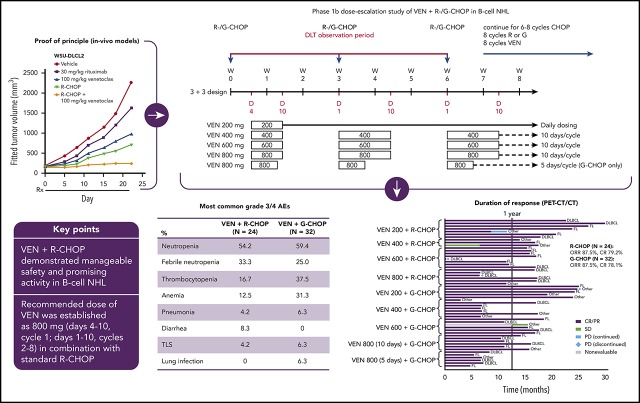
Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
In this phase 1b study, venetoclax plus R-CHOP demonstrated manageable safety and promising activity in B-cell NHL.
Recommended dose of venetoclax was established as 800 mg (days 4-10, cycle 1; days 1-10, cycles 2-8) in combination with standard R-CHOP.
Abstract
Novel strategies, such as chemosensitization with targeted agents, that build on the success of standard immunochemotherapy show promise for the treatment of non-Hodgkin lymphoma (NHL). Here, we report a phase 1b study investigating dose escalation of the BCL2 inhibitor, venetoclax, in combination with rituximab or obinutuzumab and cyclophosphamide, doxorubicin, vincristine, and prednisone (R-/G-CHOP) chemotherapy in B-cell NHL. Objectives included safety assessment and determination of a recommended phase 2 dose (RP2D). Fifty-six patients were enrolled, most with follicular lymphoma (43%) or diffuse large B-cell lymphoma (DLBCL; 32%). Dose-limiting toxicities were reported in 3/14 patients at the first venetoclax dose (200 mg/d), after which dosing was changed from daily to 10 days per cycle and escalated to 800 mg. A further reduction to 5 days per cycle occurred at the 800-mg dose level in the G-CHOP arm. Cytopenias were predominant among grade 3/4 events and reported at a higher rate than expected, particularly in the G-CHOP arm; however, safety was manageable. Overall response rates were 87.5% (R-CHOP and G-CHOP combinations); complete response (CR) rates were 79.2% and 78.1%, respectively. Most double-expressor (BCL2+ and MYC+) DLBCL patients (87.5%; n = 7/8) achieved CR. Although the maximum tolerated dose was not reached, the RP2D for venetoclax with R-CHOP was established at 800 mg days 4 to 10 of cycle 1 and days 1 to 10 of cycles 2 to 8; higher doses were not explored, and this dosing schedule demonstrated an acceptable safety profile. This regimen is subsequently being evaluated in first-line DLBCL in the phase 2 portion of the study. This trial was registered at www.clinicaltrials.gov as #NCT02055820.
Visual Abstract
Introduction
BCL2 is an important prosurvival molecule and a key member of a family of proteins that governs the intrinsic apoptosis pathway.1 Overexpression of BCL2 due to t(14;18) chromosomal translocation is found in ∼90% of cases of follicular lymphoma (FL).2,3 The same translocation is present in 15% to 30% of patients with diffuse large B-cell lymphoma (DLBCL), with 8% to 30% exhibiting BCL2 amplification.4-9 BCL2 overexpression confers resistance to the proapoptotic activities of chemotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and is associated with poor prognosis in patients with first-line (1L) DLBCL.10,11 In particular, patients with concurrent overexpression of BCL2 and MYC proteins (“double-expressor” lymphoma; DE) or concurrent translocations of both MYC and BCL2 genes (“double-hit” lymphoma) have inferior outcomes relative to other groups.6,10-14 Inhibition of BCL2 is therefore an attractive therapeutic target for B-cell malignancies, particularly because it acts independently of the often dysfunctional tumor suppressor protein, TP53, which lies upstream and renders B cells resistant to chemotherapy.15,16
Venetoclax is a highly selective, potent, oral BCL2 inhibitor that is approved in >50 countries, including in the United States, for the treatment of adult patients with chronic lymphocytic leukemia with or without 17p deletion (del[17p]), who have received at least 1 prior therapy, and in the European Union for adult chronic lymphocytic leukemia patients with del(17p) or TP53 mutation, who are unsuitable for or have failed a B-cell receptor pathway inhibitor, or without del(17p) or TP53 mutation who have failed both chemoimmunotherapy and a B-cell receptor pathway inhibitor.17-19 Recently, a single-agent dose-escalation trial of venetoclax in relapsed/refractory non-Hodgkin lymphoma (NHL) reported an overall response rate (ORR) of 38% (complete response [CR] rate, 14%) and 18% (CR rate, 12%) in patients with FL and DLBCL, respectively.20
Obinutuzumab (GA101; G) is a glycoengineered, type II monoclonal anti-CD20 antibody, with greater direct cell death induction, antibody-dependent cellular cytotoxicity, and phagocytosis than rituximab (R).21 In the phase 3 GALLIUM trial, FL patients treated with G plus chemotherapy had longer progression-free survival (PFS) than patients treated with R plus chemotherapy, but end-of-induction response rates were similar in both groups (88.5% vs 86.9%, respectively).22 In the phase 3 GOYA study in 1L DLBCL patients, G-CHOP and R-CHOP demonstrated similar activity (with CR rates of 56.7% and 59.5%, respectively); the primary end point of improved PFS with G-CHOP over R-CHOP was not met.23
Preclinical data demonstrated synergy when venetoclax was combined with R24 or G in vitro and increased efficacy of venetoclax plus R when combined with CHOP in vivo in DLBCL xenograft models (supplemental Appendix, available on the Blood Web site). Based on these findings and mode of action, venetoclax may have potential as a chemosensitizing agent.
The CAVALLI study explored the safety and efficacy of combining venetoclax with R-CHOP or G-CHOP chemotherapy in patients with NHL. Here, we report results of the phase 1b part of CAVALLI.
Methods
Preclinical materials and methods
Details of the preclinical analyses of venetoclax with R and G, with and without CHOP, in NHL models are available in the supplemental Appendix.
Study design and patients
This phase 1b/2 multicenter, open-label study was conducted to assess oral administration of venetoclax in combination with IV R or G plus standard doses of CHOP in patients with B-cell NHL (phase 1b) and 1L DLBCL (phase 2) (clinicaltrials.gov identifier: #NCT02055820; European Union Clinical Trials Register identifier: 2013-003749-40). The study was conducted in 2 stages: the dose-escalation phase 1b stage followed a modified 3+3 design to guide dose and schedule selection for the phase 2 expansion stage in 1L DLBCL. The phase 1b stage was conducted at 15 sites across North America, Europe, and Australia.
The study protocol was approved by the institutional review board or ethics committee at participating institutions in accordance with the International Conference on Harmonization guidelines, including Good Clinical Practice and the ethical principles originating from the Declaration of Helsinki.25,26 Informed consent was obtained from all patients. A scientific oversight committee, including 3 experts in NHL, reviewed safety and efficacy data regularly during the conduct of the study, along with the study internal monitoring committee. All authors had access to study data.
Eligible patients were aged 18 years or older, with histologically confirmed B-cell NHL (transformed lymphomas, including Richter syndrome cases, were considered following discussion with the Medical Monitor); untreated or with 1 prior therapy (excluding R-CHOP); 1 or more bidimensionally measurable lesions >1.5 cm; Eastern Cooperative Oncology Group (ECOG) performance status ≤2; and adequate organ and hematologic function, defined as hemoglobin ≥9 g/dL, absolute neutrophil count ≥1.5 × 109/L, and platelet count ≥75 × 109/L (unless due to underlying disease, as evidenced by extensive bone marrow [BM] involvement).
