Abstract
neurogenin2 (ngn2) encodes a basic helix–loop–helix transcription factor and plays an important role in neurogenesis from migratory neural crest cells. Its role in retinal development is poorly understood. We observed that in the developing chick retina, ngn2 was expressed in a subpopulation of proliferating progenitor cells. Ectopic expression of ngn2 in nonneural, retinal pigment epithelial cell culture triggered de novo generation of cells that expressed neural-specific markers and exhibited neuronal morphologies. Further molecular and morphological analyses showed that the main products of the induced neurogenesis were cells resembling young photoreceptor cells and cells resembling retinal ganglion cells. The generation of multiple cell types suggests that ngn2 induces various retinal pathways. Thus, unlike in the peripheral nervous system where ngn2 specifies one type of sensory neuron, ngn2 in the retina is likely involved in a common step leading to different cellular pathways. Our finding that ngn2 can instruct nonneural retinal pigment epithelial cells to differentiate toward retinal neurons demonstrates one possible way to induce de novo retinal neurogenesis.
The vertebrate retina contains five major types of neurons: photoreceptor, horizontal, bipolar, amacrine, and ganglion cells. Each cell type is functionally and morphologically distinct and resides at a stereotyped histological location. After receiving photons, photoreceptor cells initiate electrophysiological signals that are modulated and relayed by interneurons (horizontal, bipolar, and amacrine cells) to ganglion cells. Retinal ganglion cells send the information to the brain (1). Degeneration of the retina results in irreversible visual loss. Several common eye diseases, such as retinitis pigmentosa, age-related macular degeneration, and glaucoma, are caused by death of either photoreceptors or ganglion cells (2–4). Replacing the faulty retinal neurons with healthy ones might restore vision (5). Yet, inducing mature retinal neurons to regenerate remains an elusive task, because they, like other neurons, are terminally differentiated and will not reenter the cell cycle.
Elucidation of the molecular mechanisms underlying retinal development bears clinical implications as it might lead to genes capable of inducing the genesis of retinal neurons for tissue or cell replacement therapies. Studies with various experimental approaches have shown that the different types of retinal neurons are generated from common multipotent progenitor cells under the instruction of intrinsic and extrinsic factors (for a recent review, see ref. 6). Recent reports indicate that the intrinsic factors may include a number of basic helix–loop–helix (bHLH) genes with proneural activities, such as ash-1 (7), nurogenin1 (ngn1) (8), ath3 (8, 9), ath-5 (10–15), and neuroD (16–18).
ngn2 is a member of the neurogenin subfamily of bHLH genes homologous to the Drosophila proneural gene atonal. During mouse neurogenesis, ngn2 and ngn1 are expressed in distinct progenitor populations in the central and the peripheral nervous systems (19, 20). Their expression patterns partially overlap in some areas but are distinct in others. Targeted mutational analyses showed that ngn1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia (21), and ngn2 is essential for the determination of precursors for epibranchial placode-derived sensory neurons (22). In the peripheral nervous system of the chick, ngn1 and ngn2 are expressed in a subset of neural crest cells early during crest cell migration (23, 24). ngn2 specifies one type (trkB+/trkC+) of sensory neurons and ngn1 specifies another (trkA+), and both genes induce neuroD (23). Mouse and chick ngn2 is expressed in the retina (14, 15), and the promoter/reporter assay indicates that ngn2 can activate ath5 (15). However, how ngn2 participates in retinal neurogenesis remains largely unknown.
The retinal pigment epithelium (RPE) consists of nonneural cells in a monolayer structure that lies immediately outside the neural retina. Unlike retinal neurons, RPE cells from several species, including human, can reenter the cell cycle when stimulated. Furthermore, their progenies may, under appropriate conditions, differentiate into cell types other than RPE (25–27). One of the most intriguing phenomena reported is the transdifferentiation of RPE tissue into a neural retina in vivo (28–30) or in vitro (31), under the induction of basic fibroblast growth factor (bFGF). However, bFGF-induced transdifferentiation into a neural retina can occur only in young RPE up to embryonic day (E) 4.5 in chick (29, 31) and up to E13 in rat (27). Furthermore, bFGF has failed to induce neuronal transdifferentiation when RPE is cultured as dissociated cells (31).
We have recently observed that dissociated RPE cells derived from an E6 chick can be guided toward distinct pathways with various stimuli. For example, ectopic expression of neuroD triggers de novo production of photoreceptor cells (16, 17). In the presence of basic fibroblast growth factor, on the other hand, cultured RPE cells will transdifferentiate into cells that express RA4 (32), which is a marker for ganglion cells in the chick retina (33). Because it shares the same origin as the retina, it can reenter the cell cycle (i.e., can be cultured) and its progenies display developmental plasticity, RPE might serve as a source of cells to regenerate retinal neurons.
