Abstract
The circadian clock is an endogenous, time-tracking system that directs multiple metabolic and physiological functions required for homeostasis. The master or “central” clock located within the suprachiasmatic nucleus (SCN) in the hypothalamus governs peripheral clocks present in all systemic tissues, contributing to their alignment and ultimately to temporal coordination of physiology. Accumulating evidence reveals the presence of additional clocks in the brain and suggests the possibility that circadian circuits may feedback to these from the periphery. Here, we highlight recent advances on the communications between clocks and discuss how they relate to circadian physiology and metabolism.
Graphical Abstract
In addition to the central pacemaker, the mammalian brain contains additional circadian clocks whose function illustrates how systemic homeostasis relies on the coordinated communication between clocks.
Circadian rhythms govern an extensive variety of behavioral, physiological and metabolic functions in virtually all life forms, from bacteria to plants, invertebrates and mammals. These rhythms are largely controlled by the circadian clock, a molecular machinery operating in all cells (Figure 1). The organization of the mammalian circadian clock is based on transcriptional-translational feedback loops. Central to the core clock are the transcription factors CLOCK and BMAL1, which heterodimerize and drive the expression of a large number of clock-controlled genes (CCGs) by binding to E-boxes, the most common promoter element on the genome. Because of this, the molecular clock directs the expression of an estimated 10-15 % genes in all organs and tissues1, 2. Importantly, through the interplay between the clock and tissue-specific transcriptional pathways, the overlap of CCGs in each organ is relatively small, underscoring the concept that a very large fraction of the genome has the potential of being regulated in a circadian manner3. Among the CCGs there are the genes encoding the repressors period (PER) and cryptochrome (CRY) whose accumulation results in inhibition of CLOCK:BMAL1-driven transcription. PER and CRY repressors are subsequently degraded through clock-dedicated proteasome circuits, leading to new transcription cycles. In addition to this central circuit, the orphan nuclear receptors ROR and REV-ERB contribute to the clock mechanism by generating an additional regulatory loop. Finally, a variety of signaling pathways influence core clock regulators by inducing several post-translational modifications that ultimately lead to changes in clock control4.
Figure1: Molecular Organization of the Mammalian Circadian Clock.
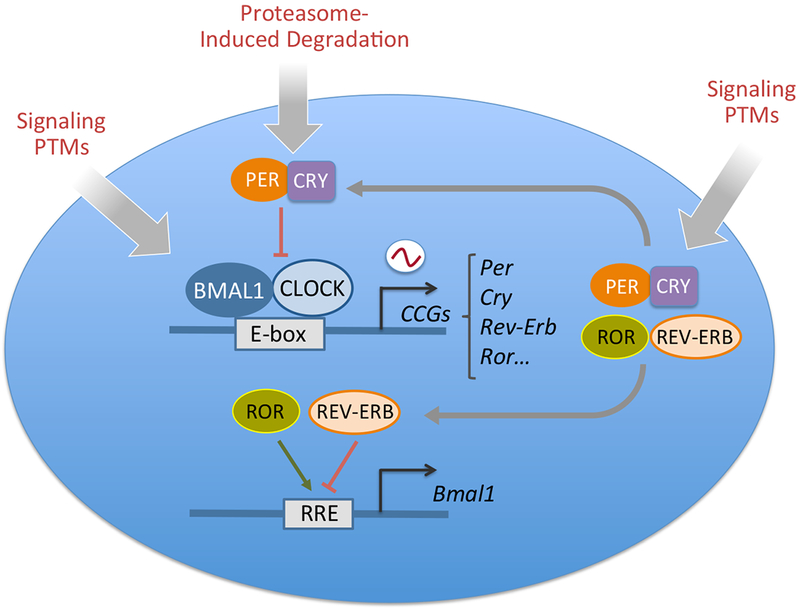
The mammalian molecular clock consists of a positive loop driven by the transcriptional activators CLOCK and BMAL1 and a negative feedback loop driven by the repressors period (PER) and cryptochrome (CRY) proteins. In mammals there are three PER proteins and two CRYs. CLOCK and BMAL1 activate the expression of clock-controlled genes (CCGs) through binding to E-box elements in their promoters. Among the CCGs are Per and Cry genes whose products dimerize and translocate into the nucleus where they inhibit CLOCK:BMAL1 activity. PERs and CRYs undergo a number of post-translational modifications that result in proteasome-induced degradation with a 24 hour rhythmicity, ultimately allowing the start of a new circadian cycle. CLOCK:BMAL1 also induce the activation of Rev-Erb and Ror genes that give rise to a secondary loop by binding to responsive promoter elements (RRE ) and inhibit and activate respectively Bmal1 transcription. Most of the molecular clock components are additionally regulated through various signaling pathways that post-translationally modify the core clock. Post-translational modifications (PTMs) include acetylation, phosphorylation, O-GlcNAcylation and SUMOylation (See Ref 181 for an overview). Together these transcriptional-translational regulatory loops generate the circadian output.
 indicates oscillation.
indicates oscillation.
The exquisite control of circadian gene expression by the clock is associated to chromatin remodeling. The very first observation of circadian chromatin transitions illustrated that H3-Ser10 phosphorylation occurs in SCN neurons in response to a light stimulus and is linked to the activation of clock genes5. Subsequently, a number of chromatin remodelers have been found to display circadian activity6. Among the chromatin remodelers involved in circadian control, the nicotinamide adenine dinucleotide (NAD+)-dependent SIRT1 deacetylase deserves special mention. Indeed, SIRT1 and other members of the so-called ‘sirtuin’ family provide a relevant molecular link between metabolism, epigenetics and the circadian clock7.
Virtually every tissue in our body harbors a functional molecular clock and coordination among clocks is crucial for optimal timekeeping and physiology. Here, we discuss the relationship between circadian clocks and metabolic homeostasis. First we describe some evidence on newly discovered brain clock functions and their implication for circadian physiology. We then examine the complex network of output and feedback signals that couples brain clocks to the peripheral metabolic framework. We conclude by discussing the current understanding of how nutrition affects circadian physiology and how this relates to brain functions.
Clocks in the brain
Mechanisms of SCN entrainment
The mammalian circadian system is a hierarchical network of oscillators, where the master or “central” clock is in the suprachiasmatic nuclei (SCN) of the hypothalamus that receives photic information via the retino-hypothalamic tract. The central clock connects with peripheral clocks present in all systemic tissues, contributing to their coordinated functions. The combination of this interplay and environmental cues ensures temporally coordinated physiology. The SCN comprises about 20,000 interconnected neurons capable of generating diurnal rhythms in neuronal activity, which persist in the absence of external timing cues. The SCN is sub-divided into two main nuclei, the “core” and “shell”, diverse for neuronal projections, expression of neuronal peptides and activation in response to light. “Core” and “shell” neurons form circuits that are coupled via firing of action potentials and release of neuropeptides. Well-characterized SCN neuropeptides are vasoactive intestinal polypeptide (VIP) synthesized by ventrolateral “core” neurons and arginine vasopressin (AVP) released from neurons in the dorsal “shell”8, 9. Signaling of both VIP and AVP is critical for establishing and synchronizing firing as well as behavioral rhythms10–13.
A feature of the central circadian system is its heterogeneity. Transcriptional analysis of single-cell SCN neurons highlighted the complexity of the signaling network underlying synchronization of the master clock14. Hence, in addition to the known coupling mechanism within the SCN, numerous others are likely to help regulate circadian period. In this respect, astrocytes, which make up a substantial fraction of the cells in the SCN, are responsible for a variety of functions within the CNS. Astrocytes provide energy supply through glucose/glycogen pathways15, regulating neurovascular coupling via release of vasoactive molecules16 and playing an active role in synaptic transmission by actively releasing synaptically active molecules including glutamate, ATP and GABA17. Rat astrocytes display rhythmic expression of clock genes18, 19 as well as cyclic ATP release in culture20, indicating that astrocytes indeed have a functional clock. Moreover, pharmacological inhibitors of glial activity affect rhythms of SCN neuronal firing and of diurnal behavioral rhythms21, thereby suggesting that glial cells could play a role as synchronizers of circadian networks within the SCN. In support of this notion, several animal models have proven the importance of SCN astrocytes for rhythmic entrainment and circadian physiology22–24. The behavioral phenotypes highlighted by these studies denote the importance of reciprocal interactions between glial and neuronal cells in the context of circadian circuitry. Indeed, in vitro co-culture experiments showed that synchronous astrocytes are able to entrain rhythmicity in neurons with a mechanism that is mediated by GABA (γ-aminobutyric acid) and GABAA receptor signaling22. GABA is the most abundant neurotransmitter in the SCN, where it acts as a primary synchronizing signal25 and is able to communicate phase information to distinct SCN populations26. Astrocytes also express GABA transporters and receptors and activation of both results in increased intracellular Ca2+ concentration27, 28, a marker of metabolic activation associated with the release of various gliotransmitters, including glutamate29. Notably SCN astrocytic intracellular Ca2+ levels rise during the circadian night displaying an oscillatory pattern that is anti-phasic with that of SCN neurons. Astrocyte Ca2+ oscillation is coupled with release of high levels of glutamate into the extracellular space which leads to inhibition of SCN neuronal activity through pre-synaptic activation of GABAergic neurons23.
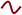
Another fundamental feature of astrocytes is to supply metabolic substrates to neurons through the proposed “astrocyte to neuron lactate shuttle” (ANLS) mechanisms30. Glucose consumption in the SCN is rhythmic31 and brain lactate concentrations show daily variations peaking during the active phase32, possibly suggesting the existence of a rhythmic “neurometabolic coupling” mechanism among astrocytes and neurons throughout the SCN (Figure 2). Moreover, given that glutamate is a key mediator of astrocyte time keeping23, it is worthy to note that glutamate uptake by astrocytes can trigger a cascade of events ultimately leading to production and release of lactate into the extracellular space15. Further underlying the link between astrocyte glutamate metabolism and circadian physiology, the astrocytic glutamate transporter EAAT1 displays rhythmic expression in the SCN that is deregulated in Per2 mutant mice33. Importantly, astrocyte lactate trafficking is crucial for the activity of orexin neurons and for the regulation of sleep-wake cycles34. Thus, it is tempting to speculate that lactate shuttling may also be involved in promoting SCN neuronal firing and circadian pacemaking.
