Abstract
Long-range interactions between cortical areas are undoubtedly key to the computational power of the brain. For healthy human subjects the premier method for measuring brain activity on fast timescales is EEG, and coherence between EEG signals is often used to assay functional connectivity between different brain regions. However, the nature of the underlying brain activity that is reflected in EEG coherence is currently the realm of speculation, because seldom have EEG signals been recorded simultaneously with intracranial recordings near cell bodies in multiple brain areas. Here we take the early steps towards narrowing this gap in our understanding of EEG coherence by measuring local field potentials with microelectrode arrays in two brain areas (extrastriate visual area V4 and dorsolateral prefrontal cortex) simultaneously with EEG at the nearby scalp in rhesus macaque monkeys. Although we found inter-area coherence at both scales of measurement, we did not find that scalp-level coherence was reliably related to coherence between brain areas measured intracranially on a trial-to-trial basis, despite that scalp-level EEG was related to other important features of neural oscillations, such as trial-to-trial variability in overall amplitudes. This suggests that caution must be exercised when interpreting EEG coherence effects, and new theories devised about what aspects of neural activity long-range coherence in the EEG reflects.
Graphical Abstract
We measured local field potentials with microelectrode arrays in two brain areas (V4 and PFC) simultaneously with EEG at nearby scalp in rhesus macaque monkeys. Although we found inter-area coherence at both measurement scales, we found no evidence that long-range coherence at the scalp-level related to long-range coherence intracranially measured on the scale of our arrays. Our results suggest the need for new theories about what scalp EEG coherence tells us about underlying brain activity.
Introduction
Since Paul Broca’s pioneering studies of disfluent aphasias in the 1860s, neuroscientists have appreciated that different cortical areas are specialized for processing particular types of information (Broca, 1861a; b; Lorch, 2011). Coordination between functionally specialized cortical areas through long-range connections is critical for guiding complex cognitive behaviors, and the disruption of such coordination had been implicated in a number of neurological disorders, which have been collectively referred to as “disconnection syndromes” – a theory developed by neurologist Norman Geschwind that linked seemingly distinct neurological disorders (Geschwind, 1965b; a). These include Alzheimer’s disease (Delbeuck et al., 2003), schizophrenia (Friston & Frith, 1995), multiple sclerosis (Calabrese & Penner, 2007), attention deficit disorders (Niedermeyer & Naidu, 1997; Mazaheri et al., 2010), and autism spectrum disorders (Geschwind & Levitt, 2007; Melillo & Leisman, 2009), among others (Geschwind, 1965b; a; Catani & ffytche, 2005; Cronin-Golumb, 2010; Kleinschmidt & Vuilleumier, 2013).
Some sixty years after Broca’s discovery, the German psychiatrist Hans Berger recorded the first human electroencephalogram (EEG) using electrodes placed on the scalp (Berger, 1929). One of Berger’s earliest observations was the occurrence of salient band-limited oscillations in the EEG. Since that time, neuroscientists have hypothesized that oscillatory coherence may play a critical role in the functional connectivity between brain areas (Singer & Gray, 1995; Singer, 1999; Varela et al., 2001; Buzsaki & Draguhn, 2004; Fries, 2005; Tallon-Baudry, 2009; Hahn et al., 2014; Bastos et al., 2015; Fries, 2015; McLelland & VanRullen, 2016).
Disruption of typical patterns of coherence between the EEG measured at scalp sites is observed in human patients with disconnection syndromes (Leocani et al., 2000; Spencer et al., 2003; Babiloni et al., 2016; Duffy et al., 2017; Schwartz et al., 2017; Shou et al., 2017). In experiments with non-human primates, coherence in local field potentials (LFPs) between brain areas measured with microelectrodes positioned intracranially near cell bodies has been linked to a number of cognitive functions such as selective attention (Gregoriou et al., 2009), working memory (Salazar et al., 2012), and decision-making (Nacher et al., 2013). Despite these converging lines of evidence regarding the importance of oscillatory coherence between brain areas for cognition, there is currently no direct evidence about the precise ways in which coherence as measured at the scalp can be used to index the strength of correlation in the activity of underlying brain areas on a moment-to-moment basis. Some of the current open questions include: what type of brain activity might be reflected in coherence between EEG channels? Is it correlated spiking activity, coherence in local field potentials (LFPs), spike-field coherence or something else entirely? How strong is the relationship between such coherent brain activity and the coherence that can be measured at the scalp? What is the spatial scale of the brain structures that contribute to EEG coherence? Are the answers to the previous questions invariant across different brain regions and tasks contexts, or are they context-dependent? Here we take the first empirical steps towards answering these questions by comparing inter-area coherence measured with EEG electrodes on the scalp to LFP coherence measured simultaneously using microelectrode arrays implanted into cortex.
The current understanding of EEG makes three key predictions that we tested in the current study. The first prediction we tested concerns the type of signal which EEG coherence might reflect. It is commonly supposed that EEG signals are generated by post-synaptic potentials, via constructive summation of synaptic currents in the geometrically arranged apical dendrites of pyramidal neurons (Logothetis, 2003; Nunez & Srinivasan, 2006). Thus, one might reasonably predict that EEG coherence would most likely be a reflection of coherence between LFPs, which are also dominated by post-synaptic currents (Buzsaki et al., 2012). To test this prediction, we compared the coherence between EEG electrodes to the coherence of the LFPs recorded on microelectrode arrays implanted in nearby cortical tissue.
The second prediction we tested concerns the expanse of cortical activation that might contribute to coherent EEG signals. It is commonly supposed that EEG signals reflect coordinated activity of a reasonably expansive patch of contiguous cortex, since such coordination would provide the summation needed for small currents to be detectable at the scalp (Juergens et al., 1999; Nunez & Srinivasan, 2006). Our approach enabled us to tap into this issue by examining both coherence between single LFP channels in each brain area of interest, as well as coherence between each microelectrode array as a whole. It is thus sensible to anticipate that the average activity across an entire array might be more strongly related to what is measured by EEG, since this average activity represents the type of summation that is assumed to underlie EEG signals.
The third prediction we tested concerns the spatial specificity needed to guide inferences about the cortical contributors to coherence measured with EEG. It is generally accepted that focal current sources in the brain generate diffuse voltage topographies at the level of the scalp. This is supported both by computational modeling studies (Nunez & Srinivasan, 2006), and also by studies in which small focal currents of known location and intensity were injected directly into brain tissue (Tao et al., 2005; Ramantani et al., 2016). Given these observations, one straightforward prediction is that coherence between neural sources should be fairly estimable from a range of locations across the scalp. For this study, we used an EEG montage in which electrodes were close to (yet not directly above) the areas where we implanted our microelectrode arrays, which enabled us to test this prediction. If we found that intracranial LFP coherence was related to the coherence measured with these nearby EEG electrodes, this would support the notion that coherence between brain areas can be assayed via their diffuse voltage topographies. In contrast, if we were unable to relate intracranial LFP coherence to our scalp-level measurements, this would suggest that the inferences that can be made from EEG coherence estimates might require more precise spatial specificity than is true for other types of EEG metrics, such as evoked potentials.
Taking these predictions together, our current study sought to directly test the hypothesis that coherence between EEG electrodes at the scalp tracks the coherence in LFPs between cortical areas recorded intracranially on a moment-to-moment basis. Moreover, we aimed to quantify the strength of any such relationship in order to gauge the robustness of EEG coherence as an index of the type of intracortical coordination that we measured; to be useful, any such relationship would ideally be fairly reliable, beyond being merely statistically significant. To this end, we implanted two microelectrode arrays, each spanning 16 mm2 of cortex: one in extrastriate visual area V4 and the other in dorsolateral prefrontal cortex (PFC), areas which have each been implicated in the performance of cognitively demanding visual tasks (Petrides, 2005; Roe et al., 2012; Gregoriou et al., 2014). Then, we tasked animals with a difficult visual change-detection task and assayed whether coherence as measured with EEG electrodes near the brain areas of interest was related to coherence between the neural populations as recorded intracranially. We found that coherence between EEG electrodes was not strongly related to coherence in LFPs recorded from the underlying brain tissue, and in a battery of control analyses demonstrated that the introduction of a craniotomy did not have an appreciable impact on scalp EEG signals. We conclude that caution is warranted when inferring the underlying neural sources of coherence in the scalp EEG.
Materials and Methods
Experimental procedures were approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh and were performed in accordance with the United States National Institutes of Health’s guidelines for the care and use of laboratory animals.
Analysis Strategy.
Our general approach was to record LFPs from chronically implanted microelectrode arrays in V4 and PFC of macaque monkeys simultaneously with EEG recorded at their scalps to test whether the coherence between the average voltages on the arrays was related to the coherence between nearby EEG electrodes. To address this question we performed four main analyses: (1) we measured the strength of coherence between the relevant EEG electrodes; (2) we measured the strength of coherence between LFPs (both at short and long distances); (3) we measured the strength of coherence between LFPs in one brain area with the EEG signals recorded nearby; and (4) we tested whether coherence between EEG channels was correlated on a trial-to-trial basis with coherence between the LFPs in the nearby underlying brain areas.
The first two steps, measuring coherence between brain areas at each measurement scale, enabled us to get a sense of the functional connectedness of the brain regions of interest. If we found both pairs of signals consistently had weak coherence, then one might not expect to find a relationship between the two scales. If, however, both pairs of signals consistently, or even occasionally, had strong coherence, then we might expect these periods of strong coherence to be shared across the measurement scales. The third step is a more direct test of the link between signals measured at the scalp and those measured intracranially. If an EEG electrode was not coherent with the nearby underlying LFPs, then it would not be surprising if inter-area coherence was also not related across the measurement scales. However, such an observation would be an important contribution to our understanding of EEG signals by highlighting the tenuousness of generalizability between LFPs and the EEG, even if those LFPs are averaged over a sizeable patch of cortex. If, on the other hand, we found that EEG electrodes were coherent with nearby LFPs, this would have important implications. First, it would reinforce the notion that focal current sources in cortex generate diffuse scalp topographies, and indicate that the sensitivity of scalp EEG to moment-to-moment fluctuations in underlying current is robust across a range of scalp locations. Second, this tight temporal correspondence between measurement scales would make it more likely that scalp-level EEG coherence could be used to index coherence between nearby underlying LFPs. If we still failed to find a relationship in this case, it would suggest that the circumstances that link coherence measurements across recording scales are quite particular, and inferences from scalp-level measurements about the functional connectivity of intracranial sources should be made narrowly and carefully pending further research. The fourth step is the key direct test of the relationship between scalp-level EEG coherence and intracranial LFP coherence.
We considered that introducing craniotomies in order to implant microelectrode arrays might have dramatically altered the way current flows to the scalp, potentially affecting the generalizability of our findings to human subjects. Thus, we performed an additional analysis of the effect of craniotomy on the EEG. We compared the effect of craniotomy on the reliability of EEG signals to the reliability of EEG signals between different subjects and the reliability across repeated sessions within a subject. We reasoned that if the reliability of EEG signals before and after craniotomy fell in a comparable range of that for these other two, well-known sources of variation in EEG signals, then the impact of the craniotomy was tolerable.