Exclusion criteria included mantle cell lymphoma or small lymphocytic lymphoma histology; primary mediastinal DLBCL; primary central nervous system lymphoma or secondary involvement; chemotherapy or other investigational therapy within 28 days prior to start of cycle 1; significant cardiovascular or liver disease that could affect compliance with the protocol or interpretation of results or that could increase risk to the patient; or known HIV infection. A full list of inclusion/exclusion criteria is provided in the supplemental Appendix.
Treatments
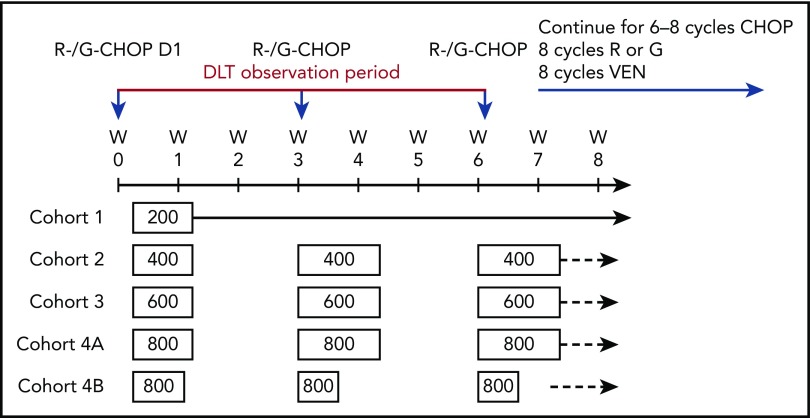
The phase 1b portion of the study included 2 parallel treatment arms, with planned venetoclax doses ranging from 200 to 800 mg once daily orally on 21 days per cycle plus standard cycles of R-CHOP (arm A) or G-CHOP (arm B). Standard CHOP chemotherapy consisted of IV cyclophosphamide 750 mg/m2, IV doxorubicin 50 mg/m2, and IV vincristine 1.4 mg/m2 (with a 2.0-mg cap) on day 1, and prednisone 100 mg/d orally on days 1 to 5. In arm A, venetoclax was combined with R (375 mg/m2 IV) on day 1 for 8 cycles. In arm B, venetoclax was combined with G (1000 mg IV) on days 1, 8, and 15 of cycle 1 and day 1 of cycles 2 to 8. Venetoclax was initiated on day 4 of cycle 1 and then on day 1 for cycles 2 to 8 to mitigate the potential risk and allow distinction of tumor lysis syndrome (TLS) induced by venetoclax from that caused by R-CHOP or G-CHOP. CHOP chemotherapy was given for 6 or 8 cycles on a patient-by-patient basis after discussion between the Investigator and Medical Monitor. Patients receiving only 6 cycles of CHOP received 2 additional 21-day cycles of venetoclax plus anti-CD20 antibody on day 1 of the cycle to ensure equal duration of therapy in all patients. Protocol-mandated treatment modifications are summarized in supplemental Table 1.
Venetoclax dose escalation was under a modified 3+3 design, with at least 3 patients per arm enrolled into each cohort. A 6-week (ie, 2-cycle) observation period was allowed for evaluation of dose-limiting toxicities (DLTs). If none of the first 3 evaluable patients experienced a DLT, the next dose cohort was opened. If a DLT was observed in 1 patient, additional patients were enrolled at that dose level until at least 6 evaluable patients had completed the DLT observation window or a second DLT occurred. If no additional DLTs were reported, the next dose could be evaluated. If an additional DLT was observed after cohort expansion, further enrollment on that dosing schedule was halted and that dose was either declared as exceeding the maximum tolerated dose (MTD) or a different dosing regimen was implemented, in which case dose escalation could resume.
Granulocyte-colony stimulating factor was mandated in later protocol versions for all cycles with CHOP (administration formulation and length of administration by Investigator’s choice). Anti-infective prophylaxis for viral, fungal, bacterial, or Pneumocystis jirovecii infections was permitted. A short (<7 days) course of steroids (up to 100 mg of prednisone or equivalent daily) was allowed before initiation of study treatment of palliation of lymphoma-related symptoms. Warfarin could be administered under the guidance of the Medical Monitor.
Mandatory prophylaxis for TLS included hydration and administration of a uric acid–reducing agent (eg, allopurinol), orally beginning 72 hours prior to the first venetoclax dose (details in the supplemental Appendix). Patients with bulky disease (ie, any lymph node or mass more than either 8 or 10 cm on the screening computed tomography [CT] depending on the protocol version) and/or lymphocytosis due to circulating lymphoma cells were considered to be at higher TLS risk and required hospitalization for more intensive prophylaxis (eg, rasburicase) and monitoring during the initial dose of venetoclax. Supportive measures were permitted per local standard of care.
Objectives and outcomes
The primary objective was to determine the MTD of venetoclax when given in combination with R-CHOP or G-CHOP, defined as the highest dose resulting in DLTs in less than one-third of patients in a cohort of 6 or more patients. The MTD and dosing schedule for venetoclax when combined with R-CHOP and G-CHOP were to be taken forward as the recommended phase 2 dose (RP2D) for the respective arm, unless there was any safety or tolerability indication that a lower dose would be more appropriate. Secondary objectives were to assess efficacy (response, duration of response, PFS, and overall survival) and characterize the pharmacokinetic profiles of venetoclax plus R-CHOP or G-CHOP. Exploratory objectives included biomarker assessments and preliminary assessment of the efficacy of venetoclax plus R-CHOP in different potential prognostic subgroups. See the supplementary Appendix for additional methodology on pharmacokinetics and exploratory objectives.
Safety
Laboratory assessments and adverse event (AE) monitoring were used to assess safety. AEs were coded using the most recent version of the Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0 (NCI CTCAE v4.0). The MTD of venetoclax plus R-CHOP or G-CHOP was determined separately for each arm. DLTs were any grade ≥3 AEs attributed to venetoclax plus R-CHOP or G-CHOP, as well as grade 3/4 neutropenia or thrombocytopenia, identified on day 1 of cycle 2 or 3, resulting in delay of that cycle. The DLT window was defined as the first 2 cycles, because venetoclax administration started on day 4 of cycle 1. The following AEs were excluded as DLTs: grade 3/4 neutropenia not accompanied by fever and improved to grade 2 by day 1 of next cycle; grade 3 febrile neutropenia; grade 3 TLS without manifestations of clinical TLS according to Howard criteria27; grade 3 nausea or vomiting ≤7 days.
Hematologic assessment to monitor for cytopenias occurred throughout treatment, including complete blood counts performed on days 1, 4, 5, 8, and 15 of cycle 1, days 1 and 10 of cycles 2 to 6, and day 1 of cycles 7 and 8.
Efficacy
Responses were assessed by the investigators and an independent review committee according to a modified version of Lugano 2014 criteria,28 which required confirmation of a partial response (PR) by a decrease in lesion size by 50% using CT and confirmation of a CR by clearing of BM on biopsy/aspirate if involved with lymphoma at diagnosis. Response assessment by clinical evaluation and imaging occurred after cycle 4 and at end of treatment. Positron emission tomography (PET)-CT was mandatory at screening and end of treatment, but diagnostic CT could be used at the cycle 4 assessment. After end of treatment, clinical assessment occurred every 3 months until progressive disease (PD) or end of study, with CT scans every 6 months for 2 years and as clinically indicated.