Figure 2: Metabolic coupling of neurons and astrocytes.
Glutamate is released at neuronal excitatory synapses and up-taken by astrocytes through glial-specific glutamate transporters (EAATs). Glutamate uptake by astrocytes triggers an increase in glycolysis, enhancing influx of glucose from blood vessels via glucose transporters (GLUTs) expressed in astrocytes. Part of the glucose is metabolized to lactate via pyruvate by the lactate dehydrogenase isoenzyme A (LDHA) and transported outside the astrocytes via monocarboxylate transporters (MCTs). Once transferred to neurons, lactate is oxidized to produce ATP. Both neurons and astrocytes have an autonomous circadian clock. Several of these enzymes display daily rhythms in the SCN (see the Circadiomics web portal as a reference182). Additionally, lactate levels show diurnal variations. We propose a model where circadian neurometabolic coupling contributes to SCN function.
Clocks in other hypothalamic nuclei
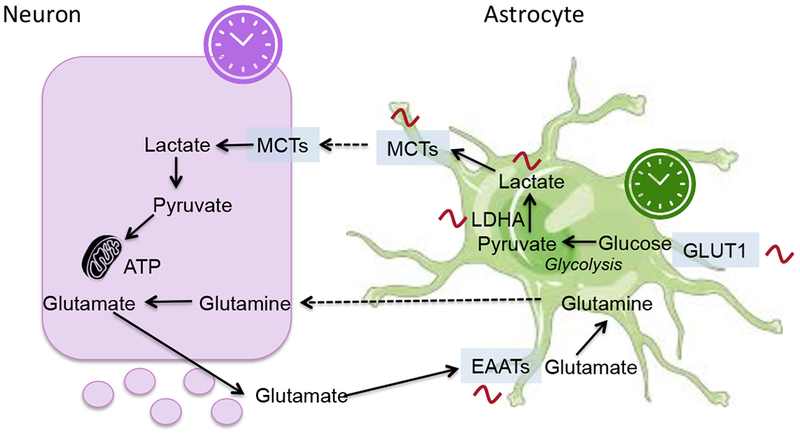
In addition to the SCN, the hypothalamus is subdivided into several interconnected nuclei, which include the dorsomedial nucleus of the hypothalamus (DMH), ventromedial nucleus of the hypothalamus (VMH), arcuate nucleus (ARC) and paraventricular nucleus of the hypothalamus (PVH). These nuclei control numerous physiological processes that display diurnal rhythmicity, including sleep-wake cycles, energy expenditure, thermoregulation, glucose and lipid metabolism, and feeding behavior35. In addition to receiving projections from the SCN, extra-SCN hypothalamic sites harbor their own circadian oscillator capable of sustaining autonomous circadian rhythms36. Although the functional significance of these non-SCN brain clocks remains elusive it is thought that they play a central role in gating some behavioral and physiological processes during the 24 hours, acting in synchrony or independently from the master clock (Figure 3).
Figure 3: Network of hypothalamic oscillators.
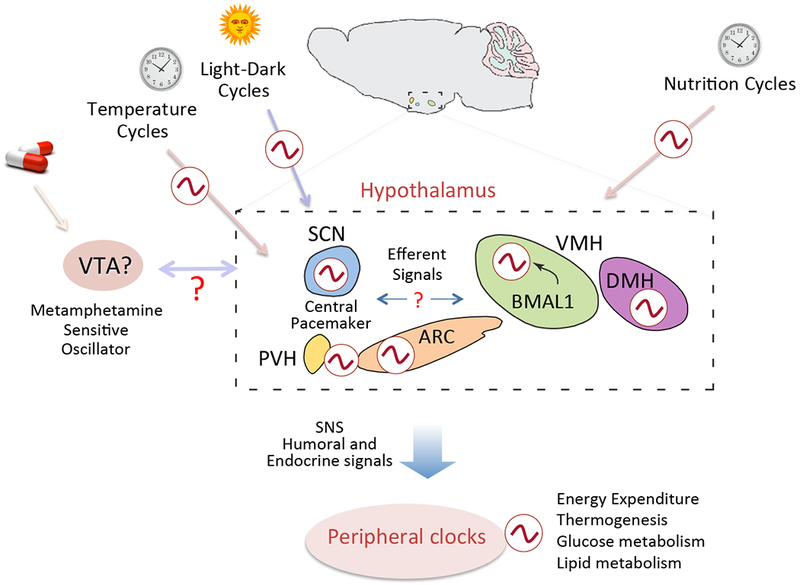
Representative illustration of how external cues and output signals are integrated within the hypothalamic network of oscillators. The hypothalamic SCN is the master regulator, whereas extra-SCN hypothalamic nuclei harbour their own independent circadian oscillator. It is hypothesized that extra-SCN clocks operate in concert with the SCN to gate circadian physiology during the 24 hour cycle. Clocks in the arcuate nucleus (ARC), dorsomedial hypothalamus (DMH) and ventromedial hypothalamus (VMH) integrate external cues such as temperature cycles and nutrition cycles. Central output signals are conveyed to peripheral tissues via the sympathetic nervous system (SNS) as well as through humoral factors thereby regulating peripheral metabolism and energy homeostasis. Light is the main entrainment signal for the suprachiasmatic nucleus (SCN). Additionally, oscillators sensitive to other external cues, such as food and drugs of abuse, have been hypothesized. The location and functional relationships among brain oscillators remains to be elucidated. PVH, paraventricular nucleus of the hypothalamus; VTA, ventral tegmental area.
Both ARC and DMH are critical in governing feeding behavior37 and most likely act in synchrony with the SCN to generate feeding rhythms. Circadian activity of α-MSH neurons in the ARC is controlled by the SCN38 and, vice versa ventromedial ARC neurons convey feeding related signals to the SCN39. In the ARC orexigenic and anorexigenic peptides neuropeptide Y (Npy), agouti-related peptide (Agrp), pro-opiomelanocortin (Pomc) as well as cocaine and amphetamine regulated transcript (Cart) are expressed in a diurnal manner40, 41. Notably, targeted disruption of NPY signaling in the ARC leads to profound effects on daily patterns of activity and feeding42,43. In the DMH NPY is important for the maintenance of energy homeostasis through a mechanism that involves both food intake and energy expenditure modulation44. Additionally, NPY regulates rhythmic expression of a subset of genes in the liver45. Yet, it is unclear whether the observed changes in cyclic gene expression are driven by central or peripheral NPY signaling. Further studies may help clarify whether this neuropeptide may play a key role in coupling central and peripheral clocks.
While a direct link between rhythmic expression of these neuropeptides and the molecular clock has not been proven, targeted genetic alteration of clock core genes generates animals with disrupted circadian rhythms, characterized by alteration in feeding behavior as well as energy homeostasis (Table 1). CLOCK mutant mice are hyperphagic and display significant increased food intake during the day, which is coupled with decreased expression of orexigenic transcripts in the hypothalamus46. Likewise, global loss or mutation of other core clock components, including Bmal1, Per1, Per2 and Cry1/2, leads to disruption in feeding behavior47–52. Moreover, central clocks are not solely implicated in rhythmic feeding behavior regulation, but appear to be involved in the control of energy expenditure as well. For example, mice with ablation of either Rev-erbα or Per2 genes display a faulty thermogenic response due to impaired activity of brown adipose tissue (BAT)53, 54. These mice mutants carry the ablation of the circadian gene in all cells, thereby being unable to distinguish between peripheral or centrally controlled regulatory mechanisms. While this type of genetic models of clock disruption have been instrumental for the understanding of circadian physiology, a major limitation stands in their inability to characterize distinct functions of the clock in specific areas of the brain. In the case of Rev-erbα, brain specific deletion results in constant elevated body temperature55, suggesting that REV-ERBα may be instrumental for central regulation of circadian energy balance.
Table1.
Effect of clock disruption on behavior and metabolism
| Gene | Animal Model | Phenotype | Comment | Reference |
|---|---|---|---|---|
| CLOCK | Clock Δ19 mutant | Hyperphagic Obese | Decreased expression of orexigenic transcripts | 46 |
| BMAL1 | Null mice | Arrhythmic feeding | No effect on FAA | 47 |
| BMAL1 | VMH specific deletion | Decreased body weight | Affects BAT circadian activity | 56 |
| BMAL1 | Liver specific deletion | Hypoglycemia | Arrhythmic expression of hepatic glucose regulatory genes | 102 |
| Per1 | S714G mutant | Advanced food intakeIncreased sensitivity to obesity | Feeding uncoupled from energy expenditure | 52 |
| Per2 | Null mice(Per2Brdm1) | Absence of diurnal feeding rhythmsImpaired BAT activity | Loss of α-MSH diurnal rhythmsAltered lipid metabolism | 48, 53 |
| Cry1/Cry2 | shRNA,Null mice | Absence of diurnal feeding rhythmsHyperglycemia | Feeding behavior rescued by TRF | 50, 148 |
| Reverbα | Null mice | Absence of diurnal rhythms of BAT activity | Higher Ucp1 expression in BAT | 54 |
FAA, food anticipatory activity; BAT, brown adipose tissue; α-MSH, α–melanocyte-stimulating hormone; TRF, Time restricted feeding
The strategy of ablating clock function uniquely in specific neuronal populations has remarkable potential to unravel specialized circadian circuits. Indeed, a targeted approach to delete Bmal1 specifically in Sf1-neurons of the hypothalamic VMH led to the discovery of an unrecognized function of the hypothalamic clock in regulating circadian energy expenditure56. This finding most likely relates to the well-known diurnal oscillations in body temperature57, though it remains unclear how this rhythmicity is achieved. Activity of the BAT, the main thermogenic organ, is under the control of a central neuronal circuitry that resides in several hypothalamic nuclei58. While the existence of a SCN-SNS (Sympathetic nervous system)-BAT circuitry implicated in rhythmic body temperature control has been suggested59, coordinated activity between the SCN and ARC has been shown to be critical for BAT thermogenesis60. In this respect, the consequences of ablating Bmal1 in the VMH represent a demonstration that a specialized, SCN-independent hypothalamic clock orchestrates diurnal energy expenditure and thermogenesis56. Given the intimate relationship between the VMH and glucose/insulin homeostasis61, it is tempting to speculate that the clock within the VMH could be also involved in generating daily rhythms in glucose levels and/or tolerance and integrate these with thermogenesis.
