Abstract
Objective To compare the efficacy of opt-in versus opt-out recruitment methods in pediatric weight management clinical trials. Methods Recruitment of preschoolers and school-age children across two obesity randomized controlled trials (RCTs) were compared using the same opt-in recruitment approach (parents contact researchers in response to mailings). Opt-in and opt-out strategies (parents send decline postcard in response to mailings if they do not want to participate) were then compared across two preschool obesity RCTs. Results Opt-in strategies yielded a significantly lower overall recruitment rate among preschoolers compared with school-age children. Among preschoolers, an opt-out strategy demonstrated a significantly higher overall recruitment rate compared with an opt-in strategy with the main advantage in the number of families initially contacted. Conclusions Opt-out recruitment strategies may be more effective in overcoming the barriers of recruitment in the preschool age-group because it does not rely on parent recognition of obesity.
Keywords: children, obesity, weight management.
Childhood obesity, defined as being at or above the 95th percentile for body mass index (BMI) for age and gender (Kuczmarski et al., 2000), was estimated at 8.4% of 2- to 5-year-old children in 2012, which equates to nearly 2 million preschoolers with obesity in the United States (Ogden, Carroll, Kit, & Flegal, 2014). While a slight decrease in obesity prevalence from 12.1% in 2010 (Ogden Carroll, Kit, & Flegal, 2012) to 8.4% in 2012 (Ogden et al., 2014) has been documented within the 2- to 5-year-old age-group, the prevalence of severe obesity (BMI ≥ 99th percentile) and obesity in children <2 years has continued to increase (Ogden et al., 2014).
Preschoolers with obesity experience negative medical and psychosocial risks including an increased risk of elevated blood pressure, asthma, sleep apnea, type 2 diabetes (Daniels, 2009; Gopinath et al., 2011) as well as poor health-related quality of life and low self-esteem (Kuhl, Rausch, Varni, & Stark, 2012). Obesity in the preschool years dramatically increases the risk of being overweight, obese, and even severely obese in later childhood and adulthood (Cunningham, Kramer, & Narayan 2014; Nader et al., 2006), making these children at greater risk for comorbid health conditions as adults. Improvement of weight status and associated health-related risk factors through obesity treatment has the potential to decrease poor health trajectories by reducing overweight and obesity in preschoolers. Further, the formation of healthy eating behaviors learned through obesity treatment in the preschool years may promote healthy eating behaviors across the lifespan (Birch & Fisher, 1998). Therefore, the American Heart Association (2015) cited early interventions focused on weight management, developing healthy lifestyle habits, and returning children to a normal weight growth trajectory as critical because these behaviors are hypothesized to be more susceptible to change during the preschool years.
Barriers to Recruitment of Preschoolers for Obesity Treatment
Despite the need for efficacious obesity treatment interventions in preschoolers, research with this age-group has been limited (Foster, Farragher, Parker, & Sosa, 2015). A systematic review of treatment interventions for early childhood obesity by Foster and colleagues (2015) identified only six studies, all of which were conducted in the past six years with only half of these studies demonstrating successful outcomes. Owing to the limited number of obesity treatment studies with preschoolers, there is a great opportunity for conducting research; however, unique challenges associated with recruiting children in this age-group have emerged as barriers to conducting weight management interventions with preschoolers.
Recruitment of preschoolers with overweight and obesity is particularly difficult owing to lack of recognition of overweight and/or obesity in the preschool years by both primary care physicians and parents (Dilley et al., 2007; Duncan, Hansen, Wang, Yan, & Zhang, 2015). In general, obesity is often inadequately assessed and diagnosed in the primary care setting, and this is particularly true among young children (Dilley et al., 2007). Pediatricians are more likely to diagnose overweight and obesity in children >5 years of age and in children with higher BMI percentiles (Dilley et al., 2007). Parents of preschoolers have also been found to incorrectly perceive child weight as healthy, for example, in one study with nearly 80% of parents misperceiving their preschoolers with obesity as being at a healthy weight (Duncan et al., 2015). Parent misperception of weight status appears to be more prevalent in preschoolers compared with older children with parents acknowledging obesity as problematic in later childhood and adulthood, but not during the preschool years (Eli, Howell, Fisher, & Nowicka, 2014). The combination of low identification of obesity in preschoolers at the primary care office and parent misperceptions about child weight represent unique challenges to recruitment with this age-group.
Recruitment Strategies
Opt-in Approaches
Recruitment methods commonly used in school-age obesity trials use an opt-in approach, which requires families to take action and contact the research team if interested in participating in a study (Raynor et al., 2009). For example, if a family receives a flyer in the mail with information about a study, opt-in methodology requires a family to contact the research team if interested in learning more about a study or participating (Juengst & Goldenberg, 2008). Opt-in recruitment can involve active methods (e.g., research team directly providing information to potential participants, such as a pediatrician sending a letter to a family describing the study) or passive methods (e.g., researcher making information available on a flyer or Web site; Raynor et al., 2009). The cornerstone of opt-in methodology is the requirement for participants to take action to contact the research team to learn more about a study. Active opt-in recruitment methods (e.g., a pediatrician directly giving a letter to a family) have been shown to yield better recruitment rates and be more cost-effective than passive strategies of flyers (Raynor et al., 2009). This contrast in recruitment success in active versus passive opt-in methods suggests that the more steps of action a potential participant must take to learn about and enroll in a study may be a barrier to recruitment.
