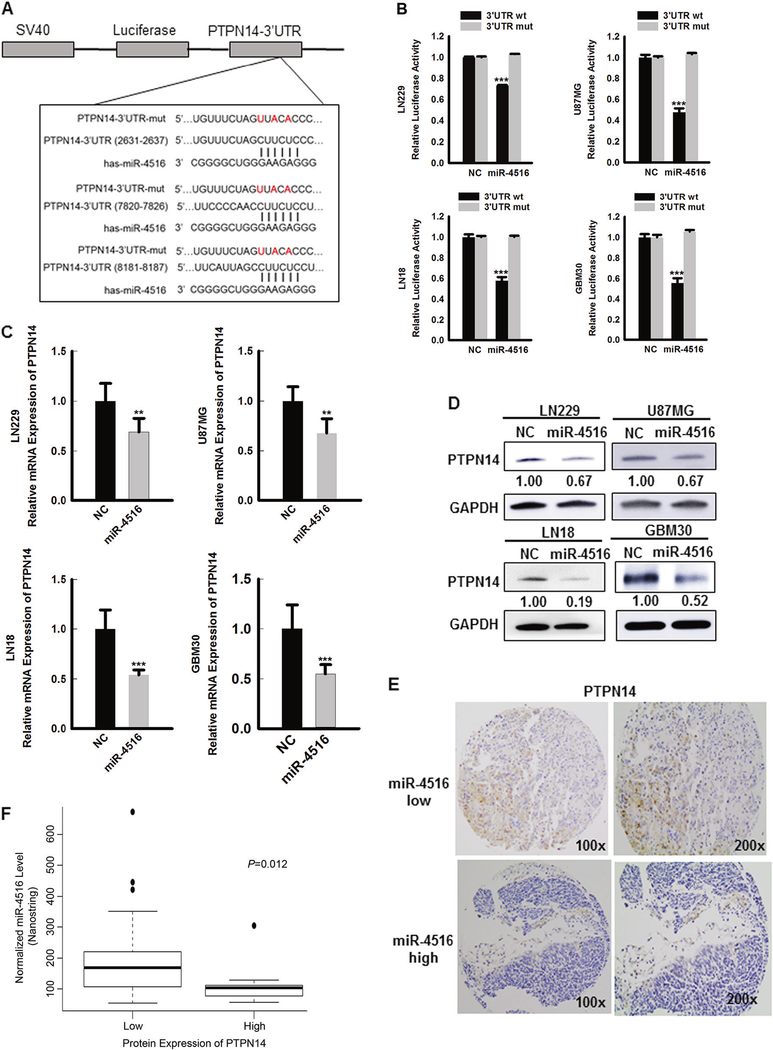
To identify putative mRNA targets of miR-4516, we performed bioinformatics analyses employing three different micro-RNA target prediction tools and found several candidate targets (Figure S3A). Among them, the 3’UTR of PTPN14 mRNA contains sequences complementary to the miR-4516 seed sequence (Figure 3A). To verify whether PTPN14 is a putative direct target of miR-4516, LN229, U87MG, LN18, and GBM30 cells were co-transfected with luciferase reporter constructs containing 3 putative miR-4516 targeting sequences in the 3’UTR of PTPN14, and NC/miR-4516 mimics. Consistent reduction in luciferase activity by miR-4516 was observed in all GBM cell lines examined (Figure 3B). To further validate target specificity, we generated mutated form of the 3’UTR (Figure 3A), where all the three binding sites of miR-4516 were destroyed using Q5 Site-Directed Mutagenesis Kit (NEB) that altered 3 key nucleotides. Co-transfection of NC or miR-4516 mimics with mutated forms of the 3’UTR (all three binding sites) significantly restored the luciferase activity (Figure 3B). Next, we examined the effects of miR-4516 on both mRNA and protein levels of PTPN14. Overexpression of miR-4516 markedly reduced the expression of PTPN14 in LN229, LN18, U87MG, and GBM30 cells at both mRNA and protein levels (Figures 3C–D). Conversely, the expression of PTPN14 at both mRNA and protein levels was increased after inhibition of miR-4516 in U87MG and LN18 cells (Figures S3B–D). To confirm that PTPN14 is a putative target of miR-4516 in GBM tissues, the protein levels of PTPN14 were examined using IHC with a tissue microarray containing 56 GBM tissues. Overall, the majority of the GBM tissues (82.1% (46/56)) showed low cytoplasmic expression of PTPN14 (Figure 3E). Most importantly, we observed a highly significant negative correlation between the expression of miR-4516 and PTPN14 (by Nanostring, p=0.012, Figure 3F and by qPCR, p=0.0007, Figure S4, respectively). Simultaneously, we validated the miR-4516 expression levels obtained from Nanostring using qPCR in these GBM tissues and as expected, there is a strong positive expression correlation between Nanostring and qPCR (r=0.602, p=2.315e-06, Figure S5). Collectively, these data suggest that miR-4516 suppresses the expression of PTPN14 by directly targeting its 3’-UTR in GBM cells both in vitro and in vivo.
Figure 3. PTPN14 is the putative direct target of miR-4516.
(A) Luciferase reporter containing 3 putative miR-4516 targeting sequences in the 3’UTR of PTPN14 (PTPN14 3’UTR wild-type) and mutated versions, containing 3 altered nucleotides (red). (B) LN229, U87MG, LN18, and GBM30 cells were co-transfected with the luciferase reporter constructs containing wild type (wt) or mutated PTPN14 3’UTR (mut) and NC or miR4516 mimic into. n=3, ***P<0.001. 72 hours post-transfection luciferase activity was measured and normalized. (C-D) qPCR and Western blotting to measure PTPN14 mRNA and protein levels in LN229, U87MG, LN18, and GBM30 cells transfected with NC or miR-4516 mimic. 72 hours post-transfection, cells were harvested for RNA and protein extractions. n=3, **P<0.01, ***P<0.001. (E) Immunohistochemical staining of PTPN14 on TMA showed the differential expression of PTPN14 in GBM tissues with low or high miR-4516 expression. Magnification: ×100 and ×200. (F) An inverse relationship between miR-4516 expression level and the PTPN14 protein level in 56 GBM tissues. P=0.012. Score 0/1 was considered as low expression of PTPN14, whereas score 2/3 together as high expression of PTPN14.