Clocks intrinsic to other hypothalamic nuclei are likely to contribute to various aspects of circadian physiology. For example, a recent study highlighted the importance of the enzyme O-GlcNAc transferase (OGT) in the PVN for feeding behavior regulation62. In peripheral tissues OGT rhythmically O-GlcNAlates and stabilizes BMAL1 and CLOCK, ultimately affecting circadian oscillations63. Thus, OGT could act as nutritional sensor conveying information to the clock machinery in the CNS and thereby play a role in circadian regulation of energy balance and feeding behavior.
Further studies aimed at deciphering the relative roles of extra-SCN hypothalamic clocks with appropriate genetic models will be critical to unravel the relationship between circadian rhythms and metabolic homeostasis.
Metabolic coupling of central and peripheral clocks
As illustrated above, the central clock within the SCN appears to operate within a network of hypothalamic oscillators to orchestrate many aspects of physiology and behavior. Several pathways governing cellular and systemic metabolism are intimately interconnected to the circadian system64, 65. Indeed, optimal timing of diurnal metabolic processes is obtained via tight control of central output signals, whereas peripheral cellular metabolism is suspected to feedback to the hypothalamus66.
SCN output to peripheral clocks
Together with the autonomic nervous system, endocrine signals are key mediators of rhythmic physiology. The first clue suggesting that SCN output signals may operate through paracrine endocrine signaling was obtained by transplanting SCN grafts into animals lacking a central pacemaker67–69. Subsequently, parabiosis experiments between SNC-lesioned and intact animals further confirmed that circulating factors could drive rhythms in peripheral tissues70. These findings are in keeping with the notion that secretion of several endocrine factors is diurnal and driven by the central clock71. One of the best examples is the release of cortisol from the hypothalamic-pituitary-adrenal (HPA) axis. In fact, under normal physiological conditions blood glucocorticoid levels oscillate as a result of endogenous clock function72. The central clock in the SCN regulates HPA rhythms via two main mechanisms: projections from the SCN to the paraventricular nucleus (PVN) drive rhythmic secretion of adrenocorticotropin hormone (ACTH)73, which in turn regulates circadian release of corticoids from the adrenal cortex; additionally, the SCN is connected to the adrenal gland via the autonomous nervous system (ANS), enabling the central clock to modulate adrenal sensitivity to ACTH, as well as light-induced adrenal activation74, 75. Additionally, circadian production of glucocorticoid is gated through the peripheral adrenal clock76–78. Glucocorticoids play a central role in energy balance and have been implicated in circadian metabolic control because of their ability to entrain peripheral clocks78–81. In the liver, glucocorticoids synchronize circadian expression by directly activating clock genes via glucocorticoid response elements (GRE) present in their regulatory regions80. Moreover, glucocorticoids can activate BAT thermogenesis in humans82 and a recent study determined that human BAT activity is rhythmic83. Interestingly, the peak of thermogenic activity in BAT is in phase with the secretion of cortisol, suggesting that glucocorticoids might play a role in driving rhythmic body temperature. Peripheral oscillators are extremely sensitive to temperature changes; hence temperature cycles can also function as output entraining signals for peripheral clocks84–86. Accordingly, temperature increase triggers heat shock factor 1 (HSF1) mediated transcription of the Per2 gene by binding to a HSE site within its promoter87. Conversely, low temperatures induce expression of the RNA binding protein CIRBP, which post-transcriptionally regulates CLOCK and possibly Rorα and Per388.
Central coordination of peripheral metabolism is also obtained through secretion of the sleep-promoting hormone melatonin. Melatonin is released by the pineal gland throughout the day-night cycle under tight control of the SCN89. Underlying the ability of melatonin to entrain peripheral rhythms, melatonin receptors are expressed in several peripheral tissues90 and rhythmic exposure of cultured adipocytes to melatonin is sufficient to synchronize the expression of clock genes91. In pancreatic beta cells melatonin regulates insulin secretion by reducing cytosolic cAMP and cGMP levels, thus directly influencing diurnal glucose and insulin blood levels92–94. Accordingly, pinealectomized animals loose diurnal rhythmicity of insulin secretion and glucose tolerance95, 96. More recently, human mutations in the melatonin receptor 1b gene MTNR1B have been identified. Subjects with the MTNR1B variant have hyper activation of melatonin signaling, resulting in impaired insulin release, hyperglycemia and increased risk to develop type 2 diabetes (T2D)97.
Together these findings demonstrate that the SCN relies on a variety of output signals to synchronize peripheral clocks and contributes to a large network of oscillators working together to drive circadian physiology.
Daily rhythms in glucose metabolism
Many aspects of glucose homeostasis are circadian: first, glucose plasma levels are rhythmic both in rodents and humans98; also, it is well established that glucose uptake into the brain follows a circadian rhythm31; lastly, glucose tolerance displays daily variations99. Supporting the notion that the central clock drives circadian plasma glucose metabolism, SCN-lesioned mice display no rhythm in glucose nor insulin levels100. Importantly, peak of plasma glucose levels at the onset of the active period is detected even when mice receive 6 meals per day every 4 hours101, indicating that glucose oscillations are driven by the central clock and are thereby independent from feeding rhythms.
Nevertheless, diurnal glucose homeostasis involves not only the central clock but also peripheral clocks in liver, pancreas, muscle and adipose tissue. Via the gluconeogenic pathway, the liver is the main source of endogenous glucose, and is thus the most plausible candidate for diurnal glucose control. Loss of hepatic circadian rhythms by liver-specific ablation of Bmal1 causes severe hypoglycemia during the active phase, suggesting that the liver clock is necessary for diurnal glucose homeostasis102. Moreover, hypothalamic clocks signal to the liver through the sympathetic and parasympathetic nervous system103, 104. It has been demonstrated that central administration of the GABAA receptor antagonist bicuculline induces activation of hypothalamic orexin neurons, which in turn triggers hepatic glucose production105. Orexins are critical modulators of the sleep/wake cycle as well as of energy and glucose metabolism106 and are thus thought to operating by connecting the clock and glucose rhythmicity. In fact, by signaling to orexin neurons in the perifornical area (PF) via the DMH, the SCN controls rhythmic release of orexin107, 108. Notably, levels of orexin in the cerebrospinal fluid (CSF) peak at the onset of the active phase109 alongside increase in hepatic glucose production. Recent evidence further supports the essential nature of the orexin system for glucose plasma rhythms: intracerebroventricular delivery of orexin modulates plasma glucose levels in a time-dependent manner by affecting cyclic hepatic gluconeogenesis110. Additionally, orexin-null mice, while maintaining a normal feeding behavior, completely loose daily rhythms in glucose levels110. In keeping with this observation, narcoleptic patients are characterized by a total lack of the orexin system111 and show features of metabolic syndrome including impaired glucose tolerance and obesity112, 113.
Metabolic feedback from peripheral tissues to the central clock
As discussed above, the SCN coordinates a wide array of physiological and metabolic processes, including feeding behavior. By driving 24-h feeding rhythms, the central clock regulates the nutritional status of peripheral organs. In turn, feeding schedule can function as potent timing cue for peripheral clocks, modulating local metabolism and signaling. Because the clock needs to continuously adjust to external stimuli to maintain correct timing of circadian physiology, it needs to rely on and integrate feedback signals from peripheral clocks. Indeed, it is thought that autonomous rhythms of the SCN are fortified and enhanced through central and peripheral feedback mechanisms (Figure 4).
Figure 4: Interplay between peripheral and central clocks.
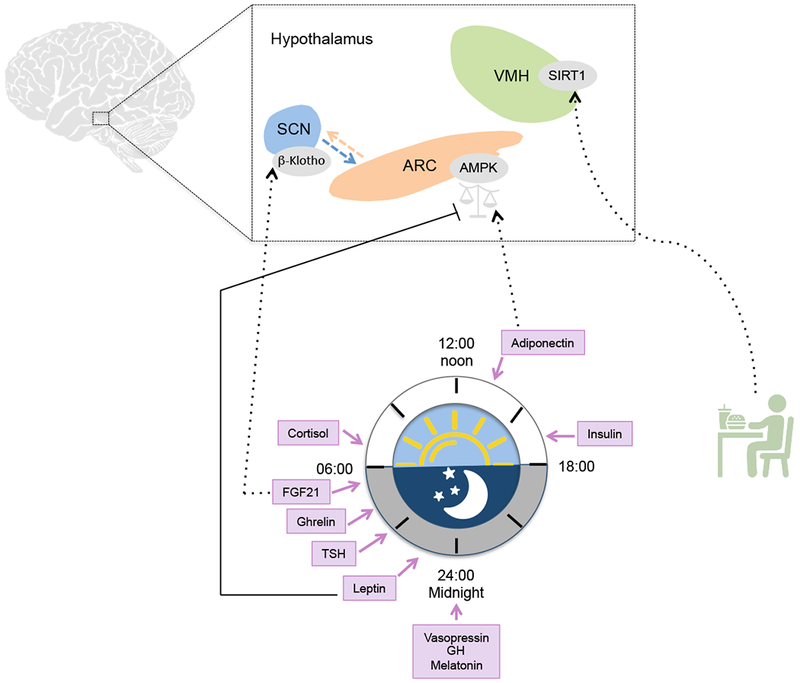
Endocrine signals are central mediators of circadian physiology and circulating levels of several endocrine factors oscillate over the course of the day, including cortisol, insulin, ghrelin, TSH, vasopressin, growth hormone (GH) and melatonin. Peripheral tissues coordinate the diurnal secretion of factors that provide positive and negative feedback inputs to the brain clocks. Hypothalamic diurnal activity of AMP-activated protein kinase (AMPK) is finely tuned via the opposite action of two adipokines: leptin and adiponectin. The metabolic regulator fibroblast growth factor 21 (FGF21) is secreted from the liver and modulates directly the central clock in the SCN by inducing activation of β–Klotho receptors. Oscillation of other humoral factors such as ghrelin may also provide peripheral feedback to central clocks. Metabolic cues are also conveyed to the brain through the action of the metabolic sensor SIRT1. In the ventromedial hypothalamus (VMH) SIRT1 is key for the adaptation of the central clock to feeding. ARC, arcuate nucleus. Dotted arrows indicate activation. Solid arrows indicate inhibition.