Opt-out Approaches
In contrast, opt-out recruitment procedures are an alternative recruitment technique that decreases the action that potential participants need to take to learn about and join a research trial. Grounded in behavioral economics (BE) theory, an opt-out approach capitalizes on the “status quo” cognitive bias in human behavior such that individuals are more likely to not take an action and/or go with the default option that requires no action (Johnson & Goldstein, 2013). Opt-out approaches require potential participants to make contact with the research team only if they do not want to be provided with additional information about a study. For example, an opt-out approach would involve sending a letter to a family describing a study and asking them to send a “decline” postcard back if they do not want to be contacted by the research team about the study. Using this method, potential participants do not need to take any action to learn more about a study. In contrast to opt-in procedures, opt-out methods require action by the family to not have future contact with the research team (i.e., if the family does not perform any actions or behaviors, additional contact occurs; Juengst & Goldenberg, 2008). See Table I for examples of opt-in and opt-out methodology.
Table I.
Descriptions of Opt-in and Opt-out Recruitment Methodology
| Recruitment methodology | Description | Examples |
|---|---|---|
| Opt-in recruitment | Potential participants receive additional information about a study only if they take action to contact the research team or “opt-in” to being in contact with the research team. | Active: Pediatrician sends a letter to a family that describes a clinical trial. Contact information for the research team is included in the letter. Requires participants to contact research team if interested. Passive: Flyers about the clinical trial are posted in schools and community centers. Contact information for the research team is included. Requires participants to contact research team if interested. |
| Opt-out recruitment | Potential participants receive information about a study and if they do not take action to “opt-out” of being contacted, the research team reaches out to contact them. | Pediatrician sends a letter to a family that describes a clinical trial. Included with the letter is a self-addressed postcard that families are asked to send back to the pediatrician’s office if they are not interested in learning more information about the study. The letter states the research team will call the family if the postcard is not returned within a specified time frame. |
Opt-out recruitment has been used by our research team to successfully recruit several pediatric chronic illness populations into clinical trials including children with cystic fibrosis (CF; Stark et al., 2009), irritable bowel syndrome (IBD; Stark et al., 2005), and juvenile rheumatoid arthritis (JRA; Stark et al., 2006). Opt-out strategies have worked well because the population is typically well-defined through medical subspecialty clinics in academic medical centers or children’s hospitals. In designing a randomized controlled trial (RCT) to address obesity in preschool-age children (Stark et al., 2011), we incorporated this successful opt-out recruitment strategy by partnering with community pediatricians to identify and recruit children meeting criteria for obesity. We hypothesized that as children without chronic illness are routinely seen by pediatricians, partnerships with pediatricians could improve recruitment in pediatric weight management trials by allowing the use of similar opt-out strategies.
To date, studies directly comparing recruitment rates using opt-out and opt-in approaches within the medical setting have focused exclusively on adult populations. Within these studies, results have consistently shown that relative to opt-in approaches, opt-out approaches are associated with higher recruitment rates (Hunt, Shlomo, & Addington-Hall, 2013; Junghans, Feder, Hemingway, Timmis, & Jones, 2005; Nelson et al., 2002). For example, an RCT with adults in a medical setting showed that recruitment rates, defined by attendance at the first clinic session, were significantly higher when using an opt-out (50%) compared with an opt-in (38%) approach to recruiting participants (Junghans et al., 2005). Opt-out approaches have also yielded recruitment of patients that are significantly less healthy and more functionally impaired than those recruited with opt-in strategies (Junghans et al., 2005). The extra step involved in “opting in” creates a barrier to participation for certain subsets of patients and may lead to samples unrepresentative of the general population. This poses a significant threat to the generalizability of findings by limiting participants to those who actively seek out participation in studies to those who are healthier.
A review of the literature revealed that only two studies have explicitly used opt-out methods for recruitment of preschool participants into pediatric weight management trials (Stark et al., 2011; Taveras et al., 2011), though this may be an underestimate as many studies do not provide enough detail to determine what type of recruitment was used. No studies, however, have directly compared outcomes of opt-in recruitment approaches with opt-out recruitment in a pediatric population or specifically within preschool weight management interventions to determine whether opt-out methods may overcome the barriers associated with recruitment in this age-group.
Current Study
Given the success of the opt-out recruitment method with chronic illness populations, we were interested in understanding if an opt-out method would be successful in recruitment for preschool weight management interventions compared with more traditional opt-in recruitment methods used in weight management trials. Using secondary data analysis from a school-age and two preschool weight management trials, we sought to examine the following: (1) whether opt-in recruitment is particularly problematic for the preschool population by comparing the recruitment and retention rates of preschoolers and school-age children into two separate weight management clinical trials using the same opt-in strategy; (2) if opt-out strategies yield higher recruitment rates of preschoolers by comparing the recruitment and retention rates of two simultaneous preschool weight management clinical trials that used opt-in and opt-out approaches, respectively. Based on previous research showing that parents and physicians often do not recognize overweight/obesity in preschoolers (Duncan et al., 2015), we hypothesize that relative to school-age children, there will be a lower number of preschool-age children initially contacted (phone screened) and recruited (meeting intent-to-treat) into a weight management trial. Based on previous studies with adults, we also hypothesize that an opt-out recruitment strategy will yield a higher number of participants contacted (phone screened) and recruited (meeting intent-to-treat) into a weight trial compared with an opt-in approach for preschoolers.
Methods
Study Description
School-Age Opt-in Group
Data for the school-age opt-in group were collected as part of an institutional review board (IRB) approved behavioral family-based obesity intervention for school-age children aged 8–12 years who were overweight or obese (BMI ≥ 85th percentile) and living in rural communities. The Extension Family Lifestyle intervention project for Kids (E-FLIP for Kids) was a three-arm, randomized, controlled trial to test the effectiveness of a behavioral family-based intervention compared with a behavioral parent-only intervention and an education control condition in community settings. All assessment and treatment contacts were conducted at Cooperative Extension Services offices (i.e., a federally funded network which provides the infrastructure to support research, education, and programming in local communities around agriculture, health, and well-being, Janicke et al., 2011) across 10 participating rural counties in north central Florida. All three treatment conditions included two phases: an initial 4-month intervention phase requiring 12 group meetings over 16 weeks, and then an 8-month maintenance phase with monthly group meetings. Assessments occurred at baseline, posttreatment (12 months), and follow-up (24 months) (Janicke et al., 2011). Recruitment took place between August 2009 and March 2012.