PTPN14 plays an essential role in the miR-4516-induced phenotype of GBM cells
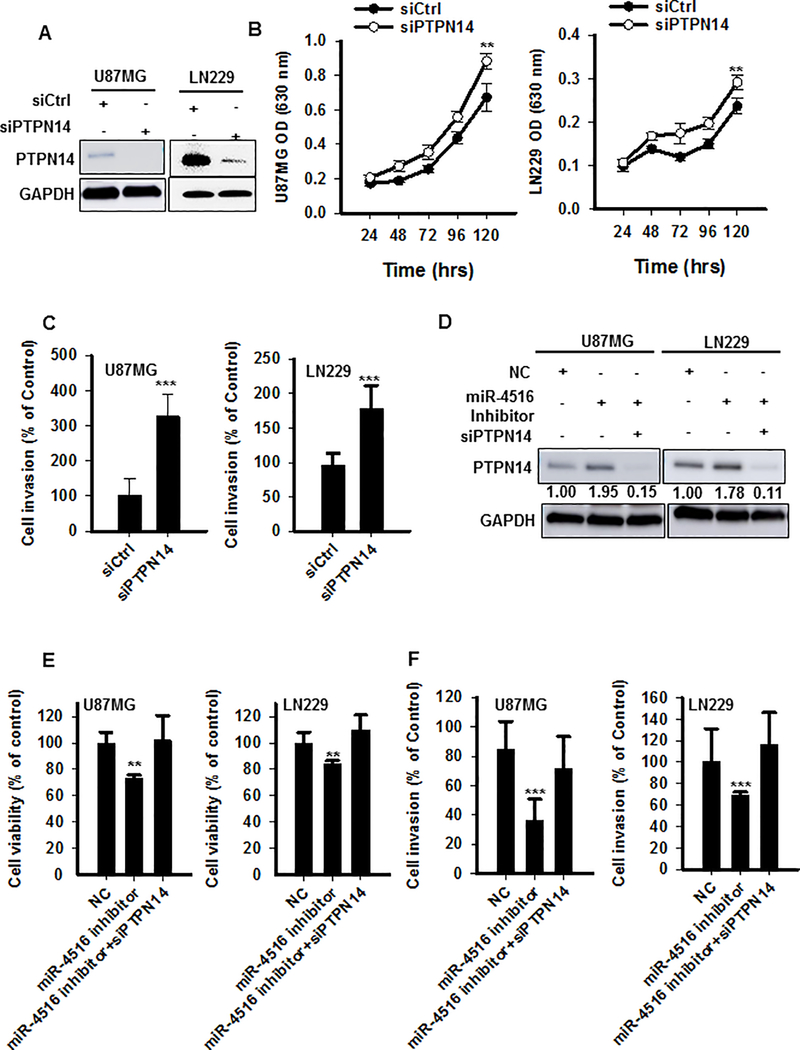
Downregulation of PTPN14 is regarded as a trigger of cancer metastasis (14). However, the role of PTPN14 in GBM is not fully understood. In this study, we hypothesized that the oncogenic function of miR-4516 is mediated via direct targeting of the tumor suppressor PTPN14. To explore whether PTPN14 has tumor suppressive function in GBM cells, we first overexpressed PTPN14 in three different GBM cell lines (LN18, U87MG, and LN229), and confirmed that overexpression of PTPN14 inhibited GBM cell proliferation (Figures S6A–D). Then, we examined whether downregulation of PTPN14 would mimic the effect of overexpression of miR-4516; PTPN14 was knocked down in both U87MG and LN229 cells by using RNAi (siRNA) (Figure 4A). As expected, blocking PTPN14 expression significantly increased proliferative and invasive ability of both U87MG and LN229 cells (Figures 4B–C). Additionally, U87MG and LN229 cells were transfected with NC or miR-4516-inhibitor alone, or co-transfected with miR-4516-inhibitor and PTPN14 siRNA. Western blot analyses showed that miR-4516 inhibition enhanced expression of PTPN14 at protein level (Figure 4D). Further, functional assays demonstrated that miR-4516 inhibition significantly suppressed cell proliferation and invasion which were significantly reversed by knockdown of PTPN14 expression (Figures 4E–F). Collectively, these data confirmed that the oncogenic potential of miR-4516 is largely mediated through the suppression of PTPN14 expression in GBM cells.
Figure 4. PTPN14 plays a crucial role in miR-4516-induced cell growth and invasion.
(A) Western blot analysis for PTPN14 in U87MG/LN229 cells transfected with siRNA control or PTPN14 siRNA for 48hrs. (B) Cell proliferation assay for U87MG/LN229 cells transiently transfected with PTPN14 siRNA. The cell proliferation rates were measured by methylene blue. n=5, **P<0.01. (C) Cell invasion assay for U87MG/LN229 cells transiently transfected with PTPN14 RNAi using matrigel transwell membranes. n=3, ***P<0.001. (D-F) U87MG/LN229 cells were transiently transfected with NC or miR-4516 inhibitor alone, or simultaneously with PTPN14 RNAi. The protein expression of PTPN14 was determined using westen blotting (D). Cell proliferation at 72 hours was determined using methylene blue (E). n=5, **, P < 0.01. The matrigel transwell invasion assay was carried out to quantify the invaded cells (F). n=3, ***, P < 0.001.