How peripheral metabolic signals may be conveyed to the central clock? The hypothalamus serves as the principal relay center for metabolic feedback from the periphery. AMP-activated protein kinase (AMPK) is an essential cellular energy sensor and within the hypothalamus AMPK is involved in regulating whole-body energy status114. Moreover, hypothalamic activity of AMPK is responsive to feeding-fasting cycles115. In the arcuate hypothalamic nucleus (ARC), AMPK activity is inhibited by leptin115, an anorexigenic hormone whose levels display diurnal variations116 and whose rhythms are governed by the clock in the adipose tissue117. More importantly, the central clock in the SCN finely tunes sensitivity of ARC neurons to circulating leptin117. Additionally to leptin another adipokine, namely adiponectin, exhibits diurnal rhythms in WAT and serum and is controlled in a circadian manner in adipose tissue118, 119. Via its receptor AdipoR1 expressed in the ARC, adiponectin is also able to elicit central effects that are similar but opposite to those induced by leptin. In fact, in the hypothalamus adiponectin activates AMPK and this activation is associated with increased food intake and suppressed energy expenditure120. The opposite nature of these two adipokines argues for a complementary role of leptin and adiponectin in driving rhythmic activation of AMPK. Accordingly, kinase activity of AMPK is robustly rhythmic in the hypothalamus121 as well as in the liver where it phosphorylates and triggers degradation of CRY1122. Activation of AMPK may be then conveyed to the SCN via neuronal projections from the ARC, which constitute the main communication route of metabolic information to the central clock39. Thus, a leptin/adiponectin/AMPK axis may signal metabolic feedback cues to the SCN via the ARC. In support of this scenario, targeted deletion of AMPK, as well as adiponectin and leptin receptor expressing neurons in the ARC, lead to profound disruption of feeding behavior42, 115, 120, 121. Additionally, AMPK regulates the mammalian-target-of-rapamycin (mTOR) pathway, which is a major nutrient sensing pathway that displays rhythmic activity in several tissues, including the SCN123. Notably, work in fruit flies124 and in mammals125 illustrates that manipulation of TOR/mTOR signaling directly affects the endogenous clock in the SCN, thereby linking nutrient sensing mechanisms to circadian timekeeping.
Another metabolic sensor capable of conveying metabolic inputs to the SCN is SIRT1. In peripheral tissues SIRT1 influences several metabolic processes by deacetylating key metabolic factors including proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), peroxisome proliferators activated receptor (PPAR)-γ and forkhead box O1 (FOXO1)126. SIRT1 also deacetylates the core clock proteins BMAL1 and PER2 and is thus intimately coupled to circadian regulation127, 128. This coupling is further strengthened by the fact that sirtuins use the cellular metabolite nicotinamide adenine dinucleotide (NAD+) as a cofactor, the levels of which oscillate and consequently define cyclic activity of SIRT1129, 130. SIRT1 is highly expressed in several hypothalamic nuclei where it plays a key role in energy homeostasis. In POMC neurons, SIRT1 is required for long-term control of body weight balance131 and in the ARC it regulates feeding behavior as well as body weight132. In support of a role of SIRT1 as a metabolic sensor communicating the nutritional status to the endogenous clock, SIRT1 expressed in the VMH has been shown to be crucial for the adaptation of the central clock to feeding cues133. This concept is further corroborated by the fact that hypothalamic SIRT1 is induced by fasting134, suggesting that activity of this deacetylase changes as a consequence of feeding-fasting cycles. Peripheral activity of SIRT1 may also modulate circadian function in the brain. Indeed, a study reports that SIRT1 controls the secretion of extracellular NAMPT (eNAMPT) from the adipose tissue into the circulation. Circulating eNAMPT appears to maintain NAD+ levels in the hypothalamus and adipose tissue-specific deletion of eNAMPT has profound effects on hypothalamic function. In line with the rhythmic activity of SIRT1, circulating levels of eNAMPT seem to display diurnal oscillation in mice135 and may thus affect rhythmic levels of NAD+ in the hypothalamus.
Feeding-fasting cycles can additionally modulate the levels of other metabolic regulators associated to the circadian system. Importantly, fasting rapidly increases the circulating levels of fibroblast growth factor 21 (FGF21), a metabolic regulator produced in the liver under the control of PPARα and that plays an central role in the adaptation to fasting136, 137. However, it is yet unclear whether hepatic expression and circulating concentrations of Fgf21 are rhythmic. A study reported that Fgf21 expression is circadian in the liver, peaking during the fasting phase and under direct control of the circadian machinery138. However, transactivation of Fgf21 by BMAL1/CLOCK was not confirmed by an independent study139 and serum levels of FGF21 are relatively stable under normal feeding regimens137, displaying a 24-h oscillatory pattern only following prolonged fasting140, 141. Nevertheless, FGF21 is able to modulate directly the central clock by activating β-Klotho signaling in the SCN. This activation induces reduction of insulin levels, increase of systemic corticosterone and changes in locomotor activity142. Therefore, FGF21 signaling appears to be critical for the central clock to detect and consequently gate adaptive responses to nutritional states.
Overall, how the SCN responds to peripheral metabolic signals remains ill-defined and needs further investigation. Nonetheless, experimental indications argue for a system where the SCN is reciprocally connected to other biological clocks via a complex network of output and feedback signals. These reciprocal interactions seem to be crucial for maintaining circadian rhythmicity and metabolic homeostasis. Misalignement and consequent loss of synchrony has pronounced consequences on health143.
Food and biological clocks
Food acts as a potent synchronizer for peripheral clocks. Restricting food access to rodents exclusively during the day shifts the phase of circadian gene expression within peripheral tissues, whereas the SCN remains entrained to the light-dark cycle144. Consequently, changes in feeding habits may lead to the uncoupling of peripheral tissues from the master clock, possibly causing metabolic alterations145. These metabolic perturbations are similar to those occurring in shift workers who are subjected to abnormal eating schedules and have a misalignment of the sleep/wake and feeding/fasting cycles to the light-dark cycle. Importantly, mounting data indicates that misalignment of the internal clocks can have negative effects on health and possibly contribute to the development of a variety of pathologies, including neurodegerative disorders.
Feeding time
Numerous evidences from mouse studies highlight the importance of feeding time for peripheral rhythmicity and metabolic fitness64 (Table 2). Mice receiving a high fat diet (HFD) display blunted rhythms in feeding behavior consuming the majority of food intake during the rest period. Changes in feeding behavior occur before the increase in body weight, arguing that circadian disruption is a key factor in the development of obesity and metabolic disease146. Accordingly, CLOCK mutant mice also exhibit increased food consumption during the day and have a larger predisposition to develop metabolic syndrome46. Rescue of CLOCK in the liver of mutant mice restores hepatic circadian rhythms as well as diurnal feeding rhythms and reduces their sensitivity to HFD147. Imposing a feeding rhythm to Cry1/Cry2-deficient mice by restricting food access exclusively to the night is sufficient to rescue hepatic transcriptional rhythms148. Similarly, Per1S714G mutated mice that exhibit altered feeding patterns and increased sensitivity to obesity, greatly benefit from restricting food access to nighttime52. Indeed restoring feeding/fasting rhythms with light/dark cycle has many metabolic benefits. In mice fed a HFD, time-restricted feeding (TRF) completely prevents and reverts the adverse metabolic effects of HFD even though the mice consume equivalent levels of calories149, 150. Further consolidating the implications of feeding time on metabolic fitness, 30% caloric restriction ad libitum has different outcomes on body weight depending on if the diet is consumed in the day-time or night, with the latter being associated with reduced body weight 151.
Table2.
Effects of dietary interventions and diurnal rhythms
| Dietary Intervention | Model | Circadian Phenotype | Molecular Effectors | Reference |
|---|---|---|---|---|
| Day-time restricted feeding | WT micecry1−/−; cry2−/− mice | Restored 24-h rhythms of gene expression in liver | Metabolic responsive TFs | 148 |
| Fat supplemented diet restricted to 12-h/day | WT Drosophila clk, cyc, per, tim mutant Drosophila | Increased amplitude of oscillating transcriptsImproved cardiac function | Endogenous clock | 177 |
| 5-h delay in meal time | Male human subjects | Phase delay of plasma glucose rhythmsPhase shift Per2 expression in adipose tissue | NR | 152 |
| HFD | WT mice | Lengthening of circadian periodBlunted rhythmic gene expression | NR | 146 |
| HFD | WT mice | Loss and gain of oscillation of transcripts and metabolites in the liver | BMAL1 PPARγ |
156 |
| HFD | WT mice Scap−/− mice Pparα−/− mice |
Remodeling of circadian enhancers Gain of oscillation of de novo lipogenesis and FA oxidation | SREBP PPARα |
180 |
| HFD restricted to 8-h/day | WT mice | Reduced BW gainImproved hepatic glucose metabolismReduced hepatic steatosisEnhanced oscillation of clock genes and of metabolic sensors | NR | 149 |
| DR | WT Drosophilaper, tim mutant Drosophila | Enhanced amplitude of cycling genesGain of oscillation of medium chain triglycerides | Endogenous clock | 178 |
| CR | Young and old WT mice | Enhanced amplitude of clock genes in liverIncreased protein acetylationChanges in NAD+ metabolism | SIRT1 | 159 |
| CR | Young and old WT mice | Phase advance of clock genes in SCs “Adult” like rhythmic gene expression in aged SCs | NR | 160 |
| KD | WT mice | Lower amplitude of clock genes in the brainHigher amplitude of clock genes in liverIncreased basal locomotor activity | NR | 169 |
| KD | WT mice CLOCK−/− mice | Loss of RER rhythmsGain of oscilation of liver cholesterol levelsGain of oscillation of free FAs in the gutEnhanced amplitude of CCGsGain of oscillation of serum βOHB levels | BMAL1 PPARα |
171 |
NR non reported; WT wild-type; TFs transcription factors; clk clock; cyc cycle; tim timeless; per period; BW body weight; NAD+ nicotinamide adenine dinucleotide; SCs stem cells; RER respiratory exchange ratio; FAs fatty acids; CCGs clock controlled genes; βOHB β–Hydroxybutyrate; HFD high-fat diet; DR dietary restriction; CR calorie restriction; KD ketogenic diet;
Recent evidence translates the murine observations on restricted feeding and circadian synchrony to humans. A 13-day laboratory protocol explored the effects of meal timing on human physiological rhythms. In this study, delaying mealtime by 5 hours did not affect daily rhythms of plasma melatonin and cortisol, indicating that the SCN was not affected. However, the authors were able to observe significant changes in the phase of plasma glucose rhythms as well as a 1-hour delay of Per2 expression in WAT152. Results from this study thus provide convincing evidence that meal timing can affect diurnal rhythms in peripheral tissues uncoupling them from the central clock in human subjects. In another study, the eating pattern of healthy adults was monitored and correlated with metabolic parameters such as caloric intake and BMI. Whereas there was no correlation between daily eating duration and BMI, restricting the eating interval from about 14 hours to 10 hours over 16 weeks led to a reduction in body weight as well as BMI153. Therefore, adjusting daily behavior favoring alignment of activity, food intake and light exposure can support beneficial outcomes on metabolic homeostasis.