Preschool Opt-in Group
Data for the opt-in group were collected as part of an IRB-approved behavioral family-based obesity intervention for preschoolers, defined as aged 3–7 years, who were overweight or obese (BMI ≥ 85th percentile) and living in rural communities. Community Health Lifestyle Intervention for Rural Preschoolers (CHIRP) was planned as a two-arm RCT to test the effectiveness of a behavioral family intervention compared with a waitlist control condition in community settings. All assessment and treatment contacts were conducted at Cooperative Extension Services offices across four participating rural counties in north central Florida. The treatment arm included 12 group treatment sessions over 16 weeks. Assessments occurred at baseline, posttreatment (4 months), and follow-up (10 months) (Janicke et al., 2013). Recruitment took place between August 2012 and February 2014.
Preschool Opt-out Group
Data for the opt-out preschool group were collected as part of an IRB-approved family and behavioral-based obesity intervention that is still in progress (Learning about Activity and Understanding Nutrition for Child Health, LAUNCH) for preschoolers, defined as aged 2–5 years, who met criteria for obesity (BMI ≥ 95th percentile; Stark et al., In Preparation). LAUNCH was a three-arm, randomized, parallel group, controlled trial to test a behavioral family-based clinical and home-based intervention to motivational interviewing arm and standard care arm. Treatment was 24 weeks long (12-week intensive treatment, 12-week maintenance for a total of 18 sessions) with assessments at baseline, posttreatment (6 months), and follow-ups (12 months and 18 months). Recruitment took place between February 2012 and April 2015.
Recruitment Procedures
Opt-in Recruitment Approach for Both Preschool and School-age Studies
Recruitment for both the preschool and school-age opt-in studies was identical and entailed direct solicitation methods including (a) direct mailings from purchased mailing lists of families with children within the study age range (3–7 years for preschool study and 8–12 for school-age study); (b) distribution of brochures through local preschools, elementary schools, and local physician offices; (c) newspaper press releases; and (d) presentations at community events. Materials invited potential participants to learn more about the study by calling the study’s toll-free telephone number. A trained member of the research team made return phone calls to families who contacted the study in which they described the study and performed a brief screening for eligibility. Inclusion criteria included (1) children aged 3 years 0 months to 7 years 9 months (for CHIRP) or 8 years 0 months to 12 years 11 months (for E-FLIP for Kids); (2) ≥ 85th percentile for age- and gender-specific BMI; (3) child must have lived in the same home with his/her participating parent in a rural county in North Central Florida; and (4) participating parent or legal guardian was ≤75 years of age. Exclusion criteria included (1) child or participating parent/legal guardian had dietary or exercise restrictions or a medical condition that contraindicates mild energy restriction or moderate physical activity; (2) child had resting blood pressure ≥ 140/90 mm Hg; (3) child or participating parent on antipsychotic agents; monoamine oxidase inhibitors; systemic corticosteroids; antibiotics for HIV or Tuberculosis; chemotherapeutic drugs; or use of prescription weight-loss drugs within 6 months; and (4) child or parent reported or exhibited conditions or behaviors likely to affect the conduct of the trial (e.g., learning difficulties, speech delays, or Autism Spectrum Disorder were excluded).
Opt-out Recruitment Approach with Preschool Study
Children were identified via systematic chart review of all children within ages 2–5 years at 27 pediatric practices by practice and study personnel (under a Health Insurance Portability and Accountability Act [HIPAA] waiver or business associate agreement) for inclusion and exclusion criteria. Inclusion criteria included (1) children aged 2 years 0 months to 5 years 11 months; (2) ≥ 95th percentile for age- and gender-specific BMI but no more than 100% above the median BMI for age and gender; (3) child having medical clearance; and (4) child having a well-child check-up within a year of the chart review. Exclusion criteria included (1) child having a medical condition known to promote obesity (e.g., Prader-Willi syndrome); (2) parent or child having a medical condition, disability, or illness that may preclude full participation in program (e.g., developmental disability that may affect understanding material); (3) child participating in another weight control program; (4) child taking weight-affecting medication; (5) parent or child not being English-speaking; and (6) family living >50 miles from the medical center conducting the study. Parents of children meeting these screening criteria for the study were sent a letter from their pediatrician or nurse practitioner explaining the study and why their child may be appropriate to participate. The letter included a flyer describing the study and a return addressed, stamped “do not contact” postcard for a family to mail to the pediatrician office if they did not want to be contacted by research personnel to learn more about the study. Study personnel then attempted to call families from whom a postcard was not received within 10 days of the date of mailing to explain the study and assess the family’s interest in participation. All families whose preschoolers continued to meet eligibility after the phone screen and were interested in participating were then scheduled for a baseline clinic visit, held at a medical center that was separate from their pediatrician’s office, where informed consent was obtained before any study measures were taken.
Dependent Measures
Table II provides definitions and calculations for each of the following dependent measures: (1) percent of eligible families phone screened; (2) percent agreed to in-person screening; (3) percent consented; (4) percent eligible and enrolled; (5) percent randomized; (6) percent meeting intent-to-treat; and (7) overall recruitment rate.
Table II.