miR-4516 regulates the Hippo pathway through targeting of PTPN14
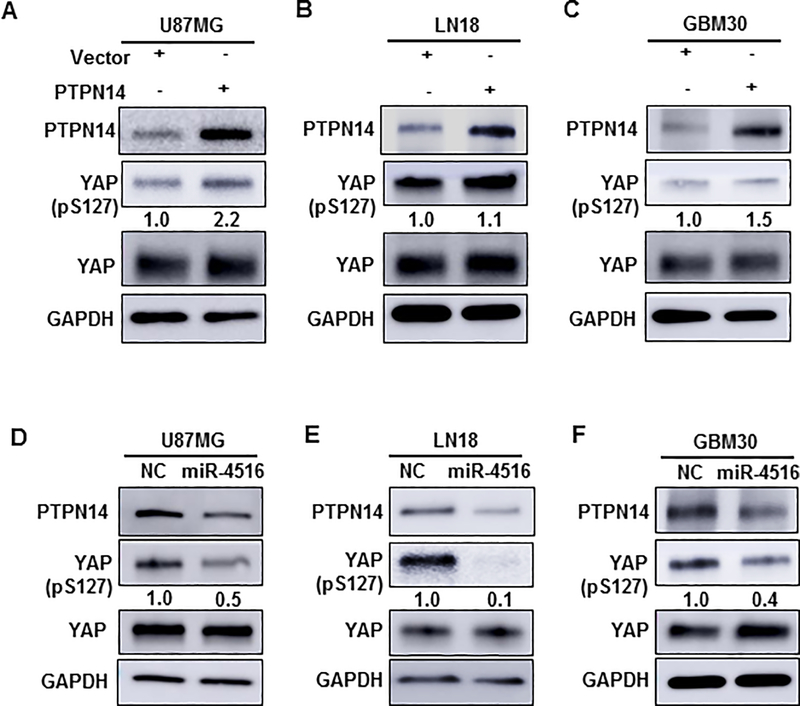
PTPN14 was found to be involved in the Hippo signaling pathway through regulation of the Hippo effector YAP in 2012 (19, 20). Recently, studies have identified the Hippo/YAP signaling pathway as a key mechanism that controls cell growth and proliferation (21, 22). YAP, a pivotal effector of the Hippo pathway, is a transcriptional co-activator that is amplified in human cancers, and promotes epithelial-to-mesenchymal transition (EMT) and malignant transformation (23). Therefore, we sought to determine whether miR-4516 contributes to cell proliferation and invasion by regulating the Hippo pathway in GBM. It is known that Ser127-phosphorylation in YAP promotes its binding to 14–3-3 proteins, which causes cytoplasmic retention YAP, and upon de-phosphorylation, YAP moves to the nucleus and activates genes that promote cell proliferation and inhibits apoptosis (24). Accordingly, to investigate whether the Hippo pathway is affected by PTPN14 in GBM, we determined the phosphorylation of YAP in cytoplasm of U87MG, LN18, and GBM30 cell lines transiently overexpressing PTPN14. As expected, overexpression of PTPN14 resulted in an increase in cytoplasmic YAP levels of LN229, U87MG, LN18, and GBM30 cells (Figures 5A–C). Furthermore, we confirmed the translocation of phospho-YAP (Ser127) into the cytoplasm in PTPN14-overexpressing GBM30 cells (Figures S7A–B) as previously reported (16). To further determine whether miR-4516 regulates the Hippo pathway by affecting the expression of PTPN14, we overexpressed miR-4516 in U87MG, LN18, and GBM30 cell lines and determined the change of phosphorylation of YAP. As shown in Figures 5D–F, overexpression of miR-4516 considerably decreased S127-phospho-YAP in the cytoplasm, concomitant with downregulation of PTPN14. Taken together, these data indicate that miR-4516 regulates the Hippo pathway by targeting the expression of PTPN14.
Figure 5. miR-4516 regulates the Hippo pathway by targeting PTPN14.
(A-C) U87MG, LN18, and GBM30 cells were transfected with either control (pcDNA3) or pcDNA3/PTPN14 plasmid. Forty-eight hours post-transfection, western blotting was performed to determine the protein expression of PTPN14, pYAP, YAP, and GAPDH using respective antibodies. The intensity of each band was quantified using ImageJ and normalized to GAPDH and then to pcDNA3-transfected cells. (D-F) U87MG, LN18, and GBM30 cells were transiently transfected with either miRNA negative control (NC) or miR-4516 mimic. After 48 hours, western blotting was performed to determine the protein level of PTPN14, pYAP, YAP, and GAPDH using respective antibodies. The intensity of each band was quantified using ImageJ and normalized to GAPDH and then to NC-transfected cells.