Statistical analysis
Sample size for the dose-finding stage was based on a modified 3+3 design in order to guide dose and schedule selection for the phase 2 portion of the study on the basis of DLTs. The expected enrollment for the dose-finding stage was 24 to 60 patients. The intent-to-treat population, which included all enrolled patients regardless of any treatment delivered, was used to evaluate efficacy. All patients who received venetoclax, R-CHOP, or G-CHOP in any amount were included in the safety and pharmacokinetic populations. Efficacy and safety evaluations were summarized descriptively. Analysis was performed at Roche Products Ltd (Welwyn Garden City, UK).
Results
Preclinical data
Preclinical in vitro and in vivo studies in NHL cell lines and xenograft models showing enhanced cell death induction and antitumoral efficacy for the combination of venetoclax and G (supplemental Figures 1 and 2) provided proof of principle for the clinical investigation of this combination in a phase 1b study in NHL patients. Significantly increased direct cell death was observed with the addition of venetoclax to R or G; furthermore, venetoclax did not impact antibody-dependent cellular cytotoxicity compared with the antibodies alone. In in vivo models, reduction in tumor volume was greatest in animals treated with the combination of R or G (± CHOP) plus venetoclax.
Patient disposition and demographics
Data cutoff was June 30, 2017. Fifty-six patients (arm A with R-CHOP, n = 24; arm B with G-CHOP, n = 32) were enrolled in this phase 1b study. All patients were included in both the efficacy and the safety analysis populations. Patient demographics and baseline characteristics are shown in Table 1. Most patients had FL or DLBCL. Most (91.1%) patients had no prior therapy; of the 5 patients with prior therapy, 4 had FL and 1 had marginal zone lymphoma.
Table 1.
Patient demographics and baseline characteristics
| Daily VEN administration | VEN administration for 10 d per 21-d cycle | |||||
|---|---|---|---|---|---|---|
| Characteristic, n (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | ||
| Cohort 1 (n = 7) | Cohort 2 (n = 3) | Cohort 3 (n = 8) | Cohort 4 (n = 6) | Total (N = 24) | ||
| Arm A: VEN + R-CHOP | ||||||
| Mean age, y (range) | 67.0 (57-79) | 61.0 (52-76) | 57.0 (37-70) | 56.3 (44-77) | 60.3 (37-79) | |
| Sex | ||||||
| Male | 4 (57.1) | 1 (33.3) | 6 (75.0) | 4 (66.7) | 15 (62.5) | |
| Female | 3 (42.9) | 2 (66.7) | 2 (25.0) | 2 (33.3) | 9 (37.5) | |
| ECOG performance status | ||||||
| 0-1 | 7 (100) | 3 (100) | 7 (87.5) | 6 (100) | 23 (95.8) | |
| 2 | 0 | 0 | 1 (12.5) | 0 | 1 (4.2) | |
| BM involvement | ||||||
| Yes | 1 (14.3) | 1 (33.3) | 3 (37.5) | 1 (16.7) | 6 (25.0) | |
| No | 6 (85.7) | 1 (33.3) | 4 (50.0) | 4 (66.7) | 15 (62.5) | |
| Unknown | 0 | 1 (33.3) | 1 (12.5) | 1 (16.7) | 3 (12.5) | |
| Histology | ||||||
| DLBCL | 3 (42.9) | 0 | 2 (25.0) | 5 (83.3) | 10 (41.7) | |
| FL | 3 (42.9) | 1 (33.3) | 6 (75.0) | 0 | 10 (41.7) | |
| Other* | 1 (14.3) | 2 (66.7) | 0 | 1 (16.7) | 4 (16.7) | |
| Number of patients with prior therapies† | 1 | 0 | 1 | 0 | 2 | |
| Number of patients with bulky disease (cutoff 10 cm) | 0 | 0 | 1 | 0 | 1 | |
| Number of patients with lymphocytosis | 0 | 0 | 1 | 1 | 2 | |
| Daily VEN administration | VEN administration for 10 d per 21-d cycle/5 d for Cohort 4B | |||||
|---|---|---|---|---|---|---|
| Characteristic, n (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | VEN 800 mg, | |
| Cohort 1 (n = 7) | Cohort 2 (n = 7) | Cohort 3 (n = 6) | Cohort 4A (n = 6) | Cohort 4B (n = 6) | Total (N = 32) | |
| Arm B: VEN + G-CHOP | ||||||
| Mean age, y (range) | 52.1 (24-69) | 60.9 (55-73) | 66.7 (61-71) | 60.5 (39-76) | 66.8 (58-74) | 61.1 (24-76) |
| Sex | ||||||
| Male | 5 (71.4) | 2 (28.6) | 2 (33.3) | 2 (33.3) | 4 (66.7) | 15 (46.9) |
| Female | 2 (28.6) | 5 (71.4) | 4 (66.7) | 4 (66.7) | 2 (33.3) | 17 (53.1) |
| ECOG performance status | ||||||
| 0-1 | 7 (100) | 7 (100) | 6 (100) | 6 (100) | 5 (83.3) | 31 (96.9) |
| 2 | 0 | 0 | 0 | 0 | 1 (16.7) | 1 (3.1) |
| Number of patients with prior therapies† | 1 | 0 | 1 | 1 | 0 | 3 |
| Number of patients with bulky disease (cutoff 10 cm) | 0 | 1 | 1 | 0 | 0 | 2 |
| Number of patients with lymphocytosis | 2 | 1 | 0 | 1 | 0 | 4 |
| BM involvement | ||||||
| Yes | 2 (28.6) | 2 (28.6) | 3 (50.0) | 3 (50.0) | 2 (33.3) | 12 (37.5) |
| No | 5 (74.1) | 4 (57.1) | 3 (50.0) | 3 (50.0) | 4 (66.7) | 19 (59.4) |
| Unknown | 0 | 1 (14.3) | 0 | 0 | 0 | 1 (3.1) |
| Histology | ||||||
| DLBCL | 0 | 1 (14.3) | 2 (33.3) | 2 (33.3) | 3 (50.0) | 8 (25.0) |
| FL | 3 (42.9) | 3 (42.9) | 3 (50.0) | 3 (50.0) | 2 (33.3) | 14 (43.8) |
| Other* | 4 (57.1) | 3 (42.9) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 10 (31.3) |
VEN, venetoclax.
Transformed lymphoma, marginal zone lymphoma, composite lymphoma, and Waldenström macroglobulinemia. Given the mixed population of NHL patients enrolled in the study, risk categories for the International Prognostic Index (DLBCL patients) and Follicular Lymphoma International Prognostic Index (FL patients) were not collected on the case report form.
One patient with FL received 2 prior therapies (1L rituximab and then second-line cyclophosphamide, vincristine, and prednisone followed by rituximab maintenance); however, this was considered a protocol violation. Four (7%) additional patients received 1 prior therapy, including 1 patient with FL who received rituximab, 2 patients with FL who received radiotherapy, and 1 patient with marginal zone lymphoma who received rituximab, cyclophosphamide, vincristine, and prednisone.
DLTs, modification of venetoclax dosing schedule, and RP2D
In both arms, the initial starting dose (cohort 1) of venetoclax was 200 mg/d for 8 cycles (21-day cycles). One patient in arm A (R-CHOP) cohort 1 and 2 patients in arm B (G-CHOP) cohort 1 experienced a DLT (supplemental Table 2). The incidence of cytopenias (Table 2; supplemental Table 3) and DLTs led the scientific oversight committee to recommend a shorter venetoclax dosing schedule of 400 to 800 mg on days 4 to 10 of cycle 1 and days 1 to 10 of cycles 2 to 8 in both arms to mitigate the unexpectedly high hematologic toxicity (Figure 1). Details of DLTs in subsequent cohorts are provided in supplemental Table 2. High levels of cytopenias, predominantly midcycle thrombocytopenia, in the first 6 patients in cohort 4 (800 mg venetoclax) of arm B led to further shortening of the venetoclax dosing schedule (cohort 4B); these 6 patients received venetoclax 800 mg on days 4 to 8 of cycle 1 and days 1 to 5 of cycles 2 to 8, resulting in reduced incidence of all-grade and grade 4 thrombocytopenia. Two patients in arm B cohort 4A who experienced midcycle thrombocytopenia also subsequently had their venetoclax therapy reduced to 5 days per cycle. Platelet counts for 3 patients in arm B cohort 4A are shown in supplemental Figure 3; lower platelet counts were observed in later cycles.