Dependent Measures and Recruitment Rate Calculations
| Dependent measure | Calculation |
|---|---|
| Percent phone screened | Number of families reached by phone and screened ÷ Total number of families screened as eligible |
| Percent agreed to in-person screening | Number of families phone screened who agreed to attend in-person screening ÷ Total number of families phone screened |
| Percent consented | Number of families who signed a consent form ÷ Total number of families who agreed to attend in-person screening |
| Percent eligible and enrolled | Number of families consented who were eligible and enrolled ÷ Total number of families who consented |
| Percent randomized | Number of families who were randomized ÷ Total number of families who were eligible and enrolled |
| Percent met intent-to-treat (ITT) | Number of families who met ITT (attended the 1st treatment session) ÷ Total number of families randomized |
| Percent overall recruitment rate | Number of families who met ITT ÷ Total number of families sent fliers or letters |
To calculate these recruitment rates, the number of families eligible to participate in each weight management clinical trial first needed to be established. For the opt-out preschool study (i.e., LAUNCH), this was a straightforward calculation of the number of preschoolers in the pediatric practices screened via chart review as meeting inclusion/exclusion criteria divided by the total population of preschoolers in the practices. The base rate of eligible families for the studies using opt-in recruitment (i.e., CHIRP and E-FLIP), however, had to be estimated based on the number of brochures mailed to families within each age range: 49,000 for school age and 44,000 for preschool. We then used the national estimates of overweight and obesity from the most recent National Health and Nutrition Examination Survey (NHANES 2011–2012; Ogden et al., 2014) to estimate the children in each age-group that received brochures who would be expected to meet criteria for overweight and obesity. Specifically, 22.8% of the 44,000 2–7-year-olds sent brochures and 34.2% of the 49,000 8–12-year-olds sent brochures were estimated to be ≥ 85th BMI percentile (Ogden et al., 2014) based on national data. We then reduced this estimate based on the percent eligible for the opt-out preschool group (i.e., LAUNCH) owing to exclusion criteria (4.7% of the medical charts screened for eligibility were deemed eligible) compared with the NHANES incidence of obesity in preschoolers aged 2–5 years (8.4% of preschoolers classified as obese in NHANES), which equates to a 45% reduction from the NHANES estimate. Therefore, to be consistent with the opt-out preschool study (i.e., LAUNCH) eligibility, we reduced the estimated eligibility for opt-in studies (i.e., CHIRP and E-FLIP) by 45%, which yielded an estimated eligibility of 12.5% for the opt-in CHIRP preschool study (5,565) and 18.8% for the opt-in E-FLIP school-age study (9,384).
Statistical Analysis
Chi square analyses were first used to compare the opt-in recruitment strategy with school-age children (i.e., E-FLIP) to preschoolers (i.e., CHIRP) on all of the dependent measures described in Table II. The same variables were then compared between the opt-out preschool sample (i.e., LAUNCH) and the opt-in preschool sample (i.e., CHIRP).
Results
Comparing Opt-in Approach for Preschoolers and School-Age Children
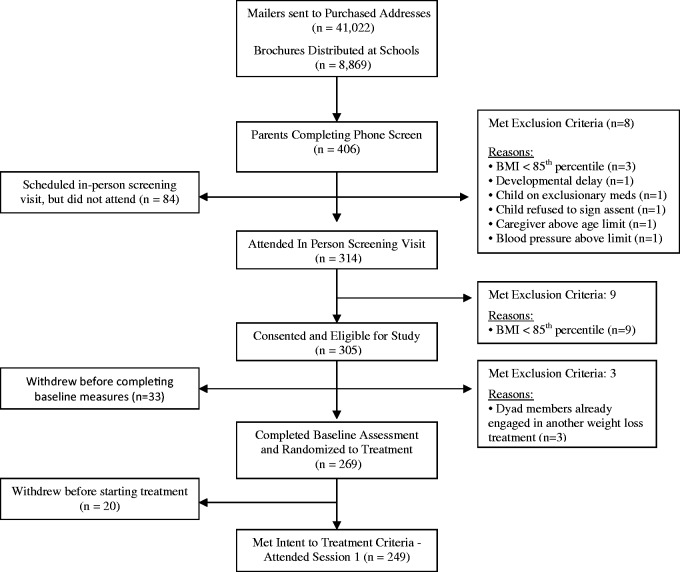
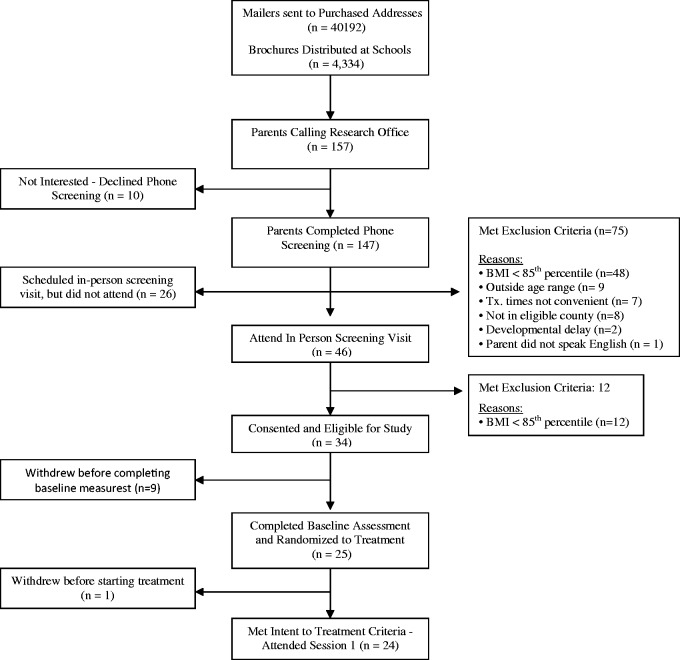
Figure 1 shows the flow of participants through all phases of school-age opt-in (i.e., E-FLIP) and Figure 2 shows the flow of participants through all phases of preschool opt-in (i.e., CHIRP). Table III shows the comparison between families of school-age children (E-FLIP) and preschoolers (CHIRP) on the outcomes of interest. Overall, the population of school-age children was slightly larger than the population of preschoolers both in the number of brochures distributed (49,000 vs. 44,000) and the proportion of each population we would expect to meet the criteria for being ≥ 85th percentile BMI and eligible to be in the study (18.8% for school-age vs. 12.5% for preschoolers), which are estimates based on several assumptions described in the Dependent Measures section.