In this study, we examined the retinal expression of ngn2 and tested whether ngn2 could induce the genesis of retinal neurons from RPE cells. We found that during retinal neurogenesis in the chick, a subpopulation of proliferating progenitors expressed ngn2. When ectopically expressed in cultured RPE cells, ngn2 elicited de novo generation of neuronal cells, which resembled young photoreceptor cells and young ganglion cells. We propose that ngn2 in the retina, unlike in the peripheral nervous system, is likely to be involved in a common developmental event that precedes cell fate specification and can induce the genesis of different types of retinal neurons from cultured RPE cells.
Materials and Methods
Cloning of Chick ngn2.
The coding region of mouse ngn1 (also called neuroD3) was PCR-amplified from mouse genomic DNA with primers (5′-TAC ACC ATG GCT GCC CCT TTG GAG ACC TG-3′ and 5′-CTC TGG ATC CTT ACA AAG GCC TAG TGG TA-3′) based on a published sequence (20, 34). After sequence verification, the cloned PCR product was used as a probe to screen a λZAP II (Stratagene) cDNA library constructed with mRNA isolated from an E8 chick brain. Three full-length primary clones were obtained; the longest clone contained a longer 3′ untranslated sequence of ngn2 (GenBank accession no. AF109014). Chick ngn2 was also independently isolated by Perez et al. (24) and Matter-Sadzinski et al. (15).
In Situ Hybridization.
To avoid potential cross hybridization with other bHLH genes, the coding sequence for the C′ terminal nonconserved 61-aa and 3′ untranslated sequence was used to synthesize digoxigenin-labeled antisense RNA probes (788 nucleotides long) against ngn2. Preparations of digoxigenin-labeled probes against other genes and the details of in situ hybridization with retinal cryosections have been described (16, 17). In situ hybridization of cultured cells was also as described (32). For double-labeling, dishes were subjected to in situ hybridization first, followed by immunocytochemistry.
Immunocytochemistry.
MAb against microtubule associate proteins (MAP2) was purchased from Sigma (clone HM-2) and used at a 1:100 dilution. Polyclonal anti-AP2 Ab (1:500) was purchased from Santa Cruz Biotechnology. Affinity-purified anti-Calretinin (1:2000) was purchased from Chemicon. MAb against proliferating cell nuclear antigen (PCNA) was purchased from Dako and used at a dilution of 1:200. Three mAbs were obtained from the Developmental Studies Hybridoma Bank (Iowa University, Ames): Ab against neural cell adhesion molecule (N-CAM) (clone 5e; 1:200; developed by U. Rutishauser), anti-BrdUrd (clone G3G4; 1:100; developed by S. J. Kaufman), and anti-Visinin (clone 7G4; 1:500; developed by C. Cepko). RA4 (1:500) was a gift from S. McLoon (Univ. of Minnesota, Minneapolis). Standard immunocytochemistry was performed. For double-labeling experiments, secondary Abs conjugated with fluorescent chromophore were used.
Pulse-Labeling of Chick Embryos with BrdUrd.
BrdUrd (50 μg) was dropped onto E7 chick embryos through a window in the shell. Four hours later, the eyes were fixed. Cryosections were subjected to in situ hybridization with antisense RNA probes against ngn2, followed by immunocytochemistry with a mAb against BrdUrd, as described (35).
Generation of Retrovirus Expressing ngn2.
The coding region of chick ngn2 was PCR-amplified with primers based on the sequence of the full-length primary clone and subcloned into shuttle vector ClaI2Nco, from which a ClaI fragment was inserted into proviral DNA vector replication-competent retrovirus (RCAS) (36). Recombinant viral DNA was introduced into chick embryonic fibroblast cells through transfection. Retrovirus particles were harvested as described (16). The titer of the concentrated viral stock was 1–2 × 108 pfu/ml.
RPE Transdifferentiation Assay.
RPE tissues were dissected from E6 chick embryos, and dissociated RPE cells were cultured as described (16). After 3–4 days in culture, when ≈50% confluence was reached, 5 μl of the retrovirus stock was added to each 35-mm dish, and the culture was kept for an additional 8–12 days. At the end of culturing, cells in the dish were washed twice with PBS and fixed with 4% paraformaldehyde for 20 min. After washing with PBS, the cells were subjected to immunocytochemistry or in situ hybridization. All experiments were independently repeated at least 3 times in their entirety, from RPE isolation to cell labeling.
Results
Expression of ngn2 in Proliferating Retinal Cells.