Table 2.
All-grade AEs occurring in ≥20% of patients overall in either arm
| Arm A: VEN + R-CHOP, n (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | Total (N = 24) |
|---|---|---|---|---|---|
| Cohort 1 (n = 7) | Cohort 2 (n = 3) | Cohort 3 (n = 8) | Cohort 4 (n = 6) | ||
| Neutropenia | 4 (57.1) | 2 (66.7) | 5 (62.5) | 2 (33.3) | 13 (54.2) |
| Nausea | 3 (42.9) | 2 (66.7) | 1 (12.5) | 5 (83.3) | 11 (45.8) |
| Diarrhea | 3 (42.9) | 2 (66.7) | 2 (25.0) | 3 (50.0) | 10 (41.7) |
| Fatigue | 5 (71.4) | 0 | 3 (37.5) | 2 (33.3) | 10 (41.7) |
| Neuropathy peripheral | 2 (28.6) | 1 (33.3) | 3 (37.5) | 3 (50.0) | 9 (37.5) |
| Constipation | 1 (14.3) | 2 (66.7) | 3 (37.5) | 2 (33.3) | 8 (33.3) |
| Peripheral sensory neuropathy | 3 (42.9) | 1 (33.3) | 2 (25.0) | 2 (33.3) | 8 (33.3) |
| Anemia | 4 (57.1) | 1 (33.3) | 1 (12.5) | 2 (33.3) | 8 (33.3) |
| Febrile neutropenia | 3 (42.9) | 0 | 2 (25.0) | 3 (50.0) | 8 (33.3) |
| Thrombocytopenia | 4 (57.1) | 1 (33.3) | 2 (25.0) | 0 | 7 (29.2) |
| Cough | 2 (28.6) | 0 | 3 (37.5) | 1 (16.7) | 6 (25.0) |
| Infusion-related reaction | 1 (14.3) | 2 (66.7) | 1 (12.5) | 2 (33.3) | 6 (25.0) |
| Stomatitis | 2 (28.6) | 1 (33.3) | 1 (12.5) | 2 (33.3) | 6 (25.0) |
| Asthenia | 2 (28.6) | 1 (33.3) | 3 (37.5) | 0 | 6 (25.0) |
| Hypokalemia | 3 (42.9) | 1 (33.3) | 2 (25.0) | 0 | 6 (25.0) |
| Dyspnea | 3 (42.9) | 1 (33.3) | 0 | 1 (16.7) | 5 (20.8) |
| Headache | 1 (14.3) | 0 | 3 (37.5) | 1 (16.7) | 5 (20.8) |
| Vomiting | 0 | 2 (66.7) | 1 (12.5) | 2 (33.3) | 5 (20.8) |
| Arm B: VEN + G-CHOP, n (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | VEN 800 mg, | Total (N = 32) |
|---|---|---|---|---|---|---|
| Cohort 1 (n = 7) | Cohort 2 (n = 7) | Cohort 3 (n = 6) | Cohort 4A (n = 6) | Cohort 4B (n = 6) | ||
| Neutropenia | 2 (28.6) | 4 (57.7) | 5 (83.3) | 6 (100.0) | 2 (33.3) | 19 (59.4) |
| Nausea | 3 (42.9) | 4 (57.1) | 3 (50.0) | 5 (83.3) | 4 (66.7) | 19 (59.4) |
| Constipation | 6 (85.7) | 3 (42.9) | 2 (33.3) | 2 (33.3) | 1 (16.7) | 14 (43.8) |
| Vomiting | 5 (71.4) | 4 (57.1) | 0 | 4 (66.7) | 1 (16.7) | 14 (43.8) |
| Diarrhea | 5 (71.4) | 3 (42.9) | 2 (33.3) | 2 (33.3) | 1 (16.7) | 13 (40.6) |
| Fatigue | 5 (71.4) | 2 (28.6) | 2 (33.3) | 3 (50.0) | 1 (16.7) | 13 (40.6) |
| Thrombocytopenia | 2 (28.6) | 3 (42.9) | 2 (33.3) | 4 (66.7) | 2 (33.3) | 13 (40.6) |
| Infusion-related reaction | 4 (57.1) | 1 (14.3) | 2 (33.3) | 2 (33.3) | 3 (50.0) | 12 (37.5) |
| Anemia | 1 (14.3) | 1 (14.3) | 1 (16.7) | 5 (83.3) | 4 (66.7) | 12 (37.5) |
| Cough | 3 (42.9) | 3 (42.9) | 0 | 3 (50.0) | 2 (33.3) | 11 (34.4) |
| Pyrexia | 1 (14.3) | 5 (71.4) | 2 (33.3) | 1 (16.7) | 2 (33.3) | 11 (34.4) |
| Dysgeusia | 1 (14.3) | 3 (42.9) | 1 (16.7) | 2 (33.3) | 4 (66.7) | 11 (34.4) |
| Febrile neutropenia | 2 (28.6) | 4 (57.1) | 0 | 1 (16.7) | 1 (16.7) | 8 (25.0) |
| Decreased appetite | 2 (28.6) | 2 (28.6) | 1 (16.7) | 2 (33.3) | 1 (16.7) | 8 (25.0) |
| Headache | 3 (42.9) | 3 (42.9) | 1 (16.7) | 0 | 0 | 7 (21.9) |
| Peripheral sensory neuropathy | 0 | 1 (14.3) | 1 (16.7) | 1 (16.7) | 1 (16.7) | 4 (12.5) |
| Dyspnea | 0 | 1 (14.3) | 0 | 1 (16.7) | 1 (16.7) | 3 (9.4) |
| Stomatitis | 0 | 0 | 1 (16.7) | 0 | 1 (16.7) | 2 (6.3) |
Percentages represent the incidence of that specific AE per cohort in each arm.
AEs were coded using the most recent version of the Medical Dictionary for Regulatory Activities and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0 (NCI CTCAE v4.0). The protocol-specified safety reporting window was 30 d after the last dose of VEN or CHOP, or 90 d after the last dose of rituximab or obinutuzumab. After this period, only serious AEs, AEs of special interest, and AEs that were deemed by the investigator to be related to study drug were reported.
Figure 1.
Dose-escalation cohorts (3+3 design): modified dosing schema. Four dosing cohorts received venetoclax ranging from 200 to 800 mg every day plus R-CHOP or G-CHOP. Standard CHOP chemotherapy was administered consisting of IV cyclophosphamide 750 mg/m2, IV doxorubicin 50 mg/m2, and IV vincristine 1.4 mg/m2 (with a 2.0-mg cap) on day 1, and prednisone 100 mg/day orally on days 1 to 5. A 6-week (ie, 2-cycle) observation period was allowed for evaluation of DLTs. Patients who experienced responses without excessive toxicity were allowed up to 8 cycles of CHOP at the investigator’s discretion after discussion with the Medical Monitor. D, day; W, week.