Figure 1.
CONSORT flow diagram: opt-in school-age clinical trial (E-FLIP for Kids).
Figure 2.
CONSORT flow diagram: preschool opt-in clinical trial (CHIRP).
Table III.
Recruitment Rates and Chi-Square Comparisons Between Opt-in School Age (E-FLIP), Opt-in Preschool (CHIRP), and Opt-out Preschool (LAUNCH) Studies Expressed as Percentages or Frequencies
| Recruitment Stage | Opt-in school age (E-FLIP) | Opt-in preschool (CHIRP) | Comparing E-FLIP to CHIRP (χ2) | Opt-out preschool (LAUNCH) | Comparing CHIRP to LAUNCH (χ2) |
|---|---|---|---|---|---|
| Medical charts screened for eligibility/mailed brochure | 49,891 | 44,526 | n/a | 42,684 | n/a |
| Percent eligible | 18.8%a (9,384) | 12.5%b (5,565) | 700** | 3.7%c (1,627) | 1,981** |
| Percent phone screened | 4.3% (406) | 2.8% (157) | 21** | 46.5% (758) | 2,205** |
| Percent agreed to in- person screening | 98% (398) | 45.8% (72) | 219** | 42% (319) | 0.55 |
| Percent consented | 78.9% (314) | 63.8% (46) | 6.9** | 71% (227) | 1.2 |
| Percent eligible and enrolled | 97% (305) | 74% (34) | 34** | 75.7% (172) | 0.57 |
| Percent randomized | 88% (269) | 73.5% (25) | 4.3* | 94.8% (165) | 13.7** |
| Percent met intent-to-treat | 92.5% (249) | 96% (24) | .05 | 90.3% (149) | 0.30 |
| Percent overall recruitment rate | .50% (249) | .054% (24) | 160** | .34% (149) | 94** |
aThe percent eligible for E-FLIP was based on 55% of the 34.2% incidence rate of children aged 8–12 years > 85th percentile BMI (Ogden, 2014) to be comparable to the eligibility of the children in LAUNCH who were screened on additional criteria such as developmental disorders. The identification of eligible preschoolers in LAUNCH was 55% of the incidence of 8.4% of obesity (>95th percentile BMI) in children 2–5 years.
bThe percent eligible for CHIRP was based on 55% of the 22.8% incidence rate of children aged 2–5 years > 85th percentile BMI (Ogden, 2014) to be comparable to the eligibility of the children in LAUNCH who were screened on additional criteria such as developmental disorders. The identification of eligible preschoolers in LAUNCH was 55% of the incidence of 8.4% of obesity (>95th percentile BMI) in children 2–5 years. **p < .01; * p < .05.
c Number with Medical Release and/or with siblings eliminated who were sent recruitment letters.
As detailed in Table III, families of preschoolers were significantly less likely to contact the study and be phone screened, to agree to in-person screening, to consent to be in the study, meet inclusion criteria and enroll in the study, and to be retained from enrollment to randomization compared with families of school-age children (p’s < .05). However, there were no differences in retention rate of families from randomization to meeting intent-to-treat for families of preschoolers compared with families of school-age children (p > .05). See Table III.
Overall Recruitment
For the opt-in strategy, 249 families of 49,891 school-age children in the population met intent-to-treat for an estimated recruitment rate of 0.50%. Twenty-four families of 44,526 preschoolers in the population met intent-to-treat for a recruitment rate of 0.054%. Families of preschoolers were significantly less likely to be recruited into a weight management study than families of school-age children, χ2 (1) = 160, p < .01.
Comparing Opt-out and Opt-in Recruitment for Preschoolers
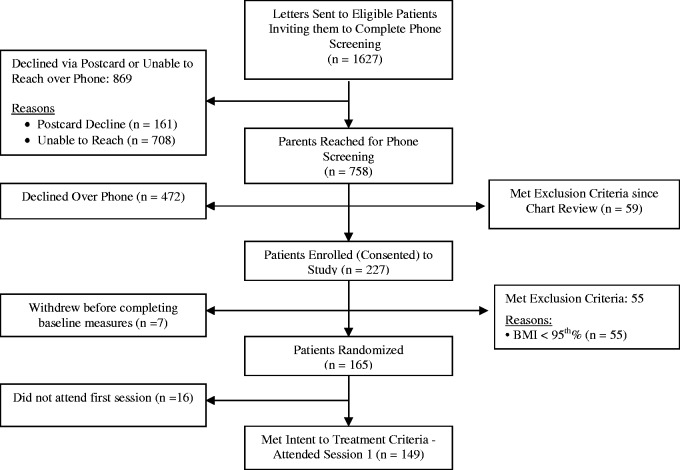
Figures 2 and 3 show the flow of participants through all phases of the preschool studies CHIRP and LAUNCH, respectively. Table III shows the comparison between opt-out (i.e., LAUNCH) and opt-in (i.e., CHIRP) recruitment strategies on the outcomes of interest. Overall, both studies had a similar population of preschoolers from which they were drawing, approximately 43,000–44,000.