Effect of miR-4516 on in vivo tumorigenicity
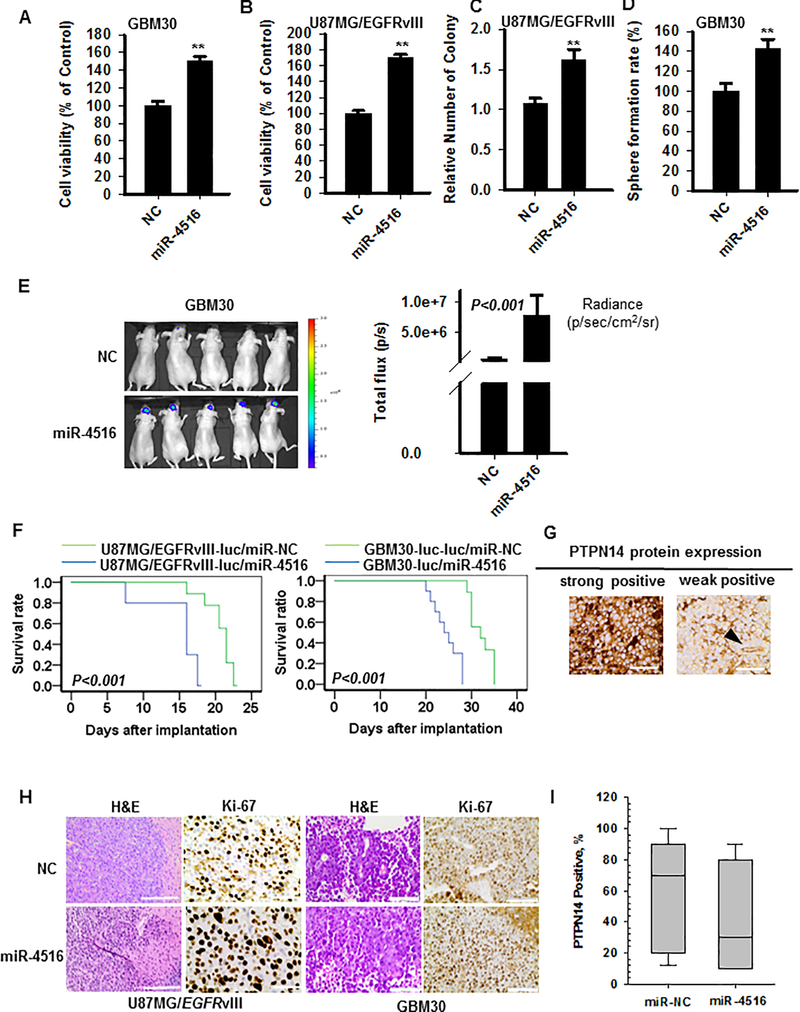
To determine the role of miR-4516 on tumor growth in mouse model, we generated stable U87MG/EGFRvIII-luc/miR-4516 and GBM30-luc/miR-4516 cell lines by lentiviral transduction. In response to overexpression of miR-4516, the ability of proliferation was increased in both U87MG/EGFRvIII-luc/miR-4516 and GBM30-luc/miR-4516 cells (Figures 6A–B). In addition, the ability of colony formation in U87MG/EGFRvIII-luc/miR-4516 and sphere formation rate in GBM30-luc/miR-4516 cells were increased (Figures 6C–D and Figures S8A–B). Then, an athymic nude xenograft mouse model was established, in which stable U87MG/EGFRvIII-luc/miR-4516 or GBM30-luc/miR-4516 cells were stereotactically implanted into the brain. Mice were sacrificed when they became moribund. As shown in Figure 6E, increased brain tumor formation and growth were observed with GBM cells after overexpression of miR-4516 as assessed by luminescent imaging, as well as reduced overall survival of mice (Figure 6F). Representative H&E staining is shown in Figure 6H. Compared with NC tumors, more aggressive tumors with infiltrative borders and higher mitotic index were found in tumors derived from miR-4516-overexpressing cells. Immunohistochemical staining for Ki-67 and PTPN14 showed increased expression of Ki-67 and decreased expression of PTPN14 in miR-4516 overexpressing tumors compared to NC tumors (Figures 6G–I). Taken together, these data clearly demonstrated that miR-4516 promotes GBM tumor growth and negatively correlated with expression of PTPN14 in mouse xenograft models of human GBM patient-derived primary and established cell lines
Figure 6. miR-4516 promotes tumorigenicity in vivo.
(A-B) Cell proliferation assay for U87MGvIII-luc/GBM30-luc cells stably transfected with lentivirus control or lentivirus miR-4516. The cell proliferation rates at 72 hours were measured by methylene blue. n=5, **P<0.01 (C) U87MG/EGFRvIII-luc/NC cells and U87MG/EGFRvIII-luc/miR-4516 cells (100 cells/well) were seeded into 6-well plates for colony formation assay and cultured for ~2 weeks to allow colony formation. The number of detectable colonies was counted. n=3, **P<0.0. (D) GBM30-luc/NC cells and GBM30-luc/miR-4516 cells (1000 cells/well) were seeded into 6-well plates for sphere formation assay and cultured for ~2 weeks. The number of detectable colonies was counted. n=3, **P<0.01. (E) Luminescent imaging for mice with intracranial tumor formed from GBM30-luc/NC and GBM30-luc/miR-4516 cells on day 10 after implanting (left). Quantification of luminescence signal intensity (right). Student t test was carried out for statistical analysis (mean ±SEM, n=9). (F) Kaplan-Meier survival analysis shows that overall survival of U87MGvIII-luc or GBM30-luc cells with stable overexpression of miR-4516 in comparison with miRNA-NC (n=9) (G) Representative images of strongly positive or weakly positive PTPN14 in GBM30-luc/NC and GBM30-luc/miR-4516 tissue are presented. Arrow head identifies a blood vessel with positive PTPN14 expression in endothelial cells as opposed to lack of expression on neoplastic cells. Magnification: ×400. (H) Tumors were formalin-fixed, paraffin-embedded, sliced and stained with H&E. Representative images of each group are presented. Magnification: left: ×200, right: ×400. Cell proliferation was determined by Ki-67 using immunohistochemistry. Magnification: ×400. (I) The PTPN14 protein in GBM30-luc/NC and GBM30-luc/miR-4516 tissues displayed an inverse correlation with the expression of miR-4516.
DISCUSSION
Numerous therapeutic challenges are contributing to poor survival times in GBM patients, including aggressive tumor growth rates, heterogeneity, and therapeutic resistance (25). In order to find novel approaches for GBM treatment, it is imperative that current studies identify new biomarkers and therapeutic strategies (26). In this article, we report, for the first time to our knowledge, that 1) miR-4516 is associated with worse OS independent of MGMT methylation status in GBM patients; 2) miR-4516 promotes proliferation and invasion via targeting PTPN14-mediated regulation of the Hippo pathway in GBM.
Accumulated evidence indicates that miRNA abnormalities are involved in cancer development by negative regulation of tumor suppressor genes (27). Interestingly, our previous study, for the first time, identified a predictive model for the recurrence after salvage RT in prostate cancer and demonstrated that miR-4516 was overexpressed in patients that experienced treatment failure after salvage RT (28). Moreover, another study shows that high level of miR-4516 is associated with infiltrative growth of follicular variant of papillary thyroid carcinomas (29). However, little is known about the function of miR-4516 in GBM.