Cytopenias, including febrile neutropenia, as well as diarrhea were reported at increased frequency over that expected with the R-CHOP backbone and were considered to be related to combination therapy. Although these toxicities were adequately managed with medical intervention and dose interruptions, the scientific oversight committee did not recommend further dose escalation beyond 800 mg. Therefore, although the MTD of venetoclax plus R-CHOP was not reached, the RP2D for this combination was determined to be 800 mg on days 4 to 10 of cycle 1 and days 1 to 10 of cycles 2 to 8.
The alternative 5-day dosing regimen for 800 mg venetoclax plus G-CHOP resulted in acceptable toxicity; however, arm B was not expanded to phase 2 because the GOYA study (from which results became available while this study was ongoing) did not demonstrate superiority of G-CHOP over R-CHOP.23
Safety
The most common all-grade AEs in treatment arms A and B together were neutropenia (57.1%) and nausea (53.6%). Table 2, supplemental Table 3, and supplemental Table 4 summarize AEs and serious AEs, in each treatment arm, and supplemental Figure 4 provides an overview of the incidence of cytopenia per cycle. No deaths due to AEs were reported. The most common grade 3/4 AEs were neutropenia, febrile neutropenia, thrombocytopenia, and anemia, reported in 54.2%, 33.3%, 16.7%, and 12.5% of patients, respectively, with R-CHOP, and 59.4%, 25.0%, 37.5%, and 31.3% of patients with G-CHOP (Table 3; supplemental Table 5). Thrombocytopenia occurred without hemorrhage and recovered before the start of the next cycle. The percentage of patients receiving prophylactic granulocyte-colony stimulating factor during cycles 1 to 6 in arm A ranged from 85.7% to 100% (cohort 1), 66.7% to 100% (cohorts 2 and 4), and 87.5% to 100% (cohort 3), and in arm B from 85.7% to 100% (cohorts 1 and 2), 83.3% to 100% (cohorts 3 and 4a), and all patients in cohort 4b.
Table 3.
Grade 3/4 AEs occurring in 2 or more patients in either study arm
| Arm A: VEN + R-CHOP, n (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | Total | |
|---|---|---|---|---|---|---|
| Cohort 1 (n = 7) | Cohort 2 (n = 3) | Cohort 3 (n = 8) | Cohort 4 (n = 6) | (N = 24) | ||
| Neutropenia | 4 (57.1) | 2 (66.6) | 5 (62.5) | 2 (33.3) | 13 (54.2) | |
| Febrile neutropenia | 3 (42.9) | 0 | 2 (25.0) | 3 (50.0) | 8 (33.3) | |
| Thrombocytopenia | 3 (42.9) | 0 | 1 (12.5) | 0 | 4 (16.7) | |
| Anemia | 2 (28.6) | 0 | 0 | 1 (16.7) | 3 (12.5) | |
| Pneumonia | 0 | 0 | 0 | 0 | 1 (4.2) | |
| Diarrhea | 1 (14.3) | 1 (33.3) | 0 | 0 | 2 (8.3) | |
| TLS | 0 | 0 | 1 (12.5) | 0 | 1 (4.2) | |
| Arm B: VEN + G-CHOP, n (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | VEN 800 mg, | Total (N = 32) |
|---|---|---|---|---|---|---|
| Cohort 1 (n = 7) | Cohort 2 (n = 7) | Cohort 3 (n = 6) | Cohort 4A (n = 6) | Cohort 4B (n = 6) | ||
| Neutropenia | 2 (28.6) | 4 (57.1) | 5 (83.3) | 6 (100) | 2 (33.3) | 19 (59.4) |
| Febrile neutropenia | 2 (28.6) | 4 (57.1) | 0 | 1 (16.7) | 1 (16.7) | 8 (25.0) |
| Thrombocytopenia | 2 (28.6) | 2 (28.6) | 2 (33.3) | 4 (66.7) | 2 (33.3) | 12 (37.5) |
| Anemia | 1 (14.3) | 1 (14.3) | 1 (16.7) | 4 (66.7) | 3 (50.0) | 10 (31.3) |
| Pneumonia | 1 (14.3) | 1 (14.3) | 0 | 0 | 0 | 2 (6.3) |
| Diarrhea | 0 | 0 | 0 | 0 | 0 | 0 |
| TLS | 0 | 0 | 1 (16.7) | 1 (16.7) | 0 | 2 (6.3) |
| Lung infection | 0 | 1 (14.3) | 0 | 1 (16.7) | 0 | 2 (6.3) |
Percentages represent the incidence of that specific AE per cohort in each arm.
Three patients (total 5.4%), all with FL, developed laboratory TLS without clinical sequelae on cycle 1 day 4, after the first dose of venetoclax and having shown no TLS after R-CHOP or G-CHOP administration. The patient with grade 4 TLS defined as a DLT (arm A cohort 3 [venetoclax 600 mg]) was able to reinitiate venetoclax 200 mg and escalate back to venetoclax 600 mg without a further occurrence. The 2 patients with grade 3 TLS were in arm B cohorts 3 and 4, assigned to 600 and 800 mg venetoclax. All 3 patients were at high risk for TLS per protocol.
Median duration of treatment was 154 days in arm A (venetoclax plus R-CHOP) and 152 days in arm B (venetoclax plus G-CHOP). Three patients overall received >6 cycles of CHOP, all in arm B: 2 in cohort 4A (venetoclax 800 mg, 10-day administration) and 1 in cohort 4B (venetoclax 800 mg, 5-day administration). More than two-thirds of patients in both treatment arms received ≥90% overall dose intensity (supplemental Table 6) for each drug, with the exception of venetoclax in arm B (22% of patients received ≥90% dose intensity). Venetoclax dosing was modified (reduced or interrupted) per protocol due to AEs for 37 patients (66.1%), with neutropenia (9/37; 24.3%) and febrile neutropenia (8/37; 21.6%) accounting for nearly half of these cases. Overall, a higher percentage of patients in arm B (71.9%) than in arm A (58.3%) experienced AEs leading to venetoclax dose reduction or interruption. Seventeen patients (30.3%) discontinued venetoclax treatment early (16 patients discontinued due to AEs [supplemental Table 7]; 1 patient discontinued due to PD).
Two of 24 patients (8.3%) in arm A and 6 of 32 patients (18.8%) in arm B, respectively, were unable to complete ≥6 cycles of CHOP because of AEs. The rate of dose reduction due to AEs for each study drug is shown in supplemental Table 8. Further details of treatment modifications are available in the supplemental Appendix.
Efficacy
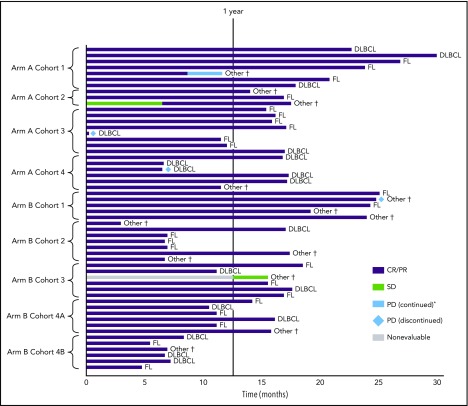
Fifty-six patients were evaluable for end-of-treatment response (Table 4). The median follow-up time for patients with CR/PR at end of treatment (n = 49) was 22 months (range 11.4-36.4). In the intent-to-treat population, the ORR (CR/PR at end of treatment) was 87.5% for the entire study population, similar in both arms, with CR rates of 79.2% and 78.1% in arms A and B, respectively (Table 4). PD occurred in a small number of patients, including 2 patients with 1L DLBCL (6 days from the start of cycle 1 and 42 days after the last dose of venetoclax [cycle 8]) (Figure 2); of these, 1 patient had DE DLBCL and the other had MYC-positive DLBCL.