Food composition
Besides to the time of feeding also the quality and quantity of food we consume can affect our circadian and metabolic physiology. There are a number of studies delineating how food composition can affect and rewire the molecular clock. We will provide a brief summary on how these nutritional regimens reprogram circadian biology.
High-fat diet
An early study had shown that feeding mice HFD ad libitum altered both the behavior as well as the expression of clock genes in peripheral tissues146. It has been shown that excessive caloric intake can affect the SCN leading to a disrupted response to light154, though it is yet unclear how nutritional cues affect the central clock. Remarkably, HFD rewires circadian metabolism in several tissues, including the medial prefontal cortex (mPFC) and the SCN in the brain155. In the liver HFD generates a widespread remodeling of the hepatic circadian clock, leading to profound reprogramming of specific metabolic pathways. Specifically HFD affects the liver circadian transcriptome and metabolome in three ways: abolition of previously oscillating transcripts and metabolites, shift in phase of metabolites that oscillated in both feeding conditions and induction of newly oscillating transcripts and metabolites. Mechanistically, HFD causes the impairment of CLOCK-BMAL1 chromatin recruitment at CCGs promoters, and induces de novo oscillation of surrogate transcriptional pathways, such for example the regulator PPARγ156. In the CNS activation of PPARγ is linked to energy balance regulation and contributes to the hyperphagic phenotype observed in animals fed HFD157. Interestingly the PPARγ ligand arachidonate is strongly increased in the serum of HFD-fed animals158; continuous activation of central PPARγ could explain why mice fed a HFD display dampening of their feeding rhythms.
Calorie restriction
Calorie restriction (CR) is best known for its beneficial effects on aging and lifespan. While aging in associated with changes in circadian physiology, how the aging process affects circadian rhythms remains poorly understood. Given that CR is able to reverse aging-associated homeostatic decay, it is a potent tool to try to dissect molecular mechanism occurring during aging and lifespan extension. This is exemplified by two studies that employed CR to investigate how aging influences circadian rhythms in peripheral tissues159, 160. Aging was associated with a tissue-specific rewiring of circadian function, mainly involving DNA repair mechanisms in adult stem cells160 and protein deubiquitination and cell-cycle biological processes in the liver159, whereas the expression of core clock genes remained unchanged in all the examined cell types. Moreover, the authors found that CR generated a widespread reprogramming of circadian transcription and that a portion of the reprogrammed genes overlapped with those oscillating in young mice. In the liver, CR exerted most of its action on NAD+ metabolism and global protein acetylation. Decline in total NAD+ levels is a common feature of aging and has been observed in peripheral tissues161 as well as in the brain162. Stressing the relationship between NAD+ and aging is the observation that supplementation with precursors that replenish the NAD pool can restore and reverse some aspects of age-associated decay163.
Most of the aspects of CR are mediated by the sirtuins. Accordingly, many hepatic genes regulated by CR overlapped with SIRT1 target genes, suggesting that the CR-mediated circadian rewiring may go through SIRT1159. Additionally to its role in regulating circadian physiology in peripheral tissues164, SIRT1 has also been implicated in circadian control in the SCN where it acetylates and consequently regulates the activity of BMAL1165. Aged mice show a decline of the levels of SIRT1 in the SCN that is associated with an impaired adaptation to a “jet lag” paradigm. This phenotype is rescued in mice with brain-targeted overexpression of SIRT1164. It would thus be of interest to explore the outcomes of CR on the central clock and SIRT1 activity in the SCN. One would predict that CR might modulate NAD metabolism also in the CNS, leading to activation of SIRT1 and improved entrainment fitness of the SCN. Intriguingly, a report suggests that CR may affect the temporal organization of the SCN and its response to light166. Moreover, CR was recently shown to generate a self-imposed temporal restriction of food intake as well as changes in locomotor activity151.
Ketogenic Diet
Ketogenic diet (KD) consists in a low carbohydrate and high-fat diet that induces endogenous ketogenesis. KD stimulates a metabolic response similar to that induced by fasting and calorie restriction: dependence on fatty acids and production of ketone bodies, which are used as an energy source by the brain and other tissues.
Ketone bodies can be sensed by the hypothalamus and stimulate food intake by inducing the expression of hypothalamic orexigenic neuropeptides167. Interestingly, ketone bodies released from the liver seem to be required for daily food anticipation via feedback to the hypothalamus168. Additionally, βOHB can modulate phase and amplitude of clock genes in the brain169.
Notably, ketone bodies are not only a source of energy, but can also have a role as epigenetic regulators. Specifically, β-hydroxyl-butyrate (βOHB) operates as an endogenous inhibitor of HDACs170, linking its changing levels to the degree of histone acetylation. Moreover, KD can modulate circadian oscillations in peripheral tissues via a βOHB-driven chromatin remodeling mechanism171. Indeed, robust serum βOHB oscillation induces rhythmic histone acetylation in the gut by inhibiting HDAC activity171. It is interesting to note that βOHB not only influences protein acetylation, but it also may function as a cofactor for another histone modification associated with transcriptional activation, namely β-hydroxyl-butyrylation. This newly discovered histone modification is induced upon fasting and it has been shown to mark several clock genes in the liver172.
KD is used in children to treat recurrent epilepsy. Recent evidences suggest that it could be additionally used as a therapeutic strategy for neuronal pathologies characterized by abnormal energy utilization, including neurodegenerative disorders like Alzheimer and Parkinson disease173. Accordingly, feeding mice KD for a long period of time improves memory in aged mice174. Of note, the presence of a functional circadian oscillator in the hippocampus, argues for a role of the clock in neuronal plasticity and memory formation175. This is further underscored by the fact that uncoupling of SCN oscillators leads to memory processing impairment22, 176. Whether health-promoting effects of KD in the brain are linked to central clock mechanisms remains to be elucidated.
Altogether, feeding regimens powerfully influence rhythmic transcription by enhancing and disrupting oscillations, or even by inducing de novo oscillations of otherwise non-cyclic genes. Yet, the specific contribution of the molecular clock to these processes is not fully elucidated. Two independent studies carried out in Drosophila melanogaster investigated whether the beneficial effects of TRF177 and dietary restriction (DR)178 on aging are dependent on a functional endogenous oscillator. Notably, flies carrying mutations for clock components showed attenuated responses to both dietary interventions, arguing that in Drosophila a functional clock is required for optimal response to feeding cues. Experiments in the mouse show that food-driven rhythmic gene under TRF seems to be in large part independent of a functional clock148. Additionally, a functional clock cannot generate rhythmic expression of nutrient responsive genes in the absence of nutritional cues148. Importantly, in the absence of a functional clock, TRF is not able to restore completely oscillation and amplitude of food-driven transcripts and KD-mediated circadian reprogramming of a subset of genes is blunted171. Thus, the endogenous clock is able to integrate nutritional cues. Therefore, food-driven circadian rewiring is likely to be mediated by both clock-independent metabolic regulators and the molecular clock, suggesting that adaptation to nutritional challenges involves the synergy of the two. Further investigations carried out on clock mutant animals and under free-running conditions are thus warranted to shed light on the reciprocal interaction between nutrition and endogenous clocks.
Conclusions and future directions
The past two decades have witnessed extensive advances in characterizing the molecular mechanisms of circadian clocks. While the connections between the clock and cellular metabolism have revealed some conceptually novel leads65, 179, the comprehension of how peripheral clocks connect to the brain and neuronal metabolism remains limited. In addition, the presence of several extra-SCN brain oscillators begs the question of how these connect to the SCN and possibly with the periphery. Recent technological advances have allowed editing circadian regulators in specific brain areas and cell types thus helping the identification of novel circadian circuits. In light of these recent discoveries, important questions need to be addressed: how are brain clocks aligned for proper cyclic function? Are these brain clocks operating in concert to convey messages to the periphery? How does this network of brain clocks respond to extrinsic inputs such as metabolic signals, drugs and food? We have highlighted a number of potential links coupling central brain clocks to metabolic signaling pathways. Studies aimed at investigating how brain clocks adapt to environmental cues will provide further insights on how this complex network operates. High-throughput approaches will again prove valuable to dissect the specificity, plasticity and communications between clocks.
Acknowledgements
We thank all members of the Sassone-Corsi laboratory for helpful discussion. Funding for C.M.G was provided by the National Cancer Institute of the US National Institutes of Health (NIH T32 2T32CA009054-36A1) and by European Research Council (ERC MSCA-IF-2016 MetEpiClock 749869). Financial support for P.S.-C. was provided by the National Institute of Health, the INSERM (Institut National de la Sante et de la Recherche Medicale, France), a KAUST-UCI partnership and a Novo Nordisk Challenge Grant.