In this study, we are the first to investigate the association of miR-4516 expression and clinical outcome in GBM patients. In a comprehensive approach, miR-4516 was identified as one of the top miRNAs that associated with GBM patient OS among several miRNA candidates as assessed by both univariable and multivariable analyses. To our knowledge, this is the first study that indicates overexpression of miR-4516 is related with poor prognosis in GBM patients. Importantly, miR-4516 holds an independent prognostic value even in the context of MGMT methylation status in our cohort of patients and warrants further validation to assess its clinical utility as a prognostic biomarker.
To further substantiate the clinical significance of miR-4516 and investigate its biological mechanism for this observation, we performed in vitro and in vivo studies after overexpression and inhibition of miR-4516 in GBM cells. Our study characterized a possible biological role of miR-4516 in GBM, in which our results suggest that miR-4516 promotes GBM cell proliferation and invasion. Overexpressing miR-4516 in GBM cells induced tumor growth and invasion both in vitro and in vivo. As we know, the infiltrative nature of GBM is an important factor that limits treatment efficacy. Our study might open a new therapeutic strategy; however, the value of miR-4516 inhibitors as potential agents to inhibit tumor growth and invasion should be further tested in preclinical models.
To elucidate possible molecular mechanisms regulated by miR-4516, we performed a comprehensive in silico analysis to predict its putative targets. One of them, PTPN14 (also called Pez, PTP36, PTPD2), a non-receptor tyrosine phosphatase, which is frequently mutated in a variety of human cancers, has been previously reported to be involved in inhibiting metastasis through reducing intracellular protein trafficking (14) It is reported to be a tumor suppressor in colorectal cancer (15), and acts as a regulator of oncogene YAP, which is a critical component of the mammalian Hippo pathway (30). The Hippo pathway controls cell number by inhibiting cellular proliferation and promoting apoptosis through phosphorylation of the transcriptional co-activator YAP (23). However, the cell signaling pathways regulated by PTPN14 in GBM largely remain to be elucidated. The lack of information prompted us to focus on PTPN14. Importantly, for the first time, we found that miR-4516 exerts its oncogenic function by targeting PTPN14-mediated regulation of Hippo pathway in GBM. Additionally, we provided evidence from the luciferase activity assay that miR-4516 regulates PTPN14 through directly binding to the predicted miRNA-mRNA binding site in the 3’UTR region of the PTPN14 mRNA. These data confirmed the tumor suppressive role of PTPN14 in GBM cells and provide evidence that PTPN14 is an important mediator of miR-4516 induced regulation of the Hippo pathway. Importantly, we also confirmed that miR-4516 is negatively correlated with PTPN14 protein expression in our GBM patient samples. Furthermore, Chowdhari et al. have suggested that miR-4516 is important in PUVA-mediated apoptosis (18). However, in GBM cells, the underlying mechanisms through which miR-4516 functions as an onco-miRNA need to be further investigated. Notably, this is the first study indicating that miR-4516 can promote proliferation and invasion of GBM cells in part via directly targeting of PTPN14. In addition, a better understanding of the role of PTPN14 in GBM, which has been proposed for the first time in this study, might also help to develop new therapeutic strategies for GBM patients. However, to restore tumor suppressive function of protein-tyrosine phosphatase function is a challenging task in translational research. It is important to highlight the limitations of the study. First, the prognostic value of miR-4516 should be further tested in additional cohorts as well as prospective biomarker studies; however, there are no data available from the TCGA database for miR-4516. Second, Chowdhari et al. (18) have shown that miR-4516 directly targets STAT3 and TP53 by binding to their cognate 3’UTR in HaCaT cells, and downregulates STAT3, CDK6, and UBE2N proteins. However, we did not see the effect of miR-4516 on STAT3 expression (Figures S9A–B). Third, in this study, only one of the direct targets of miR-4516 was thoroughly investigated; therefore, more putative targets, such as PIM2, MECP2, COL5A1, and CDKN1A (Figures S9C–F) and their downstream signaling pathways need to be investigated in future studies. In conclusion, this study indicates that miR-4516 represents an independent negative prognostic factor in GBM patients and plays a novel oncogenic role in GBM; moreover, miR-4516 promotes proliferation and invasion via targeting PTPN14-mediated regulation of the Hippo pathway in glioblastoma.
MATERIALS AND METHODS
Patient Cohort, Ethics Statement, and RNA Isolation
FFPE tumor specimens from 268 newly diagnosed GBM patients who do not have IDH1R132H mutation were used in the study. These patients underwent tumor biopsy or resection at the University of Utrecht (The Netherlands) during 2005 to 2014. The study was approved by the institutional review boards of The Ohio State University and Utrecht University Medical Center. Total RNA was isolated from the tumor specimens as described previously (28).
miRNA Expression Analysis
miRNA data were generated from 268 tumors as previously described (28) and R statistical computing software was used for data analyses (31). Raw NanoString data were log2 transformed for the filtering step. Per sample thresholds for missing values were calculated as 1.5 standard deviations above the mean of the negative controls. miRNAs were removed if > 90% of the values were below the threshold, and samples were removed if, after miR filtering, samples had > 60% missing values. The miRNA data were normalized by the geometric mean. This was accomplished using the filtered raw counts data set, by multiplying the expression of each miRNAs for each sample by the ratio of the mean of the geometric means across samples to the geometric mean of the sample. The filtered geometric mean normalized data were then log2 transformed for further analysis.
MGMT Promoter Methylation Analyses
DNA (250 ng) input was used per sample and the MGMT-STP27 model was used to calculate MGMT promoter methylation status from Illumina EPIC data (32). EPIC data were SWAN (33) normalized and M-value transformed using the minfi (34) package in R.