Subjects.
We used four adult male rhesus macaques (Macaca mulatta) for this study. A replication of findings in at least two animals is the minimum to ensure reliable results. In the case of this study, all of the animals were engaged in other ongoing experiments that enabled EEG electrodes to be placed and these observations made with minimal additional effort. Since all of our monkey subjects were actively involved in multiple projects with different experimental constraints, each subject had a different subset of data available for the analyses in this report. For one subject (Monkey P) we did not record EEG before performing a craniotomy, so that subject could not be included in the analysis of the effect of craniotomy on EEG signals, but this subject was used to measure inter-area LFP coherence. Two other subjects (Monkeys R and B) only had microelectrode arrays implanted in one brain area, and so could not be included in the analysis of inter-area coherence, but were used to measure the effect of the craniotomy procedure on EEG signals. Data from Monkey W was included in all the analyses.
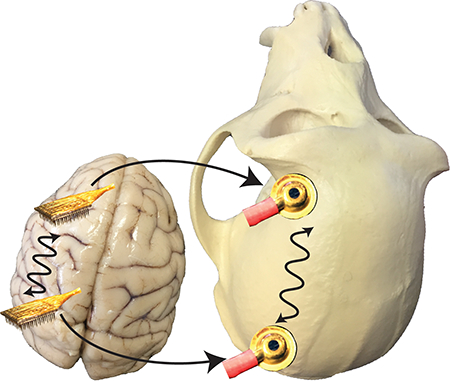
Surgeries were performed in aseptic conditions under isoflurane anesthesia. Opiate analgesics were used to minimize pain and discomfort perioperatively. A titanium head post was attached to the skull with titanium screws to immobilize the head during experiments. After each subject was trained to perform one or both of the behavioral tasks described below, we implanted one or two 100-electrode “Utah” arrays (Blackrock Microsystems, Salt Lake City, UT). Each animal was implanted with one array in visual area V4 using methods described previously (Smith & Sommer, 2013), and in two of the animals a second array in dorsolateral PFC of the same hemisphere. The PFC arrays were situated directly rostral to the arcuate sulcus and medial to the principle sulcus in cytoarchitectonic area 8ad (Petrides & Pandya, 1999). The implantation of each array involved introducing a craniotomy approximately 20 mm in diameter in the skull overlying the relevant cortical area (Figure 1), which we subsequently covered with titanium mesh secured with titanium screws. Monkey P and Monkey W were each implanted with two arrays: one in V4 and one in PFC. Both arrays were implanted in the right hemisphere for Monkey P and both arrays were implanted in the left hemisphere for Monkey W. Monkey R and Monkey B were implanted with arrays in V4 of the right hemisphere only. However, for these subjects we analyzed only the effect of craniotomy on EEG signals, and intracranial array data from these subjects are not included in this report as those recordings could not be used to assay inter-area coherence.
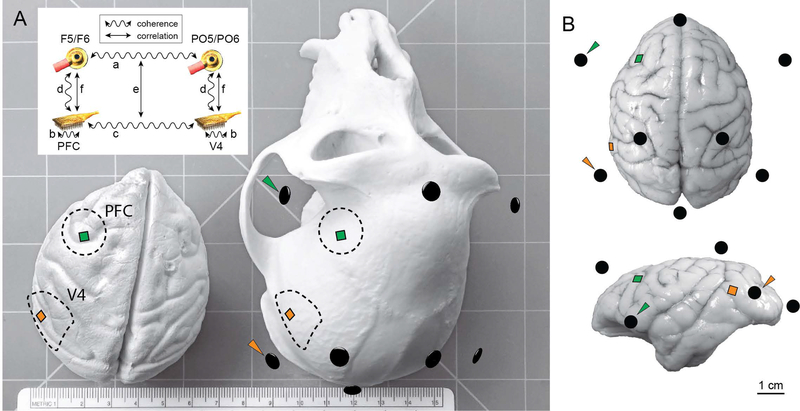
Figure 1.
Schematic of electrode arrays and EEG electrodes. A) Model of a rhesus macaque brain (left) and skull (right). Dotted lines indicate craniotomy locations for Monkey W (other subjects had craniotomies on their right sides). Squares represent electrode arrays in PFC and V4. Black ellipsoids correspond to EEG electrode locations from a representative session co-registered in three-dimensional space with anatomical landmarks on the skull, and arrowheads indicate EEG electrodes used in the coherence analysis. The ruler on the bench shows scale in centimeters (15.3 cm across). Inset depicts the analysis strategy. First, we analyzed coherence between EEG electrodes (a). Next, we analyzed coherence between LFPs both within (b) and between (c) arrays. We then analyzed coherence between the LFP arrays and the nearby EEG electrodes (d). Our key analysis was to measure trial-to-trial correlation between coherence estimates at the two measurement scales (e). We also measured trial-to-trial correlations of oscillatory amplitudes between measurement scales (f). B) Dorsal (top) and lateral (bottom) views of a rhesus brain superimposed with electrode locations. The brain depicted was not from a subject of this study and is only for illustration purposes; the array icons are positioned according to anatomical landmarks, which vary between individual animals.
Behavioral tasks.
Two tasks were used for the experiments described in this report: 1) a cognitively demanding, visual change-detection task; with the aim of eliciting interesting, task-relevant activity in both V4 and PFC; and 2) a simpler, passive visual stimulation paradigm, which was easier for the subjects to perform. Data from the visual change-detection task were used to test the relationship between inter-area EEG coherence and inter-area LFP coherence. Data from the passive visual stimulation task were used to assay the impact of craniotomy on EEG signals.
Visual change-detection task.
In brief: the subjects maintained central fixation as sequences of Gabor stimuli were presented in one or both of the visual hemifields, and were rewarded with water or juice for detecting a change in orientation of one of the stimuli in the sequence (the “target”) and making a saccade to that stimulus. The probable target location was block-randomized such that 90% of the targets would occur in one hemifield until the subject made 80 correct detections in that block, at which point the probable target location was changed to the opposite hemifield.
The fixation point was a 0.6° yellow dot at the center of a flat-screen cathode ray tube monitor positioned 36 cm from the subjects’ eyes. The background of the display was 50% gray. We measured monitor luminance gamma functions by photometer and linearized the relationship between input voltage and output luminance using lookup tables. We tracked the gaze of the subjects using an infrared eye tracking system (EyeLink 1000; SR Research, Ottawa, Ontario). Gaze was monitored online by the experimental control software to ensure fixation within ~1° of the central fixation point throughout each trial. We excluded data segments during which a subject’s gaze left the fixation window from analysis.
After fixating for a randomly-chosen duration of 300 to 500 ms (uniformly distributed), a visual stimulus was presented for 400 ms, or until the subjects’ gaze left the fixation window, whichever came first. For the initial trials within a block, a Gabor stimulus was presented only in the hemifield that was chosen to have a high probability of target occurrence for the block. These “cue” trials were to alert the subjects to a change in the probable target location and were excluded from the analysis. The initial cue location was counterbalanced across recording sessions. Once a subject correctly detected five orientation changes during the cue trials, bilateral Gabor stimuli were presented for the remainder of the block.
Each trial consisted of a sequence of 400 ms stimulus presentations separated by 300 – 500 ms interstimulus intervals (uniformly distributed). Stimulus sequences continued until the subject made an eye-movement, or a target was presented but the subject did not respond to it within 700 ms (i.e., a “miss”). For the first presentation in a sequence, the orientation of the stimulus at the cued location was randomly chosen to be 45 or 135 degrees and the orientation of the stimulus in the opposite hemifield, if present, was orthogonal to this. Subsequent stimulus presentations in the sequence each had a 40% probability (uniform hazard function) of containing a target, i.e. a change in orientation of one of the Gabor stimuli compared to the preceding stimulus presentations in the trial. Within a block, 90% of targets (randomly chosen) occurred in one hemifield (“valid” targets) and 10% of targets occurred in the opposite hemifield (“invalid” targets). For valid targets, the orientation change was randomly chosen to be 1, 3, 6, or 15 degrees in either the clockwise or anti-clockwise direction. For invalid targets, the orientation change was always the near-threshold value of 3 degrees, clockwise or anti-clockwise. We excluded target stimulus presentations from the analysis of neural data.
From the continuous recording we extracted data segments for analysis from 100 ms preceding each stimulus onset to 400 ms following stimulus onset. We analyzed six sessions of this task for Monkey W and 22 sessions for Monkey P.
Passive visual stimulation task.
In the passive visual stimulation task, subjects began each trial by fixating their gaze on a centrally-presented 0.6° blue dot for 200 ms. Then, a full-contrast sinusoidal grating stimulus was presented within a circular aperture 3.52° in diameter located 5.67° below the horizontal meridian and 11.61° to either the left (one third of trials) or right (one third of trials) of the fixation point for 600 ms. Because this task was performed before any intracranial arrays were implanted, the parameters and location of the grating stimuli were not optimized to drive any particular neurons in visual cortex. At the conclusion of the 600 ms stimulus presentation interval, the fixation point was moved 8.76° in a random direction, and the subjects were rewarded with water or juice for making a saccade to the new fixation location. As a control for effects of the task structure in the EEG that were unrelated to the visual stimulus, on one third of trials no grating stimulus was presented and the subject was required to maintain fixation for 600 ms, after which the fixation point moved and the subject was rewarded for making a saccade to the new fixation location.
Three monkeys performed the passive visual stimulation task. We excluded sessions with excessively non-stationary EEG data (see section “Benchmarking craniotomy effect sizes on VEPs and power spectra” below). Ultimately, we analyzed one session of this task from Monkey R before and after introducing a craniotomy over right V4; one session of this task from Monkey B before and three sessions after introducing a craniotomy over right V4; and three sessions of this task from Monkey W before and three sessions after introducing craniotomies over left V4 and PFC. Monkey W also performed the change-detection task described above.
Electrophysiological recordings.