Glossary
- Promoter element:
proximal DNA regulatory element immediately upstream of the transcription start site (TSS)
- Chromatin transitions:
Promoter elements are governed by specific epigenetic modifications that can shift from an “active” accessible state to a “repressed” state and vice versa.
- Oscillator:
timing system composed of transcriptional and translational feedback loops with an endogenous periodicity of approximately 24 hours
- Hyperphagic:
abnormal increase of food consumption
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Zhang R, Lahens NF, Ballance HI, Hughes ME & Hogenesch JB A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A 111, 16219–24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mure LS et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science 359 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Masri S & Sassone-Corsi P Plasticity and specificity of the circadian epigenome. Nat Neurosci 13, 1324–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehra A, Baker CL, Loros JJ & Dunlap JC Post-translational modifications in circadian rhythms. Trends Biochem Sci 34, 483–90 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crosio C, Cermakian N, Allis CD & Sassone-Corsi P Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci 3, 1241–7 (2000). [DOI] [PubMed] [Google Scholar]
- 6.Aguilar-Arnal L & Sassone-Corsi P Chromatin landscape and circadian dynamics: Spatial and temporal organization of clock transcription. Proc Natl Acad Sci U S A 112, 6863–70 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masri S & Sassone-Corsi P Sirtuins and the circadian clock: bridging chromatin and metabolism. Sci Signal 7, re6 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Mai JK, Kedziora O, Teckhaus L & Sofroniew MV Evidence for subdivisions in the human suprachiasmatic nucleus. J Comp Neurol 305, 508–25 (1991). [DOI] [PubMed] [Google Scholar]
- 9.Abrahamson EE & Moore RY Suprachiasmatic nucleus in the mouse: retinal innervation, intrinsic organization and efferent projections. Brain Res 916, 172–91 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Harmar AJ et al. The VPAC(2) receptor is essential for circadian function in the mouse suprachiasmatic nuclei. Cell 109, 497–508 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Aton SJ, Colwell CS, Harmar AJ, Waschek J & Herzog ED Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci 8, 476–83 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mieda M et al. Cellular clocks in AVP neurons of the SCN are critical for interneuronal coupling regulating circadian behavior rhythm. Neuron 85, 1103–16 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Mieda M, Okamoto H & Sakurai T Manipulating the Cellular Circadian Period of Arginine Vasopressin Neurons Alters the Behavioral Circadian Period. Curr Biol 26, 2535–2542 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Park J et al. Single-Cell Transcriptional Analysis Reveals Novel Neuronal Phenotypes and Interaction Networks Involved in the Central Circadian Clock. Front Neurosci 10, 481 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petit JM & Magistretti PJ Regulation of neuron-astrocyte metabolic coupling across the sleep-wake cycle. Neuroscience 323, 135–56 (2016). [DOI] [PubMed] [Google Scholar]
- 16.Iadecola C & Nedergaard M Glial regulation of the cerebral microvasculature. Nat Neurosci 10, 1369–76 (2007). [DOI] [PubMed] [Google Scholar]
- 17.Perea G, Navarrete M & Araque A Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32, 421–31 (2009). [DOI] [PubMed] [Google Scholar]
- 18.Prolo LM, Takahashi JS & Herzog ED Circadian rhythm generation and entrainment in astrocytes. J Neurosci 25, 404–8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yagita K, Yamanaka I, Emoto N, Kawakami K & Shimada S Real-time monitoring of circadian clock oscillations in primary cultures of mammalian cells using Tol2 transposon-mediated gene transfer strategy. BMC Biotechnol 10, 3 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Womac AD, Burkeen JF, Neuendorff N, Earnest DJ & Zoran MJ Circadian rhythms of extracellular ATP accumulation in suprachiasmatic nucleus cells and cultured astrocytes. Eur J Neurosci 30, 869–76 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prosser RA, Edgar DM, Heller HC & Miller JD A possible glial role in the mammalian circadian clock. Brain Res 643, 296–301 (1994). [DOI] [PubMed] [Google Scholar]
- 22.Barca-Mayo O et al. Astrocyte deletion of Bmal1 alters daily locomotor activity and cognitive functions via GABA signalling. Nat Commun 8, 14336 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is one of three studies demonstrating the central role of astrocyte signaling for circadian pacemaking in the SCN.
- 23.Brancaccio M, Patton AP, Chesham JE, Maywood ES & Hastings MH Astrocytes Control Circadian Timekeeping in the Suprachiasmatic Nucleus via Glutamatergic Signaling. Neuron 93, 1420–1435 e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is one of three studies demonstrating the central role of astrocyte signaling for circadian pacemaking in the SCN
- 24.Tso CF et al. Astrocytes Regulate Daily Rhythms in the Suprachiasmatic Nucleus and Behavior. Curr Biol 27, 1055–1061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This article is one of three studies demonstrating the central role of astrocyte signaling for circadian pacemaking in the SCN
- 25.Liu C & Reppert SM GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron 25, 123–8 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Albus H, Vansteensel MJ, Michel S, Block GD & Meijer JH A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Curr Biol 15, 886–93 (2005). [DOI] [PubMed] [Google Scholar]
- 27.Yoon BE, Woo J & Lee CJ Astrocytes as GABA-ergic and GABA-ceptive cells. Neurochem Res 37, 2474–9 (2012). [DOI] [PubMed] [Google Scholar]
- 28.Doengi M et al. GABA uptake-dependent Ca(2+) signaling in developing olfactory bulb astrocytes. Proc Natl Acad Sci U S A 106, 17570–5 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araque A et al. Gliotransmitters travel in time and space. Neuron 81, 728–39 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pellerin L & Magistretti PJ Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A 91, 10625–9 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz WJ & Gainer H Suprachiasmatic nucleus: use of 14C-labeled deoxyglucose uptake as a functional marker. Science 197, 1089–91 (1977). [DOI] [PubMed] [Google Scholar]
- 32.Dash MB, Bellesi M, Tononi G & Cirelli C Sleep/wake dependent changes in cortical glucose concentrations. J Neurochem 124, 79–89 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spanagel R et al. The clock gene Per2 influences the glutamatergic system and modulates alcohol consumption. Nat Med 11, 35–42 (2005). [DOI] [PubMed] [Google Scholar]
- 34.Clasadonte J, Scemes E, Wang Z, Boison D & Haydon PG Connexin 43-Mediated Astroglial Metabolic Networks Contribute to the Regulation of the Sleep-Wake Cycle. Neuron 95, 1365–1380 e5 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Myers MG Jr. & Olson DP Central nervous system control of metabolism. Nature 491, 357–63 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Abe M et al. Circadian rhythms in isolated brain regions. J Neurosci 22, 350–6 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]; This was one of the first studies to show that extra-SCN brain regions harbor an autonomous circadian oscillator
- 37.Williams KW & Elmquist JK From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15, 1350–5 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guzman-Ruiz M et al. The suprachiasmatic nucleus changes the daily activity of the arcuate nucleus alpha-MSH neurons in male rats. Endocrinology 155, 525–35 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Yi CX et al. Ventromedial arcuate nucleus communicates peripheral metabolic information to the suprachiasmatic nucleus. Endocrinology 147, 283–94 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Akabayashi A, Levin N, Paez X, Alexander JT & Leibowitz SF Hypothalamic neuropeptide Y and its gene expression: relation to light/dark cycle and circulating corticosterone. Mol Cell Neurosci 5, 210–8 (1994). [DOI] [PubMed] [Google Scholar]
- 41.Xu B, Kalra PS, Farmerie WG & Kalra SP Daily changes in hypothalamic gene expression of neuropeptide Y, galanin, proopiomelanocortin, and adipocyte leptin gene expression and secretion: effects of food restriction. Endocrinology 140, 2868–75 (1999). [DOI] [PubMed] [Google Scholar]
- 42.Li AJ et al. Leptin-sensitive neurons in the arcuate nuclei contribute to endogenous feeding rhythms. Am J Physiol Regul Integr Comp Physiol 302, R1313–26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiater MF et al. Circadian integration of sleep-wake and feeding requires NPY receptor-expressing neurons in the mediobasal hypothalamus. Am J Physiol Regul Integr Comp Physiol 301, R1569–83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chao PT, Yang L, Aja S, Moran TH & Bi S Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab 13, 573–83 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Erion R, King AN, Wu G, Hogenesch JB & Sehgal A Neural clocks and Neuropeptide F/Y regulate circadian gene expression in a peripheral metabolic tissue. Elife 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Turek FW et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–5 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Storch KF & Weitz CJ Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci U S A 106, 6808–13 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang S et al. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150, 2153–60 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feillet CA et al. Lack of food anticipation in Per2 mutant mice. Curr Biol 16, 2016–22 (2006). [DOI] [PubMed] [Google Scholar]
- 50.Zhang EE et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med 16, 1152–6 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iijima M et al. Altered food-anticipatory activity rhythm in Cryptochrome-deficient mice. Neurosci Res 52, 166–73 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Liu Z et al. PER1 phosphorylation specifies feeding rhythm in mice. Cell Rep 7, 1509–1520 (2014). [DOI] [PubMed] [Google Scholar]
- 53.Chappuis S et al. Role of the circadian clock gene Per2 in adaptation to cold temperature. Mol Metab 2, 184–93 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gerhart-Hines Z et al. The nuclear receptor Rev-erbalpha controls circadian thermogenic plasticity. Nature 503, 410–413 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delezie J et al. Rev-erbalpha in the brain is essential for circadian food entrainment. Sci Rep 6, 29386 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orozco-Solis R et al. The Circadian Clock in the Ventromedial Hypothalamus Controls Cyclic Energy Expenditure. Cell Metab 23, 467–78 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study identified a novel function of the VMH clock for circadian energy expenditure control