Table 4.
Disease response at end of treatment by PET/CT
| Arm A: VEN + R-CHOP, n/N (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg, | Total | |
|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4 | |||
| Responders | 6/7 (85.7) | 3/3 (100) | 7/8 (87.5) | 5/6 (83.3) | 21/24 (87.5) | |
| CR | ||||||
| Overall | 6/7 (85.7) | 2/3 (66.7) | 6/8 (75.0) | 5/6 (83.3) | 19/24 (79.2) | |
| DLBCL | 3/3 (100) | 0/0 | 1/2 (50.0) | 4/5 (80.0) | 8/10 (80.0) | |
| FL | 2/3 (66.7) | 1/1 (100) | 5/6 (83.3) | 0/0 | 7/10 (70.0) | |
| Other* | 1/1 (100) | 1/2 (50.0) | 0/0 | 1/1 (100) | 3/4 (75.0) | |
| PR | ||||||
| Overall | 0/7 | 1/3 (33.3) | 1/8 (12.5) | 0/6 | 2/24 (8.3) | |
| DLBCL | 0/3 | 0/0 | 0/2 | 0/5 | 0/10 | |
| FL | 0/3 | 0/1 | 1/6 (16.7)† | 0/0 | 1/10 (10.0) | |
| Other* | 0/1 | 1/2 | 0/0 | 0/1 | 1/4 (25.0) | |
| PD | ||||||
| Overall | 0/7 | 0/3 | 1/8 (12.5) | 1/6 (16.7) | 2/24 (8.3) | |
| DLBCL | 0/3 | 0/0 | 1/2 (50.0) | 1/5 (20.0) | 2/10 (20.0) | |
| FL | 0/3 | 0/1 | 0/6 | 0/0 | 0/10 | |
| Other* | 0/1 | 0/2 | 0/0 | 0/1 | 0/4 | |
| Missing | ||||||
| Overall | 1/7 (14.3) | 0/3 | 0/8 | 0/6 | 1/24 (12.5) | |
| DLBCL | 0/3 | 0/0 | 0/2 | 0/5 | 0/10 | |
| FL | 1/3 (33.3) | 0/1 | 0/6 | 0/0 | 1/10 (10.0) | |
| Other* | 0/1 | 0/2 | 0/0 | 0/1 | 0/4 | |
| Arm B: VEN + G-CHOP, n/N (%) | VEN 200 mg, | VEN 400 mg, | VEN 600 mg, | VEN 800 mg (10 d), | VEN 800 mg (5 d), | Total |
|---|---|---|---|---|---|---|
| Cohort 1 | Cohort 2 | Cohort 3 | Cohort 4A | Cohort 4B | ||
| Responders | 5/7 (71.4) | 6/7 (85.7) | 5/6 (83.3) | 6/6 (100) | 6/6 (100) | 28/32 (87.5) |
| CR | ||||||
| Overall | 3/7 (42.9) | 6/7 (85.7) | 4/6 (66.7) | 6/6 (100) | 6/6 (100) | 25/32 (78.1) |
| DLBCL | 0/0 | 1/1 (100) | 2/2 (100) | 2/2 (100) | 3/3 (100) | 8/8 (100) |
| FL | 2/3 (66.7) | 3/3 (100) | 2/4 (50.0) | 3/3 (100) | 2/2 (100) | 12/15 (80.0) |
| Other* | 1/4 (25.0) | 2/3 (66.7) | 0/1 | 1/1 (100) | 1/1 (100) | 5/10 (50.0) |
| PR | ||||||
| Overall | 2/7 (28.6) | 0/7 | 1/6 (16.7) | 0/6 | 0/6 | 3/32 (9.4) |
| DLBCL | 0/0 | 0/1 | 0/2 | 0/2 | 0/3 | 0/8 |
| FL | 0/3 | 0/3 | 1/3 (33.3) | 0/3 | 0/2 | 1/14 (7.1) |
| Other* | 2/4 (50.0)† | 0/3 | 0/1 | 0/1 | 0/1 | 2/10 (20.0) |
| Missing | ||||||
| Overall | 2/7 (28.6) | 1/7 (14.3) | 1/6 (16.7) | 0/6 | 0/6 | 4/32 (12.5) |
| DLBCL | 0/0 | 0/1 | 0/2 | 0/2 | 0/3 | 0/8 |
| FL | 1/3 (33.3) | 0/3 | 0/2 | 0/3 | 0/2 | 1/13 (7.7) |
| Other* | 1/4 (25.0) | 1/3 (33.3) | 1/1 (100) | 0/1 | 0/1 | 3/10 (30.0) |
If no PET-CT was performed at end of treatment, available CT results were included instead. Responses were assessed by the investigators and central review based on imaging studies and BM examinations using a modified version of the Lugano Classification described by Cheson et al28 with the following modifications: (1) For CR, if the BM was involved by lymphoma prior to treatment, the infiltrate must have cleared on repeat BM biopsy or aspirate; (2) For PET-CT–based PR, CT criteria for PR (or CR) must also be met. Nonmissing end-of-treatment (EOT) CT response was used if EOT PET response was missing. Five (8.9%) patients discontinued early due to toxicity (3 in cohort 1) and have missing EOT response assessments (2 discontinued in cycle 5 and responded in cycle 4; 1 discontinued in cycle 1 and responded during follow-up).
Transformed lymphoma, marginal zone lymphoma, composite lymphoma, and Waldenström macroglobulinemia.
Three patients (1 in arm A, 2 in arm B) with BM involvement at baseline who achieved radiologic CR were classified as having PR due to missing BM data at EOT with all other evidence of CR. Percentages within bars were calculated per cohort; those above bars were calculated per arm.
Figure 2.
Duration of disease response by PET-CT or CT. The combined response for CR/PR can be contributed by either PET-CT or CT, whichever is available. If both PET-CT and CT results were available and discordant, PET-CT results were used. *Patient continued on study therapy after PD. †Five transformed DLBCL (all CR); 1 composite lymphoma with DLBCL and FL portions (response not available); 1 Waldenström macroglobulinemia (PR); 5 marginal zone lymphoma (3 CR, response not available in 2 patients). SD, stable disease.
Across both treatment arms, ORR was 88.9% (all CR) in DLBCL, 83.3% (75.0% CR; 8.3% PR) in FL, and 71.4% (57.1% CR; 14.3% PR) in other histologic types. Responses with venetoclax plus G-CHOP and venetoclax plus R-CHOP by histologic subtype of NHL are shown in Table 4.
Median PFS was not reached in either treatment arm. Patients with DLBCL achieved 1-year PFS rates of 70% and 100% in arms A and B, respectively; for those with FL, PFS was 100% and 90% in arms A and B, respectively. One-year PFS estimates across cohorts are shown in supplemental Figure 5 and by cohort and histology in supplemental Table 9a-b. Kaplan-Meier curves for overall survival according to treatment arm are shown in supplemental Figure 6.
Exploratory data on BCL2/MYC biomarker expression were available for 39 of 56 patients, of whom 74.4% (29/39) were BCL2 positive. Analysis in DLBCL patients revealed that 57% (8/14) were DE (supplemental Table 10a). Nine DLBCL samples were classified by cell-of-origin (COO) subtype (NanoString Technologies) as activated B-cell-like (n = 5), germinal center B-cell-like (n = 3), or unclassified (n = 1). Most DLBCL (87.5%) DE patients achieved CR (supplemental Table 10b). One DLBCL patient had double-hit status according to fluorescence in situ hybridization; this patient achieved a CR and remained progression free as of the data cutoff.