We recorded signals from up to three electrode arrays: a Utah array implanted in V4, a Utah array implanted in PFC, and an array of eight Ag/AgCl EEG electrodes (Grass Technologies, Warwick, RI) adhered to the scalp with an electrically conductive paste. Signals from the arrays were band-pass filtered (0.3 – 250 Hz), digitized at 1 kHz and amplified by a Grapevine system (Ripple, Salt Lake City, UT). The EEG electrodes were positioned roughly according to a scaled version of the 10–20 system at these locations: Fz, Iz, CP3, CP4, F5 F6, PO7, and PO8 (Figure 1). Each electrode array had a separate reference electrode and was amplified by an electrically isolated port in the amplifier. The reference electrode for the EEG was attached with a steel screw to the titanium head post (roughly at position CZ in the 10–20 system). The intracranial arrays each had a reference wire positioned subdurally and distant from the corresponding recording sites; using separate references for the two arrays reduces biased overestimation of coherence that could result if a shared reference was used (Shirhatti et al., 2016). Because the intracranial reference wires were inside the skull, this also likely mitigated the potential “floating reference” contamination that might occur with a relatively electrically independent reference on the scalp (cf. Hu et al., 2007). We focused our analysis of the EEG data only on the pair of frontal and parietal electrodes ipsilateral to the Utah arrays (i.e., PO8 and F6 for Monkeys P, R and B, whose arrays were in the right hemisphere; and PO7 and F5 for Monkey W, whose arrays were in the left hemisphere), reasoning that the signals on these electrodes would be most likely to relate to the signals measured on the intracranial arrays because of their proximity. We also considered that measurements of EEG coherence between these electrodes might be affected by the use of the common head post reference (Hu et al., 2010). Signal processing techniques such as independent components analysis or the derivation of current source densities can ameliorate this potential issue (Hu et al., 2007; Shirhatti et al., 2016), but the sparsity of our electrode montage prohibited the application of these methods for this study. Another potential approach is to use bipolar references (Shirhatti et al., 2016), which we tested during a preliminary analysis. Specifically, we re-referenced each EEG electrode of interest to the nearby midline electrode (e.g., we referenced F5 to Fz and PO5 to Oz). We found that the results using bipolar references were qualitatively similar to what we observed using the common head post reference, albeit noisier and less reliable, likely attributable to the noise-doubling effect of the re-referencing procedure (Supplemental Figure 1). Thus, we proceeded with the initial common reference as the most straightforward approach.
Receptive field (RF) mapping.
Prior to beginning the visual change-detection experiment, we mapped the RFs of the spiking neurons recorded on the V4 arrays by presenting small (~1°) sinusoidal gratings at four orientations positioned one at a time on the vertices of a lattice covering the likely RF area per the anatomical location of the implant. We subsequently used a Gabor size and position to roughly cover the aggregate RF area. For Monkey P this was 7.02° full-width at half-maximum (FWHM) centered 7.02° below and 7.02° to the left of fixation, and for Monkey W this was 4.70° FWHM centered 2.35° below and 4.70° to the right of fixation. For each subject we used full-contrast Gabor stimuli with a temporal and spatial frequency that evoked a robust response from the population overall (i.e., our stimulus was not optimized for any single neuron). For the change-detection task, we presented a Gabor stimulus at the estimated RF location, at the mirror-symmetric location in the opposite hemifield, or at both locations simultaneously.
Coherence.
We estimated coherence using the multitaper method implemented by the Chronux Toolbox (Mitra & Bokil, 2008) for MATLAB (The Mathworks, Natick, MA). We calculated spectral densities for the time window starting 100 ms following stimulus onset and ending at stimulus offset (i.e., 100 – 400 ms for the change detection task, and 100 – 600 ms for the passive fixation task). Each data segment was tapered using the first five discrete prolate spheroidal sequences with a time-bandwidth product of 3. We then zero-padded the tapered data segments to 2048 samples, derived the two-sided Fourier transform, and then averaged the resultant spectra across the tapers used to derive spectral density estimates. Finally, we calculated the coherence as the magnitude of the ratio of the complex-valued cross-spectral density to the product of the two auto-spectral densities for each of pair of signals of interest.
Two brain areas that are not, in fact, functionally connected may appear coherent if they each respond consistently, albeit independently, to a visual stimulus. Therefore, we also examined a stricter coherence measurement that asked whether two signals were coherent beyond this amount that could be accounted for by the consistent yet independent activation of the two sources. Specifically, we subtracted from each coherence measure of interest an estimate of coherence calculated on surrogate data in which we randomly permuted the trial order for one of the two signals of interest. We refer to this trial-shuffle-corrected coherence estimate as “excess coherence” (Δ coherence). Significant excess coherence is a strong indication that the two signals are functionally related on a moment-to-moment basis. This approach is analogous to the use of shuffle correction in spike cross-correlograms (Smith & Kohn, 2008; Smith & Sommer, 2013), and removes coherence that could be attributed to consistent, but independent, stimulus responses. However, we should emphasize that a lack of excess coherence does not mean that the two signals are not coupled. It may still be the case that the signals are both coupled and also following a consistent time course across trials (i.e., both signals are phase-locked to the stimulus onset). To summarize, our trial-shuffled comparison is a stringent test that can rule in that two areas are functionally connected, but cannot determine that two areas are functionally independent.
Data pre-processing and artifact rejection for coherence analysis.
Because the estimation of coherence is strongly affected by the presence of artifacts that are shared across the signals being compared, we sought to exclude Utah array channels with low signal-to-noise ratios. We estimated these signal-to-noise ratios by first deriving the average visual-evoked local field potentials in response to the visual stimuli that we presented, and then calculating the ratio of the standard deviation during the visual response (50 to 350 ms post-stimulus onset) to the standard deviation in the baseline period (−100 to 0 ms pre-stimulus). We excluded the 20% of channels with the lowest signal-to-noise ratios, and averaged over the remaining channels to obtain a single voltage measurement for each Utah array. The 20% threshold was quite liberal (i.e., some relatively “clean” channels were discarded in addition to clearly noisy ones), and our results and conclusions were not meaningfully altered by moderate variation of the precise value of this rejection criterion.
To mitigate the effect of slow voltage drifts on the EEG signals, we subtracted the best-fitting linear trend for each channel on each data segment. Next, to identify excessively noisy EEG signals, we measured the standard deviation of the voltage on each of the eight EEG channels on each data segment. We rejected data segments for which any channel had a standard deviation less than 300 nanovolts (indicating a flat signal), or for which the standard deviation was outlying (determined as 10 standard deviations above the average for the entire session). We iteratively excluded data segments in which any channel had such outlying variance until no data segments met this criterion. This resulted in 95.7 ± 105.0 (mean ± SD over sessions) data segments excluded for Monkey W (1091.7 ± 930.0 data segments included in the analysis) and 136.3 ± 303.0 data segments excluded for Monkey P (1940.0 ± 561.0 data segments included). Our results and conclusions were not meaningfully altered by moderate variation of the precise values of these rejection criteria.
Visual-evoked potentials (VEPs).
To investigate the potential impact of craniotomy on the measurement of evoked EEG responses, we derived VEPs for the passive visual stimulation task using the Fieldtrip toolbox (Oostenveld et al., 2011) for MATLAB, and custom MATLAB scripts. First, we extracted data segments from the passive visual stimulation task from 2 s prior to stimulus onset to 2 s following stimulus onset. Next, we band-pass filtered the EEG data between 1 and 50 Hz using a fourth-order, two-pass Butterworth filter, then baseline-corrected each data segment by subtracting the average voltage over a window from −100 to 0 ms prior to stimulus onset and finally averaged over data segments. For plotting, we truncated the data segment to −50 ms preceding stimulus onset until stimulus offset (600 ms after stimulus onset). The number of data segments per session included in this analysis for each animal was (mean ± SD): Monkey R: 2160.5 ± 369.8; Monkey B: 1694.0 ± 540.6; Monkey W: 1327.3 ± 731.3. None of the available data segments was excluded from this analysis.
Power spectral analysis.
We also asked whether introducing craniotomies might have altered the spectral characteristics of the EEG. Unlike evoked potentials, which are only defined with respect to a specific event (such as a visual stimulus onset), the EEG power spectrum can be measured in ongoing EEG independent of task context. Thus, for the analysis of power spectra only (i.e., Figure 9), we divided the full continuous recordings from the passive visual stimulation task into non-overlapping 30 s segments (spanning across trial and inter-trial periods) and applied the FFT to each segment. This approach enabled us to use the maximum amount of data for the power spectral analysis and also provided high frequency resolution (0.03 Hz). We normalized the power spectrum of each segment to have a unit integral from 1 to 50 Hz, and then averaged over all segments for each recording session. The number of 30 s data segments per session included in the power spectral analysis for each animal was (mean ± SD): Monkey R: 247.5 ± 13.4; Monkey B: 223.0 ± 49.6; Monkey W: 226.8 ± 74.0. None of the available data segments was excluded from this analysis.
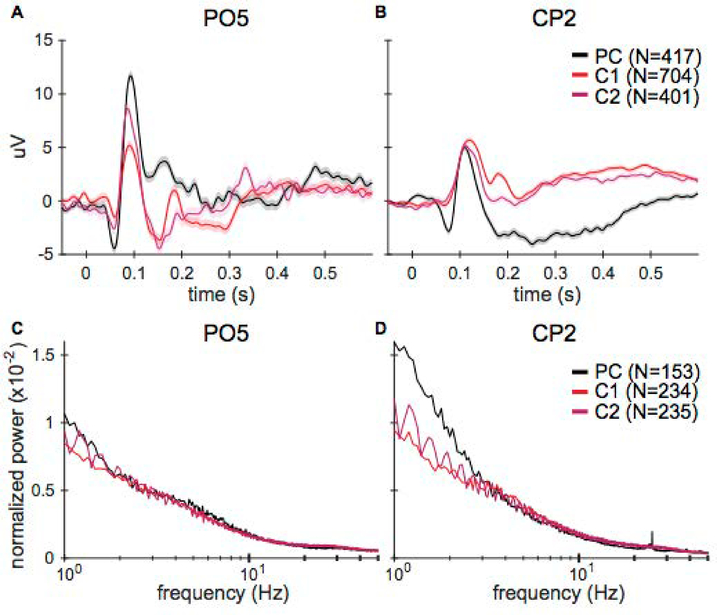
Figure 9.
VEP magnitudes differed on some electrodes before and after the craniotomy and between recording sessions. The overall power spectrum was similar for all sessions. A) VEPs of electrode PO5 for one subject (Monkey B) on a pre-craniotomy (PC) and two post-craniotomy sessions (C1, C2). Shading indicates ± 1 SEM (total number of trials per day is noted in the legend). The VEPs have similar time courses but there are magnitude differences between sessions. B) VEPs for the CP2 electrode. These VEPs have similar time courses and magnitudes. C-D) fast Fourier transforms (FFTs) of the EEG data on electrodes PO5 and CP2, computed by averaging the FFTs of 30 second data segments and normalizing by total average power. The frequency axis is logarithmically scaled. Standard error is within the lines shown (total number of segments per day is noted in the legend). The power across all frequencies is very similar between days and before and after surgery, especially for frequencies greater than 2 Hz.
Statistical methods.
To test the significance of coherence, we first subtracted the average coherence obtained when the trial order was randomly permuted for one of the two signals being compared from the observed coherence values. Then, we tested statistical significance with one-sample t-tests at each frequency point against a null-hypothesis of zero excess coherence with recording sessions as the degrees of freedom. Our overall type I error rate for the t-tests was α = 0.05, which we corrected for inflation due to multiple comparisons using Bonferroni’s method.
To estimate trial-to-trial correlation between coherence or amplitude values, we used Spearman’s rank correlation at each frequency point, applied Fisher’s r-to-z transformation to the resulting coefficient values, and then smoothed the resulting correlation coefficients over frequency using a 1.95 Hz moving average. We tested statistical significance with one-sample t-tests at each frequency point against a null-hypothesis of zero correlation with recording sessions as the degrees of freedom. Our overall type I error rate for the t-tests was α = 0.05, which we corrected for inflation due to multiple comparisons using Bonferroni’s method. Note that since the trial-shuffled correction for coherence values involves only subtracting a constant value at each frequency point, this procedure has no effect on correlation coefficients.