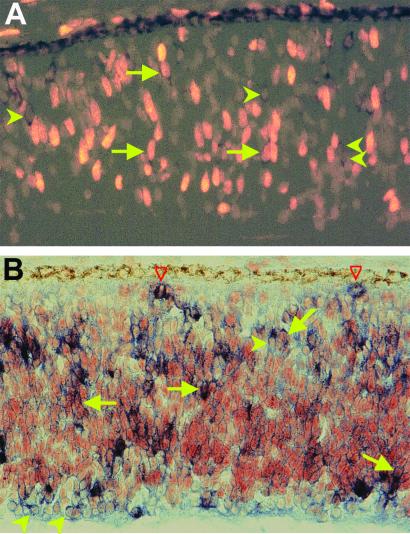
The retinal neuroepithelium is a pseudostratified structure lacking a conspicuous demarcation between the ventricular zone, which contains proliferating cells, and the mantle zone, which contains differentiating neurons. Nevertheless, within the pseudostratified structure, young neurons accumulate in their prospective locations. In the developing chick retina, ngn2 mRNA was detected in cells scattered across the retinal neuroepithelium. Double-labeling showed that cells expressing ngn2 also incorporated BrdUrd (Fig. 1A) or expressed PCNA (Fig. 1B), indicating that proliferating neuroblasts expressed ngn2. Note that only a subpopulation of BrdUrd+ cells or PCNA+ cells contained detectable levels of ngn2 mRNA. Some M-phase cells were also found to contain ngn2 mRNA (open arrows in Fig. 1B).
Figure 1.
Expression of ngn2 in proliferating cells of E7 chick retinal neuroepithelium. (A) Doubling-labeling for ngn2 mRNA (dark stains in the cytoplasm) and BrdUrd incorporation (bright nuclei). (B) Doubling-labeling for ngn2 mRNA (blue stains in the cytoplasm) and PCNA immunoreactivity (red/brown stains of the nuclei). Arrows point to double-labeled cells. Arrowheads point to ngn2+/BrdUrd− or ngn2+/PCNA− cells. Red open arrowheads point to ngn2+ cells that were in M-phase of the cell cycle. Magnification: ×100.
De Novo Generation of Neural Cells in RPE Cultures Under the Induction of ngn2.
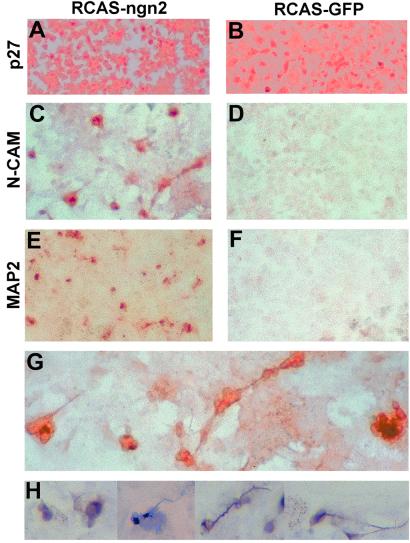
The RPE cell culture system we recently developed (16) was used to study whether ngn2 could induce de novo generation of retinal neurons. E6 chick RPE cells were dissociated and cultured for 2–4 days to reach about 50% confluence. At this point, RCAS expressing ngn2, or the green fluorescent protein (GFP), was added. Retroviral infection was monitored by immunocytochemistry with a specific Ab against a viral protein, p27. Four days after administration of the retrovirus, more than 80% of the cells in the dish became infected with the virus (Fig. 2 A and B).
Figure 2.
De novo generation of neurons in RPE cell culture under the induction of ngn2. (A and B) Immunostaining of RPE culture infected with RCAS-ngn2 (A) or RCAS-GFP (B) with anti-p27 Abs. (C and D) Immunostaining with anti-N-CAM Ab. (E and F) Immunostaining with anti-MAP2 Abs. (G) Higher magnification of C. (H) Individual MAP2+ cells or neural clusters at a higher magnification. Magnification: A–F, ×50; G, ×100; H, ×120.
To detect the presence of neural cells, RPE cultures were examined 8–12 days after administration of the retrovirus with mAbs against two general neural markers, N-CAM and MAP2. In dishes infected with RCAS-ngn2, tens of thousands per dish of 8 cm2 were N-CAM+ and MAP2+ (Fig. 2 C and E). No such positive cells were present in control dishes infected with RCAS-GFP (Fig. 2 D and F). N-CAM+ cells were often found in clusters, and the clusters were sometimes interconnected by a long cellular process characteristic of neurons (Fig. 2G). Morphologically, both N-CAM+ and MAP2+ cells were neural-like (Fig. 2 G and H). Their morphologies were clearly different from the cuboidal-hexagonal morphology of nondividing RPE cells or the fibroblast-like morphology of actively dividing RPE cells.