- 57.Refinetti R & Menaker M The circadian rhythm of body temperature. Physiol Behav 51, 613–37 (1992). [DOI] [PubMed] [Google Scholar]
- 58.Morrison SF, Madden CJ & Tupone D Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab 19, 741–756 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bartness TJ, Song CK & Demas GE SCN efferents to peripheral tissues: implications for biological rhythms. J Biol Rhythms 16, 196–204 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Guzman-Ruiz MA et al. Role of the Suprachiasmatic and Arcuate Nuclei in Diurnal Temperature Regulation in the Rat. J Neurosci 35, 15419–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Grayson BE, Seeley RJ & Sandoval DA Wired on sugar: the role of the CNS in the regulation of glucose homeostasis. Nat Rev Neurosci 14, 24–37 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lagerlof O et al. The nutrient sensor OGT in PVN neurons regulates feeding. Science 351, 1293–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li MD et al. O-GlcNAc signaling entrains the circadian clock by inhibiting BMAL1/CLOCK ubiquitination. Cell Metab 17, 303–10 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asher G & Sassone-Corsi P Time for food: the intimate interplay between nutrition, metabolism, and the circadian clock. Cell 161, 84–92 (2015). [DOI] [PubMed] [Google Scholar]
- 65.Panda S Circadian physiology of metabolism. Science 354, 1008–1015 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Masri S & Sassone-Corsi P The circadian clock: a framework linking metabolism, epigenetics and neuronal function. Nat Rev Neurosci 14, 69–75 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.LeSauter J, Romero P, Cascio M & Silver R Attachment site of grafted SCN influences precision of restored circadian rhythm. J Biol Rhythms 12, 327–38 (1997). [DOI] [PubMed] [Google Scholar]
- 68.Silver R, LeSauter J, Tresco PA & Lehman MN A diffusible coupling signal from the transplanted suprachiasmatic nucleus controlling circadian locomotor rhythms. Nature 382, 810–3 (1996). [DOI] [PubMed] [Google Scholar]
- 69.Lehman MN et al. Circadian rhythmicity restored by neural transplant. Immunocytochemical characterization of the graft and its integration with the host brain. J Neurosci 7, 1626–38 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guo H, Brewer JM, Champhekar A, Harris RB & Bittman EL Differential control of peripheral circadian rhythms by suprachiasmatic-dependent neural signals. Proc Natl Acad Sci U S A 102, 3111–6 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gamble KL, Berry R, Frank SJ & Young ME Circadian clock control of endocrine factors. Nat Rev Endocrinol 10, 466–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Spiga F, Walker JJ, Terry JR & Lightman SL HPA axis-rhythms. Compr Physiol 4, 1273–98 (2014). [DOI] [PubMed] [Google Scholar]
- 73.Simpson ER & Waterman MR Regulation of the synthesis of steroidogenic enzymes in adrenal cortical cells by ACTH. Annu Rev Physiol 50, 427–40 (1988). [DOI] [PubMed] [Google Scholar]
- 74.Buijs RM et al. Anatomical and functional demonstration of a multisynaptic suprachiasmatic nucleus adrenal (cortex) pathway. Eur J Neurosci 11, 1535–44 (1999). [DOI] [PubMed] [Google Scholar]
- 75.Ishida A et al. Light activates the adrenal gland: timing of gene expression and glucocorticoid release. Cell Metab 2, 297–307 (2005). [DOI] [PubMed] [Google Scholar]
- 76.Oster H et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab 4, 163–73 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Son GH et al. Adrenal peripheral clock controls the autonomous circadian rhythm of glucocorticoid by causing rhythmic steroid production. Proc Natl Acad Sci U S A 105, 20970–5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.So AY, Bernal TU, Pillsbury ML, Yamamoto KR & Feldman BJ Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proc Natl Acad Sci U S A 106, 17582–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Balsalobre A et al. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–7 (2000). [DOI] [PubMed] [Google Scholar]
- 80.Reddy AB et al. Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 45, 1478–88 (2007). [DOI] [PubMed] [Google Scholar]
- 81.Yamamoto T et al. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. J Biol Chem 280, 42036–43 (2005). [DOI] [PubMed] [Google Scholar]
- 82.Stimson RH et al. Acute physiological effects of glucocorticoids on fuel metabolism in humans are permissive but not direct. Diabetes Obes Metab 19, 883–891 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee P et al. Brown Adipose Tissue Exhibits a Glucose-Responsive Thermogenic Biorhythm in Humans. Cell Metab 23, 602–9 (2016). [DOI] [PubMed] [Google Scholar]
- 84.Brown SA, Zumbrunn G, Fleury-Olela F, Preitner N & Schibler U Rhythms of mammalian body temperature can sustain peripheral circadian clocks. Curr Biol 12, 1574–83 (2002). [DOI] [PubMed] [Google Scholar]
- 85.Buhr ED, Yoo SH & Takahashi JS Temperature as a universal resetting cue for mammalian circadian oscillators. Science 330, 379–85 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Saini C, Morf J, Stratmann M, Gos P & Schibler U Simulated body temperature rhythms reveal the phase-shifting behavior and plasticity of mammalian circadian oscillators. Genes Dev 26, 567–80 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tamaru T et al. Synchronization of circadian Per2 rhythms and HSF1-BMAL1:CLOCK interaction in mouse fibroblasts after short-term heat shock pulse. PLoS One 6, e24521 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Morf J et al. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science 338, 379–83 (2012). [DOI] [PubMed] [Google Scholar]
- 89.Borjigin J, Zhang LS & Calinescu AA Circadian regulation of pineal gland rhythmicity. Mol Cell Endocrinol 349, 13–9 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Slominski RM, Reiter RJ, Schlabritz-Loutsevitch N, Ostrom RS & Slominski AT Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol Cell Endocrinol 351, 152–66 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alonso-Vale MI et al. Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J Pineal Res 45, 422–9 (2008). [DOI] [PubMed] [Google Scholar]
- 92.Peschke E, Bach AG & Muhlbauer E Parallel signaling pathways of melatonin in the pancreatic beta-cell. J Pineal Res 40, 184–91 (2006). [DOI] [PubMed] [Google Scholar]
- 93.Peschke E et al. Receptor (MT(1)) mediated influence of melatonin on cAMP concentration and insulin secretion of rat insulinoma cells INS-1. J Pineal Res 33, 63–71 (2002). [DOI] [PubMed] [Google Scholar]
- 94.Muhlbauer E, Albrecht E, Hofmann K, Bazwinsky-Wutschke I & Peschke E Melatonin inhibits insulin secretion in rat insulinoma beta-cells (INS-1) heterologously expressing the human melatonin receptor isoform MT2. J Pineal Res 51, 361–72 (2011). [DOI] [PubMed] [Google Scholar]
- 95.Lima FB et al. Pinealectomy causes glucose intolerance and decreases adipose cell responsiveness to insulin in rats. Am J Physiol 275, E934–41 (1998). [DOI] [PubMed] [Google Scholar]
- 96.Picinato MC, Haber EP, Carpinelli AR & Cipolla-Neto J Daily rhythm of glucose-induced insulin secretion by isolated islets from intact and pinealectomized rat. J Pineal Res 33, 172–7 (2002). [DOI] [PubMed] [Google Scholar]
- 97.Tuomi T et al. Increased Melatonin Signaling Is a Risk Factor for Type 2 Diabetes. Cell Metab 23, 1067–1077 (2016). [DOI] [PubMed] [Google Scholar]
- 98.La Fleur SE Daily rhythms in glucose metabolism: suprachiasmatic nucleus output to peripheral tissue. J Neuroendocrinol 15, 315–22 (2003). [DOI] [PubMed] [Google Scholar]
- 99.la Fleur SE, Kalsbeek A, Wortel J, Fekkes ML & Buijs RM A daily rhythm in glucose tolerance: a role for the suprachiasmatic nucleus. Diabetes 50, 1237–43 (2001). [DOI] [PubMed] [Google Scholar]
- 100.Yamamoto H, Nagai K & Nakagawa H Role of SCN in daily rhythms of plasma glucose, FFA, insulin and glucagon. Chronobiol Int 4, 483–91 (1987). [DOI] [PubMed] [Google Scholar]
- 101.Ruiter M et al. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 52, 1709–15 (2003). [DOI] [PubMed] [Google Scholar]
- 102.Lamia KA, Storch KF & Weitz CJ Physiological significance of a peripheral tissue circadian clock. Proc Natl Acad Sci U S A 105, 15172–7 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nonogaki K New insights into sympathetic regulation of glucose and fat metabolism. Diabetologia 43, 533–49 (2000). [DOI] [PubMed] [Google Scholar]
- 104.Kalsbeek A, La Fleur S, Van Heijningen C & Buijs RM Suprachiasmatic GABAergic inputs to the paraventricular nucleus control plasma glucose concentrations in the rat via sympathetic innervation of the liver. J Neurosci 24, 7604–13 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yi CX et al. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes 58, 1998–2005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tsuneki H, Wada T & Sasaoka T Role of orexin in the regulation of glucose homeostasis. Acta Physiol (Oxf) 198, 335–48 (2010). [DOI] [PubMed] [Google Scholar]
- 107.Yoshida K, McCormack S, Espana RA, Crocker A & Scammell TE Afferents to the orexin neurons of the rat brain. J Comp Neurol 494, 845–61 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang S et al. Lesions of the suprachiasmatic nucleus eliminate the daily rhythm of hypocretin-1 release. Sleep 27, 619–27 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Gotter AL et al. The duration of sleep promoting efficacy by dual orexin receptor antagonists is dependent upon receptor occupancy threshold. BMC Neurosci 14, 90 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tsuneki H et al. Hypothalamic orexin prevents hepatic insulin resistance via daily bidirectional regulation of autonomic nervous system in mice. Diabetes 64, 459–70 (2015). [DOI] [PubMed] [Google Scholar]
- 111.Nishino S, Ripley B, Overeem S, Lammers GJ & Mignot E Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355, 39–40 (2000). [DOI] [PubMed] [Google Scholar]
- 112.Schuld A, Hebebrand J, Geller F & Pollmacher T Increased body-mass index in patients with narcolepsy. Lancet 355, 1274–5 (2000). [DOI] [PubMed] [Google Scholar]
- 113.Honda Y, Doi Y, Ninomiya R & Ninomiya C Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep 9, 254–9 (1986). [DOI] [PubMed] [Google Scholar]
- 114.Lopez M, Nogueiras R, Tena-Sempere M & Dieguez C Hypothalamic AMPK: a canonical regulator of whole-body energy balance. Nat Rev Endocrinol 12, 421–32 (2016). [DOI] [PubMed] [Google Scholar]
- 115.Minokoshi Y et al. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428, 569–74 (2004). [DOI] [PubMed] [Google Scholar]
- 116.Kalsbeek A et al. The suprachiasmatic nucleus generates the diurnal changes in plasma leptin levels. Endocrinology 142, 2677–85 (2001). [DOI] [PubMed] [Google Scholar]
- 117.Kettner NM et al. Circadian Dysfunction Induces Leptin Resistance in Mice. Cell Metab 22, 448–59 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barnea M, Chapnik N, Genzer Y & Froy O The circadian clock machinery controls adiponectin expression. Mol Cell Endocrinol 399, 284–7 (2015). [DOI] [PubMed] [Google Scholar]