Benchmarking craniotomy effect sizes on VEPs and power spectra.
To determine whether differences between EEG VEPs recorded before craniotomy and those recorded after craniotomy were best explained by the craniotomy or other sources of variability, we calculated the effect of three sources of variability in the EEG: (1) trial-to-trial differences within a session, (2) day-to-day differences between sessions, and (3) differences due to the visual stimulus location (left versus right hemifield). Then, we compared the variability in pre-craniotomy (PC) versus craniotomy (C) sessions to the magnitude of variability due to the other sources. We used Pearson’s correlation (details below) as a measure of similarity between pairs of VEPs.
Before computing correlations, we performed two additional preprocessing steps. First, to control for non-visual-stimulation-related signals (e.g., stereotyped eye movements to achieve fixation in the baseline), we subtracted the average response to the blank condition (trials where no stimulus was shown) from each trial on which a visual stimulus was presented. Then, to account for day-to-day variability in the signal-to-noise ratio of our EEG recordings, we calculated a z-score equivalent for each trial using the mean and standard deviation of the voltage 100 ms prior to stimulus onset in the trial-averaged response.
Since these correlations were a measure of similarity in our data, we grouped them according to the source of variation in the VEP pair: within-session, between-sessions, between-stimuli (left vs. right), and craniotomy. The correlations measuring within-session similarity included all correlations between VEP pairs of trial subsets from the same recording session (i.e., a random partitioning of trials from each session). The between-session similarity correlations compared VEP pairs where both trial subsets were recorded before the craniotomy or both were recorded after the craniotomy. We only had repeated recording sessions for Monkey B (3 craniotomy sessions) and Monkey W (3 pre-craniotomy sessions and 3 craniotomy sessions), so we could not compute between-session similarity for Monkey R. The correlations that measured similarity between stimulus locations included correlations between opposite hemifield stimuli VEPs for every trial subset, regardless of whether the trial subset was collected before or after the craniotomy. These comparisons were the only correlations between VEPs for different stimuli. Finally, the correlations reflecting similarity before and after craniotomy included all comparisons between a pre-craniotomy VEP trial subset and a craniotomy VEP trial subset. For each comparison group, we found the mean of the r-to-z-transformed correlations within the group and 95% bootstrap confidence intervals. To bootstrap confidence intervals for correlations between pairs of VEPs, we randomly divided the full set of z-scored trials from each session into two subsets and calculated a VEP for each subset. Then, we computed the correlation between pairs of VEPs for comparisons of interest. We repeated this procedure 1000 times for each comparison, and performed Fisher’s r-to-z transformation on each correlation coefficient before finding the mean across iterations. Finally, we applied the inverse z-to-r transformation on the mean values for presentation purposes. Data from sessions that had low average within-session correlations (r < 0.5), indicating excessive variability across trials, were deemed to have excessively non-stationary EEG data and were excluded from analysis (2 of 8 sessions excluded from Monkey W, leaving the 6 sessions listed above). An equivalent analysis was performed to assess craniotomy effect sizes on FFTs of the EEG data from pre-craniotomy and craniotomy sessions. Since the FFTs were evaluated over multiple trials regardless of stimulus, there was no between-stimuli comparison.
Results
We recorded local field potentials (LFPs) from chronically implanted Utah arrays in V4 and PFC of macaque monkeys simultaneously with EEG recorded at their scalps to test whether the coherence between the average voltages on the arrays was related to the coherence between nearby EEG electrodes. To address this question we performed four main analyses: (1) we tested that the relevant EEG electrodes were, in fact, coherent; (2) we tested for coherence between LFPs (both at short and long distances); (3) we tested whether LFPs in one brain area were coherent with the EEG signals recorded nearby; and (4) we tested whether coherence between EEG channels was correlated on a trial-to-trial basis with coherence between the LFPs in the nearby underlying brain areas.
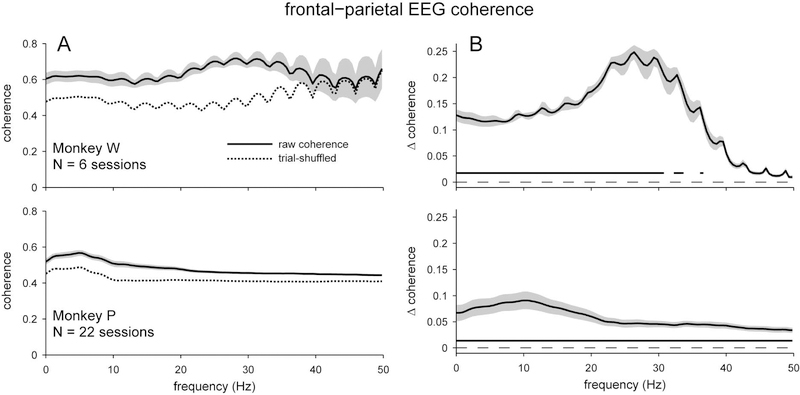
We found that the EEG electrodes of interest were more coherent with each other than would be expected if the trial order was randomly shuffled independently for the two signals (Figure 2). Both subjects had greater excess coherence at low frequencies (< 40 Hz), which diminished with increasing frequency. We noted intriguing inter-individual differences, also. For example, Monkey W had much greater excess coherence (peaking around a value of 0.25) than Monkey P (peaking around a value of 0.10). Also, excess coherence peaked for Monkey W around the beta band (25 Hz), whereas excess coherence peaked for Monkey P around the alpha band (10 Hz). Despite these substantial differences, the idiosyncratic features of the coherence spectra were fairly consistent across repeated sessions within an individual (standard errors in Figure 2 are across sessions, see also Figure 11 which shows separate traces for repeated sessions). This result is in line with the finding that, for human subjects, spectral features of the EEG are both distinct enough between subjects and reliable enough within subjects to be able to identify specific individuals (Napflin et al., 2007; La Rocca et al., 2014).
Figure 2.
Coherence between frontal and parietal scalp EEG electrodes during visual change-detection task. A) Overall coherence (solid), and the average coherence when trial order was shuffled (dashed). B) Excess coherence (Δ coherence) when the trial-shuffled value was subtracted from the overall coherence. Both subjects had significant excess coherence, particularly at frequencies < 30 Hz. Shading represents ± 1 SEM. Underlining represents frequencies with significant, Bonferroni-corrected, one-sample t-test results at α = 0.05.
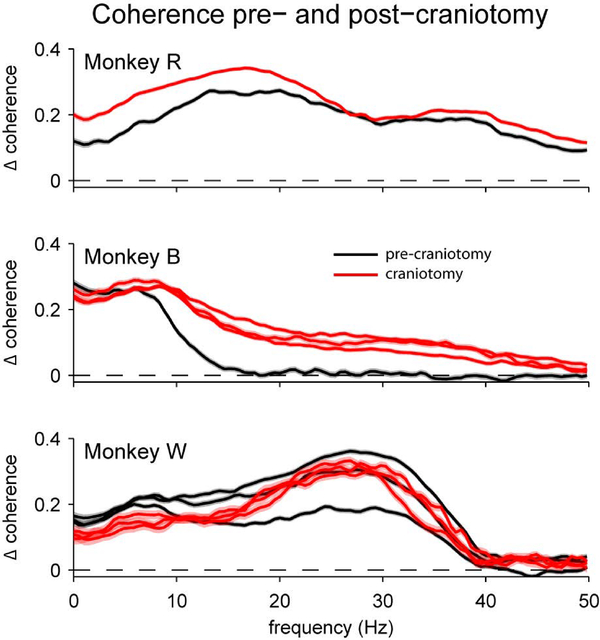
Figure 11.
Effect of craniotomy on frontal-parietal EEG coherence. While some effects of the craniotomy are evident (e.g., higher coherence for Monkeys R and B), the magnitude of these effects appear lesser than the effects of inter-individual variability in the coherence spectra, or in some cases, test-retest reliability within a subject (e.g., for Monkey W). Each line represents a separate recording session. Shading represents ± 1 SEM over trials within a session.
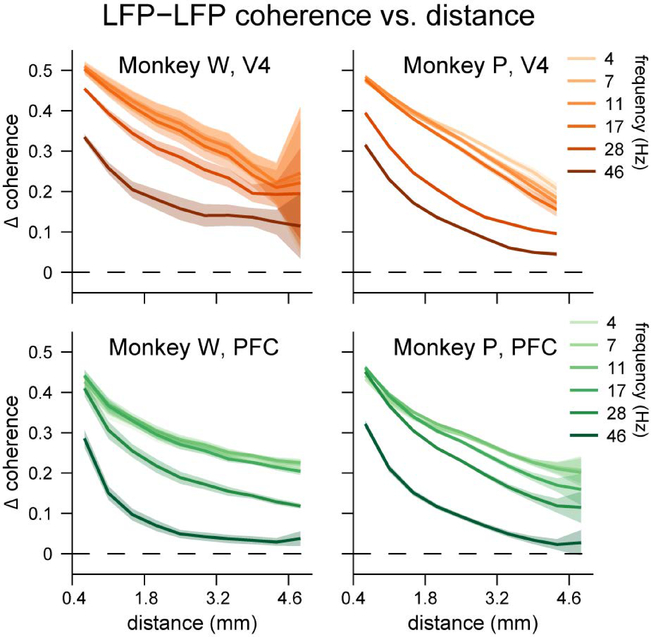
Next we measured the strength of coherence between LFPs recorded on different channels within a given microelectrode array. We found substantial excess coherence between channels within an array (Figure 3), again, relative to a trial-shuffled control analysis (see Supplemental Figure 2 for raw coherence). Coherence was greatest at low frequencies and at close proximity, and decayed roughly exponentially with increasing distance or frequency. Moreover, the rate of decrease in coherence over distance seemed to accelerate with increasing frequency. At around 46 Hz, coherence did not exceed the trial-shuffled values for the most distant pairs of electrodes on the arrays (those separated by 5 mm); but even at frequencies as low as 4 Hz, excess coherence diminished from a peak of around 0.5 for adjacent electrodes by nearly half to around 0.25 for the most distant pairs of electrodes. If spatial proximity was the only determinant of inter-electrode coherence, then given these rates of decay, one would expect negligible excess coherence between the two arrays, which were separated by more than three centimeters (Figure 1). In contrast, if excess coherence were found between the arrays, this would be a strong indicator of the functional connectedness of the two brain areas.
Figure 3.
Excess coherence (Δ coherence) between electrodes within a brain area. A-B) Excess coherence between electrodes in V4. C-D) Excess coherence between electrodes in PFC. For both brain areas we binned pairs of electrodes based on the distance between them, and averaged coherence values within each distance bin. Excess coherence within each brain area was greatest at low frequencies and at close proximity, and decreased with increasing frequency or distance. Shading represents ± 1 SEM.