Figure 3.
CONSORT flow diagram: preschool opt-out clinical trial (LAUNCH).
As shown in Table III, when using an opt-out strategy, families of preschoolers were significantly more likely to have contact with the study staff, be phone screened, and to be retained from enrollment to randomization compared with an opt-in strategy (p’s < .01). However, the opt-out and opt-in strategies yielded a similar percentage of families who agreed to attend an in-person screening, who consented to be in the study, met inclusion criteria and enrolled in the study, and who were retained from randomization to meeting intent-to-treat (p’s > .05).
Overall Recruitment
For the opt-out strategy, 149 families of 43,421 preschoolers in the population met intent-to-treat for a recruitment of 0.34%. For the opt-in strategy, 24 families of 44,526 in the population met intent-to-treat for a recruitment of 0.054%. Families in the opt-out group were significantly more likely to be recruited into the study compared with families in the opt-in group, χ2 (1) = 94, p < .01.
Discussion
As the need for intervention at younger ages is increasingly recognized as necessary to address the obesity epidemic (AHA, 2015), it is becoming critical to identify the most effective approaches for engaging families of young children in clinical trials addressing weight management. Our research team has consistently used opt-out strategies for recruitment of chronic illness populations and applied it to recruitment of preschoolers for a weight management trial. Using secondary data analysis, this article was the first to compare an opt-in strategy to an opt-out strategy in preschoolers to demonstrate how the methodology used impacts recruitment rates for weight management trials in this age-group. However, before comparing recruitment rates of opt-out versus opt-in for preschoolers, we first sought to establish that preschoolers were a more difficult population to recruit when using opt-in method by comparing school-age to preschool children using the same opt-in strategy for weight management trials.
As hypothesized, the opt-in strategy resulted in a lower percentage of families of preschool-age children recruited into a weight management program at each phase of recruitment from the initial contact through randomization compared with school-age children. No differences, however, emerged between samples in percentage of families retained through intent-to-treat. We hypothesize that these age differences in recruitment rates using the same opt-in strategy highlight the barriers associated with recruitment of young children into clinical trials, namely parents and physicians being less likely to identify obesity in preschoolers compared with school-age children (Duncan et al., 2015) and thus less likely to contact a study. These age differences suggest that preschool populations need to be approached with a recruitment method that improves identification of obesity in this age-group and facilitates an awareness of the importance of early intervention, such as an opt-out approach that allows researchers to make the initial contact with families and explain how participation may benefit their child and family.
Using an opt-out methodology for recruitment, which we have previously used with chronic illness populations, yielded a greater percentage of families of preschoolers recruited into a weight management program compared with the opt-in strategy. Our findings suggest that the main advantage that the opt-out strategy conferred was increasing the number of families who were reached by the research team to explain the study in greater detail and invite participation. While we do not have data in the current study to document the reason increased contact would yield increased recruitment, we hypothesize that the outreach from study personnel allowed us to more fully explain to families why their child’s weight may be a health concern and to identify benefits to their child and family in participating in the trial. Anecdotally, parents often expressed surprise their child was identified as eligible to participate and appreciated the opportunity to talk through their perceptions of their child’s weight as well as the benefits of developing healthier diet and activity patterns for their child and family. Because the research team could reach out to families, a greater number of children were recruited into the opt-out study (LAUNCH), with six times as many children recruited using the opt-out methodology compared with opt-in during the same time frame.
The field of BE provides a theoretical framework to explain why opt-out approaches may reduce barriers to recruitment in preschoolers. BE research shows that individuals possess cognitive biases that influence their decision-making (Stevens, 2014). Status quo is one such cognitive bias in which individuals are more likely to not take an action or go with the default option that requires no action (Johnson & Goldstein, 2013). Using a BE theoretical framework, opt-out recruitment methods take advantage of the status quo bias in human behavior and reduce barriers to recruitment because individuals do not need to take action to learn more about participation in a study. Even someone who is interested in participating in a study could be deterred by the numerous steps associated with using an opt-in methodology. For example, consider the number of actions required for families to simply learn about a study with an opt-in methodology. After receiving a study letter/brochure/flyer, an individual would need to (1) open and read the letter/brochure/flyer thoroughly; (2) reach out to other family members to determine interest; (3) remember to contact the research team; (4) contact the research team via phone, email, or letter; and (5) wait for call back from study team if call is not answered directly. The opt-out approach assumes that individuals prefer to not take action, thus, requires minimal to no action on the part of the participant to learn more information and enroll in the study if interested.
Notably, our data would indicate that while opt-out methods resulted in significantly higher recruitment rates in preschoolers compared with opt-in methodology, this strategy did not completely overcome the reluctance of parents of preschoolers to enroll in a weight management study. This is shown in that the preschool opt-out recruitment of 0.35% was still below the school-age opt-in recruitment rate of 0.50%. This begs the question of how we can improve recruitment rates among this population. One published preschool obesity RCT (Quattrin et al., 2012) demonstrated an impressive recruitment rate (70%) by reducing the steps that families needed to take to enroll in the study. After healthcare professionals identified preschoolers who were overweight and provided parents with information about the study, they provided a “warm hand off” by introducing the family to a member of the research team who was located in the office and able to enroll and consent participants immediately. These findings suggest that it may be important to not only remove barriers to learning more about the study, but also to reduce the number of steps necessary for parents of preschoolers to actually enroll in the study. One would have to weigh the costs associated with having research personnel on site at a pediatrician’s office, though, as the number of well-visits for preschoolers on a given day is low and would require staffing for long periods.