Because bHLH proteins are known to form homo- or heterodimers, experimental manipulation of a bHLH gene expression always creates concerns about the specificity. In the RPE transdifferentiation assay, we performed ectopic expression of two other bHLH genes, cNSCL1 and cNSCL2, and found neither could induce neural transdifferentiation (data not shown).
Presence of Young Photoreceptor Cells.
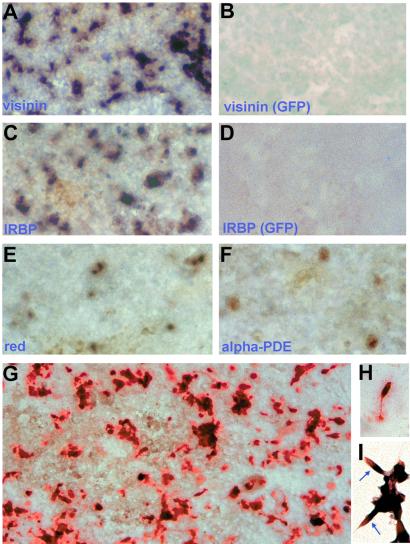
To identify photoreceptor neurons in the RPE cultures, we examined the expression of photoreceptor-specific genes and their cellular morphologies. We found tens of thousands (per dish) in RCAS-ngn2-infected cultures expressed visinin (Fig. 3 A and G), a gene encoding a calcium-binding protein present only in cone photoreceptor cells (37). These visinin-expressing cells were generated de novo under the induction of ngn2, because no such cells were present in any of the control dishes infected with RCAS-GFP (Fig. 3B). Interphotoreceptor retinoid-binding protein (IRBP) was also expressed in similar numbers of cells in dishes infected with RCAS-ngn2 (Fig. 3C), but not in the control (Fig. 3D). In the retina, IRBP is expressed in photoreceptor cells and is involved in shuttling retinoids between photoreceptors and RPE cells. Expression of the red pigment gene, a hallmark of cone photoreceptor cells that sense red light, was also detected in RPE cultures forced to express ngn2 (Fig. 3E). We also detected the expression of α-phosphodiesterase (PDE; Fig. 3F), which is involved in phototransduction. About 10–100-fold more cells expressed the early photoreceptor markers (visinin and IRBP) than the later photoreceptor markers (red pigment and α-PDE). This finding might be a result of different detection sensitivities or the result of in vitro conditions being unfavorable for photoreceptor maturation.
Figure 3.
Expression of photoreceptor-specific genes in RPE cultures under ngn2 induction. (A, C, E, and F) In situ hybridization of E6 RPE culture infected with RCAS-ngn2 with digoxigenin-labeled antisense RNA probes against visinin (A), IRBP (C), the red pigment gene (E), and α-phosphodiesterase (PDE) (F). (B and D) In situ hybridization of cultured E6 RPE cells infected with RCAS-GFP with antisense RNA probes against visinin (B) and IRBP (D). (G) Immunostaining of RPE culture infected with RACS-ngn2 with anti-Visinin protein. (H) A Visinin+ cell with cone photoreceptor morphology. (I) Double-labeling for IRBP mRNA (blue/purple) and Visinin protein (red). Arrows point to double-labeled cells. Magnification: A–G, ×50; H and I, ×200.
To better preserve cellular morphology, immunocytochemistry was used instead of in situ hybridization. Expression of visinin was detected with a mAb against Visinin protein. Like N-CAM+ cells, Visinin+ cells often appeared as clusters (Fig. 3G). Within each cluster, a large number of cells, but not all of the cells, were immunostained. The Visinin+ cells exhibited morphologies typical of young photoreceptor cells under culture conditions (38). Some cells developed an inner segment-like process at the apical end and an axonal process with terminal arboration at the basal end (Fig. 3H). Double-labeling showed that IRBP mRNA and Visinin protein were colocalized to the same cells, with the former restricted to the inner segment-like compartment and the latter distributed in the cell body (Fig. 3I).
Expression of Retinal Ganglion Cell Markers.
We then asked whether the induction of the photoreceptor pathway was the sole downstream event of ngn2 expression. RPE cultures infected with RCAS-ngn2 were examined for the presence of other retinal neurons. To identify ganglion cells, we used both morphological criteria and molecular markers. Retinal ganglion cells typically have long axons and can be identified with mAb RA4 (33), or mAb against Calretinin (unpublished observations), or cNSCL1, which is transiently expressed in ganglion cells (39).
