Abstract
In this issue of Blood, Ganuza et al describe decreased clonal contributions to hematopoiesis during steady-state aging and after transplant-induced stress in mice. Using an in vivo Confetti tracking model, they attribute murine clonal dominance to mutations in genes not previously associated with clonal hematopoiesis.1
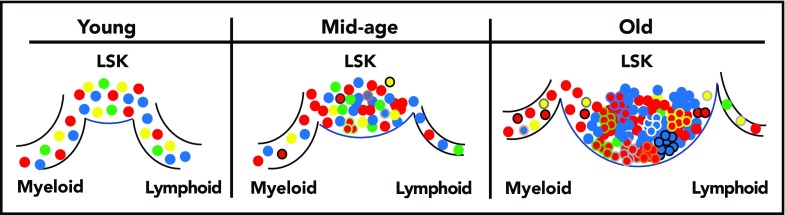
Natural aging affects the clonal diversity within the primitive compartment as well as the clones that contribute to differentiation. Young Confetti-labeled HSCs and multipotent progenitors within the most primitive compartment (LSK) give rise to myeloid and lymphoid cells in a balanced output, with significant clonal diversity contributing to the differentiated cells. By mid-age, the number of LSK cells increases, but the overall clonal diversity begins to wane and there are changes in the clones contributing to differentiation. By 26 months, there is a large expansion of the LSK compartment with even greater loss of clonal diversity. Clonal contribution to the myeloid and lymphoid lineages is decreased but does not reflect the clonal distribution shifts in the LSK compartment, suggesting that many LSK cells may be unable to significantly contribute to downstream hematopoiesis. This loss of potential is also correlated with accumulation of clones that have acquired somatic mutations (represented by different colored cell borders).
Aging hematopoiesis is associated with a decline in lymphoid potential but a significant increase in the number of phenotypic hematopoietic stem cells (HSCs) in both murine and human studies.2 The expanded HSC compartment in humans also corresponds to clonal hematopoiesis, in which there is selective expansion of clones contributing to hematopoiesis. Clonal hematopoiesis has now been described in several human cohorts, and somatic mutations in epigenetic modifiers such as DNMT3A, TET2, and ASXL1 have been identified as key genes associated with the expanded presence of specific clones.3 Experiments in the murine system have demonstrated loss of potential for functional reconstitution as well as increased bias toward myeloid lineages from aged HSCs with an expansion of myeloid-biased HSCs; however, these studies relied heavily on transplantation stress to assay the functional potential of the aged stem cells. Thus, it remains unclear whether native hematopoiesis in the murine system recapitulates age-associated alterations such as clonal hematopoiesis seen in humans.
Ganuza et al used a Cre-recombinase inducible multicolor allele system (Confetti) they described previously4 to label cells at various stages of development, and examined the clonal color distribution of hematopoietic progenitor cells (subsets of ckit+ cells) or terminally differentiated peripheral blood cells during aging. There was a reduction in the clonal diversity starting at mid-age (16 months) that was pronounced in aged mice (26 months old). The most strikingly affected compartments were the most primitive stem cells (HSCs) and multipotent progenitors, suggesting an accumulation of clones that acquired selective self-renewal advantages because the HSC compartment size expands concomitant with the loss of clonal diversity (see figure). Although decreases in clonal distributions were also seen in the peripheral blood of aged mice, the magnitude of clonal diversity loss was not as robust and did not seem to directly correspond to the changes in clonal distributions of the primitive cells. One interpretation of these data is aged murine HSCs have selectively expanded clones that preferentially self-renew, which drives the expanded size of the compartment, whereas a different group of aged HSCs contribute to downstream differentiation (see figure). Analysis of Confetti color correlation between the primitive compartment and more differentiated cells shows age-associated erosion, which supports restriction of functional potential within the expanded HSC compartment. Single-cell analysis of aged HSCs also suggests increased functional constraint, because significant loss of multilineage output was seen in myeloid-differentiation–promoting media compared with young HSCs.
The results from the Ganuza et al study, similar to those in studies of barcoded HSCs transplanted into unconditioned hosts,5 transposon-labeled cells,6 and single-color genetic labeling,7 show the contribution of stem cells to bone marrow and blood is largely polyclonal in native hematopoiesis. In addition, those results provide support for a model in which clones contributing to differentiation during aging are not permanent, but instead fluctuate throughout life (see figure). However, because of limitations of the Ganuza et al model, which requires pooled-cell color analysis, the distinction between an individual clone that contributes at one point during the lifespan and is then exhausted versus a unique clone recruited at multiple time points throughout life cannot be ascertained.
Although the authors were somewhat restricted by having to use pooled clones because of the limitations of the Confetti system, they present an elegant study of native, aged hematopoiesis and effects of stress. The murine model shared similarities with age-associated human clonal hematopoiesis, but this study highlights features that are perhaps unique to aged human hematopoiesis. Several groups have reported that mutations in murine Dnmt3a and Tet2 lead to clonal expansion of HSCs and drive significant self-renewal advantages8,9 (similar to clonal hematopoiesis of indeterminate potential), but mutations in these epigenetic regulators were not detected in the bone marrow analyzed in the Ganuza et al study. This does not exclude the possibility that these mutations were present, but they do not seem to be the dominant mutations that accrue in aged murine hematopoiesis. This might indicate limitations of the C57BL/6 mouse as a model of human aging or, alternatively, it might provide novel gene candidates that drive clonal expansion restricted to less-accessible human progenitor cell compartments that reside in the bone marrow. The authors also introduced replication stress resulting from transplants that caused clonal collapse, which may be similar to that reported in geriatric individuals with very few clones contributing to overall hematopoiesis.10 Thus, it will be interesting to determine whether similar clonal collapse features are seen in geriatric mice.
Footnotes
Conflict-of-interest disclosure: I.B. declares no competing financial interests.
REFERENCES
- 1.Ganuza M, Hall T, Finkelstein D, et al. . The global clonal complexity of the murine blood system declines throughout life and after serial transplantation. Blood. 2019;133(18):1927-1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beerman I, Maloney WJ, Weissmann IL, Rossi DJ. Stem cells and the aging hematopoietic system. Curr Opin Immunol 2010;22(4):500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bowman RL, Busque L, Levine RL. Clonal hematopoiesis and evolution to hematopoietic malignancies. Cell Stem Cell. 2018;22(2):157-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganuza M, Hall T, Finkelstein D, Chabot A, Kang G, McKinney-Freeman S. Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny. Nat Cell Biol. 2017;19(10):1153-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu R, Czechowicz A, Seita J, Jiang D, Weissman IL. Clonal-level lineage commitment pathways of hematopoietic stem cells in vivo. Proc Natl Acad Sci USA. 2019;116(4):1447-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sun J, Ramos A, Chapman B, et al. . Clonal dynamics of native haematopoiesis. Nature. 2014;514(7522):322-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Busch K, Klapproth K, Barile M, et al. . Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature. 2015;518(7540):542-546. [DOI] [PubMed] [Google Scholar]
- 8.Moran-Crusio K, Reavie L, Shih A, et al. . Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeong M, Park HJ, Celik H, et al. . Loss of Dnmt3a immortalizes hematopoietic stem cells in vivo. Cell Reports. 2018;23(1):1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holstege H, Pfeiffer W, Sie D, et al. . Somatic mutations found in the healthy blood compartment of a 115-yr-old woman demonstrate oligoclonal hematopoiesis. Genome Res. 2014;24(5):733-742. [DOI] [PMC free article] [PubMed] [Google Scholar]