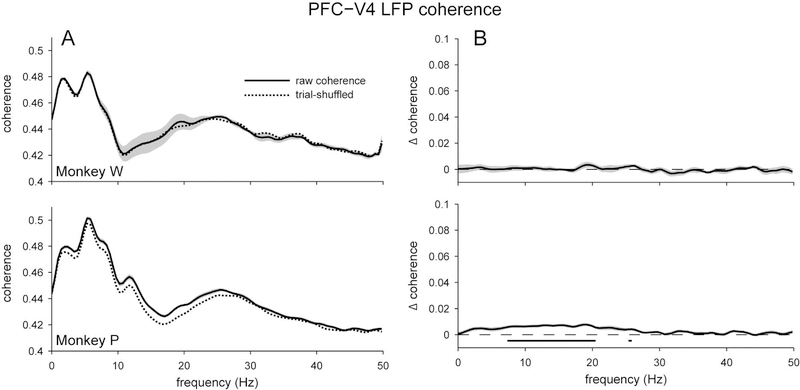
When we examined LFP coherence between the two arrays, we found strong inter-area coherence (Figure 4a). The coherence spectra were similar for the two subjects, with the greatest peak in the theta band (~7 Hz), and a smaller peak around the beta band (~25 Hz). We also found significant excess coherence, albeit only for Monkey P (Figure 4b). This excess coherence was around the alpha band (~10 Hz), and was a relatively small effect (Δ coherence = 0.0077) compared to the excess coherence that we observed for the EEG signals (Δ coherence = 0.09). This measurement was made by averaging the LFPs across the channels of each array; we also performed an analysis in which we searched for the pair of individual electrodes from each brain area with the greatest excess coherence, and found that the effect size was similarly weak (Supplementary Figure 3). Despite the small effect size, the presence of excess coherence supports the hypothesis that the two brain areas of interest were indeed functionally connected.
Figure 4.
Coherence between local field potentials (LFPs) recorded intracranially in V4 and PFC. A) Overall coherence (solid), and the average coherence when trial order was shuffled (dashed). Both subjects showed strong inter-area coherence and had similar coherence spectra, with peaks in the theta and beta bands. B) Excess coherence (Δ coherence) when the trial-shuffled value was subtracted from the overall coherence. For both subjects, there was little excess coherence between brain areas, especially when compared to the magnitude of coherence between nearby EEG electrodes (Figure 2). For Monkey P, significant excess coherence was found between 7 to 20 Hz, whereas no significant excess coherence was found for Monkey W. Shading represents ± 1 SEM. Underlining represents frequencies with significant, Bonferroni-corrected, one-sample t-test results at α = 0.05.
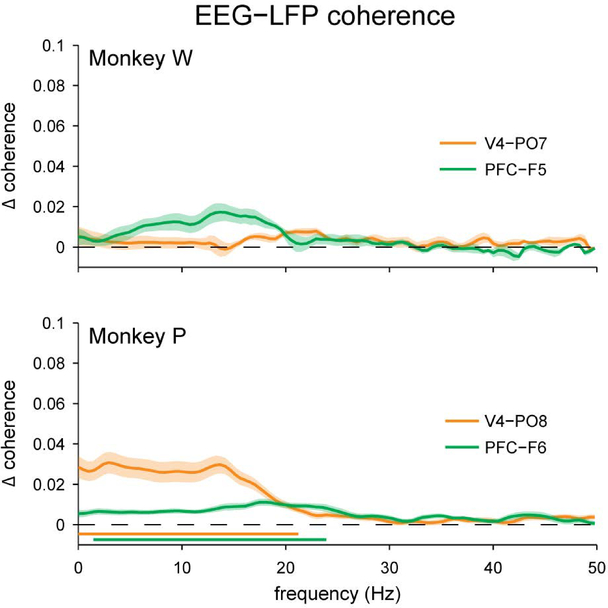
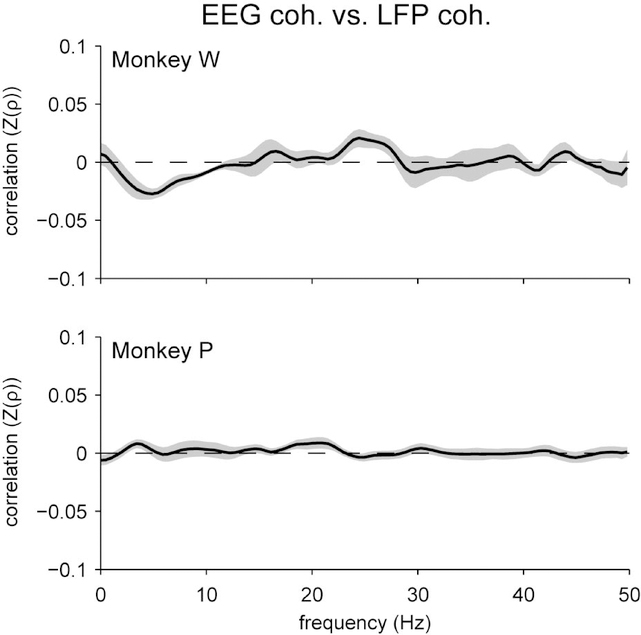
That long-range LFP-LFP coherence was so much lesser than EEG-EEG coherence suggested that EEG signals might not be tightly coupled to nearby LFP signals. We confirmed this by measuring the excess coherence between the average LFP signal in each brain area and the nearby EEG electrode. We found that EEG-LFP coherence was not much greater than the trial-shuffled case (Figure 5; Supplemental Figure 4); this excess coherence was significant only for Monkey P. It is important to note, however, that for this subject EEG-LFP coherence peaked in the same frequency range (< 25 Hz) in which we observed significant EEG-EEG coherence and significant LFP-LFP coherence. Since this excess coherence indicates that EEG signals can be related to nearby LFPs on a moment-to-moment basis, one might predict it would be possible to also relate coherence between the two measurement scales from moment-to-moment. This prediction needs to be explicitly tested, however, as the relationship between EEG and nearby LFPs may be not be robust enough to index inter-area coherence in practice. In fact, when we directly measured the trial-to-trial correlation between inter-area EEG coherence and inter-area LFP coherence, we did not find any statistically significant relationship for either subject (Figure 6). This was true even if we specifically selected pairs of individual LFP channels from each brain area that had the strongest coherence for each session (Supplementary Figure 5a), or if we restricted our analysis to the 20% of trials from each session with the greatest inter-area LFP coherence (Supplementary Figure 5b), which suggests that our failure to find a relationship between coherence measurements at the two recording scales could not be attributed merely to weak inter-area coherence.
Figure 5.
Excess coherence (Δ coherence) between local field potentials measured intracranially and EEG signals measured nearby at the scalp. For both subjects, there was little excess coherence between local field potentials and nearby scalp EEG, especially when compared to the magnitude of coherence between EEG electrodes (Figure 2). For Monkey P, significant excess coherence was found at frequencies below 24 Hz, whereas no significant coherence was found for Monkey W. Shading represents ± 1 SEM. Underlining represents frequencies with significant, Bonferroni-corrected, one-sample t-test results at α = 0.05.
Figure 6.
Spearman’s rank correlation between frontal-parietal coherence measured at the scalp with EEG and PFC-V4 coherence measured intracranially with local field potentials. We did not find any significant trial-to-trial correlation between coherences at these two measurement scales. Fisher’s r-to-z transformation was applied to the correlation coefficients prior to averaging, signified as “Z(ρ)”. Shading represents ± 1 SEM.
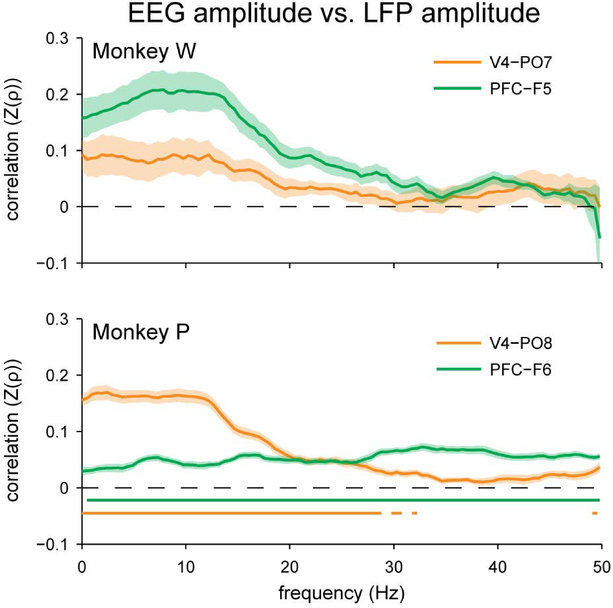
It was not the case, however, that oscillatory features in the EEG were completely unrelated to LFP oscillations measured intracranially on a trial-to-trial basis. When we measured the correlation between the amplitude of oscillations at the scalp and intracranially we found a substantial correlation at both recording sites of interest (Figure 7). This is important not only because it demonstrates our ability to detect some relationship between EEG and LFP oscillations, but because amplitude correlations are also a contributor to the measurement of phase coherence (Lachaux et al., 1999; Srinath & Ray, 2014). Nevertheless we were unable to reliably link coherence across the two measurement scales. Although these amplitude correlations were significant after correction for multiple comparisons only for Monkey P, this general result replicates a previously published finding by our group in two other monkeys (Snyder & Smith, 2015). Thus, while EEG can reliably reflect important oscillatory features of nearby underlying cortex such as overall amplitude, it seems that the link between inter-area coherence in EEG and LFPs might not be as robust, at least on the scale of our 16 mm2 microelectrode arrays.
Figure 7.
Spearman’s rank correlation between EEG amplitude measured at the scalp and local field potential amplitude measured intracranially. Compared to the results for coherence (Figures 4 and 5), there was a stronger trial-to-trial relationship between the amplitude of local field potential oscillations and the amplitude of nearby EEG oscillations, particularly at low frequencies. This relationship was significant for Monkey P, but did not survive Bonferroni correction for Monkey W. Fisher’s r-to-z transformation was applied to the correlation coefficients prior to averaging, signified as “Z(ρ)”. Shading represents ± 1 SEM. Underlining represents frequencies with significant, Bonferroni-corrected, one-sample t-test results at α = 0.05.
A salient difference between our EEG recordings and those that are more typically used with human subjects is that we introduced craniotomies in order to implant microelectrode arrays, and this may raise concerns that the alteration of the skull may have fundamentally changed the nature of the EEG signal that we recorded and its relation to underlying neural activity. In anticipation of this potential concern, we collected EEG data from three subjects using a passive visual stimulation paradigm before and after the craniotomy procedure. We then analyzed three key features of the EEG signals to assay the magnitudes of effects that the craniotomy may have had on our measurements: 1) the time-course, amplitude and topography of the VEP; 2) the overall power spectrum; and 3) the coherence between the EEG electrodes nearest the eventual microelectrode array implants.