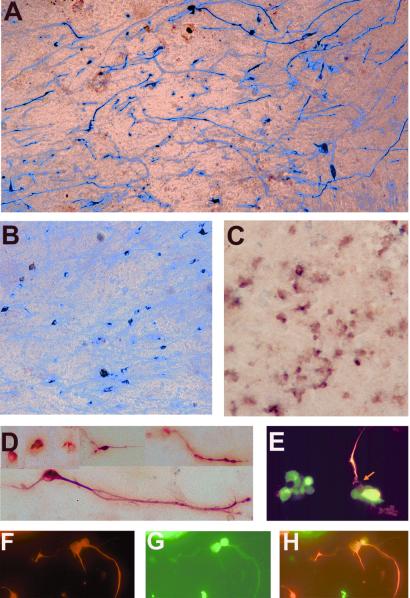
RA4+ were present in RPE cultures infected with RCAS-ngn2 (Fig. 4A) but not in a control culture infected with RCAS-GFP (Table 1). Calretinin+ cells also were present in the experimental dishes (Fig. 4B) but not in the control. We also detected the expression of cNSCL1 specifically in ngn2-expressing cultures (Fig. 4C). Many of the RA4+ and Calretinin+ cells displayed rather long cellular processes (Fig. 4 A and B). Photomicrographs of RA4+ cells at a higher magnification (Fig. 4D) showed that these cells resembled young retinal ganglion cells under similar culture conditions. Double-labeling revealed that RA4 and Calretinin immunoreactivities were colocalized to the same cells (Fig. 4 F–H).
Figure 4.
Expression of ganglion cell markers in RPE cultures under ngn2 induction. (A) Immunocytochemistry with RA4. (B) Immunocytochemistry with anti-Calretinin. (C) In situ hybridization with digoxigenin-labeled RNA probes for cNSCL1. (D) Higher magnification of RA4+ cells from ngn2-expressing RPE culture. (E) Double-labeling with RA4 (in red) and anti-Visinin Ab (in green). Arrow points to an RA4+/Visinin− cell in a cluster of Visinin+ cells. (F–H) Double-labeling with RA4 (in red, F) and anti-Calretinin Ab (in green, G). H is a merge of F and G. Magnification: A–C, ×50; D and E, ×100; F–H, ×80.
Table 1.
Expression of various neural markers in RPE cell cultures infected with retrovirus expressing GFP or ngn2
| Photoreceptor
|
Ganglion
|
Horizontal
and amacrine
|
Neurons in general
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Visinin | IRBP | Red | α-PDE | RA4 | cNSCL1 | Calretinin | AP2 | cNSCL2 | N-CAM | MAP2 | |
| RCAS-GFP | − | − | − | − | + | − | + | − | − | − | − |
| RCAS-ngn2 | +++ | +++ | ++ | + | +++ | ++ | +++ | + | + | +++ | +++ |
No. of positive cells per dish (8 cm2) are indicated by “+” for hundreds, “++” for thousands, and “+++” for equal or more than tens of thousands or too many to count. The data presented were the average of data collected from at least three independent experiments with three dishes used/counted for each marker in one experiment. PDE, phosphodiesterase.
Although cells reminiscent of photoreceptor cells often appeared as clusters, cells with ganglion cell traits were more physically separated. The morphologies of the two populations were also different: the former had short processes typical of young photoreceptor cells and the latter had long processes typical of ganglion cells, indicating that the two types of cells were distinct. We directly addressed the question of whether individual cells expressed both photoreceptor and ganglion cell markers. Double-labeling was carried out to determine whether cells coexpressed visinin, the most abundantly expressed photoreceptor marker among those tested, and RA4, the most abundantly expressed ganglion cell marker. Within a cluster, the two markers labeled different cells (Fig. 4E), further indicating that different types of neural cells were generated in RPE cultures infected with RCAS-ngn2.
Markers that label horizontal cells and amacrine cells were also used in the analysis. AP2 immunoreactivity and expression of cNSCL2, a bHLH gene homologous to Drosophila atonal, label horizontal and amacrine cells in the chick retina (40). Both markers were induced by ngn2 in the RPE culture and they were not expressed in control cultures infected with RCAS-GFP (Table 1). Nonetheless, fewer cells expressed these markers, compared with the photoreceptor and ganglion cell markers (Table 1).
Induction of neuroD.
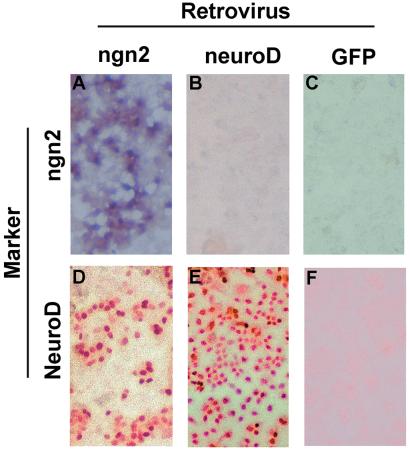
In the chick retina, neuroD appears to be specifically expressed in photoreceptor cells and their precursors (ref. 16; unpublished data). Ectopic expression of neuroD triggers selective de novo generation of photoreceptor cells from RPE cell cultures (16, 17). We examined whether ngn2-induced RPE cell transdifferentiation into photoreceptor cells was mediated by neuroD, and whether there is a linear or circular relationship between ngn2 and neuroD in our RPE culture system. We detected ngn2 mRNA in cultures infected with RCAS-ngn2 (Fig. 5A) but not in cultures infected with RCAS-neuroD (Fig. 5B) or RCAS-GFP (Fig. 5C), indicating that neuroD did not induce ngn2 expression.