Pharmacokinetics
Pharmacokinetic data from 49 patients indicate that venetoclax exposures (Cmax and area under the curve0-8h) tested at 200, 400, 600, and 800 mg were similar when coadministered with R-CHOP or G-CHOP (eg, 1.43 vs 1.98 μg/mL for Cmax, and 6.82 vs 6.85 h·μg /mL for area under the curve0-8h, following the administration of an 800 mg venetoclax dose on day 4; supplemental Table 11). Venetoclax plasma concentrations peaked at ∼4 to 8 hours, whereas trough concentrations (cycle 1, day 8) remained comparable between patients who received either R or G.
Discussion
CAVALLI is the first study to evaluate the combination of standard CHOP plus an anti-CD20 antibody with the BCL2 inhibitor, venetoclax, in patients with DLBCL and FL. The phase 1b dose-finding part of the study, which was based on preclinical findings, identified the RP2D for venetoclax in combination with standard R-CHOP as 800 mg on days 4 to 10 of cycle 1 and days 1 to 10 of cycles 2 to 8. Although the MTD was not reached, venetoclax doses beyond 800 mg were not planned because less than dose-proportional changes in plasma exposure at high doses were seen in an earlier phase 1 study, where 1200 mg was the highest dose explored.20
The main safety signal to arise in this study overall was for cytopenias; in particular, a higher incidence of febrile neutropenia, thrombocytopenia, and anemia was associated with addition of venetoclax to G- or R-CHOP than has been reported for either G- or R-CHOP.23 Other novel targeted agents combined with chemoimmunotherapy have shown similar increased rates of grade 3/4 hematologic AEs.29,30 Although the rate of cytopenias, including neutropenia and thrombocytopenia, was higher than expected (particularly with venetoclax plus G-CHOP), it should be noted that midcycle laboratory sampling per the protocol may have picked up additional cases. Nonetheless, the dose intensity of the backbone remained intact, and the regimen was tolerated overall. Moreover, the myelosuppressive effects of venetoclax plus R-CHOP or G-CHOP were manageable with prophylaxis, supportive measures, and dose modifications or delays (applied first to venetoclax). There were no fatal AEs. Further assessment of increased myelosuppressive effects in the larger phase 2 part of the CAVALLI study is needed for more accurate prophylactic recommendations.
After modification of initial daily venetoclax dosing to a shorter schedule in response to toxicity, no patients in cohorts 2 to 4 discontinued from arm A (venetoclax plus R-CHOP). Thrombocytopenia appeared to be cumulative over treatment, with average platelet levels decreasing in later cycles, although there was no excess of bleeds, and only 5 patients received platelet transfusions.
Response rates for venetoclax plus R-CHOP or G-CHOP were promising in both DLBCL and FL, comparing favorably with historical rates from studies such as GOYA, GAUDI, and GALLIUM.22,23,31 The high CR rate (88.9%) observed in the poor-prognosis population of DE and BCL2-high DLBCL is a particularly promising finding, but requires validation in a larger group of patients, which is ongoing in the phase 2 portion of this study. Insufficient material was available for COO analysis in some cases, limiting the interpretation of the data in DLBCL patients in this context.
TLS predominantly occurs after therapy in hematologic malignancies and has been reported in the literature in some patients with DLBCL or FL, particularly those with a high tumor burden.32,33 Of note, although all 3 cases of laboratory TLS in CAVALLI occurred in patients who were considered at high risk, events did not occur until after initiation of venetoclax, providing evidence for the increased antitumor properties of the combination over R-/G-CHOP alone. This provides additional support for further testing of this combination in NHL, particularly as effective TLS mitigation strategies for venetoclax therapy are established.34
In conclusion, the phase 1b CAVALLI study demonstrated manageable safety and promising efficacy for the combination of venetoclax with R-/G-CHOP in NHL and established a dosing regimen for venetoclax plus R-CHOP that has been implemented in the ongoing phase 2 study in 1L DLBCL. This will provide further information on the benefit-risk profile of this combination in 1L DLBCL, including higher-risk patient subgroups identified by BCL2, MYC, and COO biomarker analysis.
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors especially thank the patients and their families, investigators, study coordinators, and support staff, and the CAVALLI study team members.
Venetoclax is being developed in collaboration between Genentech Inc and AbbVie. Genentech and AbbVie provided financial support for the study and participated in the design, study conduct, analysis, and interpretation of data as well as the writing, review, and approval of the manuscript. Medical writing support was provided by Tara Miller of Envision Pharma Group and Kate Rijnen of Gardiner-Caldwell Communications and funded by F. Hoffmann-La Roche Ltd.
Footnotes
Qualified researchers may request access to individual patient level data through the clinical study data request platform (www.clinicalstudydatarequest.com). Further details on Roche’s criteria for eligible studies are available at https://clinicalstudydatarequest.com/Study-Sponsors/Study-Sponsors-Roche.aspx. For further details on Roche’s Global Policy on the Sharing of Clinical Information and how to request access to related clinical study documents, see https://www.roche.com/research_and_development/who_we_are_how_we_work/clinical_trials/our_commitment_to_data_sharing.htm.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: F.M., M.M., A.M.P., D. Sampath, G.S., C.K., and A.D.Z. designed the study; C.C., G.C., S.H., E.I., M.K., P.J.L., S.L.G., F.M., K.D.M., M.M., D. Samineni, D. Sampath, G.S., E.S.-G., C.S.T., H.T., A.D.Z., L.H.S., M.E.D.C., and A.G. collected and assembled data; C.C., S.H., C.K., M.K., P.J.L., F.M., M.M., A.M.P., D. Samineni, D. Sampath, G.S., A.S., E.S.-G., H.T., A.D.Z., L.H.S., M.E.D.C., and A.G. analyzed and interpreted data; C.K., M.K., F.M., M.M., D. Sampath, G.S., and A.D.Z. wrote the manuscript; and all authors participated in manuscript development and final approval.
Conflict-of-interest disclosure: A.D.Z. serves on advisory committees/consults for Adaptive Biotechnologies, Amgen, Boehringer Ingelheim, Celgene, Roche-Genentech, Gilead, Hospira, NanoString Technologies, Novartis, Pharmacyclics, Portola Pharmaceuticals, and Takeda and has stock holdings in Adaptive Biotechnologies (options, not exercised); G.S. consults for Roche, Gilead, Celgene, Novartis, and Amgen, receives research funding from Roche, and receives honoraria from Roche, BMS, Merck, Servier, Gilead, Celgene, Novartis, Amgen, and Janssen; C.C. consults for Infinity, receives research funding from Celgene, and receives honoraria from Infinity; S.L.G. serves on advisory boards for Roche, Janssen, and Celgene and receives research funding from Roche and Janssen; L.H.S. consults for Roche/Genentech, Amgen, Gilead, Lundbeck, Seattle Genetics, Janssen, AbbVie, TG Therapeutics, and Celgene, and receives honoraria from Seattle Genetics, AbbVie, and TG Therapeutics; H.T. receives honoraria from Roche, BMS, and Servier and serves on advisory committees for Roche and Karyopharm; G.C. consults for Roche and Celgene and receives honoraria from Roche, Celgene, Gilead, and Janssen; M.E.D.C. serves on advisory boards for Roche, Gilead, and Celgene; A.G. consults for Celgene, Pharmacyclics/J&J, Acerta, Takeda, and Infinity, receives research funding from Celgene, Pharmacyclics/J&J, and Genentech, receives honoraria from Celgene, Takeda, Pharmacyclics/J&J, and Acerta, serves on speakers bureaus for Takeda and Pharmacyclics/J&J, and has received writing support from Takeda; C.S.T. receives honoraria from Roche and AbbVie and research funding from Roche; P.J.L. consults for Roche‐Genentech, Celgene, Jansen‐Cilag, Servier, Takeda, and Mundipharma, receives research support from Roche‐Genentech, and receives travel expenses from Roche‐Genentech; A.M.P. is an employee of AbbVie; A.S. is an employee of Roche; D. Samineni is an employee of Genentech; S.H. is an employee of Roche; E.I. is an employee of Genentech; E.S.-G. is an employee of Genentech; C.K. is an employee and equity holder of Roche; D. Sampath is an employee of Genentech; M.K. is an employee and equity holder of Roche; M.M. was an employee of Genentech and is equity holder of Roche; F.M. consults for Celgene and Gilead and receives honoraria from Celgene, Roche, Janssen, Gilead, and BMS; K.D.M. declares no competing financial interests.