Cell Lines and Cell Culture
Normal human Astrocytes (NHAs) were purchased from Lonza (Walkersville, MD). Human GBM cell lines U87MG, LN18, LN229, U251, and T98G were from ATCC. U87MG/EGFRvIII cells were provided by Dr. Deliang Guo (The Ohio State University). U87MG/EGFRvIII-luc and primary GBM patient-derived GBM30-luc cells were provided by Dr. Balveen Kaur (The Ohio State University). U87MG/EGFRvIII-luc cells were grown in DMEM (Gibco) with 10% (v/v) FBS (Invitrogen), 100U/ml penicillin/streptomycin (Sigma), in the presence of 5% CO2 at 37°C. GBM30-luc cells were cultured in neurobasal medium with EGF (20ng/ml), FGF (20ng/ml), B27 (1×), and Heparin (2μg/ml).
qRT-PCR Analysis of mRNA Expression and miRNA expression
Total RNA was isolated from GBM cells with TRIzol reagent as previously described (35). RNA (1000 ng) was subjected to reverse transcription to cDNA using high capacity RNA to cDNA kit (Applied Biosytems) and amplified by qPCR using SYBR Green (Qiagen). Resultant values were normalized against the internal control gene GAPDH for each replicate. The primers used for qPCR were listed in Supplementary Table 1. For miRNA expression analysis, RNA (100 ng) was reverse transcribed using the TaqMan Advanced MicroRNA Assay kit (Applied Biosystems) with miRNA-specific primers. Primers of Taqman MicroRNA Assays for hsa-miR-4516 (Cat. #A25576, 478303_mir) and hsa-miR-361–5p (Cat. #A25576, 478056_mir) were purchased from Applied Biosystems. Relative expression levels of miR-4516 were calculated by normalization with miR-361–5p expression in GBM cells, using 24 RNAs isolated from glioma tissues (Utrecht patient cohort, WHO grades II-IV). All the experiments were performed in triplicate.
Western Blot Analysis
Protein extraction and Western blot analysis were performed as previously described (35). The antibodies: PTPN14 (1:1000 dilution), pYAP (s127, 1:1000 dilution), total YAP (1:1000 dilution), c-parp/parp (1:1000) and GAPDH (1:1000 dilution) were purchased from Cell Signaling Technology (Denvers, MA). Polyclonal goat anti-rabbit antibody (Cell Signaling Technology) and Western Blotting Detection System (Millipore) were used for exposure.
Immunohistochemistry (IHC)
A human GBM tissue array containing 56 cases was constructed at the University of Utrecht. IHC was carried out as previously described (36). Primary mouse antibody against PTPN14 (1:500, R&D Systems) was used. The percentage of positive cytoplasm-stained cells was scored as score 0 (0%), score 1 (1–10%), score 2 (11–50%), or score 3 (>50%). Score 0 and 1 was considered as low expression of PTPN14, whereas score 2 and 3 together as high expression of PTPN14 for statistical evalution.
Immunofluorescence
Twenty-four hours post-transfection, cells were re-seeded onto 4-well cell culture slides, and then fixed and washed with PBS as previously described (35, 37). Primary p-YAP (s127, 1:100) antibody and fluorescence-labeled secondary antibody goat anti-rabbit Alexa Fluor 594 (1:1000, Invitrogen) were used. Samples were analyzed under a confocal microscopy (Carl Zeiss MicroImaging).
Transfection of miR-4516 Mimics and Inhibitors, siRNA Treatment, Plasmids, and Lentiviral Transduction
For functional studies, a specific miR-4516 mimic/inhibitor (Ambion) and a respective negative control (Ambion) were transfected into the GBM cells. For siRNA transfection, a specific PTPN14 siRNA (50 nmol/L) (OriGene) and a negative control were used. pcDNA3/PTPN14 plasmid containing PTPN14 open reading frame (plasmid #61003) was purchased from Addgene (Cambridge, MA). For studying in vivo effects, stable miR-4516-overexpressing U87MG/EGFRvIII-luc and GBM30-luc cells were generated by lentiviral transduction (Sigma). Cells were selected in the presence of 2μg/ml Puromycin (Sigma) for 7 days.
Luciferase Reporter Assay
The 3’UTR of PTPN14 was synthesized, annealed, and then inserted into the Xho l and Not I sites of the pCheck2-reporter luciferase vector downstream of the luciferase gene. To introduce mutations, the sequences complementary to the binding sites of miR-4516 in the 3’UTR (site 1: 5’-UGUUUCUAGCUUCUCCC-3’, site 2: 5’-UUCCCCAACCUUCUCCU-3’, site 3: 5’-UUCAUUAGCCUUCUCCU-3’) were replaced by site 1: 5’- UGUUUCUAGUUACACCC-3’, site 2: 5’- UGUUUCUAGUUACACCC-3’, site 3: 5’-UGUUUCUAGUUACACCC-3’. The constructs were confirmed by nucleotide sequencing. Cells were co-transfected with the wild-type or mutated constructs, pCheck2 plasmid, and equal amount of negative control or miR-4516 mimic. Luciferase assay was performed using Dual Luciferase Reporter Assay System (Promega).
Cell Proliferation and Invasion Assays
Twenty-four hours post-transfection, cells (1000 cells/well) were seeded in 96-well plates, washed, fixed, and stained after indicated time points as previously described (37). For GBM30 cells, the MTS assay (Promega) was used to measure cell proliferation. Cell invasion assays were performed as previously described (35). All the experiments were performed in triplicate.
Colony Formation Assay
GBM cells (100 cells/well) were used for colony formation assay as described previously (37). Briefly, 100 cells were plated onto 6-well tissue culture plates. Visible colonies were fixed and stained with 0.1% crystal violet in 20% methanol after 10–14 days. All the experiments were performed in triplicate.