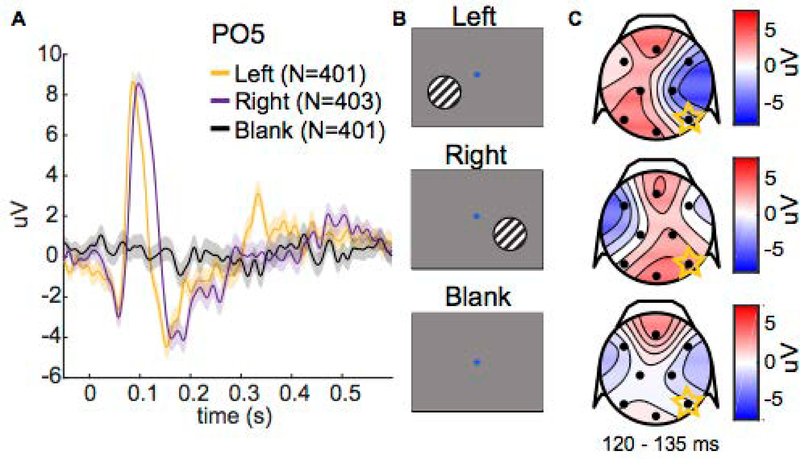
For each recording session, we calculated a VEP in response to a stimulus in the left visual hemifield (L), a stimulus in the right visual hemifield (R), and no stimulus (“blank” control condition) on each electrode (Figure 8). The time-course of the pre-craniotomy (PC) and craniotomy (C) VEPs were qualitatively similar; however, the amplitudes during certain noteworthy periods, such as during the “P1” component (50 to 150 ms), were different on particular electrodes (Figure 9a,b). We noticed comparable amplitude differences between VEPs from repeated experimental sessions within a given surgical condition (i.e., repeated sessions before or after the craniotomy; Figure 9a,b). Thus, a simple comparison of VEPs from different craniotomy conditions, which were always from different sessions, did not allow us to distinguish between effects arising from day-to-day variability and potential effects from introducing a craniotomy.
Figure 8.
Visual-evoked potentials (VEPs). A) VEPs for one subject (Monkey B) on the PO5 electrode for different stimulus conditions. Stimulus onset occurs at 0 s. Shading represents ± 1 SEM (total number of trials per condition is noted in the legend). B) Depictions of different stimuli in the passive visual stimulation task. The subject fixated on the central dot, then a sinusoidal grating appeared on one side of the screen at 0 s (left/right condition), or no grating appeared (blank condition). The grating remained on for 600 ms. C) Topographical scalp voltage maps from 120 to 135 ms. Black dots show the approximate location of EEG electrodes.
We sought to understand whether the observed differences in the VEPs were the result of the craniotomy or some other source of variability in our EEG recordings. Similarity between VEPs from different sessions was limited by the test-retest reliability of our EEG recordings between sessions, which was affected by day-to-day variability. We quantified how similarity between VEPs was influenced by (1) trial-to-trial variability within a single recording session, (2) day-to-day variability between recording sessions, and (3) differences between visual stimulation conditions. Then, we tested whether the similarity between a pre-craniotomy session VEP and craniotomy session VEP was within the confidence interval of typical within-craniotomy VEP similarity.
We used Pearson’s correlation between VEPs as a measure of similarity. For this analysis we bootstrapped confidence intervals by randomly dividing the full set of trials for each session into two subsets, computing the correlation between VEPs for pairs of trial subsets during the “P1” component using all eight electrodes, and then repeating 1000 times with different random subsets. We predicted correlations between VEPs would be strongest within a session, slightly less strong between sessions, and weakest between different stimulus conditions, because visual stimuli in the left and right hemifield evoke characteristically different visual responses.
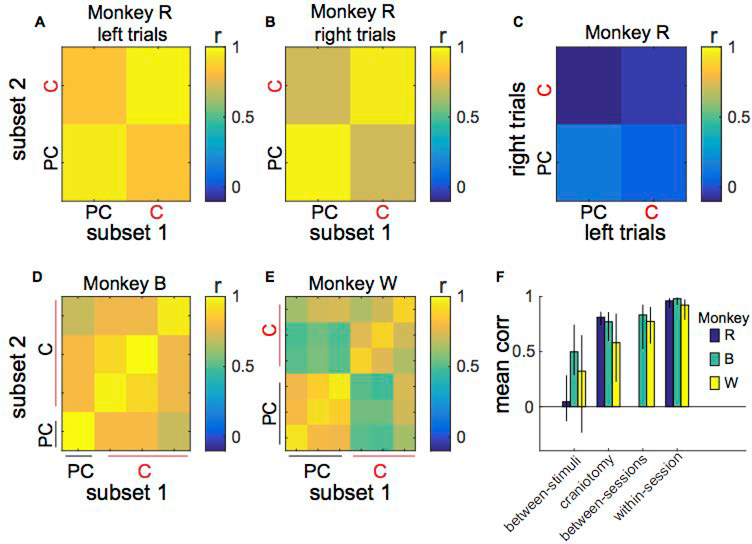
First, we analyzed the variability within a day by comparing VEPs generated from trial subsets of a recording session. VEPs from random subsets of trials within a single recording session were highly correlated as we predicted (Figure 10a,b,d,e). Secondly, we asked how day-to-day variability affected VEPs. We calculated the correlations between VEPs from pairs of sessions that were either both collected before the craniotomy or both collected afterwards. Compared to the correlations within-session, correlations between repeated sessions were slightly weaker, regardless of whether the pair came from before or after the craniotomy (Figure 10d,e). Third, we computed the correlations between VEPs for opposite hemifield stimuli (left hemifield versus right hemifield). We found these to be the weaker than both within-session and between-session correlations (Figure 10c–e), which was also in line with our predictions.
Figure 10.
Pre-craniotomy (PC) and craniotomy (C) VEPs are more correlated than left and right stimulus VEPs. A-B) Subset 1 versus subset 2 correlations for Monkey R’s left (L) and right (R) stimulus VEPs. Subset 1 and 2 are partitions of the entire set of VEP trials for a particular session and stimulus. Each square is the correlation across the P1 component using all eight electrodes between all combinations of trial subsets for the same stimulus. VEPs from the same session are strongly correlated (r > 0.95). VEPs from pre-craniotomy and craniotomy sessions are only slightly less correlated (r ≈ 0.80). C) Opposite hemifield stimuli VEP correlations for Monkey R. Each square depicts the correlation between the left and right stimulus VEPs from all combinations of trial subsets for the same stimulus. VEPs to opposite hemifield stimuli are relatively weakly correlated (r < 0.22). D-E) Subset 1 versus subset 2 correlations for Monkey B and W (VEP correlations for left hemifield stimuli averaged with VEP correlations for right hemifield stimuli). F) Mean of correlations for different sources of variability (between-stimuli, craniotomy, between sessions, within-session) for all subjects (Monkeys R, B and W). We applied Fisher’s r-to-z transformation to correlation coefficients prior to averaging, then inverse-transformed the resultant averages for presentation. Error bars indicate the 95% bootstrapped confidence intervals. A between-sessions comparison was not available for Monkey R.
Finally, we compared VEP pairs where one was derived from data collected before the craniotomy and the other was derived from data collected afterwards. Correlations between VEPs collected before and after the craniotomy were very similar to correlations between sessions that were both collected before the craniotomy or both collected afterwards. That is, apparent differences in the VEPs before and after the craniotomy were within the range of test-retest reliability between sessions for all subjects (Figure 10f).
Since we were especially interested in oscillatory characteristics of the EEG, we analyzed the overall power spectra before and after the craniotomy, derived on non-overlapping 30 s segments of the continuous recording from the passive visual stimulation task. We observed that the power spectra appeared similar before and after craniotomy (Figure 9c,d). Additionally, we assessed the influence of the craniotomy, day-to-day variability, and trial-to-trial variability on the FFTs via the same procedure we used to benchmark the effects of those sources of variability on the VEP amplitudes (Supplemental Figure 6). We found the correlation between a power spectrum from a session before the craniotomy and a power spectrum from a session after the craniotomy was as high as correlations between power spectra from the same session, and correlations between power spectra from within a craniotomy condition (i.e. both from pre-craniotomy sessions or both from craniotomy sessions). These results are in line with the qualitative similarity between FFTs of EEG data collected before and after the craniotomy.
Most critically for validating our main result, we analyzed the effect of the craniotomies on the coherence between the frontal and parietal EEG electrodes ipsilateral to the surgical sites during the passive visual stimulation task (Figure 11; Supplemental Figure 7). Three subjects were included in this analysis, one of which (Monkey W) also performed the change-detection task. Our first observation was that there was substantial inter-individual variability, much as we observed for the change detection task (Figure 2). Notably, the shape and magnitude of the coherence spectrum for Monkey W for this passive visual stimulation task (Figure 11, bottom) was similar to what we observed for the same individual during the change-detection task in different experimental sessions (Figure 2, top). This suggests that individual characteristics may be a greater determinant of EEG coherence than task demands. Our second observation was that the introduction of craniotomies did not change the overall shape of the coherence spectra. As an example, for Monkey W (for whom we had data from multiple repeated sessions both before and after the craniotomy), the coherence spectra we measured after craniotomy all lied within the range of test-retest reliability of the pre-craniotomy sessions. The other two subjects seemed to show slight increases in coherence after the craniotomies, but these differences were much less extreme than the differences between individuals.
Taken together these results suggest that any alteration of the EEG signal engendered by our surgical intervention is well within the bounds of variation due to factors typical for EEG studies (test-retest reliability, inter-individual differences, etc.) and does not invalidate the results of our efforts to relate scalp-level coherence to coherence measured intracranially.
Discussion
We used simultaneous scalp EEG and intracranial recordings to test the hypothesis that scalp-level coherence reflects LFP coherence between disparate cortical areas. Previously we reported (Snyder & Smith, 2015) that scalp EEG was related to important oscillatory features of the LFP recorded nearby, such as trial-to-trial variation in amplitude, particularly at low frequencies –a finding that we replicated with the current study. Despite these promising links between the two signal types, we did not find evidence in the current study that long-range scalp EEG coherence was related to long-range LFP coherence recorded intracranially. Both long-range EEG coherence and long-range LFP coherence have been linked to a number of important cognitive functions (Kelly et al., 2003; Deeny et al., 2009; Gregoriou et al., 2009; Salazar et al., 2012; Nacher et al., 2013; van Driel et al., 2014; Fleck et al., 2016; van Driel et al., 2017). Our results do not invalidate this existing literature, but rather bring into question the way (or degree) in which coherence is related between these two types of electrophysiological measurements. Continuing investigation into this relationship is necessary to understanding how scalp-level EEG signals, of essential importance to the study of human neurophysiology, are related to micro- and meso-scale neural networks that can be measured intracranially.