Figure 5.
Induction of neuroD by ngn2. (A–C) In situ hybridization with antisense RNA probes against ngn2 in RPE cultures infected with RCAS-ngn2 (A), RCAS-neuroD (B), or RCAS-GFP (C). (D–F) Immunostaining with anti-NeuroD Ab in RPE cultures infected with RCAS-ngn2 (D), RCAS-neuroD (E), or RCAS-GFP (F). Magnification: ×50.
Expression of neuroD in the ngn2 cultures was examined with affinity-purified Abs against the C-terminal nonconserved sequence of chick NeuroD (unpublished data). In control cultures infected with RCAS-GFP, no cells were found to be positive for NeuroD protein (Fig. 5F). In cultures infected with RCAS-neuroD, essentially all cells were stained with anti-NeuroD Ab (Fig. 5E). The Ab also labeled many cells in cultures infected with RCAS-ngn2 (Fig. 5D), demonstrating that ngn2 induced neuroD expression in cultured RPE cells.
Discussion
When ectopically expressed in cultured RPE cells, ngn2 induced the expression of genes associated with photoreceptor production and differentiation (both general neuron markers and photoreceptor-specific markers). Furthermore, ngn2 induced morphological changes from nonneuronal to photoreceptor-like cells. These observations argue strongly that ngn2 is capable of triggering a cascade of gene expression events leading to a photoreceptor phenotype.
Photoreceptor cells were not the only product of ngn2-induced neurogenesis in cell cultures derived from RPE. In fact, de novo generation of ganglion cells, judged by morphological and molecular criteria, was observed. In addition, markers of other types of retinal neurons (amacrine and/or horizontal cells) were also detected in RPE cultures ectopically expressing ngn2. These findings suggest that ngn2 induced at least two, and probably more, retinal pathways.
The presence of nonphotoreceptor neurons indicated that ngn2 exhibited a general proneural activity. However, this does not necessarily rule out the possibility that ngn2 in the developing retina specifies one type of cells. Studies have shown that some proneural genes exhibit flexibility or genetic compensation, and often double-knockouts of proneural genes are needed to demonstrate phenotypic changes in neurogenesis (9). It is possible that when present alone in RPE cells, ngn2 exerted functions that would otherwise be inhibited by other proneural genes present in the retina. Presently, little is known about how proneural genes interact to regulate retinal neurogenesis. In any event, our observations indicate that ngn2 alone could induce cells in an RPE culture to differentiate along at least two different neural pathways.
In the developing chick retina, ngn2 was expressed in retinal neuroepithelium lacking a recognizable histological demarcation. Thus, it is difficult to judge, based on the spatial expression pattern, whether cells expressing ngn2 would develop into a single cell type or multicell types. Expression of ngn2 in BrdUrd+ or PCNA+ cells suggests that it may be involved in a developmental step before terminal mitosis. Considering the current concept that retinal cell fate specification is a postmitotic event (41–43), expression in proliferating cells suggests that ngn2 is involved in a developmental event that precedes retinal cell fate specification. Thus, it is likely that ngn2 plays a role in the genesis of retinal neurons in general, and not a single cell type in particular. Consistent with this scenario was our observation that ngn2 led to the genesis of at least two types of retinal neurons from RPE cell cultures.
Apparently, the detailed molecular events of retinal neurogenesis are different from those of peripheral nervous system (PNS) neurogenesis. During PNS development, ngn2 specifies one type of neuron and ngn1 specifies another type. Both ngn1 and ngn2 induce the expression of neuroD and NSCL1, which are commonly expressed in both types of neurons (23). However, in the chick retina, neuroD and NSCL1 are expressed in different types of cells (16, 39), and neuroD plays a role in specifying a photoreceptor cell fate (16). Furthermore, data in this report suggest that ngn2 is likely to be involved in a general aspect of retinal neurogenesis, not the specification of a single retinal cell type.
An interesting and potentially important observation was the presence of neural clusters, a typical feature of neural stem cells, in RPE cultures ectopically expressing ngn2. Not all of the cells in a cluster were labeled with anti-Visinin Ab. Visinin− cells could be young rod cells or young nonphotoreceptor neurons. They could also be undifferentiated progenitor cells. This finding raises a possibility that ngn2 might induce the generation of retinal stem cells from cultured RPE, which possibility warrants further investigation.