Sphere formation assay
A total of 1,000 cells were incubated in neurobasal medium with B27 (1×), Heparin (2μg/ml), EGF (20ng/ml), and FGF (20ng/ml) in a humidified atmosphere with 5% CO2 at 37°C. The number of spheres was counted two weeks after cell seeding. All the experiments were performed in triplicate.
Apoptosis assay
Apoptosis was detected using a FITC Apoptosis Detection Kit (Invitrogen). Cells were processed using flow cytometry according to the manufacturer’s protocol. All the experiments were performed in triplicate.
Intracranial Mouse Model, Luminescence Imaging, and Mouse Survival Analysis
Six to eight week old athymic nude mice, obtained from the Target Validation Shared Resource at The Ohio State University, were used for the intracranial xenograft models. GBM cells (1 × 105 U87MG/EGFRvIII-luc and GBM30-luc in 2 μl PBS), transduced with miR-4516 or control were implanted into the mouse brain. For live tumor imaging, luciferin (Perkin Elmer) solution (100mg/kg) was injected and after 10 min, a group of 5 mice were placed into a clear Plexiglas anesthesia box (2.5% isoflurane). The IVIS Lumina II imaging platform was used. The experiments were performed at the Ohio State University (OSU) Small Animal Imaging Core. Mouse survival time and tumor size were recorded and analyzed. All procedures were approved by the Subcommittee on Research Animal Care at The Ohio State University.
Target Analysis
Bioinformatic analysis was performed by using these specific programs: miRDB (38), Targetscan (39), miRTarBase (40).
Statistical Analysis
Analysis of Nanostring data was carried out in R. Cox regression was used to identify the association between the NanoString miRs (continuous) and overall survival with age as a covariable. P-values were adjusted by the false discovery rate method (41). miR-4516 was then median dichotomized and the log rank test was employed to visualize the association between the expression and overall survival. Cox regression was used for a comprehensive multivariable analysis of the continuous expression. Covariables included in the multivariable model were gender (categorical), age (continuous), KPS (categorical; < 70 vs 70 and above), treatment (categorical; “none”, “monotherapy TMZ or radiation only”, or “TMZ + radiation (Stupp protocol)”), and MGMT methylation status (methylated vs unmethylated). The SPSS 19.0 (SPSS, Chicago, USA) was used for other statistical analyses. Spearman’s rank correlation was performed to detect the correlation between qPCR and Nanostring data. The Wilcoxon rank sums test was employed to test the association between Nanostring/qPCR and PTPN14 protein expression level in tissues. Student’s t-test was carried out to detect the different miRNA and protein expression in vitro. P values were calculated two-sided and less than 0.05 were defined as statistically significant.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the T&P Bohnenn Fund for Neuro-Oncology Research (grant to P.A.R.), the Ohio State University (OSU) Comprehensive Cancer Center Small Animal Imaging Core, and the Ohio State University (OSU) Comprehensive Cancer Center Pathology Core Facility supported in part by grant P30 CA016058, National Cancer Institute, Bethesda, MD.
GRANT SUPPORT
This work was supported by National Cancer Institute [R01CA169368 (to A.C), R01CA11522358 (to A.C), R01CA1145128 (to A.C), R01CA108633 (to A.C), R01CA188228 (to A.C., R.B., K.L., and J.B.), 1RC2CA148190 (to A.C), and U10CA180850-01 (to A.C.)]; A Brain Tumor Funders Collaborative Grant (to A.C.); Ohio State University Comprehensive Cancer Center Award (to A.C.).
Footnotes
CONFLICT OF INTERST
The authors declare no conflict of interest.
Supplementary information is available at Oncogene’s website.
REFERENCES
- 1.Ostrom QT, Gittleman H, Fulop J, Liu M, Blanda R, Kromer C, et al. CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008–2012. Neuro-oncology. 2015;17 Suppl 4:iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Losman JA, Kaelin WG Jr. What a difference a hydroxyl makes: mutant IDH, (R)-2-hydroxyglutarate, and cancer. Genes & development. 2013;27(8):836–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lenos K, Grawenda AM, Lodder K, Kuijjer ML, Teunisse AF, Repapi E, et al. Alternate splicing of the p53 inhibitor HDMX offers a superior prognostic biomarker than p53 mutation in human cancer. Cancer research. 2012;72(16):4074–84. [DOI] [PubMed] [Google Scholar]
- 4.Zougman A, Hutchins GG, Cairns DA, Verghese E, Perry SL, Jayne DG, et al. Retinoic acid-induced protein 3: identification and characterisation of a novel prognostic colon cancer biomarker. European journal of cancer. 2013;49(2):531–9. [DOI] [PubMed] [Google Scholar]
- 5.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nature reviews Genetics. 2004;5(7):522–31. [DOI] [PubMed] [Google Scholar]
- 6.Garzon R, Fabbri M, Cimmino A, Calin GA, Croce CM. MicroRNA expression and function in cancer. Trends in molecular medicine. 2006;12(12):580–7. [DOI] [PubMed] [Google Scholar]
- 7.Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–8. [DOI] [PubMed] [Google Scholar]
- 8.Shenouda SK, Alahari SK. MicroRNA function in cancer: oncogene or a tumor suppressor? Cancer metastasis reviews. 2009;28(3–4):369–78. [DOI] [PubMed] [Google Scholar]
- 9.Zhang B, Pan X, Cobb GP, Anderson TA. microRNAs as oncogenes and tumor suppressors. Developmental biology. 2007;302(1):1–12. [DOI] [PubMed] [Google Scholar]
- 10.Xia H, Yan Y, Hu M, Wang Y, Wang Y, Dai Y, et al. MiR-218 sensitizes glioma cells to apoptosis and inhibits tumorigenicity by regulating ECOP-mediated suppression of NF-kappaB activity. Neuro-oncology. 2013;15(4):413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li S, Zeng A, Hu Q, Yan W, Liu Y, You Y. miR-423–5p contributes to a malignant phenotype and temozolomide chemoresistance in glioblastomas. Neuro-oncology. 2017;19(1):55–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang W, Liu M, Xu J, Fu H, Zhou B, Yuan T, et al. MiR-377 inhibits the proliferation of pancreatic cancer by targeting Pim-3. Tumour biology : the journal of the International Society for Oncodevelopmental Biology and Medicine. 2016;37(11):14813–24. [DOI] [PubMed] [Google Scholar]