Correspondence: Andrew D. Zelenetz, Memorial Sloan Kettering Cancer Center, 1275 York Ave, Box 330, New York, NY 10065; e-mail: zeleneta@mskcc.org.
REFERENCES
- 1.Hata AN, Engelman JA, Faber AC. The BCL2 family: key mediators of the apoptotic response to targeted anticancer therapeutics. Cancer Discov. 2015;5(5):475-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stenner F, Renner C. Cancer immunotherapy and the immune response in follicular lymphoma. Front Oncol. 2018;8:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zelenetz AD, Chu G, Galili N, et al. . Enhanced detection of the t(14;18) translocation in malignant lymphoma using pulsed-field gel electrophoresis. Blood. 1991;78(6):1552-1560. [PubMed] [Google Scholar]
- 4.Huang JZ, Sanger WG, Greiner TC, et al. . The t(14;18) defines a unique subset of diffuse large B-cell lymphoma with a germinal center B-cell gene expression profile. Blood. 2002;99(7):2285-2290. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal J, Meyer PN, Smith LM, et al. . BCL2 predicts survival in germinal center B-cell-like diffuse large B-cell lymphoma treated with CHOP-like therapy and rituximab. Clin Cancer Res. 2011;17(24):7785-7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson NA, Slack GW, Savage KJ, et al. . Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452-3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valera A, López-Guillermo A, Cardesa-Salzmann T, et al. ; Grup per l’Estudi dels Limfomes de Catalunya i Balears (GELCAB). MYC protein expression and genetic alterations have prognostic impact in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Haematologica. 2013;98(10):1554-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ennishi D, Mottok A, Ben-Neriah S, et al. . Genetic profiling of MYC and BCL2 in diffuse large B-cell lymphoma determines cell-of-origin-specific clinical impact. Blood. 2017;129(20):2760-2770. [DOI] [PubMed] [Google Scholar]
- 9.Bellas C, García D, Vicente Y, et al. . Immunohistochemical and molecular characteristics with prognostic significance in diffuse large B-cell lymphoma. PLoS One. 2014;9(6):e98169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hu S, Xu-Monette ZY, Tzankov A, et al. . MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program. Blood. 2013;121(20):4021-4031, quiz 4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sehn LH, Oestergaard MZ, Trněný M, et al. . Prognostic impact of BCL2 and MYC expression and translocation in untreated DLBCL: results from the phase III GOYA study. Hematol Oncol. 2017;35(S2):131-133. [Google Scholar]
- 12.Friedberg JW. Double-hit diffuse large B-cell lymphoma. J Clin Oncol. 2012;30(28):3439-3443. [DOI] [PubMed] [Google Scholar]
- 13.Rosenthal A, Younes A. High grade B-cell lymphoma with rearrangements of MYC and BCL2 and/or BCL6: Double hit and triple hit lymphomas and double expressing lymphoma. Blood Rev. 2017;31(2):37-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson NA, Savage KJ, Ludkovski O, et al. . Lymphomas with concurrent BCL2 and MYC translocations: the critical factors associated with survival. Blood. 2009;114(11):2273-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davids MS. Targeting BCL-2 in B-cell lymphomas. Blood. 2017;130(9):1081-1088. [DOI] [PubMed] [Google Scholar]
- 16.Tessoulin B, Eveillard M, Lok A, et al. . p53 dysregulation in B-cell malignancies: More than a single gene in the pathway to hell. Blood Rev. 2017;31(4):251-259. [DOI] [PubMed] [Google Scholar]
- 17.AbbVie Inc/Genentech Inc. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/208573s009lbl.pdf. Accessed 20 March 2019.
- 18.AbbVie Deutschland GmBH & Co. Available at: https://www.ema.europa.eu/en/documents/product-information/venclyxto-epar-product-information_en.pdf. Accessed 20 March 2019.
- 19.Seymour JF, Kipps TJ, Eichhorst B, et al. . Venetoclax-rituximab in relapsed or refractory chronic lymphocytic leukemia. N Engl J Med. 2018;378(12):1107-1120. [DOI] [PubMed] [Google Scholar]
- 20.Davids MS, Roberts AW, Seymour JF, et al. . Phase I first-in-human study of venetoclax in patients with relapsed or refractory non-Hodgkin lymphoma. J Clin Oncol. 2017;35(8):826-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mössner E, Brünker P, Moser S, et al. . Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393-4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcus R, Davies A, Ando K, et al. . Obinutuzumab for the first-line treatment of follicular lymphoma. N Engl J Med. 2017;377(14):1331-1344. [DOI] [PubMed] [Google Scholar]
- 23.Vitolo U, Trněný M, Belada D, et al. . Obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone in previously untreated diffuse large B-cell lymphoma. J Clin Oncol. 2017;35(31):3529-3537. [DOI] [PubMed] [Google Scholar]
- 24.Souers AJ, Leverson JD, Boghaert ER, et al. . ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19(2):202-208. [DOI] [PubMed] [Google Scholar]
- 25.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) adopts Consolidated Guideline on Good Clinical Practice in the Conduct of Clinical Trials on Medicinal Products for Human Use. Int Dig Health Legis. 1997;48(2):231-234. [PubMed] [Google Scholar]
- 26.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. [DOI] [PubMed] [Google Scholar]
- 27.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364(19):1844-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheson BD, Fisher RI, Barrington SF, et al. ; United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Younes A, Thieblemont C, Morschhauser F, et al. . Combination of ibrutinib with rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) for treatment-naive patients with CD20-positive B-cell non-Hodgkin lymphoma: a non-randomised, phase 1b study. Lancet Oncol. 2014;15(9):1019-1026. [DOI] [PubMed] [Google Scholar]
- 30.Nowakowski GS, LaPlant B, Macon WR, et al. . Lenalidomide combined with R-CHOP overcomes negative prognostic impact of non-germinal center B-cell phenotype in newly diagnosed diffuse large B-cell lymphoma: a phase II study. J Clin Oncol. 2015;33(3):251-257. [DOI] [PubMed] [Google Scholar]
- 31.Radford J, Davies A, Cartron G, et al. . Obinutuzumab (GA101) plus CHOP or FC in relapsed/refractory follicular lymphoma: results of the GAUDI study (BO21000). Blood. 2013;122(7):1137-1143. [DOI] [PubMed] [Google Scholar]
- 32.Cheson BD, Heitner Enschede S, Cerri E, et al. . Tumor lysis syndrome in chronic lymphocytic leukemia with novel targeted agents. Oncologist. 2017;22(11):1283-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Belay Y, Yirdaw K, Enawgaw B. Tumor lysis syndrome in patients with hematological malignancies. J Oncol. 2017;2017:9684909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kater AP, Seymour JF, Hillmen P, et al. . Fixed duration of venetoclax-rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post-treatment follow-up of the MURANO phase III study. J Clin Oncol. 2019;37(4):269-277. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.