Though opt-out methods appear to be effective in overcoming some barriers associated with recruitment of preschoolers into weight management trials, some IRBs do not permit their use in clinical studies. These IRBs appear to be in the minority as Higgerson and colleagues (2014) found that only 33% of IRBs across a sample of 24 hospital-based institutions did not permit opt-out methods owing to their interpretation of the HIPAA Privacy Rule, which sets limits on the uses that may be made of private health information without patient consent. Of note, 67% of IRBs did allow opt-out strategies. It should be noted, however, that the HIPAA privacy rule makes allowances for the use of a passive consent strategy and thus not permitting opt-out methods is an overly narrow interpretation of the rule. The current study indicates that prohibiting opt-out approaches not only impacts researchers, but denies families the opportunity to learn more about and potentially join a clinical trial, if only it were less time- and effort-consuming or more apparent why a given trial may be applicable to them. At the very least, the results of this study contribute to the ongoing discussion of the ethical trade-off to using opt-out methodology by highlighting that a greater number of families can be reached using this method that may not have otherwise had contact with the research team even if they would like to participate or would benefit from learning more about a study. On a broader level, the results highlight the importance of capitalizing on the status quo bias through using alternative recruitment methods that may be more acceptable to IRBs that do not allow opt-out strategies, such as pairing the return of the consent form with the child’s well-child visit as van Grieken and colleagues (2013) did in their preschool weight management trial.
In summary, the current study demonstrated preschoolers as a more difficult population to recruit into weight control trials compared with school-age children. This study also showed that using an opt-out approach with this population reduced barriers to recruitment and yielded higher recruitment rates than the traditional opt-in method. However, opt-out methodology did not reduce all barriers as recruitment into the preschool opt-out study was still lower than the school-age opt-in study. Limitations of the current study include that recruitment rates may have been influenced by the setting in which children were recruited. For the opt-in studies (i.e., CHIRP and E-FLIP), children were recruited solely from rural settings, while the opt-out study (i.e., LAUNCH) recruited from rural and urban settings. As recruitment in rural settings is associated with challenges of reaching families, this may have resulted in a lower recruitment rate of preschoolers in the opt-in sample. Comparison of the same opt-in strategy between preschoolers and school-age children in rural settings, though, still indicates the unique challenges associated with recruiting preschool-age children into obesity RCTs. Another limitation is that the actual number of eligible children could only be estimated for the opt-out studies. While a conservative approach was taken in devising the method for estimating the percentage of children ≥ 85th BMI percentile in the opt-in studies, our estimates may be over or under the actual incidence. Children in the opt-out group were also known to have had a well-child checkup within the previous 12 months, while the prior well-child visits of the opt-in group are unknown. It may be that the samples were different in the healthcare contact and that this difference contributed to their likelihood of participating in a weight management trial.
Despite these limitations, the results provide important implications for how we conceptualize and develop recruitment strategies to best reach challenging populations and show the benefit of using an opt-out approach with preschoolers for weight management trials in particular. Given the status quo bias, opt-out methodology could also be effectively used to recruit other pediatric populations, as evidenced by our research team successfully recruiting children with CF, IBD, and JRA into studies. Future studies designed to directly compare the opt-in and opt-out recruitment strategies simultaneously with a preschool population and recruiting from similar settings (e.g., rural or urban) and with other pediatric populations would be beneficial in identifying the strategies most beneficial to families and researchers.
Acknowledgments
Recruitment for these studies would not have been possible without the tireless efforts of our clinical research coordinators, Jared Connor, Angela Moore, and Megan Richardson.
Funding
Studies in this article were supported by the following grants funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: T32DK063929): R01DK091251, R18DK082374, R21DK095269.
Conflicts of interest: None declared.
References
- American Heart Association. (AHA) (2015). Policy recommendations for obesity prevention in early care and education settings. Retrieved from https://www.heart.org/idc/groups/heart-public/@wcm/@adv/documents/downloadable/ucm_473791.pdf
- Birch L. L., Fisher J. O. (1998). Development of eating behaviors among children and adolescents. Pediatrics, 101, 539–549. [PubMed] [Google Scholar]
- Cunningham S. A., Kramer M. R., Narayan K. M. (2014). Incidence of childhood obesity in the United States. New England Journal of Medicine, 370, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels S. R. (2009). Complications of obesity in children and adolescents. International Journal of Obesity, 33, S60–S65. [DOI] [PubMed] [Google Scholar]
- Dilley K. J., Martin L. A., Sullivan C., Seshadri R., Binns H. J.; Pediatric Practice Research Group. (2007). Identification of overweight status is associated with higher rates of screening for comorbidities of overweight in pediatric primary care practice. Pediatrics, 119, e148–e155. [DOI] [PubMed] [Google Scholar]
- Duncan D. T., Hansen A. R., Wang W., Yan F., Zhang J. (2015). Change in misperception of child's body weight among parents of American preschool children. Childhood Obesity, 11, 384–393. [DOI] [PubMed] [Google Scholar]