There are several potential explanations for the null result we obtained. First, as with any null finding, we may have had insufficient statistical power to detect a small effect. However, based on the trends in our data, this interpretation would suggest that the true relationship between long-range EEG coherence and long-range LFP coherence is so weak that the utility of this tenuous link for interpreting the results of EEG studies would still be questionable. For example, Monkey P, for whom we had the most experimental sessions, showed a peak mean correlation between scalp coherence and LFP coherence of only Z(ρ) = 0.0088. In comparison, the relationship in overall amplitude between EEG and LFP for that subject was much stronger, peaking at Z(ρ) = 0.1695 between the V4 array and the nearby scalp electrode. This suggests that EEG can be sensitive enough to reliably index the trial-to-trial variation in amplitude of nearby oscillatory sources, but not the trial-to-trial phase relationships of those same sources.
A second potential explanation for the null result is that our EEG electrodes were not optimally placed. One might argue that if only we had placed the electrodes in a different location (e.g., as close as physically possible to the arrays) that we might have detected a relationship. We believe we can rule out this potential objection for several reasons. First of all, modelling studies (Nunez & Srinivasan, 2006) and studies where small amounts of electrical current have been injected into patients undergoing surgery for severe epilepsy (Cohen et al., 1990; Cuffin et al., 1991; Krings et al., 1999; van Burik & Peters, 1999) have shown that focal current sources lead to diffuse voltage topographies on the scalp, suggesting that precise electrode placement is not necessary to detect the influence of distant current sources. Secondly, because the EEG electrodes were applied to the scalp anew each session, there was inherent day-to-day variation in electrode placement (in a prior report, we measured the standard deviation of our EEG electrode placement method to be about 0.5 cm in any direction (Snyder et al., 2015)), yet the results in the current study were highly consistent across sessions within a subject, suggesting that exact electrode placement was not critical. Third, in another prior report (Snyder & Smith, 2015), we examined how each of the eight EEG electrodes in our montage related to the amplitude and spatial coherence of LFPs at a single intracortical location and found significant relationships at distances exceeding 5 cm. Finally, in at least one subject for the current study (Monkey P) we observed significant temporal coherence at both scalp sites of interest with the underlying LFPs, which suggests that our electrodes were appropriately placed to detect potential coherence effects. Of course, we cannot rule out that a different EEG montage might have yielded a different outcome. Our current results point to two possible interpretations about the relationship between scalp-level coherence and LFP coherence as we measured it here, either it is (1) weak or absent or (2) so spatially sensitive, at a level beyond current assumptions of EEG signal propagation, as to be easily missed due to small variations in EEG electrode position.
A third potential explanation for our inability to find a relationship between long-range coherence at the scalp and long-range coherence intracranially is that we may have misestimated scalp-level coherence due to volume conduction effects. The influence of volume conduction on coherence estimates of EEG is a topic well-covered in the literature (Nunez et al., 1997; Nunez & Srinivasan, 2006; Brunner et al., 2016). A related concern is the choice of recording reference (Nunez et al., 1997; Nunez & Srinivasan, 2006; Hu et al., 2007; Hu et al., 2010; Hindriks et al., 2016), which was the head-holder implant in our case. These are legitimate concerns that we are somewhat limited in our ability to address due to the sparsity of our EEG electrode montage. For example, one option for mitigating the potential impact of volume conduction on coherence estimates is to first derive the current source density or to estimate an inverse source solution (Nunez et al., 1997), neither of which are feasible with our EEG configuration. One thing we tried during preliminary analyses to mitigate this issue was to re-reference each EEG electrode of interest to another nearby EEG electrode at the midline to create a pair of bipolar recordings. In this case we found that the results were not meaningfully altered, and in fact noise levels were increased by re-referencing to the other, noisy scalp electrodes (Supplemental Figure 1). In the end we decided to proceed with the most straightforward analysis using the original recording reference. Another rebuff to concerns of volume conduction for our analysis is that the LFP recordings are also subject to volume conduction (Herreras, 2016). Although the reference wires for the Utah arrays were not positioned precisely, they were several centimeters away from the arrays themselves. The critical point is that the LFP arrays would be embedded in the same “volume” as the EEG electrodes (i.e., the head), and thus would be subject to the same volume conduction effects. To put it another way, volume-conducted current would have to first pass the LFP arrays on the way to the EEG electrodes on the scalp. Therefore, if anything, volume conduction might lead to spuriously strong relationships between coherence measured intracranially and at the scalp, rather than spuriously weak relationships.
The fourth, and potentially most interesting, explanation for our negative result is that the functional connectivity reflected by scalp EEG coherence operates between cortical areas that themselves span a greater spatial scale than that of our 16 mm2 Utah arrays (we can rule out the alternative possibility that the proper scale of measurement is smaller than that of the array, since our analysis with the most promising single electrode from each area also turned up no relationship with the EEG; Supplemental Figure 3). Further research will be needed to determine the area of cortex we must observe for which long-range intracranial coherence begins to reflect coherence measured at the scalp. In the meantime, we can look for guidance to modeling studies (Nunez & Srinivasan, 2006) as well as studies using simultaneous electrocorticography and scalp-level EEG in people awaiting surgery for epilepsy (Tao et al., 2005; Ramantani et al., 2016). These studies suggest that coordinated activity across a patch of cortex with at least 6 cm2 of gyral surface area is necessary to detect temporally distinct cortical events (such as epileptic spikes) with EEG. Six square centimeters is a substantial piece of cortex. For context, Van Essen and colleagues (2012) estimated the surface area of 52 human cortical areas (following established parcellation schemes based on cytoarchitecture, retinotopy or somatotopy) and found that more than two-thirds of them (67.3%) had areas less than 4 cm2. Extrapolating their measurements to the entire cortex, they surmised that the average size of a human cortical area is 4.8 cm2. All together, these observations suggest that long-range EEG coherence could imply not only coordination between distant brain areas, but also synchrony across multiple adjacent areas. We should emphasize that this synchrony between adjacent areas would have to occur specifically at the centimeter scale and not be detectable on the subordinate scale of several millimeters, or else we might have found stronger coherence between our Utah arrays. If this is true, then EEG coherence studies are likely tapping into fundamentally different sorts of processes than are plumbed by studies of long-range LFP coherence, for which activity in very focal regions of cortex are measured. In the current study, we found that coherence decreased rapidly over distance on the scale of our arrays (i.e., decreasing by more than half over distances from 0.4 to 5 mm in both V4 and PFC). This is similar to the decay of coherence over distance in V1 reported previously (Frien & Eckhorn, 2000; Leopold et al., 2003). Also, in line with our finding, Kellis and colleagues (2016) found that correlation between electrocorticography electrodes in humans also decreased by half over a distance of 5 mm. Given these observations, it is truly striking that synchrony on the scales needed to generate measureable long-range coherence at the scalp, likely an order of magnitude larger, occurs regularly enough to be commonly detected in EEG studies.
To summarize, we found no evidence that long-range coherence between scalp EEG electrodes relates to long-range coherence between local field potentials measured on the scale of our Utah arrays, despite finding other relationships between the two scales of measurement. Naturally, further research is needed to determine the scale at which long-range EEG coherence can be related to long-range coherence measured intracranially. Until then, we believe our observations imply that different theoretical frameworks are needed to interpret coherences at these different scales, and that this disparity presents an opportunity to devise a new unifying theory that reconciles the differences in neural communication across these two important types of electrophysiological signals.
Supplementary Material
Acknowledgements
ACS was supported by NIH grant K99EY025768. DI was supported by NIH grant R90DA023426. MAS was supported by NIH grants R01EY022928 and P30EY008098, Research to Prevent Blindness, and the Eye and Ear Foundation of Pittsburgh. The authors thank Ms. Samantha Schmitt for help with data collection.
Abbreviations
- C
Craniotomy
- EEG
Electroencephalography
- FFT
Fast Fourier transform
- FWHM
Full-width at half-maximum
- L
Left visual stimulus
- LFP
Local field potential
- PC
Pre-craniotomy
- PFC
Prefrontal cortex
- R
Right visual stimulus
- RF
Receptive field
- SD
Standard deviation
- SEM
Standard error of the mean
- VEP
Visual-evoked potential
Footnotes
Conflict of Interest Statement
The authors declare no competing financial interests.
Data Accessibility Statement
Data and analysis code are available from the authors upon request.