Various approaches have recently been explored to identify renewable sources of cells that have the potential to differentiate into particular types of neurons or neural precursors for cell-based therapies. The possibility of harnessing multipotent stem cells for neural transplantation has recently been demonstrated (44). Among the many possible cells to use, embryonic stem cells and neural stem cells, particularly retinal stem cells, hold promise as renewable sources of cells for generating retinal neurons. Our study showed that cultured RPE cells could be guided toward retinal neuron pathways by a bHLH gene, ngn2, offering a potential source of cells for generating retinal neurons.
Acknowledgments
We thank Dr. Stephen Hughes (National Cancer Institute) for shuttle vector ClaI2Nco and proviral vector RCAS, and Dr. Steven McLoon for mAb RA4. This work was supported by National Institutes of Health Grant EY11640, the EyeSight Foundation, and by Research to Prevent Blindness (unrestricted grant to Department of Ophthalmology, University of Alabama at Birmingham).
Abbreviations
- RPE
retinal pigment epithelium
- bHLH
basic helix–loop–helix
- En
embryonic day n
- PCNA
proliferating cell nuclear antigen
- N-CAM
neural cell adhesion molecule
- RCAS
replication-competent retrovirus
- GFP
green fluorescent protein
- IRBP
interphotoreceptor retinoid-binding protein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Dacheux R F, Raviola E. In: Principles and Practice of Ophthalmology. Albert D M, Jacobiec F A, editors. Philadelphia: Saunders; 2000. pp. 1601–1624. [Google Scholar]
- 2.Stone J L, Barlow W E, Humayun M S, de Juan E, Jr, Milam A H. Arch Ophthalmol. 1992;110:1634–1639. doi: 10.1001/archopht.1992.01080230134038. [DOI] [PubMed] [Google Scholar]
- 3.Curcio C A, Medeiros N E, Millican C L. Invest Ophthalmol Visual Sci. 1996;37:1236–1249. [PubMed] [Google Scholar]
- 4.Quigley H A, Dunkelberger G R, Green W R. Am J Ophthalmol. 1989;107:453–464. doi: 10.1016/0002-9394(89)90488-1. [DOI] [PubMed] [Google Scholar]
- 5.Berson E L, Jakobiec F A. Ophthalmology. 1999;106:445–446. doi: 10.1016/S0161-6420(99)90134-3. [DOI] [PubMed] [Google Scholar]
- 6.Livesey F J, Cepko C L. Nat Rev Neurosci. 2001;2:109–118. doi: 10.1038/35053522. [DOI] [PubMed] [Google Scholar]
- 7.Tomita K, Ishibashi M, Nakahara K, Ang S L, Nakanishi S, Guillemot F, Kageyama R. Neuron. 1996;16:723–734. doi: 10.1016/s0896-6273(00)80093-8. [DOI] [PubMed] [Google Scholar]
- 8.Perron M, Opdecamp K, Butler K, Harris W A, Bellefroid E J. Proc Natl Acad Sci USA. 1999;96:14996–15001. doi: 10.1073/pnas.96.26.14996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatakeyama J, Tomita K, Inoue T, Kageyama R. Development (Cambridge, UK) 2001;128:1313–1322. doi: 10.1242/dev.128.8.1313. [DOI] [PubMed] [Google Scholar]
- 10.Wang S W, Kim B S, Ding K, Wang H, Sun D, Johnson R L, Klein W H, Gan L. Genes Dev. 2001;15:24–29. doi: 10.1101/gad.855301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown N L, Patel S, Brzezinski J, Glaser T. Development (Cambridge, UK) 2001;128:2497–2508. doi: 10.1242/dev.128.13.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu W, Mo Z, Xiang M. Proc Natl Acad Sci USA. 2001;98:1649–1654. doi: 10.1073/pnas.98.4.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutcheson D A, Vetter M L. Dev Biol. 2001;232:327–338. doi: 10.1006/dbio.2001.0178. [DOI] [PubMed] [Google Scholar]
- 14.Brown N L, Kanekar S, Vetter M L, Tucker P K, Gemza D L, Glaser T. Development (Cambridge, UK) 1998;125:4821–4833. doi: 10.1242/dev.125.23.4821. [DOI] [PubMed] [Google Scholar]
- 15.Matter-Sadzinski L, Matter J M, Ong M T, Hernandez J, Ballivet M. Development (Cambridge, UK) 2001;128:217–231. doi: 10.1242/dev.128.2.217. [DOI] [PubMed] [Google Scholar]
- 16.Yan R-T, Wang S-Z. J Neurobiol. 1998;36:485–496. [PMC free article] [PubMed] [Google Scholar]