- Eli K., Howell K., Fisher P. A., Nowicka P. (2014). “A little on the heavy side”: A qualitative analysis of parents' and grandparents' perceptions of preschoolers' body weights. BMJ Open, 4, e006609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster B. A., Farragher J., Parker P., Sosa E. T. (2015). Treatment interventions for early childhood obesity: A systematic review. Academy of Pediatrics, 15, 353–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopinath B., Baur L. A., Garnett S., Pfund N., Burlutsky G., Mitchell P. (2011). Body mass index and waist circumference are associated with blood pressure in preschool-aged children. Annals of Epidemiology, 21, 351–357. [DOI] [PubMed] [Google Scholar]
- Higgerson R. A., Olsho L. E., Christie L. M., Rehder K., Doksum T., Gedeit R., Giuliano J. S., Jr., Brennan B., Wendlandt R., Randolph A. G.; PALISI PICFlu Study Investigators. (2014). Variability in IRBs regarding parental acceptance of passive consent. Pediatrics, 134, e496–e503. [DOI] [PubMed] [Google Scholar]
- Hunt K. J., Shlomo N., Addington-Hall J. (2013). Participant recruitment in sensitive surveys: A comparative trial of “opt in” versus “opt out” approaches. BMC Medical Research Methodology, 13, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke D. M., Lim C. S., Mathews A. E., Shellnut K., Boggs S. R., Silverstein J. H., Brumback B. A. (2013). The community-based healthy-lifestyle intervention for rural preschools (CHIRP) study: Design and methods. Contemporary Clinical Trials, 34, 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke D. M., Lim C. S., Perri M. G., Mathews A., Bobroff L., Silverstein J. H., Brumback B. A., Dumont-Driscoll M. (2011). Extension family lifestyle intervention project (E-FLIP for Kids): Design and methods. Contemporary Clinical Trials, 32, 50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E.G., Goldstein D.G. (2013). Decisions by default In Shafir E. (Ed.), The behavioral foundations of public policy (pp. 417–427). Princeton, NJ: Princeton University Press. [Google Scholar]
- Junghans C., Feder G., Hemingway H., Timmis A., Jones M. (2005). Recruiting patients to medical research: Double blind randomised trial of “opt-in” versus “opt-out” strategies. BMJ, 331, 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juengst E. T., Goldenberg A. (2008). Genetic diagnostic, pedigree, and screening research In Emanual E. J. (Ed.), The Oxford textbook of clinical research ethics (pp. 298–313). New York, NY: Oxford University Press. [Google Scholar]
- Kuczmarski R. J., Ogden C. L., Grummer-Strawn L. M., Flegal K. M., Guo S. S., Wei R., Mei Z., Curtin L. R., Roche A. F., Johnson C. L. (2000). CDC growth charts: United States. Advance Data, 314, 1–27. [PubMed] [Google Scholar]
- Kuhl E. S., Rausch J. R., Varni J. W., Stark L. J. (2012). Impaired health-related quality of life in preschoolers with obesity. Journal of Pediatric Psychology, 37, 1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader P. R., O'brien M., Houts R., Bradley R., Belsky J., Crosnoe R., Friedman S., Mei Z., Susman E. J.; Human Development Early Child Care Research, Network. (2006). Identifying risk for obesity in early childhood. Pediatrics, 118, e594–e601. [DOI] [PubMed] [Google Scholar]
- Nelson K., Garcia R. E., Brown J., Mangione C. M., Louis T. A., Keeler E., Cretin S. (2002). Do patient consent procedures affect participation rates in health services research? Medical Care, 40, 283–288. [DOI] [PubMed] [Google Scholar]
- Ogden C. L., Carroll M. D., Kit B. K., Flegal K. M. (2012). Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. JAMA, 307, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C. L., Carroll M. D., Kit B. K., Flegal K. M. (2014). Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA, 311, 806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quattrin T., Roemmich J. N., Paluch R., Yu J., Epstein L. H., Ecker M. A. (2012). Efficacy of family-based weight control program for preschool children in primary care. Pediatrics, 130, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor H. A., Osterholt K. M., Hart C. N., Jelalian E., Viver P., Wing R. R. (2009). Evaluation of active and passive recruitment methods used in randomized controlled trials targeting pediatric obesity. Pediatric Obesity, 4, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark L. J., Davis A. M., Janicke D. M., Mackner L. M., Hommel K. A., Bean J. A., Lovell D., Heubi J. E., Kalkwarf H. J. (2006). A randomized clinical trial of dietary calcium to improve bone accretion in children with juvenile rheumatoid arthritis. The Journal of Pediatrics, 148, 501–507. [DOI] [PubMed] [Google Scholar]
- Stark L. J., Hommel K. A., Mackner L. M., Janicke D. M., Davis A. M., Pfefferkorn M., Crandall W., Heubi J. (2005). Randomized trial comparing two methods of increasing dietary calcium intake in children with inflammatory bowel disease. Journal of Pediatric Gastroenterology and Nutrition, 40, 501–507. [DOI] [PubMed] [Google Scholar]
- Stark L. J., Quittner A. L., Powers S. W., Opipari-Arrigan L., Bean J. A., Duggan C., Stallings V. A. (2009). Randomized clinical trial of behavioral intervention and nutrition education to improve caloric intake and weight in children with cystic fibrosis. Archives of Pediatrics and Adolescent Medicine, 163, 915–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark L. J., Spear S., Boles R., Kuhl E., Ratcliff M., Scharf C., Bolling C., Rausch J. (2011). A pilot randomized controlled trial of a clinic and home-based behavioral intervention to decrease obesity in preschoolers. Obesity, 19, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens J. (2014). Behavioral economics as a promising framework for promoting treatment adherence to pediatric regimens. Journal of Pediatric Psychology, 39, 1097–1103. [DOI] [PubMed] [Google Scholar]
- Taveras E. M., Gortmaker S. L., Hohman K. H., Horan C. M., Kleinman K. P., Mitchell K., Price S., Prosser L. A., Rifas-Shiman S. L., Gilman M. W. (2011). Randomized controlled trial to improve primary care to prevent and manage childhood obesity: The High Five for Kids Study. JAMA Pediatrics, 165, 714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Grieken A., Veldhuis L., Renders C. M., Borsboom G. J., van der Wouden J. C., Hirasing R. A., Raat H. (2013). Population-based childhood overweight prevention: outcomes of the ‘Be active, eat right’study. PloS One, 8, e65376. [DOI] [PMC free article] [PubMed] [Google Scholar]