- 119.Gavrila A et al. Diurnal and ultradian dynamics of serum adiponectin in healthy men: comparison with leptin, circulating soluble leptin receptor, and cortisol patterns. J Clin Endocrinol Metab 88, 2838–43 (2003). [DOI] [PubMed] [Google Scholar]
- 120.Kubota N et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab 6, 55–68 (2007). [DOI] [PubMed] [Google Scholar]
- 121.Um JH et al. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS One 6, e18450 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lamia KA et al. AMPK regulates the circadian clock by cryptochrome phosphorylation and degradation. Science 326, 437–40 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cao R et al. Translational control of entrainment and synchrony of the suprachiasmatic circadian clock by mTOR/4E-BP1 signaling. Neuron 79, 712–24 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zheng X & Sehgal A AKT and TOR signaling set the pace of the circadian pacemaker. Curr Biol 20, 1203–8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ramanathan C et al. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet 14, e1007369 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brooks CL & Gu W How does SIRT1 affect metabolism, senescence and cancer? Nat Rev Cancer 9, 123–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Asher G et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 134, 317–28 (2008). [DOI] [PubMed] [Google Scholar]
- 128.Nakahata Y et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 134, 329–40 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nakahata Y, Sahar S, Astarita G, Kaluzova M & Sassone-Corsi P Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science 324, 654–7 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ramsey KM et al. Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science 324, 651–4 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ramadori G et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 12, 78–87 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cakir I et al. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One 4, e8322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Orozco-Solis R, Ramadori G, Coppari R & Sassone-Corsi P SIRT1 Relays Nutritional Inputs to the Circadian Clock Through the Sf1 Neurons of the Ventromedial Hypothalamus. Endocrinology 156, 2174–84 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ramadori G et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci 28, 9989–96 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yoon MJ et al. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab 21, 706–17 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Badman MK et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5, 426–37 (2007). [DOI] [PubMed] [Google Scholar]
- 137.Galman C et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 8, 169–74 (2008). [DOI] [PubMed] [Google Scholar]
- 138.Tong X et al. Transcriptional repressor E4-binding protein 4 (E4BP4) regulates metabolic hormone fibroblast growth factor 21 (FGF21) during circadian cycles and feeding. J Biol Chem 285, 36401–9 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Chavan R et al. REV-ERBalpha regulates Fgf21 expression in the liver via hepatic nuclear factor 6. Biol Open 6, 1–7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Oishi K, Uchida D & Ishida N Circadian expression of FGF21 is induced by PPARalpha activation in the mouse liver. FEBS Lett 582, 3639–42 (2008). [DOI] [PubMed] [Google Scholar]
- 141.Andersen B, Beck-Nielsen H & Hojlund K Plasma FGF21 displays a circadian rhythm during a 72-h fast in healthy female volunteers. Clin Endocrinol (Oxf) 75, 514–9 (2011). [DOI] [PubMed] [Google Scholar]
- 142.Bookout AL et al. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat Med 19, 1147–52 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a nice example of how a peripheral metabolic regulator can modulate the central clock in the SCN
- 143.Bass J & Lazar MA Circadian time signatures of fitness and disease. Science 354, 994–999 (2016). [DOI] [PubMed] [Google Scholar]
- 144.Damiola F et al. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev 14, 2950–61 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mukherji A et al. Shifting eating to the circadian rest phase misaligns the peripheral clocks with the master SCN clock and leads to a metabolic syndrome. Proc Natl Acad Sci U S A 112, E6691–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kohsaka A et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6, 414–21 (2007). [DOI] [PubMed] [Google Scholar]
- 147.Meyer-Kovac J et al. Hepatic gene therapy rescues high-fat diet responses in circadian Clock mutant mice. Mol Metab 6, 512–523 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Vollmers C et al. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A 106, 21453–8 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Hatori M et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab 15, 848–60 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study is a nice demonstration of how feeding time can impact peripheral circadian rhythms and metabolic fitness
- 150.Chaix A, Zarrinpar A, Miu P & Panda S Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab 20, 991–1005 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Acosta-Rodriguez VA, de Groot MHM, Rijo-Ferreira F, Green CB & Takahashi JS Mice under Caloric Restriction Self-Impose a Temporal Restriction of Food Intake as Revealed by an Automated Feeder System. Cell Metab 26, 267–277 e2 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wehrens SMT et al. Meal Timing Regulates the Human Circadian System. Curr Biol 27, 1768–1775 e3 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that meal timing affects daily rhythms also in humans
- 153.Gill S & Panda S A Smartphone App Reveals Erratic Diurnal Eating Patterns in Humans that Can Be Modulated for Health Benefits. Cell Metab 22, 789–98 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mendoza J, Pevet P & Challet E High-fat feeding alters the clock synchronization to light. J Physiol 586, 5901–10 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Dyar KA et al. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell 174, 1571–1585 e11 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides a comprehensive analysis of circadian metabolic profiles across multiple tissues
- 156.Eckel-Mahan KL et al. Reprogramming of the circadian clock by nutritional challenge. Cell 155, 1464–78 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]; This study provides the first comprehensive evidence that nutritional challenges can rewire peripheral clocks
- 157.Ryan KK et al. A role for central nervous system PPAR-gamma in the regulation of energy balance. Nat Med 17, 623–6 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abbondante S, Eckel-Mahan KL, Ceglia NJ, Baldi P & Sassone-Corsi P Comparative Circadian Metabolomics Reveal Differential Effects of Nutritional Challenge in the Serum and Liver. J Biol Chem 291, 2812–28 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sato S et al. Circadian Reprogramming in the Liver Identifies Metabolic Pathways of Aging. Cell 170, 664–677 e11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Solanas G et al. Aged Stem Cells Reprogram Their Daily Rhythmic Functions to Adapt to Stress. Cell 170, 678–692 e20 (2017). [DOI] [PubMed] [Google Scholar]
- 161.Verdin E NAD(+) in aging, metabolism, and neurodegeneration. Science 350, 1208–13 (2015). [DOI] [PubMed] [Google Scholar]
- 162.Zhu XH, Lu M, Lee BY, Ugurbil K & Chen W In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc Natl Acad Sci U S A 112, 2876–81 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yoshino J, Baur JA & Imai SI NAD(+) Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metab (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Masri S et al. Partitioning circadian transcription by SIRT6 leads to segregated control of cellular metabolism. Cell 158, 659–72 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Chang HC & Guarente L SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell 153, 1448–60 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mendoza J, Drevet K, Pevet P & Challet E Daily meal timing is not necessary for resetting the main circadian clock by calorie restriction. J Neuroendocrinol 20, 251–60 (2008). [DOI] [PubMed] [Google Scholar]
- 167.Carneiro L et al. Evidence for hypothalamic ketone body sensing: impact on food intake and peripheral metabolic responses in mice. Am J Physiol Endocrinol Metab 310, E103–15 (2016). [DOI] [PubMed] [Google Scholar]
- 168.Chavan R et al. Liver-derived ketone bodies are necessary for food anticipation. Nat Commun 7, 10580 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Genzer Y, Dadon M, Burg C, Chapnik N & Froy O Ketogenic diet delays the phase of circadian rhythms and does not affect AMP-activated protein kinase (AMPK) in mouse liver. Mol Cell Endocrinol 417, 124–30 (2015). [DOI] [PubMed] [Google Scholar]
- 170.Shimazu T et al. Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science 339, 211–4 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Tognini P et al. Distinct Circadian Signatures in Liver and Gut Clocks Revealed by Ketogenic Diet. Cell Metab 26, 523–538 e5 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Xie Z et al. Metabolic Regulation of Gene Expression by Histone Lysine beta-Hydroxybutyrylation. Mol Cell 62, 194–206 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Paoli A, Rubini A, Volek JS & Grimaldi KA Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets. Eur J Clin Nutr 67, 789–96 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Newman JC et al. Ketogenic Diet Reduces Midlife Mortality and Improves Memory in Aging Mice. Cell Metab 26, 547–557 e8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Valnegri P et al. A circadian clock in hippocampus is regulated by interaction between oligophrenin-1 and Rev-erbalpha. Nat Neurosci 14, 1293–301 (2011). [DOI] [PubMed] [Google Scholar]
- 176.Fernandez F et al. Circadian rhythm. Dysrhythmia in the suprachiasmatic nucleus inhibits memory processing. Science 346, 854–7 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Gill S, Le HD, Melkani GC & Panda S Time-restricted feeding attenuates age-related cardiac decline in Drosophila. Science 347, 1265–9 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Katewa SD et al. Peripheral Circadian Clocks Mediate Dietary Restriction-Dependent Changes in Lifespan and Fat Metabolism in Drosophila. Cell Metab 23, 143–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Eckel-Mahan K & Sassone-Corsi P Metabolism and the circadian clock converge. Physiol Rev 93, 107–35 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Guan D et al. Diet-Induced Circadian Enhancer Remodeling Synchronizes Opposing Hepatic Lipid Metabolic Processes. Cell 174, 831–842 e12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Gallego M & Virshup DM Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol 8, 139–48 (2007). [DOI] [PubMed] [Google Scholar]
- 182.Ceglia N et al. CircadiOmics: circadian omic web portal. Nucleic Acids Res 46, W157–W162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