- 17.Yan R-T, Wang S-Z. Neurosci Lett. 2000;280:83–86. doi: 10.1016/s0304-3940(99)01003-4. [DOI] [PubMed] [Google Scholar]
- 18.Morrow E M, Furukawa T, Lee J E, Cepko C L. Development (Cambridge, UK) 1999;126:23–36. doi: 10.1242/dev.126.1.23. [DOI] [PubMed] [Google Scholar]
- 19.Sommer L, Ma Q, Anderson D J. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- 20.Ma Q, Kintner C, Anderson D J. Cell. 1996;87:43–52. doi: 10.1016/s0092-8674(00)81321-5. [DOI] [PubMed] [Google Scholar]
- 21.Ma Q, Chen Z, del Barco Barrantes I, de la Pompa J L, Anderson D J. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- 22.Fode C, Gradwohl G, Morin X, Dierich A, LeMeur M, Goridis C, Guillemot F. Neuron. 1998;20:483–494. doi: 10.1016/s0896-6273(00)80989-7. [DOI] [PubMed] [Google Scholar]
- 23.Ma Q, Fode C, Guillemot F, Anderson D J. Genes Dev. 1999;13:1717–1728. doi: 10.1101/gad.13.13.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez S E, Rebelo S, Anderson D J. Development (Cambridge, UK) 1999;126:1715–1728. doi: 10.1242/dev.126.8.1715. [DOI] [PubMed] [Google Scholar]
- 25.Eguchi G. In: Current Topics in Developmental Biology. Moscona A A, Monroy A, editors. New York: Academic; 1986. pp. 21–37. [Google Scholar]
- 26.Dutt K, Scott M, Sternberg P P, Linser P J, Srinivasan A. DNA Cell Biol. 1993;12:667–673. doi: 10.1089/dna.1993.12.667. [DOI] [PubMed] [Google Scholar]
- 27.Zhao S, Rizzolo L J, Barnstable C J. Int Rev Cytol. 1997;171:225–265. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- 28.Orts-Llorca F, Genis-Galvez J M. Acta Anat. 1960;42:31–70. doi: 10.1159/000141635. [DOI] [PubMed] [Google Scholar]
- 29.Coulombre J L, Coulombre A J. Dev Biol. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- 30.Park C M, Hollenberg M J. Dev Biol. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- 31.Pittack C, Jones M, Reh T A. Development (Cambridge, UK) 1991;113:577–588. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- 32.Yan R-T, Wang S-Z. Visual Neurosci. 2000;17:157–164. doi: 10.1017/s0952523800171172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waid D K, McLoon S C. Neuron. 1995;14:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- 34.McCormick M B, Tamimi R M, Snider L, Asakura A, Bergstrom D, Tapscott S J. Mol Cell Biol. 1996;16:5792–5800. doi: 10.1128/mcb.16.10.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li C-M, Yan R-T, Wang S-Z. Dev Dyn. 1999;215:238–247. doi: 10.1002/(SICI)1097-0177(199907)215:3<238::AID-AJA6>3.0.CO;2-F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hughes S H, Greenhouse J J, Petropoulos C J, Sutrave P. J Virol. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamagata K, Goto K, Kuo C H, Kondo H, Miki N. Neuron. 1990;4:469–476. doi: 10.1016/0896-6273(90)90059-o. [DOI] [PubMed] [Google Scholar]
- 38.Madreperla S A, Edidin M, Adler R. J Cell Biol. 1989;109:1483–1493. doi: 10.1083/jcb.109.4.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li C-M, Yan R-T, Wang S-Z. Mol Cell Neurosci. 1999;14:17–27. doi: 10.1006/mcne.1999.0765. [DOI] [PubMed] [Google Scholar]
- 40.Li, C.-M., Yan, R.-T. & Wang, S.-Z. (2001) Invest. Ophthalmol. Visual Sci., in press.
- 41.Ezzeddine Z D, Yang X, DeChiara T, Yancopoulos G, Cepko C L. Development (Cambridge, UK) 1997;124:1055–1067. doi: 10.1242/dev.124.5.1055. [DOI] [PubMed] [Google Scholar]
- 42.Belliveau M J, Young T L, Cepko C L. J Neurosci. 2000;20:2247–2254. doi: 10.1523/JNEUROSCI.20-06-02247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Adler R. Prog Retina Eye Res. 2000;19:529–557. doi: 10.1016/s1350-9462(00)00008-2. [DOI] [PubMed] [Google Scholar]
- 44.Brustle O, Jones K N, Learish R D, Karram K, Choudhary K, Wiestler O D, Duncan I D, McKay R D. Science. 1999;285:754–756. doi: 10.1126/science.285.5428.754. [DOI] [PubMed] [Google Scholar]