References
- Babiloni C, Lizio R, Marzano N, Capotosto P, Soricelli A, Triggiani AI, Cordone S, Gesualdo L & Del Percio C (2016) Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 103, 88–102. [DOI] [PubMed] [Google Scholar]
- Bastos AM, Vezoli J & Fries P (2015) Communication through coherence with inter-areal delays. Curr Opin Neurobiol, 31, 173–180. [DOI] [PubMed] [Google Scholar]
- Berger H (1929) Über das elektrenkephalogramm des menschen. European archives of psychiatry and clinical neuroscience, 87, 527–570. [Google Scholar]
- Broca P (1861a) Perte de la parole; ramollissement chronique et destruction partielle du lobe antérieure gauche du cerveau. Bulletin de la Société d’Anthropologie de Paris, 2, 235–238. [Google Scholar]
- Broca P (1861b) Remarque sur le siège de la faulté du langage articulé, suivie d’une observation d’aphemie (perte de la parole). Bulletin de la Société d’Anthropologie de Paris, 36. [Google Scholar]
- Brunner C, Billinger M, Seeber M, Mullen TR & Makeig S (2016) Volume Conduction Influences Scalp-Based Connectivity Estimates. Frontiers in computational neuroscience, 10, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Anastassiou CA & Koch C (2012) The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nature reviews. Neuroscience, 13, 407–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G & Draguhn A (2004) Neuronal oscillations in cortical networks. Science, 304, 1926–1929. [DOI] [PubMed] [Google Scholar]
- Calabrese P & Penner IK (2007) Cognitive dysfunctions in multiple sclerosis--a “multiple disconnection syndrome”? Journal of neurology, 254 Suppl 2, II18–21. [DOI] [PubMed] [Google Scholar]
- Catani M & ffytche DH (2005) The rises and falls of disconnection syndromes. Brain : a journal of neurology, 128, 2224–2239. [DOI] [PubMed] [Google Scholar]
- Cohen D, Cuffin BN, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy JG & Schomer DL (1990) MEG versus EEG localization test using implanted sources in the human brain. Annals of neurology, 28, 811–817. [DOI] [PubMed] [Google Scholar]
- Cronin-Golumb A (2010) Parkinson’s Disease as a Disconnection Syndrome. Neuropsychology review, 20, 191–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuffin BN, Cohen D, Yunokuchi K, Maniewski R, Purcell C, Cosgrove GR, Ives J, Kennedy J & Schomer D (1991) Tests of EEG localization accuracy using implanted sources in the human brain. Annals of neurology, 29, 132–138. [DOI] [PubMed] [Google Scholar]
- Deeny SP, Haufler AJ, Saffer M & Hatfield BD (2009) Electroencephalographic coherence during visuomotor performance: a comparison of cortico-cortical communication in experts and novices. Journal of motor behavior, 41, 106–116. [DOI] [PubMed] [Google Scholar]
- Delbeuck X, Van der Linden M & Collette F (2003) Alzheimer’s disease as a disconnection syndrome? Neuropsychology review, 13, 79–92. [DOI] [PubMed] [Google Scholar]
- Duffy FH, Shankardass A, McAnulty GB & Als H (2017) A unique pattern of cortical connectivity characterizes patients with attention deficit disorders: a large electroencephalographic coherence study. BMC medicine, 15, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleck JI, Kuti J, Brown J, Mahon JR & Gayda-Chelder C (2016) Frontal-posterior coherence and cognitive function in older adults. International journal of psychophysiology : official journal of the International Organization of Psychophysiology, 110, 217–230. [DOI] [PubMed] [Google Scholar]
- Frien A & Eckhorn R (2000) Functional coupling shows stronger stimulus dependency for fast oscillations than for low-frequency components in striate cortex of awake monkey. The European journal of neuroscience, 12, 1466–1478. [DOI] [PubMed] [Google Scholar]
- Fries P (2005) A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends in cognitive sciences, 9, 474–480. [DOI] [PubMed] [Google Scholar]
- Fries P (2015) Rhythms for Cognition: Communication through Coherence. Neuron, 88, 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ & Frith CD (1995) Schizophrenia: a disconnection syndrome? Clin Neurosci, 3, 89–97. [PubMed] [Google Scholar]
- Geschwind DH & Levitt P (2007) Autism spectrum disorders: developmental disconnection syndromes. Curr Opin Neurobiol, 17, 103–111. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1965a) Disconnexion syndromes in animals and man. I. Brain : a journal of neurology, 88, 237–294. [DOI] [PubMed] [Google Scholar]
- Geschwind N (1965b) Disconnexion syndromes in animals and man. II. Brain : a journal of neurology, 88, 585–644. [DOI] [PubMed] [Google Scholar]
- Gregoriou GG, Gotts SJ, Zhou H & Desimone R (2009) High-frequency, long-range coupling between prefrontal and visual cortex during attention. Science, 324, 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriou GG, Rossi AF, Ungerleider LG & Desimone R (2014) Lesions of prefrontal cortex reduce attentional modulation of neuronal responses and synchrony in V4. Nat Neurosci, 17, 1003–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn G, Bujan AF, Fregnac Y, Aertsen A & Kumar A (2014) Communication through resonance in spiking neuronal networks. PLoS computational biology, 10, e1003811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herreras O (2016) Local Field Potentials: Myths and Misunderstandings. Frontiers in neural circuits, 10, 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindriks R, Arsiwalla XD, Panagiotaropoulos T, Besserve M, Verschure PF, Logothetis NK & Deco G (2016) Discrepancies between Multi-Electrode LFP and CSD Phase-Patterns: A Forward Modeling Study. Frontiers in neural circuits, 10, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Stead M, Dai Q & Worrell GA (2010) On the recording reference contribution to EEG correlation, phase synchrony, and coherence. IEEE transactions on systems, man, and cybernetics. Part B, Cybernetics : a publication of the IEEE Systems, Man, and Cybernetics Society, 40, 1294–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Stead M & Worrell GA (2007) Automatic identification and removal of scalp reference signal for intracranial EEGs based on independent component analysis. IEEE transactions on bio-medical engineering, 54, 1560–1572. [DOI] [PubMed] [Google Scholar]
- Juergens E, Guettler A & Eckhorn R (1999) Visual stimulation elicits locked and induced gamma oscillations in monkey intracortical- and EEG-potentials, but not in human EEG. Exp Brain Res, 129, 247–259. [DOI] [PubMed] [Google Scholar]
- Kellis S, Sorensen L, Darvas F, Sayres C, O’Neill K 3rd, Brown RB, House P, Ojemann J & Greger B (2016) Multi-scale analysis of neural activity in humans: Implications for micro-scale electrocorticography. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 127, 591–601. [DOI] [PubMed] [Google Scholar]
- Kelly SP, Dockree P, Reilly RB & Robertson IH (2003) EEG Alpha Power and Coherence Time Courses in a Sustained Attention Task. Proceedings of the First International IEEE EMBS Conference on Neural Engineering, 83–86. [Google Scholar]
- Kleinschmidt A & Vuilleumier P (2013) Disconnecting cognition. Current opinion in neurology, 26, 333–338. [DOI] [PubMed] [Google Scholar]
- Krings T, Chiappa KH, Cuffin BN, Cochius JI, Connolly S & Cosgrove GR (1999) Accuracy of EEG dipole source localization using implanted sources in the human brain. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 110, 106–114. [DOI] [PubMed] [Google Scholar]
- La Rocca D, Campisi P, Vegso B, Cserti P, Kozmann G, Babiloni F & De Vico Fallani F (2014) Human brain distinctiveness based on EEG spectral coherence connectivity. IEEE transactions on bio-medical engineering, 61, 2406–2412. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J & Varela FJ (1999) Measuring phase synchrony in brain signals. Human brain mapping, 8, 194–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leocani L, Locatelli T, Martinelli V, Rovaris M, Falautano M, Filippi M, Magnani G & Comi G (2000) Electroencephalographic coherence analysis in multiple sclerosis: correlation with clinical, neuropsychological, and MRI findings. Journal of neurology, neurosurgery, and psychiatry, 69, 192–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leopold DA, Murayama Y & Logothetis NK (2003) Very slow activity fluctuations in monkey visual cortex: implications for functional brain imaging. Cereb Cortex, 13, 422–433. [DOI] [PubMed] [Google Scholar]
- Logothetis NK (2003) The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci, 23, 3963–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch M (2011) Re-examining Paul Broca’s initial presentation of M. Leborgne: understanding the impetus for brain and language research. Cortex; a journal devoted to the study of the nervous system and behavior, 47, 1228–1235. [DOI] [PubMed] [Google Scholar]
- Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, Berry AS & Corbett BA (2010) Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biological psychiatry, 67, 617–623. [DOI] [PubMed] [Google Scholar]
- McLelland D & VanRullen R (2016) Theta-Gamma Coding Meets Communication-through-Coherence: Neuronal Oscillatory Multiplexing Theories Reconciled. PLoS computational biology, 12, e1005162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo R & Leisman G (2009) Autistic spectrum disorders as functional disconnection syndrome. Reviews in the neurosciences, 20, 111–131. [DOI] [PubMed] [Google Scholar]
- Mitra P & Bokil H (2008) Observed brain dynamics. Oxford University Press, Oxford; New York. [Google Scholar]
- Nacher V, Ledberg A, Deco G & Romo R (2013) Coherent delta-band oscillations between cortical areas correlate with decision making. Proceedings of the National Academy of Sciences of the United States of America, 110, 15085–15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napflin M, Wildi M & Sarnthein J (2007) Test-retest reliability of resting EEG spectra validates a statistical signature of persons. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology, 118, 2519–2524. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E & Naidu SB (1997) Attention-deficit hyperactivity disorder (ADHD) and frontal-motor cortex disconnection. Clin Electroencephalogr, 28, 130–136. [DOI] [PubMed] [Google Scholar]
- Nunez PL & Srinivasan R (2006) Electric fields of the brain : the neurophysics of EEG. Oxford University Press, Oxford; New York. [Google Scholar]
- Nunez PL, Srinivasan R, Westdorp AF, Wijesinghe RS, Tucker DM, Silberstein RB & Cadusch PJ (1997) EEG coherency. I: Statistics, reference electrode, volume conduction, Laplacians, cortical imaging, and interpretation at multiple scales. Electroencephalography and clinical neurophysiology, 103, 499–515. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E & Schoffelen JM (2011) FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Computational intelligence and neuroscience, 2011, 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M (2005) Lateral prefrontal cortex: architectonic and functional organization. Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 360, 781–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M & Pandya DN (1999) Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. The European journal of neuroscience, 11, 1011–1036. [DOI] [PubMed] [Google Scholar]
- Ramantani G, Maillard L & Koessler L (2016) Correlation of invasive EEG and scalp EEG. Seizure, 41, 196–200. [DOI] [PubMed] [Google Scholar]
- Roe AW, Chelazzi L, Connor CE, Conway BR, Fujita I, Gallant JL, Lu H & Vanduffel W (2012) Toward a unified theory of visual area V4. Neuron, 74, 12–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salazar RF, Dotson NM, Bressler SL & Gray CM (2012) Content-specific fronto-parietal synchronization during visual working memory. Science, 338, 1097–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Kessler R, Gaughan T & Buckley AW (2017) Electroencephalogram Coherence Patterns in Autism: An Updated Review. Pediatric neurology, 67, 7–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirhatti V, Borthakur A & Ray S (2016) Effect of Reference Scheme on Power and Phase of the Local Field Potential. Neural computation, 28, 882–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shou G, Mosconi MW, Wang J, Ethridge LE, Sweeney JA & Ding L (2017) Electrophysiological signatures of atypical intrinsic brain connectivity networks in autism. Journal of neural engineering, 14, 046010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer W (1999) Neuronal synchrony: a versatile code for the definition of relations? Neuron, 24, 49–65, 111–125. [DOI] [PubMed] [Google Scholar]
- Singer W & Gray CM (1995) Visual feature integration and the temporal correlation hypothesis. Annual review of neuroscience, 18, 555–586. [DOI] [PubMed] [Google Scholar]
- Smith MA & Kohn A (2008) Spatial and temporal scales of neuronal correlation in primary visual cortex. J Neurosci, 28, 12591–12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA & Sommer MA (2013) Spatial and temporal scales of neuronal correlation in visual area V4. J Neurosci, 33, 5422–5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC, Morais MJ, Willis CM & Smith MA (2015) Global network influences on local functional connectivity. Nat Neurosci, 18, 736–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder AC & Smith MA (2015) Stimulus-dependent spiking relationships with the EEG. J Neurophysiol, 114, 1468–1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME & McCarley RW (2003) Abnormal neural synchrony in schizophrenia. J Neurosci, 23, 7407–7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinath R & Ray S (2014) Effect of amplitude correlations on coherence in the local field potential. J Neurophysiol, 112, 741–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C (2009) The roles of gamma-band oscillatory synchrony in human visual cognition. Front Biosci (Landmark Ed), 14, 321–332. [DOI] [PubMed] [Google Scholar]
- Tao JX, Ray A, Hawes-Ebersole S & Ebersole JS (2005) Intracranial EEG substrates of scalp EEG interictal spikes. Epilepsia, 46, 669–676. [DOI] [PubMed] [Google Scholar]
- van Burik M & Peters M (1999) EEG and implanted sources in the brain. Archives of physiology and biochemistry, 107, 367–375. [DOI] [PubMed] [Google Scholar]
- van Driel J, Gunseli E, Meeter M & Olivers CN (2017) Local and interregional alpha EEG dynamics dissociate between memory for search and memory for recognition. NeuroImage, 149, 114–128. [DOI] [PubMed] [Google Scholar]
- van Driel J, Knapen T, van Es DM & Cohen MX (2014) Interregional alpha-band synchrony supports temporal cross-modal integration. NeuroImage, 101, 404–415. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J & Coalson T (2012) Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex, 22, 2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E & Martinerie J (2001) The brainweb: phase synchronization and large-scale integration. Nature reviews. Neuroscience, 2, 229–239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.