- 13.Du W, Liu X, Chen L, Dou Z, Lei X, Chang L, et al. Targeting the SMO oncogene by miR-326 inhibits glioma biological behaviors and stemness. Neuro-oncology. 2015;17(2):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Belle L, Ali N, Lonic A, Li X, Paltridge JL, Roslan S, et al. The tyrosine phosphatase PTPN14 (Pez) inhibits metastasis by altering protein trafficking. Science signaling. 2015;8(364):ra18. [DOI] [PubMed] [Google Scholar]
- 15.Laczmanska I, Sasiadek MM. Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer. Acta biochimica Polonica. 2011;58(4):467–70. [PubMed] [Google Scholar]
- 16.Liu X, Yang N, Figel SA, Wilson KE, Morrison CD, Gelman IH, et al. PTPN14 interacts with and negatively regulates the oncogenic function of YAP. Oncogene. 2013;32(10):1266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang LJ, He CC, Sui X, Cai MJ, Zhou CY, Ma JL, et al. MiR-21 promotes intrahepatic cholangiocarcinoma proliferation and growth in vitro and in vivo by targeting PTPN14 and PTEN. Oncotarget. 2015;6(8):5932–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chowdhari S, Saini N. hsa-miR-4516 mediated downregulation of STAT3/CDK6/UBE2N plays a role in PUVA induced apoptosis in keratinocytes. Journal of cellular physiology. 2014;229(11):1630–8. [DOI] [PubMed] [Google Scholar]
- 19.Michaloglou C, Lehmann W, Martin T, Delaunay C, Hueber A, Barys L, et al. The tyrosine phosphatase PTPN14 is a negative regulator of YAP activity. PloS one. 2013;8(4):e61916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang W, Huang J, Wang X, Yuan J, Li X, Feng L, et al. PTPN14 is required for the density-dependent control of YAP1. Genes & development. 2012;26(17):1959–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan D The hippo signaling pathway in development and cancer. Developmental cell. 2010;19(4):491–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu FX, Zhao B, Guan KL. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell. 2015;163(4):811–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao B, Li L, Lei Q, Guan KL. The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes & development. 2010;24(9):862–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Varelas X The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141(8):1614–26. [DOI] [PubMed] [Google Scholar]
- 25.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. The Lancet Oncology. 2009;10(5):459–66. [DOI] [PubMed] [Google Scholar]
- 26.McNamara MG, Sahebjam S, Mason WP. Emerging biomarkers in glioblastoma. Cancers. 2013;5(3):1103–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 28.Bell EH, Kirste S, Fleming JL, Stegmaier P, Drendel V, Mo X, et al. A novel miRNA-based predictive model for biochemical failure following post-prostatectomy salvage radiation therapy. PloS one. 2015;10(3):e0118745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Borrelli N, Denaro M, Ugolini C, Poma AM, Miccoli M, Vitti P, et al. miRNA expression profiling of ‘noninvasive follicular thyroid neoplasms with papillary-like nuclear features’ compared with adenomas and infiltrative follicular variants of papillary thyroid carcinomas. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc 2017;30(1):39–51. [DOI] [PubMed] [Google Scholar]
- 30.Wilson KE, Li YW, Yang N, Shen H, Orillion AR, Zhang J. PTPN14 forms a complex with Kibra and LATS1 proteins and negatively regulates the YAP oncogenic function. The Journal of biological chemistry. 2014;289(34):23693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Team RC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. [Google Scholar]
- 32.Bady P, Sciuscio D, Diserens AC, Bloch J, van den Bent MJ, Marosi C, et al. MGMT methylation analysis of glioblastoma on the Infinium methylation BeadChip identifies two distinct CpG regions associated with gene silencing and outcome, yielding a prediction model for comparisons across datasets, tumor grades, and CIMP-status. Acta neuropathologica. 2012;124(4):547–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maksimovic J, Gordon L, Oshlack A. SWAN: Subset-quantile within array normalization for illumina infinium HumanMethylation450 BeadChips. Genome biology. 2012;13(6):R44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fortin JP, Triche TJ Jr., Hansen KD. Preprocessing, normalization and integration of the Illumina HumanMethylationEPIC array with minfi. Bioinformatics. 2017;33(4):558–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cui T, Chen Y, Yang L, Knosel T, Huber O, Pacyna-Gengelbach M, et al. The p53 target gene desmocollin 3 acts as a novel tumor suppressor through inhibiting EGFR/ERK pathway in human lung cancer. Carcinogenesis. 2012;33(12):2326–33. [DOI] [PubMed] [Google Scholar]
- 36.Han C, Zhao R, Liu X, Srivastava A, Gong L, Mao H, et al. DDB2 suppresses tumorigenicity by limiting the cancer stem cell population in ovarian cancer. Molecular cancer research : MCR. 2014;12(5):784–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui T, Srivastava AK, Han C, Yang L, Zhao R, Zou N, et al. XPC inhibits NSCLC cell proliferation and migration by enhancing E-Cadherin expression. Oncotarget. 2015;6(12):10060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic acids research. 2015;43(Database issue):D146–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agarwal V, Bell GW, Nam JW, Bartel DP. Predicting effective microRNA target sites in mammalian mRNAs. eLife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsu SD, Lin FM, Wu WY, Liang C, Huang WC, Chan WL, et al. miRTarBase: a database curates experimentally validated microRNA-target interactions. Nucleic acids research. 2011;39(Database issue):D163–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 1995;57(1):289–300. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.